Abstract
Background
Insomnia is a common problem in modern society. It is associated with reduced quality of life and impairments in physical and mental health. Listening to music is widely used as a sleep aid, but it remains unclear if it can actually improve insomnia in adults. This Cochrane Review is an update of a review published in 2015.
Objectives
To assess the effects of listening to music on sleep in adults with insomnia and to assess the influence of specific variables that may moderate the effect.
Search methods
For this update, we searched CENTRAL, MEDLINE, Embase, nine other databases and two trials registers up to December 2021. In addition, we handsearched reference lists of included studies, and contacted authors of published studies to identify additional studies eligible for inclusion, including any unpublished or ongoing trials.
Selection criteria
Randomised controlled trials comparing the effects of listening to music with no treatment or treatment as usual (TAU) in adults complaining of sleep difficulties.
Data collection and analysis
Two review authors independently screened records for eligibility, selected studies for inclusion, extracted data and assessed risk of bias of the included studies. We assessed the certainty of the evidence using GRADE. The primary outcomes were sleep quality, insomnia severity, sleep‐onset latency, total sleep time, sleep interruption, sleep efficiency and adverse events. Data on the predefined outcome measures were included in meta‐analyses when consistently reported by at least two studies that were homogeneous in terms of participants, interventions and outcomes. We undertook meta‐analyses using random‐effects models.
Main results
We included 13 studies (eight studies new to this update) comprising 1007 participants. The studies examined the effect of listening to prerecorded music daily, for 25 to 60 minutes, for a period of three days to three months. The risk of bias within the studies varied, with all studies being at high risk of performance bias, because of limited possibilities to blind participants to the music intervention. Some studies were at high risk of detection bias or other bias. Four studies reported funding from national research councils, three studies reported financial support from university sources and one study reported a grant from a private foundation. Five studies did not report any financial support.
At the end of the intervention, we found moderate‐certainty evidence for improved sleep quality measured with the Pittsburgh Sleep Quality Index (PSQI) in themusic groups compared to no intervention or TAU (mean difference (MD) −2.79, 95% confidence interval (CI) −3.86 to −1.72; 10 studies, 708 participants). The PSQI scale ranges from 0 to 21 with higher scores indicating poorer sleep. The size of the effect indicates an increase in sleep quality of the size of about one standard deviation in favour of the intervention. We found no clear evidence of a difference in the effects of listening to music compared to no treatment or TAU on insomnia severity (MD −6.96, 95% CI −15.21 to 1.28; 2 studies, 63 participants; very low‐certainty evidence). We found low‐certainty evidence that, compared to no treatment or TAU, listening to music may reduce problems with sleep‐onset latency (MD −0.60, 95% CI −0.83 to −0.37; 3 studies, 197 participants), total sleep time (MD −0.69, 95% CI −1.16 to −0.23; 3 studies, 197 participants) and sleep efficiency (MD −0.96, 95% CI −1.38 to −0.54; 3 studies, 197 participants), but may have no effect on perceived sleep interruption (MD −0.53, 95% CI −1.47 to 0.40; 3 studies, 197 participants). In addition, three studies (136 participants) included objective measures of sleep‐onset latency, total sleep time, sleep efficiency and sleep interruption and showed that listening to music may not improve these outcomes compared to no treatment or TAU. None of the included studies reported any adverse events.
Authors' conclusions
The findings of this review provide evidence that music may be effective for improving subjective sleep quality in adults with symptoms of insomnia. More research is needed to establish the effect of listening to music on other aspects of sleep as well as the daytime consequences of insomnia.
Plain language summary
Music for insomnia in adults
Review question
This review assessed the effects of listening to music on insomnia (sleep problems) in adults and the impact of factors that may influence the effect.
Key messages
We found a beneficial effect of music on sleep quality. For all the other outcomes, we did not find enough good‐quality evidence as there were too few participants and the people doing the scoring of the data were aware of the treatment.
What is insomnia?
Worldwide, millions of people experience insomnia. People can have difficulties getting to sleep, staying asleep or may experience poor sleep quality.
Poor sleep affects people's physical and mental health. The consequences of poor sleep are costly, for both individuals and society. Many people listen to music to improve their sleep, but the effect of listening to music is unclear.
What did we do?
We searched electronic databases to identify relevant studies. We included 13 studies with 1007 participants. The studies compared the effect of listening to music to treatment as usual or no treatment. Treatment as usual could be sleep hygiene education (learning a set of rituals to help with sleep) or standard care for participants with insomnia related to chronic medical conditions. The studies examined the effect of listening to prerecorded music daily, for 25 to 50 minutes, for three days to three months. Seven of the included studies reported funding from national research councils or university sources, and one study reported funding from a private foundation. Five studies did not report any funding sources.
What did we find?
Ten studies measured sleep quality, and the results showed that music probably facilitates a large improvement in the quality of sleep compared to no treatment or treatment as usual. We do not know if listening to music has an effect on the severity of insomnia (difficulty in falling or staying asleep) or the number of times a person wakes up (broken sleep) compared to no treatment or treatment as usual. Listening to music may improve slightly sleep‐onset latency (how quickly a person falls asleep), sleep duration (length of time a person is asleep) and sleep efficiency (amount of a time a person is asleep compared to the total time spent in bed), compared to no treatment or treatment as usual. None of the studies reported any negative effects caused by listening to music.
What are the limitations of the evidence?
The quality of evidence from the 10 studies that examined sleep quality was moderate. Our confidence in the evidence for the quality of sleep is only moderate because the people in the studies were aware of which treatment they were getting and the people scoring the data were also sometimes aware of which treatment the participants were getting, which could introduce bias. We have little confidence in the evidence for the severity of insomnia because the studies were very small and were done in different types of people who knew which treatment they were getting. Our confidence in the evidence on sleep‐onset latency, sleep duration and sleep efficiency is low because the studies used very different methods to measure these outcomes, and the people in the studies were aware of the nature of the treatment. We have little confidence in the evidence on sleep interruption because the studies used different methods and showed different results. Furthermore, the participants in the studies knew which treatment they were getting.
Future studies should assess other aspects of sleep as well as measures of daytime functioning, such as mood, fatigue, concentration, and quality of life.
How up to date is this evidence?
The evidence is current to 31 December 2021.
Summary of findings
Summary of findings 1. Listening to music compared to no treatment or treatment as usual for adults with insomnia.
| Listening to music compared to no treatment or treatment as usual for adults with insomnia | ||||||
| Patient or population: adults with insomnia Settings: home, sleep laboratory or rehabilitation centre Intervention: listening to music Comparison: no treatment (including waitlist controls) or TAU (i.e. sleep hygiene education or standard care for participants with insomnia related to chronic medical conditions) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment or TAU | Listening to music | |||||
|
Sleep quality – immediately post‐treatment
Assessed with: PSQIa Follow‐up: 14–90 days |
The mean score in the control groups ranged from 4.8 to 14.22 | The mean score in the intervention groups was 2.79 lower (3.86 lower to 1.72 lower) | — | 708 (10 RCTs) | ⊕⊕⊕⊝ Moderateb | A lower score indicates better sleep quality (i.e. fewer sleep problems). The change is approaching the size of 1 standard deviation (SMD −0.86, CI −0.54 to −1.19), which is considered a clinically relevant change. |
|
Insomnia severity – immediately post‐treatment Assessed with: ISIc Follow‐up: 21–42 days |
The mean score in the control groups ranged from 16.5 to 19.9 | The mean score in the intervention groups was 6.96 lower (15.21 lower to 1.28 higher) | — | 63 (2 RCTs) |
⊕⊝⊝⊝
Very lowb,d,e |
A lower score indicates less severe insomnia. |
|
SOL – immediately post‐treatment
Assessed with: PSG and PSQIa subscale Follow‐up: 3–21 days for PSG measures and 21–90 days for PSQI subscale |
3 studies (136 participants) measuring objective SOL with PSG found no effect of the intervention. | — | 457 (8 RCTs) | ⊕⊕⊝⊝ Lowb,f | Data from 2 studies reporting objective SOL were presented in a format that did not allow for inclusion in a meta‐analysis. |
|
| 5 studies (321 participants) measured SOL with the PSQI subscale, and 4 of these found shortened SOL with the music intervention. The mean score in the intervention group was 0.60 lower (0.83 lower to 0.37 lower; 3 studies, 197 participants). | ||||||
|
Total sleep time – immediately post‐treatment
Assessed with: PSG and PSQIa subscale Follow‐up: 3–21 days for PSG measures and 21–90 days for PSQI subscale |
3 studies (136 participants) measuring total sleep time with PSG found no effect of the intervention. | — | 611 (9 RCTs) | ⊕⊕⊝⊝ Lowb,f | Data from 2 studies reporting objective total sleep time were presented in a format that did not allow for inclusion in a meta‐analysis. | |
| 5 studies (321 participants) used the PSQI subscale, and 4 studies found significant improvement in sleep duration. The mean score in the intervention group was 0.69 lower (1.16 lower to 0.23 lower; 3 studies, 197 participants). 1 study (154 participants) reported improved sleep duration using a categorical approach. | ||||||
|
Sleep interruption – immediately post‐treatment
Assessed with: PSG and PSQIa subscale Follow‐up: 3–21 days for PSG measures and 21–90 days for PSQI subscale |
3 studies (136 participants) measuring wake time after sleep and number of awakenings with PSG found no effect of the intervention. | — | 457 (8 RCTs) | ⊕⊝⊝⊝ Very lowb,f,g | Data from 2 studies reporting objective sleep interruption were presented in a format that did not allow for inclusion in a meta‐analysis. |
|
| 5 studies (321 participants) used the PSQI subscale. 3 studies found significant reduction in experienced sleep disturbance, whereas 2 studies found no effect. A meta‐analysis found no effect (MD −0.53, 95% CI −1.47 to 0.40; 3 studies, 197 participants). | ||||||
|
Sleep efficiency – immediately post‐treatment
Assessed with: PSG and PSQIa subscale Follow‐up: 3–21 days for PSG measures and 21–90 days for PSQI subscale |
3 studies (136 participants) measuring sleep efficiency with PSG found no effect of the intervention. | — | 457 (8 RCTs) | ⊕⊕⊝⊝ Lowb,f | Data from 2 studies reporting objective sleep efficiency were presented in a format that did not allow for inclusion in a meta‐analysis. |
|
| 5 studies (321 participants) used the PSQI subscale, and found improved sleep efficiency with the intervention. The mean score in the intervention group was 0.96 lower (1.38 lower to 0.54 lower; 3 studies, 197 participants). | ||||||
| Adverse events | None of the 10 included studies reported any adverse events. | — | — | — | — | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; ISI: Insomnia Severity Index; MD: mean difference; PSG: polysomnography; PSQI: Pittsburgh Sleep Quality Index; RCT: randomised controlled trial; SMD: standardised mean difference; SOL: sleep‐onset latency; TAU: treatment as usual. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aPSQI. Global score 0 indicates good sleep quality and 21 indicates severe sleep problems. Clinical cut off greater than 5 (Buysse 1989). Seven subscales including sleep latency, sleep duration, sleep efficiency and sleep disturbance. bDowngraded one level due to risk of bias: no blinding of participants and personnel (not possible), and sometimes no or unclear blinding of outcome assessment. cISI. Score from 0 to 28 with higher scores indicating more severe insomnia. dDowngraded one level due to inconsistency: I2 = 95%. eDowngraded one level due to imprecision: small number of participants and CIs include both benefit and harm. fDowngraded one level due to inconsistency: data were too heterogeneous to pool in a statistical synthesis. gDowngraded one level due to inconsistency: high heterogeneity and different directions of the effect.
Background
Description of the condition
Sleep difficulties are highly prevalent in modern society with around 27% of the general population reporting symptoms of insomnia (Perlis 2020). Insomnia can be defined as a subjective complaint of disturbed sleep in the presence of adequate opportunity and circumstance for sleep (NIH 2005). It is characterised by dissatisfaction with the quality, duration or continuity of sleep, such as problems falling asleep, maintaining sleep, early morning awakenings or complaints of non‐restorative sleep (Morin 2013). When sleep difficulties persist in a severe form, they are characterised as insomnia disorder, which is the most common sleep disorder. To fulfil the diagnostic criteria of insomnia disorder, one must experience sleep difficulties at least three nights per week for a minimum of three months with associated impairments in daytime functioning or well‐being (AASM 2014; APA 2013). As such, insomnia disorder can be considered a subgroup within the insomnia definition stated above.
Insomnia is common in people with medical or psychiatric illness and trials have found consistent relationships between insomnia and depression, anxiety disorders, and other psychiatric disorders, as well as substance abuse and dependence. Furthermore, insomnia is associated with a number of somatic problems such as decreased immune functioning (Taylor 2003), cardiovascular disorders, hypertension, chronic pain, breathing difficulties, and gastrointestinal and urinary problems (Taylor 2007). Insomnia itself can have a number of negative daytime consequences and it is well recognised that people with insomnia experience impairments in everyday life such as fatigue and greater irritability (Riedel 2000; Shekleton 2010). People with insomnia report significantly lower quality of life than those without insomnia, and the reduction in quality of life is correlated with symptom severity (Léger 2001). Insomnia affects occupational functioning and social relations and is associated with higher work absenteeism and increased risk of accidents, and therefore represents a condition with great costs for both the individual and society (Walsh 2004).
Estimates of the prevalence of insomnia vary according to the definitions used. One review of epidemiological trials revealed that about one third of the general population experiences symptoms of insomnia, such as difficulties initiating or maintaining sleep (Ohayon 2002). When adding daytime consequences to the definition of insomnia, the prevalence rate drops to about 9% to 15%. Using the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5) criteria for insomnia disorder, there is a prevalence of 10.8% (Chung 2015). Thus, insomnia disorder can be considered a subgroup within the larger group of people experiencing insomnia. The prevalence of insomnia increases with age and is generally higher in women (NIH 2005).
Description of the intervention
When individuals experience insomnia, they most often do not seek professional help (Léger 2008). Instead, many people use various self‐help strategies to improve sleep. Some use herbal or dietary products to facilitate sleep, others follow sleep hygiene advice, exercise or relaxation strategies (Aritake‐Okada 2009; Morin 2006; Urponen 1988). If the insomnia persists and turns into insomnia disorder with associated daytime impairments, the likelihood of seeking professional help increases, and the most common step is to consult a general practitioner (Morin 2006). Healthcare professionals may offer sleep hygiene advice, pharmacotherapy, or psychological and behavioural interventions as treatments for insomnia disorder. Despite widespread use of hypnotics, cognitive‐behavioural therapy for insomnia (CBT‐I) is recommended as first‐line treatment for insomnia disorder (Riemann 2017). CBT‐I usually consists of several elements, including sleep hygiene, relaxation training, stimulus control therapy, sleep restriction therapy and cognitive therapy. In spite of good results, psychological and behavioural treatments appear to be underutilised, perhaps because they require considerable time and effort for the patient (Krystal 2004). Furthermore, there is a problem of availability, with relatively few well‐trained professionals in the field (Wilson 2019). Online solutions are being tested, but are still not broadly available. Therefore, pharmacotherapeutic interventions are still commonly used (NIH 2005). Benzodiazepine receptor agonists have been approved for the treatment of insomnia disorder by the US Food and Drug Administration (FDA; FDA 2022), and trials report beneficial short‐term effects of these agents (Riemann 2017). With the exception of eszopiclone, approved use is limited to 35 days or less (NIH 2005). However, there are some concerns about the long‐term use of these medications, due to risk of abuse, dependence, and adverse effects such as residual daytime sedation, cognitive impairments, and reduced motor co‐ordination. Furthermore, the use of hypnotics has been associated with increased mortality (Frandsen 2014; Jennum 2015; Jennum 2018; Kripke 2012).
Given the current limitations of psychological and pharmacological treatments for insomnia disorder and the tendency of most people to not seek professional treatment when experiencing difficulties initiating or maintaining sleep, self‐help strategies are the most common approach to combat insomnia in adults. Among these, listening to music is commonly used by adults to improve sleep (Aritake‐Okada 2009; Morin 2006; Urponen 1988), and a simple Google search on 'music' and 'sleep' reveals a huge market of music that is promoted for its sleep‐inducing properties. However, the efficiency of music as an intervention for insomnia remains unclear. Music is used as a therapeutic intervention in a number of areas, including pain relief (Cepeda 2006), psychiatry (Aalbers 2017; Geretsegger 2017), neuro‐rehabilitation (Magee 2017), and for improving psychological outcomes in medical conditions such as cancer and heart disease (Bradt 2021; Bradt 2013). Experimental and clinical studies show that music can affect autonomous nervous system functioning (Hodges 2009), as well as psychological factors such as mood and attention (Garza‐Villarreal 2014; Juslin 2001); as such, it may potentially impact sleep (see How the intervention might work). Research on the impact of music on sleep has evolved since the early 2000s, and methods for applying music listening to improve sleep quality vary across trials. Generally, the intervention involves the use of prerecorded music in relation to sleep initiation. Music listening can be used passively, or it can be used actively with specific instructions (e.g. relaxation instructions). The duration of the intervention period and the time spent listening to music may vary. The choice of music may be determined by the researcher or by the participants themselves.
How the intervention might work
Music has been found to influence human beings on many levels (Juslin 2001), and the impact of music listening on sleep has been attributed to different mechanisms. Several authors argue that improvement of sleep is obtained because slow soothing music enhances relaxation (Deshmukh 2009; Hernández‐Ruiz 2005; Jespersen 2019; Lai 2005). This suggestion is substantiated by trials showing reduced levels of cortisol as an effect of music listening (Koelsch 2011; Nilsson 2009), and changes in autonomous measures such as heart rate and blood pressure (Korhan 2011; Su 2013; Trappe 2010). These trials show that music can affect various physiological measures that reflect autonomic nervous system activity, and as such, slow soothing music may lead to a decrease in sympathetic arousal and thus improve sleep (Su 2013). From a psychological perspective, trials have shown that listening to music can reduce anxiety and stress responses (Dileo 2007; Zhang 2012), which can lead to greater relaxation and improvement of sleep. Another possible mechanism for the effect of music on sleep is the distracting power of music. Hernández‐Ruiz 2005 suggests that music can function as a focal point of attention that distracts from stressful thoughts and thereby improves sleep. Other researcher‐proposed mechanisms include entrainment, masking of noise, enjoyment, expectations (positive or negative beliefs about the effect) and conditioning (building up an association between the music and sleep) (Dickson 2019; Dickson 2020). However, the relevance of the mechanisms have not yet been tested empirically. A number of individual factors are also likely to influence the music experience, such as age and sex (Juslin 2011; Nieminen 2012), music preference (Vuust 2010), musical training (Brattico 2009; Vuust 2006), and culture (Hargreaves 1997). Therefore, different effects may be found depending on the type of music used, the aetiology of insomnia symptoms, and the length and duration of the intervention.
Why it is important to do this review
Music is commonly used to relieve sleep problems and the use of music as a non‐pharmacological intervention offers potential advantages of easy administration, low cost and safety. Clinical trials have been performed to investigate the effect of music on sleep (Amiri 2019; Cai 2015; Chan 2010; Deshmukh 2009; Harmat 2008; Hérnandez‐Ruíz 2005; Huang 2017; Jespersen 2012; Koenig 2013; Kullich 2003; Lazic 2007; Shobeiri 2016; Street 2014; Wang 2016), but it remains unclear if the existing evidence is rigorous enough to reach conclusions about the general efficacy of the intervention. A systematic review is needed to establish the efficacy of music listening for improvement of sleep quality and thereby refute or validate the popular belief that music is helpful to promote sleep. This review is an update of the Cochrane Review published in 2015 (Jespersen 2015). The first version included only six trials. As there are several new RCTs published, an update has become necessary to provide a solid and up‐to‐date overview of the effect of music for insomnia.
Objectives
To assess the effects of listening to music on sleep in adults with insomnia and to assess the influence of specific variables that may moderate the effect.
Methods
Criteria for considering studies for this review
Types of studies
The methods of this review were prespecified in the protocol (Jespersen 2013). See Differences between protocol and review, for information on any adjustments to the methods.
We considered randomised controlled trials (RCTs) for inclusion in the review. Since it is not possible to blind participants to the treatment (music), we included unblinded or single‐blinded trials.
Types of participants
We included adults with a complaint of sleep difficulties, as documented by standardised measures (e.g. Pittsburgh Sleep Quality Index (PSQI; Buysse 1989), or reports or diaries kept by participants, relatives or other informants; or poor sleep documented by objective measures (e.g. polysomnography (PSG) or actigraphy); or individuals diagnosed with an insomnia disorder by standard diagnostic criteria, such as the International Classification of Diseases (ICD; WHO 1992), the Diagnostic and Statistical Manual of Mental Disorders (DSM; APA 2013) or International Classification of Sleep Disorders (ICSD; AASM 2014).
Types of interventions
We included any intervention that comprised listening to prerecorded music with or without relaxation instructions. The intervention could be self‐administered or administered by research or clinical personnel. Interventions included music listening compared with a no music control group or treatment as usual (TAU), and music listening added to TAU compared to TAU alone. No intervention control groups could be waitlist controls, and TAU could be sleep hygiene education or standard care for participants with insomnia related to chronic medical conditions.
Types of outcome measures
Primary outcomes
Our outcomes of interest were sleep‐ and insomnia‐related symptoms as measured by sleep diaries, PSG, actigraphy or by standardised scales for the assessment of sleep and insomnia symptoms (e.g. PSQI or Insomnia Severity Index (ISI)). Furthermore, to establish the safety of the intervention, we considered the reporting of adverse events as a primary outcome. The primary outcomes were:
sleep quality;
insomnia severity;
sleep‐onset latency;
total sleep time;
sleep interruption (number of awakenings and waking after sleep onset);
sleep efficiency (percent of time in bed spent asleep);
adverse events (as reported by trialists; e.g. discomfort or hearing loss).
Secondary outcomes
Secondary outcomes of interest were waking‐related correlates and daytime consequences of insomnia. The relevant measures were:
-
psychological outcomes:
depression;
anxiety;
quality of life;
-
physical outcomes:
fatigue;
daytime sleepiness;
pain;
-
physiological outcomes:
heart rate;
heart rate variability;
blood pressure.
We included trials that measured psychological outcomes by standardised questionnaires with established reliability and validity (e.g. Beck Depression Inventory (BDI; Beck 1996), State‐Trait Anxiety Inventory (STAI; Spielberger 1983), 36‐item Short‐Form (SF‐36) health survey (Ware 1992)). We included trials that measured physical outcomes with standardised procedures such as the Multiple Sleep Latency Test (MSLT) or validated rating scales. We included trials that measured physiological outcomes with standardised procedures such as an electrocardiogram (ECG).
We considered the trial period and follow‐up as described in the included trials. When assessing outcomes in relation to time points, we grouped the data as: immediate postintervention, short‐term (postintervention to one month), medium‐term (between one and three months' follow‐up), and long‐term (more than three months' follow‐up) effects.
We reported all primary outcomes in Table 1.
Search methods for identification of studies
For this update, we revised the previous search strategies to take account of new indexing terms in MEDLINE, and included some additional free‐text terms (see Differences between protocol and review). Following the guidelines in Chapter 4 of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2021), we searched each source from inception, and applied no restrictions on date, language, or publication status when searching for trials or when deciding on trial inclusion. Details of the previous search strategies are available in Jespersen 2015.
Electronic searches
The Cochrane Information Specialist for Developmental Psychosocial and Learning Problems ran the searches for this update in January 2021 and top‐up searches in December 2021 for the electronic databases listed below.
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 12) part of the Cochrane Library, and includes the Cochrane Developmental Psychosocial and Learning Problems Group Specialised Register. Searched 13 December 2021.
MEDLINE Ovid (1946 to November week 5 2021).
MEDLINE In‐Process & Other Non‐Indexed Citations Ovid (1946 to 10 December 2021).
MEDLINE Epub Ahead of Print Ovid (10 December 2021).
Embase Ovid (1974 to 10 December 2021).
CINAHL EBSCOhost (1937 to 13 December 2021).
APA PsycINFO Ovid (1806 to November week 5 2021).
Web of Science Clarivate (Science Citation Index Expanded, Social Sciences Citation Index, Arts and Humanities Citation Index, Conference Proceedings Citation Index – Science, and Conference Proceedings Citation Index – Social Science and Humanities) (1970 to 13 December 2021).
SCOPUS Elsevier (all available years). Searched 13 December 2021.
RILM Abstracts of Music Literature EBSCOhost (Répertoire International de Littérature Musicale; 1969 to 13 December 2021).
Cochrane Database of Systematic Reviews (CDSR; 2021 Issue 12), part of the Cochrane Library. Searched 13 December 2021.
Epistemonikos (www.epistemonikos.org). Searched 13 December 2021.
ClinicalTrials.gov (ClinicalTrials.gov). Searched 13 December 2021.
World Health Organization International Clinical Trials Registry Platform (trialsearch.who.int/). Searched 13 December 2021.
The search strategies for this update are reported in Appendix 1.
Searching other resources
We checked relevant reviews and the reference lists of the included studies to identify additional trials missed by the electronic searches. We also contacted authors and experts in the field for additional information on unpublished trials or to request additional data.
For this update, we did not handsearch specialist journals, since most are now indexed in the electronic databases. Furthermore, our handsearch for the first version did not yield any additional trials.
Data collection and analysis
Selection of studies
Two review authors (KVJ and VPN) independently screened all titles and abstracts using Covidence. We retrieved all papers for which the title or abstract referred to an RCT on music and sleep in full text. In cases where there was insufficient information in the title or abstract to determine the relevance of a paper, we retrieved the full text. Both review authors independently reviewed the full‐text papers against the previously defined inclusion criteria (Criteria for considering studies for this review), to assess the trial's eligibility for inclusion. We discussed disagreements until we reached consensus. We recorded excluded articles and the reason for their exclusion (see Characteristics of excluded studies table). We reported the selection process in a PRISMA diagram (Page 2021).
Data extraction and management
Two review authors (KVJ and VPN), who were blinded to each other's assessment, extracted data using Covidence. The Covidence template was adjusted and piloted prior to use, to ensure it matched the nature of our outcomes. We resolved disagreements by consensus. If outcome data were not available, we contacted the authors of the trial.
From each trial, we extracted the following information.
1. General information
Author
Year of publication
Title
Journal (title, volume, pages) or if unpublished source
Country
Language of publication
2. Trial design
Design (e.g. parallel or cross‐over design)
Method of randomisation (and concealment)
Nature of the control group (e.g. no treatment or TAU)
Losses to follow‐up
Blinding of trial evaluators
Washout period in cross‐over design
Inclusion criteria
Exclusion criteria
3. Participants
Total sample size
Number in experimental group (number randomised and number completed)
Number in control group (number randomised and number completed)
Age
Gender
Ethnicity
Diagnosis
Comorbidities
Sleep quality (and reason for poor sleep)
Duration of disorder
Previous or additional treatments
4. Intervention
Type of music employed (characteristics)
Music selection (selected by participant or researcher)
Who provided the music (participant or research personnel)
Length and frequency of intervention sessions
Intervention period (duration of intervention)
How participants were exposed to music (e.g. headphones or loudspeakers)
Listening instructions
5. Outcomes
Methods of sleep assessment
Secondary outcome measures
Pretest means and post‐test means or change scores and standard deviations (SD), for all groups for all outcomes in Primary outcomes and Secondary outcomes
Baseline differences
Follow‐up period
Assessment of risk of bias in included studies
Two review authors (KVJ and VPN) independently assessed the risk of bias using the tool described (and the criteria outlined) in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). They solved disagreements by discussion with the fourth review author (PV). When information for evaluating methodological criteria was absent, we contacted the study authors to request further information.
We rated each trial at high, low or unclear risk of selection bias (random sequence generation, allocation concealment); performance bias (blinding of participants and personnel); detection bias (blinding of outcome assessment); attrition bias (incomplete outcome data); reporting bias (selective reporting) and risk of other bias (see Appendix 2 for judgement criteria). With reference to each of these domains, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact the findings. We explored the impact of the level of bias by undertaking sensitivity analyses – see subsection in Sensitivity analysis. We used this information to evaluate the impact of risk of bias for each outcome in the GRADE assessment, so that high risk of bias in one or more domains resulted in a reduced GRADE rating. Similarly, an unclear risk of bias in one or more domains could reduce the GRADE rating if it presented limitations that would lower confidence in the estimate of the effect.
Measures of treatment effect
We used Review Manager Web for data entry and analyses (Review Manager Web 2021).
Continuous data
We analysed continuous outcomes measured on the same scale between trials (e.g. PSQI) using the mean difference (MD) and 95% confidence intervals (CI).
Ordinal data
We analysed ordinal data measured on scales (i.e. sleep quality on visual analogue scales) as continuous data and the intervention effect was expressed as MDs with 95% CIs.
When possible, we checked the distributions for normality.
See Jespersen 2013 and Appendix 3 for additional methods archived for future updates of this review.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials. For further information on how these types of studies will be dealt with in future updates of this review, see Jespersen 2013 and Appendix 3.
Cross‐over trials
We did not identify any cross‐over trials. For further information on how these types of studies will be dealt with in future updates of this review, see Jespersen 2013 and Appendix 3.
Trials with more than two treatment arms
If a trial reported multiple treatment arms, we only used comparisons between the music intervention and the control or TAU group. For further information on how we will deal with other trials with more than two treatment arms, see Jespersen 2013 and Appendix 3.
Dealing with missing data
We noted levels of attrition in the incomplete outcome data section of the risk of bias tables (within the Characteristics of included studies table). Where information about the presented data set was missing in the trial reports, or if there was a lack of detail or a discrepancy between different reports, or clarification was needed, we tried to retrieve relevant information by contacting the authors of the trial. Where data were missing due to loss to follow‐up or dropout, we attempted to obtain complete outcome data from trial authors to include all participants randomised to each group in the analyses. If any outcome data remained missing, or if trial authors did not respond within a reasonable time, we analysed data on an available‐case basis, based on the numbers of participants for whom outcome data (continuous and dichotomous) were known. We did not impute missing data. For more information on how we will deal with missing data in future updates of this review, see Jespersen 2013 and Appendix 3.
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by examining the characteristics of the trials. The similarities between interventions (e.g. dose, frequency), participants (e.g. age), trial design (e.g. allocation concealment, blinding, losses to follow‐up) and the outcomes are reported in the Included studies subsection. We assessed heterogeneity of treatment response visually from the forest plot of the MD and the Chi² test. In addition, we assessed heterogeneity statistically according to the standard method using the I² statistic, calculated for each comparison on each outcome. There was substantial heterogeneity if the I² statistic was greater than 50%, indicating that 50% of the variability in the outcome cannot be explained by sampling variation. For further information on how we will deal with heterogeneity in future updates of this review, see Jespersen 2013 and Appendix 3.
Assessment of reporting biases
We attempted to minimise the potential for publication bias by our comprehensive search strategy that included evaluating published and unpublished literature.
Where we suspected reporting bias, we contacted trial authors asking them to provide missing outcome data.
For further information on how we will deal with reporting bias in future updates of this review, see Jespersen 2013 and Appendix 3.
Data synthesis
We entered all trials included in the systematic review into Review Manager Web (Review Manager Web 2021), and checked for data entry errors. We conducted a meta‐analysis using the inverse variance method when there were data from at least two included trials. We undertook meta‐analyses using both fixed‐effect and random‐effects models. Where there was agreement between the results of both analyses, we reported the results from random‐effects model, as it conveys the variability better. If fixed‐effect and random‐effects models revealed different results, we investigated possible sources of heterogeneity or inconsistency among trials in the magnitude or direction of effects.
When data were not available for a meta‐analysis, we synthesised the results narratively.
Subgroup analysis and investigation of heterogeneity
We conducted the following subgroup analyses (ranked in order of importance).
Duration of the intervention (short: one to four days, medium: five to 20 days, long: 21 days or more).
Aetiology of insomnia.
Researcher‐selected music versus participant choice among preselected music.
Music listening alone versus music listening with relaxation instructions.
The subgroup analyses were exploratory and conducted as recommended in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (McKenzie 2021). The subgroup analyses are presented in the Effects of interventions section with each outcome.
For further information on other intended subgroup analyses, see Jespersen 2013.
Sensitivity analysis
We conducted sensitivity analyses to determine the impact of risk of bias on the results of the meta‐analyses by excluding trials rated at unclear risk of bias for random sequence generation, allocation concealment and blinding of outcome assessment as recommended in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2021).
For additional information on other intended sensitivity analyses, see Jespersen 2013 and Appendix 3.
Summary of findings and assessment of the certainty of the evidence
We summarised the primary outcomes in Table 1. The primary outcomes included sleep quality, insomnia severity, sleep‐onset latency, total sleep time, sleep interruption, sleep efficiency and adverse events. The table included end‐of‐treatment effects of the comparison between the music intervention and TAU or no‐intervention control group. We rated the certainty of the evidence using the GRADE approach for each outcome (Guyatt 2011). Two review authors (KVJ and VPN) independently performed assessments and resolved disagreements by discussion until reaching consensus. We gave evidence from RCTs an initial high‐certainty rating, but downgraded the assessment if the trial methodology was at risk of bias, if there was substantial inconsistency among the results, if the evidence was indirect or imprecise or if there was evidence of publication bias. We used GRADEpro GDT software to produce the table (GRADEpro GDT). The GRADE rating reflects how certain we are that the estimate reflects the true effect of the intervention.
Results
Description of studies
Results of the search
The searches for this update found 2654 records, resulting in 1358 records after removing duplicates. We identified four additional reports that were ongoing or awaiting classification in the previous version of the review (see Figure 1).
1.
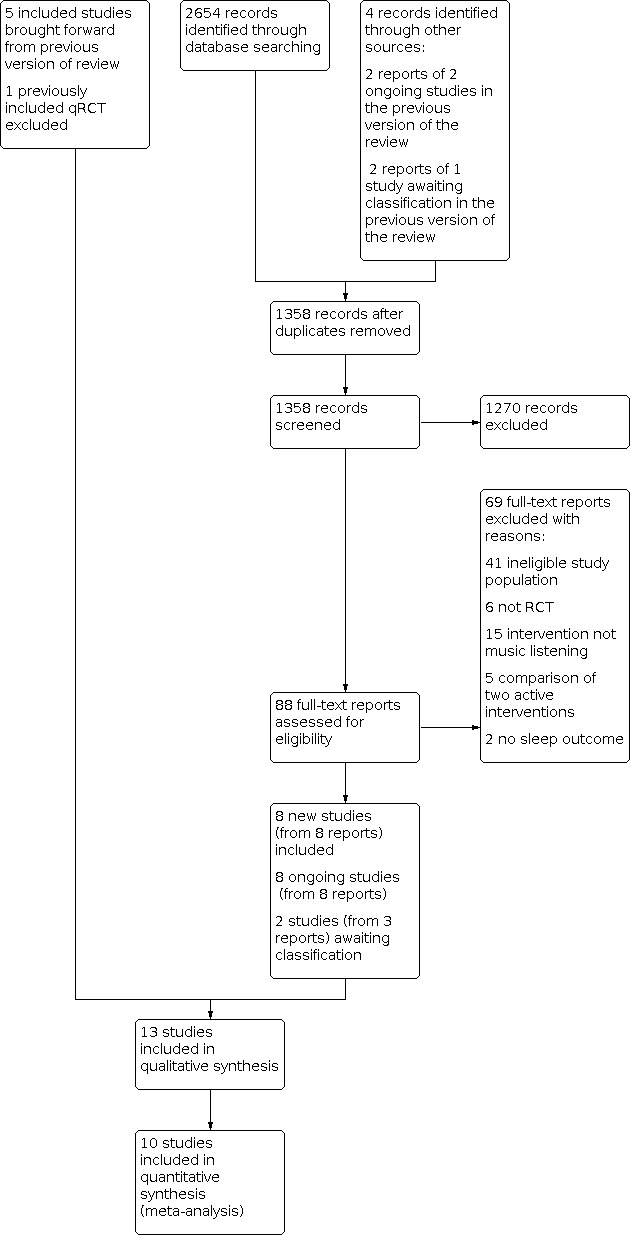
PRISMA flow diagram
After screening titles and abstracts, we identified 88 records that were considered potentially relevant and retrieved them for further examination. One potentially relevant trial was published by two of the authors of this review. To avoid the influence of dual authorship, two authors with no involvement in the trial (JK and VPN) assessed the eligibility, extracted data and evaluated the certainty of evidence from this trial, including risk of bias assessment. We excluded 69 full‐text reports (see Excluded studies), and included eight new studies (from nine reports) in the review.
In addition, we found eight protocols for relevant ongoing studies, and two studies (from three reports) are awaiting classification because of insufficient information to assess inclusion.
In total, we included 13 trials in this updated version of the review. We included six trials in the first version of this review (Jespersen 2015), one of which was excluded in this version because it is a quasi‐RCT (Jespersen 2012).
Included studies
In the present review, we included 13 trials (1007 participants) (see Characteristics of included studies table). Five of these were included in the first version of the review (Chang 2012; Harmat 2008; Kullich 2003; Lai 2005; Shum 2014) (Jespersen 2015), and we added eight new studies in the current update (Amiri 2019; Burrai 2020; Cai 2015; Huang 2017; Jespersen 2019; Liu 2016; Momennasab 2018; Wang 2016). All trials used a parallel‐group design.
The trials were conducted in eight different countries. Four were conducted in Taiwan (Chang 2012; Huang 2017; Lai 2005; Liu 2016), two in Iran (Amiri 2019; Momennasab 2018), two in China (Cai 2015; Wang 2016), one in Singapore (Shum 2014), one in Hungary (Harmat 2008), one in Denmark (Jespersen 2019), one in Italy (Burrai 2020), and one in Austria (Kullich 2003).
Trial size
The 13 included trials comprised 1007 participants. Trial sample sizes ranged from 30 to 159 participants, with a mean sample size of 77 (median 64). One trial had a small sample size of 30 participants (Amiri 2019), three trials included between 121 and 159 participants (Burrai 2020; Cai 2015; Liu 2016), and the remaining nine studies had sample sizes between 48 and 68 (Chang 2012; Harmat 2008; Huang 2017; Jespersen 2019; Kullich 2003; Lai 2005; Momennasab 2018; Shum 2014; Wang 2016).
Setting
In 10 of the included trials, the participants used the music listening intervention in their own home (Amiri 2019; Burrai 2020; Harmat 2008; Huang 2017; Jespersen 2019; Lai 2005; Liu 2016; Momennasab 2018; Shum 2014; Wang 2016). One trial offered participants a live music session once a week in addition to listening to music at home (Amiri 2019). Three trials telephoned participants once or twice a week to ensure compliance with the protocol (Lai 2005; Momennasab 2018; Shum 2014). One study used twice weekly telephone calls to ensure compliance (Wang 2016), one study called participants every second day (Huang 2017), and one study sent daily text reminders to the participants (Amiri 2019). One trial telephoned the intervention group but not the control group once a week to assess compliance (Harmat 2008).
Of the remaining three trials, one was conducted in a sleep laboratory (Chang 2012), and two trials implemented the intervention at an inpatient rehabilitation facility for people with low‐back pain (Kullich 2003) and poststroke rehabilitation (Cai 2015).
Participants
The participants in the included trials were between 18 and 83 years of age.
One trial did not report gender (Lai 2005), and one study included only men (Amiri 2019), whereas another focused on pregnancy‐related insomnia and included only women (Liu 2016). Two studies had an even gender distribution (Cai 2015; Momennasab 2018), but in most studies, the majority of participants were women (70% to 80%). One study had mostly men (Burrai 2020).
Two studies included participants with insomnia disorder according to the DSM‐5 or ICSD (Amiri 2019; Jespersen 2019). The remaining studies stated that they recruited participants with complaints of insomnia. Most trials used scores greater than five on the PSQI as evidence of sleep difficulties. One trial did not include sleep difficulties as an inclusion criterion, but all participants had PSQI scores greater than five, indicating sleep problems (Kullich 2003). The severity of the sleep difficulties varied, with mean PSQI scores at baseline ranging from 6.8 to 14.9. Six trials reported mean baseline scores around 10 (Amiri 2019; Chang 2012; Huang 2017; Kullich 2003; Lai 2005; Shum 2014).
The target populations in the 13 trials were diverse. Two studies included adults with insomnia disorder (Amiri 2019; Jespersen 2019). Three studies focused on age‐related sleep problems (Lai 2005; Shum 2014; Wang 2016), whereas four studies focused on insomnia related to medical conditions (Burrai 2020; Cai 2015; Kullich 2003; Momennasab 2018). One study focused on pregnancy‐related insomnia (Liu 2016), another included students with poor sleep (Harmat 2008), and two studies did not specify their population beyond adults with a complaint of insomnia (Chang 2012; Huang 2017).
Interventions
All included trials used listening to prerecorded music as the main intervention. Most trials examined the effects of listening to music only; two trials examined listening to music with relaxation instructions (Kullich 2003; Lai 2005), and one trial included weekly live music sessions (Amiri 2019). All trials used music once a day. Most trials instructed participants to listen to the music at bedtime, and only four trials did not specify what time of the day to listen to the music (Burrai 2020; Cai 2015; Kullich 2003; Shum 2014). The length of the music listening sessions ranged from 25 to 60 minutes, with a mean of 36 minutes. The duration of the intervention ranged from three to 90 days, with two trials having an intervention period of three to four days (Chang 2012; Huang 2017), and two trials having an intervention period of 90 days (Burrai 2020; Wang 2016). The remaining trials had intervention periods between 14 and 42 days.
Seven trials used researcher‐selected music where all participants received the same intervention music (Amiri 2019; Burrai 2020; Cai 2015; Harmat 2008; Huang 2017; Kullich 2003; Momennasab 2018). In four trials, the participants had a choice among four or six researcher‐created playlists of music in different genres (Jespersen 2019; Lai 2005; Liu 2016; Shum 2014). Similarly, one trial gave the participants access to a large music database with 169 pieces of slow music in various genres and encouraged participants to find their preferred music (Wang 2016). One trial encouraged participants to bring their own preferred music for bedtime listening (Chang 2012). Those who did not bring their own music listened to music prepared by the researchers. In total, 10 participants listened to their own preferred music and 149 participants listened to music chosen by the researcher.
All trials provided information on the music used in the study. The genres reported were Western and Chinese classical music, Buddhist songs, new age, lullabies, Persian traditional music, Chinese five Elements tone music, eclectic, ambient, popular oldies and jazz. Five trials gave information on the specific recordings used (composer, composition title and recording information) (Harmat 2008; Huang 2017; Kullich 2003; Lai 2005; Momennasab 2018). Two trials stated the pieces of music used, but did not give performance‐specific information (Chang 2012; Shum 2014). Four trials described characteristics of the music (Chang 2012; Lai 2005; Shum 2014; Wang 2016). These shared common features such as low tempo (52 beats per minute to 85 beats per minute), stable dynamic structure and no strong rhythmic accentuation.
Seven trials compared the music‐listening intervention to a no‐treatment control group (Amiri 2019; Chang 2012; Harmat 2008; Huang 2017; Jespersen 2019; Lai 2005; Shum 2014), and six trials compared music listening adjunctive to TAU versus TAU alone (Burrai 2020; Cai 2015; Kullich 2003; Liu 2016; Momennasab 2018; Wang 2016). Four trials had two active intervention groups, but we included only data from the music listening group compared to the no‐treatment control group in this review (Harmat 2008; Huang 2017; Jespersen 2019; Momennasab 2018; see Characteristics of included studies table for all interventions used).
Outcomes
Ten trials reported on sleep quality using the PSQI (Amiri 2019; Burrai 2020; Harmat 2008; Jespersen 2019; Kullich 2003; Lai 2005; Liu 2016; Momennasab 2018; Shum 2014; Wang 2016). The PSQI is a commonly used self‐report questionnaire with 19 items. From these items, seven component scores are calculated, each with a score from 0 (no problems) to 3 (severe problems), leading to a total score ranging from 0 to 21 (Buysse 1989). Higher scores indicate more sleep problems, and a total score greater than 5 indicates poor sleep quality. The seven component scores address specific sleep parameters, including sleep latency, total sleep time, sleep efficiency, etc.
Two studies assessed insomnia severity using the ISI (Amiri 2019; Jespersen 2019). The ISI is a well‐validated questionnaire consisting of seven items addressing insomnia symptoms that are each rated from 0 to 4. The total score range from 0 to 28 with higher scores indicating more severe insomnia (Bastien 2001).
Three trials used electroencephalogram (EEG) or full PSG to objectively measure sleep‐onset latency, total sleep time, sleep interruption and sleep efficiency (Chang 2012; Huang 2017; Jespersen 2019). PSG is considered the gold standard of sleep assessment allowing for the scoring of different sleep stages and the transitions between them. This method allows objective measure of the amount of time it takes to fall asleep (sleep‐onset latency, measured in minutes) and the total sleep time (measured in minutes). Furthermore, the amount of wake time after sleep onset (measured in minutes) is a measure of sleep interruption, and sleep efficiency refers to the percentage of time spend asleep while in bed (i.e. total sleep time divided by time in bed) (Kryger 2017). Five trials measured these outcomes subjectively with the PSQI subscales described above (Harmat 2008; Kullich 2003; Lai 2005; Momennasab 2018; Wang 2016).
No trials reported adverse events or deterioration of outcomes during the intervention period. This lack of reporting of adverse events could both reflect that there were no adverse events or that researchers neglected to report them. A few studies reported some of the secondary outcomes. Two studies reported on depressive symptoms, using the Depression, Anxiety and Stress Scale (DASS‐21) (Amiri 2019), and the Hospital Anxiety and Depression Scale (HADS) (Burrai 2020). Three studies reported on anxiety (Amiri 2019; Burrai 2020; Liu 2016), where Liu 2016 used the State section of the State‐Trait Anxiety Inventory, Amiri 2019 used the anxiety scale of the DASS‐21 and Burrai 2020 used the anxiety scale of the HADS. Finally, two studies reported the effect on quality of life using the 12‐item Short Form Health Survey (SF‐12) (Burrai 2020) and the psychological subscale of the World Health Organization Quality‐of‐Life Scale (WHOQOL‐BREF) (Jespersen 2019).
Funding sources‐item
Eight trials were funded or partly funded by a grant from a national research council, university, government or foundation (Amiri 2019; Cai 2015; Chang 2012; Harmat 2008; Huang 2017; Jespersen 2019; Kullich 2003; Momennasab 2018). Five trials reported no information on funding sources (Burrai 2020; Lai 2005; Liu 2016; Shum 2014; Wang 2016).
Excluded studies
We excluded 69 reports identified by the updated searches (see Figure 1). Of these 69, we excluded six trials because they did not have an RCT design (e.g. no control group or no randomisation procedure), and 41 trials because the participants were not adults with insomnia as defined in the Types of participants section (some trials enrolled participants with no sleep problems, some included both good and poor sleepers, and some had no clear documentation of the insomnia problems). We excluded a further 15 trials because the intervention was not listening to music (e.g. choir singing), five trials because they compared two active interventions (e.g. music versus muscle relaxation techniques), and two trials because they did not evaluate any sleep outcome measures. In addition, we excluded one trial included in the original version of the review because it was a quasi‐RCT (see Differences between protocol and review). Twenty‐eight excluded trials were ongoing as reported in a trial registry. We selected 27 studies identified for the original review for this update, and reported why they did not meet our eligibility criteria in the Characteristics of excluded studies table. These include the previously included study, Jespersen 2012.
Studies awaiting classification
We identified two potentially relevant trials that could not be assessed due to limited information. One studies was identified in the previous version of this review, and it is still awaiting classification. It is an unpublished trial on pain‐related sleep difficulties (Miller 2002), but the trial is referred to in published material (Bernatzky 2011). Still, there is insufficient information to assess the trial for inclusion or exclusion from this review. We contacted the author, who has yet to respond (see Characteristics of studies awaiting classification table). For this update, we identified another potentially relevant study on personalised music interventions for people with sleep disorders (Zhu 2018). However, the report included too little information to determine inclusion or exclusion. There were no other publications of the trial found and the author information could not be obtained.
Ongoing studies
Eight relevant studies were still ongoing when this review was written. Three studies focus on sleep problems in elderly people (IRCT2015051822141N1; IRCT20150519022320N10; NCT04157244), and two focus on insomnia and depression (NCT02376686; NCT03676491). Three trials focus on sleep problems in general medicine (NCT04578860), sleep‐onset insomnia (NCT04585425), and pregnancy‐related insomnia (NCT04633395).
Risk of bias in included studies
We assessed the 13 included trials for risk of bias across the following domains: random sequence generation (selection bias); allocation concealment (selection bias); blinding of participants and personnel (performance bias); blinding of outcome assessment (detection bias); incomplete outcome data (attrition bias); selective reporting (reporting bias); and other bias. The results are depicted in Figure 2. Figure 3 provides a summary of the risk of bias results for each of the included trials. Reasons for the judgement are described in the risk of bias tables within the Characteristics of included studies table.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial
Allocation
Random sequence generation
Ten trials described the randomisation procedures applied and were at low risk of bias (Cai 2015; Chang 2012; Harmat 2008; Huang 2017; Jespersen 2019; Kullich 2003; Lai 2005; Momennasab 2018; Shum 2014; Wang 2016). Three trials stated that the trial was randomised, but did not describe the randomisation procedure and were judged at unclear risk of bias (Amiri 2019; Burrai 2020; Liu 2016).
Allocation concealment
Seven trials described measures taken to conceal allocation and were rated at low risk of bias (Chang 2012; Huang 2017; Jespersen 2019; Kullich 2003; Lai 2005; Shum 2014; Wang 2016). Five trials had limited information on allocation concealment and were judged at unclear risk of bias (Amiri 2019; Burrai 2020; Cai 2015; Harmat 2008; Liu 2016). One trial used a block randomisation procedure that enabled researchers to predict group allocation for some participants and was judged at high risk of bias (Momennasab 2018).
Blinding
Blinding of participants and personnel
Due to the nature of the intervention, it is not possible to blind participants and it can be difficult to blind personnel or researchers. Bias was considered likely to have occurred in those trials using subjective reports of sleep quality when participants were not blinded. Bias was considered less likely to have occurred in the trials involving objective measures of sleep, although these trials also included subjective measures of sleep (Chang 2012; Huang 2017; Jespersen 2019). Therefore, all trials were judged at high risk of performance bias.
Blinding of outcome assessment
Eight trials stated that outcome assessors where blinded to group allocation and were at low risk of bias (Burrai 2020; Chang 2012; Harmat 2008; Huang 2017; Jespersen 2019; Kullich 2003; Momennasab 2018; Wang 2016). One trial reported no information on who conducted the rating of the outcome and was judged at unclear risk of bias (Cai 2015). Four trials reported no measures taken to blind outcome assessors and were at high risk of bias (Amiri 2019; Lai 2005; Liu 2016; Shum 2014).
Incomplete outcome data
Twelve trials were at low risk of bias because they either reported no attrition and no missing data, or accounted well for attrition and missing data that was low and balanced between groups. Harmat 2008 and Kullich 2003 did not include the information on attrition in the published report but the authors provided it at our request. One trial had unclear information on attrition and was at unclear risk of bias (Lai 2005).
Selective reporting
There was some uncertainty in two trials about the full reporting of outcomes, so these were at unclear risk of reporting bias (Chang 2012; Shum 2014). One trial did not include outcome measures for the no‐treatment control group in the published report, but the authors provided this information at our request and it did not change the results or conclusions of the published paper (Harmat 2008). Hence, we did not suspect reporting bias and judged the trial at low risk of reporting bias. There was no indication of selective reporting in the remaining 10 trials, which were at low risk of reporting bias.
Overall, we found publicly available protocols for five studies. Three of these were published before study initiation (Burrai 2020; Jespersen 2019; Momennasab 2018), one was registered during recruitment (Amiri 2019), and one was registered retrospectively (Huang 2017). These are the five most recent trials, and the findings may reflect a growing awareness of the importance of making study protocols of clinical trials available.
Other potential sources of bias
Three trials had other potential sources of bias (Chang 2012; Harmat 2008; Lai 2005). Two trials reported baseline differences between the intervention and control group (Chang 2012; Lai 2005), and one trial reported methods of data collection differed between the intervention and control group (Harmat 2008). Thus, these trials were at high risk for other biases. The remaining 10 trials had no risks of other bias and were at low risk of bias.
Effects of interventions
See: Table 1
For an overview, see Table 1. All outcomes are reported as immediate post‐treatment effects. Since we did not have individual participant data, we were unable to verify the distribution of data for continuous outcomes.
Primary outcomes
Sleep quality
Ten trials, comprising 708 participants, reported on sleep quality and were pooled in a meta‐analysis (Analysis 1.1). All trials measured sleep quality using the PSQI. The PSQI total scores range from 0 to 21 with higher scores indicating more sleep problems. The results of the analysis revealed an effect in favour of the intervention compared to no treatment or TAU (MD −2.79, 95% CI −3.86 to −1.72, P < 0.001; moderate‐certainty evidence; Figure 4). This shows that the music intervention likely reduces sleep problems by 1.72 to 3.86 points in the PSQI score, and the size of the effect indicates a reduction of sleep problems of approximately one SD in favour of the intervention compared to no treatment or TAU. Heterogeneity was high (I² = 81%) and this was investigated separately using subgroup analyses and sensitivity analyses.
1.1. Analysis.

Comparison 1: Sleep quality: listening to music versus control – Pittsburgh Sleep Quality Index (PSQI) – global score, Outcome 1: Sleep quality: Pittsburgh Sleep Quality Index (PSQI) – immediately postintervention
4.

Forest plot of comparison: 1 Sleep quality: listening to music versus control ‐ Pittsburgh Sleep Quality Index (PSQI) ‐ global score, outcome: 1.1 Sleep quality: Pittsburgh Sleep Quality Index (PSQI) ‐ immediately post‐treatment.
Subgroup analyses
For the sleep quality outcome, the number of included trials enabled us to conduct four of the predefined subgroup analyses.
Duration of the intervention
We explored the influence of the duration of the intervention period in a subgroup analysis comparing medium duration (eight to 21 days) with long duration (22 days and more). There were no studies with a short duration (one to seven days) reporting this outcome. The results of the analysis showed a likely effect of the intervention compared to controls with both intervention durations (medium: MD −2.24, 95% CI −2.90 to −1.58; 5 studies, 343 participants; long: MD −3.36, 95% CI −5.63 to −1.10; 5 studies, 365 participants). There was no clear difference between the two subgroups (Chi² = 0.86, degrees of freedom (df) = 1 (P = 0.35), I² = 0%; Analysis 1.2). Heterogeneity among the medium duration studies was low (I2 = 13%), but very high among the long duration studies (I2 = 90%). The high heterogeneity seems to be mainly due to a larger effect in the Momennasab 2018 study compared to the other studies.
1.2. Analysis.

Comparison 1: Sleep quality: listening to music versus control – Pittsburgh Sleep Quality Index (PSQI) – global score, Outcome 2: Subgroup (PSQI) by duration of intervention – immediately postintervention
Insomnia aetiology
We explored if the size of the effect was related to the insomnia aetiology in a subgroup analysis. We compared sleep quality in participants with age‐related insomnia (Lai 2005; Shum 2014; Wang 2016), insomnia related to a medical condition (Burrai 2020; Kullich 2003; Momennasab 2018), participants with insomnia disorder (Amiri 2019; Jespersen 2019), and pregnancy‐related insomnia (Liu 2016). The analysis showed evidence for a likely reduction in sleep problems in favour of the intervention in all four groups and no evidence of a difference in the effect between the subgroups compared to controls (Chi² = 4.59, df = 3 (P = 0.20), I² = 34.6%, 9 studies, 644 participants; Analysis 1.3).
1.3. Analysis.

Comparison 1: Sleep quality: listening to music versus control – Pittsburgh Sleep Quality Index (PSQI) – global score, Outcome 3: Subgroup (PSQI) by aetiology – immediately postintervention
Researcher‐selected music versus participant‐selected music
Ten studies were included in the subgroup analysis comparing researcher‐selected music (Amiri 2019; Burrai 2020; Harmat 2008; Kullich 2003; Momennasab 2018) and participants' choice among researcher selected playlists (Jespersen 2019; Lai 2005; Liu 2016; Shum 2014; Wang 2016). The results of the analysis revealed that, compared to no treatment or TAU, music likely results in a reduction of sleep problems both if the music was selected by the researchers (MD −3.31, 95% CI −5.32 to −1.29; 5 studies, 370 participants), and by the participants based on a preselected choice (MD −2.33, 95% CI −3.37 to −1.29; 5 studies, 338 participants). However, we found no evidence of a difference between the effect of the different subgroups (Chi² = 0.71, df = 1 (P = 0.40), I² = 0%; Analysis 1.4).
1.4. Analysis.

Comparison 1: Sleep quality: listening to music versus control – Pittsburgh Sleep Quality Index (PSQI) – global score, Outcome 4: Subgroup (PSQI) by music selection – immediately postintervention
Music listening alone versus music listening and relaxation instructions
We were able to compare trials that applied music listening alone (Amiri 2019; Burrai 2020; Harmat 2008; Jespersen 2019; Liu 2016; Momennasab 2018; Shum 2014; Wang 2016) to trials that used music listening and relaxation instructions (Kullich 2003; Lai 2005). The results of the analysis revealed a likely reduction in sleep problems compared to controls, regardless of whether the intervention was applied without relaxation instructions or with relaxation instructions (without: MD −2.85, 95% CI −4.18 to −1.51; 8 studies, 583 participant; with: MD −2.64; 95% CI −3.74 to −1.54; 2 studies, 125 participants). However, we found no evidence of a difference in the effect of the two subgroups (Chi² = 0.05, df = 1 (P = 0.82), I² = 0%; Analysis 1.5).
1.5. Analysis.

Comparison 1: Sleep quality: listening to music versus control – Pittsburgh Sleep Quality Index (PSQI) – global score, Outcome 5: Subgroup (PSQI) by relaxation instructions – immediately postintervention
Summary
In summary, the subgroup analyses do not indicate a crucial role of the duration of the intervention, the aetiology of the sleep problems, the music selection procedures or relaxation instructions on the effect of music for improving sleep quality. Furthermore, the subgroup analyses do not explain the heterogeneity in the meta‐analysis including all studies reporting on sleep quality. Mainly, it seems that Momennasab 2018 represents an outlier result in the sense that the effect reported by this study is larger than what is found in the other studies (see Analysis 1.1). The subgroup analyses do not suggest that this larger effect is related to any of the parameters explored here, as there are other studies with similar duration, aetiology and music selection showing smaller effect. See Sensitivity analysis section for further exploration of heterogeneity.
Insomnia severity
Two studies measured insomnia severity including 63 participants with insomnia disorder (i.e. diagnosed according to the DSM‐5 or ICSD2 criteria (Amiri 2019; Jespersen 2019). Both studies used the ISI to evaluate insomnia severity, with scores ranging from 0 to 28 and higher scores indicating more severe insomnia symptoms. The evidence is very uncertain about the effect of music on insomnia severity. A meta‐analysis showed no clear evidence of an effect, as the CIs included both a clinically relevant reduction in insomnia severity and a small increase (MD −6.96, 95% CI −15.21 to 1.28; P = 0.10; very low‐certainty evidence; Analysis 2.1). Heterogeneity was very high (I2 = 95%). This may relate to the longer intervention period in the study showing the largest effect (Amiri 2019), or it could relate to different demographic variables. The study by Amiri 2019 included male students with a mean age of 27 (SD 2.5) years, whereas the participants included in Jespersen 2019 were older (mean 48.4 (SD 8.8) years), with a majority of women (79%).
2.1. Analysis.

Comparison 2: Insomnia severity: listening to music versus control – Insomnia Severity Index (ISI), Outcome 1: Insomnia severity: Insomnia Severity Index (ISI) – immediately postintervention
Sleep‐onset latency
Eight trials reported on sleep‐onset latency (SOL) including 457 participants. Three trials reported objective SOL measured with PSG (Chang 2012; Huang 2017; Jespersen 2019), and five studies reported experienced SOL measured using the PSQI subscale (Harmat 2008; Kullich 2003; Lai 2005; Momennasab 2018; Wang 2016).
The three studies using PSG included 136 participants. None of the studies found evidence of an effect of the intervention on objective SOL compared to no treatment or TAU (Chang 2012; Huang 2017; Jespersen 2019). We could not conduct a meta‐analysis because two studies reported the data in a format that did not allow for inclusion (Chang 2012; Huang 2017).
Five studies, including 321 participants, measured SOL using the PSQI subscale 'Sleep latency'. The scores of this subscale range from 0 to 3 with higher scores reflecting more problems. Three studies reported the results in a format that allowed inclusion in a meta‐analysis (Kullich 2003; Momennasab 2018; Wang 2016). The results of this analysis, which included 197 participants, indicate an effect in favour of the music intervention compared to no treatment or TAU (MD −0.60, 95% CI −0.83 to −0.37; P < 0.001; low‐certainty evidence; Analysis 3.1). This evidence suggests that listening to music may reduce experienced SOL between 0.37 and 0.83 points on this PSQI subscale, with low heterogeneity of the results (I2 = 0%).
3.1. Analysis.

Comparison 3: Sleep onset latency: listening to music versus control, Outcome 1: Sleep onset latency: Pittsburgh Sleep quality Index (PSQI) – immediately postintervention
A narrative summary of all five studies reporting subjective measures of SOL showed that four of these studies reported improved SOL with the music intervention compared to no treatment or TAU (Harmat 2008; Lai 2005; Momennasab 2018; Wang 2016), whereas Kullich and colleagues found no difference between the groups (Kullich 2003).
Total sleep time
Nine trials, including 611 participants, registered total sleep time. Three studies used PSG, five studies used the PSQI subscale 'Sleep duration' and one study performed a categorial assessment of improvement in sleep duration.
Three studies, including 136 participants, measured objective total sleep time with PSG and found no effect of the music intervention compared to controls (Chang 2012; Huang 2017; Jespersen 2019). Two studies reported the data in a format that did not allow for inclusion in a meta‐analysis.
Five studies reported the results of the PSQI subscale 'Sleep duration' (Harmat 2008; Kullich 2003; Lai 2005; Momennasab 2018; Wang 2016). A meta‐analysis including three of these studies with 197 participants found evidence that music listening may improve sleep duration compared to no treatment or TAU (MD −0.69, 95% CI −1.16 to −0.23; P = 0.004; low‐certainty evidence; Analysis 4.1). This suggests a reduction between 0.23 and 1.16 points on this subscale ranging from 0 to 3. Heterogeneity was substantial in this analysis (I2 = 70%).
4.1. Analysis.

Comparison 4: Total sleep time: listening to music versus control, Outcome 1: Total sleep time: Pittsburgh Sleep Quality Index – immediately postintervention
A narrative summary of all five studies reporting subjective measures of total sleep time with the PSQI subscale showed that four of these studies found an effect of the intervention (Harmat 2008; Kullich 2003; Lai 2005; Momennasab 2018), and one study showed no effect (Wang 2016), compared with no treatment or TAU. In addition, one study including 154 participants with poststroke insomnia reported ratings of the effect of the intervention in four categories depending on the degree of improvement in sleep duration (Cai 2015). They found that more participants in the music group showed improved sleep duration than the control group.
Sleep interruption
Eight trials with 457 participants reported sleep interruption.
Three trials, including 136 participants, measured sleep using PSG and reported wake time after sleep onset and number of awakenings (Chang 2012; Huang 2017; Jespersen 2019). They found no effect of the intervention on these objective sleep measures compared to no treatment or TAU.
Five trials, including 321 participants, measured sleep interruption using the PSQI subscale 'Sleep disturbance'. A meta‐analysis including data from three of these studies (197 participants), showed that music may have no effect on sleep interruption compared with no treatment or TAU, but the evidence is very uncertain (MD −0.53, 95% CI −1.47 to 0.40; P = 0.26; very low‐certainty evidence; Analysis 5.1). This analysis showed very high heterogeneity (I2 = 97%). The cause of this heterogeneity seemed to be the discrepancy between the relatively large reduction seen in Momennasab 2018, whereas the two other studies showed no difference in the effect between music and control groups (see Analysis 5.1).
5.1. Analysis.

Comparison 5: Sleep interruption: listening to music versus control, Outcome 1: Sleep interruption: Pittsburgh Sleep Quality Index – immediately postintervention
A narrative summary of all five studies also showed inconsistency in the results. Three studies reported a reduction in sleep interruption in the music group (Harmat 2008; Kullich 2003; Momennasab 2018), whereas two studies found no effect of the intervention (Lai 2005; Wang 2016), compared to no treatment or TAU. The two studies with no effect included elderly people with sleep problems, and it may be that music is less efficient for improving sleep interruption with this population.
Sleep efficiency
Eight studies measured sleep efficiency using PSG and the PSQI subscale 'Sleep efficiency'.
The three studies, including 136 participants, using PSG reported no effect of the intervention compared to no treatment or TAU (Chang 2012; Huang 2017; Jespersen 2019).
A meta‐analysis with three studies using the PSQI subscale showed that music listening may improve sleep efficiency compared to no treatment or TAU (MD −0.96, 95% CI −1.38 to −0.54; P < 0.001; 197 participants; low‐certainty evidence; Analysis 6.1). The evidence suggests a reduction in sleep efficiency problems between 0.54 and 1.38 points on this scale ranging from 0 to 3 in the music group compared to controls. The analysis showed moderate heterogeneity (I2 = 62%).
6.1. Analysis.

Comparison 6: Sleep efficiency: listening to music versus control, Outcome 1: Sleep efficiency: Pittsburgh Sleep Quality Index (component score) – immediately postintervention
A narrative summary including all five studies measuring experienced sleep efficiency showed that all studies reported an effect of the intervention compared to no treatment or TAU (Harmat 2008; Kullich 2003; Lai 2005; Momennasab 2018; Wang 2016).
Adverse events
No trials reported a deterioration of a primary outcome or reported any other adverse events.
Secondary outcomes
The secondary outcomes were sleep‐related psychological outcomes (depression, anxiety and quality of life), physical outcomes (fatigue, daytime sleepiness and pain), and physiological outcomes (heart rate, heart rate variability and blood pressure). Trials reported three of these outcomes (depression, anxiety and quality of life.
Depression
Two studies, including 173 participants, reported the effect of the intervention on depressive symptoms (Amiri 2019; Burrai 2020). A meta‐analysis showed no clear effect of the music intervention on depressive symptoms compared with no treatment or TAU, but the evidence is very uncertain (SMD −2.04, 95% CI −4.45 to 0.37; P = 0.10; Analysis 7.1). The evidence suggests a large mean reduction in depressive symptoms, but the CIs were very broad and covered both large reductions and slight increases. Statistical heterogeneity was very high (I2 = 94%). This may relate to differences in the included populations; Burrai 2020 included adults with chronic heart failure and sleep problems, whereas Amiri 2019 included students with insomnia disorder. Furthermore, Burrai 2020 had an intervention period of 90 days compared to 42 days in Amiri 2019.
7.1. Analysis.

Comparison 7: Depression: listening to music versus control, Outcome 1: Depression – immediately postintervention
Anxiety
Three studies, including 294 participants, measured anxiety (Amiri 2019; Burrai 2020; Liu 2016). A meta‐analysis found evidence that listening to music may reduce anxiety compared to no treatment or TAU (SMD −0.52, 95% CI −0.75 to −0.28; P < 0.001; Analysis 8.1). This evidence suggests a medium effect size reduction in anxiety symptoms with the music intervention compared to controls. Heterogeneity was low (I2 = 0%).
8.1. Analysis.

Comparison 8: Anxiety: listening to music versus control, Outcome 1: Anxiety – immediately postintervention
Quality of life
Two studies, including 177 participants, reported the effect on quality of life (Burrai 2020; Jespersen 2019). There was evidence that music may increase quality of life compared to no treatment or TAU (SMD 0.55, 95% CI 0.25 to 0.85; P < 0.001; Analysis 9.1). This indicates a small‐to‐large effect of the music intervention compared to no intervention or TAU. Heterogeneity was low (I2 = 0%).
9.1. Analysis.

Comparison 9: Quality of life: listening to music versus control, Outcome 1: Quality of life – immediately postintervention
Sensitivity analyses
We conducted a series of sensitivity analyses to determine the impact of risk of bias on the results of the meta‐analysis for the outcome of sleep quality. Other outcomes of interest were not considered as there was an insufficient number of studies reporting the outcomes.
First, we excluded trials potentially indicating publication bias as illustrated in Figure 5 (Momennasab 2018). Excluding the study decreased heterogeneity (I² = 36%) and the overall effect size estimate remained similar (MD −2.22, 95% CI −2.83 to −1.62; P < 0.001; analysis not shown). The funnel plot clearly identified Momennasab 2018 as an outlier, but whether this is due to publication bias was unclear from the plot. It could equally well be due to methodological limitations or clinical aspects (Sterne 2011). Next, we excluded all studies with potential risk of selection bias (random sequence generation and allocation concealment) from analyses (Amiri 2019; Burrai 2020; Harmat 2008; Liu 2016; Momennasab 2018). Heterogeneity was substantially decreased (I² = 7%) and the overall effect size estimate remained the same (MD −2.71, 95% CI −3.46 to −1.97; P = 0.0001; analysis not shown). Further excluding two studies with risk of detection bias (Lai 2005; Shum 2014) resulted in lowest heterogeneity (I² = 0%) while the overall effect size estimate remained similar (MD −2.03, 95% CI −3.08 to −0.98; P = 0.0002; analysis not shown). Overall, the sensitivity analyses revealed that accounting for publication bias, and excluding studies with risk of selection bias and detection bias did not change the results of the meta‐analyses.
5.

Discussion
Summary of main results
We found 13 trials that met the inclusion criteria for this review comprising 1007 participants. These trials evaluated the effect of listening to music for insomnia in adults compared to no treatment or TAU. We conducted a meta‐analysis using a random‐effects model for the primary outcome of sleep quality, which 10 trials reported. We found moderate‐certainty evidence that listening to music probably improves sleep quality compared to no treatment or TAU (Analysis 1.1; 708 participants). The analysis showed a large effect of about one SD in favour of the intervention (Figure 4). The direction of the results was consistent across the included trials and sensitivity analyses showed that the beneficial effect of the intervention remained unchanged when excluding trials carrying potential risk of selection bias (Amiri 2019; Burrai 2020; Harmat 2008; Liu 2016; Momennasab 2018), or detection bias (Lai 2005; Shum 2014). Subgroup analyses revealed no difference depending on the duration of the intervention (Analysis 1.2; 708 participants), the aetiology of insomnia (Analysis 1.3; 644 participants), whether the music was selected by research personnel or the participant (Analysis 1.4; 708 participants), or whether listening to music was accompanied by relaxation instructions or not (Analysis 1.5; 708 participants). The evidence for the additional primary outcomes was of low or very low certainty. Two studies reported insomnia severity, and the meta‐analysis showed no clear evidence of a difference between the intervention and control groups (Analysis 2.1; 63 participants). Sleep‐onset latency, sleep duration, sleep interruption and sleep efficiency were measured both objectively by three studies and subjectively by five studies. The objective measures indicated that listening to music may not improve any of these outcomes compared to no treatment or TAU (Chang 2012; Huang 2017; Jespersen 2019). Three studies with subjective measures were included in a meta‐analysis (Kullich 2003; Momennasab 2018; Wang 2016), which showed evidence that listening to music may reduce problems with sleep‐onset latency (Analysis 3.1; 197 participants), sleep duration (Analysis 4.1; 197 participants), and sleep efficiency (Analysis 6.1; 197 participants), but may have no effect on sleep interruption (Analysis 5.1; 197 participants), compared to no treatment or TAU. None of the trials reported adverse events. A few studies reported some of the secondary outcomes. Compared with no treatment or TAU, listening to music may result in little to no difference in depressive symptoms (Analysis 7.1; 2 studies, 173 participants), but may improve anxiety (Analysis 8.1; 3 studies, 294 participants) and quality of life (Analysis 9.1; 2 studies, 177 participants). For an overview of the results see Table 1.
Overall completeness and applicability of evidence
Outcomes
Ten trials reported the primary outcome of sleep quality measured with the same questionnaire (PSQI), giving substantial weight for a meta‐analysis. The fact that people experienced improvement in sleep quality is important, and the improvement is large enough to be considered clinically relevant. As most trials focused narrowly on subjective sleep quality, there is limited information on other aspects of sleep that might be affected by the intervention. However, five trials reported the PSQI subscales on 'sleep‐onset latency', 'total sleep time', 'sleep disturbance' and 'sleep efficacy'. Three of these studies reported the data in a format that could be included in a meta‐analysis. Additionally, three studies reported these outcomes using objective sleep measures such as PSG. However, the data format in two of these studies did not allow for inclusion in a meta‐analysis. Overall, none of the studies using objective sleep measures reported any effect of the intervention (Chang 2012; Huang 2017; Jespersen 2019). This is in contrast to the questionnaire data showing a beneficial effect on sleep‐onset latency, total sleep time and sleep efficiency. Discrepancies between subjective and objective measures of sleep are commonly reported, particularly among people with sleep difficulties, and it is recommended to document treatment efficacy with multiple outcomes and multiple assessment modalities (Morin 2003). The current evidence suggests no effect of music on objective sleep measures, but with only three studies reporting this outcome, it remains unclear if this is a true estimate or related to lack of power to detect more subtle effects of the music intervention or the very short intervention period in two of the studies using objective sleep measures.
The included trials reported three of the predefined secondary outcomes. Two studies reported depression, three reported anxiety and two reported on quality of life. This gives us very limited information and reflects a lack of data on how the music intervention may affect the waking correlates and consequences of insomnia, such as mood, quality of life, daytime fatigue, pain, heart rate or blood pressure. These measures are important for determining effects beyond the reduction of insomnia symptoms. Insomnia is associated with considerable daytime dysfunction and an effective treatment should improve both sleep and daytime functioning.
No trial reported a deterioration of a primary outcome or other adverse events. Even though adverse events were not among the primary outcomes of the individual trials, it is considered unethical not to report such events. Therefore, the absence of these reports may support the safety of the intervention. However, since it is not reported that no adverse events occurred, we cannot be sure if adverse events did occur, and authors just neglected to report them.
Population
The trials were heterogeneous with regard to participant characteristics. Most trials did not describe the sleep problems of the participants in sufficient detail. Few studies reported information the nature or duration of the sleep difficulties. All participants experienced insomnia as defined in this review as dissatisfaction with the quality, duration or continuity of sleep. However, the studies used different wording to describe the condition (insomnia, poor sleep, sleep problems), and most trials relied on the PSQI for the identification of sleep problems. The PSQI is a well‐validated tool to measure sleep problems, with a clear cut‐off score distinguishing good and poor sleepers (Buysse 1989). However, it is not a specific screening tool for insomnia and the exact nature of the sleep problems are not revealed by the global PSQI score. Therefore, it is unclear if the participants experienced difficulties initiating sleep, maintaining sleep, non‐restorative sleep or any combination of these. Furthermore, the amount of daytime dysfunction resulting from the insomnia was not described. It could be argued that other tools, such as the ISI (Bastien 2001), would make a better screening tool for insomnia. Two studies included participants with a clinical diagnosis of insomnia disorder, but they were quite heterogeneous and more studies are needed to determine the effect of listening to music for insomnia disorder. Furthermore, very few of the included trials reported screening for other sleep disorders, and it cannot be excluded that some of the sleep complaints of the participants were due to other sleep disorders such as sleep apnoea or restless legs syndrome. Some trials gave no information on the underlying cause of insomnia; others related insomnia to a wide range of different conditions, such as pregnancy, chronic medical conditions or old age. The subgroup analyses found no difference in the effect on sleep quality based on condition and it seems that the effect of the music intervention was consistent across these diverse populations. However, the results show no evidence for an effect on sleep interruption and this suggests that music is less efficient for sleep maintenance problems although this hypothesis remains to be tested. As mentioned in the Description of the condition, insomnia symptoms are associated with a number of disorders and may be seen as a precursor to depression (Baglioni 2011), as a factor affecting the long‐term outcomes in neurological diseases (Mayer 2011), or a factor contributing to risk of falling in the elderly population (Latimer Hill 2007). Therefore, early and safe interventions may be of great importance to both healthy and diseased populations.
Intervention
All trials used prerecorded music for the intervention, which reflects the common use of listening to music in many clinical and at‐home settings. We were interested in the effect of music interventions that could easily be used by the general population or in clinical settings, and, therefore, we did not include studies using live music interventions. Most of the included trials used researcher‐selected music without any clear rationale for the choice of the music. Some trials provided information on the specific characteristics of the music, and these features (e.g. slow tempo and low rhythmic accentuation) fit well with the literature describing the characteristics of potentially sedative music (Scarratt 2021; Wigram 2002). Such detailed description of the music should be obligatory when reporting these types of trials since they can help clinicians make well‐informed music selections. At the same time, we also know that musical taste varies widely among individuals and preferences as well as familiarity with the music may influence the efficacy of the intervention. In five trials, participants could choose among researcher‐selected playlists of different genres or select tracks from a researcher‐provided music database. To some degree this approach takes individual preferences into account and may enhance the participants' sense of control, which can be an important factor, especially in institutional or hospital settings where people can feel disempowered. When exploring the impact of giving participants a choice among preselected music versus the use of researcher‐selected music, we found no difference in the effect on sleep quality (see Analysis 1.4). However, there was a limited number of studies giving the participants a choice, and no studies used fully participant‐selected music.
Some trials added relaxation instructions to the music listening intervention, but subgroup analyses showed no evidence of a difference in effect between trials with and without relaxation instructions (see Analysis 1.5). The music interventions used in these types of trials were called several names, including music listening, music intervention, music therapy or music. A common distinction is made between music medicine and music therapy, with music therapy involving an active therapeutic process between the patient and therapist, including the use of music (Bruscia 1998). This is not the case in any of the included trials, and the interventions in this review fall within the music medicine domain.
Duration and setting
Based on this review, listening to music daily for 14 to 90 days results in improved subjective sleep quality. The trials using only three or four days of intervention found no evidence of an effect of music on objective measures of SOL, total sleep time, sleep interruption or sleep efficiency (Chang 2012; Huang 2017). However, it is unclear if this lack of effect is related to the duration of the intervention or the use of objective measures of sleep. The subgroup analysis exploring the impact of the duration of the intervention found no effect (Analysis 1.2), despite the fact that this has previously been suggested (Chen 2021; Dickson 2020). The relationship between the duration of the intervention and the effect of the intervention remains unclear, and more research is needed to establish optimal duration of music interventions for adults with insomnia.
The included trials used daily for about 45 minutes. This frequency and dosage of the intervention seems beneficial, given the reported effects on sleep quality. However, we cannot conclude if changes in these parameters would affect the effect of the intervention. Similarly, there was limited information on the significance of the timing of the intervention, even though most trials reported the use of music at bedtime. One included study had an active control group comparing daytime music listening to the use of music at bedtime (Momennasab 2018). These results suggest a larger effect in the group listening to music at bedtime, but it remains to be tested in more studies. Most trials administered the intervention by participants in their own homes. This indicates that music listening can be effective as a self‐administered intervention. However, it is important to note that these trials often included weekly contact from researchers to ensure compliance. This may be particularly important with elderly populations or populations with comorbid disorders.
Quality of the evidence
All included trials were at high risk of bias on at least one of the rated items; consequently, the results of this review need to be interpreted with caution. Due to the nature of the intervention, blinding of the participants was not possible, and not all trials reported blinding of outcome assessors. This may result in overestimation of the treatment effects, especially since most trials used a self‐report questionnaire to assess the main outcome of sleep quality. A placebo effect cannot be excluded. It will be important to have more studies with objective outcome measures of sleep since these are less sensitive to the placebo effect than subjective measures. Furthermore, the results are limited by the small sample sizes in many of the studies, resulting in a relatively small number of participants included in this review. Sensitivity analyses revealed no impact of inadequate randomisation, allocation concealment or blinding of outcome assessors on the results. The positive effect on sleep quality was consistent across all trials, with narrow CIs in most trials. For some trials, we received additional methodological and statistical information from the principal investigators, which improved the quality of the review. When summarising the assessment of risk of bias of individual studies, the results of the sensitivity analyses, and taking into account GRADE judgements of the overall certainty of the evidence (see Table 1), there is moderate‐certainty evidence that, compared to no treatment or TAU, listening to music likely results in a large increase in sleep quality; and low‐certainty evidence that it may improve sleep‐onset latency, total sleep time and sleep efficiency. The evidence is very uncertain about the effect of listening to music on insomnia severity and sleep interruption.
Potential biases in the review process
We conducted extensive electronic searches and handsearches, and we contacted first authors and relevant experts for information on unpublished trials. Therefore, it seems unlikely that we missed important trials within this field. However, one can never be completely sure that all trials have been identified. Since there are still relatively few studies in this field, it may be that future studies can change the estimates of the effect. For example, the search identified eight ongoing studies, but the results were not yet published and could not be included. In this update, we decided to include only RCTs. The intention was to improve the certainty of the evidence now that more RCT were available. However, this decision also led to a number of quasi‐randomised studies to be excluded, and we cannot rule out the possibility that they contain relevant information.
Agreements and disagreements with other studies or reviews
We identified five other systematic reviews on the efficacy of music listening for improvement of sleep quality in adults with sleep problems (Chen 2021; De Niet 2009; Feng 2018; Petrovsky 2021; Wang 2014). These reviews had different inclusion and exclusion criteria and, therefore, included a different set of trials. The major difference was the population under review and the definition of the music intervention. Compared to other reviews, our inclusion criteria were stricter with regard to population, intervention and study design. We included only studies that documented the sleep problems of the participants at baseline. Furthermore, we focused on listening to prerecorded music and included only RCTs in this 2021 update.
De Niet 2009 included five trials (308 participants). They only included trials in which the participants were adults with sleep complaints; however, it seems that they did not apply this inclusion criteria strictly, since they included one trial in which only some participants had poor sleep (Hérnandez‐Ruíz 2005), and one trial with no clear documentation of the participants' sleep problems (Zimmerman 1996). They conducted a meta‐analysis on sleep quality showing a beneficial effect similar to our results (SMD −0.74, 95% CI −0.96 to −0.46) (De Niet 2009).
Wang 2014 focused on acute and chronic sleep disorder, and they did not require documentation of the sleep problems at baseline, resulting in a broader range of included trials. They included 10 trials (557 participants) that also included sleep problems related to hospitalisation. Their main outcome was sleep quality, and they reported a positive effect of the intervention (SMD −0.63, 95% CI −0.92 to −0.34). These results are similar to our meta‐analysis of the sleep quality outcome, but the effect size is slightly smaller.
Feng 2018 conducted a network meta‐analysis, including both randomised and non‐randomised trials with combined interventions involving music, exercise, acupuncture, stimulus control and relaxation instructions. The study claimed to focus on adults with primary insomnia, but several of the studies included populations with no diagnosis of primary insomnia. The review included 20 studies (1339 participants), and they reported a meta‐analysis on the effect of listening to music compared with usual care on sleep quality measured using the PSQI (SMD −0.61, 95% CI −1.01 to −0.20; 10 studies; 541 participants). These results are in line with our analysis, even though the population under study may be slightly different.
Recently, two reviews have been published, focusing on the effect of music for sleep improvement in older adults (Chen 2021; Petrovsky 2021). Petrovsky 2021 included both randomised and non‐randomised trials (16 studies, 812 participants). The studies included adults aged 50 years or older, and 11 studies used music listening interventions and five used combined interventions including music. Some studies had no control group and others compared music listening to active interventions such as walking exercise, meditation or progressive muscle relaxation. The review did not conduct a meta‐analysis due to high clinical and methodological heterogeneity, but in their narrative found mixed evidence regarding an effect on sleep quality and other sleep parameters measured subjectively or objectively. These results differ from our results where we found evidence of a beneficial effect on sleep quality. However, we also found mixed evidence for an effect on other sleep outcomes, similar Petrovsky 2021. Chen 2021 focused on music for sleep improvement in adults aged 60 years or older and only included RCTs. They included five studies (288 participants) that included both music listening interventions and active music making. The meta‐analysis showed a beneficial effect of the music interventions on sleep quality measured using the PSQI (MD −1.96, 95% CI −3.23 to −0.69). In a subgroup analysis, they found that sedative music was more beneficial than rhythm‐centred music. Another subgroup analysis suggested that using the music listening intervention for four weeks or longer was more efficient than shorter intervention periods (Chen 2021). In our review, we did not find this effect of a longer intervention period, and future studies should clarify the impact of the duration of the intervention. Overall, the present review adds to the robustness of the findings by following rigorous methodology, including an extensive search strategy, clear inclusion criteria, and careful assessment and reporting of risk of bias.
Authors' conclusions
Implications for practice.
The findings of the meta‐analysis suggest that listening to music may have a moderate to large beneficial effect on sleep quality in different populations experiencing insomnia symptoms. Limited conclusions can be drawn on the effect of music listening on other aspects of sleep or on related physiological and psychological aspects of daytime function, since few trials reported these outcomes. With the available evidence, this review provides no evidence of an effect of the music intervention on objective measures of sleep‐onset latency, total sleep time, sleep interruption and sleep efficiency. However, subjective measures of these outcomes suggest that participants may experience improvements in sleep‐onset latency, total sleep time and sleep efficiency with the music intervention with broad confidence intervals ranging from small to large effect sizes. We found no evidence of an effect on experienced sleep interruption. Since the studies report limited information on the nature of participants' sleep problems, it is not possible to draw any conclusions with regard to the effect on insomnia subtypes such as difficulties with sleep initiation, sleep maintenance or non‐restorative sleep. Two studies diagnosed the participants with insomnia, and they both showed improved sleep quality with the music intervention, but no change in insomnia severity.
All included trials used music that was characterised as sedative or relaxing. However, these included a number of different musical styles (e.g. classical, new age, jazz, etc.), and at this point, it is not clear if some types of music may be more effective than others. In the literature, it is often recommended that participants are allowed to choose their own preferred music. In this review, there was no difference in the effect on sleep quality between trials using researcher‐selected music and trials giving the participants a choice among a number of preselected types of music. Very few participants were offered the possibility to bring their own preferred music, and the effect of purely participant‐selected music could not be investigated.
Implications for research.
More high‐quality randomised controlled trials are needed to assess the effectiveness of music listening for treating insomnia. The quality of the studies has improved from the 2015 review to this 2021 update, but blinding of researchers and outcome assessors should still be prioritised to minimise performance and detection biases.
Future research should consider a wider range of outcomes. In particular, more research should include objective measures of sleep, such as polysomnography and actigraphy, that are less sensitive to detection bias. The use of objective measures of sleep would reduce the impact of any placebo effect. Furthermore, there is limited knowledge of the effect of listening to music on daytime consequences and waking correlates of insomnia. It is important to know if the reported changes in sleep patterns or sleep quality are sufficient to affect daytime function. Furthermore, longer follow‐up periods are important to genuinely establish the effectiveness of music and its long‐term effect.
More research is needed to establish the effect of the intervention on different insomnia subgroups. Insomnia is a highly heterogeneous condition with different aetiology and severity. Future trials should take care to define and appropriately measure sleep disturbances and provide detailed information on the cause, duration and severity of symptoms, as well as any comorbid conditions. Participants should be screened for other sleep disorders to clarify the nature of the sleep complaint. It is also recommended that researchers employ well‐defined criteria for insomnia such as the diagnostic criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM) or ICSD. This would improve the precision of the clinical diagnosis and hence improve comparability across trials.
The music therapy literature recommends that music used for sedative purposes should be characterised by a slow tempo and an absence of abrupt changes and rhythmic complexity (Wigram 2002). These recommendations are supported by experimental research in the field of music psychology (Scarratt 2021), but more controlled clinical trials are needed to examine which aspects of music are important to achieve an improvement in sleep. In addition, the relationship between the objective characteristics of the music and the subjective preferences of the individual remain unclear, and more trials are needed to investigate potential differences in effect between music selected by the researcher and that selected by the participant. Another aspect of the intervention that remains unclear is the optimal frequency, timing and duration of the intervention. Further research into these domains is important for assessing the effectiveness of the intervention and for providing the best treatment options for people with insomnia.
What's new
| Date | Event | Description |
|---|---|---|
| 17 June 2022 | New citation required but conclusions have not changed | We included eight new studies in this updated review. One previously included study was excluded as it was a quasi‐randomised controlled trial (RCT) and this version of the review was restricted to RCTs only. The conclusions have not changed. |
| 13 December 2021 | New search has been performed | Updated following a new search in January 2021 and a top‐up search in December 2021 |
History
Protocol first published: Issue 3, 2013 Review first published: Issue 8, 2015
| Date | Event | Description |
|---|---|---|
| 27 November 2015 | Amended | Typographical error corrected |
Acknowledgements
We would like to thank the editorial team of the Cochrane Developmental, Psychosocial and Learning Problems Group (CDPLPG) for their excellent advice and support. We are also grateful to Information Specialist Margaret Anderson for her assistance with the search process and to PhD student Mia Dong for translations. We thank Dr Kullich, Dr Bernatzky, Dr Harmat and Dr Burrai for kindly providing additional information about their trials. Thanks also to the Danish National Research Foundation for providing the fundament for our research center (DNRF117).
The CRG Editorial Team are grateful to the following peer reviewers for their time and comments: Dr Bei Bei, Monash University, Australia; Colin Espie, Professor of Sleep Medicine, University of Oxford, UK; Dr Helen McAneney, School of Nursing, Midwifery and Health Systems, University College Dublin, Ireland; and Ngenjang Melvis BSc Nursing/Midwifery, University of Bamenda, Cameroon.
In addition, the CRG Editorial Team are grateful to Anne Lawson, Copy Edit Support, Cochrane, for copyediting this review.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL), part of the Cochrane Library
#1 [mh Music] #2 [mh "Music therapy"] #3 music* #4 {or #1‐#3} #5 [mh Sleep] #6 [mh "Sleep Wake Disorders"] #7 (pre NEXT sleep* or presleep*) #8 sleep* #9 insomnia* #10 dyssomn* #11 (awake* or wake* or waking or awaking) #12 {or #5‐#11} #13 #4 AND #12 in Trials
MEDLINE Ovid
1 Music/ 2 music therapy/ 3 music$.mp. 4 or/1‐3 5 exp Sleep/ 6 exp Sleep Wake Disorders/ 7 (pre‐sleep$ or presleep$).tw,kf. 8 sleep$.tw,kf. 9 insomnia$.tw,kf. 10 dyssomn$.tw,kf. 11 (awake$ or wake$ or waking or awaking).tw,kf. 12 or/5‐11 13 4 and 12 14 randomized controlled trial.pt. 15 controlled clinical trial.pt. 16 randomi#ed.ab. 17 placebo$.ab. 18 drug therapy.fs. 19 randomly.ab. 20 trial.ab. 21 groups.ab. 22 or/14‐21 23 exp animals/ not humans.sh. 24 22 not 23 25 13 and 24
MEDLINE In‐Process & Other Non‐Indexed Citations Ovid
1 music$.mp. 2 (pre‐sleep$ or presleep$).tw,kf. 3 sleep$.tw,kf. 4 insomnia$.tw,kf. 5 dyssomn$.tw,kf. 6 (awake$ or wake$ or waking or awaking).tw,kf. 7 or/2‐6 8 1 and 7 9 (random$ or trial$ or control$ or group$ or placebo$ or blind$ or prospectiv$ or longitudinal$ or meta‐analys$ or systematic review$).tw. 10 8 and 9
MEDLINE Epub Ahead of Print Ovid
1 music$.mp. 2 (pre‐sleep$ or presleep$).tw,kf. 3 sleep$.tw,kf. 4 insomnia$.tw,kf. 5 dyssomn$.tw,kf. 6 (awake$ or wake$ or waking or awaking).tw,kf. 7 or/2‐6 8 1 and 7 9 (random$ or trial$ or control$ or group$ or placebo$ or blind$ or prospectiv$ or longitudinal$ or meta‐analys$ or systematic review$).tw. 10 8 and 9
Embase Ovid
1 music therapy/ 2 music/ 3 music$.mp. 4 or/1‐3 5 exp sleep disorder/ 6 exp sleep quality/ 7 exp sleep disorder assessment/ 8 sleep$.tw,kw. 9 (pre‐sleep$ or presleep$).tw,kw. 10 insomnia$.tw,kw. 11 dyssomn$.tw,kw. 12 (awake$ or wake$ or waking or awaking).tw,kw. 13 or/5‐12 14 4 and 13 15 Randomized controlled trial/ 16 Controlled clinical study/ 17 random$.ti,ab. 18 randomization/ 19 intermethod comparison/ 20 placebo.ti,ab. 21 (compare or compared or comparison).ti. 22 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 23 (open adj label).ti,ab. 24 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 25 double blind procedure/ 26 parallel group$1.ti,ab. 27 (crossover or cross over).ti,ab. 28 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 29 (assigned or allocated).ti,ab. 30 (controlled adj7 (study or design or trial)).ti,ab. 31 (volunteer or volunteers).ti,ab. 32 human experiment/ 33 trial.ti. 34 or/15‐33 35 (random$ adj sampl$ adj7 ("cross section$" or questionnaire$1 or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.) 36 Cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.) 37 (((case adj control$) and random$) not randomi?ed controlled).ti,ab. 38 (Systematic review not (trial or study)).ti. 39 (nonrandom$ not random$).ti,ab. 40 "Random field$".ti,ab. 41 (random cluster adj3 sampl$).ti,ab. 42 (review.ab. and review.pt.) not trial.ti. 43 "we searched".ab. and (review.ti. or review.pt.) 44 "update review".ab. 45 (databases adj4 searched).ab. 46 (rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/ 47 Animal experiment/ not (human experiment/ or human/) 48 or/35‐47 49 34 not 48 50 14 and 49
CINAHL EBSCOhost
S1 MH randomized controlled trials S2 MH double‐blind studies S3 MH single‐blind studies S4 MH random assignment S5 MH pretest‐posttest design S6 MH cluster sample S7 TI (randomised OR randomized) S8 AB (random*) S9 TI (trial) S10 MH (sample size) AND AB (assigned OR allocated OR control) S11 MH (placebos) S12 PT (randomized controlled trial) S13 AB (control W5 group S14 MH (crossover design) OR MH (comparative studies) S15 AB (cluster W3 RCT) S16 MH animals+ S17 MH (animal studies) S18 TI (animal model*) S19 S16 OR S17 OR S18 S20 MH (human) S21 S19 NOT S20 S22 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 S23 S22 NOT S21 S24 (MH "Music") S25 (MH "Music Therapy") S26 music* S27 (MH "Sleep") S28 (MH "Sleep Disorders+") S29 (presleep* or pre‐sleep*) S30 sleep* S31 insomnia* S32 dyssomn* S33 awake* or wake* or waking or awaking S34 S24 OR S25 OR S26 S35 S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 S36 S23 AND S34 AND S35
APA PsycINFO Ovid
1 exp Music/ 2 music therapy/ 3 music$.mp. 4 or/1‐3 5 exp sleep wake disorders/ 6 exp sleep/ 7 wakefulness/ 8 sleep onset/ 9 Sleep Deprivation/ 10 sleep$.tw. 11 insomnia$.tw. 12 (pre‐sleep$ or presleep$).tw. 13 dyssomn$.tw. 14 (awake$ or wake$ or waking or awaking).tw. 15 or/5‐14 16 4 and 15 17 exp clinical trials/ 18 longitudinal studies/ 19 exp program evaluation/ 20 exp Treatment Effectiveness Evaluation/ 21 random$.tw. 22 trial$.tw. 23 group$.ab. 24 ((singl$ or doubl$ or tripl$ or trebl$) adj1 (blind$ or mask$)).tw. 25 prospective.tw. 26 factorial$.tw. 27 control.ab. 28 ("treatment as usual" or "usual treatment" or "usual care" or tau).ab. 29 placebo.ab. 30 (crossover or cross‐over).tw. 31 exp program evaluation/ 32 exp treatment outcomes/ 33 ((effectiveness or evaluat$) adj3 (stud$ or research$)).tw. 34 or/17‐33 35 16 and 34
Web of Science Clarivate (Science Citation Index Expanded, Social Sciences Citation Index, Arts and Humanities Citation Index, Conference Proceedings Citation Index – Science, and Conference Proceedings Citation Index – Social Science and Humanities)
# 5 #4 AND #3 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 4 TI=( random* OR trial* OR control* OR ( ( allocat* OR assign* ) near/3 group* ) OR placebo* OR blind* OR "treatment as usual" OR tau OR "usual care" OR prospectiv* OR longitudinal* OR rct* ) OR AB=( random* OR trial* OR control* OR ( ( allocat* OR assign* ) near/3 group* ) OR placebo* OR blind* OR "treatment as usual" OR tau OR "usual care" OR prospectiv* OR longitudinal* OR rct* ) # 3 #2 AND #1 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 2 TI=( presleep* OR "pre‐sleep*" OR sleep* OR dyssomnia* OR insomnia* OR awake* OR wake* OR waking OR awaking ) OR AB= ( presleep* OR "pre‐sleep*" OR sleep* OR dyssomnia* OR insomnia* OR awake* OR wake* OR waking OR awaking ) # 1 172,810 TI=( music* ) OR AB=( music* ) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years
SCOPUS Elsevier
(TITLE‐ABS‐KEY ( music* ) ) W/15 ( TITLE‐ABS‐KEY ( presleep* OR "pre‐sleep*" OR sleep* OR dyssomnia* OR insomnia* OR awake* OR wake* OR waking OR awaking ) ) AND ( TITLE‐ABS‐KEY ( random* OR trial* OR control* OR ( ( allocat* OR assign* ) W/3 group* ) OR placebo* OR blind* OR "treatment as usual" OR tau OR "usual care" OR prospectiv* OR longitudinal* OR rct* ) )
RILM Abstracts of Music Literature EBSCOhost
S10 S8 NOT S9 S9 "In the wake of" S8 S6 AND S7 S7 TI(random* or trial* or control* or ((allocat* or assign*) N3 group*) or placebo* or "treatment as usual" or TAU or "usual care" or prospectiv* or longitudinal* or meta‐analys* or "systematic review*" or RCT*) OR AB(random* or trial* or control* or ((allocat* or assign*) N3 group*) or placebo* or "treatment as usual" or TAU or "usual care" or prospectiv* or longitudinal* or meta‐analys* or "systematic review*" or RCT*) S6 S1 OR S2 OR S3 OR S4 OR S5 S5 (TI(awake* or wake* or waking or awaking) OR AB(awake* or wake* or waking or awaking)) AND (TI(music*) OR AB(music*)) S4 TI(dyssomn*) OR AB(dyssomn*) S3 (TI(insomnia*) OR AB(insomnia*)) S2 (TI(sleep*) OR AB(sleep*)) AND TI ((music*) OR AB(music*)) S1 TI(presleep* or "pre‐sleep*") OR AB(presleep* or "pre‐sleep*")
Cochrane Database of Systematic Reviews, part of the Cochrane Library
#1 [mh Music] #2 [mh "Music therapy"] #3 music*:ti,ab,kw #4 {or #1‐#3} #5 [mh Sleep] #6 [mh "Sleep Wake Disorders"] #7 (pre NEXT sleep* or presleep*):ti,ab,kw #8 sleep*:ti,ab,kw #9 insomnia*:ti,ab,kw #10 dyssomn*:ti,ab,kw #11 (awake* or wake* or waking or awaking):ti,ab,kw #12 {or #5‐#11} #13 #4 AND #12 in Cochrane Reviews
Epistemonikos
(title:(music*) OR abstract:(music*)) AND (title:(sleep* OR presleep* OR insomnia* OR dyssomnia* OR awake* OR wake* OR waking OR awaking) OR abstract:(sleep* OR presleep* OR insomnia* OR dyssomnia* OR awake* OR wake* OR waking OR awaking)) .Limited to systematic reviews
ClinicalTrials.gov
CONDITION| insomnia OR dyssomnia OR sleep OR sleepless OR awake OR wake OR awaken OR waken OR awaking OR waking AND Interventions| music | Studies that accept healthy volunteers
World Health Organization International Clinical Trials Registry Platform
Basic search: music AND insomnia OR music AND sleep OR music AND wake OR music AND awaken
Appendix 2. Criteria for assigning risk of bias judgements
1. Random sequence generation (checking for possible selection bias)
We assessed the method used to generate the allocation sequence for each included trial in sufficient detail to allow an assessment of whether it produced comparable groups. We rated the risk of bias as follows.
Low risk of bias: adequate method of random sequence generation (e.g. any truly random process such as random number table; computer random number generator). Block randomisation was considered low risk of bias if block size varied randomly.
High risk of bias: inadequate method of random sequence generation (e.g. any non‐random process such as odd or even date of birth; hospital or clinic record number).
Unclear risk of bias: insufficient information about the method of random sequence generation to permit a judgement of low risk or high risk of bias.
2. Allocation concealment (checking for possible selection bias)
We assessed the method used to conceal the allocation sequence for each included trial and determined whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment. We rated the risk of bias as follows.
Low risk of bias: adequate method of allocation concealment (e.g. telephone or central randomisation); consecutively numbered sealed opaque envelopes.
High risk of bias: inadequate method of allocation concealment (e.g. open random allocation); unsealed or non‐opaque envelopes; alternation; date of birth.
Unclear risk of bias: insufficient information to permit a judgement of low risk or high risk of bias.
3. Blinding of participants and personnel (checking for possible performance bias)
We assessed the different methods used to blind personnel from knowledge of which intervention a participant received for each included trial. Since it is not possible to blind a participant to the treatment (music), we assessed trials at low risk of bias if we judged that the lack of blinding was not affecting the results. We assessed blinding of participants and personnel separately for different outcomes or classes of outcomes, since we expected certain outcomes (e.g. laboratory measurements and physiological data such as heart rate or blood pressure) to be unaffected by blinding of participants and personnel. We rated the risk of bias as follows.
Low risk of bias: adequate method of blinding; outcome unlikely to be influenced by lack of blinding.
High risk of bias: inadequate method of blinding; outcome likely to be influenced by lack of blinding.
Unclear risk of bias: insufficient information to permit a judgement of low risk or high risk of bias.
4. Blinding of outcome assessment (checking for possible detection bias)
We assessed the methods used to blind outcome assessment for each included trial. We assessed blinding separately for different outcomes or classes of outcomes, as stated above. We rated the risk of bias as follows.
Low risk of bias: adequate method of blinding; outcome unlikely to be influenced by lack of blinding.
High risk of bias: inadequate method of blinding; outcome likely to be influenced by lack of blinding.
Unclear risk of bias: insufficient information to permit a judgement of low risk or high risk of bias.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We assessed data on attrition, exclusions and reasons to determine if they introduced bias. We described the completeness of data for each included trial and for each outcome or class of outcomes. We assessed whether attrition and exclusions were reported, the numbers of participants included at each stage of the analysis (compared with the total participants randomised), whether reasons for attrition or exclusion were reported, and whether missing data were balanced across groups or were likely to be related to outcomes. We judged whether incomplete data were dealt with adequately and rated the risk of bias as follows.
Low risk of bias: no missing outcome data; balanced missing outcome data; appropriate methods of imputing missing data.
High risk of bias: missing outcome data; unbalanced missing outcome data; inappropriate methods of imputing missing data.
Unclear risk of bias: insufficient information to permit a judgement of low risk or high risk of bias.
6. Selective reporting bias
We investigated the possibility of selective outcome reporting bias for each included trial. We conducted electronic searches to identify protocols of respective trials as a source to judge selective reporting. We rated the risk of bias as follows.
Low risk of bias: all prespecified and expected outcomes were reported.
High risk of bias: not all prespecified and expected outcomes were reported; outcome that was not prespecified was reported; outcome was reported incompletely.
Unclear risk of bias: insufficient information to permit a judgement of low risk or high risk of bias.
7. Other bias
We assessed other risks of bias, specifically a risk of bias from baseline differences and a risk of bias from carry‐over or period effects for cross‐over trials. We rated the risk of bias as follows.
Low risk of bias: trial appeared free of other sources of bias.
High risk of bias: there was at least one high risk of bias.
Unclear risk of bias: insufficient information to permit a judgement of low risk or high risk of bias.
Appendix 3. Additional methods archived for use in future updates of this review
| Analysis | Methods |
| Measures of treatment effect |
Dichotomous data For dichotomous data, we would have presented the results as summary odd ratios (OR) with 95% confidence intervals (CI). |
|
Continuous data The standardised mean difference (SMD) would have been used to combine trials that measured the same outcome, but used different scales. All outcomes would have been presented with 95% CIs. If a trial had provided multiple interchangeable measures of the same construct at the same time point, we would have calculated the mean SMD across these outcomes and the mean of their estimated variances. Where trials had reported the same outcomes using continuous and dichotomous measures, we would have re‐expressed ORs as SMDs, thereby allowing dichotomous and continuous data to be pooled together, as described in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). | |
|
Ordinal data Ordinal data measured on shorter scales would have been analysed as dichotomous data by combining categories, and the intervention effect would have been expressed using OR. | |
| Unit of analysis issues |
Cluster‐randomised trials We anticipated that trials using clustered randomisation would have controlled for clustering effects. In case of doubt, we would have contacted the first authors to ask for individual participant data to calculate an estimate of the intracluster correlation coefficient (ICC). Had this not been possible, we would have obtained external estimates of the ICC from a similar trial or from a study of a similar population, as described in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). When the ICC was established, we would have used it to reanalyse the trial data. If ICCs from other sources were used, we would have reported this and conducted sensitivity analyses to investigate the effect of variation in the ICC. |
|
Cross‐over trials Cross‐over trials would have been analysed using combined data from all study periods, or using first period data if combined data were not available. | |
|
Trials with > 2 treatment arms Had > 1 of the interventions been a music intervention, and there had been sufficient information in the trial to assess the similarity of the interventions, we would have combined similar music interventions to allow for a single pair‐wise comparison. | |
| Dealing with missing data | We would have explored the impact of including studies with high levels of missing data by performing sensitivity analyses based on consideration of best‐case and worst‐case scenarios. The potential impact of missing data on the findings of the review would have been addressed in the 'Discussion' section of the review. |
| Assessment of heterogeneity | Had there been significant heterogeneity, we would have investigated it by conducting a subgroup analysis based on the participants' clinical characteristics and the interventions used in the included studies (see subsection on 'Subgroup analyses' below). |
| Assessment of reporting bias | Had sufficient study data been available for individual outcomes, we would have drawn and inspected funnel plots for evidence of reporting or publication bias. We would have assessed funnel plot asymmetry visually and statistically using the Bee and Mazumdar (Begg 1994) and the Egger tests (Egger 1997); 10 or more studies are recommended. Had asymmetry been suggested by visual assessment or detected in any of these tests, we would have performed exploratory analyses to investigate if it reflected publication bias or a true relationship between trial size and effect size. |
| Subgroup analyses | We would have conducted the following subgroup analyses.
|
| Sensitivity analysis | We would have conducted a sensitivity analysis excluding trials using inadequate methods of blinding personnel. |
Data and analyses
Comparison 1. Sleep quality: listening to music versus control – Pittsburgh Sleep Quality Index (PSQI) – global score.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Sleep quality: Pittsburgh Sleep Quality Index (PSQI) – immediately postintervention | 10 | 708 | Mean Difference (IV, Random, 95% CI) | ‐2.79 [‐3.86, ‐1.72] |
| 1.2 Subgroup (PSQI) by duration of intervention – immediately postintervention | 10 | 708 | Mean Difference (IV, Random, 95% CI) | ‐2.79 [‐3.86, ‐1.72] |
| 1.2.1 Medium duration (8–21 days) | 5 | 343 | Mean Difference (IV, Random, 95% CI) | ‐2.24 [‐2.90, ‐1.58] |
| 1.2.2 Long duration (22–90 days) | 5 | 365 | Mean Difference (IV, Random, 95% CI) | ‐3.36 [‐5.63, ‐1.10] |
| 1.3 Subgroup (PSQI) by aetiology – immediately postintervention | 9 | 644 | Mean Difference (IV, Random, 95% CI) | ‐2.82 [‐4.09, ‐1.56] |
| 1.3.1 Age‐related sleep problems | 3 | 184 | Mean Difference (IV, Random, 95% CI) | ‐2.78 [‐3.97, ‐1.58] |
| 1.3.2 Insomnia related to medical conditions | 3 | 276 | Mean Difference (IV, Random, 95% CI) | ‐3.87 [‐7.66, ‐0.08] |
| 1.3.3 Insomnia disorder | 2 | 63 | Mean Difference (IV, Random, 95% CI) | ‐2.47 [‐4.18, ‐0.76] |
| 1.3.4 Pregnancy‐related insomnia | 1 | 121 | Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐2.35, ‐0.01] |
| 1.4 Subgroup (PSQI) by music selection – immediately postintervention | 10 | 708 | Mean Difference (IV, Random, 95% CI) | ‐2.79 [‐3.86, ‐1.72] |
| 1.4.1 Researcher‐selected | 5 | 370 | Mean Difference (IV, Random, 95% CI) | ‐3.31 [‐5.32, ‐1.29] |
| 1.4.2 Participant‐selected (choice among researcher‐selected playlists) | 5 | 338 | Mean Difference (IV, Random, 95% CI) | ‐2.33 [‐3.37, ‐1.29] |
| 1.5 Subgroup (PSQI) by relaxation instructions – immediately postintervention | 10 | 708 | Mean Difference (IV, Random, 95% CI) | ‐2.79 [‐3.86, ‐1.72] |
| 1.5.1 Music listening alone | 8 | 583 | Mean Difference (IV, Random, 95% CI) | ‐2.85 [‐4.18, ‐1.51] |
| 1.5.2 Music listening and relaxation instructions | 2 | 125 | Mean Difference (IV, Random, 95% CI) | ‐2.64 [‐3.74, ‐1.54] |
Comparison 2. Insomnia severity: listening to music versus control – Insomnia Severity Index (ISI).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Insomnia severity: Insomnia Severity Index (ISI) – immediately postintervention | 2 | 63 | Mean Difference (IV, Random, 95% CI) | ‐6.96 [‐15.21, 1.28] |
Comparison 3. Sleep onset latency: listening to music versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Sleep onset latency: Pittsburgh Sleep quality Index (PSQI) – immediately postintervention | 3 | 197 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.83, ‐0.37] |
Comparison 4. Total sleep time: listening to music versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Total sleep time: Pittsburgh Sleep Quality Index – immediately postintervention | 3 | 197 | Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.16, ‐0.23] |
Comparison 5. Sleep interruption: listening to music versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Sleep interruption: Pittsburgh Sleep Quality Index – immediately postintervention | 3 | 197 | Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.47, 0.40] |
Comparison 6. Sleep efficiency: listening to music versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Sleep efficiency: Pittsburgh Sleep Quality Index (component score) – immediately postintervention | 3 | 197 | Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐1.38, ‐0.54] |
Comparison 7. Depression: listening to music versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Depression – immediately postintervention | 2 | 173 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.04 [‐4.45, 0.37] |
Comparison 8. Anxiety: listening to music versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Anxiety – immediately postintervention | 3 | 294 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.75, ‐0.28] |
Comparison 9. Quality of life: listening to music versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Quality of life – immediately postintervention | 2 | 177 | Std. Mean Difference (IV, Random, 95% CI) | 0.55 [0.25, 0.85] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Amiri 2019.
| Study characteristics | ||
| Methods |
Study type: RCT Design: 2‐arm, parallel group design Blinding: not blinded |
|
| Participants |
Sample: adults with insomnia disorder based on the criteria in the second edition of the International Classification of Sleep Disorders n: 30 randomised, 30 completed and included in analyses Age: mean 27 (SD 2.5) years Sex: 30 men, 0 women Setting: participants' homes + 1 live session per week Country: Iran |
|
| Interventions |
Intervention (n = 15): music group. Participants listened to researcher‐selected Persian music administered themselves at home. The listening device was not reported. Music characteristics: the music included the Dastgahs of Nava and Bayat‐e Esfahan. The instruments used for playing were setar, tar, tonbak, kamancheh, oud and daf. The recorded music included songs from the works of Mohammad Reza Shajarian, Parviz Meshkatian, Hossein Alizadeh, Hossein Behroozinia, Ali Pajooheshgar, Masoud Shaari, Mohammad Reza Lotfi, Faramarz Payvar, Alireza Eftekhari, Salar Aghili, Amir Motavalli, and Gholam Hossein Banan. Length of sessions: 60 minutes Frequency of sessions: daily between 10 pm and 11 pm Duration of intervention period: 6 weeks (42 days) Control (n = 15): waitlist |
|
| Outcomes |
|
|
| Notes |
Trial start and end dates: no information provided Funding sources: sponsored by Kermanshah University of Medical Sciences, Iran Protocol registration: Iranian Registry of Clinical Trials (IRCT2017040425817N3) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "30 patients (mean age: 27) were enrolled and randomly assigned to the intervention (N = 15) and control (N = 15) groups." (Amiri 2019, p 2). Comment: no information on randomisation method. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on randomisation procedure or measures taken to conceal allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not possible to blind participants to the music intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no attrition and no indication of missing data. |
| Selective reporting (reporting bias) | Low risk | Comment: results reported corresponded to protocol. |
| Other bias | Low risk | Comment: no indication of other risk of bias. |
Burrai 2020.
| Study characteristics | ||
| Methods |
Study type: RCT Design: 2‐arm, parallel group design Blinding: single blind; outcome assessors were blinded to group allocation. |
|
| Participants |
Sample: adults with heart failure and sleep difficulties documented by PSQI scores > 5 n: 159 randomised, 141 completed and included in analyses Age: mean age reported by group; music group: mean 71.6 (SD 12) years, control group: mean 74.6 (SD 10.9) years Sex: 99 men, 60 women Setting: participants' homes Country: Italy |
|
| Interventions |
Intervention (n = 82): music group. TAU plus listening to a researcher‐selected playlist of 80 classical pieces. Participants administered the music themselves and received an MP3 music player to use for the intervention. Music characteristics: the playlist consisted of 80 predefined classical tracks. The tempo/rhythm was setup at 60–80 bpm. Length of sessions: 30 minutes Frequency of sessions: daily Duration of intervention period: 90 days Control (n = 77): TAU |
|
| Outcomes |
aOutcome not included in this review. |
|
| Notes |
Trial start and end dates: no information provided Funding sources: no information provided Protocol registration: ClinicalTrials.gov (NCT02394938) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients … were randomly assigned to a music listening group or to a control group with a 1:1 ratio" (Burrai 2020, p 542). Comment: trial described as a multicentre RCT but method of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on randomisation procedure or measures taken to conceal allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not possible to blind participants to music intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "outcomes assessors and investigators were blinded and unaware of treatment assignment" (Burrai 2020, p 542). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: attrition reported and balanced. |
| Selective reporting (reporting bias) | Low risk | Comment: protocol available at ClinicalTrials.gov. No indication of selective outcome reporting. |
| Other bias | Low risk | Comment: no indication of additional bias. |
Cai 2015.
| Study characteristics | ||
| Methods |
Study type: RCT Design: 2‐arm, parallel group design Blinding: not blinded |
|
| Participants |
Sample: adults who experienced insomnia after stroke. Insomnia diagnosed according to the seventh edition textbook of Internal Medicine of Traditional Chinese Medicine n: 154 randomised, 154 completed and included in analyses Age: mean age reported by group; music group: 63.9 (SD 10.4) years, control group: 64.5 (SD 12.6) years Sex: 83 men, 71 women Setting: rehabilitation clinic Country: China |
|
| Interventions |
Intervention (n = 77): music group. TAU (auricular acupoint sticking) plus listening to researcher‐selected music. The music was administered by the health professionals. The listening device was not reported. Music characteristics: music of traditional Five Elements tones (including Gong tone, Shang tone, Jue tone, Zhi tone, and Yu tone) was chosen in accordance with different traditional Chinese medicine patterns of body constitutions and insomnia condition. Length of sessions: 30 minutes Frequency of sessions: daily Duration of intervention period: 30 days Control (n = 77): TAU (auricular acupoint sticking) |
|
| Outcomes |
|
|
| Notes |
Trial start and end dates: January 2013 to August 2014 Funding sources: supported by Project of Zhejiang Provincial Administration of Traditional Chinese Medicine Protocol registration: none found |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The above patients were randomly divided into an observation group and a control group by the random digital table, 77 cases in each group." (Cai 2015, p 228). |
| Allocation concealment (selection bias) | Unclear risk | Comment: trial described as a multicentre RCT. No information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: trial described as a multicentre RCT. Not possible to blind participants to music intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: trial described as a multicentre RCT. No information on who performed the assessment of sleep improvement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: trial described as a multicentre RCT. No sign of attrition. |
| Selective reporting (reporting bias) | Low risk | Comment: trial described as a multicentre RCT. We found no protocol for this study, but there was no indication of reporting bias. |
| Other bias | Low risk | Comment: trial is described as a multicentre RCT. No indication of additional bias. |
Chang 2012.
| Study characteristics | ||
| Methods |
Study type: RCT Design: 2‐arm, parallel group design Blinding: single blinded; technician scoring PSG and researchers responsible for statistical analysis unaware of group allocation |
|
| Participants |
Sample: adults who experienced insomnia for ≥ 1 month documented by a PSQI score > 5 n: 50 randomised, 50 completed and included in analyses Age: mean 32 (SD 11, range 22–58) years Sex: 3 men, 47 women Setting: sleep laboratory Country: Taiwan |
|
| Interventions |
Intervention (n = 25): music group. Participants were encouraged to bring their own preferred music to listen to (n = 10) and those who did not bring their own music, listened to researcher‐selected music (n = 15). The music was administered by the researchers using a CD player (AZ‐1836, Philips, the Netherlands) Music characteristics: Rural Spring Field, Woman under the Moon (Chinese music), Going Home (Czech music), Destiny, Heart Lotus (Taiwanese music), and Memory (composed by the authors). Tempos were 60–85 bpm, minor tonalities, smooth melodies and no dramatic changes in volume or rhythm. The music was expected to be familiar to participants. Length of sessions: 45 minutes Frequency of sessions: daily at bedtime Duration of intervention period: 3 consecutive days Control (n = 25): no intervention |
|
| Outcomes |
We contacted the author 16 December 2014 to obtain data on the raw postintervention scores, but we have not yet received a reply. aOutcome not included in this review |
|
| Notes |
Trial start and end dates: May 2010 to June 2011 Funding sources: National Science Council, Taiwan Protocol registration: none found |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomly assigned …, using the drawing of lots" (Chang 2012, p 924). |
| Allocation concealment (selection bias) | Low risk | Quote: "All lots (labels) are packed in a jar that was prepared by another person. Researchers therefore did not know beforehand which group each participant would be assigned to" (Chang 2012, p 924). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: due to the nature of the intervention, blinding of participants was not possible. It is unclear if this affected the objective sleep measures, but likely that it affected the subjective measures of sleep. Blinding of personnel at the sleep laboratory was not reported. Since the intervention was music, it is likely that they were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: the technician scoring the PSG and the researchers doing the statistical analyses were not aware of which group the data belonged to. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropouts and no missing data. |
| Selective reporting (reporting bias) | Unclear risk | Comment: we found no published protocol on this study. Sleep efficiency, based on a self‐report questionnaire, was not reported. All other measures of interest were included in the analysis. |
| Other bias | High risk | Comment: there were baseline differences in measures of depression and self‐reported number of awakenings, with the music group experiencing significantly more depression and arousals than the control group. |
Harmat 2008.
| Study characteristics | ||
| Methods |
Study type: RCT Design: 3‐arm, parallel group design Blinding: single blind; group allocation was coded for the person performing the statistics (Harmat 2014 [pers comm]) |
|
| Participants |
Sample: students with poor sleep documented by PSQI scores > 5 n: 94 randomised (64 included in this review). 94 completed and included in the analyses Age: mean 22.6 (SD 2.9, range 19–28) years Sex: 21 men, 73 women Setting: participants' homes Country: Hungary |
|
| Interventions |
Intervention 1 (n = 35): music group. Participants listened to researcher‐selected classical music. Participants administered the music themselves. The listening device was not reported. Music characteristics: The Most Relaxing Classical (2 CD, Edited by Virgin 1999). Popular pieces from Baroque to Romantic Length of sessions: 45 minutes Frequency of sessions: daily at bedtime Duration of intervention period: 3 weeks Intervention 2 (n = 30): audiobook group (not included in review). Participants listened to researcher‐selected audio books. Control (n = 29): no intervention |
|
| Outcomes |
aOutcome not included in this review since it was not measured in the control group. |
|
| Notes |
Trial start and end dates: 2006 Funding sources: supported by the Hungarian Ministry of Education, the National Research Fund (Hungary), the Ferenc Faludi Academy, and the János Bolyai Research Fellowship of the Hungarian Academy of Sciences Protocol registration: none found |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: used a computerised randomisation table and variable block randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: due to the nature of the intervention, blinding of participants was not possible. It is likely that this affected the subjective outcome measures. The intervention was used at home with no personnel involved. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: group allocation was coded (Harmat 2014 [pers comm]). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no attrition in the included groups (Harmat 2014 [pers comm]). |
| Selective reporting (reporting bias) | Low risk | Comment: we found no published protocol on this study. Outcomes from the no‐intervention control group were not reported in the publication, but were provided by the first author on request (Harmat 2014 [pers comm]). These data did not alter the results or conclusions of the trial. |
| Other bias | High risk | Comment: the trial design involved a difference between the intervention and control groups. The intervention group registered sleep quality once a week, whereas the control group only registered sleep quality before and after the intervention period. In addition, the intervention group, but not the control group, was contacted weekly by telephone to assess compliance with the protocol. |
Huang 2017.
| Study characteristics | ||
| Methods |
Study type: RCT Design: 3‐arm, parallel group design Blinding: single blind; assessors were blinded to group allocation |
|
| Participants |
Sample: adults with poor sleep documented by PSQI scores > 5, sleep‐onset latency > 30 minutes, wake time after sleep onset > 30 minutes, or TST ≤ 6.5 hours n: 71 randomised (48 included in this review). 71 completed and included in analyses Age: mean 41 (SD 16.7, range 22–67) years Sex: 9 men, 39 women Setting: participants' homes Country: Taiwan |
|
| Interventions |
Intervention 1 (n = 24): music group. Participants listened to researcher‐selected Buddhist music. Participants administered the music themselves. The music was installed on the mobile phones of the participants. Music characteristics: 3 peaceful Buddhist songs: Praise Buddha, Song of Praise Sambo, and Namo Shakyamuni Buddha (Jing Si Publications, Taipei, Taiwan) Length of sessions: 30 minutes Frequency of sessions: daily at bedtime Duration of intervention period: 4 days Intervention 2 (n = 23): music video group (not included in review). Participants watched researcher‐selected religious films. Control (n = 24): no intervention |
|
| Outcomes |
aOutcome not included in this review We contacted the author 25 May and 29 June 2021 to obtain data on the raw postintervention scores, but we have not yet received a reply. |
|
| Notes |
Trial start and end dates: September 2014 to June 2016 Funding sources: funded by the National Science Council, Taiwan (NSC102‐2628‐B‐320‐001‐MY3) Protocol registration: WHO ICTRP (ISRCTN94971645) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: random allocation sequence was consecutively numbered for the participants and sealed, opaque envelopes determining groups were generated using a random numbers generator (Microsoft Excel) by a statistician (Yang, Minzi). |
| Allocation concealment (selection bias) | Low risk | Comment: the statistician was not involved in the rest of the study. The researchers and research assistant were all blinded to the randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it is not possible to blind participants to the music intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: a licensed sleep technician, blinded to group assignment, visually analysed sleep polygraphs using standard procedures. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no sign of attrition. |
| Selective reporting (reporting bias) | Low risk | Comment: protocol was retrospectively registered (ISRCTN94971645). There was no indication of selective reporting. |
| Other bias | Low risk | Comment: no indication of additional bias. |
Jespersen 2019.
| Study characteristics | ||
| Methods |
Study type: RCT Design: 3‐arm, parallel group design Blinding: single blind; data assessors blinded to group allocation |
|
| Participants |
Sample: adults with insomnia disorder according to DSM‐5 diagnostic criteria n: 57 randomised (38 included in this review). 50 completed, 57 included in analyses Age: mean 50.2 (SD 11.6, range 18–65) years Sex: 12 men, 45 women Setting: participants' homes Country: Denmark |
|
| Interventions |
Intervention 1 (n = 19): music group. Participants could choose between 4 playlists of slow music of different genres (classical, new age, jazz and ambient). Participants administered the music themselves. They received an audio player designed to be used in bed (Audiocura M2). Music characteristics: all music was instrumental, with a slow tempo, stable dynamics and a simple structure. The specific music of each playlist is provided in the publication. Length of sessions: minimum 30 minutes Frequency of sessions: daily at bedtime Duration of intervention period: 21 days Intervention 2 (n = 19): audiobook group (not included in review). Participants could choose between 4 audiobooks of different genres (short stories, tales and fairy tales, autobiographical novel, magical realism). Control (n = 19): waitlist control group with no intervention |
|
| Outcomes |
|
|
| Notes |
Trial start and end dates: March 2015 to April 2017 Funding sources: TrygFonden, Grant/Award No 109461 Protocol registration: ClinicalTrials.gov (NCT02321826) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Fifty‐seven persons were randomly allocated to one of the three groups by the drawing of lots." (Jespersen 2019, p 3). |
| Allocation concealment (selection bias) | Low risk | Quote: "The bowl was prepared by administrative staff with no knowledge of the study." (Jespersen 2019, p 3). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it is not possible to blind participants to the music intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: assessor‐blinded RCT. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol was available and all of the study's prespecified (primary and secondary) outcomes that are of interest in the review were reported in the prespecified way. |
| Other bias | Low risk | Comment: no indication of additional bias. |
Kullich 2003.
| Study characteristics | ||
| Methods |
Study type: randomised using a computer‐based randomisation list (Kullich 2014a [pers comm]) Design: 2‐arm, parallel group design Blinding: single blind; data assessment performed by non‐trial personnel (Kullich 2014a [pers comm]) |
|
| Participants |
Sample: adults with low back pain and sleep difficulties documented by PSQI scores > 5 n: 65 randomised, 65 completed and included in analyses Age: mean age reported by group (range 21–68 years); music group: mean 47.0 (SD 9.7) years, control group: mean 49.7 (SD 7.9) years Sex: 41 men, 24 women Setting: rehabilitation facility Country: Austria |
|
| Interventions |
Intervention (n = 32): music group. Participants administered the music intervention themselves. They listened to researcher‐selected music and relaxation instructions through headphones and received TAU. Music characteristics: CD 'Entspannung bei Schmerzen' (Mentalis Verlag, ISBN: 3‐932239‐95‐4). No further information provided Length of sessions: 25 minutes Frequency of sessions: once a day, no time specified Duration of intervention period: 3 weeks ± 2 days Control (n = 33): TAU |
|
| Outcomes |
aOutcome not included in this review |
|
| Notes |
Trial start and end dates: not provided. Funding sources: supported by the Ludwig Boltzmann Institut (Saalfelden), the Herbert von Karajan Centrum (Wien), Salzburg University, and the Mozart University (Salzburg). Protocol registration: none found |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer‐based randomisation list (Kullich 2014a [pers comm]) |
| Allocation concealment (selection bias) | Low risk | Comment: allocation done by another person (not the doctor) who referred the participant to the trial (Kullich 2014a [pers comm]) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: due to the nature of the intervention, blinding of participants was not possible. It is likely that this affected the subjective measures of sleep. There was no information on the blinding of the personnel at the rehabilitation facility. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: data were assessed by non‐trial personnel (secretary). Data analysis was performed by a researcher who was aware of group allocation, but did not know the patients (Kullich 2014a [pers comm]). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no attrition or missing data (Kullich 2014a [pers comm]) |
| Selective reporting (reporting bias) | Low risk | Comment: we found no published protocol on this study, but there was no indication of selective reporting. Measures on sleep quality were reported without SDs in the publication, but these were provided by the first author on request (Kullich 2014b [pers comm]). These data did not alter the conclusions of the trial. |
| Other bias | Low risk | Comment: no other risk of bias detected |
Lai 2005.
| Study characteristics | ||
| Methods |
Study type: RCT Design: 2‐arm, parallel group design Blinding: not blinded |
|
| Participants |
Sample: older adults with sleep problems documented by PSQI scores > 5 n: 60 randomised, 60 completed and included in analyses Age: mean 67 (SD 5, range 60 to 83) years Sex: not reported Setting: participants' homes Country: Taiwan |
|
| Interventions |
Intervention (n = 30): music group. Participants could choose among 6 types of researcher‐selected sedative music. Participants administered the music themselves. The music was recorded to an audiotape and participants could use earphones or not as preferred. Music characteristics: the choices of music included 5 types of Western music (new age, eclectic, popular oldies, classical, and slow jazz), and 1 type of Chinese music (folk music). Tempos ranged from 60 to 80 bpm without accented beats, percussive characteristics or syncopation. The music was expected to be familiar to the participants. Length of sessions: 45 minutes Frequency of sessions: daily at bedtime Duration of intervention period: 3 weeks Control (n = 30): no intervention |
|
| Outcomes |
|
|
| Notes |
Trial start and end date: trial conducted in 2000 Funding sources: no information provided Protocol registration: none found |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: permuted block randomisation with sealed envelopes stratified on gender |
| Allocation concealment (selection bias) | Low risk | Quote: "The envelopes were prepared by a different person so that the investigator (first author) was blind to block size and order of assignment" (Lai 2005, p 235) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: due to the nature of the intervention, blinding of participants was not possible. It is likely that this affected the subjective outcome measures. The intervention was used at home with no personnel involved. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: unclear information on attrition. 1 man was withdrawn due to hospitalisation. No information on completeness of data |
| Selective reporting (reporting bias) | Low risk | Comment: we found no published protocol on this study, but there was no indication of selective reporting. |
| Other bias | High risk | Comment: baseline differences in 2 sleep component scores, with the music group experiencing shorter sleep duration and more daytime dysfunction |
Liu 2016.
| Study characteristics | ||
| Methods |
Study type: RCT Design: 2‐arm, parallel group design Blinding: not blinded |
|
| Participants |
Sample: pregnant women of 18–34 weeks of gestation, with sleep problems documented by PSQI scores > 5 n: 128 randomised, 121 completed and included in the analyses Age: not reported Sex: women Setting: participants' homes Country: Taiwan |
|
| Interventions |
Intervention (n = 65): music group. Standard prenatal care and music listening. Participants could choose among 5 types of researcher‐selected sedative music. They administered the music themselves. The listening device was not reported. Music characteristics: the choices of music included 5 CDs of different genres (Taiwanese orchestral music, western classical music, nature sounds, lullabies and crystal music). The music had a slow tempo (60–80 bpm) and the relaxing properties were confirmed by pregnant women in a pilot investigation. Length of sessions: minimum 30 minutes Frequency of sessions: daily at bedtime Duration of intervention period: 14 days Control (n = 63): standard prenatal care |
|
| Outcomes | Sleep quality (assessed with PSQI)
aOutcome not included in this review |
|
| Notes |
Trial start and end dates: no information provided Funding sources: funded by the National Science Council of Taiwan Protocol registration: none found |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomisation procedure not clearly described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear information on the randomisation procedure and no information on measures taken to conceal allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it was not possible to blind participants to the music intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: attrition was low, balanced and well reported. |
| Selective reporting (reporting bias) | Low risk | Comment: no study protocol was found. There was no indication of reporting bias. |
| Other bias | Low risk | Comment: no indication of additional bias. |
Momennasab 2018.
| Study characteristics | ||
| Methods |
Study type: multicentre, RCT Design: 3‐arm, parallel group design Blinding: single blind |
|
| Participants |
Sample: adults with chronic renal failure n: 105 randomised (68 included in this review). 102 completed and included in the analyses Age: mean 49.86 (SD 11.12; range 18–60) years Sex: 56 men, 46 women (reported only for the 102 participants completing the trial) Setting: participants' homes Country: Iran |
|
| Interventions |
Intervention 1 (n = 33): bedtime music group. Standard treatment and listening to music at bedtime. Researcher‐chosen music. Participants administered the music themselves. They received a CD with the intervention music. The listening device was not reported. Music characteristics: 6‐piece piano improvisation created by Taylor Mesple (2015) in New Age (relaxation) genre Length of sessions: 50 minutes Frequency of sessions: daily at bedtime Duration of intervention period: 4 weeks Intervention 2 (n = 34): daytime music group (not included in review). Standard treatment and listening to music during haemodialysis Control (n = 35): standard treatment |
|
| Outcomes |
|
|
| Notes |
Trial start and end dates: May to December 2016 Funding sources: financially supported by the Vice‐Chancellor for Research Affairs, Shiraz University of Medical Sciences, Iran (Grant No 10571). Protocol registration: Iranian Registry of Clinical Trials (IRCT2016050217546N5) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: participants were allocated into 2 interventions and 1 control group by block randomisation with a block size of 3. |
| Allocation concealment (selection bias) | High risk | Comment: with 3 groups and a fixed block size of 3, you would be able to foresee every third participant. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it was not possible to blind participants to the music intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Data collection and analysis were carried out by a research assistant and a statistician who were blinded to the patient allocation groups" (Momennasab 2018, p 88). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: low attrition rate that is reported satisfactorily. |
| Selective reporting (reporting bias) | Low risk | Comment: protocol available and outcomes well reported. |
| Other bias | Low risk | Comment: no indication of additional bias. |
Shum 2014.
| Study characteristics | ||
| Methods |
Study type: RCT Design: 2‐arm, parallel group design Blinding: not blinded |
|
| Participants |
Sample: older adults with poor sleep quality documented by PSQI scores > 5 n: 60 randomised, 60 completed and included in the analyses Age: mean 64 (range 57–68) years Sex: 20 men, 40 women Setting: participants' homes Country: Singapore |
|
| Interventions |
Intervention (n = 28): music group. Participants could choose among 4 types of researcher‐selected music. Participants administered the music themselves and received an MP4 player with earphones Music characteristics: the 4 types of researcher‐selected music included Western classical (Bach: Allemande, Sarabande; Mozart: Romance from Eine kleine Nachtmusik; Chopin: Nocturne); Chinese classical (Spring River in the Moonlight; Variation on Yang Pass); New Age (Shizuki, Lord of the Wind) and jazz (Everlasting; Winter Wonderland; In Love in Vain). All compositions were soft, with no lyrics, tempos were 60–80 bpm. Length of sessions: 40 minutes Frequency of sessions: once a day, no time specified Duration of intervention period: 5 weeks Control (n = 32): uninterrupted rest at weekly visit, otherwise no intervention |
|
| Outcomes |
|
|
| Notes |
Trial start and end dates: January 2012 to January 2013 Funding sources: no information provided Protocol registration: none found |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Two cards were put inside a bag in each draw, with one labelled as "intervention" and the other as "control". Each participant was asked to draw one card from the bag to allocate him or her into either the intervention or control group" (Shum 2014, p 51). |
| Allocation concealment (selection bias) | Low risk |
Quote: "Two cards were put inside a bag in each draw, with one labelled as "intervention" and the other as "control". Each participant was asked to draw one card from the bag to allocate him or her into either the intervention or control group" (Shum 2014, p 51). Comment: this procedure makes it unlikely that the allocation was foreseen. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: due to the nature of the intervention, blinding of participants was not possible. It is likely that this affected the subjective outcome measures. The intervention was used at home with no personnel involved. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropouts and no missing data. |
| Selective reporting (reporting bias) | Unclear risk | Comment: we found no published protocol for this study. The primary outcome of sleep quality (PSQI – global scale) was fully reported, but the results of the component scores were not reported, which was common in other trials using the PSQI. |
| Other bias | Low risk | Comment: no other risk of bias detected. |
Wang 2016.
| Study characteristics | ||
| Methods |
Study type: RCT Design: 2‐arm, parallel group design Blinding: single blind |
|
| Participants |
Sample: older adults (> 60 years), with poor sleep quality documented by PSQI scores > 7 n: 64 randomised, 64 completed and included in the analyses Age: mean 69 (SD 5.46) years Sex: 13 men, 55 women (unexplained discrepancy with total number of participants in the original report) Setting: participants' homes Country: China |
|
| Interventions |
Intervention (n = 32): music group. Sleep hygiene and music listening. Participants administered the music intervention themselves. They received an MP3 player with music database stored. Music characteristics: a music database of various types of music, including Chinese instrumental classic, Western classic, natural sounds music and classical songs without lyrics. The participants could find their preferred music from this database. All selected music was soft and sedative, with stable melodies at a tempo of 60–80 bpm. Length of sessions: 30–45 minutes Frequency of sessions: daily at bedtime Duration of intervention period: 3 months Control (n = 32): sleep hygiene |
|
| Outcomes |
|
|
| Notes |
Trial start and end dates: October 2011 to January 2012 Funding sources: no information provided Protocol registration: none found |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were assigned to the invention or control group by opening a sealed opaque envelope with a computer‐generated randomisation number indicating the group allocation" (Wang 2016, p 578–579). |
| Allocation concealment (selection bias) | Low risk | Comment: with this randomisation procedure, the group allocation seemed well concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it was not possible to blind participants to the music intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The follow‐up measurements were conducted by a research assistant, who had received training for administering PSQI and was blinded with the group allocations" (Wang 2016, p 579). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no attrition. |
| Selective reporting (reporting bias) | Low risk | Comment: protocol was not available, but there was no indication of selective reporting. |
| Other bias | Low risk | Comment: no indication of additional bias. |
BDI: Beck Depression Inventory, range 0–63, higher scores indicate more severe depression; bpm: beats per minute CD: compact disc; DASS‐21: Depression, Anxiety and Stress Scale, range 0–56, higher scores indicate more severe symptoms; DSM‐5: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; EEG: electroencephalogram; GEE: generalised estimating equation; HADS: Hospital Anxiety and Depression Scale, range 0–21, higher scores indicate more anxiety or depression; ISI: Insomnia Severity Index, range 0–28, higher scores indicate more severe insomnia; MLHFQ: Minnesota Living with Heart Failure Questionnaire, range 0–105, higher scores indicate poorer quality of life; MoCA: Montreal Cognitive Assessment, range 0–30, higher scores indicate cognitive impairment; n: number of participants; PSG: polysomnography; PSQI: Pittsburgh Sleep Quality Index, range 0–21, higher scores indicate poorer sleep quality; PSS: Perceived Stress Scale, range 0–40, higher scores indicate more perceived stress; R‐MDQ: Roland‐Morris Disability Questionnaire, range 0–24, higher scores indicate more disability; RCT: randomised controlled trial; REM: rapid eye movement; SD: standard deviation; SF‐12: 12‐item Short Form Health Survey, range 0–100, higher scores indicate better physical and mental health functioning; STAI: State Trait Anxiety Inventory, range 20–80, higher scores indicate more anxiety; TAU: treatment as usual; TST: total sleep time; VAS: visual analogue scale, range 0–10, higher scores indicate more symptoms, e.g. pain; WHO: World Health Organization; WHOQOL‐BREF: World Health Organization Quality of Life Scale, range 0–100, higher scores indicate better quality of life.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bang 2019 | Ineligible comparator |
| Blanaru 2012 | Ineligible comparator (comparison of 2 interventions, music listening versus muscle relaxation techniques) |
| Chan 2010 | Ineligible population (not all participants had insomnia. No inclusion criteria of insomnia and PSQI < 5) |
| Chen 2014 | Ineligible population (young adults with different sleep latencies. Poor sleepers (PSQI < 5) excluded) |
| Deshmukh 2009 | Ineligible comparator (comparison of 2 interventions, music listening versus hypnotic medications) |
| Garcia‐Molina 2019 | Ineligible population |
| Hausenblas 2019 | Ineligible comparator |
| Hérnandez‐Ruíz 2005 | Ineligible population (some participants were 'good sleepers') |
| Jespersen 2012 | Ineligible study design (quasi‐randomised trial) |
| KaradaĞ 2015 | Ineligible population |
| Kayumov 2003 | Ineligible comparator (individualised versus non‐individualised 'brain music') |
| Koenig 2013 | Ineligible population (healthy university students with no sleep problems) |
| Lafçi 2015 | Ineligible population |
| Lai 2012 | Ineligible comparator (live music with nursing presence versus prerecorded music) |
| Lai 2015 | Ineligible intervention (music video) |
| Lazic 2007 | Ineligible population (healthy university students) |
| Lio 2018 | Ineligible intervention |
| Lu 2020 | Ineligible population |
| Mottaghi 2016 | Ineligible intervention |
| Oxtoby 2013 | Ineligible population (university students. About 50% of participants experienced no sleep problems) |
| Qin 2020 | Ineligible intervention |
| Shobeiri 2016 | Ineligible intervention (music combined with counselling) |
| Sithinamsuwan 2012 | Ineligible population (some participants were 'good sleepers' with PSQI scores < 5) |
| Srikolcheep 2017 | Ineligible intervention |
| Weise 2020 | Ineligible population |
| Yamasato 2020 | Ineligible study design |
| Ziv 2008 | Ineligible comparator (music listening versus progressive muscular relaxation) |
PSQI: Pittsburgh Sleep Quality Index.
Characteristics of studies awaiting classification [ordered by study ID]
Miller 2002.
| Methods | Design: randomised controlled trial |
| Participants | Sample: postoperative patients |
| Interventions |
Intervention: music programme Control: comparison group |
| Outcomes |
|
| Notes | This is an unpublished trial. On 9 September 2014, we requested further information from the author, but have yet to receive a response. When trying to contact the author in 2021, we did not manage to find valid contact information. |
Zhu 2018.
| Methods | Design: randomised controlled trial |
| Participants | Sample: 180 people with sleep disorders |
| Interventions |
Intervention: personalised music Control: no music |
| Outcomes |
|
| Notes | Trial start and end dates: March 2017 to March 2018 |
PSQI: Pittsburgh Sleep Quality Index.
Characteristics of ongoing studies [ordered by study ID]
IRCT2015051822141N1.
| Study name |
Public title: comparison between effect of music and relaxation on sleep Scientific title: comparison of the effects of music and muscle relaxation on sleep quality in elderly people referring to the Jahandidegan center in Shiraz 2014–2015 |
| Methods | Design: RCT |
| Participants |
Sample: elderly people with poor sleep Sample size: 105 (target) |
| Interventions |
Intervention 1: music Intervention 2: muscle relaxation Control: no intervention |
| Outcomes |
|
| Starting date | 16 May 2015 (estimated; registered 18 May 2015) |
| Contact information |
Name: Roya Razavi E‐mail: razaviroya41@yahoo.com |
| Notes | Funding: Shiraz University of Medical Sciences |
IRCT20150519022320N10.
| Study name |
Public title: the effect of traditional music on sleep quality in elderlies Scientific title: the effect of traditional music on sleep quality in elderlies |
| Methods | Design: RCT |
| Participants |
Sample: adults with poor sleep Sample size: 70 (target) |
| Interventions |
Intervention: music intervention Control: no music |
| Outcomes |
|
| Starting date | 21 May 2016 (estimated; registered 9 March 2018) |
| Contact information |
Name: Dr Tayyebeh mirzaei E‐mail: t.mirzaei@rums.ac.ir |
| Notes | Funding: Rafsanjan University of Medical Sciences |
NCT02376686.
| Study name |
Public title: music intervention in the treatment of sleep disorders for depressed patients Scientific title: Musik als nicht‐pharmakologische intervention zur behandlung von schlafstörungen bei patienten mit depressiven erkrankungen |
| Methods | Design: RCT |
| Participants |
Sample: inpatients with depression and insomnia Sample size: 50 (estimated) |
| Interventions |
Intervention: music intervention Control: treatment as usual |
| Outcomes |
|
| Starting date | April 2014 (not specified if this is the actual or estimated start date) |
| Contact information |
Name: Katja Cattapan, MD E‐mail: k.cattapan@sanatorium‐kilchberg.ch |
| Notes | Funding: none stated |
NCT03676491.
| Study name |
Public title: music to improve sleep quality in adults with depression and insomnia (MUSTAFI) Scientific title: music to improve sleep quality in adults with depression and insomnia: a randomised controlled trial using mixed methods |
| Methods | Design: RCT |
| Participants |
Sample: adults with depression and sleep complaints Sample size: 112 (actual) |
| Interventions |
Intervention: music and treatment as usual Control: treatment as usual |
| Outcomes |
|
| Starting date | 23 May 2018 (actual) |
| Contact information |
Name: Helle Nystrup Lund E‐mail: not stated, but affiliation is Aalborg University Hospital |
| Notes | Funding: Health Research Foundation, The Obel Family Foundation, Aase & Ejnar Danielsens Foundation |
NCT04157244.
| Study name |
Public title: the music, sleep and dementia study Scientific title: the feasibility of a tailored music intervention to reduce symptoms of sleep disruption in older adults with dementia |
| Methods | Design: RCT |
| Participants |
Sample: adults with dementia and sleep problems Sample size: 32 (actual) |
| Interventions |
Intervention: tailored music listening Control: wait‐list control |
| Outcomes |
|
| Starting date | 12 March 2019 (actual) |
| Contact information |
Name: Darina Petrovsky E‐mail: not stated, but affiliation is University of Pennsylvania |
| Notes | Funding: none stated |
NCT04578860.
| Study name |
Public title: effectiveness of music therapy on sleep disorders Scientific title: a 3‐months, controlled and double‐blind trial of the effectiveness of music therapy in the treatment of sleep disorders in general medicine |
| Methods | Design: RCT |
| Participants |
Sample: adults with insomnia Sample size: 120 (estimated) |
| Interventions |
Intervention: listening to music with the music care app Control 1: sound therapy with white noise Control 2: treatment as usual for sleep disorders |
| Outcomes |
|
| Starting date | 2 March 2020 (actual) |
| Contact information |
Name: Elsa Musso E‐mail: etudesommeil20@gmail.com |
| Notes | Funding: none stated |
NCT04585425.
| Study name |
Public title: music for sleep‐onset insomnia Scientific title: god nat – god dag. A randomised controlled trial of bedtime music as early intervention for sleep‐onset insomnia |
| Methods | Design: RCT |
| Participants |
Sample: adults with sleep‐onset insomnia Sample size: 70 (estimated) |
| Interventions |
Intervention: music and sleep hygiene Control: sleep hygiene alone |
| Outcomes |
|
| Starting date | September 2021 (estimated) |
| Contact information |
Name: Kira Vibe Jespersen E‐mail: kira@clin.au.dk |
| Notes | Funding: Sygekassernes Helsefond |
NCT04633395.
| Study name |
Public title: treating pregnancy related insomnia with music Scientific title: treating pregnancy related insomnia with music: a randomised control trial |
| Methods | Design: RCT |
| Participants |
Sample: pregnant women with sleep problems Sample size: 240 (estimated) |
| Interventions |
Intervention: music and sleep hygiene Control: sleep hygiene |
| Outcomes |
|
| Starting date | 1 December 2020 (actual) |
| Contact information |
Name: Nadia F Hoegholt, MD E‐mail: nadiafh@clin.au.dk |
| Notes | Funding: none stated |
ISI: Insomnia Severity Index; MUSTAFI: MUsic STAr For Insomnia; PSQI: Pittsburgh Sleep Quality Index; RCT: randomised controlled trial.
Differences between protocol and review
Differences between protocol and review
We made the following three adjustments to the protocol (Jespersen 2013).
We edited the title and the background section on 'Description of the condition' based on the comments of the peer‐reviewers.
We added a section to the methods describing the assessment of the quality of the evidence using the GRADE approach, as per Cochrane requirements.
We specified and ensured that trials involving any of the review authors were assessed by two other review authors with no involvement in the trial to reduce the potential for bias.
Differences between original review and update
We edited the title and the background section on 'Description of the condition' based on the comments of the peer reviewers and the scientific progress in the field.
For the 2021 update, more studies were available, and therefore, we included only randomised controlled trials and not quasi‐randomised controlled trials.
We included 'Insomnia severity' as a primary outcome, to ensure an outcome evaluating the perceived severity of insomnia symptoms in addition to the more general sleep outcomes. This is important since the insomnia criteria relate to a subjective complaint of poor sleep.
We revised the search strategies by adding some additional free‐text terms, and updated the old MeSH term Sleep disorders with the updated term Sleep Wake Disorders.
We added two daily updated segments of MEDLINE (MEDLINE Epub Ahead of Print and MEDLINE In‐Process & Other Non‐indexed Citations, and a source of systematic reviews (Cochrane Database of Systematic Reviews).
We replaced the trials register Current Controlled Trials with the World Health Organization International Clinical Trials Registry Platform.
For the 2021 update, we did not handsearch journals, as the large majority of journals are now digitally available and indexed in the databases. Furthermore, the handsearch of journals did not yield any additional studies in the 2015 literature search.
We conducted meta‐analyses of studies that were homogeneous in terms of participants, interventions and outcomes despite substantial statistical heterogeneity. Where there was substantial heterogeneity, we explored methodological, clinical and statistical factors underlying the heterogeneity.
Contributions of authors
Co‐ordinated the review: KVJ.
Conception and design of the review: KVJ, JK and PV.
Developed the search strategy: KVJ and PV.
Selected which trials to include: KVJ and VPN (JK replaced KVJ for the study she was involved in).
Risk of bias assessment: KVJ and VPN (JK replaced KVJ for the study she was involved in).
Arbitrated in the event of dispute regarding study selection and risk of bias assessment: PJ.
Extracted data from trials: KVJ and VPN (JK replaced KVJ for the study she was involved in).
Entered data into RevMan software: KVJ and JK.
Carried out the analysis: KVJ and JK.
Interpreted the analysis: KVJ, VPN, JK, PJ and PV.
Assessment of the certainty in the body of evidence: KVJ and VPN.
Drafted the final review: KVJ and JK.
Guarantor: KVJ.
Sources of support
Internal sources
-
Kira Vibe Jespersen: Department of Clinical Medicine, Aarhus University, Denmark
Salary support
-
Julian Koenig: Department of Psychology, The Ohio State University, USA
Salary support
-
Poul Jennum: Danish Centre for Sleep Medicine, Glostrup University Hospital, Denmark
Salary support
-
Peter Vuust: Department of Clinical Medicine, Aarhus University and the Royal Academy of Music, Aarhus, Denmark
Salary support
External sources
No sources of support provided
Declarations of interest
KVJ: reports being the primary author of one study included in the reviewa; the study was supported by Trygfonden, Denmark (grant covered equipment and running costs), but the researchers retained complete control over the study design, methods, analysis, interpretation and dissemination of the results; paid to Center for Music in the Brain, Aarhus University.
VPN: reports no known conflicts of interest.
JK: works as a health professional at the University Hospital Cologne, Clinic and Polyclinic for Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, Cologne Germany, where he leads the specialised outpatient clinic for treatment resistant psychiatric disorders in children and adolescents.
PJ: reports no known conflicts of interest.
PV: reports being a co‐author of a trial that is included in the reviewa; the study was supported by Trygfonden, Denmark (grant covered equipment and running costs), but the researchers retained complete control over the study design, methods, analysis, interpretation and dissemination of the results.
aKVJ and PV are authors on the Jespersen 2019 trial, therefore, two other review authors (VPN and JK), with no involvement in the study, assessed the trial.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Amiri 2019 {published data only}
- Amiri S, Parvizi Fard A, Khaledi-Paveh B, Foroughi A, Bavafa A, Bazani M, et al. The effectiveness of music therapy on insomnia using Persian traditional music. Journal of Kermanshah University of Medical Sciences 2019;23(2):e86914. [DOI: 10.5812/jkums.86914] [DOI] [Google Scholar]
Burrai 2020 {published data only}
- Burrai F, Sanna GD, Moccia E, Morlando F, Cosentino ER, Bui V, et al. Beneficial effects of listening to classical music in patients with heart failure: a randomized controlled trial. Journal of Cardiac Failure 2020;26(7):541-9. [DOI: 10.1016/j.cardfail.2019.12.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cai 2015 {published data only}
- Cai X-M, Zhang X-P, Tang X. Observation on clinical effect of auricular acupoint sticking plus music therapy for post-stroke insomnia. Journal of Acupuncture and Tuina Science 2015;13(4):227-31. [DOI: 10.1007/s11726-015-0858-2] [DOI] [Google Scholar]
Chang 2012 {published data only}
- Chang E-T, Lai H-L, Chen P-W, Hsieh Y-M, Lee L-H. The effects of music on the sleep quality of adults with chronic insomnia using evidence from polysomnographic and self-reported analysis: a randomized control trial. International Journal of Nursing Studies 2012;49(8):921-30. [DOI: 10.1016/j.ijnurstu.2012.02.019] [PMID: ] [DOI] [PubMed] [Google Scholar]
Harmat 2008 {published and unpublished data}
- Harmat L, Takács J, Bódizs R. Music improves sleep quality in students. Journal of Advanced Nursing 2008;62(3):327-35. [DOI: 10.1111/j.1365-2648.2008.04602.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Huang 2017 {published data only}
- Huang C-Y, Chang E-T, Hsieh Y-M, Lai H-L. Effects of music and music video interventions on sleep quality: a randomized controlled trial in adults with sleep disturbances. Complementary Therapies in Medicine 2017;34:116-22. [DOI: 10.1016/j.ctim.2017.08.015] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jespersen 2019 {published data only}
- Jespersen KV, Otto M, Kringelbach M, Someren E, Vuust P. A randomized controlled trial of bedtime music for insomnia disorder. Journal of Sleep Research 2019;28(4):e12817. [DOI: 10.1111/jsr.12817] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT02321826. Music for insomnia [Better night – better day: a randomized controlled trial of listening to music for improving insomnia]. clinicaltrials.gov/ct2/show/NCT02321826 (first received 22 December 2014).
Kullich 2003 {published and unpublished data}
- Kullich W, Bernatzky G, Hesse HP, Wendtner F, Likar R, Klein G. Music therapy – effect on pain, sleep, and quality of life in low back pain [Musiktherapie – wirkung auf schmerz, schlaf und lebensqualität bei low back pain]. Wiener Medizinische Wochenschrift 2003;153(9-10):217-21. [DOI: 10.1046/j.1563-258x.2003.02081.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lai 2005 {published data only}
- Lai H-L, Good M. Music improves sleep quality in older adults. Journal of Advanced Nursing 2005;49(3):234-44. [DOI: 10.1111/j.1365-2648.2004.03281.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Lai H-L. The Effects of Music Therapy on Sleep Quality in Elderly People [PhD thesis]. Ann Arbor (MI): UMI Dissertation Services, 2004. [Google Scholar]
Liu 2016 {published data only}
- Liu Y-H, Lee CS, Yu C-H, Chen C-H. Effects of music listening on stress, anxiety, and sleep quality for sleep-disturbed pregnant women. Women & Health 2016;56(3):296-311. [DOI: 10.1080/03630242.2015.1088116] [PMID: ] [DOI] [PubMed] [Google Scholar]
Momennasab 2018 {published data only}
- Momennasab M, Ranjbar M, Najafi SS. Comparing the effect of listening to music during hemodialysis and at bedtime on sleep quality of hemodialysis patients: a randomized clinical trial. European Journal of Integrative Medicine 2018;17:86-91. [DOI: 10.1016/j.eujim.2017.12.001] [DOI] [Google Scholar]
Shum 2014 {published data only}
- Shum A, Taylor BJ, Thayala J, Chan MF. The effects of sedative music on sleep quality of older community-dwelling adults in Singapore. Complementary Therapies in Medicine 2014;22(1):49-56. [DOI: 10.1016/j.ctim.2013.11.003] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wang 2016 {published data only}
- Wang Q, Chair SY, Wong EM, Li X. The effects of music intervention on sleep quality in community-dwelling elderly. Journal of Alternative and Complementary Medicine 2016;22(7):576-84. [DOI: 10.1089/acm.2015.0304] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bang 2019 {published data only}
- Bang YR, Choi HY, Yoon IY. Minimal effects of binaural auditory beats for subclinical insomnia a randomized double-blind controlled study. Journal of Clinical Psychopharmacology 2019;39(5):499-503. [DOI: 10.1097/JCP.0000000000001097] [PMID: ] [DOI] [PubMed] [Google Scholar]
Blanaru 2012 {published data only}
- Blanaru M, Bloch B, Vadas L, Arnon Z, Ziv N, Kremer I, et al. The effects of music relaxation and muscle relaxation techniques on sleep quality and emotional measures among individuals with posttraumatic stress disorder. Mental Illness 2012;4(2):59-65. [DOI: 10.4081/mi.2012.e13] [PMCID: PMC4253375] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimov I, Blanaro M, Arnon Z, Ziv N, Bloch B, Reshef A, et al. P1056 The effects of music and muscle relaxation therapies on sleep quality in individuals with post-traumatic stress disorder. Journal of Sleep Research 2010;19(Suppl 2):364. [URL: onlinelibrary.wiley.com/doi/epdf/10.1111/j.1365-2869.2010.00868.x] [Google Scholar]
Chan 2010 {published data only}
- Chan MF, Chan EA, Mok E. Effects of music on depression and sleep quality in elderly people: a randomised controlled trial. Complementary Therapies in Medicine 2010;18(3-4):150-9. [DOI: 10.1016/j.ctim.2010.02.004] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Chan MF. A randomised controlled study of the effects of music on sleep quality in older people. Journal of Clinical Nursing 2011;20(7-8):979-87. [DOI: 10.1111/j.1365-2702.2010.03368.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chen 2014 {published data only}
- Chen CK, Pei YC, Chen NH, Huang LT, Chou SW, Wu KP, et al. Sedative music facilitates deep sleep in young adults. Journal of Alternative and Complementary Medicine 2014;20(4):312-7. [DOI: 10.1089/acm.2012.0050] [PMID: ] [DOI] [PubMed] [Google Scholar]
Deshmukh 2009 {published data only}
- Deshmukh AD, Sarvaiya AA, Seethalakshmi R, Nayak AS. Effect of Indian classical music on quality of sleep in depressed patients: a randomized controlled trial. Nordic Journal of Music Therapy 2009;18(1):70-8. [DOI: 10.1080/08098130802697269] [DOI] [Google Scholar]
Garcia‐Molina 2019 {published data only}
- Garcia-Molina G, Patel V, Kalyan B, Tsang K. 0009 Can electroencephalogram-modulated music facilitate falling asleep? Sleep 2019;42(Suppl 1):A402. [DOI: 10.1093/sleep/zsz067.996] [DOI] [Google Scholar]
Hausenblas 2019 {published data only}
- Hausenblas H, Hooper S, Hooper D, Coyle K, Lynch T. Efficacy of Wholetones® 2Sleep and classical music on sleep and health behaviors of adults with insomnia symptoms: a single blind, randomized, controlled, crossover pilot trial. Sleep Science 2019;12(4):302-6. [DOI: 10.5935/1984-0063.20190091] [PMCID: PMC7159072] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hérnandez‐Ruíz 2005 {published data only}
- Hérnandez-Ruíz E. Effect of music therapy on the anxiety levels and sleep patterns of abused women in shelters. Journal of Music Therapy 2005;42(2):140-58. [DOI: 10.1093/jmt/42.2.140] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jespersen 2012 {published data only}
- Jespersen KV, Vuust P. The effect of relaxation music listening on sleep quality in traumatized refugees: a pilot study. Journal of Music Therapy 2012;49(2):205-29. [DOI: 10.1093/jmt/49.2.205] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Jespersen KV. The Impact of Music on Sleep: a Review and Empirical Study of Music Listening as Intervention for Sleep Improvement [Master thesis]. Aarhus (Denmark): Department of Psychology and Behavioural Sciences, Aarhus University, 2010. [Google Scholar]
KaradaĞ 2015 {published data only}
- KaradaĞ E, Karadakovan A. The effect of music on the sleep quality and vital signs of the chronic renal failure patients who are getting hemodialysis treatment. Turkiye Klinikleri [Journal of Nursing Sciences] 2015;7(2):79-89. [DOI: 10.5336/nurses.2013-34224] [DOI] [Google Scholar]
Kayumov 2003 {published data only}
- Kayumov L, Moller HJ. Brain music: a novel somatic treatment for insomnia and anxiety. In: 156th Annual Meeting of the American Psychiatric Association; 2003 May 17-22; San Francisco (CA). 2003:Nr405.
Koenig 2013 {published data only}
- Koenig J, Jarczok MN, Warth M, Harmat L, Hesse N, Jespersen KV, et al. Music listening has no positive or negative effects on sleep quality of normal sleepers: results of a randomized controlled trial. Nordic Journal of Music Therapy 2013;22(3):233-42. [Google Scholar]
Lafçi 2015 {published data only}
- Lafçi D, Öztunç G. The effect of music on the sleep quality of breast cancer patients. International Journal of Caring Sciences 2015;8(3):633-40. [URL: internationaljournalofcaringsciences.org/docs/14_Laftci_original_8_3.pdf] [Google Scholar]
Lai 2012 {published data only}
- Lai HL, Li YM, Lee LH. Effects of music intervention with nursing presence and recorded music on psycho-physiological indices of cancer patient caregivers. Journal of Clinical Nursing 2012;21(5-6):745-56. [DOI: 10.1111/j.1365-2702.2011.03916.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lai 2015 {published data only}
- Lai HL, Chang ET, Li YM, Huang CY, Lee LH, Wang HM. Effects of music videos on sleep quality in middle-aged and older adults with chronic insomnia: a randomized controlled trial. Biological Research for Nursing 2015;17(3):340-7. [DOI: 10.1177/1099800414549237] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lazic 2007 {published data only}
- Lazic SE, Ogilvie RD. Lack of efficacy of music to improve sleep: a polysomnographic and quantitative EEG analysis. International Journal of Psychophysiology 2007;63(3):232-9. [DOI: 10.1016/j.ijpsycho.2006.10.004] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lio 2018 {published data only}
- Lio Y, Jiang Y, Liu. Influence of Chinese pestle therapy combined with five tone therapy on sleep quality in patients with digestive system malignancy with heart and spleen deficiency. Chinese Nursing Research 2018;32(22):3553-7. [URL: cdutcm.irtree.com/articles/article_detail.aspx?id=b9c53c945fb94130bbec2e78d1f81db0] [Google Scholar]
Lu 2020 {published data only}
- Lu Y, Nie Z, Yu C. Effect of paroxetine combined with music relaxation therapy on psychology and sleep state of patients with depression. Chinese Nursing Research 2020;34(20):3625-8. [URL: jglobal.jst.go.jp/en/detail?JGLOBAL_ID=202102266167537868] [Google Scholar]
Mottaghi 2016 {published data only}
- Mottaghi R, Kamkar A, Maredpoor A. Effectiveness of targeted musical therapy on sleep quality and overcoming insomnia in seniors. Salmand [Iranian Journal of Ageing] 2016;11(2):348-57. [URL: doaj.org/article/5728c8a6fb804ce5862af6c60ea276f4] [Google Scholar]
Oxtoby 2013 {published data only}
- Oxtoby J, Sacre S, Lurie-Beck J, Pedersen IN. The impact of relaxing music on insomnia-related thoughts and behaviours. Australian Journal of Music Therapy 2013;24:67-86. [DOI: 10.3316/INFORMIT.878304921746988] [DOI] [Google Scholar]
Qin 2020 {published data only}
- Qin Y, Xiaobin Z, Wenxia H, Xie W, Qian M, Yanli L. The application of self-made Chinese medicine pillow combined with five elements music therapy on insomnia due to disharmony between heart and kidney in patients with chronic renal failure. Nursing of Integrated Traditional Chinese and Western Medicine 2020;6(8):112-7. [URL: www.zxyjhhl.com.cn/EN/abstract/abstract7572.shtml] [Google Scholar]
Shobeiri 2016 {published data only}
- Shobeiri F, Khaledi S, Masoumi SZ, Roshanaei G. The effect of music therapy counseling on sleep quality in pregnant women. International Journal of Medical Research & Health Sciences 2016;5(Suppl 9):408-16. [URL: www.ijmrhs.com/medical-research/the-effect-of-music-therapy-counseling-on-sleep-quality-in-pregnant-women.pdf] [Google Scholar]
Sithinamsuwan 2012 {published and unpublished data}
- Sithinamsuwan P, Saengwanitch S, Pinidbunjerdkool A, Ukritchon S, Mungthin M. The effect of Thai traditional music on cognitive function, psychological health and quality of sleep among older Thai individuals with dementia. Journal of the American Geriatrics Society 2012;60(Suppl 4):S61. [DOI: 10.1111/j.1532-5415.2012.04000.x] [DOI] [Google Scholar]
Srikolcheep 2017 {published data only}
- Srikolcheep N, Sittiprapaporn P. Efficacy of the Integrated Listening Systems' Dreampad™ device to sleep quality in insomnia patient. In: International Conference on Digital Arts, Media and Technology (ICDAMT); 2017 Mar 1-4; Chiang Mai, Thailand. 2017:356-9. [DOI: 10.1109/ICDAMT.2017.7904991] [DOI]
Weise 2020 {published data only}
- Weise L, Töpfer NF, Deux J, Wilz G. Feasibility and effects of individualized recorded music for people with dementia: a pilot RCT study. Nordic Journal of Music Therapy 2020;29(1):39-56. [DOI: 10.1080/08098131.2019.1661507] [DOI] [Google Scholar]
Yamasato 2020 {published data only}
- Yamasato A, Kondo M, Hoshino S, Kikuchi J, Ikeuchi M, Yamazaki K, et al. How prescribed music and preferred music influence sleep quality in university students. Tokai Journal of Experimental and Clinical Medicine 2020;45(4):207-13. [PMID: ] [PubMed] [Google Scholar]
Ziv 2008 {published data only}
- Ziv N, Rotem T, Arnon Z, Haimov I. The effect of music relaxation versus progressive muscular relaxation on insomnia in older people and their relationship to personality traits. Journal of Music Therapy 2008;45(3):360-80. [DOI: 10.1093/jmt/45.3.360] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Miller 2002 {published and unpublished data}
- Bernatzky G, Presch M, Anderson M, Panksepp J. Emotional foundations of music as a non-pharmacological pain management tool in modern medicine. Neuroscience & Biobehavioral Reviews 2011;35(9):1989-99. [DOI: 10.1016/j.neubiorev.2011.06.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Miller K, Bernatzky G, Wendtner F. The purpose of music and relaxation for health promotion after surgical procedures – results of a prospective, randomized study. In: O'Dostrovsky J, Carr DB, Koltzenburg M , editors(s). Proceedings of the 10th World Congress on Pain; 2002 Aug 17-22; San Diego (CA). Seattle (WA): International Association for the Study of Pain (IASP) Press, 2003.
Zhu 2018 {published data only}
- Zhu Y. 200 Research on effects of personalized music intervention on sleep quality of people. Basic & Clinical Pharmacology & Toxicology 2018;123 (Suppl 3):110. [URL: onlinelibrary.wiley.com/doi/epdf/10.1111/bcpt.13100] [Google Scholar]
References to ongoing studies
IRCT2015051822141N1 {published data only}IRCT2015051822141N1
- IRCT2015051822141N1. Comparison between effect of music and relaxation on sleep [Comparison of the effects of music and muscle relaxation on sleep quality in elderly people referring to the Jahandidegan center in Shiraz 2014-2015]. www.irct.ir/trial/19172 (first received 18 May 2015).
IRCT20150519022320N10 {published data only}IRCT20150519022320N10
- IRCT20150519022320N10. The effect of traditional music on sleep quality in elderlies. www.irct.ir/trial/29507 (first received 9 March 2018).
NCT02376686 {published data only}
- NCT02376686. Music intervention in the treatment of sleep disorders for depressed patients [Musik als nicht-pharmakologische intervention zur behandlung von schlafstörungen bei patienten mit depressiven erkrankungen]. clinicaltrials.gov/ct2/show/NCT02376686 (first received 3 March 2015).
NCT03676491 {published data only}
- NCT03676491. Music to improve sleep quality in adults with depression and insomnia (MUSTAFI) [Music to improve sleep quality in adults with depression and insomnia: a randomised controlled trial using mixed methods]. clinicaltrials.gov/ct2/show/NCT03676491 (first received 18 September 2018).
NCT04157244 {published data only}
- NCT04157244. The music, sleep and dementia study [The feasibility of a tailored music intervention to reduce symptoms of sleep disruption in older adults with dementia]. clinicaltrials.gov/ct2/show/NCT04157244 (first received 8 November 2019).
NCT04578860 {published data only}
- NCT04578860. Effectiveness of music therapy on sleep disorders [A 3-months, controlled and double-blind trial of the effectiveness of music therapy in the treatment of sleep disorders in general medicine]. clinicaltrials.gov/ct2/show/NCT04578860 (first received 9 October 2020).
NCT04585425 {published data only}
- NCT04585425. Music for sleep-onset insomnia [God Nat – God Dag. A randomized controlled trial of bedtime music as early intervention for sleep-onset insomnia]. clinicaltrials.gov/ct2/show/NCT04585425 (first received 14 October 2020).
NCT04633395 {published data only}
- NCT04633395. Treating pregnancy related insomnia with music [Treating pregnancy related insomnia with music: a randomised control trial]. clinicaltrials.gov/ct2/show/NCT04633395 (first received 18 November 2020).
Additional references
Aalbers 2017
- Aalbers S, Fusar-Poli L, Freeman RE, Spreen M, Ket JC, Vink AC, et al. Music therapy for depression. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD004517. [DOI: 10.1002/14651858.CD004517.pub3] [PMCID: PMC6486188] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
AASM 2014
- American Academy of Sleep Medicine. ICSD3: the International Classification of Sleep Disorders: Diagnostic and Coding Manual. 3rd edition. Rochester (MN): American Academy of Sleep Medicine, 2014. [URL: tinyurl.com/5x8bwxxz] [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th edition. Arlington (VA): American Psychiatric Association, 2013. [Google Scholar]
Aritake‐Okada 2009
- Aritake-Okada S, Kaneita Y, Uchiyama M, Mishima K, Ohida T. Non-pharmacological self-management of sleep among the Japanese general population. Journal of Clinical Sleep Medicine 2009;5(5):464-9. [PMCID: PMC2762720] [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Baglioni 2011
- Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. Journal of Affective Disorders 2011;135(1-3):10-9. [DOI: 10.1016/j.jad.2011.01.011] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bastien 2001
- Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine 2001;2(4):297-307. [DOI: 10.1016/s1389-9457(00)00065-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Beck 1996
- Beck AT, Steer RA, Brown GK. BDI-II: Beck Depression Inventory. 2nd edition. San Antonio (TX): Psychological Corporation, 1996. [Google Scholar]
Begg 1994
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088-101. [PMID: ] [PubMed] [Google Scholar]
Bernatzky 2011
- Bernatzky G, Presch M, Anderson M, Panksepp J. Emotional foundations of music as a non-pharmacological pain management tool in modern medicine. Neuroscience & Biobehavioral Reviews 2011;35(9):1989-99. [DOI: 10.1016/j.neubiorev.2011.06.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bradt 2013
- Bradt J, Dileo C, Potvin N. Music for stress and anxiety reduction in coronary heart disease patients. Cochrane Database of Systematic Reviews 2013, Issue 12. Art. No: CD006577. [DOI: 10.1002/14651858.CD006577.pub3] [PMCID: PMC8454043] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bradt 2021
- Bradt J, Dileo C, Myers-Coffman K, Biondo J. Music interventions for improving psychological and physical outcomes in people with cancer. Cochrane Database of Systematic Reviews 2021, Issue 10. Art. No: CD006911. [DOI: 10.1002/14651858.CD006911.pub4] [PMCID: PMC8510511 (available on 12 October 2022)] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brattico 2009
- Brattico E, Pallesen KJ, Varyagina O, Bailey C, Anourova I, Järvenpää M, et al. Neural discrimination of nonprototypical chords in music experts and laymen: an MEG study. Journal of Cognitive Neuroscience 2009;21(11):2230-44. [DOI: 10.1162/jocn.2008.21144] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bruscia 1998
- Bruscia KE. Defining Music Therapy. 2nd edition. Gilsum (NH): Barcelona Publishers, 1998. [Google Scholar]
Buysse 1989
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research 1989;28(2):193-213. [DOI: 10.1016/0165-1781(89)90047-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cepeda 2006
- Cepeda MS, Carr DB, Lau J, Alvarez H. Music for pain relief. Cochrane Database of Systematic Reviews 2006, Issue 2. Art. No: CD004843. [DOI: 10.1002/14651858.CD004843.pub2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chen 2021
- Chen CT, Tung HH, Fang CJ, Wang JL, Ko NY, Chang YC, et al. Effect of music therapy on improving sleep quality in older adults: a systematic review and meta-analysis. Journal of the American Geriatrics Society 2021;69(7):1925-32. [DOI: 10.1111/jgs.17149] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chung 2015
- Chung KF, Yeung WF, Ho FY, Yung KP, Yu YM, Kwok CW. Cross-cultural and comparative epidemiology of insomnia: the Diagnostic and Statistical Manual (DSM), International Classification of Diseases (ICD) and International Classification of Sleep Disorders (ICSD). Sleep Medicine 2015;16(4):477-82. [DOI: 10.1016/j.sleep.2014.10.018] [PMID: ] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed 25 January 2022. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
De Niet 2009
- De Niet GJ, Tiemens BG, Kloos MW, Hutschemaekers GJ. Review of systematic reviews about the efficacy of non-pharmacological interventions to improve sleep quality in insomnia. International Journal of Evidence-Based Healthcare 2009;7(4):233-42. [DOI: 10.1111/j.1744-1609.2009.00142.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dickson 2019
- Dickson GT, Schubert E. How does music aid sleep? Literature review. Sleep Medicine 2019;63:142-50. [DOI: 10.1016/j.sleep.2019.05.016] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dickson 2020
- Dickson GT, Schubert E. Music on prescription to aid sleep quality: a literature review. Frontiers in Psychology 2020;11:1695. [DOI: 10.3389/fpsyg.2020.01695] [PMCID: PMC7399370] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dileo 2007
- Dileo C, Bradt J. Music therapy: applications to stress management. In: Lehrer PM, Woolfolk RL, Sime WE, editors(s). Principles and Practice of Stress Management. 3rd edition. New York (NY): Guilford Press, 2007:519-44. [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI: 10.1136/bmj.315.7109.629] [PMCID: PMC2127453] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
FDA 2022
- Food & Drug Administration. Benzodiazepine Drug Information . www.fda.gov/drugs/information-drug-class/benzodiazepine-drug-information (accessed 18 August 2022).
Feng 2018
- Feng F, Zhang Y, Hou J, Cai J, Jiang Q, Li X, et al. Can music improve sleep quality in adults with primary insomnia? A systematic review and network meta-analysis. International Journal of Nursing Studies 2018;77:189-96. [DOI: 10.1016/j.ijnurstu.2017.10.011] [PMID: ] [DOI] [PubMed] [Google Scholar]
Frandsen 2014
- Frandsen R, Baandrup L, Kjellberg J, Ibsen R, Jennum P. Increased all-cause mortality with psychotropic medication in Parkinson's disease and controls: a national register-based study. Parkinsonism & Related Disorders 2014;20(11):1124-8. [DOI: 10.1016/j.parkreldis.2014.07.012] [PMID: ] [DOI] [PubMed] [Google Scholar]
Garza‐Villarreal 2014
- Garza-Villarreal EA, Wilson AD, Vase L, Brattico E, Barrios FA, Jensen TS, et al. Music reduces pain and increases functional mobility in fibromyalgia. Frontiers in Psychology 2014;5:90. [DOI: 10.3389/fpsyg.2014.00090] [PMCID: PMC3920463] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Geretsegger 2017
- Geretsegger M, Mössler KA, Bieleninik Ł, Chen XJ, Heldal TO, Gold C. Music therapy for people with schizophrenia and schizophrenia-like disorders. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No: CD004025. [DOI: 10.1002/14651858.CD004025.pub4] [PMCID: PMC6481900] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 17 August 2021. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Guyatt 2011
- Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 2011;64(4):380-2. [DOI: 10.1016/j.jclinepi.2010.09.011] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hargreaves 1997
- Hargreaves DJ, North AC, editor(s). The Social Psychology of Music. Oxford (UK): Oxford University Press, 1997. [Google Scholar]
Harmat 2014 [pers comm]
- Harmat L. RE: page proofs and question [personal communication]. Email to: J Koenig 26 September 2014.
Hernández‐Ruiz 2005
- Hernández-Ruiz E. Effect of music therapy on the anxiety levels and sleep patterns of abused women in shelters. Journal of Music Therapy 2005;42(2):140-58. [DOI: 10.1093/jmt/42.2.140] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021
- Higgins JP, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Hodges 2009
- Hodges DA. Bodily responses to music. In: Hallam S, Cross I, Thaut M, editors(s). The Oxford Handbook of Music Psychology. Oxford (UK): Oxford University Press, 2009:121-30. [Google Scholar]
Jennum 2015
- Jennum P, Baandrup L, Ibsen R, Kjellberg J. Increased all-cause mortality with use of psychotropic medication in dementia patients and controls: a population-based register study. European Neuropsychopharmacology 2015;25(11):1906-13. [DOI: 10.1016/j.euroneuro.2015.08.014] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jennum 2018
- Jennum P, Baandrup L, Tønnesen P, Ibsen R, Kjellberg J. Mortality and use of psychotropic medication in sleep apnoea patients: a population-wide register-based study. Sleep Medicine 2018;43:19-24. [DOI: 10.1016/j.sleep.2017.11.1142] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jespersen 2012
- Jespersen KV, Vuust P. The effect of relaxation music listening on sleep quality in traumatized refugees: a pilot study. Journal of Music Therapy 2012;49(2):205-29. [DOI: 10.1093/jmt/49.2.205] [PMID: ] [DOI] [PubMed] [Google Scholar]
Juslin 2001
- Juslin PN, Sloboda JA. Music and Emotion: Theory and Research. Oxford (UK): Oxford University Press, 2001. [Google Scholar]
Juslin 2011
- Juslin PN, Liljeström S, Laukka P, Västfjäll D, Lundqvist LO. Emotional reactions to music in a nationally representative sample of Swedish adults: prevalence and causal influences. Musicae Scientiae 2011;15(2):174-207. [DOI: 10.1177/1029864911401169] [DOI] [Google Scholar]
Koelsch 2011
- Koelsch S, Fuermetz J, Sack U, Bauer K, Hohenadel M, Wiegel M, et al. Effects of music listening on cortisol levels and propofol consumption during spinal anesthesia. Frontiers in Psychology 2011;2:58. [DOI: 10.3389/fpsyg.2011.00058] [PMCID: PMC3110826] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Korhan 2011
- Korhan EA, Khorshid L, Uyar M. The effect of music therapy on physiological signs of anxiety in patients receiving mechanical ventilatory support. Journal of Clinical Nursing 2011;20(7-8):1026-34. [DOI: 10.1111/j.1365-2702.2010.03434.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kripke 2012
- Kripke DF, Langer RD, Kline LE. Hypnotics' association with mortality or cancer: a matched cohort study. BMJ Open 2012;2(1):e000850. [DOI: 10.1136/bmjopen-2012-000850] [PMCID: PMC3293137] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kryger 2017
- Kryger MH, Roth T, Dement WC. Principles and Practice of Sleep Medicine. Philadelphia (PA): Elsevier, 2017. [DOI: 10.1016/C2012-0-03543-0] [DOI] [Google Scholar]
Krystal 2004
- Krystal AD. The changing perspective on chronic insomnia management. Journal of Clinical Psychiatry 2004;65(Suppl 8):20-5. [PMID: ] [PubMed] [Google Scholar]
Kullich 2014a [pers comm]
- Kullich W. Re: data request / Cochrane Review [personal communication]. Email to: J Koenig 25 September 2014.
Kullich 2014b [pers comm]
- Kullich W. Fw: Data Request / Cochrane Review [personal communication]. Email to: J Koenig 15 September 2014.
Latimer Hill 2007
- Latimer Hill E, Cumming RG, Lewis R, Carrington S, Le Couteur DG. Sleep disturbances and falls in older people. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2007;62(1):62-6. [DOI: 10.1093/gerona/62.1.62] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Léger 2001
- Léger D, Scheuermaier K, Philip P, Paillard M, Guilleminault C. SF-36: evaluation of quality of life in severe and mild insomniacs compared with good sleepers. Psychosomatic Medicine 2001;63(1):49-55. [DOI: 10.1097/00006842-200101000-00006] [PMID: ] [DOI] [PubMed] [Google Scholar]
Léger 2008
- Léger D, Poursain B, Neubauer D, Uchiyama M. An international survey of sleeping problems in the general population. Current Medical Research and Opinion 2008;24(1):307-17. [DOI] [PubMed] [Google Scholar]
Magee 2017
- Magee WL, Clark I, Tamplin J, Bradt J. Music interventions for acquired brain injury. Cochrane Database of Systematic Reviews 2017, Issue 1. Art. No: CD006787. [DOI: 10.1002/14651858.CD006787.pub3] [PMCID: PMC6464962] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mayer 2011
- Mayer G, Jennum P, Riemann D, Dauvilliers Y. Insomnia in central neurologic diseases – occurrence and management. Sleep Medicine Reviews 2011;15(6):369-78. [DOI: 10.1016/j.smrv.2011.01.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
McKenzie 2021
- McKenzie JE, Brennan SE, Ryan RE, Thomson HJ, Johnston RV. Chapter 9: Summarizing study characteristics and preparing for synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Morin 2003
- Morin CM. Measuring outcomes in randomized clinical trials of insomnia treatments. Sleep Medicine Reviews 2003;7(3):263-79. [DOI: 10.1053/smrv.2002.0274] [PMID: ] [DOI] [PubMed] [Google Scholar]
Morin 2006
- Morin CM, LeBlanc M, Daley M, Gregoire JP, Mérette C. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Medicine 2006;7(2):123-30. [DOI: 10.1016/j.sleep.2005.08.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Morin 2013
- Morin CM, Jarrin DC. Epidemiology of insomnia: prevalence, course, risk factors, and public health burden. Sleep Medicine Clinics 2013;8(3):281-97. [DOI: 10.1016/j.jsmc.2013.05.002] [DOI] [PubMed] [Google Scholar]
Nieminen 2012
- Nieminen S, Istók E, Brattico E, Tervaniemi M. The development of the aesthetic experience of music: preference, emotions, and beauty. Musicae Scientiae 2012;16(3):372-91. [DOI: 10.1177/1029864912450454] [DOI] [Google Scholar]
NIH 2005
- National Institutes of Health (NIH). National Institutes of Health State of the Science conference statement on Manifestations and Management of Chronic Insomnia in Adults, June 13-15, 2005. Sleep 2005;28(9):1049-57. [DOI: 10.1093/sleep/28.9.1049] [PMID: ] [DOI] [PubMed] [Google Scholar]
Nilsson 2009
- Nilsson U. The effect of music intervention in stress response to cardiac surgery in a randomized clinical trial. Heart & Lung: The Journal of Critical Care 2009;38(3):201-7. [DOI: 10.1016/j.hrtlng.2008.07.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ohayon 2002
- Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Medicine Reviews 2002;6(2):97-111. [DOI: 10.1053/smrv.2002.0186] [PMID: ] [DOI] [PubMed] [Google Scholar]
Page 2021
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI: 10.1136/bmj.n71] [PMCID: PMC8005924] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perlis 2020
- Perlis ML, Vargas I, Ellis JG, Grandner MA, Morales KH, Gencarelli A, et al. The natural history of insomnia: the incidence of acute insomnia and subsequent progression to chronic insomnia or recovery in good sleeper subjects. Sleep 2020;43(6):zsz299. [DOI: 10.1093/sleep/zsz299] [PMCID: PMC7294401] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Petrovsky 2021
- Petrovsky DV, Ramesh P, McPhillips MV, Hodgson NA. Effects of music interventions on sleep in older adults: a systematic review. Geriatric Nursing 2021;42(4):869-79. [DOI: 10.1016/j.gerinurse.2021.04.014] [PMCID: PMC8316320 (available on 2022-07-01)] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager Web 2021 [Computer program]
- Review Manager Web (RevMan Web). The Cochrane Collaboration, 2021. Available at revman.cochrane.org.
Riedel 2000
- Riedel BW, Lichstein KL. Insomnia and daytime functioning. Sleep Medicine Reviews 2000;4(3):277-98. [DOI: 10.1053/smrv.1999.0074] [PMID: ] [DOI] [PubMed] [Google Scholar]
Riemann 2017
- Riemann D, Baglioni C, Bassetti C, Bjorvatn B, Dolenc Groselj L, Ellis JG, et al. European guideline for the diagnosis and treatment of insomnia. Journal of Sleep Research 2017;26(6):675-700. [DOI: 10.1111/jsr.12594] [PMID: ] [DOI] [PubMed] [Google Scholar]
Scarratt 2021
- Scarratt RJ, Heggli OA, Vuust P, Jespersen KV. The music people use to sleep: universal and subgroup characteristics. psyarxiv.com/5mbyv/ (accessed 1 September 2021). [DOI: 10.31234/osf.io/5mbyv] [DOI]
Shekleton 2010
- Shekleton JA, Rogers NL, Rajaratnam SM. Searching for the daytime impairments of primary insomnia. Sleep Medicine Reviews 2010;14(1):47-60. [DOI: 10.1016/j.smrv.2009.06.001] [PMID: ] [DOI] [PubMed] [Google Scholar]
Spielberger 1983
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto (CA): Consulting Psychologists Press, 1983. [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI: 10.1136/bmj.d4002] [PMID: ] [DOI] [PubMed] [Google Scholar]
Street 2014
- Street W, Weed D, Spurlock A. Use of music in the treatment of insomnia: a pilot study. Holistic Nursing Practice 2014;28(1):38-42. [DOI: 10.1097/HNP.0000000000000005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Su 2013
- Su CP, Lai HL, Chang ET, Yiin LM, Perng SJ, Chen PW. A randomized controlled trial of the effects of listening to non-commercial music on quality of nocturnal sleep and relaxation indices in patients in medical intensive care unit. Journal of Advanced Nursing 2013;69(6):1377-89. [DOI: 10.1111/j.1365-2648.2012.06130.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Taylor 2003
- Taylor DJ, Lichstein KL, Durrence HH. Insomnia as a health risk factor. Behavioral Sleep Medicine 2003;1(4):227-47. [DOI: 10.1207/S15402010BSM0104_5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Taylor 2007
- Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep 2007;30(2):213-8. [DOI: 10.1093/sleep/30.2.213] [PMID: ] [DOI] [PubMed] [Google Scholar]
Trappe 2010
- Trappe HJ. The effects of music on the cardiovascular system and cardiovascular health. Heart 2010;96(23):1868-71. [DOI: 10.1136/hrt.2010.209858] [PMID: ] [DOI] [PubMed] [Google Scholar]
Urponen 1988
- Urponen H, Vuori I, Hasan J, Partinen M. Self-evaluations of factors promoting and disturbing sleep: an epidemiological survey in Finland. Social Science & Medicine 1988;26(4):443-50. [DOI: 10.1016/0277-9536(88)90313-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Vuust 2006
- Vuust P, Roepstorff A, Wallentin M, Mouridsen K, Østergaard L. It don't mean a thing…keeping the rhythm during polyrhythmic tension, activates language areas (BA47). NeuroImage 2006;31(2):832-41. [DOI: 10.1016/j.neuroimage.2005.12.037] [PMID: ] [DOI] [PubMed] [Google Scholar]
Vuust 2010
- Vuust P, Gebauer L, Hansen NC, Jørgensen SR, Møller A, Linnet J. Personality influences career choice: sensation seeking in professional musicians. Music Education Research 2010;12(2):219-30. [DOI: 10.1080/14613801003746584] [DOI] [Google Scholar]
Walsh 2004
- Walsh JK. Clinical and socioeconomic correlates of insomnia. Journal of Clinical Psychiatry 2004;65(Suppl 8):13-9. [PMID: ] [PubMed] [Google Scholar]
Wang 2014
- Wang CF, Sun YL, Zang HX. Music therapy improves sleep quality in acute and chronic sleep disorders: a meta-analysis of 10 randomized studies. International Journal of Nursing Studies 2014;51(1):51-62. [DOI: 10.1016/j.ijnurstu.2013.03.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Medical Care 1992;30(6):473-83. [PMID: ] [PubMed] [Google Scholar]
WHO 1992
- World Health Organization. ICD-10: International Statistical Classification of Diseases and Related Health Problems Tenth Revision. Vol. 1. Geneva (CH): World Health Organization, 1992. [Google Scholar]
Wigram 2002
- Wigram T, Pedersen IN, Bonde LO. A Comprehensive Guide to Music Therapy: Theory, Clinical Practice, Research and Training. London (UK): Jessica Kingsley Publishers, 2002. [Google Scholar]
Wilson 2019
- Wilson SJ, Anderson K, Baldwin D, Dijk D-K, Epsie A, Espie C, et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders: an update. Journal of Psychopharmacology 2019;33(8):923-47. [DOI: 10.1177/0269881119855343] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhang 2012
- Zhang JM, Wang P, Yao JX, Zhao L, Davis MP, Walsh D, et al. Music interventions for psychological and physical outcomes in cancer: a systematic review and meta-analysis. Supportive Care in Cancer 2012;20(12):3043-53. [DOI: 10.1007/s00520-012-1606-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zimmerman 1996
- Zimmerman L, Nieveen J, Barnason S, Schmaderer M. The effects of music interventions on postoperative pain and sleep in coronary artery bypass graft (CABG) patients. Scholarly inquiry for nursing practice 1996;10(2):153-70; discussion 171-4. [PubMed] [Google Scholar]
References to other published versions of this review
Jespersen 2013
- Jespersen KV, Koenig J, Jennum P, Vuust P. Listening to music for improving sleep in adults with insomnia. Cochrane Database of Systematic Reviews 2013, Issue 3. Art. No: CD010459. [DOI: 10.1002/14651858.CD010459] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jespersen 2015
- Jespersen KV, Koenig J, Jennum P, Vuust P. Music for insomnia in adults. Cochrane Database of Systematic Reviews 2015, Issue 8. Art. No: CD010459. [DOI: 10.1002/14651858.CD010459.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]


