Abstract
Background
The monovalent type 2 oral poliovirus vaccine (mOPV2) stockpile is low. One potential strategy to stretch the existing mOPV2 supply is to administer a reduced dose: 1 drop instead of 2.
Methods
We conducted a randomized, controlled, open-label, noninferiority trial (10% margin) to compared immunogenicity after administration of 1 versus 2 drops of mOPV2. We enrolled 9–22-month-old infants from Mocuba district of Mozambique. Poliovirus neutralizing antibodies were measured in serum samples collected before and 1 month after mOPV2 administration. Immune response was defined as seroconversion from seronegative (<1:8) at baseline to seropositive (≥1:8) after vaccination or boosting titers by ≥4-fold for those with titers between 1:8 and 1:362 at baseline. The trial was registered at anzctr.org.au (no. ACTRN12619000184178p).
Results
We enrolled 378 children, and 262 (69%) completed per-protocol requirements. The immune response of mOPV2 was 53.6% (95% confidence interval, 44.9%–62.1%) and 60.6% (52.2%–68.4%) in 1-drop and 2-drop recipients, respectively. The noninferiority margin of the 10% was not reached (difference, 7.0%; 95% confidence interval, −5.0% to 19.0%).
Conclusion
A small loss of immunogenicity of reduced mOPV2 was observed. Although the noninferiority target was not achieved, the Strategic Advisory Group of Experts on Immunization recommended the 1-drop strategy as a dose-sparing measure if mOPV2 supplies deteriorate further.
Keywords: monovalent type 2 oral poliovirus vaccine (mOPV2), immunogenicity, 1-drop, Mozambique
Immunization with 1 drop instead of the customary 2 drops of monovalent type 2 oral poliovirus vaccine results in only slightly reduced immunogenicity; this approach should be considered to stretch a limited supply of this vaccine when responding to outbreaks.
The Global Polio Eradication Initiative (GPEI) has made steady progress toward the eradication target since its inception in 1988 [1]. The number of paralytic cases due to wild poliovirus has declined worldwide by >99.9%, and 2 of the 3 wild poliovirus serotypes have been declared eradicated by an independent Global Certification Commission: serotype 2 in 2015 and serotype 3 in 2019 [2, 3].
The GPEI has implemented the endgame Strategic Plan 2013–2018 to accelerate the eradication of wild poliovirus type 1 from its last endemic zones in Pakistan and Afghanistan. [4]. In addition, the plan called for the sequential removal of the Sabin strains from the oral poliovirus vaccine (OPV) and the concomitant addition of ≥1 dose of inactivated poliovirus vaccine (IPV) in routine national immunization programs. The removal was needed because the continuing use of live viral vaccines is incompatible with eradication, since these viruses can mutate and recombine, thus reacquiring the neurovirulence and transmission characteristics of wild poliovirus [5]. The burden of paralytic disease caused by vaccine-related polioviruses would not be accepted as the world approaches eradication of wild poliovirus. For example, Sabin type 2 was responsible for approximately 40% of the vaccine-associated paralytic poliomyelitis burden and caused 91% of circulating vaccine-derived poliovirus (cVDPV) cases between 2000 and 2016 [6].
Therefore, in April 2016, trivalent OPV (serotypes 1, 2, and 3) was globally withdrawn and replaced by bivalent types 1 and 3 OPV plus ≥1 dose of IPV in routine immunization schedule [7]. While 1 dose of IPV protects about 50% of vaccine recipients against paralytic poliomyelitis and primes the vast majority of remaining IPV recipients, it provides limited mucosal intestinal immunity to prevent infection and transmission of poliovirus [8]. Therefore, the GPEI created a global stockpile of monovalent type 2 OPV (mOPV2) to ensure the availability of an effective outbreak response tool in the post-OPV2 withdrawal period [1].
Since April 2016, the number of cVDPV2 outbreaks has substantially exceeded what was forecasted. In 2019 alone, there have been >40 cVDPV2 outbreaks affecting 17 countries, of which the majority were in Africa [9]. These outbreaks are largely continuing and require large quantities of mOPV2 for control. The original stockpile is low, and the additional bulk stored by manufacturers is being procured and converted into the final product [10].
As a contingency measure for responding to such a scenario, the Bill & Melinda Gates Foundation established the New OPV Consortium to develop a more genetically stable novel OPV2 (nOPV2) vaccine. Preclinical research and development of nOPV2 have been ongoing since 2011, with an acceleration of the development in the past 2 years [11]. Until nOPV2 is available, the depleting mOPV2 stockpile is the only option available to respond to cVDPV2 outbreaks [12], and dose-sparing strategies must be considered to stretch finite mOPV2 supplies to control the increasing number and geographic scope of cVDPV2 outbreaks.
One dose-sparing strategy is to reduce the volume of administered vaccines, from the standard 2 drops of mOPV2 (≥105 50% cell culture infective dose [CCID50]) to a single drop (≥104.9 CCID50) as an immunizing dose. In our trial, we compared the immunogenicity of a 1-drop single dose with a standard 2-drop single dose of mOPV2.
METHODS
This noninferiority, randomized controlled trial, was conducted during the cVDPV2 outbreak response in Zambézia Province, Mozambique, with the primary objective to assess the antibody response induced by 1 drop of mOPV2 compared with the standard 2 drops. The study had to be conducted as part of the cVDPV2 outbreak response, since any use of mOPV2 is restricted to outbreak response and must be authorized directly by the director-general of the World Health Organization (WHO). The outbreak of cVDPV2 in Mozambique provided an opportunity to conduct this study; however, the timing and logistic complexities of the outbreak response led to limitations in our ability to enroll and follow-up on the selected study cohort.
The study was nested in a specific geographic area within the outbreak response area for cVDPV2 in Molumbo District, Zambézia Province, Mozambique. The outbreak response was conducted between January and March 2019 and comprised 3 rounds of supplemental immunization activities with mOPV2 in children <5 years old in Zambézia Province and surrounding districts in Nampula and Niassa Provinces. Following the standard operating procedures for outbreak response [12], 3 rounds of mOPV2 (round 0 in the immediate area of the detection of cVDPV2; rounds 1 and 2 in a larger geographic area) were conducted. The study was carried out during round 1 (the first of the 2 larger rounds) of mOPV2 in Zambézia. The trial was approved by the ethics review committees of WHO, and by the Institutional Health Bioethics Committee and the National Health Bioethics Committee (IRB00002657; reference no. 62/CNBS/19), before administrative approval by the Ministry of Health of Mozambique was sought.
This open-label, noninferiority, 2-arm, randomized controlled trial was conducted in Mocuba, Zambézia, Mozambique. The district of Mocuba was chosen because it was included in the larger outbreak response but not in round 0 and was located at a considerable distance from the residence of the confirmed polio case patient (>200 km).
Study participants were enrolled from 8 sites in the Mocuba district. Inclusion criteria were healthy children between 9 and 22 months of age whose parents did not intend to travel within the next 6 weeks. This age group had not been previously vaccinated with mOPV2 (the most recent mOPV2 campaign in Mozambique was in May 2017). Children underwent a clinical examination to confirm eligibility and written informed consent was obtained from the child’s guardian. Children with severe illness on physical examination, fever defined as a body temperature of ≥37.5°C, history of coagulation disorder, or diagnosis or suspicion of immunodeficiency disorder were excluded from the study. Eligible children were randomized to the intervention arm (1- drop; arm A) or control arm (2 drops; arm B) of mOPV2. Randomization was done at block sizes of 4:6:12, with equal distribution for both study arms. The randomization list was available only to the lead researcher and the study coordinator.
At the first visit, after confirmation of eligibility for enrollment and obtaining of informed consent, 1 mL of venous blood was collected; the vaccine was subsequently administered orally, according to study arm allocation. The second visit was conducted 4 weeks after the first visit and a second 1-mL sample of venous blood was collected. After the second blood sample was obtained, all children were administered 2 drops of mOPV2 and a single dose of IPV to provide maximum protection against polio to all enrolled children; this was a requirement of the ethics review committee. These additional vaccines were not part of the study. The mid–upper arm circumference was measured during the second visit to assess the presence of severe acute malnutrition [13]. This measurement was added to the study procedures after the first visit because the study staff reported a high perceived malnutrition rate in this population during the first visit.
The vaccine used in the study was mOPV2 from GlaxoSmithKline with a reported titer ≥105 CCID50, manufactured in 2017. The actual prerelease titer for this vaccine lot, reported to WHO, was 105.5, corresponding to 316 227 CCID50, approximately 3 times the potency minimum levels required for release; this allows for the use of a reduced dose.
All blood specimens collected were transported to the laboratory of Mocuba District Hospital and centrifuged to separate serum. Specimens were stored at 2ºC–8ºC for 72 hours before shipment to the Instituto Nacional de Saúde in Marracuene, where they were stored at −40ºC until shipment to the Centers for Disease Control and Prevention in Atlanta, Georgia, which tested serum samples for the presence of poliovirus-neutralizing antibodies, using standard neutralization assays [14]. The final dilution tested was 1:1024, and the maximum reported titer was ≥1:1448.
The primary outcome of the study was the poliovirus type 2 immune response 4 weeks after receipt of mOPV2. The secondary outcome was seropositivity for poliovirus serotypes 1 and 3 and median antibody titers. For each serotype, seropositivity was defined as a detectable titer of poliovirus neutralizing antibodies of ≥1:8. Seroconversion was defined as a change from a nondetectable to a detectable titer (ie, from <1:8 to ≥1:8). For subjects with reciprocal titer ≥1:8 at baseline, boosting was defined as a 4-fold increase in titer. Because the upper detection limit of the test was 1:1448, children with baseline antibody titers >1:362 had to be excluded from analysis for boosting. Immune response was defined as the presence of either seroconversion or boosting. The distribution of antibody titers is reported as a reverse cumulative curve.
The target sample size for each arm was calculated to be 176 children. This was based on the assumptions of type 2 immune response of 85%, a 10% noninferiority margin, and a 5% level of significance with 80% power. In addition, we assumed that 10% of children would have high baseline antibody titers and would need to be excluded from the boosting and immune response end points. Noninferiority was set as ≤10% of the lower bound of 95% CI of the difference in the proportion of immune response between study arms.
We report the per-protocol analysis; an intention-to-treat analysis was also performed with the same results. Baseline categorical factors were compared using Fisher exact tests and normal tests for proportion between 1-drop and 2-drop arms, and Mann-Whitney U tests were used for age (in months). The 95% exact confidence intervals (CIs) were calculated for the differences between the groups, and noninferiority was assessed based on these CIs. Median antibody titers were computed, along with interquartile ranges. Titer distributions were compared using the Mann-Whitney U test. Adverse events and severe adverse events were noted and reported as percentages in each arm. All P values were 2 sided, and all analyses were performed using SAS 9.4 software. This trial was registered at anzctr.org.au (no. ACTRN12619000184178p) on 8 February 2019.
RESULTS
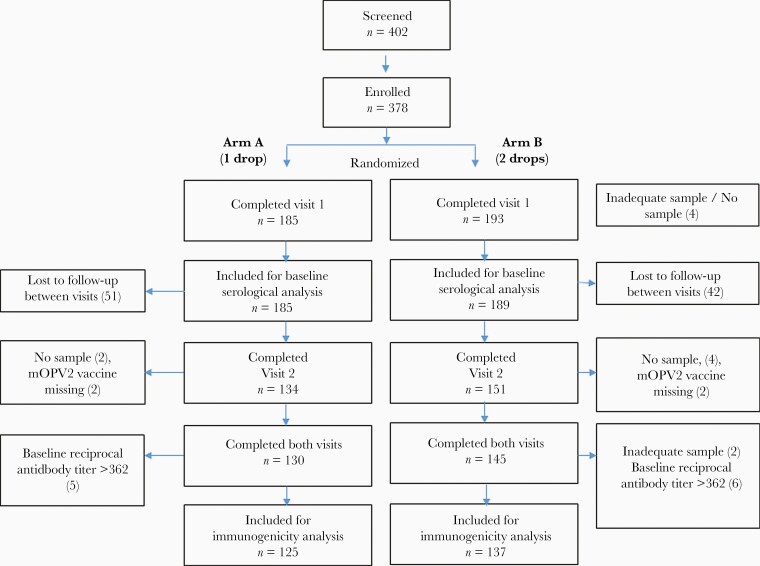
From March to April 2019, a total of 402 children aged 9–22 months were screened and 378 children were enrolled (Figure 1). Of those, 275 children (73%) completed all study procedures; however, 13 of 275 (5%) children were further excluded from the immunogenicity analysis because 11 had initial titers >1:362, which did not allow for evaluation of boosting, and 2 did not provide enough serum for analysis. The final sample for analysis of immunogenicity in the per-protocol population was 262 children (125 in arm A and 137 in arm B).
Figure 1.
Trial profile. Abbreviation: mOPV2, monovalent oral poliovirus vaccine type 2.
The demographic characteristics are shown in Table 1. There were no significant differences between arms A and B with regard to sex, prior vaccination history with IPV, or the number of bivalent OPV doses received.
Table 1.
Baseline Characteristics of Study Population
| Characteristic | Study Participants, No./Total (%)a | P Value | |
|---|---|---|---|
| Arm A (1-Drop mOPV2) | Arm B (2-Drop mOPV2) | ||
| Age, median (IQR), mo | 14 (11–18) | 14 (11–17) | .29b |
| Male sex | 92/185 (49.7) | 104/193 (53.9) | .47c |
| bOPV doses receivedd | |||
| 0 | 11/164 (6.7) | 4/160 (2.5) | .21c |
| 1–3 | 77/164 (47.0) | 76/160 (47.5) | |
| >3 | 76/164 (46.3) | 80/160 (50.0) | |
| Receipt of 1 IPV dosed | 107/139 (77.0) | 114/133 (85.7) | .09c |
| Nutritional status based on MUAC | |||
| Normal (MUAC >13.5 cm) | 85/134 (63.4) | 104/151 (68.9) | .59e |
| Mild malnutrition (MUAC 12.5 to ≤13.5 cm) | 34/134 (25.4) | 34/151 (22.5) | .28e |
| Moderate/severe malnutrition (MUAC <12.5 cm) | 15/134 (11.2) | 13/151 (8.6) | .36e |
| MUAC, median (IQR), cm | 14 (13.2–14.6) | 14 (13.5–14.9) | .32b |
Abbreviations: bOPV, bivalent OPV; IPV, inactivated poliovirus vaccine; IQR, interquartile range; mOPV2, monovalent oral poliovirus vaccine type 2; MUAC, mid–upper arm circumference.
aData represent no./total (%) of study participants unless otherwise specified.
bMann-Whitney U test.
cFisher exact test.
dBased on the information in the immunization card; children without cards were excluded.
eNormal test for proportions.
The baseline seroprevalence was 89.7%, 54.1%, and 82.7% for serotypes 1, 2, and 3, respectively; there were no significant differences in baseline seroprevalence or baseline antibody titers between study arms (Table 2). Baseline seroprevalence for poliovirus type 2 was significantly higher among children with a history of 1 dose of IPV than in those without an IPV history (62.8% [137 of 218] vs 23.5% [12 of 51]; P< .001).
Table 2.
Seroprevalence and Median Titers for Poliovirus Serotypes Before and After Vaccination in the 1-Drop and 2-Drop Arms
| Seroprevalence and Titers | Arm A (1-Drop mOPV2) | Arm B (2-Drop mOPV2) | P Value |
|---|---|---|---|
| Baseline seroprevalence, no./total (% [95% CI]) | |||
| Type 1 | 166/185 (89.7 [84.4–93.7]) | 170/189 (90.0 [84.8–93.8]) | >.99a |
| Type 2 | 100/185 (54.1 [46.6–61.4]) | 96/189 (50.8 [43.4–58.1]) | .54a |
| Type 3 | 153/185 (82.7 [76.5–87.9]) | 162/189 (85.7 [80.0–90.4]) | .48a |
| Baseline titer, median (IQR) | |||
| Type 1 | ≥1448 (455.1 to ≥1448) | 1152 (362 to ≥1448) | .46b |
| Type 2 | 9.0 (<8 to 22.6) | 9.0 (<8 to 28) | .98b |
| Type 3 | 910.2 (113.8 to ≥1448) | 910 (228 to ≥1448) | .45b |
| Final seroprevalence, no./total (% [95% CI]) | |||
| Type 1 | 117/130 (90.0 [83.5–94.6]) | 132/145 (91.0 [85.2–95.1]) | .84a |
| Type 2 | 105/130 (80.8 [72.9–87.2]) | 117/145 (80.7 [73.3–86.8]) | >.99a |
| Type 3 | 113/130 (86.9 [80.0–92.2]) | 124/145 (85.5 [78.7–90.8]) | .86a |
| Final titer, median (IQR) | |||
| Type 1 | ≥1448 (362 to ≥1448) | ≥1448 (910 to ≥1448) | .54b |
| Type 2 | 324 (11.3 to ≥1448) | 910 (11 to ≥1448) | .73b |
| Type 3 | ≥1448 (181 to ≥1448) | ≥1448 (455 to ≥1448) | .57b |
Abbreviations: CI, confidence interval; IQR, interquartile range; mOPV2, monovalent oral poliovirus vaccine type 2.
aFisher exact test.
bMann-Whitney U test.
The difference in the proportion of those with immune response between the 2-drop and 1-drop arms was 7.0% (95% CI, −5.0% to 19.0%), and the differences for seroconversion and boosting were 3.2% (95% CI, −13.4 to 19.7) and 6.5% (−10.5 to 23.5), respectively (Table 3). The noninferiority margin of 10% was not reached for any of the 3 indicators (Table 3). We did not find any significant risk factors affecting immune response (malnutrition, P = .54; prior IPV history, P = .63; age, sex, or diarrhea in the 24 hours before visit 1, P > .1 for all factors).
Table 3.
Seroconversion, Boosting, and Immune Response in the 1-Drop and 2-Drop Arms and Differences Between Arms
| Results | Participants, No./Total (% [95% CI] | Difference Between Arms (95% CI), %a | |
|---|---|---|---|
| Arm A (1-Drop mOPV2) | Arm B (2-Drop mOPV2) | ||
| Seroconversion | 35/54 (64.8 [51.5–76.2]) | 51/75 (68.0 [56.8–77.5]) | 3.2 (-13.4 to 19.7) |
| Boosting | 32/71 (45.1 [34.1–56.6]) | 32/62 (51.6 [39.5–63.6]) | 6.5 (-10.5 to 23.5) |
| Immune response | 67/125 (53.6 [44.9–62.1]) | 83/137 (60.6 [52.2–68.4]) | 7.0 (-5.0 to 19.0) |
Abbreviations: CI, confidence interval; mOPV2, monovalent oral poliovirus vaccine type 2.
aArm A minus arm B.
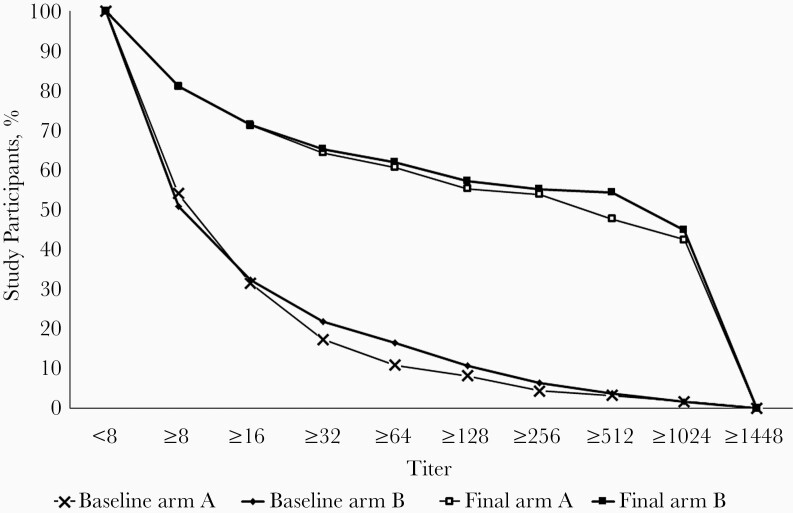
Before vaccination, the median titer (interquartile range) of type 2 antibodies was 9.0 (<8 to 22.6) and 9.0 (<8 to 28) in the 1-drop and 2-drop arms, respectively, compared with 324 (11.3 to ≥1448) and 910 (11 to ≥1448) about a month after receiving the vaccine (Table 2). The antibody titer distributions did not differ between study arms at baseline (P = .98) or after vaccination (P = .73). The distributions of antibody titers at baseline and the end of the study are shown in Figure 2.
Figure 2.
Reverse cumulative distribution of poliovirus serotype 2 antibody titers at baseline and at the final visit (4 weeks after vaccination) for arms A (1-drop monovalent oral poliovirus vaccine type 2 [mOPV2]) and B (2-drop mOPV2).
A total of 30 adverse events in the 1-drop and 45 in the 2-drop arm were reported between visits 1 and 2. The adverse events reported included fever with or without suspected malaria (63% in arm A and 62% in arm B), upper respiratory infections (10% and 16%, respectively), diarrhea (17%, and 13%), and other conditions (10% and 8%). None of the adverse events were assessed by the principal investigator to be related to the study procedures or the vaccine.
Discussion
Our study found that a 50% dose reduction of mOPV2 was marginally less immunogenic than the standard dose of mOPV2; in fact, the reverse antibody titer distribution curves were very similar to each other. However, we did find differences between the study arms. The overall immunogenicity (seroconversion and boosting) levels were somewhat lower (7% [95% CI, −5.0% to 19.0%]) in the 1-drop arm. If we restrict the analysis to subjects with no detectable antibodies at baseline, the difference in seroconversion between study arms becomes 3.2% (95% CI, −13.4% to 19.7%). Seroconversion among the standard protocol (2-drop) arm was similar to the ones reported in Lithuania in Europe and Pakistan in Asia (71% [95% CI, 54.5%–83.9%; 29 of 41] and 76% [63%–87%; 39 of 51]), respectively [15, 16]. In an outbreak response, the seronegative group (probably fully susceptible to the infection) would be of greatest interest.
As mentioned, the standard operating procedure for outbreak control requires several campaign rounds with mOPV2 [12]. Initially, round 0 includes the immediate detection zone, and then rounds 1 and 2 include a larger geographic area. In practice, however, often more campaigns with mOPV2 are needed when assessments of low supplemental immunization activity quality or breakthrough infection are found. Cumulative seroconversion calculations with 3 reduced versus full doses of mOPV2 will result in narrowing the difference in seroconversion to about 1%, a difference that is unlikely to have significant public health implications.
However, the 1-drop strategy would require substantial programmatic efforts to train the vaccinators and the supervisors. It could also possibly result in increased wastage rates. Finally, it would also involve off-label use of mOPV2. Therefore, the 1-drop strategy must be embraced with caution. Only a critical shortage of the available mOPV2 may require such drastic measures as an alternative to not doing a polio mass campaign and not protecting children. A possibly delay in the availability of the newly developed nOPV2 would lead the polio eradication program to implement this strategy.
Our study demonstrated that the routine immunization program in Mocuba district performed well and achieved high seroprevalence rates for poliovirus type 1 (90%) and type 3 (84%). The joint UNICEF/WHO-reported vaccine coverage estimate for IPV in Zambézia in 2018 was 86% [17]. We found that the baseline seroprevalence for poliovirus type 2 was 52%. Assuming that Mocuba had no documented circulation of type 2 poliovirus since 2016 and that immunogenicity of 1 dose of IPV administrated at 4 months is estimated at 63% [8] we calculated that the IPV routine immunization coverage in Mocuba was about 85%, consistent with the estimated coverage reported to UNICEF/WHO.
We did not observe any effect of malnutrition on mOPV2 immunogenicity, unlike a previous study in Pakistan [18]. We assessed acute malnutrition based only on mid–upper arm circumference, whereas chronic malnutrition in Pakistan was assessed using weight and height measurements; there, only chronic severe malnutrition interfered with the immunogenicity of mOPV2. The other issue may be our small sample size.
Our study had some limitations, the most important of which was the loss to follow-up, approaching 30%. This is because the study was conducted under emergency conditions, as mOPV2 use is allowed only in the context of an already- planned cVDPV2 response. The recruitment and follow-up had to be completed quickly to avoid overlap with the upcoming mOPV2 vaccination campaign round, which led to a high attrition rate between visits 1 and 2. In addition, in the rural population in Mocuba, rumors circulated that the blood of their children was sold for witchcraft. This led to some families’ decision to withdraw from the study. The reduced sample size resulted in reduced power of the study and consequently wide CIs around point estimates of the immune response.
Finally, we could not measure mucosal response, although it is an excellent proxy. Excretion of the virus in stool would have provided additional value, but was deemed not feasible under the emergency circumstances of this study. With the seroconversion rate of 60.6% in the 2-drop arm, the sample sizes of 125 in arm A and 137 in arm B achieved only 45% power to detect a 10% noninferiority margin, and 76% power to detect a 15% noninferiority margin. Finally, we cannot rule out secondary transmission of poliovirus type 2, because the first round of the mOPV2 vaccination campaign started on 16 March during recruitment.
The results of this study were presented to WHO’s Strategic Advisory Group of Experts on Immunizations (SAGE) in October 2019. Although SAGE noted the limitations of the study, they recommended that the global polio eradication program consider the use of a 1-drop mOPV2 strategy in the case of severe vaccine shortage [19].
In summary, our study provides important information on the possible dose-sparing effect of mOPV2. Although SAGE recommends the use of this strategy, if necessary, it has not been implemented thus far. However, if the cVDPV2 outbreaks cannot be controlled rapidly, this option may very well need to be exercised.
Notes
Presented in part: Strategic Advisory Group of Experts on Immunization, World Health Organization in Geneva, Switzerland.
Acknowledgments. The authors thank the legal guardians of the study participants, as well as the study participants and the study team, in particular, Dionísio Maitor João and Evilise Matico. They also thank the health authorities, the community leaders, and the communities involved in the study, from Zambézia province and Mocuba district, who provided all assistance required in the study implementation. Finally, thanks to Deborah Moore, Yiting Zhang, Sharla McDonald, William Hendley, Kathryn Manly, and Mario Nicolas for assistance with polio serology testing.
Disclaimer. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. This work was supported by Rotary International, through a grant from the World Health Organization (grant 2019/889177-2).
Potential conflicts of interest. All authors: No potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Nilsa de Deus, Instituto Nacional de Saúde (INS), Maputo, Mozambique.
Igor Paulo Ubisse Capitine, Instituto Nacional de Saúde (INS), Maputo, Mozambique.
Adilson Fernando Loforte Bauhofer, Instituto Nacional de Saúde (INS), Maputo, Mozambique; Instituto de Higiene e Medicina Tropical–Universidade Nova de Lisboa, Lisboa, Portugal.
Selma Marques, Instituto Nacional de Saúde (INS), Maputo, Mozambique.
Marta Cassocera, Instituto Nacional de Saúde (INS), Maputo, Mozambique; Instituto de Higiene e Medicina Tropical–Universidade Nova de Lisboa, Lisboa, Portugal.
Assucênio Chissaque, Instituto Nacional de Saúde (INS), Maputo, Mozambique; Instituto de Higiene e Medicina Tropical–Universidade Nova de Lisboa, Lisboa, Portugal.
Diocreciano Matias Bero, Instituto Nacional de Saúde (INS), Maputo, Mozambique.
José Paulo Langa, Instituto Nacional de Saúde (INS), Maputo, Mozambique.
Fernando Manuel Padama, Direcção Provincial de Saúde da Zambézia, Mozambique.
Visalakshi Jeyaseelan, Polio Eradication Department, World Health Organization, Geneva, Switzerland.
M Steven Oberste, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Concepcion F Estivariz, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Harish Verma, Polio Eradication Department, World Health Organization, Geneva, Switzerland.
Ilesh Jani, Instituto Nacional de Saúde (INS), Maputo, Mozambique.
Ondrej Mach, Polio Eradication Department, World Health Organization, Geneva, Switzerland.
Roland W Sutter, Polio Eradication Department, World Health Organization, Geneva, Switzerland.
References
- 1. Global Polio Eradication Initiative . Cases of wild poliovirus by country and year.http://www.polioeradication.org/Dataandmonitoring/Poliothisweek/Wildpolioviruslist.aspx. Accessed 6 March 2020.
- 2. Centers for Disease Control and Prevention . Global certification of eradication of indigenous wild poliovirus type 3.https://www.cdc.gov/globalhealth/immunization/stories/global-certification-of-eradication-of-indigenous-wild-poliovirus-type-3.html. Accessed 6 March 2020.
- 3. Global Polio Eradication Initiative . Global eradication of wild poliovirus type 2 declared.http://polioeradication.org/news-post/global-eradication-of-wild-poliovirus-type-2-declared/. Accessed 14 September 2020.
- 4. Global Polio Eradication Initiative . Polio eradication and endgame strategic plan 2013–2018.http://polioeradication.org/wp-content/uploads/2016/07/PEESP_EN_A4.pdf. Accessed 6 March 2020.
- 5. Sutter RW, Platt L, Mach O, Jafari H, Aylward RB. The new polio eradication end game: rationale and supporting evidence. J Infect Dis 2014; 210(suppl 1):S434–8. [DOI] [PubMed] [Google Scholar]
- 6. Platt LR, Estivariz CF, Sutter RW. Vaccine-associated paralytic poliomyelitis: a review of the epidemiology and estimation of the global burden. J Infect Dis 2014; 210(suppl 1):S380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Global Polio Eradication Initiative . Global switch in oral polio vaccines.http://maps.who.int/OPV_switch/. Accessed 7 June 2016.
- 8. Resik S, Tejeda A, Sutter RW, et al. . Priming after a fractional dose of inactivated poliovirus vaccine. N Engl J Med 2013; 368:416–24. [DOI] [PubMed] [Google Scholar]
- 9. Macklin GR, O’Reilly KM, Grassly NC, et al. . Evolving epidemiology of poliovirus serotype 2 following withdrawal of the type 2 oral poliovirus vaccine. Science 2020; 368:401–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization . WHO global action plan to minimize poliovirus facility-associated risk after type-specific eradication of wild polioviruses and sequential cessation of oral polio vaccine use.2015. http://polioeradication.org/wp-content/uploads/2016/12/GAPIII_2014.pdf. Accessed 6 March 2020.
- 11. Van Damme P, De Coster I, Bandyopadhyay AS, et al. . The safety and immunogenicity of two novel live attenuated monovalent (serotype 2) oral poliovirus vaccines in healthy adults: a double-blind, single-centre phase 1 study. Lancet 2019; 394:148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Global Polio Eradication Initiative . Strategy for the response to type 2 circulating vaccine-derived poliovirus.http://polioeradication.org/wp-content/uploads/2020/04/Strategy-for-the-response-to-type-2-circulating-Vaccine-Derived-Poliovirus-20200406.pdf. Accessed 4 April 2020.
- 13. World Health Organization . WHO child growth standards and the identification of severe acute malnutrition in infants and children.https://www.who.int/nutrition/publications/severemalnutrition/9789241598163_eng.pdf. Accessed 4 April 2020. [PubMed]
- 14. Weldon WC, Oberste MS, Pallansch MA. Standardized methods for detection of poliovirus antibodies. Methods Mol Biol 2016; 1387:145–76. [DOI] [PubMed] [Google Scholar]
- 15. Bandyopadhyay AS, Gast C, Brickley EB, et al. . A randomized phase 4 study of immunogenicity and safety after monovalent oral type 2 Sabin poliovirus vaccine challenge in children vaccinated with inactivated poliovirus vaccine in Lithuania. J Infect Dis 2021; 223:119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saleem AF, Yousafzai MT, Mach O, et al. . Evaluation of vaccine derived poliovirus type 2 outbreak response options: a randomized controlled trial, Karachi, Pakistan. Vaccine 2018; 36:1766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health Organization/UNICEF . WHO/UNICEF joint reporting process.https://www.who.int/immunization/monitoring_surveillance/routine/reporting/en/. Accessed 14 April 2020.
- 18. Saleem AF, Mach O, Quadri F, et al. . Immunogenicity of poliovirus vaccines in chronically malnourished infants: a randomized controlled trial in Pakistan. Vaccine 2015; 33:2757–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization . Meeting of the Strategic Advisory Group of Experts on Immunization, October 2019: conclusions and recommendations. Wkly Epidemiol Rec 2019; 94:541–60. [Google Scholar]