Abstract
Background
Treatment of early-stage non-small cell lung cancer (NSCLC) is rapidly evolving. When introducing novelties, real-life data on effectiveness of currently used treatment strategies are needed. The present study evaluated outcomes of stage I–IIIA NSCLC patients treated with upfront radical surgery in everyday clinical practice, between 2010–2017.
Patients and methods
Data of 539 consecutive patients were retrieved from a prospective hospital-based registry. All diagnostic, treatment and follow-up procedures were performed at the same thoracic oncology centre according to the valid guidelines. The primary outcome was overall survival (OS) analysed by clinical(c) and pathological(p) TNM (tumour, node, metastases) stage. The impact of clinicopathological characteristics on OS was evaluated using univariable (UVA) and multivariable regression analysis (MVA).
Results
With a median follow-up of 53.9 months, median OS and 5-year OS rate in the overall population were 90.4 months and 64.4%. Five-year OS rates by pTNM stage I, II and IIIA were 70.2%, 60.21%, and 49.9%, respectively. Both cTNM and pTNM stages were associated with OS; but only pTNM retained its independent prognostic value (p = 0.003) in MVA. Agreement between cTNM and pTNM was 69.0%. Next to pTNM, age (p = 0.001) and gender (p = 0.004) retained their independent prognostic value for OS.
Conclusions
The study showed favourable outcomes of resectable stage I–IIIA NSCLC treated with upfront surgery in real-life. Relatively low agreement between cTNM and pTNM stages and independent prognostic value of only pTNM, observed in real-life data, suggest that surgery remains the most accurate provider of the anatomical stage of disease and important upfront therapy.
Key words: resectable NSCLC, upfront surgery, real-life data, overall survival, prognostic factors
Introduction
Lung cancer is a major public health issue worldwide, with an estimated 2.2 million new cases and 1.8 million deaths in 2020 making it the second most common cancer and the leading cause of cancer death worldwide.1 After decades of poor control of lung cancer, the mortality rates began to decrease in the last two decades.1 This trend coincides with a slow, but steady increase in lung cancer survival rates, that was up to now mostly noticeable in localized (stage I and II) non-small cell lung cancer (NSCLC). Currently the 5-year net survival of localized lung cancer is around 60%.2, 3
Localized lung cancer accounts for around 25% of newly diagnosed lung cancers, with a vast majority of them having NSCLC histology.3 Surgery with curative intent remains fundamental treatment for stage I–II and for selected stage IIIA NSCLC patients.4 With the introduction of novel, less invasive surgical techniques, such as video-assisted thoracoscopic surgery and improved perioperative care, the outcomes of patients with resectable NSCLC improved substantially.3, 4 Platinum-based adjuvant chemotherapy, which is nowadays considered as a standard adjuvant treatment of early-stage NSCLC, further improved cure rates.5 With the incorporation of novel targeted therapies and immunotherapy with immune checkpoint inhibitors (ICIs) additional increase in overall survival is expected. Targeted therapy with osimertinib, which led to significant reduction in distant recurrence or death in a prospective phase 3 trial has already been incorporated into treatment recommendations for epidermal growth factor receptor (EGFR) positive patients.5 Based on the positive results of some recently published adjuvant trials, it is expected that ICIs will soon become a part of standard adjuvant therapy for early-stage NSCLC as well. There is growing evidence that neoadjuvant treatment with ICI leads to major or even complete pathologic responses in a substantial percentage of patients without compromising surgery for resectable NSCLC6, thus making neoadjuvant immunotherapy an appealing approach in the future.
It is expected that the percentage of patients diagnosed with resectable NSCLC will increase in the next years. Several international clinical trials, including the European NELSON study confirmed the efficacy of low-dose CT screening in decreasing lung cancer mortality in the high-risk population of heavy smokers.7, 8 With the introduction of screening programs, we expect not only an increase of patients diagnosed with localized NSCLC but it might also become necessary to redefine treatment paradigms for early-stage NSCLC.
There is no doubt that major changes in the detection and treatment of early-stage NSCLC are expected shortly. To better predict and evaluate the effectiveness of those novel strategies in everyday clinical practice and to develop individualized risk-adjusted treatment strategies for individual patients, more data on clinicopathological characteristics and outcomes of early-stage NSCLC patients treated in a real-life before the introduction of those novelties, are needed. The International Association for the Study of Lung Cancer (IASLC) recommendations for TNM classification scheme, based on a database of nearly 90.000 patients9 as well as some IASCL validation studies performed on the Caucasian population10 provide valuable data on survival of patients treated in routine clinical practice. Next to the IASLC data, there is almost complete lack of information on the outcomes of the cohorts of resectable stage I–IIIA NSCLC patients, treated in a real-life scenario in the last decade. Most of the real-life observational trials reported recently present data for specific subpopulations of resectable NSCLC, such as patients treated with adjuvant chemotherapy11 or patients with stage IIIA or N2 disease.12, 13, 14 Our study aimed to evaluate overall survival of consecutive resectable TNM stage I–IIIA NSCLC patients treated with upfront radical surgery in a real-life practice, using prospectively collected hospital-based registry data. We also assessed the impact of clinicopathological characteristics, particularly TNM stage, on survival.
Patients and methods
Data source and study population
Data were retrieved from the hospital-based lung cancer registry, which prospectively collects demographics, clinicopathological, treatment, and survival data for all lung cancer patients diagnosed and treated at the centre. In hospital follow-up data are supplemented with the death certificates provided by the National Health Institute on a regular basis. All data was collected in an anonymised fashion. For the purpose of this study, survival status was updated and the data were retrieved in January 2020.
We retrieved the data of consecutive patients with resectable cTNM stage I–III NSCLC, treated with upfront radical surgical resection at a single thoracic oncology centre in Slovenia, between January 2010 and December 2017. All patients had pathologically confirmed NSCLC. Diagnostic and treatment procedures were performed as recommended by the international guidelines valid at the time.15, 16 Lymph nodes showing (18) F-fluorodeoxyglucose (FDG) uptake on preoperative PET-CT scans, or their short axis > 1 cm on CT scans were marked as clinically positive. In patients with clinically positive mediastinal lymph nodes endobronchial ultrasound-guided lymph node biopsy (EBUS TBNB) was performed, whenever feasible. For all patients, including those with cN2 disease, the institutional multidisciplinary tumour board concluded that they have resectable NSCLC and were referred to upfront surgery.
All patients underwent radical surgical resection (R0) with lobectomy, bilobectomy, or pneumonectomy with complete lymph node dissection as a standard surgical procedur.16, 17 Adjuvant chemotherapy and/or postoperative radiotherapy were performed according to the international guidelines valid at that time.15, 16 Patients with neoadjuvant treatment were not included in the study population.
Clinical stage was defined as the last stage determined before surgical resection. All resected tissue including lymph nodes was examined by board certified pathologists. Clinical and pathological stages were assigned based on the 7th edition TNM classification for NSCLC17, valid at the time. Testing for EGFR mutations and anaplastic lymphoma kinase (ALK) rearrangements has been introduced gradually as recommended by the international societies.18 Testing was performed on formalin fixed, paraffin embedded tumour tissue specimens or different cytological specimens. For EGFR testing allele-specific PCR method with commercial kits, either Cobas EGFR mutation test (Roche, USA) or Therascreen EGFR PCR Kit (Qiagen, UK). ALK immunohistochemical detection was based on ALK CDx assay (Ventana, Roche, USA). Patients were followed-up with physical examination and chest CT scan, first biannually and after two years annually.
The hospital-based registry data collection and all subsequent analyses for academic purposes were approved by the Slovenian National Committee for Medical Ethics (approval number 135/07/09 and 40/04/12). All patients consented for data collection and subsequent analyses.
Outcome measures and statistical analyses
The primary endpoint was overall survival (OS), defined as the time in months from the date of surgery until either the date of death from any cause or the date the patient was last known to be alive (censored data). Patient and treatment characteristics were analysed using descriptive statistics. The agreement between clinical and pathological TNM staging variables was calculated as simple percent agreement to ease the interpretation of the results. Survival curves were estimated using the Kaplan-Meier estimator. The independent prognostic value of each included characteristic was tested in a Cox proportional hazards regression model. All variables with p ≤ 0.250 in univariable regression analysis (UVA) were considered for and included in the multivariable regression analysis (MVA), except EGFR and ALK status due to being applicable only to a subset of patients. A p-value below 0.05 was considered statistically significant. All reported p-values are two-tailed. All statistical analyses were carried out using IBM SPSS Statistics software (version 21).
Results
We identified 539 consecutive stage I–IIIA NSCLC patients treated with upfront radical surgery. Demographic, clinicopathological, and treatment characteristics of the study population are presented in Table 1. The median age was 64 years (range, 39–83), males accounted for 58.4% of patients. Most patients were current or former smokers, with only 12.7% of never smokers included in the study. Adenocarcinoma appeared most frequently (63.3%), followed by squamous-cell carcinoma (36.2%) and other rare types of NSCLC (0.6%). EGFR mutations and ALK rearrangements were detected in 12.3% and 5.3 % of tested patients, with low completeness of ALK testing due to the introduction of testing to routine clinical practice from 2014 onward. Lobectomy was performed in a vast majority of patients, bilobectomy or pneumonectomy was required in only 5.8% and 9.1% of patients, respectively. Adjuvant platinum doublet chemotherapy was delivered in 146 (27.1%) of patients, the vast majority of whom had pathologically confirmed lymph node involvement. Postoperative radiotherapy was used in 36 (6.7%) patients; all of them had pathological N2 disease.
Table 1.
Demographic, clinicopathological and treatment characteristics of study population
| Characteristic | N (%) |
|---|---|
|
| |
| No. of patients | 539 |
| Age in years: median (range) | 64 (39–83) |
| < 65 years | 271 (50.3) |
| ≥ 65 years | 268 (49.7) |
| Gender | |
| Male | 315 (58.4) |
| Female | 224 (41.6) |
| Smoking status (n = 537; completeness = 99.6 %) | |
| Current | 257 (47.8) |
| Former | 212 (39.5) |
| Never | 68 (12.7) |
| Histology | |
| Adenocarcinoma | 341 (63.3) |
| Squamous-cell carcinoma | 195 (36.2) |
| NSCLC other rare types | 3 (0.6) |
| EGFRa status in non-squamous NSCLC | |
| (n = 334; completeness = 99.7%) | |
| Positive | 41 (12.3) |
| Negative | 292 (87.7) |
| ALKb status in non-squamous NSCLC | |
| (n = 334; completeness = 39.2%) | |
| Positive | 7 (5.3) |
| Negative | 124 (94.7) |
| Clinical TNM stage c | |
| I | 309 (57.3) |
| II | 145 (26.9) |
| IIIA | 85 (15.8) |
| Clinical T stage | |
| T1 | 242 (44.9) |
| T2 | 193 (35.8) |
| T3 | 96 (17.8) |
| T4 | 8 (1.5) |
| Clinical N stage | |
| N0 | 393 (72.9) |
| N1 | 102 (18.9) |
| N2 | 44 (8.2) |
| Pathological TNM stagec (n = 532; completeness = 98.7%) | |
| I | 296 (55.6) |
| II | 150 (28.2) |
| III | 86 (16.2) |
| Pathological T stage (n = 537; completeness = 99.6%) | |
| T1 | 223 (41.5) |
| T2 | 248 (46.2) |
| T3 | 58 (10.8) |
| T4 | 8 (1.5) |
| Pathological N stage (n = 534; completeness = 99.1% | |
| N0 | 386 (72.3) |
| N1 | 81 (15.2) |
| N2 | 67 (12.5) |
| Surgery type | |
| Lobectomy | 459 (85.2) |
| Bilobectomy | 31 (5.8) |
| Pneumonectomy | 49 (9.1) |
| Adjuvant treatment | |
| Platinum-based chemotherapy | 146 (27.1) |
| Postoperative radiotherapy | 36 (6.7) |
EGFR: epidermal growth factor receptor; bALK: anaplastic lymphoma kinase; cstage defined by American Joint Committee on Cancer staging
PET-CT was performed in 94.8% of patients (511/539). EBUS TBNB was gradually introduced in the routine clinical practice during the study period and was applied in 112 patients, with cN1 and cN2 disease according to CT and/or PET-CT scan. Lymph node involvement was confirmed in 65.5% of the samples obtained from the patients with cN2 disease. Mediastinoscopy was performed in five patients with cN2 and negative EBUS TNBN of mediastinal nodes; all lymph node samples obtained by mediastinoscopy were negative. Most patients were diagnosed with clinical stage I (57.3%) or stage II (26.9%). Clinical stage IIIA was determined in 15.8% of patients. All patients had either a single zone cN2 involvement or cT3/T4 disease without tumour invasion to the adjacent vessels or organs. Postoperative pathological examination and staging also revealed high rate of pathological stage I (55.6%) or stage II (28.2%), with low percentage of stage IIIA disease (16.2%). However, the agreement between clinical and pathological staging was relatively low.
Table 2 shows the comparison between clinical (cTNM) and pathological (pTNM) staging according to TNM staging categories. The agreement between cTNM and pTNM stages was the highest for stage I (81%) and much lower for stage II (55%) and stage IIIA (49%). Of note, cTNM stage IIIA turned out to be pTNM stage II or stage I in 36% and 14% of patients, respectively. When analysing T and N descriptors separately, the accuracy of cT-descriptor decreased with increasing stage while for cN-descriptor the lowest accuracy rate was observed for cN1 stage. The overall agreement between clinical and pathological stage were quite similar for all three descriptors, TNM stage, T stage and N stage, i.e., 69.0%, 72.3% and 71.9%, respectively.
Table 2.
Comparison between clinical (c) and pathological (p) TNM staging
2A. Comparison between clinical and pathological TNM stage (n = 532; completeness = 98.7%)
| c Stage I (N = 303) N (%) | c Stage II (N = 144) N (%) | c Stage IIIA (N = 85) N (%) | |
|---|---|---|---|
| p Stage I | 246 (81%) | 38 (26%) | 12 (14%) |
| p Stage II | 40 (13%) | 79 (55%) | 31 (36%) |
| p Stage IIIA | 17 (6%) | 27 (19%) | 42 (49%) |
Overall agreement: 367 out of 532 cases (69.0%)
2B.
Comparison between clinical and pathological T stage (n = 537; completeness = 99.6%)
| cT1 (N = 240) N (%) | cT2 (N = 193) N (%) | cT3 (N = 96) N (%) | cT4 (N = 8) N (%) | |
|---|---|---|---|---|
| pT1 | 187 (78%) | 24 (13%) | 10 (10%) | 2 (25%) |
| pT2 | 46 (19%) | 158 (82%) | 41 (43%) | 3 (37%) |
| pT3 | 5 (2%) | 9 (4%) | 42 (44%) | 2 (25%) |
| pT4 | 2 (1%) | 2 (1%) | 3 (3%) | 1 (13%) |
Overall agreement between: 388 out of 537 cases (72.3%)
2C.
Comparison between clinical and pathological N stage (n = 534; completeness = 99.1%)
| cN0 (N = 388) N (%) | cN1 (N = 102) N (%) | cN2 (N = 44) N (%) | |
|---|---|---|---|
| pN0 | 324 (84%) | 49 (48%) | 13 (30%) |
| pN1 | 42 (11%) | 34 (33%) | 5 (11%) |
| pN2 | 22 (6%) | 19 (19%) | 26 (59%) |
Overall agreement: 384 out of 534 cases (71.9%)
The median follow-up time was 53.9 (50.9–56.9) months. At the end of follow-up, 177/539 patients (32.8%) died. The median OS (mOS) for the whole cohort of patients was 90.4 months (95% CI calculation unreliable due to few events after mOS), with an estimated 5-year OS rate of 64.4% (Figure 1). The overall survival of patients grouped by cTNM, pTNM, cN and pN stage is depicted in Figure 2. The mOS has not been reached in the majority of the subgroups. The estimated 5-year OS rates for patients with cTNM stage I, stage II, and stage IIIA were 70.6%, 56.9%, and 55.3%; while the estimated 5-year OS rates for patients with pTNM stage I, stage II, and stage IIIA were 70.2%, 60.2%, and
Figure 1.
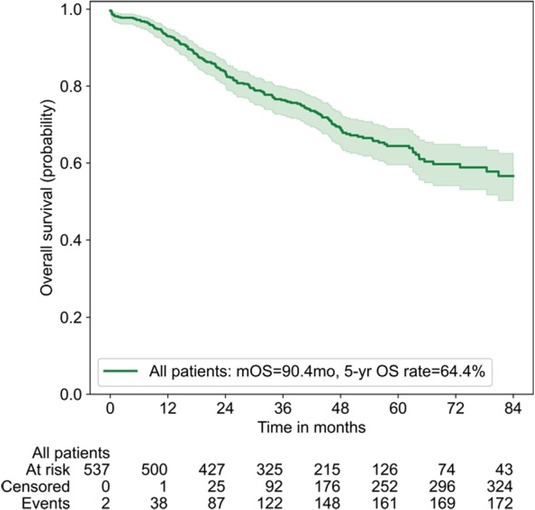
Overall survival of patients with completely resected stage I–III A non-small cell lung cancer.
Figure 2.
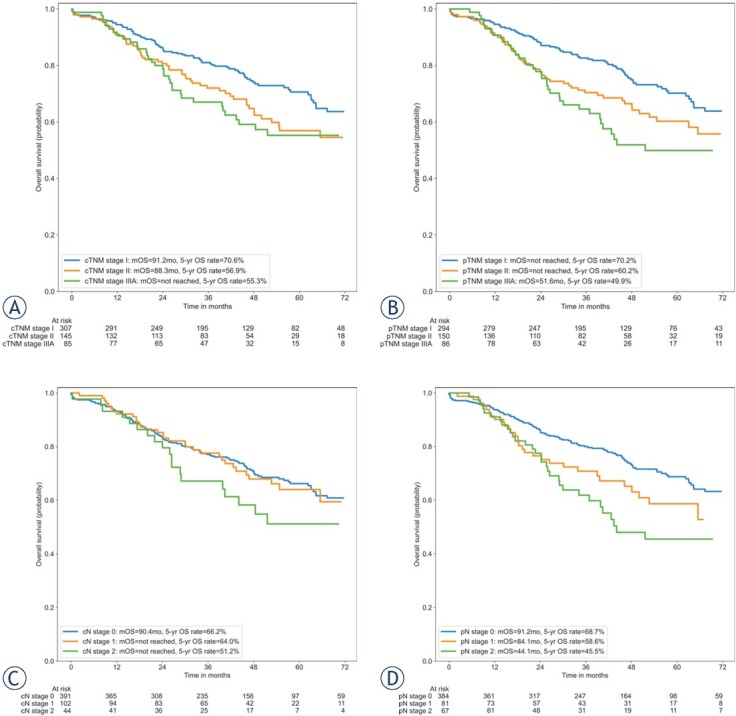
Overall survival by clinical TNM stage (A), pathological TNM stage (B), clinical N stage (C) and pathological N stage (D).
In UVA the factors significantly associated with shorter overall survival were age ≥ 65 years and male gender. Furthermore, with respect to the anatomical stages, all stage categories, except cN (p = 0.313), were significantly associated with OS in the UVA (Table 3). However, in MVA that included either cTNM or pTNM stage as a determinator of the anatomical extent of disease, pTNM retained its significant and independent impact on OS (p = 0.003), next to age and gender, while cTNM stage lost its independent prognostic value (p = 0.092) (Table 4). Of note, TNM stage (clinical or pathological) was always included in the model for multivariate analyses, while the other factors were included in the stepwise procedure (thus only the significant factors are reported in Table 4).
Table 3.
Univariate analyses of overall survival
| Factor | p-value | HR (95% CI) |
|---|---|---|
| Age | ||
| < 65 | 1 | |
| ≥ 65 | 0.002 | 1.59 (1.18 – 2.15) |
| Gender | ||
| Male | 1 | |
| Female | 0.001 | 0.59 (0.43 – 0.81) |
| Smoking status | ||
| never | 1 | |
| current or former | 0.115 | 1.50 (0.91 – 2.47) |
| Histology | ||
| adenocarcinoma or NOS | 1 | |
| squamous cell carcinoma | 0.111 | 1.28 (0.95 – 1.73) |
| EGFR statusa (positive vs negative) | ||
| negative | 1 | |
| positive | 0.111 | 0.56 (0.27 – 1.14) |
| Clinical TNM stage | 0.027* | |
| I | 1 | |
| II | 0.034 | 1.44 (1.03 – 2.02) |
| IIIA | 0.025 | 1.57 (1.06 – 2.34) |
| Clinical T stage | 0.001* | |
| T1 | 1 | |
| T2 | 0.882 | 0.97 (0.69 – 1.38) |
| T3 or T4 | 0.001 | 1.86 (1.29 – 2.68) |
| Clinical N stage | 0.317* | |
| N0 | 1 | |
| N1 | 0.958 | 0.99 (0.67 – 1.46) |
| N2 | 0.137 | 1.44 (0.89 – 2.34) |
| Pathological TNM stage | 0.003* | |
| I | 1 | |
| II | 0.030 | 1.46 (1.04 – 2.06) |
| IIIA | 0.001 | 1.90 (1.29 – 2.79) |
| Pathological T stage | 0.007* | |
| T1 | 1 | |
| T2 | 0.019 | 1.49 (1.07 – 2.07) |
| T3 or T4 | 0.004 | 1.92 (1.23 – 2.98) |
| Pathological N stage | 0.002* | |
| N0 | 1 | |
| N1 | 0.054 | 1.48 (0.99 – 2.20) |
| N2 | 0.001 | 1.93 (1.29 – 2.87) |
only in non-squamous NSCLC; *for the whole variable
Table 4.
Multivariate analyses of overall survival (separate for clinical and for pathological stage)
| Cox regression model with clinical stage | p-value | HR (95% CI) |
|---|---|---|
| Age | ||
| < 65 | 1 | |
| ≥ 65 | 0.003 | 1.58 (1.17 – 2.14) |
| Gender | ||
| Male | 1 | |
| Female | 0.006 | 0.63 (0.46 – 0.88) |
| Clinical TNM stage | 0.092* | |
| I | 1 | |
| II | 0.078 | 1.36 (0.97 – 1.91) |
| IIIA | 0.068 | 1.46 (0.97 – 2.18) |
| Cox regression model with pathological stage | p-value | HR (95% CI) |
| Age | ||
| < 65 | 1 | |
| ≥ 65 | 0.001 | 1.68 (1.24 – 2.28) |
| Gender | ||
| Male | 1 | |
| Female | 0.004 | 0.62 (0.45 – 0.86) |
| Pathological TNM stage | 0.003* | |
| I | 1 | |
| II | 0.076 | 1.37 (0.97 – 1.93) |
| IIIA | 0.001 | 1.95 (1.32 – 2.88) |
for the whole variable
Discussion
This observational cohort study presents real-life data on long-term survival and the impact of clinicopathological characteristics on overall survival of resectable stage I–IIIA NSCLC patients, treated with upfront radical surgery at a single thoracic oncology centre in the period 2010–2017. The median OS time of 90.4 months and estimated 5-year survival rate of 64.4% observed in our real-life cohort of 539 consecutive patients are encouraging. Our data exceed the median OS of 63 months observed in a German cohort of patients with radically resected stage I–IIIB NSCLC, treated at a single academic centre in a very similar period (from 2009 to 2014), which also included patients with a higher stage IIIB disease.10 When comparing by pTNM stage I, II and IIIA, the estimated 5-year survival rates of 70.2%, 60.2% and 49.9%, respectively, observed in our study, correspond very well to the 5-year survival rates in the German study.10 Our findings also slightly exceed the 5-year survival rates of 83%–71%, 57%–49% and 36% for pTNM stage IA–B, II A–B and IIIA, published by IASLC.9 Furthermore, our findings are also in line with 5-year survival rates between 37%–47%, observed in real-life cohorts of patients with resectable stage IIIA–N2 NSCLC, treated with upfront surgery in a similar period.12, 13, 14 Thus, our observation supports the idea that selected patients with stage IIIA NSCLC might have a favourable outcome when treated by upfront radical surgery followed by adjuvant chemotherapy and/or irradiation.
As expected, the observed survival rates decreased with increasing stage of all staging variables (T, N, and TNM). But of note, while significant differences in survival were observed according to both clinical and pathological T and both clinical and pathological TNM stage, clinical N stage (as opposed to pathological N stage) did not prove a significant prognostic factor already in the UVA. Furthermore, in the multivariate analyses in which only TNM stage as a comprehensive denominator of T and N stages was included, only pTNM stage retained its significant and independent impact on overall survival, while cTNM stage failed to do so (likely due to its N stage part). This clearly points towards a much stronger prognostic value of pathological compared to clinical staging variables in resectable NSCLC. Also, in many previous studies evaluating prognostic impact of clinical and pathological TNM or N stage on OS the information on pathological stage improved prognostic value of the model.9, 14, 17 There is evidence suggesting quite a high rate of disagreement between clinical and pathological staging in operable NSCLC patients treated in everyday practice. Even in studies performed after introduction of PET-CT and EBUS TBNB in routine clinical practice, relatively high rate of disagreement between clinical and pathological N and TNM staging was observed. In the Dutch observational study performed in patients with pathological stage IIIA disease, the agreement between clinical and pathological T and N stage was 57.1% and 28.5%, respectively.19 The agreement rates observed in our study were relatively high for all three descriptors T, N and TNM stage (72.3%, 71.9% and 69.0%, respectively), but still not optimal. However, EBUS TBNB have only been introduced in our everyday clinical practice during the study period. With the incoming era of neoadjuvant systemic therapy, the accurate non-surgical staging of not only mediastinal lymph nodes but also hilar lymph nodes were becoming important. In our study the lowest agreement between clinical and pathological N status was observed particularly for cN1 stage (33%). Very interesting and clinically important observation is that almost half (48%) of cN1 patients were down staged to pN0, while upgrading to pN2 was found in a smaller, 19% proportion of patients. With recent dilemmas whether more invasive mediastinal lymph node staging might change the treatment paradigm and outcomes of NSCLC patients with cN1 disease our data become even more appealing.
Notably, the survival rates observed in our current study far exceed those observed in a retrospective analysis of NSCLC patients treated at our centre in 2006.20 The latter revealed much shorter median overall survival rates for all clinical TNM stages I, II and IIIA NSCLC with the largest differences observed in stages II–IIIA. In that analysis all consecutive patients were included, regardless of whether they received treatment with curative intent or not, which is definitively one of the reasons for worse survival rates. But still, improvement in overall survival achieved over the last years is obvious. This can be attributed to major advances in diagnostic procedures, surgical techniques, postoperative care and adjuvant therapies for early NSCLC that we witnessed in the last decade and their rapid transfer into everyday clinical practice at our institution.21
The clinicopathological characteristics of our cohort of patients mirror the typical population of NSCLC patients in our country and region at the beginning of this century, with prevailing smokers and squamous-cell histology.21 Next to pTNM stage, age and gender retained their significant and independent prognostic value for OS in MVA; while smoking status and histology failed to show prognostic value already in the UVA. Our results are in concordance with the observations made on a large series of patients with NSCLC confirming older age and male gender as independent prognostic factors for worse survival.22, 23 Male gender was confirmed as an independent prognostic factor for worse survival in published trials, however this has been seen particularly in patients with advanced NSCLC and adenocarcinomas.23 In our study male gender turned out to be an independent predictor of worse survival in early-stage NSCLC and irrespective of histology, thus suggesting other probable causes of poor survival in male NSCLC patients which need to be further investigated.
Our study also provides valuable data on the frequency of EGFR mutations and their prognostic value in early-stage NSCLC. The findings are in line with the results of recently published large individual study24 which failed to confirm prognostic impact of EGFR status on survival of patients with resectable NSCLC. There are still uncertainties about the percentage of EGFR mutated tumours in early-stage NSCLC. In our study, EGFR testing performed on a large series of 334 patients with resectable non-squamous cell NSCLC, revealed a 12.3% positivity rate which is quite comparable to the 13.8% positivity rate observed in advanced NSCLC in the countries and the centres which participated in the INSIGHT registry trial.25 Similarly, ALK positivity rate of 5.3% observed in our series of resectable NSCLC corresponds very well with the positivity rates observed in advanced NSCLC.26
The results of our study should be considered in the context of its strengths and limitations. The study provides a wealth of information on clinicopathological characteristics and survival outcomes of a large cohort of resectable NSCLC patients, treated with upfront surgery in real-life practice. Additionally, all data were collected prospectively by the hospital-based lung cancer registry. Looking at potential limitations, results from a single centre study might not be generalisable to the overall population in the country or region. However, at our centre more than a half of the country’s newly diagnosed resectable NSCLC are treated, thus representing the entire population quite well. It is also encouraging that the activities on establishing a nationwide register of lung cancer patients collecting detailed data on clinicopathological characteristics and individual treatments at the Cancer Registry of Slovenia are ongoing. Since our hospital-based registry does not capture data on the cause of death, we do not present data on cancer specific survival but on overall survival, which might be influenced by comorbidities and other conditions often present in fairly old population of patients with resectable NSCLC. The hospital registry also does not collect precise data on modality of preoperative staging (imaging versus invasive procedures) to determine clinical N stage in each individual patient. Therefore, the data on mediastinal staging by EBUS TNBN and mediastinoscopy were collected retrospectively and might be subject to bias.
Our study with a lengthy follow-up, showed a favourable outcome for patients with resectable stage I–IIIA NSCLC treated with upfront surgery in a real-life setting. Particularly encouraging are the survival rates observed in patients with stage IIIA disease indicating that selected patients with N2 disease are candidates for upfront surgery. Relatively low agreement between cTNM and pT-NM stages and the independent prognostic value of pTNM but not cTNM stage observed in our study, suggest that we should aim to further improve preoperative staging. Until then we should always weight our decisions about upfront treatment of resectable NSCLC very carefully for each individual patient. Currently, surgery remains the most reliable provider of information on anatomical TNM stage as one of the strongest prognostic factors and enables us to make an informed decision on adjuvant systemic treatment in each individual patient.
Finally, it is inspiring to notice a substantial improvement in overall survival rates of early-stage NSCLC patients treated over the last decades at the same large thoracic oncology centre. With the aim of further improving our results, we are planning an additional study which will strive to evaluate preoperative staging of nodal involvement more profoundly, thus providing for better multimodality treatment selection for each individual patient.
Acknowledgement
The authors thank all doctors and other staff providing standard care for patients with NSCLC at Surgery Bitenc and at the University Clinic Golnik. Special thanks go to the staff of the hospital-based registry, especially to Ana Herzog and Tjaša Brus Pičman, for their dedicated work. This work was supported by the Slovenian Research Agency [grants number J3-4076, J3-7372] by providing the funds for establishment of the hospital-based registry. This analysis has received no funding.
The raw data underlying this article are available in the article. Due to data privacy, and hospital registry-related restrictions, the clinicopathological data cannot be made public, i.e., accessible to anyone for any purpose without a review process and without putting an agreement in place.
Disclosure
No potential conflicts of interest were disclosed.
Footnotes
Data availability statement and author contribution statement
Marko Bitenc: conceptualization, writing – original draft, formal analysis, writing – review & editing. Tanja Cufer: conceptualization, formal analysis, writing – original draft, writing – review & editing, supervision. Izidor Kern: investigation, writing—original draft, formal analysis. Martina Miklavcic: data curation, investigation, writing – original draft, writing – review & editing. Sabrina Petrovic: data curation, investigation, writing – original draft. Vida Groznik: software, data curation. Aleksander Sadikov: software, formal analysis, visualization, writing – review & editing, supervision.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. et al. [DOI] [PubMed] [Google Scholar]
- 2.Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–75. doi: 10.1016/S0140-6736(17)33326-3. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zadnik V, Žagar T, Tomšič S, Lokar K, Duratović Konjević A, Zakotnik B. [Survival of cancer patients]. [Slovenian] Ljubljana: Institute of Oncology; 2020. [Google Scholar]
- 4.Montagne F, Guisier F, Venissac N, Baste JM. The role of surgery in lung cancer treatment: present indications and future perspectives – state of the art. Cancers. 2021;13:1–24. doi: 10.3390/cancers13153711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Remon J, Soria JC, Peters S. Early and locally advanced non-small-cell lung cancer: an update of the ESMO Clinical Practice Guidelines focusing on diagnosis, staging, systemic and local therapy. Ann Oncol. 2021;32:1637–42. doi: 10.1016/J.ANNONC.2021.08.1994. [DOI] [PubMed] [Google Scholar]
- 6.Shukla N, Hanna N. Neoadjuvant and adjuvant immunotherapy in early-stage non-small cell lung cancer. Lung Cancer Targets Ther. 2021;12:51–60. doi: 10.2147/LCTT.S277717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMOA1102873/SUPPL_FILE/NEJMOA1102873_DISCLOSURES.PDF. The National Lung Screening Trial Research Team. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503–13. doi: 10.1056/nejmoa1911793. [DOI] [PubMed] [Google Scholar]
- 9.Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WEE. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM Classification for lung cancer. J Thorac Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. et al. [DOI] [PubMed] [Google Scholar]
- 10.Taber S, Pfannschmidt J. Validation of the 8th lung cancer TNM classification and clinical staging system in a German cohort of surgically resected patients. Innov Surg Sci. 2020;5:1–9. doi: 10.1515/iss-2020-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai B, Fulcher N, Boyd M, Spira A. Clinical outcomes and resource utilization after surgical resection with curative intent among patients with non-small cell lung cancer treated with adjuvant therapies in a community oncology setting: a real-world retrospective observational study. Thorac Cancer. 2021;12:2055–64. doi: 10.1111/1759-7714.14007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abrão FC, Moreira FR, de Abreu IRLB, Marciano MG, Younes RN. Real-life long-term cohort of patients with stage IIIA non-small-cell lung cancer: overall survival related to patients’ characteristics and multiple treatment models. JCO Glob Oncol. 2021;7:1572–85. doi: 10.1200/go.21.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas DC, Arnold BN, Rosen JE, Salazar MC, Detterbeck FC, Blasberg JD. The significance of upfront knowledge of N2 disease in non-small cell lung cancer. World J Surg. 2018;42:161–71. doi: 10.1007/s00268-017-4165-6. et al. [DOI] [PubMed] [Google Scholar]
- 14.Yun JK, Bok JS, Lee GD, Kim HR, Kim YH, Kim DK. Long-term outcomes of upfront surgery in patients with resectable pathological N2 non-small-cell lung cancer. Eur J Cardio-Thoracic Surg. 2020;58:59–69. doi: 10.1093/ejcts/ezaa042. et al. [DOI] [PubMed] [Google Scholar]
- 15.Crinò L, Weder W, van Meerbeeck J. Felip ESMO Guidelines Working Group Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v103–15. doi: 10.1093/annonc/mdq207. [DOI] [PubMed] [Google Scholar]
- 16.Vansteenkiste J, De Ruysscher D, Eberhardt WEE, Lim E, Senan S, Felip E. ESMO Guidelines Working Group. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi89. doi: 10.1093/annonc/mdt241. et al. –. [DOI] [PubMed] [Google Scholar]
- 17.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R. The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:706–14. doi: 10.1097/JTO.0b013e31812f3c1a. et al. [DOI] [PubMed] [Google Scholar]
- 18.Kerr KM, Bubendorf L, Edelman MJ, Marchetti A, Mok T, Novello S. Second ESMO consensus conference on lung cancer: pathology and molecular biomarkers for non-small-cell lung cancer. Ann Oncol. 2014;25:1681–90. doi: 10.1093/annonc/mdu145. et al. [DOI] [PubMed] [Google Scholar]
- 19.Heineman DJ, Beck N, Wouters MW, van Brakel TJ, Daniels JM, Schreuers WH. The dutch national clinical audit for lung cancer: a tool to improve clinical practice? An analysis of unforeseen ipsilateral mediastinal lymph node involvement in the Dutch Lung Surgery Audit (DLSA) Eur J Surg Oncol. 2018;44:830–4. doi: 10.1016/J.EJSO.2017.12.002. et al. [DOI] [PubMed] [Google Scholar]
- 20.Debevec L, Jerič T, Kovač V, Bitenc M, Sok M. Is there any progress in routine management of lung cancer patients? A comparative analysis of an institution in 1996 and 2006. Radiol Oncol. 2009;43:47–53. doi: 10.2478/V10019-009-0008-X. [DOI] [Google Scholar]
- 21.Zwitter M, Čufer T, Vrankar M, Kern I, Štupnik T, Rozman A. Lung Cancer in Slovenia. J Thorac Oncol. 2019;14:1327–31. doi: 10.1016/J.JTHO.2019.02.025. et al. [DOI] [PubMed] [Google Scholar]
- 22.Tas F, Ciftci R, Kilic L, Karabulut S. Age is a prognostic factor affecting survival in lung cancer patients. Oncol Lett. 2013;6:1507–13. doi: 10.3892/ol.2013.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Visbal AL, Williams BA, Nichols FC, Marks RS, Jett JR, Aubry MC. Gender differences in non-small-cell lung cancer survival: an analysis of 4,618 patients diagnosed between 1997 and 2002. Ann Thorac Surg. 2004;78:209–15. doi: 10.1016/J.ATHORACSUR.2003.11.021. et al. [DOI] [PubMed] [Google Scholar]
- 24.Saw SPL, Zhou S, Chen J, Lai G, Ang MK, Chua K. Association of clinicopathologic and molecular tumor features with recurrence in resected early-stage epidermal growth factor receptor-positive non-small cell lung cancer. JAMA Netw Open. 2021;4:e2131892. doi: 10.1001/jamanetworko-pen.2021.31892. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramlau R, Cufer T, Berzinec P, Dziadziuszko R, Olszewski W, Popper H. Epidermal growth factor receptor mutation-positive non-small-cell lung cancer in the real-world setting in Central Europe: the INSIGHT study. J Thorac Oncol. 2015;10:1370–4. doi: 10.1097/JTO.0000000000000621. et al. [DOI] [PubMed] [Google Scholar]
- 26.Ryska A, Berzinec P, Brcic L, Cufer T, Dziadziuszko R, Gottfried M. NSCLC molecular testing in Central and Eastern European countries. BMC Cancer. 2018;18:269. doi: 10.1186/s12885-018-4023-4. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]


