Abstract
Background and purpose:
Inflammatory bowel disease (IBD) is a chronic and multifactorial disease with unknown etiology and a decisive cure. Salvia officinalis (sage) which has anti-inflammatory, anti-oxidative, and ulcer healing properties can be useful for the treatment of IBD. Therefore, the effect of S. officinalis ethanolic extract (SOEE) and methanolic partition (SOMP) was investigated on acetic acid-induced ulcerative colitis.
Experimental approach:
Male Wistar rats (180-220 g) were used. SOEE (30, 60, and 120 mg/kg) and SOMP (50, 100, and 150 mg/kg) were prepared through maceration method. Prepared extracts, dexamethasone (1 mg/kg, i.p.), and mesalamine (100 mg/kg) as reference drugs and normal saline as control were administered by gavage, 2 h before colitis induction and preserved for four further days to animals. The colon tissues were examined for macroscopic and pathologic parameters and myeloperoxidase (MPO) and malondialdehyde (MDA) levels.
Findings/Results:
SOEE (60 and 120 mg/kg) and SOMP at all doses alleviated colitis severity and indices both in macroscopic and microscopic views. MDA and MPO activities were also significantly declined in the extracts-treated groups compared to the controls. The lowest dose of SOEE couldn’t meaningfully reduce any of the parameters compared to the control group.
Conclusion and implications:
Both extracts of S. officinalis exerted anti-colitis effects in rats, though methanolic partition was more effective, especially at the highest dose. It seems S. officinalis could exert protection against oxidative stress and inflammatory mediators in colitis tissue. More experimental and clinical studies are required to explore the exact mechanisms and active ingredients which are involved.
Keywords: Rats, Sage, Salvia officinalis, Ulcerative colitis
INTRODUCTION
Idiopathic inflammatory bowel disease (IBD) is a chronic and multifactorial disease that is divided into ulcerative colitis and Crohn’s disease. A vast variety of factors such as environmental abnormalities, genetics, immune system disorders, and changes in gut microbiota are among the most probable etiologic factors (1,2).
5-Aminosalicylic acid congeners like sulfasalazine and mesalamine that have anti- inflammatory effects, glucocorticosteroids, azathioprine, 6-mercaptopurine, and methotrexate as immunomodulatory and immunosuppressive drugs, infliximab and adalimumab (anti-tumor necrosis factor alpha, TNF-α) are among the most conventional treatments for IBD (3).
Several side effects have been reported due to current treatments including bone marrow suppression, liver and kidney toxicity, visual abnormality, headache, infectious disease, metabolic and bone disorders, hypertension, and hypersensitivity reactions (4).
Due to the adverse effects related to the current drugs and the ambiguous etiology of IBD, research to find alternative and/or complementary remedies among herbal and traditional medicines has been highly motivated (5). Salvia officinalis (common sage, garden sage) is an ancient plant native to Europe, Asia, and Latin America, however, it is planted all over the world today. Salvia is the largest genus of the Lamiaceae family and consists of about 900 species. Its shrub and aerial parts are used in cookery and traditional medicine due to their flavoring and therapeutic properties (6). In Iranian folk medicine as well as traditional medicine in Asia and America, S. officinalis has been used in the treatment of rheumatism, gout, gastrointestinal ulcers, dyspepsia and bloating, diarrhea, and inflammation suggesting its reasonable potential in the treatment of IBD (7).
The most important components of S. officinalis aerial parts are polyphenols and flavonoids (rosmarinic acid, caffeic acid, chicoric acid, and quinic acid), essential oils (camphor, 8-cineol, thujon, and borneol), tannins, polysaccharides, diterpenoids, triterpenoids (oleanolic acid and ursolic acid), and lipophilic flavons (luteolin and luteolin-7-glycoside) for which antioxidant, anti-inflammatory, hepato- protective, ulcer healing, and even immune-modulatory properties have been established (8,9,10,11,12,13,14).
S. officinalis has been shown to be a plant with ample antimicrobial capacity, acting significantly on clinical samples and prevalent bacterial and fungal species responsible for the diseases of the oral cavity (15). It has also contributed to the control of pro-inflammatory cytokine production as a mouthwash as suggested by Fawzi et al. (16). Considering the role of bacterial infections and pro-inflammatory cytokines in the pathogenesis of IBD and especially colitis with fistulae, it is assumed that S. officinalis might be useful for IBD prevention and/or treatment.
Therefore, the current study was designed and carried out to assess the ulcer healing and anti-colitis properties of S. officinalis ethanolic extract (SOEE) and methanolic partition (SOMP) in experimental colitis in rats.
MATERIALS AND METHODS
Drugs and chemicals
Mesalamine and dexamethasone powders were received as a gift from Iran Hormone Company (Tehran, Iran). O-dianisidine dihydrochloride (ODD) and hexadecyl trimethyl ammonium bromide (HTAB) were purchased from Sigma Company (St. Louis, USA). Ethyl and methyl alcohols, diethyl ether oxide, n-hexane, glacial acetic acid, and formaldehyde were procured from Merck Company (Darmstadt, Germany). Normal saline made in Shahid-Ghazi Corporation (Tabriz, Iran) was prepared from a local pharmacy.
Animals
Male Wistar rats (n = 60, weighing 180-220 g) were provided from the animal house of Isfahan School of Pharmacy, Isfahan, Iran. The rats were kept in a similar and controlled environmental situation in terms of light/dark cycle (12/12 h), relative humidity (30-50%), and temperature 20-23 °C. Pelleted chow and tap water were freely available and water was ad libitum. The research was confirmed by the Iranian Ethics Committee for biomedical studies (ethics registration code: IR.MUI.RESEARCH.REC676) and done in accordance with guidelines from the National Institute of Health Care and Use of Laboratory Animals. The investigators tried to decrease the number of animals and the experimental distress throughout the study.
Preparation of herbal extracts
S. officinalis leaves (common sage) were prepared from a trusted company; Pakan-bazr (Isfahan, Iran), and its variety was initially identified by Dr. A. Yegdaneh, a pharmacognosist while subsequently additional microscopic and macroscopic tests confirmed it. In addition, the prepared plant was visually matched with the herbarium specimen deposited at the department of Pharmacognosy which was specified as specimen #1487. SOEE was prepared by macerating the sage leaves (930 g). In this process, the leaves of the plant were first pulverized and then soaked in ethanol 70%. After 24 h the content was filtered. Soaking and filtering were repeated 5 times till almost all plant compounds were extracted into the solvent. Then the solvent was concentrated in a rotary apparatus. Then n-hexane with the least amount and methanol were added to the separator funnel and the process was repeated 3 times to separate the methanolic part. Finally, SOMP, as well as SOEE extracts, were freeze-dried to obtain full-dried contents. The yields of SOEE and SOMP were determined at 29.5% and 18.1% w/w, respectively (17).
Determination of total phenol contents of plant extracts
Total phenolic compounds of S. officinalis were evaluated through the Folin-Ciocaltue method (18). This method is briefly based on the reaction of phenolic compounds with a colorimetric reagent, which allows measurement in the visible portion of the spectrum (absorbance 765 nm). Gallic acid was used as the reference agent and a standard curve was depicted against increasing concentrations of gallic acid. Total phenols were calculated as gallic acid equivalents using the regression equation between gallic acid standards and absorbance 765.
Experimental design
Sixty rats were used and randomized in ten groups, 6 each, as follows:
(1) Sham group (normal group), normal saline/tween was administered by gavage as the vehicle (5 mL/kg, p.o.) 2 h before intra-rectal instillation of normal saline (2 mL/rat).
(2) Control (colitis) group, normal saline/tween was administered (5 mL/kg, p.o.) 2 h before instillation of acetic acid intra-rectally for induction of colitis and continued 4 days thereafter (19).
(3-5) SOEE groups including three increasing doses of SOEE (30, 60, and 120 mg/kg, p.o.) (11,14) were administered 2 h before induction of colitis and continued 4 days thereafter.
(6-8) SOMP groups including three increasing doses of SOMP (50, 100, and 150 mg/kg, p.o) were administered 2 h before induction of colitis and continued 4 days thereafter.
(9) Mesalamine group (reference group), mesalamine (100 mg/kg, p.o.) (19) was administered 2 h before induction of colitis and continued 4 days thereafter.
(10) Dexamethasone group (reference group), dexamethasone (1 mg/kg i.p.) (20) was administered 2 h before induction of colitis and continued 4 days thereafter.
SOEE and SOMP at applied doses were made as to the dispersion in normal saline/tween 80 (0.1%), and administered by gavage in a similar volume (1 mL/rat).
Induction of acute colitis
Before induction of colitis, the animals were kept in a fasting condition for 24 h. Then, the animals were lightly anesthetized with ketamine/xylazine (75/10 mg/kg) and a suitable catheter (2 mm in diameter and 8 cm in length) was applied for installation of 2 mL acetic acid 3% via the rectum. After the induction of colitis, access to water and food was free for the animals (21).
Assessment of macroscopic parameters
One day after the last dose of extracts administration, the animals were euthanized by inhalation of carbon dioxide. Colons at the distal part (8 cm in length and 3 cm above the anus) were cut, taken out, and irrigated with normal saline whereas their wet weight was immediately determined. Then, the tissue was established on a working board and multiple photographs were taken by a mobile camera for subsequent macroscopic assessment. The area of ulcers was determined by Fiji-win 32 software. The scoring of ulcer severity was done as 0: no ulcer; 1: inflammation, edema, thickness, and superficial erosions; 2: bleeding, hemorrhage, and definite ulcers; 3: deep ulcers, necrosis, and/or perforation. The index of the ulcer was calculated by summing the ulcer area and ulcer severity (21).
After macroscopic assessments, colon specimens were longitudinally sectioned into 2 similar parts. One part was immersed in formalin 10% for histopathology assessment, whereas another part was kept in freeze condition (-70 °C) for myeloperoxidase (MPO) and malondialdehyde (MDA) measurements (22).
Assessment of histopathologic parameters
For histopathological evaluation, tissue samples were embedded in paraffin, processed, and cut into 4-mm layers in diameter. Then, the samples were deparaffinized by xylene and hydrated by ethyl alcohol. Then, hematoxylin and eosin (H&E) staining was done. The severity of inflammation (0: none, 1: slight, 2: moderate, and 3: severe) and its extent (0: none, 1: mucosal, 2: mucosal and submucosal, and 3: transmural embracement), crypt damage (0: none, 1: basal 1/3 damaged, 2: basal 2/3 damaged, 3: surface epithelium was intact only, and 4: crypt and surface epithelium were distorted) and the infiltration of leukocyte (0: trace, 1: mild, 2: moderated, and 3: sever) were evaluated. The total colitis index (0-13) was measured by summing the abovementioned variables. Evaluation of histopathological parameters was done by applying a Zeiss microscope equipped with a color video camera (Sony, Japan) for digital imaging (23).
Assessment of MPO activity
One hundred mg of each rat’s colon was mixed in potassium buffer (5 mL, pH 6) containing HTAB (0.5%) and homogenized for three 45-sec periods. The mixture was transferred into a sonicator for 10 s in an ice bath, and then, it was centrifuged at 4000 rpm for 15 min. The supernatant (0.1 mL) was mixed with 2.9 mL of 50 mM phosphate buffer (pH 6) containing 0.167 mg/mL ODD and 0.0005% hydrogen peroxide. The absorbance (at 450 nm) of the resultant solution was measured by a UV-Vis spectrophotometer at zero and 3 min after that (LSI Model Alfa-1502, LW Scientific, USA). MPO activity was reported as U/g of the wet weight of the colon (19).
Assessment of MDA
As an index for lipid peroxidation, MDA content was measured in colon samples. For this purpose, 1 mL of potassium chloride 1.15% w/v was added to 100 mg of colonic tissue homogenate and then centrifuged (1200 rpm) for 15 min. The supernatant was picked up and applied for MDA measurement using an assay kit (Navand-Salamat, Iran) according to its manufacturer’s instructions. MDA content was reported as nmol/mL (19).
Statistical analysis
Statistical analyses were performed using SPSS (Version 16, Chicago. IL, USA). Parametric data are presented as mean ± SEM, and one-way ANOVA followed by Tukey as a post hoc test was used for comparisons. Nonparametric data were shown as median (range) while compared using the Kruskal-Wallis test followed by Mann-Whitney U-test. A P < 0.05 was considered a significant difference level for all the comparisons.
RESULTS
Plant dry matter and total phenol contents of extracts
The mean dry matter determined after 3 replicates were 90.1% and 73.7% for SOHE and SOMP, respectively. Besides total phenol content was obtained at 201 ± 0.67 (mg gallic acid equivalents)/g and 272 ± 1.88 (mg gallic acid equivalents)/g for SOEE and SOMP, respectively.
Effect of SOEE and SOMP on macroscopic parameters
After data analysis, no changes in the sham (normal) group were found indicating that manipulation and surgical handling had no impact on the macroscopic features. The groups treated with SOEE at 60 and 120 mg/kg and SOMP at 50, 100, and 150 mg/kg exhibited diminished ulcer index and colon weight (mg/cm) significantly in comparison with the control colitis group. Ulcer index, as well as ulcer area and ulcer severity and wet weight of the colon, were attenuated significantly in dexamethasone and mesalamine-treated groups. SOEE at 30 mg/kg was not significantly effective to diminish any macroscopic features compared to the control group (Table 1 and Fig. 1).
Table 1.
Effect of SOEE and SOMP on the macroscopic parameters of colitis induced by acetic acid in rats. Data are expressed as mean ± SEM or median (range) for scoring parameter, n = 6. *P < 0.05, **P < 0.01, and ***P < 0.001 indicate significant difference versus control.
| Groups | Ulcer severity (0-3) | Ulcer area (cm2) | Ulcer index (0-11) | Colon weight (mg/cm) |
|---|---|---|---|---|
| Sham (normal) | 0 (0-0)*** | 0.0 ± 0.0*** | 0.0 ± 0.0*** | 79.8 ± 4.1*** |
| Control (colitis) | 3 (2-3) | 5.54 ± 1.50 | 8.54 ± 1.50 | 218.9 ± 16.9 |
| SOEE (30 mg/kg) | 2.5 (1-3) | 5.14 ± 1.28 | 7.64 ± 1.52 | 204.59 ± 22.3 |
| SOEE (60 mg/kg) | 1 (1-2)** | 0.35 ± 0.12*** | 1.35 ± 0.10*** | 119.5 ± 4.5*** |
| SOEE (120 mg/kg) | 1.50 (1-3)* | 0.61 ± 0.25** | 2.11 ± 0.58*** | 134.16 ± 7.3** |
| SOMP (50 mg/kg) | 2 (1-3)* | 3.17 ± 0.8 | 5.17 ± 1.07* | 159.9 ± 14.5* |
| SOMP (100 mg/kg) | 1.00 (0-2)** | 0.34 ± 0.17*** | 1.34 ± 0.41*** | 117 ± 6.7*** |
| SOMP (150 mg/kg) | 0.5 (0-1)** | 0.31 ± 0.13*** | 0.81 ± 0.28*** | 107.1 ± 4.7*** |
| Mesalamine (100 mg/kg) | 1.5 (1-2)* | 0.58 ± 0.27** | 2.08 ± 0.5** | 130.4 ± 9.5** |
| Dexamethasone (1 mg/kg) | 0.5 (0-1)** | 0.3 ± 0.09*** | 0.8 ± 0.39*** | 99.33 ± 5.8*** |
Sham, Normal rats received normal saline/tween (5 mL/kg/day); control, rats with colitis received normal saline/tween (5 mL/kg/day); SOEE, Salvia officinalis ethanolic extract; SOMP, Salvia officinalis methanolic partition.
Fig. 1.
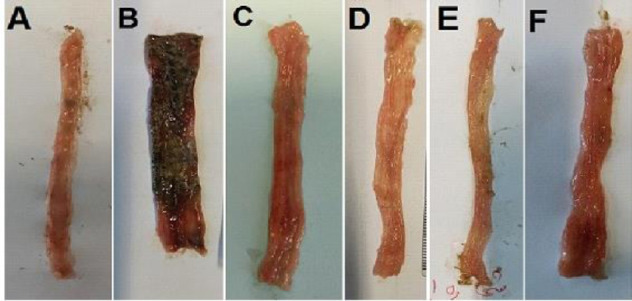
Photos of colon tissue, 5 days after acetic acid-induced colitis in rats. (A) Sham group, normal colon treated with normal saline/tween (5 mL/kg); (B) control colitis treated with normal saline/tween (5 mL/kg); (C) colitis treated with SOEE (60 mg/kg); (D) colitis treated with SOME (150 mg/kg); (E) colitis treated with dexamethasone (1 mg/kg); and (F) colitis treated with mesalamine (100 mg/kg). SOEE, Salvia officinalis ethanolic extract; SOMP, Salvia officinalis methanolic partition.
Effect of SOEE and SOMP on microscopic parameters
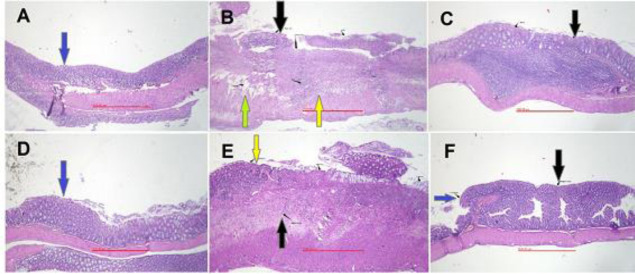
In the control group, tissue edema and thickness, sometimes bleeding, crypt damage, and infiltration of leukocytes were at the maximum level due to acute colitis developed by acetic acid and after no treatment (Table 2). In SOEE- (60 and 120 mg/kg) and SOMP- (100 and 150 mg/kg) treated groups, assessed parameters including inflammation severity and extent, crypt damage, infiltration of leucocyte as well as total colitis index were significantly diminished in comparison with the control group. As it is expected, the groups treated with dexamethasone and mesalamine exhibited the least pathologic features while the total colitis index was significantly reduced in comparison with the control group (Table 2 and Fig. 2).
Table 2.
Effect of SOEE and SOMP on the microscopic parameters of colitis induced by acetic acid in rats. Data are expressed as median (range), n = 6. *P < 0.05, **P < 0.01, and ***P < 0.001 indicate significant difference versus control.
| Groups | Inflammation severity (0-3) | Inflammation extent (0-3) | Crypt damage (0-4) | Leukocyte infiltration (0-3) | Total colitis index (0-13) |
|---|---|---|---|---|---|
| Sham (normal) | 0 (0-0)*** | 0 (0-0)*** | 0 (0-0)*** | 0 (0-0)*** | 0 (0-0)*** |
| Control (colitis) | 2.5 (2-3) | 3.0 (3-3) | 4.0 (3-4) | 3.0 (3-3) | 12.5 (11-13) |
| SOEE (30 mg/kg) | 2.5 (1-3) | 2.5 (1-3) | 3.5 (1-4) | 3.0 (2-3) | 11.5 (5-13) |
| SOEE (60 mg/kg) | 1.0 (1-2)** | 1.0 (1-2)** | 1.5 (1-2)** | 1.5 (1-2)** | 5.0 (4-8)** |
| SOEE (120 mg/kg) | 2.0 (1-3) | 2.0 (1-2)** | 1.5 (1-2)** | 1.5 (1-2)** | 7.0 (4-9)* |
| SOMP (50 mg/kg) | 2.0 (1-3) | 2.0 (1-3)* | 2.5 (1-3)* | 2.5 (2-3) | 9.0 (5-12) |
| SOMP (100 mg/kg) | 1.0 (1-1)** | 1.0 (1-2)** | 1.5 (0-2)** | 1.0 (1-2)** | 4.5 (3-6)** |
| SOMP (150 mg/kg) | 1.0 (0-1)** | 1.0 (1-2)** | 1.0 (1-2)** | 1.0 (0-1)** | 4.0 (3-6)*** |
| Mesalamine (100 mg/kg) | 1.5 (1-2)* | 1.5 (1-2)** | 1.5 (1-3)** | 1.5 (1-2)** | 6.0 (4-9)** |
| Dexamethasone (1 mg/kg) | 0.5 (0-1)*** | 0.5 (0-1)*** | 0.5 (0-1)*** | 0.5 (0-1)*** | 2.0 *** (0-4) |
Sham, Normal rats received normal saline/tween (5 mL/kg/day); control, rats with colitis received normal saline/tween (5 mL/kg/day); SOEE, Salvia officinalis ethanolic extract; SOMP, Salvia officinalis methanolic partition.
Fig. 2.
Microscopic illustration of colonic tissue in rats. (A) Sham group, normal tissue treated with normal saline/tween (5 mL/kg); (B) colitis control tissue, treated with normal saline/tween (5 mL/kg), which shows lack of epithelium, crypt damage, edema, and inflammation; (C) colitis treated with SOEE (60 mg/kg), epithelium healing occurred; (D) colitis treated with SOMP (150 mg/kg), epithelium healing occurred; (E) colitis treated with mesalamine (100 mg/kg); (F) colitis treated with dexamethasone (1 mg/kg). All images were ×40 magnified. Blue arrows indicate intact or regenerated epithelium; black arrows, crypts status; green arrows, edema; yellow arrows, inflammation status. SOEE, Salvia officinalis ethanolic extract; SOMP, Salvia officinalis methanolic partition.
MPO activity
Results obtained in this part of the study exhibited that MPO activity in groups treated with SOEE 60 and 120 mg/kg and SOMP (50, 100, and 150 mg/kg) was attenuated significantly in comparison with the untreated- control group (Fig. 3). Besides, dexamethasone and mesalamine reduced MPO activity in the related groups; whereas a decline in MPO activity at SOEE 30 mg/kg was not meaningful.
Fig. 3.
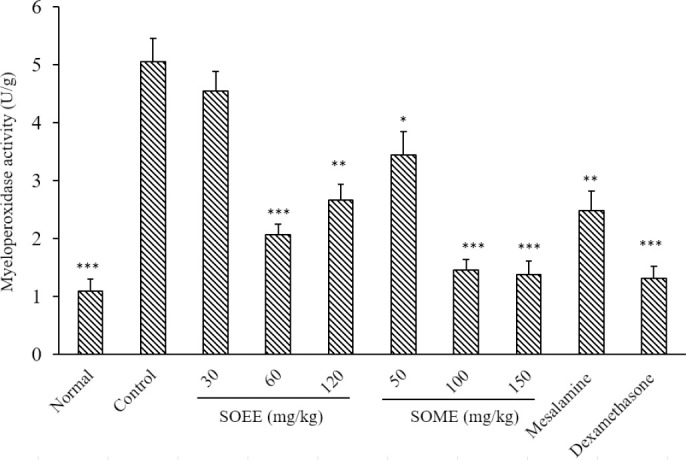
Myeloperoxidase activity in colonic tissue of rats. Normal and control rats treated with saline/tween (5 mL/kg); mesalamine was administered at 100 mg/kg, and dexamethasone at 1 mg/kg. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 indicate significant difference versus the control group. SOEE, Salvia officinalis ethanolic extract; SOMP, Salvia officinalis methanolic partition.
MDA value
MDA (thiobarbituric acid reactants) value attenuated in the groups treated with SOEE (60 and 120 mg/kg) and SOMP (50, 100, and 150 mg/kg) compared to the control group. SOEE at 30 mg/kg didn’t exhibit a meaningful change in MDA level. In addition, both dexamethasone and mesalamine caused a significant diminution in MDA value (Fig. 4).
Fig. 4.
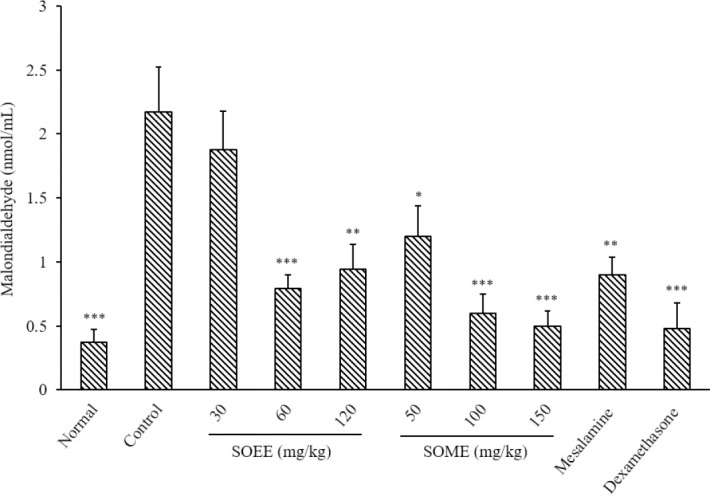
Malondialdehyde level in colonic tissue of rats. Normal and control rats treated with saline/tween (5 mL/kg); mesalamine was administered at 100 mg/kg, and dexamethasone at 1 mg/kg. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 indicate significant difference versus the control group. SOEE, Salvia officinalis ethanolic extract; SOMP, Salvia officinalis methanolic partition.
DISCUSSION
The macroscopic, microscopic, and biochemical evaluations done in this study indicated that SOEE and SOMP had therapeutic and/or preventive activity on chemically- induced colitis in rats. The findings also demonstrated an increase in weight of rats’ colon reflected as edema, thickness, and inflammation of tissue, necrosis of the colon, and infiltration of leukocytes in the control group as a result of acute colitis induced by acetic acid as an approved method for experimental colitis (21). Besides, our results exhibited an elevation of MPO and MDA values in the target tissue of the control group proposing macrophage and neutrophil migration and/or activation which in turn, caused an increase in oxidative stress and lipid peroxidation and subsequently release of inflammatory mediators in the tissue (24,25). Data analysis revealed that nearly all doses of SOMP and SOEE with the exception of SOEE at 30 mg/kg could meaningfully decrease the ulcer index and weight of the colon as main indices of the macroscopic parameters. In microscopic assessments, the total colitis index in the groups that received SOEE (60 and 120 mg/kg) and SOMP (100 and 150 mg/kg) showed a significant reduction too. Moreover, according to our results, MPO activity and MDA value of all groups with the exception of SOEE at 30 mg/kg, were significantly attenuated. It seems that ethanolic extract with the lower dose (60 mg/Kg) had a better effect on the features of colitis than the higher dose (120 mg/Kg) although the difference was not significant. There are probably harmful substances in ethanolic extract (SOEE) that oppose the beneficial effects of this plant on colitis. It is noteworthy that thujone is present in this plant as an annoying substance, the remnants of which may remain in the ethanolic extract (26,27). It seems that thujone is responsible for this pattern of action at greater doses of SOEE. In support of this hypothesis, this annoying effect is seen much less or not at all in the methanolic partition probably because hexane removes thujone from the operating environment. So the mild gradual steep between dose and effect after SOMP administration could be described. Hence, the methanolic partition of this plant extract could be considered safer and seems to have a wider therapeutic window. This is in accordance with studies suggesting that methanolic extract of sage leaf exhibited the highest antioxidant activity among different extracts obtained from various parts of the plant (28,29). Compared with the reference drugs, it seems that SOEE at 120 mg/kg and SOMP at 100 and 150 mg/kg compete easily with mesalamine (100 mg/kg) in attenuation of macroscopic, microscopic, and biochemical features of colitis. Compared to dexamethasone, on the other hand, only SOMP at 150 mg/kg is found to be competitive. In terms of MPO and MDA values, it seems that both greater doses of SOMP have a comparable effect to dexamethasone. Therefore, in terms of the effect on leukocyte migration and activation as well as oxidative stress, the methanolic extract had a similar effect to dexamethasone. However, due to the high potency of dexamethasone which is well-reflected in the consumed dose (1 mg/kg) compared to the extracts (about 100 folds), the comparison is not easily possible.
In the current study, the treatments were done by gavage and the results were promising in colitis improvement, so it could be concluded that active contents of SOEE and SOMP had acceptable bioavailability after oral administration. It is possible that some of the active ingredients in sage like tannins, glycosylated flavones and flavonoids and water soluble polysaccharides may not be absorbed enough orally and reach the colon in unchanged form. These compounds can exert a controlled and targeted effect, such as asacol and pentasa (30,31). Therefore it is logical to examine other ways of administration such as applying suppository or enema of these extracts in future studies. Sage leaf extract contains several active ingredients including polyphenols, flavonoids, triterpenoids, and lipophilic flavones for which antioxidant, anti-inflammatory, and hepatoprotective actions have been reported (8,26,27,28,32). The aqueous and butanol extracts of S. officinalis caused an analgesic effect in the hot-plate latency assay as well as in the early and late phases of formalin-induced paw licking time in rats (11). The antioxidant and anti-inflammatory effects of Italian S. officinalis leaf and flower extracts in lipopolysaccharide and tumor-mediated inflammation models also elicited a decrease in nitrite levels and pro-inflammatory cytokine expression. Interestingly, sage extracts interfered with the inflammatory activity induced by breast cancer cell-conditioned media highlighting the key role of S. officinalis in altering inflammatory processes that are related to neoplastic progression (12). The antioxidant effects of S. officinalis hydroalcoholic extract were also evaluated in an animal model of hepatotoxicity induced by isoniazid in male rats and it seems that this herbal medicine could exhibit hepatoprotective effects (14).
In general, IBD has an inflammatory identity and the cellular production of immunoglobulins and chemokine are increased which in turn motivates lymphocytes and monocytes. The production of inflammatory prostaglandins and cytokines such as interleukin 1β, interleukin 6, and TNF-α, and nuclear factor kappa-b have a pivotal role in causing inflammatory reactions in colitis (33,34). Sage leaf extract has also analgesic and anti- inflammatory effects compared to indomethacin due to its triterpenoid compounds such as ursolic acid (13).
Wound-healing property of sage was previously reported by Karimzadeh et al. (34). They revealed that topical application of S. officinalis extract, especially at a higher dose, significantly increased the percentage of wound healing and contraction, through re-epithelialization and hydroxyproline production versus the control group. Additionally, S. officeinalis significantly increased the new vessel formation and fibroblast distribution. So the investigators concluded that S. officinalis could be considered an appropriate remedy for clinical application in wound care. Additionally, the risk of colon cancer will be enhanced in patients suffering from ulcerative colitis (33). Many investigations have revealed antitumor and cytotoxic effects of S. officinalis on cancer cells both in vitro and in vivo (27,31,35). Pedro et al. reported that drinking sage tea could prevent colon cancer by decreasing DNA damage and proliferation of tumor cells (36). Taken together, it is concluded that S. officinalis not only could alleviate colitis at the experimental level but also could be effective in ulcer healing and prevention of its possible associated colon cancer.
CONCLUSION
In conclusion, S. officinalis ethanolic extract and methanolic partition improved most of the colitis features in an acute model of experimental colitis caused by a chemical agent, acetic acid. It is assumed that phenolic contents with anti-inflammatory, antioxidant, antiulcer, and antimicrobial actions are most responsible for this activity but further detailed research is needed.
Conflict of interest statement
The authors declared no conflict of interest in this study.
Authors’ contributions
M. Jalalipour executed all of the experiments and interventions under the supervision of supervisor professors; A. Yegdaneh designed and supervised all of the parts related to the identification, preparation, and evaluation of herbal materials and extracts; A. Talebi designed and supervised all the parts related to the sampling, preparation, and evaluation of tissues for histopathologic analysis; and M. Minaiyan presented the idea of research, designed, and supervised all of the parts related to the grouping of animals, determining the doses of drugs, interventions, induction of colitis, and analysis the data. All authors contributed to the writing and preparation of the manuscript and approved the final version of the article.
Acknowledgements
This study was financially supported by the Vice-Chancellor for Research and Technology in Isfahan University of Medical Sciences through Grant No. 399751.
REFERENCES
- 1.Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12(1):S3–S9. doi: 10.1097/01.mib.0000195385.19268.68. Doi: 10.1097/01.mib.0000195385.19268.68. [DOI] [PubMed] [Google Scholar]
- 2.Ekbom A. The epidemiology of IBD: a lot of data but little knowledge. How shall we proceed? Inflamm Bowel Dis. 2004;10((1)):S32–S34. doi: 10.1097/00054725-200402001-00007. [DOI] [PubMed] [Google Scholar]
- 3.McQuaid KR. Drugs Used in The Treatment of Gastrointestinal Disease. In: Katzung BG, editor. Basic and Clinical Pharmacology. 11th ed. New York: McGraw Hill Companies; 2007. pp. 1029–1035. [Google Scholar]
- 4.Triantafillidis JK, Merikas E, Georgopoulos F. Current and emerging drugs for the treatment of inflammatory bowel disease. Drug Des Devel Ther. 2011;5:185–210. doi: 10.2147/DDDT.S11290. DOI: 10.2147/DDDT.S11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahimi R, Shams-Ardekani MR, Abdollahi M. A review of the efficacy of traditional Iranian medicine for inflammatory bowel disease. World J Gastroenterol. 2010;16(36):4504–4514. doi: 10.3748/wjg.v16.i36.4504. DOI: 10.3748/wjg.v16.i36.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia CSC, Menti C, Lambert APF, Barcellos T, Mouras S, Calloni C, et al. Pharmacological perspectives from Brazilian Salvia officinalis (Lamiaceae): antioxidant, and antitumor in mammalian cells. An Acad Bras Cienc. 2016;88(1):281–292. doi: 10.1590/0001-3765201520150344. DOI: 10.1590/0001-3765201520150344. [DOI] [PubMed] [Google Scholar]
- 7.European Medicines Agency. Community Herbal Monograph on Salvia officinalis L., Folium. London: 2009. pp. 1–8. [Google Scholar]
- 8.Ovidi E, Masci VL, Zambelli M, Tiezzi A, Vitalini S, Garzoli S. Laurus nobilis, Salvia sclarea and Salvia officinalis essential oils and hydrolates: evaluation of liquid and vapor phase chemical composition and biological activities. Plants (Basel) 2021;10(4):707–723. doi: 10.3390/plants10040707. DOI: 10.3390/plants10040707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu Y, Foo LY. Flavonoid and phenolic glycosides from Salvia officinalis. Phytochemistry. 2000;55((3)):263–267. doi: 10.1016/s0031-9422(00)00309-5. [DOI] [PubMed] [Google Scholar]
- 10.Kenjeric D, Mandic ML, Primorac L, Cacic F. Flavonoid pattern of sage (Salvia officinalis L.) unifloral honey. Food Chem. 2008;110((1)):187–192. doi: 10.1016/j.foodchem.2008.01.031. DOI: 10.1016/j.foodchem.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 11.Qnais EY, Abu-Dieyeh M, Abdulla FA, Abdalla SS. The antinociceptive and anti-inflammatory effects of Salvia officinalis leaf aqueous and butanol extracts. Pharm Biol. 2010;48(10):1149–1156. doi: 10.3109/13880200903530763. DOI: 10.3109/13880200903530763. [DOI] [PubMed] [Google Scholar]
- 12.Brindisi M, Bouzidi C, Frattaruolo L, Loizzo MR, Cappello MS, Dugay A, et al. New insights into the antioxidant and anti-inflammatory effects of Italian Salvia officinalis leaf and flower extracts in lipopolysaccharide and tumor-mediated inflammation models. Antioxidants (Basel) 2021;10(2):311–329. doi: 10.3390/antiox10020311. DOI: 10.3390/antiox10020311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baricevic D, Sosa S, Loggia RD, Tubaro A, Simonovska B, Krasna A, et al. Topical antiinflammatory activity of Salvia officinalis L. leaves: the relevance of ursolic acid. J Ethnopharmacol. 2001;75((2-3)):125–132. doi: 10.1016/s0378-8741(00)00396-2. DOI: 10.1016/s0378-8741(00)00396-2. [DOI] [PubMed] [Google Scholar]
- 14.Khakpour S, Najari M, Tokazabani Belasei F, Khosravi M, Farhadi Belasei M, Jahromy Hadipour M. Hepatoprotective and antioxidant effects of Salvia officinalis L. hydroalcoholic extract in male rats. Chines Med. 2014;(5)((2)):130–136. DOI:10.4236/cm.2014.52016. [Google Scholar]
- 15.De Oliveira JR, Vilela PGDF, Almeida RBDA, Oliveira FED, Carvalho CAT, Camargo SEA, et al. Antimicrobial activity of noncytotoxic concentrations of Salvia officinalis extract against bacterial and fungal species from the oral cavity. Gen Dent. 2019;67(1):22–26. PMID: 30644826. [PubMed] [Google Scholar]
- 16.Fawzi M, Kamel Z, Farhan S. Anti-inflammatory effect of sage (Salvia officinalis) extracts abstract on oral health. Iraqi Dent J. 2017;39(1):1–6. [Google Scholar]
- 17.Ghasemi-Dehkordi N. Iranian Herbal Pharmacopoeia. 1st ed. Tehran: Ministry of Health Publications; 2002. pp. 200–205. [Google Scholar]
- 18.Makkar HPS. Measurement of Total Phenolics and Tannins Using Folin-Ciocalteu Method. In: Makkar HPS, editor. Quantification of Tannins in Tree and Shrub Foliage. 1st ed. Dordrecht: Springer; 2003. pp. 49–51. DOI: 10.1007/978-94-017-0273-7_3. [Google Scholar]
- 19.Khoramian L, Sajjadi SE, Minaiyan M. Anti- inflammatory effect of Adiantum capillus-veneris hydroalcoholic and aqueous extracts on acetic acid- induced colitis in rats. Avicenna J Phytomed. 2020;10(5):492–503. PMID: 32995327. [PMC free article] [PubMed] [Google Scholar]
- 20.Latifi G, Ghannadi A, Minaiyan M. Antiinflammatory effect of volatile oil and hydroalcoholic extract of Rosa damascena Mill. on acetic acidinduced colitis in rats. Res Pharm Sci. 2015;10((6)):514–522. PMID: 26779271. [PMC free article] [PubMed] [Google Scholar]
- 21.Mascolo N, Izzo AA, Autore G, Maiello FM, Di Carlo G, Capasso F. Acetic acid-induced colitis in normal and essential fatty acid deficient rats. J Pharmacol Exp Ther. 1995;272(1):469–475. PMID: 7815363. [PubMed] [Google Scholar]
- 22.Motavallian-Naeini A, Minaiyan M, Rabbani M, Mahzuni P. Anti-inflammatory effect of ondansetron through 5-HT3 receptors on TNBS-induced colitis in rat. EXCLI J. 2012;11:30–44. PMID: 27350767. [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69(2):238–249. PMID: 8350599. [PubMed] [Google Scholar]
- 24.Mahdavi N, Talebi A, Minaiyan M. Ameliorative effect of galantamine on acetic acid-induced colitis in rats. Res Pharm Sci. 2019;14(5):391–399. doi: 10.4103/1735-5362.268199. DOI: 10.4103/1735-5362.268199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toukap AN, Delporte C, Noyon C, Franck T, Rousseau A, Serteyn D, et al. Myeloperoxidase and its products in synovial fluid of patients with treated or untreated rheumatoid arthritis. Free Radic Res. 2014;48(4):461–465. doi: 10.3109/10715762.2014.886327. DOI: 10.3109/10715762.2014.886327. [DOI] [PubMed] [Google Scholar]
- 26.Tucker AO, Maciarello MJ. Essential oils of cultivars of Dalmatian sage (Salvia officinalis L.) J Essent Oil Res. 2011;2((3)):139–144. DOI: 10.1080/10412905.1990.9697844. [Google Scholar]
- 27.Ghorbani A, Esmaeilizadeh M. Pharmacological properties of Salvia officinalis and its components. J Tradit Complement Med. 2017;7(4):433–440. doi: 10.1016/j.jtcme.2016.12.014. DOI: 10.1016/j.jtcme.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamdy Roby MH, Sarhan MA, Selim KA-H, Khalel KI. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind Crops Prod. 2013;43:827–831. DOI: 10.1016/j.indcrop.2012.08.029. [Google Scholar]
- 29.Kontogianni VG, Tomic G, Nikolic I, Nerantzaki AA, Sayyad N, Stosic-Grujicic S, et al. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013;136(1):120–129. doi: 10.1016/j.foodchem.2012.07.091. DOI: 10.1016/j.foodchem.2012.07.091. [DOI] [PubMed] [Google Scholar]
- 30.Capek P, Hríbalova V. Water-soluble polysaccharides from Salvia officinalis L. possessing immunomodulatory activity. Phytochemistry. 2004;65((13)):1983–1992. doi: 10.1016/j.phytochem.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 31.Klotz U, Schwab M. Topical delivery of therapeutic agents in the treatment of inflammatory bowel disease. Adv Drug Deliv Rev. 2005;57(2):267–279. doi: 10.1016/j.addr.2004.08.007. DOI: 10.1016/j.addr.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Dong-Hyuck Y, Bong-Jeun A, Myung-Uk K. Biological activity of extracts from garden sage (Salvia officinalis L.) J Appl Biol Chem. 2008;51((6)):296–301. DOI.org/10.3839/jabc.2008.046. [Google Scholar]
- 33.Head KA, Jurenka JS. Inflammatory bowel disease part 1: ulcerative colitis-pathophysiology and conventional and alternative treatment options. Altern Med Rev. 2003;8(3):247–283. PMID: 12946238. [PubMed] [Google Scholar]
- 34.Karimzadeh S, Farahpour MR. Topical application of Salvia officinalis hydro-ethanolic leaf extract improves wound healing process. Indian J Exp Biol. 2017;55((2)):98–106. PMID: 30183236. [PubMed] [Google Scholar]
- 35.Mohammed HA, Eldeeb HM, Khan RA, Al-Omar MS, Mohammed SAA, Sajid MSM, et al. Sage, Salvia officinalis L., constituents, hepatoprotective activity, and cytotoxicity evaluations of the essential oils obtained from fresh and differently timed dried herbs: a comparative analysis. Molecules. 2021;26((19)):5757–5776. doi: 10.3390/molecules26195757. DOI: 10.3390/molecules26195757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pedro DFN, Ramos AA, Lima CF, Baltazar F, Pereira-Wilson C. Colon cancer chemoprevention by sage tea drinking: decreased DNA damage and cell proliferation. Phytother Res. 2016;30(2):298–305. doi: 10.1002/ptr.5531. DOI: 10.1002/ptr.5531. [DOI] [PubMed] [Google Scholar]