Abstract
Background
Platycodon grandiflorus could significantly improve the pathological results of cutaneous scald injury, reduce the release of inflammatory factors and promote angiogenesis. This study investigated the wound healing effect of luteolin, an active component of P. grandiflorus, on induced cutaneous scald injury in Sprague-Dawley (SD) rats.
Methods
The protein expression levels of TNF-α and IL-6 were detected by ELISA. QRT-PCR was adopted to detect the expression of TGF-β1 and VEGF. Histopathological changes of scald wounds were analyzed by hematoxylin-eosin staining. Cell viability and migration ability were detected by CCK-8 assay and scratch assay.
Results
Both in vivo and in vitro experiments showed that luteolin promoted wound healing of cutaneous scald injury. Gene Oncology (GO) functional analysis and rescue experiments showed that endothelial nitric oxide synthase 3 (NOS3) was the critical target of luteolin in treating cutaneous scald.
Conclusion
This study demonstrated that luteolin is an effective component of P. grandiflorus and is effective in the treatment of cutaneous scald injury.
Keywords: Platycodon grandiflorus, luteolin, scald injury, nitric oxide synthase, network pharmacology
Introduction
Skin injury caused by burns and scalds can destroy the natural barrier function of the skin, resulting in adverse consequences such as internal environment disorder, metabolic disorder, and infection.1 In severe cases, it can also affect multiple organ systems and endanger the life of patients.2 Early measures to control the disease and promote wound healing are particularly important.3–5 For burns and scalds, Silver Sulfadiazine (SSD) is one of the commonly used clinical drugs, which have anti-inflammatory, anti-bacterial and analgesic effects.6 However, in recent years, with the emergence of problems such as the abuse of antibiotics and the increased antibacterial drug resistance. Wounds become more difficult to heal, and severe infections can lead to septic shock or multiple organ failure, especially in developing countries.7 Therefore, it is necessary to seek a safe and effective treatments for burns and scalds. Traditional Chinese medicine (TCM) with few adverse reactions and multi-target effects has become one of the research hotspots in the treatment of burns and scalds.8
It had been reported that a variety of traditional Chinese herbs have been widely used to treat burns because of their broad spectrum of antiviral, antibacterial and anti-inflammatory properties.9,10 Platycodon grandiflorus is the dried root of Platycodon grandiflorus (Jacp.) A. DC., which contains many chemical compounds such as saponins, polysaccharides, flavonoids, sterols and fatty acids.11–14 Recent pharmacological studies have shown that P. grandiflorus has numerous effects, such as cough relieving, immunoregulation, anti-inflammatory, anti-tumor and hypoglycemic.15–18 Moreover, recent studies have shown that can inhibit the P. grandiflorus proliferation, migration and microvascularization of human umbilical vein endothelial cells (HUVEC), and inhibit the angiogenesis of chicken embryo allantoic membrane.19,20 P. grandiflorus root-derived saponins inhibited the development of atopic dermatitis (AD)-like skin injury by inhibiting inflammatory factors in serum, reducing epidermal/dermal thickness, inhibiting the infiltration of inflammatory cells and mast cells in skin.21 However, although P. grandiflorus has significant anti-inflammatory and skin healing effects,22,23 its role and mechanism of action in the treatment of burns and scalds is unclear.
Luteolin (3′, 4′, 5, 7-tetrahydroxyflavone), a naturally occurring flavone, is the main chemical component of P. grandiflorus. As a member of the flavonoid group, luteolin exerts multiple bioactive effects, including anti-inflammatory, anti-oxidative, as well as vasoprotective.24–28 Recent evidence has suggested that luteolin possesses protective effects on the skin. For example, luteolin can reduce adverse photobiological effects on the skin.29 Luteolin also shows potent anti-oxidative activities in keratinocytes, fibroblasts and several immune cells.25,29 Furthermore, luteolin has been shown to inhibit proinflammatory mediators (eg, TNF-α IL-1, IL-6 and IL-8) as well as regulate various signaling pathways (eg, NF-κB, JAK-STAT).27–29 Thus, as the main component of P. grandiflorus, luteolin may have played an important role in cutaneous wound healing. But the capacity of luteolin to promote skin scald repair and its interaction with key factors involved in skin scald repair has not been clarified.
The objective of our study was to investigate the protective effects of P. grandiflorus on cutaneous scald injury and explore the underlying mechanism of the critical component of P. grandiflorus.
Materials and Methods
Cell Culture and Treatment
Human Microvascular Endothelial Cell line-1 (HMEC-1) cells were obtained from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China). HMEC-1 cells were maintained in vitro at 37°C with 5% CO2 in Dulbecco’s Modified Eagle Medium (DMEM, Invitrogen, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Thermo Fisher, USA), 100 U/mL penicillin-streptomycin (Invitrogen, USA). After cells were grown to 80% confluence, the medium was changed and luteolin (Sigma, USA) with different concentrations (0, 1, 5 and 10 μM), and 10% SSD (Sigma, USA) were supplemented to induce HMEC-1 cells.
Endothelial nitric oxide synthase (NOS3) is responsible for NO generation in the vascular endothelium, and for this reason it plays an important role in vascular remodeling.30 For NOS3 knockdown, synthetic NOS3-specific siRNA, Si-NOS3 (5′-AAGUUUAACUUUCAUGUUCUC-3′), was purchased from Gene Pharma (Shanghai, China). Non-specific siRNA (Si-NC, 5′-UUCUCCGAACGUGUCACGU-3′) was used as a negative control.
HMEC-1 cells were seeded at a density of 1 × 104 cells/well of a 6-well plate and allowed to adhere for 12 h. HMEC-1 cells were transfected with 50 nM Si-NC, 10 μM luteolin and Si-NC, 10 μM luteolin and 50 nM Si-NOS3. Transfection of HMEC-1 cells was performed using lipofectamine 2000 transfection reagent (Thermo Fisher Scientific, USA) according to the manufacturer’s instructions. Cells were cultured for 48 h and then harvested for subsequent analysis.
Animals
Males specific-pathogen-free (SPF) Sprague-Dawley (SD) rats aged 6 weeks were purchased from the Animal Experiment Center of Chongqing Medical University. SD rats were housed in the SPF animal facility. The facility was kept quiet, ventilated and clean. The temperature was set at 21–25°C, the humidity was controlled at 50–70%, and the light/dark cycle was 12 h/12 h. The rats were fed with sufficient ordinary fodders and distilled water, and were free to move and eat. The experiments were conducted according to the guidelines presented by the Committee for Animal Care and Use of Laboratory Animals, Chongqing Jiulongpo People’s Hospital (No. JLZYHEC-2020-25).
Ointment Preparation
Dried tuberous root of P. grandiflorus was purchased from the herbal drug company (Tongrentang of Nanjing, China), dried and crushed for use. P. grandiflorus (1000 g) was soaked for 30 min (1000 mL distilled water), decocted for 90 min each time and extracted for 3 times in total. All extracts were incorporated, filtered and concentrated in a water bath (1 mL concentrated solution contains 10 g P. grandiflorus). The concentrated solution or luteolin was mixed with medical Vaseline (Mayinglong Pharmaceutical Group Co. Ltd) to make an ointment for the treatment of cutaneous scald.
Establishment of Cutaneous Scald Injury Rat Model and Treatment
1% sodium pentobarbital (10 μL/g) was injected intraperitoneally and the back hair of rats was depilated with 8% sodium sulfide. The 50 g weight iron was placed in boiling water for 10 min and quickly place on the back of the rat skin for 10s, resulting in the superficial II degrees scald wound with an area of 4 cm2. After establishment of the model, rats were assigned randomly to five groups (Control, Model, Silver Sulfadiazine (SSD), P. grandiflorus, luteolin, n=10). Before drug administration, the wound was cleaned with normal saline to remove the residual substance, and then the corresponding ointment was applied to the wound with a thickness of about 1.5 mm. The drug was given twice daily, at an interval of 12 h, for 21 consecutive days. Control group with no burn injury was depilated following the same method as the above model group. The depilation area of the normal group and the model group were applied with normal saline twice a day. SSD group was smeared with 1% SSD emulsifiable paste (Kunming China Resources Flame Pharmaceutical Co. Ltd). P. grandiflorus group and luteolin group was smeared with 10% P. grandiflorus and 10% luteolin mixed emulsifiable paste respectively.
Enzyme-Linked Immunosorbent (ELISA) Assay
Blood samples from each group were collected from the tail vein at the 21st day after establishment of the scald model. Blood samples were centrifuged at 8000 rpm for 40 min and culture mediums were centrifuged at 1000 g for 20 min. The supernatants were separated and stored at −70°C until analysis. The ELISA assays was performed according to the manufacturer’ instructions of an ELISA kit (Jiancheng Bioengineering Institute, Nanjing, China). Briefly, samples were added to the enzyme-labeled plate coated with TNF-α, IL-6 and NO antibody, and incubated at 37°C for 2 h. After washing with wash buffer, 100 μL of a HRP-labeled detection antibody was added to each well for 2 h at 37°C. After washing 3 times, the color-substrate solution A and B 50 μL/well was added to each well. After incubation at 37°C for 30 min, the reaction was suspended by adding 100 μL stop solution to each well. The absorbance of each well at 450 nm was measured using spectrophotometer Thermo Multiscan FC (Thermo Fisher Scientific, USA), and the TNF-α, IL-6 and NO concentration of the samples were calculated from the standard curve.
Hematoxylin Eosin (HE) Staining
Tissues were embedded in paraffin, and tissue sections were dewaxed in xylene and then immersed in gradient alcohol. After washing with distilled water, the sections were immersed in 1 mL hematoxylin solution for 5 min. Sections were washed with tap water. Then, 1% ammonia solution and eosin solution were added for 10s. At last, sections were immersed in gradient alcohol and clear in xylene solution. The pathological changes of cutaneous scald skin tissue in each group were observed under a light microscope (Leica, Solms, Germany).
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from scald tissues with Trizol reagent (Thermo Fisher, USA) and then reversely transcribed into cDNA using a RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA). RT-qPCR was performed using a SYBR-Green PCR Master Mix kit (Takara, Tokyo, Japan). Cycling was performed in a 7900 HT Fast system (Applied Biosystems, California, USA) according to the following program: 94°C, 5 min; 40 cycles of amplification (94°C, 30s and 62°C, 40s); and then 72°C, 10 min. PCR was performed with the following primer sets: TGF-β1 upstream primer: 5’ - CCACCTGCAAGACCATCGAC-3’, downstream primer: 5’ -CTGGCGAGCCTTAGTTTGGAC-3’. VEGF upstream primer: 5’ -TGCCACATATTCACGGCACCGTGC-3’, downstream primer: 5’ -GCACGTTGCACCACGGCACGTGCA-3’. GAPDH upstream primer: 5’ - AGGTCGGTGTGAACGGATTTG-3’, downstream primer: 5’ - GGGGTCGTTGATGGCAACA-3’. The 2−ΔΔCT method was used to analyze the relative expression levels of target genes.
Screening the Active Components and Target Genes of P. grandiflorus
The active components and corresponding target genes of P. grandiflorus were retrieved by Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) (https://old.tcmsp-e.com/tcmsp.php). Oral availability (OB) ≥30% and drug-likeness (DL) ≥0.18 were used as screening conditions to screen out the effective active components and their corresponding target genes of P. grandiflorus. Using Uniprot database (https://www.uniprot.org/) and the National Center for Biotechnology Information database (https://www.ncbi.nlm.nih.gov), identifying the species as “Homo sapiens”, the target was converted to the corresponding gene name.
Collecting Disease Targets
The keyword “scald” was introduced to the Human Gene Database (https://www.genecards.org) to obtain the potential target and correlation coefficient SCORE ≥5 was set as the screening criteria to obtain disease target genes.
Predicting Compounds-Disease Targets
We used the Venny 2.1.0 software (https://bioinfogp.cnb.csic.es/tools/venny/) to identify drug targets and disease targets. We also used this software to draft Venn diagrams. The overlapped targets were considered as the potential therapeutic targets of P. grandiflorum and luteolin against scald disease.
Cell Counting Kit-8 (CCK-8) Assay
The cell viability was assessed by a CCK-8 kit (Syngene, Nanjing, China). HMEC-1 cells were treated by luteolin for 24, 48 and 72 h or transfected with Si-NC, luteolin + Si-NC, luteolin + Si-NOS3 as indicated (100 μL/well). Then, CCK-8 solution (10 μL) were added to each well on the 96-well, followed by incubation for 2 h incubation at 37°C. Finally, the optical density (OD) was measured at a wavelength of 450 nm on a microplate reader (Thermo Fisher Scientific, USA).
Scratch Assay
HMEC-1 cells were treated or transfected in 24-well plates, and when the cells reached 90% confluency, the monolayers were scratched using a sterile pipette. The scratch was photographed and analysed at 0 h and 24 h post-scratching. The original opening distances were detected from three fields randomly selected each well. The opening distances in three wells of different groups were quantified and normalized by the original opening distance. The original opening distance of the scratch was set as 1. The width of the scratches in different groups was analyzed and assessed using Image Pro Plus 6.0 software (Bethesda, MD, USA). The migratory rate was calculated as follows: (treatment group cell migration distance/control group migration distance) × 100%.
Tube Formation Assay
HMEC-1 cells in the logarithmic growth phase were selected and cultured in 96-well plates containing Matrigel gel (100 μL/well) after cell density was adjusted to 3×105/mL. After the cells adhered to the wall, each well was replaced with culture medium containing different concentration of luteolin (0, 1, 5, 10 μM) and SSD or was transfected with Si-NC, luteolin + Si-NC, luteolin + Si-NOS3. Three duplicate wells were set up in each group and cultured in an incubator at 37°C and 5% CO2 for 6 h. Then, the tubule formation in each group was observed and photographed under the microscope. The experiment was repeated 3 times.
Analyzing GO Enrichment
The gene names of the overlapped gene were imported into the David 6.8 database, and the species was set as “Homo Sapiens” and the target gene was selected as the “official gene symbol”. The set value was P<0.01, the enrichment analysis of GO biological process was conducted and enrichment analysis result was visualized.
Molecular Docking Verification
The structure of the core components was downloaded from PubChem database and saved as “.pdb” format through PyMol software. The top 5 core target structures were downloaded from the PDB database and the H2O and its own ligands were removed by PyMol software. The components and target structures were hydrogenated and charged by AutoDock software, and then saved as “.pdbqt” format. Grid Box coordinates and Box sizes were set, and AutoDock software was used for molecular docking. The structure with binding energy <0 and the lowest energy was the optimal structure, and the results were saved. The “.pdbqt” format of the docking results was transformed into “.pdb” format by Open Babel GUI software. Finally, the image was processed and analyzed by PyMol software.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism software version 8.0 (GraphPad Software, San Diego, USA). The results are expressed as the means ± standard deviation (SD). Difference significance was evaluated using Student’s t-test or one-way ANOVA analysis. Each experiment was performed 3 times. *P < 0.05 was considered as of statistical significance.
Results
P. grandiflorus Promotes Scald Repair in Scald Model Rats
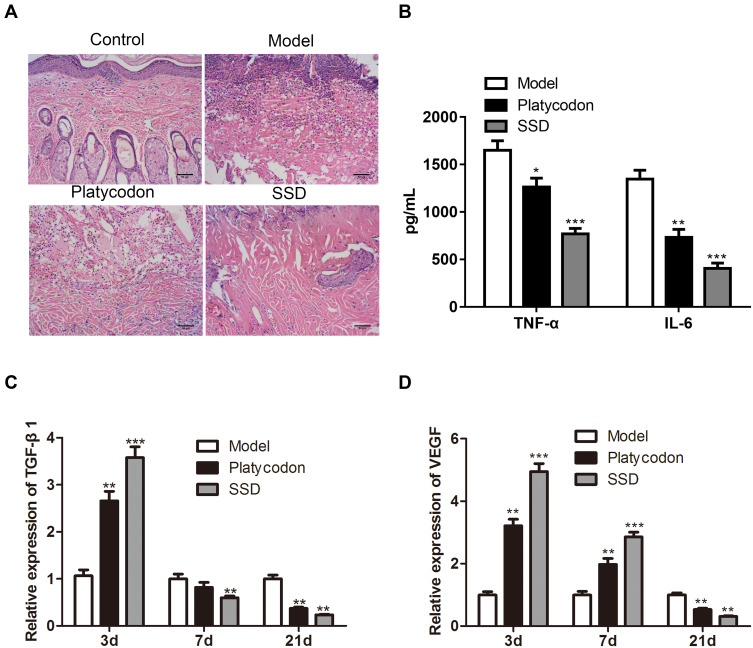
To detect the therapeutic effect of P. grandiflorus on scald model rats, we firstly observed the pathological changes of scald tissue after the treatment with P. grandiflorus. Hematoxylin-eosin staining results showed that in the control group, the whole skin layer was intact, the structure of the cuticle, epidermis and dermis were normal, and the hair follicles and accessory structures of the skin were intact. The epidermal structure of the model rats was destroyed, tissue edema and necrosis, and a large number of inflammatory cells appeared. The epidermis of rats in the P. grandiflorus group had covered most of the wound surface, and the epidermis was well repaired with a few new hair follicles under the skin. However, the recovery of scalded skin in the P. grandiflorus group was not as good as in the positive control SSD group (Figure 1A). As shown in Figure 1B, compared to the model group, ELISA analysis showed that the expression levels of TNF-α and IL-6 in the serum of P. grandiflorus group and SSD group were downregulated on day 21. Meanwhile, the relative expression levels of TGF-β1 and VEGF in scald wound tissues were detected by qRT-PCR. We found that TGF-β1 (Figure 1C) and VEGF (Figure 1D) expression were significantly increased on day 3 and then significantly decreased on day 21.
Figure 1.
The therapeutic effect of P. grandiflorus on scald model rats. (A) Hematoxylin-eosin staining analyzed the histopathological changes of scald wound. (B) The expression of TNF-α and IL-6 in serum evaluated by ELISA on day 21. The relative expression of (C) TGF-β1 and (D) VEGF in scald wound tissues detected by qRT-PCR on day 3, 7 and 21. The values were presented as mean ± SD (n=3). *P<0.05; **P<0.01 and ***P<0.001 versus the Model group. TNF-α, tumor necrosis factor-alpha; IL-6, interleukin-6; TGF-β1, transforming growth factor-β1; VEGF, vascular endothelial growth factor; SSD, silver sulfadiazine; Platycodon, P. grandiflorus.
Screening the Active Components of P. grandiflorus in the Treatment of Scald Injury
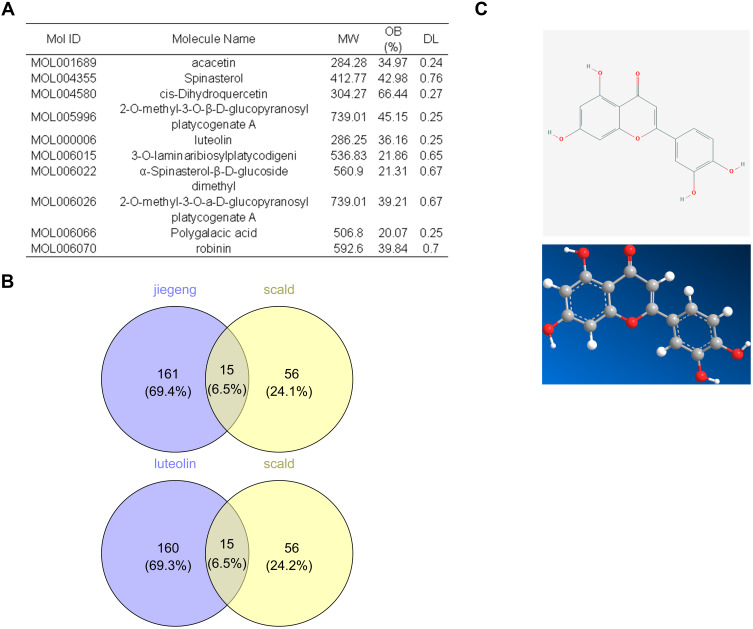
All the active components of P. grandiflorus were searched in TCMSP database. The data was shown in Supplementary Files. Oral availability (OB)≥20% and druglikeness (DL)≥0.18 were selected as screening conditions, and the basic information of the compounds was shown in Figure 2A. Ten active compounds of P. grandiflorus were screened from TCMSP database and a total of 71 disease genes were retrieved from GeneCards database. In addition, Venn diagram showed that 10 active compounds in P. grandiflorus could regulate 176 target genes while luteolin regulate the same number of target genes. The network pharmacological results preliminarily proved that luteolin was the major active component of P. grandiflorus in the treatment of scald injury (Figure 2B). The chemical structure of luteolin was shown in Figure 2C.
Figure 2.
Prediction and screening the effective monomers of P. grandiflorus in the treatment of scald. (A) The active components of p. grandiflorus were obtained by TCMSP data. (B) Venn diagram of related targets of P. grandifloras and luteolin in treating scalds. (C) Chemical structure of luteolin.
Effects of Luteolin on Scald Model Rats
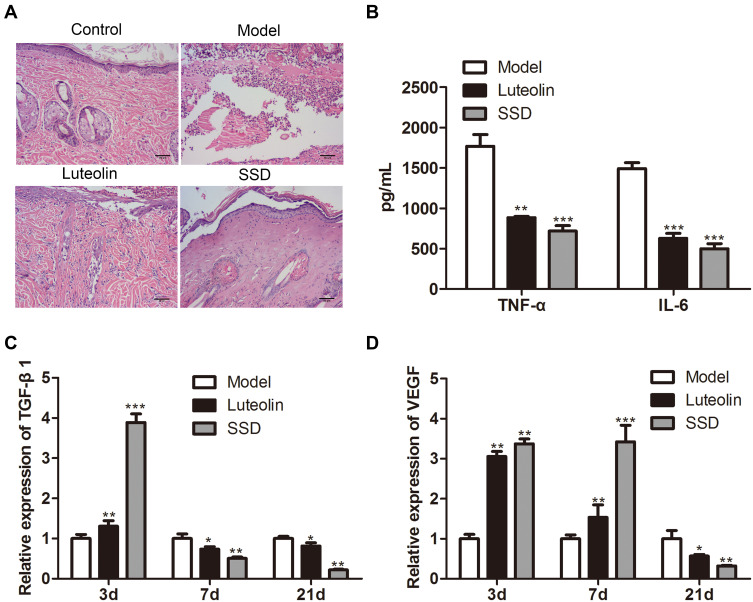
To verify whether luteolin is the main active ingredient of P. grandiflorus in the treatment of scald disease, luteolin was applied topically to the skin wound of scald rats and the pathological structure of skin was observed by Hematoxylin-eosin staining. As shown in Figure 3A, after luteolin intervention, scalded skin tissue was significantly improved and inflammation was significantly reduced. Besides, the therapeutic effect of the positive control SSD group was still better than that of the luteolin group. Compared with the model group, ELISA analysis showed that the expression levels of TNF-α and IL-6 in the serum of luteolin group and SSD group were downregulated (Figure 3B). Meanwhile, compared with the model group, TGF-β1 (Figure 3C) and VEGF expression in scalded skin tissues were significantly increased on day 3 and then significantly decreased on day 21 in both luteolin group and SSD group (Figure 3D).
Figure 3.
The therapeutic effect of luteolin on scald model rats. (A) Hematoxylin-eosin staining used to analyze the histopathological changes of the scald wound. (B) The expression of TNF-α and IL-6 in serum evaluated by ELISA on day 21. The relative expression of (C) TGF-β1 and (D) VEGF in scald wound tissues detected by qRT-PCR on day 3, 7 and 21. The values were presented as mean ± SD (n=3). *P<0.05; **P<0.01 and ***P<0.001 versus the Model group.
Luteolin Promotes Cell Proliferation, Angiogenesis, and Migration in HMEC-1
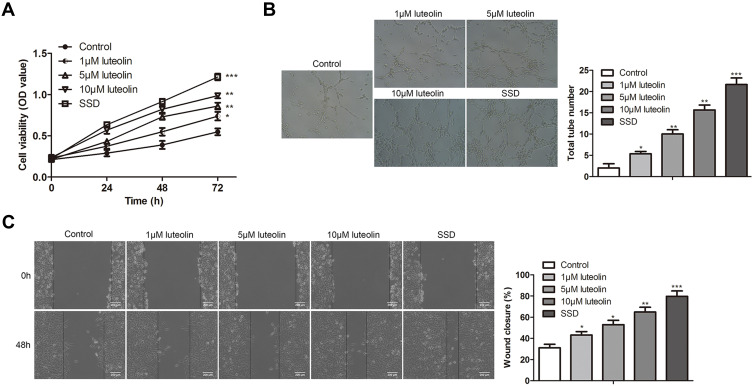
To investigate the effect of luteolin on cell proliferation, angiogenesis, and migration in HMEC-1 cells, HMEC-1 cells were stimulated with luteolin at different concentrations (1, 5, 10 μM) and SSD, which was used as a positive control. With the increase of concentration of luteolin, luteolin significantly promoted cell proliferation, angiogenesis, and migration in HMEC-1 cells, as demonstrated by the results of CCK-8, tube formation and scratch assay. The effect of SSD was more effective than luteolin (Figure 4A–C). These results indicated that luteolin played crucial roles in the treatment of scald injury.
Figure 4.
The effect of luteolin on proliferation, angiogenesis and migration in HMEC-1. (A) HMEC-1 cells were treated with different concentration of luteolin (0, 1, 5, 10 μM) and SSD for 24, 48 and 72 h, the cell viability was analyzed by CCK-8 assay. (B) HMEC-1 were treated with different concentration of luteolin (0, 1, 5, 10 μM) and SSD for 8 h, tubule formation experiment was used to observe the lumen structure of HMEC-1 cells. (C) HMEC-1 cells were treated with different concentration of luteolin (0, 1, 5, 10 μM) and SSD for 48 h, migration was analyzed by scratch assays. Scale bar =200 μm. The values were presented as mean ± SD (n=3). *P<0.05; **P<0.01 and ***P<0.001 versus the control group.
NO is the Pivotal Target of Luteolin in the Treatment of Scald Injury
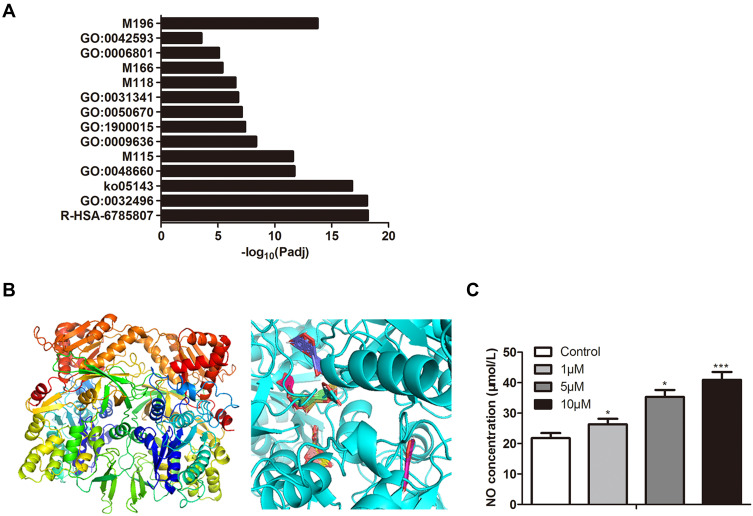
The above experiments indicated that luteolin was an effective component of P. grandifloras in the treatment of scald injury. To further explore the effect targets of luteolin, we performed GO function analysis on the targets of luteolin. Five targets that related to the regulation of muscle cell proliferation and drug response were screened (Figure 5A). NOS3 family NO enzymes may play a role in scald injury. SwissModel was used to construct a 3D structural model and Autoduck was used to calculate molecular combination. As shown in Figure 5B, the image on the left shows the model structure of the protein molecule, the image on the right shows the binding mode of the molecule. The bands represent the protein molecule, and each colored bar represents a binding mode of the chemical molecule. The content of NO in the culture medium treated with different concentrations of luteolin was determined by ELISA assay, and with the increase of luteolin stimulation concentration, the content of NO also increased (Figure 5C).
Figure 5.
Predicting and validating targets for luteolin. (A) Results of GO enrichment analysis of the potential targets. (B) Molecular docking between luteolin and main targets. (C) Effect of different concentrations of luteolin on NO concentration. The values were presented as mean ± SD (n=3). *P<0.05 and ***P<0.001 versus the control group.
NOS3 Mediates the Effects of Luteolin on Cell Proliferation, Angiogenesis, and Migration in HMEC-1
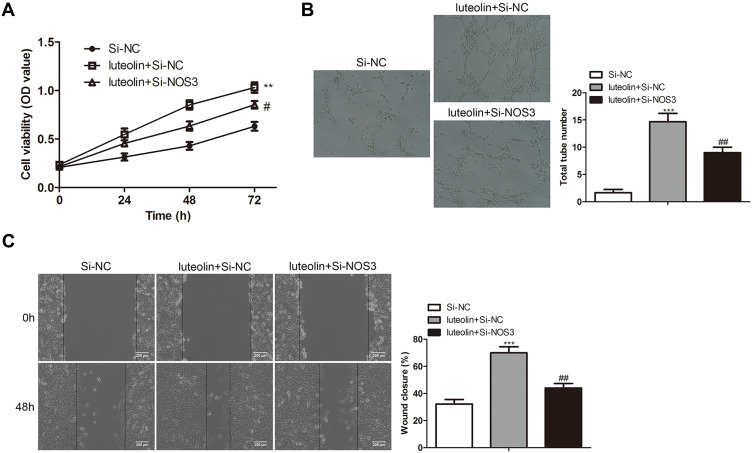
To verify the effect of NOS3 on luteolin in the treatment of scald injury, the expression of NOS3 was silenced by siRNA and then the effects of luteolin on cell proliferation, angiogenesis, and migration in HMEC-1 were detected. Compare with the Si-NC group, luteolin + Si-NC group significantly promoted the cell proliferation, angiogenesis, and migration in HMEC-1 cells. After transfection with Si-NOS3, the ability of luteolin to promote cell proliferation, angiogenesis and migration was significantly reduced (Figure 6A–C). Therefore, the rescue experiment proved that NOS3 was one of the most important targets of luteolin in the treatment of scald injury.
Figure 6.
NO mediates the effect of luteolin on proliferation, angiogenesis and migration in HMEC-1 cells. HMEC-1 cells were transfected with Si-NC and Si-NOS3, and then treated with 10 μM luteolin. (A) The cell viability was analyzed by CCK-8 assay. (B) Tube formation experiment was used to observe the lumen structure of HMEC-1 cells. (C) migration was analyzed by scratch assays. Scale bar =200μm. The values were presented as mean ± SD (n=3). **P<0.01 and ***P<0.001 versus the Si-NC group. #P<0.05, ##P<0.01 versus the luteolin + Si-NC group.
Discussion
The main manifestations of scald wounds are local redness, exudation and pain. The severity of symptoms is related to the scald site, wound area, and depth of the wound. A large number of studies believe that the healing process of burn wounds involves a variety of factors, such as immune system, cytokines and neuropeptides.31–33 Promotion of wound healing is the key to the treatment of scald injury. The appearance of new capillaries and the proliferation and migration of epidermal cells are the most important parts of wound healing and the main targets of treatment.34 The Chinese herbs used to treat scald injury usually contain plenty of complex compounds and their mechanism of treatment remains unclear.
We established a cutaneous scald injury rat model and the cutaneous scald rats were treated with 10% P. grandiflorus extract emulsifiable paste and 1% SSD. HE staining showed that the pathology of cutaneous scald was improved obviously after the intervention of P. grandiflorus and SSD. The results of ELISA showed that both P. grandiflorus and SSD inhibited the release of inflammatory cytokines in skin burn model rats, and the effect of SSD was better than P. grandiflorus. Through a series of inflammatory reactions, the immune system can recover the scalded tissue and resist bacterial infection in the initial stage of injury. However, when excessive inflammatory factors were released from the wound, high concentration of TNF-α and IL-6 become the harmful factors, causing excessive reactions in the body including necrosis of scalded tissue, excessive proliferation of repair cells, and excessive hyperplasia and scar formation.35,36 Our results suggested that P. grandiflorus prevented the damage of skin defense function and speeded up wound repair by inhibiting the inflammatory response. The wound tissues were repaired after vascular injury, increased permeability, neovascularization and excessive proliferation, and TGF-β1 and VEGF were the important regulators and promoters in the repair of injured tissue.37,38 Compared with the model group, TGF-β1 and VEGF were increased on day 3 but decreased significantly on day 21 in both P. grandiflorus group and SSD group. These results indicated that P. grandiflorus promoted wound healing by regulating the expression of TGF-β1 and VEGF.
The medication that used to treat scald injury is analgesics or topical antibiotics such as silver sulfadiazine. These medications usually have toxic side effects.6,39 Herbs are increasingly used in the treatment of burns and scalds and have a significant advantage in promoting the regeneration of wound tissues.40,41 Herbal treatments for scalds provide an effective treatment option in the clinics. The development of network pharmacology is the better solution for this problem.42–45 and we found that luteolin may be the critical components of P. grandiflorus in the treatment of scald. We verified the effect of luteolin on scald rat models, and found that luteolin has the same curative effect as P. grandiflorus, which can improve the pathological condition of scalded skin, inhibit inflammatory factors and pathological angiogenesis. In addition, in vitro experiments showed that with the increase of luteolin concentration, cell proliferation, angiogenesis and migration were enhanced. Numerous studies had shown that luteolin has a strong anti-inflammatory activity both in vivo and in vitro.46 Luteolin affects the metabolism of arachidonic acid and various inflammatory signaling pathways, such as NF-κB signaling pathway, MAPK signaling pathway, JAK and STATE signaling pathway, to inhibit the expression of inflammatory cytokines and inflammatory mediators.46–48 Network pharmacological results had predicted multiple targets of luteolin, but it was still not clear which target was effective in the treatment of cutaneous scald. Therefore, through GO functional enrichment analysis, molecular docking and validation experiments, it was proved that NOS3 plays an important role in scald injury and with the increase of luteolin concentration, luteolin can significantly increase the activity of NO. Rescue experiments revealed that inhibition of NOS3 partially weaken the ability of luteolin to promote cell proliferation, angiogenesis and migration. Nitric oxide synthetase (NOS) is the rate limiting enzyme of NO and NO is the endothelium dependent vasodilator produced and released by endothelial cells, which can relax blood vessels and inhibit platelet adhesion.49,50 Phosphorylation of NOS3 promotes the release of NO and then inhibits platelet aggregation, which is of great significance for vascular remodeling.51,52 Meanwhile, NOS3 can inhibit the release of inflammatory factors and apoptosis of vascular endothelial cells, and play a role in inhibiting vasculitis.53 These results indicated that NOS3 might be the target of luteolin to relieve cutaneous scald injury.
Conclusion
In summary, P. grandiflorus has a significant therapeutic effect on cutaneous scald injury. Using network pharmacology, verification experiments and rescue experiments, this study demonstrates that luteolin is the effective component of P. grandiflorus in the treatment of scald injury.
Funding Statement
This work is funded by the Chongqing Municipal Health Commission, Chongqing Science and Technology Bureau (NO. 2020ZY3529).
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Approval and Consent to Participate
The experimental protocol was approved by Traditional Chinese Medicine Hospital of Jiulongpo Distriction Chongqing, and adhered to the ethical guidelines of the Helsinki Declaration.
Disclosure
The authors state that there is no conflict of interest.
References
- 1.Sahu SA, Agrawal K, Patel PK. Scald burn, a preventable injury: analysis of 4306 patients from a major tertiary care center. Burns. 2016;42(8):1844–1849. doi: 10.1016/j.burns.2016.06.022 [DOI] [PubMed] [Google Scholar]
- 2.Lombardo-Quezada J, Sanclemente G, Colmenero J, et al. Mannose-binding lectin-deficient donors increase the risk of bacterial infection and bacterial infection-related mortality after liver transplantation. Am J Transplant. 2018;18(1):197–206. doi: 10.1111/ajt.14408 [DOI] [PubMed] [Google Scholar]
- 3.Ladhani HA, Yowler CJ, Claridge JA. Burn wound colonization, infection, and sepsis. Surg Infect. 2021;22(1):44–48. doi: 10.1089/sur.2020.346 [DOI] [PubMed] [Google Scholar]
- 4.Li X, Wang X, Wang T, et al. Multimethod assessing the prognosis affecting factors of hospitalized children with burns in Zunyi, southwest China. Wien Klin Wochenschr. 2021;133(5–6):194–201. doi: 10.1007/s00508-020-01676-z [DOI] [PubMed] [Google Scholar]
- 5.Patel S, Cadwell JB, Lambert WC. Comparison of adult vs. paediatric inpatients with staphylococcal scalded skin syndrome: a retrospective database analysis. Br J Dermatol. 2021;184(4):767–769. doi: 10.1111/bjd.19691 [DOI] [PubMed] [Google Scholar]
- 6.Shanmugasundaram N, Uma TS, Ramyaa Lakshmi TS, Babu M. Efficiency of controlled topical delivery of silver sulfadiazine in infected burn wounds. J Biomed Mater Res A. 2009;89(2):472–482. doi: 10.1002/jbm.a.31997 [DOI] [PubMed] [Google Scholar]
- 7.Albertyn R, Berg A, Numanoglu A, Rode H. Traditional burn care in sub-Saharan Africa: a long history with wide acceptance. Burns. 2015;41(2):203–211. doi: 10.1016/j.burns.2014.06.005 [DOI] [PubMed] [Google Scholar]
- 8.Lin LX, Wang P, Wang YT, Huang Y, Jiang L, Wang XM. Aloe vera and Vitis vinifera improve wound healing in an in vivo rat burn wound model. Mol Med Rep. 2016;13(2):1070–1076. doi: 10.3892/mmr.2015.4681 [DOI] [PubMed] [Google Scholar]
- 9.Lee K, Lee B, Lee MH, et al. Effect of Ampelopsis Radix on wound healing in scalded rats. BMC Complement Altern Med. 2015;15:213. doi: 10.1186/s12906-015-0751-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao W, Zhang R, Ma C, et al. Study on the wound healing, anti-inflammation and anti-bacterial activities of Jinjianling cream: a Chinese herbal compound. Pak J Pharm Sci. 2019;32(3Special):1361–1370. [PubMed] [Google Scholar]
- 11.Hwang KA, Hwang YJ, Im PR, Hwang HJ, Song J, Kim YJ. Platycodon grandiflorum extract reduces high-fat diet-induced obesity through regulation of adipogenesis and lipogenesis pathways in mice. J Med Food. 2019;22(10):993–999. doi: 10.1089/jmf.2018.4370 [DOI] [PubMed] [Google Scholar]
- 12.Ma G, Guo W, Zhao L, et al. Two new triterpenoid saponins from the root of Platycodon grandiflorum. Chem Pharm Bull. 2013;61(1):101–104. doi: 10.1248/cpb.c12-00713 [DOI] [PubMed] [Google Scholar]
- 13.Qiu L, Xiao Y, Liu YQ, Peng LX, Liao W, Fu Q. Platycosides P and Q, two new triterpene saponins from Platycodon grandiflorum. J Asian Nat Prod Res. 2019;21(5):419–425. doi: 10.1080/10286020.2018.1488835 [DOI] [PubMed] [Google Scholar]
- 14.Zhan Q, Zhang F, Sun L, Wu Z, Chen W. Two new oleanane-type triterpenoids from Platycodi Radix and anti-proliferative activity in HSC-T6 cells. Molecules. 2012;17(12):14899–14907. doi: 10.3390/molecules171214899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou R, Lu Z, Liu K, et al. Platycodin D induces tumor growth arrest by activating FOXO3a expression in prostate cancer in vitro and in vivo. Curr Cancer Drug Targets. 2015;14(9):860–871. doi: 10.2174/1568009614666141128104642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kong Y, Lu ZL, Wang JJ, et al. Platycodin D, a metabolite of Platycodin grandiflorum, inhibits highly metastatic MDA-MB-231 breast cancer growth in vitro and in vivo by targeting the MDM2 oncogene. Oncol Rep. 2016;36(3):1447–1456. doi: 10.3892/or.2016.4935 [DOI] [PubMed] [Google Scholar]
- 17.Li W, Tian YH, Liu Y, et al. Platycodin D exerts anti-tumor efficacy in H22 tumor-bearing mice via improving immune function and inducing apoptosis. J Toxicol Sci. 2016;41(3):417–428. doi: 10.2131/jts.41.417 [DOI] [PubMed] [Google Scholar]
- 18.Liu S, Wang X, Yang Y, Zhong Y, Chen S, Xiaowei LI. Whitening composition and the use thereof; 2017.
- 19.Luan X, Gao YG, Guan YY, et al. Platycodin D inhibits tumor growth by antiangiogenic activity via blocking VEGFR2-mediated signaling pathway. Toxicol Appl Pharmacol. 2014;281(1):118–124. doi: 10.1016/j.taap.2014.09.009 [DOI] [PubMed] [Google Scholar]
- 20.Wang W, Liu Y, You L, et al. Inhibitory effects of Paris saponin I, II, and on HUVEC cells through regulation of VEGFR2, PI3K/AKT/mTOR, Src/ eNOS, PLCgamma/ERK/MERK, and JAK2-STAT3 pathways. Biomed Pharmacother. 2020;131:110750. doi: 10.1016/j.biopha.2020.110750 [DOI] [PubMed] [Google Scholar]
- 21.Choi JH, Jin SW, Han EH, et al. Platycodon grandiflorum root-derived saponins attenuate atopic dermatitis-like skin lesions via suppression of NF-kappaB and STAT1 and activation of Nrf2/ARE-mediated heme oxygenase-1. Phytomedicine. 2014;21(8–9):1053–1061. doi: 10.1016/j.phymed.2014.04.011 [DOI] [PubMed] [Google Scholar]
- 22.Bark KM, Heo EP, Han KD, et al. Evaluation of the phototoxic potential of plants used in oriental medicine. J Ethnopharmacol. 2010;127(1):11–18. doi: 10.1016/j.jep.2009.09.058 [DOI] [PubMed] [Google Scholar]
- 23.Hwang YP, Choi JH, Kim HG, et al. Saponins, especially platycodin D, from Platycodon grandiflorum modulate hepatic lipogenesis in high-fat diet-fed rats and high glucose-exposed HepG2 cells. Toxicol Appl Pharmacol. 2013;267(2):174–183. doi: 10.1016/j.taap.2013.01.001 [DOI] [PubMed] [Google Scholar]
- 24.Cao Z, Zhang H, Cai X, et al. Luteolin promotes cell apoptosis by inducing autophagy in hepatocellular carcinoma. Cell Physiol Biochem. 2017;43(5):1803–1812. doi: 10.1159/000484066 [DOI] [PubMed] [Google Scholar]
- 25.Chen HI, Hu WS, Hung MY, et al. Protective effects of luteolin against oxidative stress and mitochondrial dysfunction in endothelial cells. Nutr Metab Cardiovasc Dis. 2020;30(6):1032–1043. doi: 10.1016/j.numecd.2020.02.014 [DOI] [PubMed] [Google Scholar]
- 26.Lin P, Tian XH, Yi YS, et al. luteolin-induced protection of h 2 o 2 -induced apoptosis in pc12 cells and the associated pathway; 2019. [DOI] [PubMed]
- 27.Nunes C, Almeida L, Barbosa RM, Laranjinha J. Luteolin suppresses the JAK/STAT pathway in a cellular model of intestinal inflammation. Food Funct. 2017;8(1):387–396. doi: 10.1039/c6fo01529h [DOI] [PubMed] [Google Scholar]
- 28.Yao ZH, Yao XL, Zhang Y, Zhang SF, Hu JC. Luteolin could improve cognitive dysfunction by inhibiting neuroinflammation. Neurochem Res. 2018;43(4):806–820. doi: 10.1007/s11064-018-2482-2 [DOI] [PubMed] [Google Scholar]
- 29.Gendrisch F, Esser PR, Schempp CM, Wolfle U. Luteolin as a modulator of skin aging and inflammation. Biofactors. 2021;47(2):170–180. doi: 10.1002/biof.1699 [DOI] [PubMed] [Google Scholar]
- 30.Oliveira-Paula GH, Lacchini R, Tanus-Santos JE. Endothelial nitric oxide synthase: from biochemistry and gene structure to clinical implications of NOS3 polymorphisms. Gene. 2016;575(2 Pt 3):584–599. doi: 10.1016/j.gene.2015.09.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friston D, Junttila S, Lemes JBP, et al. Leptin and fractalkine: novel subcutaneous cytokines in burn injury. Dis Model Mech. 2020;13(4). doi: 10.1242/dmm.042713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson BL, Rice TC, Xia BT, et al. Amitriptyline usage exacerbates the immune suppression following burn injury. Shock. 2016;46(5):541–548. doi: 10.1097/SHK.0000000000000648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang FB, Dai YL, Zhou GY, et al. Valproic acid treatment inhibits vasopermeability and improves survival in rats with lethal scald injury. J Burn Care Res. 2018;39(2):209–217. doi: 10.1097/BCR.0000000000000568 [DOI] [PubMed] [Google Scholar]
- 34.Salem A, Assaf M, Helmy A, et al. Role of vascular endothelial growth factor in keloids: a clinicopathologic study. Int J Dermatol. 2009;48(10):1071–1077. doi: 10.1111/j.1365-4632.2009.04143.x [DOI] [PubMed] [Google Scholar]
- 35.Apidianakis Y, Que YA, Xu W, et al. Down-regulation of glutatione S-transferase alpha 4 (hGSTA4) in the muscle of thermally injured patients is indicative of susceptibility to bacterial infection. FASEB J. 2012;26(2):730–737. doi: 10.1096/fj.11-192484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chai J, Sheng Z, Yang H, Diao L, Li L. Successful treatment of invasive burn wound infection with sepsis in patients with major burns. Chin Med J. 2000;113(12):1142–1146. [PubMed] [Google Scholar]
- 37.Infanger M, Schmidt O, Kossmehl P, Grad S, Ertel W, Grimm D. Vascular endothelial growth factor serum level is strongly enhanced after burn injury and correlated with local and general tissue edema. Burns. 2004;30(4):305–311. doi: 10.1016/j.burns.2003.12.006 [DOI] [PubMed] [Google Scholar]
- 38.Liu H, Lin S, Xiao D, Zheng X, Gu Y, Guo S. Evaluation of the wound healing potential of resina draconis (dracaena cochinchinensis) in animal models. Evid Based Complement Alternat Med. 2013;2013:709865. doi: 10.1155/2013/709865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rashaan ZM, Krijnen P, Kwa KAA, van der Vlies CH, Schipper IB, Breederveld RS. Flaminal(R) versus Flamazine(R) in the treatment of partial thickness burns: a randomized controlled trial on clinical effectiveness and scar quality (FLAM study). Wound Repair Regen. 2019;27(3):257–267. doi: 10.1111/wrr.12699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chusri S, Settharaksa S, Chokpaisarn J, Limsuwan S, Voravuthikunchai SP. Thai herbal formulas used for wound treatment: a study of their antibacterial potency, anti-inflammatory, antioxidant, and cytotoxicity effects. J Altern Complement Med. 2013;19(7):671–676. doi: 10.1089/acm.2012.0625 [DOI] [PubMed] [Google Scholar]
- 41.Singh S, Gupta A, Sharma D, Gupta B. Dextran based herbal nanobiocomposite membranes for scar free wound healing. Int J Biol Macromol. 2018;113:227–239. doi: 10.1016/j.ijbiomac.2018.02.097 [DOI] [PubMed] [Google Scholar]
- 42.Chen JY, Wu X. Integrative pathway modeling for drug efficacy prediction. United States; 2013. [Google Scholar]
- 43.Hu L, Chen Y, Chen T, et al. Study of mechanism of sargentodoxa cuneata and patrinia scabiosifolia against pelvic inflammatory disease with dampness-heat stasis syndrome via network pharmacology approach. Front Pharmacol. 2020;11:582520. doi: 10.3389/fphar.2020.582520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeong D, Lee J, Jeong SG, et al. Artemisia asiatica ethanol extract exhibits anti-photoaging activity. J Ethnopharmacol. 2018;220:57–66. doi: 10.1016/j.jep.2018.03.037 [DOI] [PubMed] [Google Scholar]
- 45.Wang S, Guo F, Sun X, et al. Study on the potential mechanism of fructus tribuli in the treatment of hypertensive vascular remodeling based on network pharmacology and molecular docking. Evid Based Complement Alternat Med. 2021;2021:8862176. doi: 10.1155/2021/8862176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JK, Kim SY, Kim YS, Lee WH, Hwang DH, Lee JY. Suppression of the TRIF-dependent signaling pathway of Toll-like receptors by luteolin. Biochem Pharmacol. 2009;77(8):1391–1400. doi: 10.1016/j.bcp.2009.01.009 [DOI] [PubMed] [Google Scholar]
- 47.Park CM, Song YS. Luteolin and luteolin-7-O-glucoside inhibit lipopolysaccharide-induced inflammatory responses through modulation of NF-kappaB/AP-1/PI3K-Akt signaling cascades in RAW 264.7 cells. Nutr Res Pract. 2013;7(6):423–429. doi: 10.4162/nrp.2013.7.6.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xagorari A, Papapetropoulos A, Mauromatis A, Economou M, Fotsis T, Roussos C. Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. J Pharmacol Exp Ther. 2001;296(1):181–187. [PubMed] [Google Scholar]
- 49.Costa D, Benincasa G, Lucchese R, Infante T, Nicoletti GF, Napoli C. Effect of nitric oxide reduction on arterial thrombosis. Scand Cardiovasc J. 2019;53(1):1–8. doi: 10.1080/14017431.2019.1581943 [DOI] [PubMed] [Google Scholar]
- 50.Joubert J, Malan SF. Novel nitric oxide synthase inhibitors: a patent review. Expert Opin Ther Pat. 2011;21(4):537–560. doi: 10.1517/13543776.2011.556619 [DOI] [PubMed] [Google Scholar]
- 51.Childers KC, Yao XQ, Giannakoulias S, Amason J, Hamelberg D, Garcin ED. Synergistic mutations in soluble guanylyl cyclase (sGC) reveal a key role for interfacial regions in the sGC activation mechanism. J Biol Chem. 2019;294(48):18451–18464. doi: 10.1074/jbc.RA119.011010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moore C, Tymvios C, Emerson M. Functional regulation of vascular and platelet activity during thrombosis by nitric oxide and endothelial nitric oxide synthase. Thromb Haemost. 2010;104(2):342–349. doi: 10.1160/TH09-11-0764 [DOI] [PubMed] [Google Scholar]
- 53.Ding Y, Huang L, Xian X, et al. Loss of Reelin protects against atherosclerosis by reducing leukocyte-endothelial cell adhesion and lesion macrophage accumulation. Sci Signal. 2016;9(419):ra29. doi: 10.1126/scisignal.aad5578 [DOI] [PMC free article] [PubMed] [Google Scholar]