Abstract
To study growth and cell division of anaerobic hyperthermophilic archaea in vivo, a cultivation technique using glass capillaries was developed. At temperatures of 90 to 98°C, at least 10 successive cell divisions of Pyrodictium abyssi TAG 11 were documented. Cells divide by binary fission. Visualized under a modified dark-field microscope, the formation of cannulae, which finally connected all cells, was observed. The cannulae elongated at 1.0 to 1.5 μm/min and reached final lengths of between 30 and 150 μm. A “snapping division”-like mode of cell fission was discovered for Thermoproteus tenax.
Representatives of the genus Pyrodictium have been isolated from marine hydrothermal systems at Vulcano, Italy, and the Kolbeinsey ridge north of Iceland and from deep-sea “black smoker” samples at the Guaymas basin (Mexico) and the Mid-Atlantic ridge (TAG site) (9, 12). Pyrodictium strains grow at temperatures between 80 and 110°C at neutral pH under strictly anaerobic conditions. By their mode of metabolism, members of Pyrodictium are sulfidogenic facultative chemolithoautotrophs (9, 14). Pyrodictium is unique due to the formation of a network of hollow cannulae, about 25 nm in diameter, in which the cells are embedded (10, 11). Due to their small diameter, the cannulae are invisible under the regular phase-contrast microscope. Only groups of cells with constant distances between each other can be observed (12). By using a special dark-field microscope equipped with a 500-W xenon lamp (6), it was possible to visualize the network of Pyrodictium occultum (13). However, its mode of formation during cell growth was still unknown.
Other exceptional morphological characteristics in hyperthermophilic members of the domain Archaea are the “golf clubs” of members of the order Thermoproteales (15). These are protrusions which occur usually at one cell pole. They are observed during exponential growth phase at ambient (nongrowth) temperature under a regular microscopic slide. The type strain Thermoproteus tenax is a strictly anaerobic facultatively chemolithotrophic sulfidogen, which grows at temperatures between 80 and 96°C (15).
Growth and multiplication of microorganisms can be studied by time-lapse films (5, 8). Due to the extreme growth conditions needed for Pyrodictium or Thermoproteus, several specific modifications of a dark-field microscope and adaptations of the incubation unit were necessary. Here we present for the first time results of in vivo observations of growth and cell division of Pyrodictium abyssi TAG 11 and T. tenax.
Cultivation technique.
P. abyssi TAG 11 was routinely cultivated as described previously (9, 12). Formate (0.1% [wt/vol]) was used as the electron donor. All in vivo growth studies were carried out in glass capillaries (1 mm [width] by 0.1 mm [height] by 100 mm [length]; Vitro Dynamics, Rockaway, N.J.), avoiding a gas phase. Thiosulfate (0.1% [wt/vol]) served as the electron acceptor (instead of elemental sulfur) to minimize light scattering in dark-field microscopy. T. tenax was grown according to reference 15 with thiosulfate (0.1% [wt/vol]) as the electron acceptor. The glass capillaries were coated with poly-l-lysine (7), to ensure cell adhesion. In an anaerobic chamber, culture media were inoculated with the corresponding organisms and used to fill the coated capillaries. Both ends of the capillaries were sealed by melting.
In control experiments, P. abyssi TAG 11 cultures were grown in 100-ml serum bottles and in sealed capillaries, which were kept either in an incubator (serum bottles and capillaries) or directly on the heatable stage of the microscope (capillaries, temperature = 90°C). Comparable final cell densities and doubling times (around 115 min) were obtained in all experiments. Similar to the cultures grown in serum bottles, the organisms in the capillaries formed groups of cells, embedded in a network of cannulae.
Microscope.
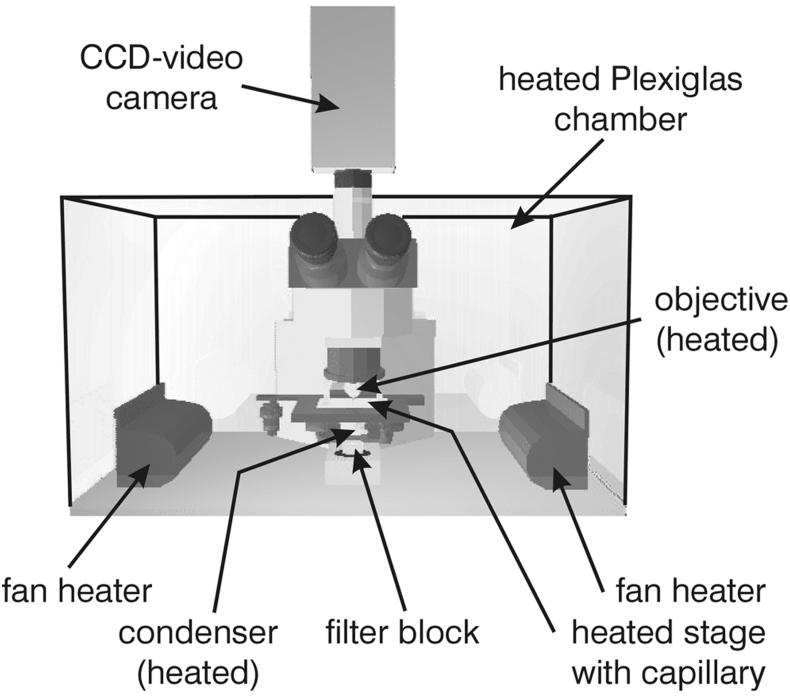
For the investigations, an Olympus BX 50 microscope was placed inside a heatable polyacrylate chamber (Fig. 1). For phase-contrast microscopy, heatable 40× and 100× phase-contrast objectives (UPLFL40× PH/0.75 and UPLFL100× PH/1.25, respectively) were used, and a halogen lamp (100 W) served as the light source. To ensure high light intensity during dark-field observation, a 100-W mercury lamp was mounted in combination with UV absorption filters (GG series; Schott, Mainz, Germany). In addition, an electromagnetic shutter was used to minimize damage to the organisms caused by high light intensities (data not shown). Both the objective (UPLFL100×; 0.60 to 1.30) and the oil-immersion condenser were heated. Furthermore, a heatable stage was designed to ensure a constant temperature in the capillaries up to 98°C (±0.2°C). A charge-coupled device video camera with image integration (PCO, Kelheim, Germany) and a video capturing board (DC20; Miro Computer Products, Braunschweig, Germany) were used for video recording and frame grabbing. The data were processed with the Adobe Premiere 4.2 software package and finally transferred onto an S-VHS videotape. For a two-dimensional reconstruction of cell division and cannula development, images were processed with Corel Photopaint, Corel Draw, and Macromedia Extreme 3D.
FIG. 1.
Scheme of the microscope including heatable polyacrylate chamber and video camera. CCD, charge-coupled device.
Cell divisions were documented by time-lapse recording with a timer control unit triggering the electromagnetic shutter and the single-frame recording function of Adobe Premiere (time base: one frame per 5 or 15 s). To document the growth of cannulae, two different recording methods were used: the cannulae were visualized in a high-intensity dark field by additional frame integration (cannula signal), while the shape of the cells was documented at low light intensities in the dark field without frame integration (cell signal). Both signals were recorded every 10 min for 2 s over a period of up to 10 h. They were mixed by using Adobe Premiere video filters to give a simultaneous impression of cells and cannulae.
Cell division and growth of cannulae of P. abyssi TAG 11.
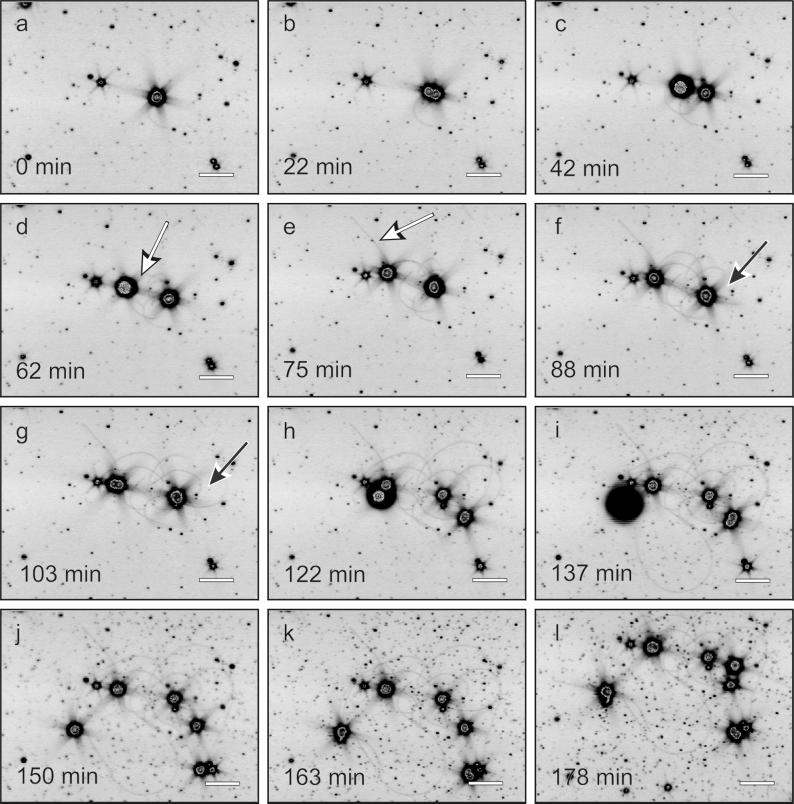
For our observations, a single cell in a capillary was selected. For about 110 to 115 min (temperature = 90°C), the cell diameter increased. During this time, one or more cannulae developed, usually forming loops with both ends attached to the cell surface, although the direct observation of insertion points was not possible. Within 2.5 min, the cell divided into two daughter cells (data not shown). After fission, both cells were connected by the cannulae (Fig. 2f and 2g, black arrow). About 2 h later, the next cell division, done nearly simultaneously by both daughter cells, took place. By elongation of the cannulae, the daughter cells increased their distance up to 30 to 150 μm. In addition, cannulae with only one insertion point were found (Fig. 2d and 2e, white arrow). Although the “free” end of such a cannula was often attached to the capillary surface, it is not clear if this attachment is artificial (e.g., as a result of the poly-l-lysine coating) or is a real function of the cannulae (3). From the experimental observations, growth at the proximal end of the cannulae can be inferred (data not shown). The elongation of the cannulae was determined to be between 1.0 and 1.5 μm/min, which was significantly faster than those of bacterial flagella (e.g., Salmonella: 0.16 μm/min, in vitro measurement [1]). In contrast, for eucaryotic microtubules (e.g., Xenopus eggs) rates of up to 20 μm/min were determined in in vitro experiments (2).
FIG. 2.
Cell division of P. abyssi TAG 11 and growth of cannulae. Frames were extracted from interval recording; for details, see the text. White arrows indicate cannulae with one insertion point and one free end; black arrows indicate cannula loops. Scale bar, 10 μm.
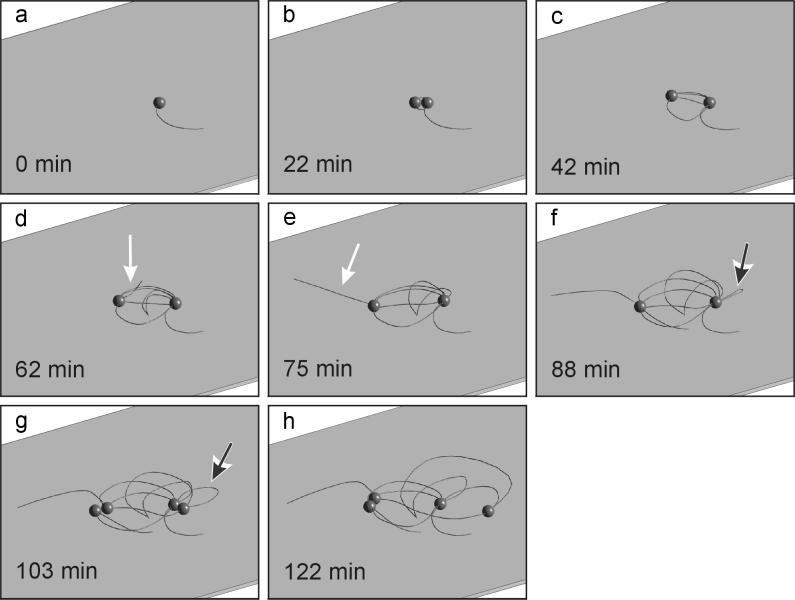
For a two-dimensional model of cell division and growth of cannulae, the data from Fig. 2a to 2h were used. For simplification, all cells were set to have the same size in this model. An animation was calculated with a time base of one frame per 30 s (Fig. 3a to 3h), including the development of loops before cell division (Fig. 3f and 3g, black arrow) and the occurrence of cannulae with free ends (Fig. 3d and 3e, white arrow).
FIG. 3.
Two-dimensional reconstruction of cell division of P. abyssi TAG 11 and development of the network. Frames a to h were calculated from the data of Fig. 2a to 2h. White arrows indicate cannulae with one insertion point and one free end; black arrows indicate cannula loops.
These results demonstrate that cells of P. abyssi TAG 11 divided exclusively by binary fission. The appearance of a new cell somewhere on a cannula was never observed, proving that the cannula network is not directly involved in cell propagation. A movement of cells along the cannulae of the final network was never observed.
The final result of cell and cannula growth was a group of cells connected by a dense network, where all cells exhibited multiple connections to their neighbors (Fig. 2i to 2l). Investigations by electron microscopy suggested that the cannulae most likely end up in the periplasmic space of the cells (10). Therefore, the periplasmic spaces of all cells are interconnected with each other. Although the function of the cannulae still remains unknown, the linkage by cannulae therefore could enable cells to exchange metabolites, genetic information, or signal compounds.
Cell division of T. tenax.
The cell division of T. tenax was observed by phase-contrast microscopy at a magnification of ×400 at a temperature of 85°C. In comparison to cultivation in serum bottles, the doubling time increased about twofold (up to 3.5 h) during growth in capillaries. After a cell of T. tenax had elongated to a final length of up to 10 μm (Fig. 4a), usually cell division initiated by an intensive vibration of the cell for about 2 min. Within a few seconds, the cell snapped off in the center (angle, 90 to 135°; Fig. 4b). As a result, the two daughter cells are arranged in a V shape, very similar to the form of cell groups commonly observed in cultures of coryneform bacteria (4). For this group of high-GC gram-positive bacteria, the “snapping postfission movement” or “snapping division” is very characteristic and is even used as a taxonomic feature (4). So far, it has not been described for members of the Archaea, and further investigations are necessary to check its distribution within this domain. In T. tenax, 2 to 5 min after snapping, the two daughter cells separated visually (Fig. 4c). For the next 3 h, they elongated again (Fig. 4d and 4e), followed by the next cell fission, lasting again only a few minutes (Fig. 4f, 4g, and 4h).
FIG. 4.
Cell division of T. tenax. Frames were obtained from time-lapse film. Scale bar, 20 μm.
T. tenax cells, observed under a regular phase-contrast microscope, are often associated with spherical bodies attached to their ends (golf clubs [15]). When such cells were used to fill capillaries and incubated at 85°C in our microscope, the golf clubs regressed within about 1 h, and the remaining rods divided normally. These experiments demonstrated that the development of golf clubs did not lead to cell lysis or cell death but that these cells were able to divide normally.
Conclusions.
In general, the combination of the modified microscope and culture technique turned out to be a powerful tool to study growth and cell division of anaerobic microorganisms up to temperatures of 98°C. Further applications can include motility of mesophilic to (hyper)thermophilic organisms or investigations with unicellular eucaryotes like flagellates or amoebae.
Acknowledgments
We thank K. O. Stetter and R. Rachel for critical and helpful discussions. Further thanks are due to J. Thienel (IWF Göttingen); to R. Knott, G. Wührl, and H. Hopf (University of Regensburg) for help in modifying the microscope; and to K. Roth for excellent technical assistance.
This work was supported by grants of the Deutsche Forschungsgemeinschaft to K. O. Stetter (Leibniz Award) and to R. Rachel, H. Huber, and G. Frey (Ra 751/1-1).
REFERENCES
- 1.Hotani H, Asakura S. Growth-saturation in vitro of Salmonella flagella. J Mol Biol. 1974;86:285–300. doi: 10.1016/0022-2836(74)90019-9. [DOI] [PubMed] [Google Scholar]
- 2.Inoue S. The role of microtubule assembly dynamics in mitotic force generation and functional organization of living cells. J Struct Biol. 1986;118:87–93. doi: 10.1006/jsbi.1996.3839. [DOI] [PubMed] [Google Scholar]
- 3.König H, Messner P, Stetter K O. The fine structure of the fibers of Pyrodictium occultum. FEMS Microbiol Lett. 1988;49:207–212. [Google Scholar]
- 4.Krulwich T A, Pate J. Ultrastructural explanation for snapping postfission movements in Arthrobacter crystallopoietes. J Bacteriol. 1971;105:408–412. doi: 10.1128/jb.105.1.408-412.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lotz G. Verzeichnis der Wissenschaftlichen Filme. Biologie. Göttingen, Germany: IWF; 1986. [Google Scholar]
- 6.Macnab R M. Examination of bacterial flagellation by dark-field microscopy. J Clin Microbiol. 1976;4:258–265. doi: 10.1128/jcm.4.3.258-265.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazia D, Schatten G, Sale W. Adhesion of cells to surfaces coated with polylysine. J Cell Biol. 1975;71:727–734. doi: 10.1083/jcb.66.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michel K. Wissenschaftlicher Film C 443/1944. Göttingen, Germany. 1944. [Google Scholar]
- 9.Pley U, Schipka J, Gambacorta A, Jannasch H W, Fricke H, Rachel R, Stetter K O. Pyrodictium abyssi, new species represents a novel heterotrophic marine archaeal hyperthermophile growing at 110°C. Syst Appl Microbiol. 1991;14:245–253. [Google Scholar]
- 10.Rieger G. Elektronenmikroskopische und biochemische Untersuchungen zum Aufbau des Netzwerkes bei Pyrodictium. Ph.D. thesis. Regensburg, Germany: University of Regensburg; 1998. [Google Scholar]
- 11.Rieger G, Rachel R, Hermann R, Stetter K O. Ultrastructure of the hyperthermophilic archaeon Pyrodictium abyssi. J Struct Biol. 1995;115:1–10. [Google Scholar]
- 12.Stetter K O. Ultrathin mycelia-forming organisms from submarine volcanic areas having an optimum growth temperature of 105°C. Nature. 1982;300:258–260. [Google Scholar]
- 13.Stetter K O. Diversity of extremely thermophilic archaebacteria. In: Brock T D, editor. Thermophiles: general, molecular and applied microbiology. New York, N.Y: John Wiley & Sons, Inc.; 1986. pp. 39–74. [Google Scholar]
- 14.Stetter K O, König H, Stackebrandt E. Pyrodictium gen. nov., a new genus of submarine disc-shaped sulphur reducing archaebacteria growing optimally at 105°C. Syst Appl Microbiol. 1983;4:535–551. doi: 10.1016/S0723-2020(83)80011-3. [DOI] [PubMed] [Google Scholar]
- 15.Zillig W, Stetter K O, Schäfer W, Janekovik D, Wunderl S, Holz I. Thermoproteales: a novel type of extremely thermoacidophilic anaerobic archaebacteria isolated from icelandic solfataras. Zentbl Bakteriol Hyg Abt 1 Orig C. 1981;2:205–227. [Google Scholar]