Abstract
Background
The impact of donor-host chimerism in post-hematopoietic stem cell transplant (HSCT) outcomes is poorly understood. We were interested in studying whether pre-HSCT variables influenced lineage-specific donor-host chimerism and how lineage-specific chimerism impacts post-HSCT outcomes.
Objective
Our main objective was to study pre-HSCT variables as predictors of lineage-specific donor-host chimerism patterns and to better characterize the relation of post-HSCT lineage-specific chimerism with adverse outcomes including graft failure and disease relapse.
Study Design
We conducted a retrospective data analysis of all patients who underwent allogeneic HSCT at the Pediatric Transplantation and Cellular Therapy service at Memorial Sloan Kettering Cancer Center between January 2010 to June 2015 and had at least 2 measurements of split-lineage chimerism. The trend of lineage-specific donor-host chimerism post-HSCT and the impact of age, disease, graft and conditioning regimen on chimerism at 3 and 12 months post-HSCT were studied. Wilcoxon signed rank tests, Mann-Whitney Wilcoxon tests, and Cox proportional hazard models were used for statistical analyses.
Results
137 patients were included (median age:11.3 years). Most patients had hematologic malignancies (n=95), while fewer had non-malignant disorders (n=27) or primary immune deficiencies (n=15). Myeloablative conditioning regimens (n=126) followed by T-cell depleted (TCD) peripheral blood or bone marrow grafts (n=101) were more commonly used. Mixed chimerism (MC) of total peripheral blood leukocytes (PBL) did not predict loss of donor chimerism in all lineages and when stable, was not associated with graft failure or rejection in this analyses. Split chimerism with complete donor chimerism (CC) of myeloid, B and NK-cells, but not T-cells occurred early post-HSCT, but full donor T-cell chimerism was achieved at 12 months post-HSCT by most patients. MC within the T-cell lineage was the major contributor to PBL MC; with lower median donor T-cell chimerism at 3 (51%) than at 12 months (91%) post-HSCT (p<0.0001). Predictors for MC at 3 and 12 months were: 1) age < 3 years (p=0.01 for PBLs and p=0.003 for myeloid lineage); 2) non-malignant disorder (p=0.007 for PBLs) and 3) the use of RIC regimens. TCD grafts produced lower donor T-cell chimerism at 3 months post-HSCT when compared to unmodified (p<0.0001), where T-cell lineage CC was achieved early post-HSCT; the donor T-cell chimerism was similar at 12 months for both types of grafts. Umbilical cord blood grafts had CC in all lineages at all time points post-HSCT. Loss of donor B-cell chimerism was associated with increased risk of relapse in hematologic malignancies (Hazard Ratio=1.33, p=0.05).
Conclusion
Age, underlying disease, conditioning regimen and graft manipulation can impact post-HSCT donor-host chimerism and be predictors for early MC. MC in total PBLs and T-cells was not related to graft failure or disease relapse. Whole blood PBL chimerism analysis is not sufficient to assess the significance of post-HSCT donor-host status, and rather lineage-specific chimerism, particularly for myeloid, T and B-cells, should be analyzed to guide interventions and inform outcomes.
Introduction
Allogeneic hematopoietic stem cell transplant (HSCT) remains one of the curative therapies for malignant and non-malignant hematologic and immune disorders.1 Long-term outcome of HSCT depends on several variables including age, disease, conditioning regimen, type of graft, graft manipulation, and graft-versus-host disease (GvHD) prophylaxis. Given recent advances in laboratory methodology, we can now study donor-host chimerism post-HCST in more detail by analyzing donor-host percentages for different hematopoietic lineages. Despite increased knowledge on donor-host chimerism status post-HSCT, its impact remains poorly understood.
Chimerism can be monitored closely post-HSCT, and increasing host lineage-specific chimerism could be the first sign of graft rejection, graft failure or disease relapse.2 Most centers monitor total peripheral blood leukocytes (PBL) donor-host chimerism post-HSCT and guide their interventions based on these results. Although the presence or progression of lineage-specific host chimerism may raise concern for developing some of the previously mentioned adverse outcomes, their true prognostic significance is not fully understood. Rapid tapering or abrupt discontinuation of immunosuppression and donor-lymphocyte infusions have been used as pre-emptive treatment of progressive host chimerism, with variable responses and significant complications such as GvHD leading to additional morbidity.3–5 Donor-host chimerism can be assessed using several methods. The analysis of short tandem repeats (STR) with polymerase chain reactions (PCR), has provided a specific and sensitive method for chimerism analysis in all donor and recipient combinations, with the exception of identical twins.6
Complete replacement of a patient’s hematopoietic and immune systems by donor cells is not essential in a number of non-malignant hematologic disorders. There are conflicting reports regarding the implications of progressive mixed chimerism (MC) in these patients, with some groups describing higher risk of graft failure and recurrent disease 7 and others describing stable MC without negative effects on long-term outcome.8 MC after HSCT for hematologic malignancies causes concern for the presence of residual leukemic or leukemic stem cells and for loss of any graft-versus-leukemia effect. Evidence is conflicting, with some investigators reporting significant association between disease relapse and re-emergence of host-derived CD38+ and CD8+ cells 9, 10 and others concluding that persistent MC is not predictive of relapse in pediatric patients who received HSCT for malignant disorders.11, 12
We retrospectively reviewed lineage-specific donor-host chimerism in recipients of allogeneic HSCT in the Pediatric Stem Cell Transplantation and Cellular Therapies (TCT) service at Memorial Sloan Kettering Cancer Center (MSKCC) and analyzed different pre-transplant variables to better understand their relation to lineage-specific chimerism and long-term post-HSCT outcomes.
Patients and Methods
Patient Characteristics
After approval of the Institutional Review Board was obtained, the medical records of 154 consecutive patients who underwent allogeneic HSCT between January 2010 to June 2015 on the Pediatric TCT Service at MSKCC were retrospectively reviewed. All patient diagnoses were included in the analysis; hematologic malignancies, non-malignant hematologic disorders and non-malignant immunologic or leukocyte disorders. Patients were excluded from analysis if they required cellular therapy from a donor other than the original HSCT donor prior to 30 days post-HSCT, died less than 3 months post-HSCT, received treatment with allogeneic cytotoxic T-cells for Epstein-Barr virus (EBV) or Cytomegalovirus (CMV) infections, or had early graft rejection or early graft failure (defined as occurring less than 30 days post-HSCT).
Conditioning Regimens and Grafts
Patients received myeloablative, non-myeloablative, or reduced-intensity conditioning (RIC) regimens depending on their underlying disease and human leukocyte antigen (HLA)-matched or mismatched grafts from related or unrelated donors. Center for International Blood & Marrow Transplant Research operational guidelines or MSKCC institutional guidelines were used to classify regimens as myeloablative, reduced intensity or non-myeloablative. Type of grafts included unmodified bone marrow (BM) or peripheral blood stem cells (PBSCs), T-cell depleted (TCD) BM or PBSCs or single umbilical cord blood (UCB). Graft selection depended on disease, donor availability and the discretion of the treating clinician. PBSCs were harvested after mobilization according to National Marrow Donor Program and institutional guidelines. Grafts were infused intravenously 36–48 hours after the last dose of chemotherapy. CD34+ cell selection from PBMSCs was performed using the CliniMACS device (Miltenyi Biotec, Bergisch Gladbach, Germany)13 or the ISOLEX 300i Magnetic Cell Selection System (Baxter Health Care Corporation, Dearfield, IL.14 followed by E-rosetting TCD for BM grafts was performed with sequential soybean lectin agglutination and sRBC rosette depletion of T-cells.15, 16
Supportive Care
MSKCC standard guidelines were used for patient’s clinical management, including blood product transfusion support, antimicrobial prophylaxis and supportive medications surrounding conditioning regimen. G-CSF was administered at 5 mcg/kg IV twice daily from day +7 post-HSCT until neutrophil engraftment. Intravenous low-dose Heparin and Ursodiol were used for veno-occlusive disease prophylaxis. Antimicrobial prophylaxis included Sulfamethoxazole/Trimethoprim or Pentamidine, Acyclovir and Micafungin. Broad-spectrum antibiotics were administered for the treatment of febrile neutropenia and infectious complications were treated based on organism sensitivities. EBV and CMV titers were monitored regularly post-HSCT to assess for viral reactivation.
Assessment of Donor-Host Chimerism
STR polymorphism analysis at the American Red Cross Blood Services (Philadelphia, PA) and, in sex mismatched cases, fluorescence in situ hybridization techniques were used to monitor donor-host chimerism post-HSCT. Genomic DNAs from whole blood or buccal swabs collected prior to HSCT and from various cell-lineages (myeloid, T, B, and NK-cells) separated from whole blood and collected within 24–48 hours at different time points were extracted with Maxwell 16 instrument according to manufacturer’s insert (Promega Corporation, Madison, WI). The CD markers used for the selection of myeloid, T, B and NK-cells were CD33/66b, CD3, CD19/20 and CD56 respectively (STEMCELL TECHNOLOGIES). To monitor donor-host chimerism post-HSCT in cell-lineages,17, 18 we used PCR based amplification of polymorphic STR markers using the GlobalFilerTM STR kit (Life Technologies, Warrington, U.K.). We utilized a pilot model for chimerism monitoring in our cohort. Lineage-specific chimerism was monitored at different time points post-HSCT. Occasionally, these time points were driven by patient and provider-specific clinical care scenarios. Patient’s immune status, absolute lymphocyte counts, T-, B- and NK-cell populations were monitored regularly post-HSCT until 12–24 months and at later time points based on immune recovery status as per institutional guidelines.
Definitions
Complete chimerism (CC) was defined as complete hematopoietic replacement of the bone marrow and peripheral blood with >90% donor cells. Whole blood PBL chimerism represents the sum of the myeloid, T-, B- and NK-cells, and is affected when the specific lineage chimerisms vary, as well as the absolute numbers of each of these lineages. A state of MC was considered when 5–95% host cells were detected in either PBLs or any single lineage. Split-chimerism was defined as the presence of one or more cell lineages with 100% of host origin, while others preserved full donor origin. We defined complete donor lineage-specific chimerism as >95% donor cells for all lineages.
Neutrophil engraftment was defined as a measurable neutrophil count > 0.5 × 109/L for three consecutive days. Primary graft failure was defined as failure to recover neutrophil count by day 28 post-HSCT without evidence of relapsed disease. Secondary graft failure was defined as graft failure occurring either after initial partial or complete engraftment and was depicted by recurrent pancytopenia with neutrophil count < 0.5 × 109/L without evidence of relapsed disease. Patients with 100% host chimerism were thought to have graft rejection and all others were thought to have graft failure.
GvHD was diagnosed clinically and confirmed by biopsy if possible. Patients were assessed for acute GvHD from engraftment day or day 14 post-HSCT, whichever was earlier, until 100 days post-HSCT. GvHD scoring was based on the International Bone Marrow Transplant Research Criteria (modified Keystone).19 Patients who survived past 100 days post-HSCT were assessed for chronic GvHD utilizing Sullivan’s et al. scoring criteria prior to December 2017 and NIH consensus criteria after that time.20
Statistics
The evolution of chimerism overall and by lineage was represented graphically using the mean at each timepoint, while the shaded area represents the 95% confidence interval (when enough data was available). Wilcoxon signed rank tests were used to compare the distribution of the chimerism percentage for each lineage at 3 months versus 12 months. The level at 3 months was defined as an average of the level between 15 days and 3 months, in order to account for missing 3-month measurements. The impact of several donor, host and transplantation related variables, namely age, disease, graft and conditioning regimens, on donor-host lineage-specific chimerism at 3 and 12 months post-HSCT was investigated. Mann-Whitney-Wilcoxon tests were used to compare the distribution of the chimerism percentage between subgroups defined by age (<3 years versus ≥3 years), disease, graft (TCD versus unmodified; UCB was described but not part of the comparison as too few patients received this type of transplantation) and conditioning regimen (myeloablative versus reduced intensity and non-myeloablative). The time to relapse was defined as the time from HSCT to the time of a registered relapse. Patients who are alive without relapse are censored at their date of last follow-up. Deaths before relapse are considered competing events. The impact of the chimerism percentage on the risk of relapse was assessed using Cox proportional hazard models with the chimerism as a time-dependent covariable whose value is updated at each measurement up to the time of relapse.
Results
Patient and Donor Characteristics
Patient and disease characteristics are detailed in Table 1. Overall, 137 of 154 patients transplanted in the study period were included in the analysis. 17 patients were excluded based on the following: early deaths (n=2), 2nd HSCT (n=7), HSCT from more than one donor (n=1), recipients of CTLs for CMV (n=5) or EBV (n=1) infections, and patient treated in the adult service (n=1) .Median age at transplant was 11.3 years (range: 0.1–32.2) and median post-HSCT follow up was 3.4 years (range: 1 month–7.4 years). Diseases included hematologic malignancies (n=95), non-malignant hematologic disorders (n=27) and primary immune deficiencies (PIDs, n=15; Table 1). The most common hematologic malignancies included acute lymphoblastic (ALL, n=43) and myeloid (AML, n=38) leukemias. Most patients with inherited bone marrow failure syndromes (IBMFS) had Fanconi anemia (n=11) and hemoglobinopathies included Thalassemia (n=3) and Sickle Cell Anemia (n=2). Most patients with PIDs had Severe Combined Immunodeficiency (SCID) (n=7) and other combined immunodeficiencies were less frequent (n=4).
Table 1:
Baseline Patient and Transplantation Characteristics
| Patient Characteristics | N | % |
|---|---|---|
| PATIENTS | 137 | |
|
| ||
| AGE (YEARS) | ||
|
| ||
| <3 | 18 | 13 |
| 3–9 | 46 | 34 |
| 10–18 | 42 | 31 |
| >18 | 31 | 23 |
|
| ||
| GENDER | ||
|
| ||
| Male/Female | 80/57 | 58/42 |
|
| ||
| DISEASE | ||
|
| ||
| Non-malignant Hematologic | 27 | 20 |
| Acquired Severe Aplastic Anemia | 6 | 4 |
| Inherited Bone Marrow Failure Syndromes | 16 | 12 |
| Hemoglobinopathies | 5 | .4 |
|
| ||
| Primary Immune Deficiencies | 15 | 11 |
| Combined Immunodeficiencies | 11 | 8 |
| White Blood Cell Disorders | 4 | 3 |
|
| ||
| Malignant Hematologic Diseases | 95 | 69 |
| Leukemia /Myelodysplastic Syndrome/ Lymphoma | 95 | 69 |
|
| ||
| CONDITIONING REGIMEN | ||
|
| ||
| Myeloablative (TBI based and non-TBI based) | 126 | 92 |
| Reduced intensity/Non-myeloablative | 9 | 7 |
| No Cytoreduction (SCID) | 2 | 1 |
|
| ||
| DONOR TYPE | ||
|
| ||
| Matched related | 43 | 31 |
| Mismatched related/haploidentical | 6 | 4 |
| Matched/Mismatched unrelated | 88 | 64 |
|
| ||
| TYPE OF GRAFT AND GRAFT MANIPULATION | ||
|
| ||
| Unmodified Marrow or Peripheral Blood | 29 | 21 |
| T-cell Depleted Bone Marrow or Peripheral Blood | 101 | 74 |
| Single Umbilical Cord Blood | 1 | 1 |
| Double Umbilical Cord Blood | 6 | 4 |
Conditioning regimen and graft characteristics are summarized in Table 2 in association with disease category and donor status. The three most frequently used myeloablative regimens included (1) Hyper-fractionated total body irradiation (TBI) (1375–1500 cGy), Thiotepa(10 mg/kg over 1 or 2 days) and either Cyclophosphamide(60 mg/kg/day x 2 doses) or Fludarabine(25 mg/m2 × 5 doses) which was used in most patients with malignant hematologic disorders (n=42), (2) Clofarabine(20–30 mg/m2/day x 5 doses), Melphalan (70 mg/m2/day x 2 doses),Thiotepa(10 mg/kg over 1 or 2 days)(n=26) and (3) Busulfan (0.8 mg/kg every 6 hours x 10 or 12 doses with dose adjusted according to pharmacokinetics on days −9 to −7 (based on analysis performed at FHCC until 10/2012 and for 7 – 9 doses after 10/2012 as per pharmacokinetics conducted at MSKCC)), Melphalan (70 mg/m2/day x 2 doses) and Fludarabine (25 mg/m2/day x 5 doses) (n=19). Non-myeloablative regimens included Fludarabine/Cyclophosphamide(n=4, Fludarabine(30 mg/m2/day x 4 doses) and Cyclophosphamide(10 mg/kg/day IV x 4 doses) and Busulfan/Cyclophosphamide/Fludarabine (n=1, Busulfan: 0.6–0.8 mg/kg IV every 12 h x 4 doses, with pharmacokinetics based adjustment, Cyclophosphamide: 10 mg/kg/day IV x 4 doses and Fludarabine: 35 mg/m2/day x 4 doses). Fludarabine/Melphalan (n=2) and low dose TBI/Fludarabine/Cyclophosphamide (n=2) were used as reduced intensity conditioning regimens. Two patients with SCID did not receive pre-HSCT conditioning based on their disease subtype.
Table 2:
Conditioning Regimen, Graft and Donor Characteristics
| Malignant Hematologic Disorders N (%) | Inherited Bone Marrow Failure Syndromes (IBMFS) N (%) | Severe Aplastic Anemia (SAA) N (%) | Hemoglobinopathies N (%) | Combined Immunodeficiencies N (%) * | White Blood Cell Disorders – other N (%) ** | |
|---|---|---|---|---|---|---|
| Patients | 95 (69) | 16 (12) | 6 (4) | 5 (4) | 11 (8) | 4 (3) |
| Donors | ||||||
| Matched Related | 28 (29) | 4 (25) | 3 (50) | 5 (100) | 2 (18) | 1 (25) |
| Matched Unrelated Mismatched Unrelated | 64 (67) | 11 (69) | 3 (50) | 0 | 8 (73) | 2 (50) |
| Mismatched Related Haploidentical | 3 (3) | 1 (6) | 0 | 0 | 1 (9) | 1 (25) |
| Conditioning Regimen | ||||||
| Myeloablative | 93 (98) | 14 (88) | 2 (33) | 5 (100) | 8 (73) | 4 (100) |
| Non Myeloablative-Reduced intensity | 2 (2) | 2 (12) | 4 (67) | 0 | 1 (9) | 0 |
| None | 0 | 0 | 0 | 0 | 2 (18) | 0 |
| Graft and Graft Manipulation | ||||||
| T-cell depletion | 75 (79) | 14 (88) | 2 (33) | 0 | 7 (64) | 3 (75) |
| Unmodified BM or PBSC | 13 (14) | 2 (12) | 4 (67) | 5 (100) | 4 (36) | 1 (25) |
| Single or double umbilical cord blood | 7 (7) | 0 | 0 | 0 | 0 | 0 |
Wiskott Aldrich Syndrome, Severe Combined Immunodeficiencies (SCID), Combined Immunodeficiencies (CID), X-linked hyper IgM
Chronic Granulomatous Deficiency (CGD), Familial Hemophagocytic lymphohistiocitosys (HLH), HLH, Lymphocyte Adhesion Deficiency
For GvHD prophylaxis, ex-vivo TCD was used in the majority (73.7%) of patients while administration of a calcineurin inhibitor with Mycophenolate Mofetil and/or Methotrexate was used with unmodified transplants.
Assessment of Donor-Host Chimerism
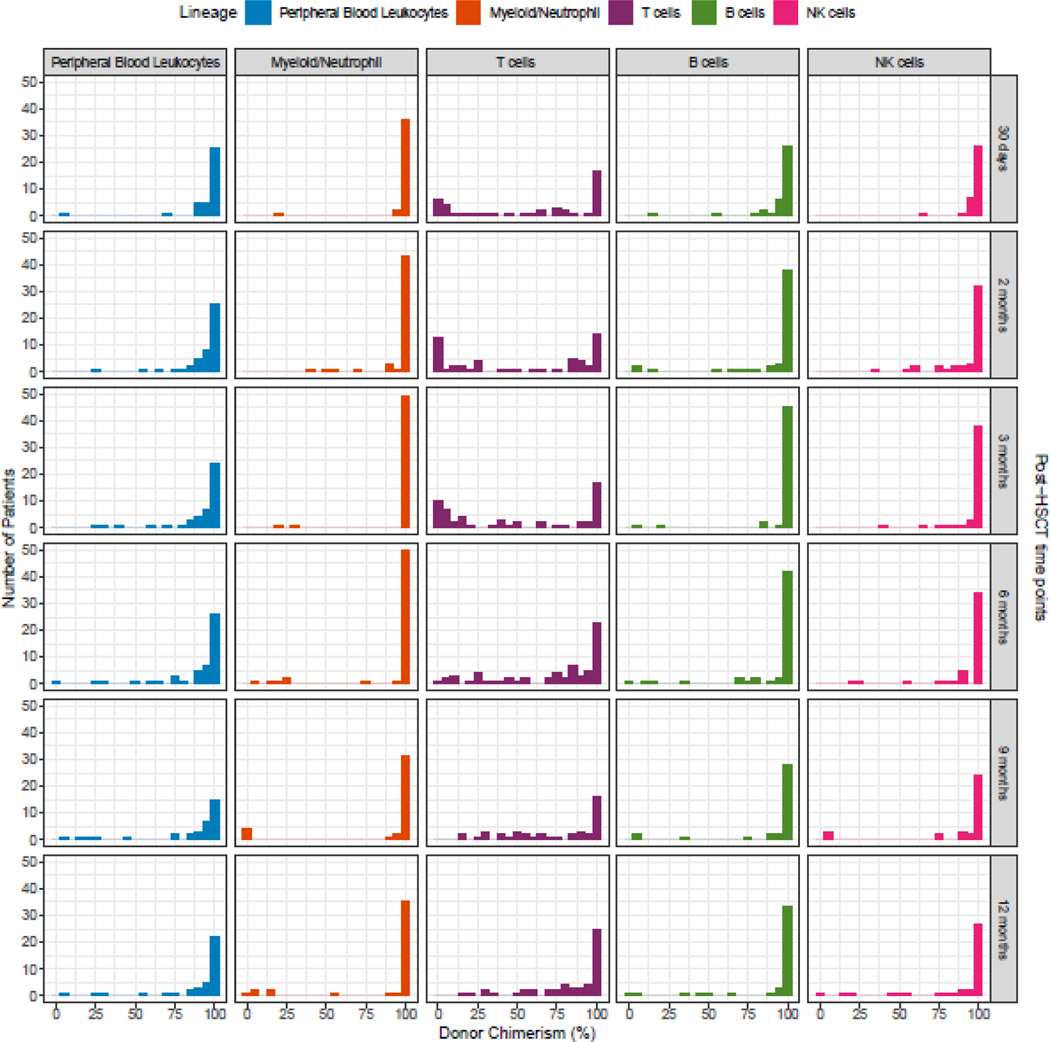
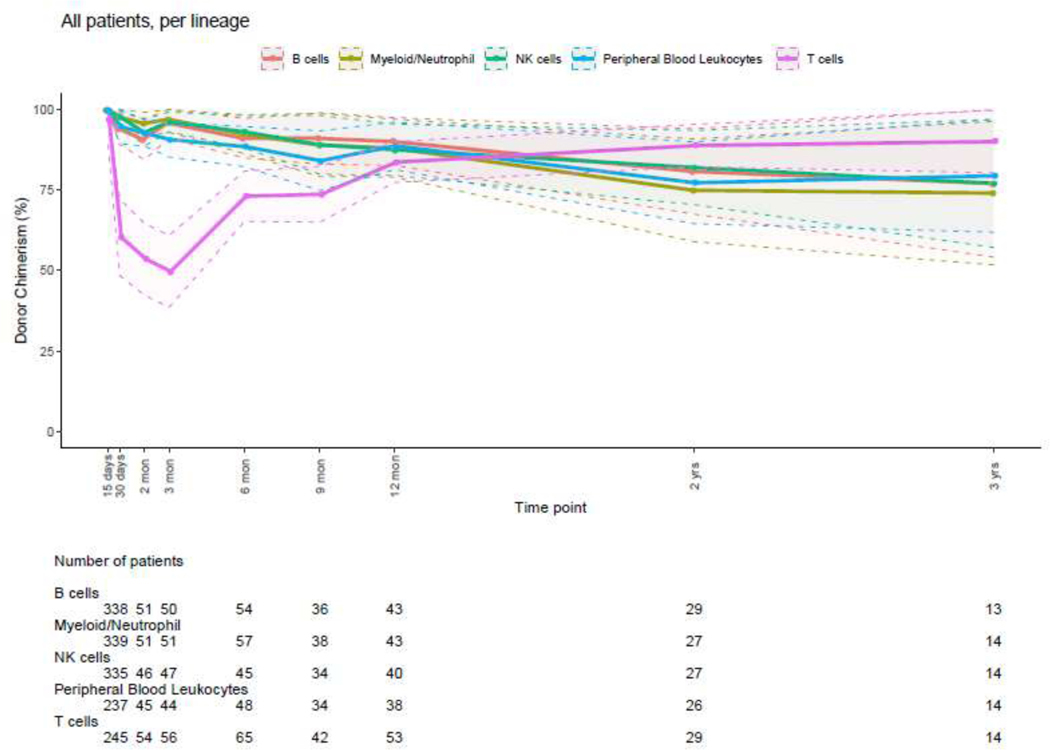
The distribution of donor chimerism percent at sequential post-HSCT timepoints for all cell lineages in the entire patient cohort is illustrated in Figure 1. The total PBL chimerism value represents the average chimerism of all lineages combined, with possible different trends of single lineages over time, as illustrated in Figure 1. The T-cell lineage had the lowest percentage of donor chimerism at all time points, although it increased with time. Observation of lineage-specific donor chimerism at different post-HSCT timepoints revealed that CC of myeloid, B- and NK-cells but not T-cells was achieved in the early post-HSCT period (Figure 2) with a large number of patients having a mixed T-cell chimerism while preserving CC for the myeloid lineage.
Figure 1: Number of Patients Studied and Donor Chimerism Percentage According to Post-HSCT Time Point - All Patient Groups, All Lineages (n=137).
The Y axis represents the number (N) of patients and the X axis represents the donor chimerism percentage for each of the lineages (columns = PBL, myeloid, T-cell, B-cell and NK-cell lineages) at different post-HSCT time points (rows = 30 days to 12 months).
Figure 2: Donor Chimerism Percents at Different Post-HSCT Time Points, All Patient Groups, All Lineages.
Dots indicate the mean values while the shaded area is the 95% confidence interval.
Donor PBL, myeloid and T-cell lineage chimerism were further compared focusing on two timepoints: 1–3 months and 12 months post-HSCT. When looking at PBL, myeloid and T-cell lineage mean chimerism percentages, we found the following: mean donor PBL chimerism was 93.6% and 88.5% at 3 vs 12 months post-HSCT respectively (p=0.40), mean myeloid chimerism was 97.9% and 90.1%, respectively (p=0.03), while T-cell donor chimerism was 48.7% and 81.2% at 3 vs 12 months respectively (p < 0.0001).
Impact of Pre-HSCT and HSCT Variables in Donor-Host Chimerism
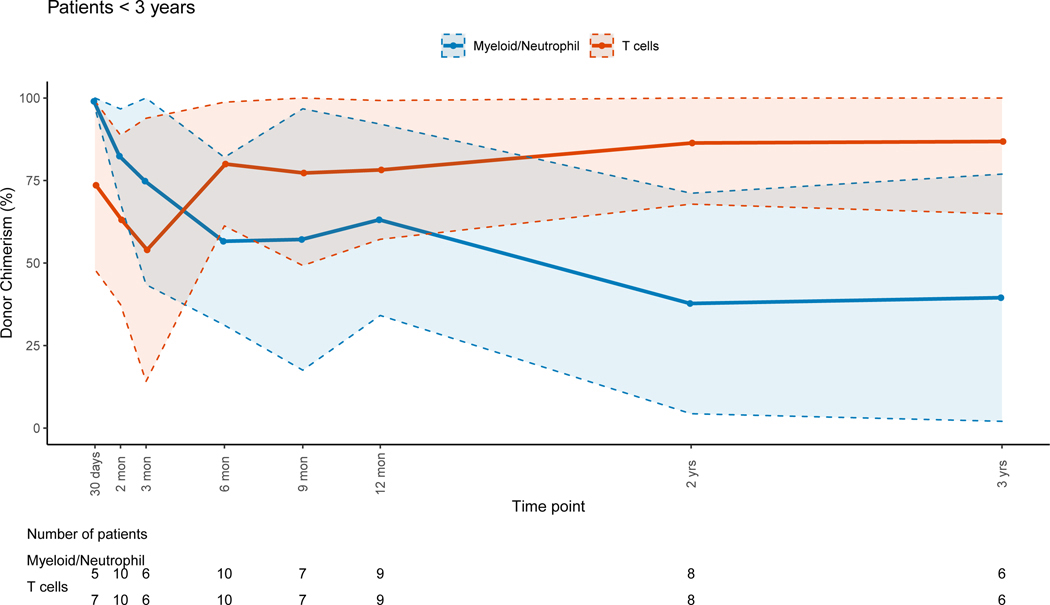
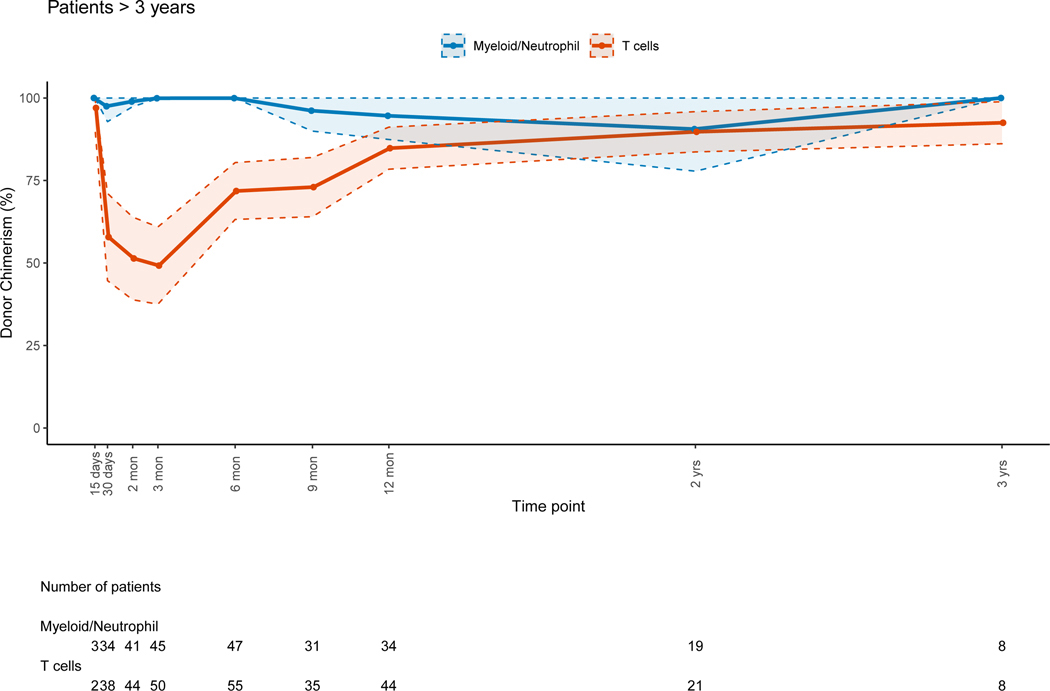
A larger decrease of median donor PBL chimerism was observed in patients < 3 years old when compared to older patients (p=0.01). Younger patients had progressive host chimerism in the myeloid lineage (figure 3A) while older patients preserved full donor chimerism; this difference was significant (p=0.003). CC or near CC was observed for the T-cell lineage at 12 months post-HSCT for the younger patients (figure 3A). Patients ≥ 3 years had CC for all lineages except for T-cells during the early and late post-HSCT period (figure 3B). It should be noted that the cohort of younger children included 11/18 patients with PIDs who were more likely to have received non-myeloablative/RIC regimens.
Figure 3: Myeloid and T-cell Lineage Donor Chimerism Percents per Age Group at Different Post-HSCT Time Points.
Figure 3A: Patients <3 Years
Figure 3B: Patients ≥ 3 Years
Dots indicate the mean values while the shaded area is the 95% confidence interval.
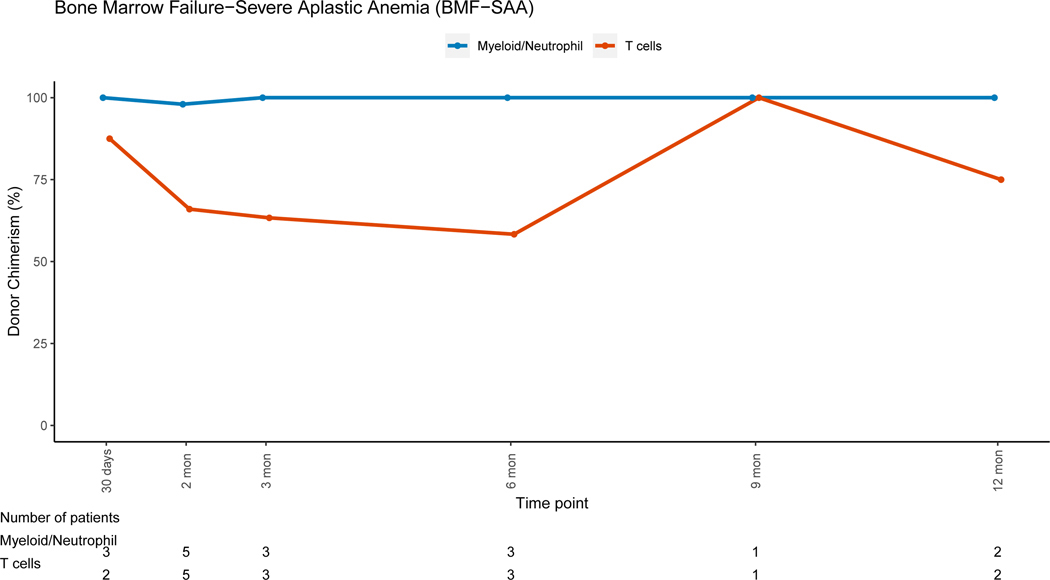
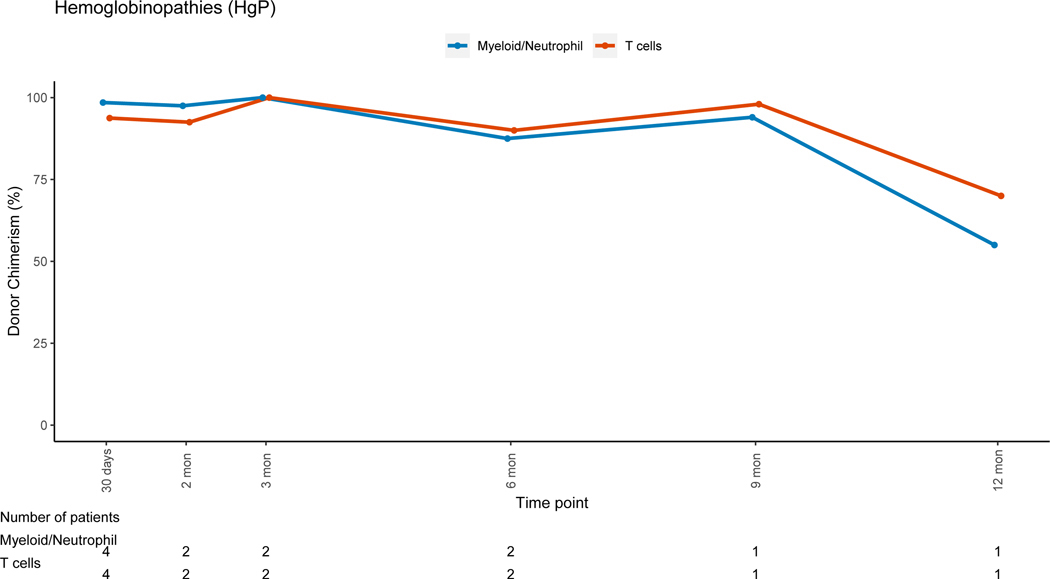
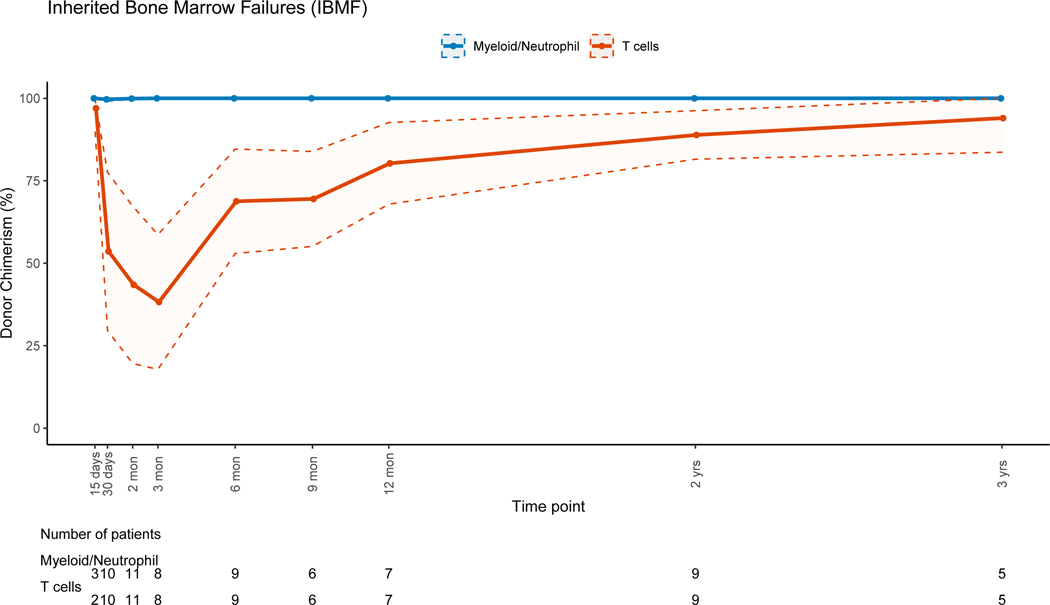
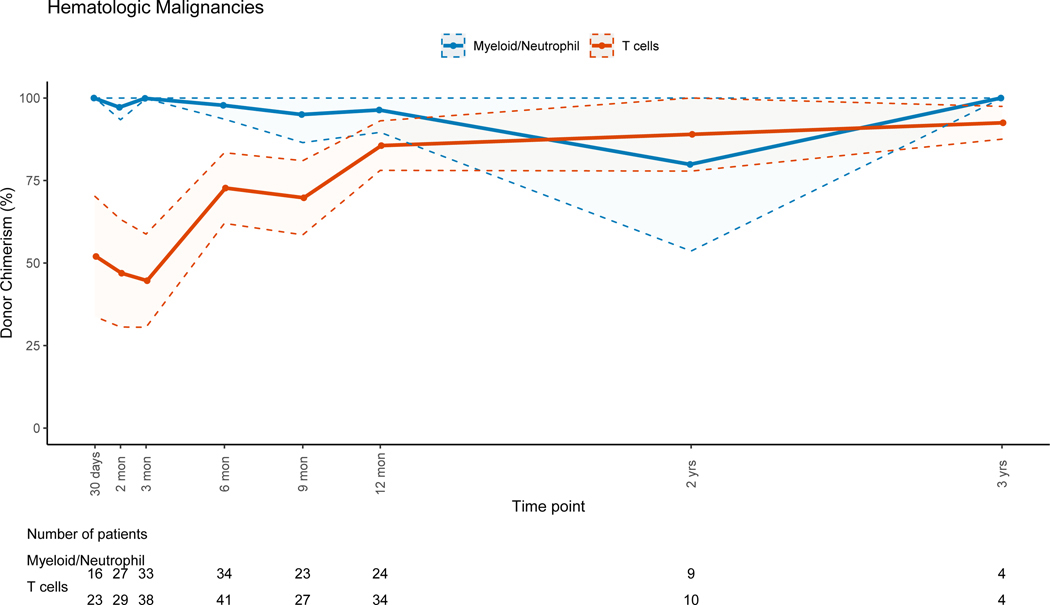
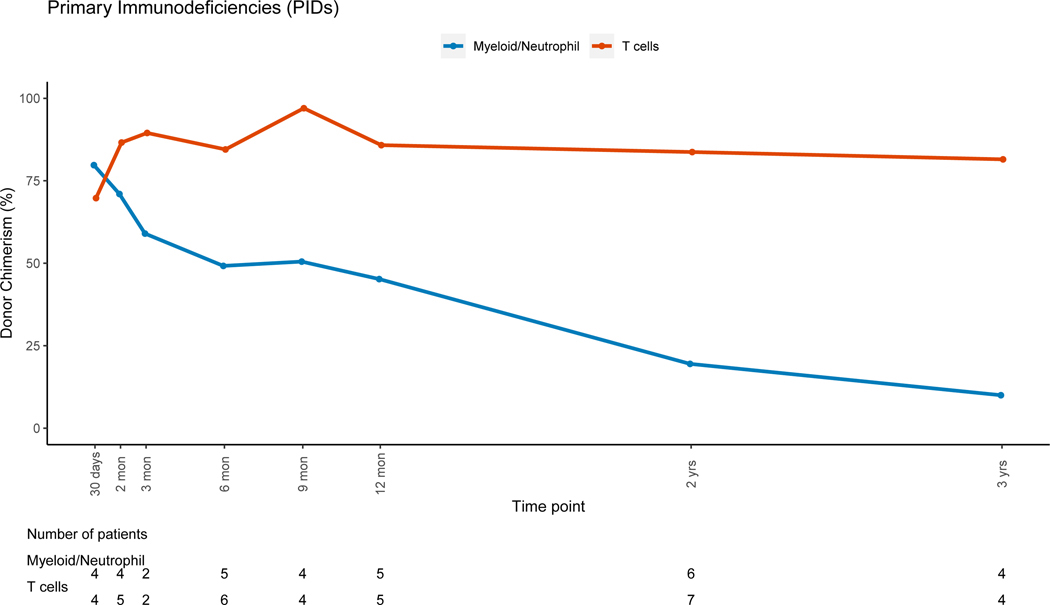
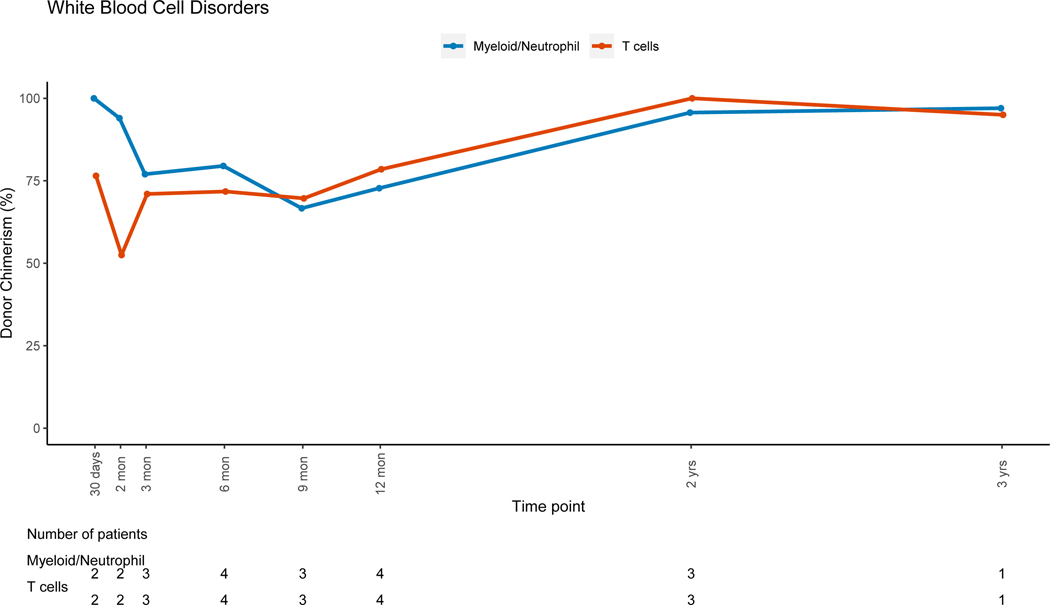
Patients with non-malignant disorders had a larger loss of donor PBL chimerism between 3 months (mean=91.7%, range: 6–100%) and 12 months (mean=80.4%, range: 3–100%, mean difference = −10.5) post-HSCT when compared to patients with malignant disorders (mean difference:+2, p=0.007). Lineage-specific donor chimerism trends for non-malignant disorders (Figures 4A–4C and 4E-4F) showed preserved CC for the myeloid, B and NK-cell lineages, while chimerism for the T-cell lineage was mixed early post-HSC with an increase in donor chimerism percent over time. Patients with hematologic malignancies had CC status in PBLs and myeloid lineage early post-HSCT, with PBL mean donor chimerism percent of 92.8% and 96.2% at 3 and 12 months post-HSCT (figure 4D). MC for the T-cell lineage went from 55.2% at 3 months (range:0 −100%), to 85.6% (range: 19–100%) at 12 months post-HSCT (figure 4D). Patients with PID had donor chimerism of the myeloid lineage early post transplant and loss of chimerism thereafter, driving a decrease in donor chimerism in PBL (figure 4E). MC in the T-cell lineage was also noted in the early post-HSCT period in this group but with an increase in donor chimerism over time.
Figure 4: Myeloid and T-cell Lineage Donor Chimerism Percents per Disease Group at Different Post-HSCT Time Points.
Dots indicate the mean values while the shaded area is the 95% confidence interval, in figures without shaded area, numbers where too small to calculate 95% confidence interval.
Figure 4A: Bone Marrow Failure-Severe Aplastic Anemia (BMF-SAA)
Figure 4B: Hemoglobinopathies (HgP)
Figure 4C: Inherited Bone Marrow Failures (IBMF)
Figure 4D: Hematologic Malignancies
Figure 4E: Primary Immunodeficiencies (PIDs)
Figure 4F: White Blood Cell Disorders
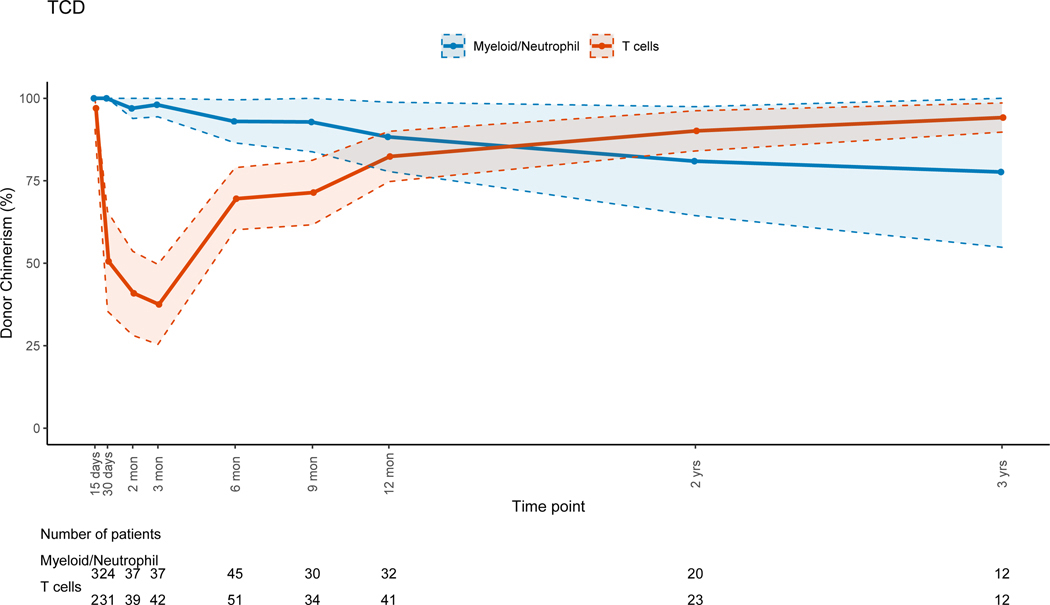
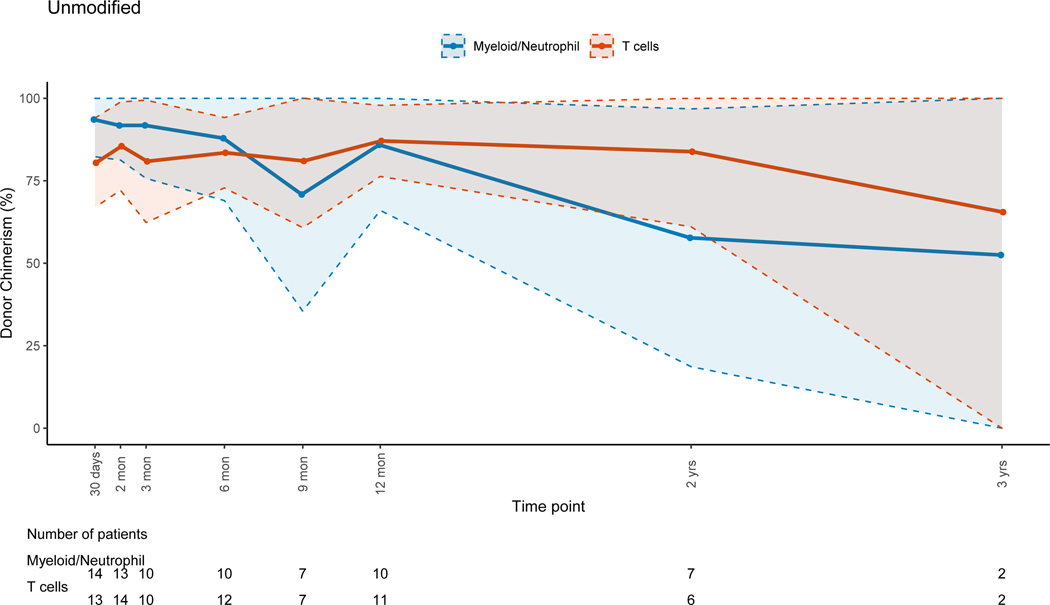
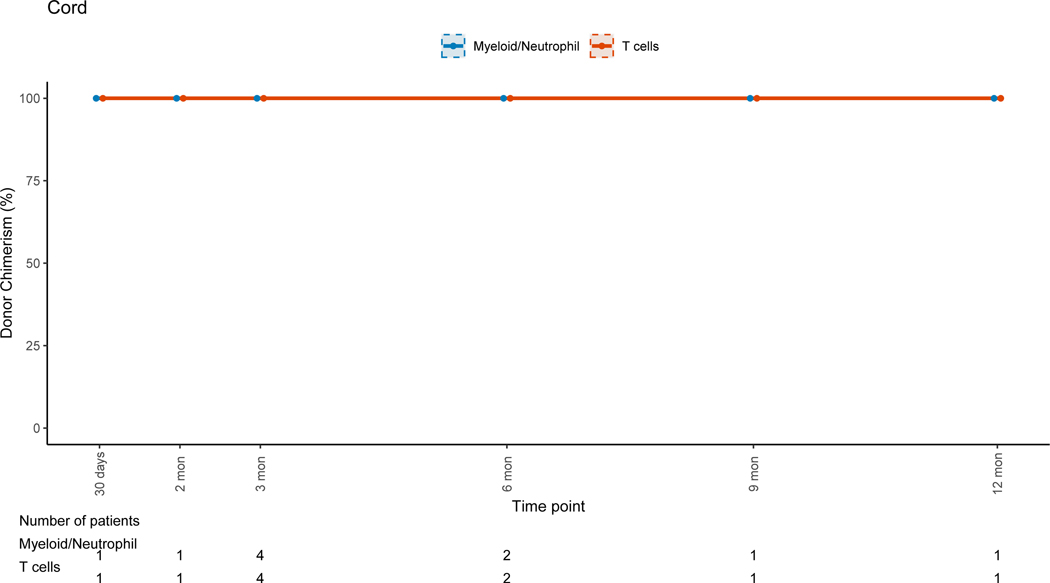
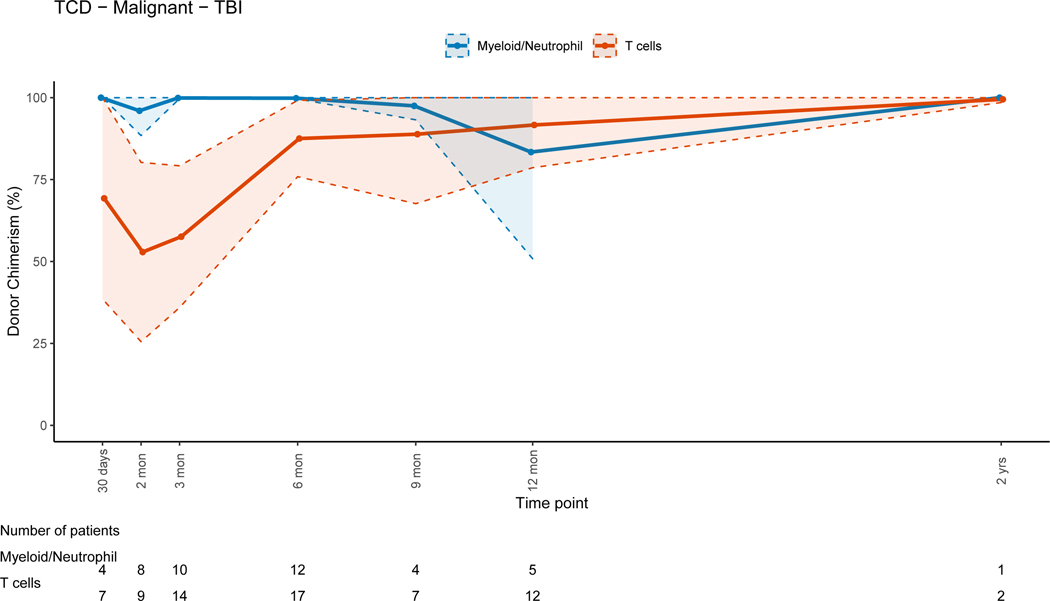
There was no evidence that the difference in donor chimerism between 3 and 12 months was significant between TCD and unmodified grafts for PBLs (p=0.17) or myeloid cells (p=0.30). For unmodified BM and PBSC grafts, T-cell lineage CC was achieved early post-HSCT (figure 5B). For TCD grafts, the trend for T-cell lineage CC took up to a year to complete (figure 5A), with mean donor T-cell chimerism of 47.3% at 3 months (range: 0–100%) and 82.4% at 12 months post-HSCT (range: 14–100). This trajectory was significantly different (p=0.02) when compared to unmodified grafts. The myeloid lineage trends revealed CC in the early post-HSCT period for all grafts, with progressive MC during the late post-HSCT period in the TCD and unmodified group (figures 5A&B). This was more pronounced in the unmodified graft group driven by non-malignant disorders. UCB grafts were associated with CC for all cell lineages throughout the entire post-HSCT period(Figure 5C).
Figure 5: Myeloid and T-cell Lineage Donor Chimerism Percents per Type of Graft at Different Post-HSCT Time Points.
Dots indicate the mean values while the shaded area is the 95% confidence interval, in figures without shaded area, numbers where too small to calculate 95% confidence interval.
Figure 5A: T-cell Depleted (TCD) Grafts
Figure 5B: Unmodified Bone Marrow (BM) or Peripheral Blood Stem Cells (PBSC) Grafts
Figure 5C: Umbilical Cord Blood Grafts
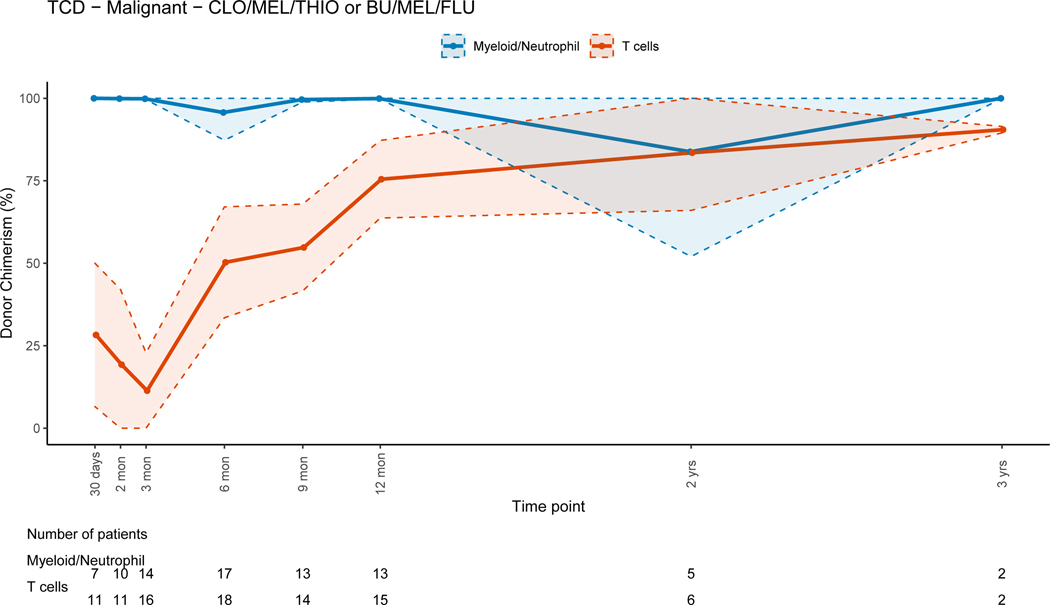
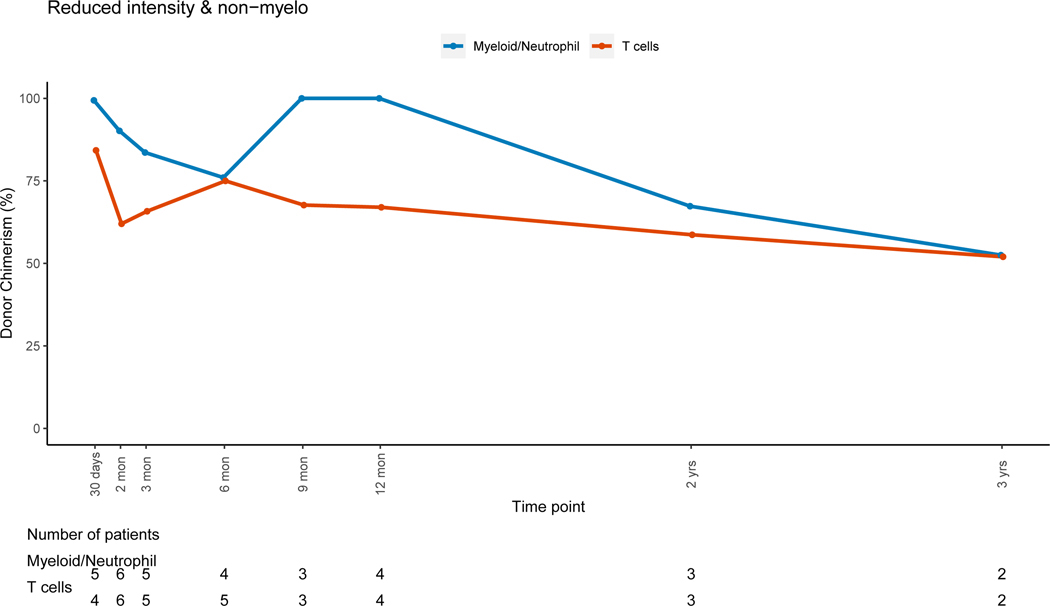
Patients who received TBI-containing regimens achieved higher T-cell lineage donor chimerism early post-HSCT compared to those receiving non-TBI containing regimens, while both groups achieved CC for the T-cell lineage late post-HSCT (figures 6A and 6B). CC for the myeloid lineage was achieved in both groups during the early post-HSCT period. Patients that received non-myeloablative/RIC regimens had progressive MC for all cell lineages with time (figure 6C). There were no significant differences in PBL (p=0.15), myeloid (p =0.08) or T-cell (p=0.28) donor chimerism change between 3 and 12 months post-HSCT between patients receiving myeloablative and non-myeloablative/RIC regimens.
Figure 6: Myeloid and T-cell Lineage Donor Chimerism Percents per Conditioning Regimen at Different Post-HSCT Time Points.
Dots indicate the mean values while the shaded area is the 95% confidence interval, in figures without shaded area, numbers where too small to calculate 95% confidence interval.
Figure 6A: Total Body Irradiation (TBI) Containing Myeloablative Regimens
Figure 6B: Non-Total Body Irradiation (TBI) Containing Myeloablative Regimens (Clofarabine/Melphalan/Thiotepa or Busulfan/Melphalan/Fludarabine)
Figure 6C: Non-myeloablative/Reduced Intensity Conditioning Regimens
Engraftment
Among the 136 (of 137) patients with evaluation for neutrophil engraftment, it occurred in the majority of patients (n=132), while primary (n=1) and late graft failure were rare (n=3). The three patients with late graft failure had progressive MC with loss of donor chimerism for all cell-lineages.
Relapse
Overall, 26 out of of 99 patients with hematologic malignancies (29.2%) had disease relapse. Diagnoses included lymphoid (n = 17; B-cell ALL, n=9, T-cell ALL, n=7 and Burkitt’s lymphoma, n=1) and myeloid (n=9; AML n=7, JMML n=1, MDS n=1) malignancies. B-cell donor chimerism data was available for 15/26 patients. Their median time between last chimerism value and relapse was 160 days (range: 20–540). For all patients in the cohort with known chimerism, the hazard ratio for disease relapse was 1.33 (95% CI: 1.00–1.76, p=0.05) for every 10% decrease in B-cell donor chimerism, reflecting a 33% increase in the risk for relapse. However, this was not the case for T-cell or myeloid chimerism, where a 10% decrease in donor chimerism had a HR of 0.98 (95% CI: 0.85–1.12, p=0.75) and 1.25 (95% CI: 0.97–1.60, p=0.09) respectively.
Discussion
Our study represents a retrospective analysis of post-HSCT lineage-specific donor-host chimerism in a large pediatric cohort who received allogeneic HSCT for diverse indications, using different types of grafts and graft manipulation. In summary, we showed that (1) persistent MC occurs more often in younger patients, after RIC transplants, or in non-malignant disorders, without necessarily being associated with graft failure, (2) early host T-cell chimerism following TCD grafts was “benign” and led to complete T-cell chimerism at 1 year post-HSCT, therefore making TCD, unmodified BM or PBSC and UCB grafts equal in terms of chimerism at one year post-HSCT, (3) the loss of donor B-cell chimerism in hematologic malignancies may be an early indicator of relapse, and (4) T and myeloid lineage chimerism are the major determinants of the whole blood PBL chimerism, and the transplant outcome.
Our study had several limitations. The number of patients with available chimerism data for all lineages at any given time point was limited given that chimerism monitoring was not standardized and on occasions driven by individual patient/provider specific circumstances. Please refer to supplemental table S1 and figure S2 in the supplementary materials for detailed characteristics and lineage specific chimerism trends of this subgroup within our cohort. Our results could guide the development of a standardized approach to post-HSCT donor-host lineage-specific chimerism monitoring that could be validated in future studies. Additionally, given the expertise with T-cell depletion techniques used for graft manipulation at our center, a large number of patients in our cohort received TCD grafts. This potentially influenced the donor-host T-cell lineage chimerism patterns in our cohort and might not be applicable to centers that routinely use conventional stem cell or UCB grafts. However, our study provides valuable information on the graft kinetics and chimerism trends of TCD grafts that has not previously been reported in the literature and could be informative to other centers.
Lineage-specific chimerism analysis appears to be more representative of the dynamic post-HSCT engraftment process compared to whole blood chimerism.21, 22 In our cohort, most patients had complete myeloid donor chimerism early post-HSCT while T-cell donor chimerism was mixed during that period and slowly increased to full donor thereafter; leading to the frequent finding of mixed whole blood PBL chimerism. This finding and the lack of evidence of graft failure and relapse with MC of the T-cell lineage highlights the importance of investigating lineage-specific chimerism to guide clinical practice and avoid unnecessary interventions that could lead to increased morbidity and mortality.
Age < 3 years at the time of HSCT significantly impacted post-HSCT donor-host chimerism. The pharmacokinetics of drugs in infants and young children differ to those of older children and young adults given age-specific physiologic and metabolic processes that influence drug deposition, dose, plasma concentration and pharmacodynamics.23, 24 Lower levels of pre-HSCT chemotherapy agents secondary to altered metabolism during conditioning likely influenced the impact of younger age on post-HSCT chimerism; where MC in the myeloid lineage and PBL at 3 and 12 months post-HSCT was observed. This was noted despite using a pharmacokinetics based approach for busulfan dosing in this population. Additionally, this age group includes a majority of patients with PID, for whom received RIC regimens are commonly used based on reports that durable split chimerism can support long term adequate immune function and survival.25
Having an underlying non-malignant disorder was associated with a steeper decrease in donor PBL chimerism from 3 to 12 months post-HSCT in our cohort. However, CC was preserved in all lineages except for T-cells, indicating that MC in the T-cell lineage was the major contributor to the global decrease in PBL donor chimerism. Only two patients in this group developed graft failure while the remainder of live patients at last follow up preserved continued engraftment and disease remission. Similar results for patients with PIDs and hemoglobinopathies have been reported, indicating that low levels of donor chimerism are sufficient to reverse the disease phenotype after allogeneic-HSCT and allow for continued engraftment despite MC.26–29
Conditioning regimen, type of graft and graft manipulation influence chimerism. Selection of conditioning regimen depends on patient diagnosis, remission status, comorbidities and donor availability.30 In general, myeloablative regimens are used in patients with hematologic malignancies and have been associated with earlier achievement of CC, while RIC regimens are frequently used for non-malignant disorders given that CC is not needed in most of these patients. RIC regimens have decreased toxicity but commonly lead to MC and concerns about graft failure remain.31–33 In our cohort, there were no differences in donor PBL chimerism when comparing TBI vs non-TBI myeloablative regimens, but persistent MC for the T-cell lineage was observed in patients who received non-TBI containing regimens. We can infer that these host T-cells lacked alloreactivity as they did not lead to graft rejection in this cohort. However, this reflects a need for closer monitoring of T-cell lineage chimerism in this group. Patients receiving RIC/non-myeloablative regimens had a trend towards mixed but stable chimerism in all lineages. The fact that no adverse post-HSCT outcomes were observed in this group indicates that MC is not necessarily detrimental when stable and therapeutic interventions such as donor-lymphocyte infusions or increased immunosuppression could be avoided in this scenario.
TCD graft recipients had significantly lower donor T-cell chimerism at 3 months post-HSCT when compared to unmodified and UCB graft recipients. They eventually achieved complete donor T-cell chimerism, indicating that this is a progressive process that takes several months to occur. UCB grafts represent an alternative source of HSCs for both children and adults lacking a matched unrelated BM or PBSC donor with similar graft-versus-leukemia effect and decreased risk of acute and chronic GvHD.34, 35 In our study, all UCB graft recipients had sustained CC for all cell lineages post-HSCT. Published data on donor chimerism status after UCB HSCT is limited. Elkaim et al. reported the cumulative incidence of near-complete donor chimerism in 94 children recipients of single UCB HSCT for malignant and non-malignant disorders to be 81.9% at 1 year post-HSCT and identified history of malignancy, older age and increased level of HLA mismatch as positive predictive factors.36 Despite our small number of UCB graft recipients, our results are consistent with published literature showing achievement of CC post-HSCT in most UCB recipients.37–39
Graft rejection and failure are rare but serious complications of allogeneic-HSCT associated with multifactorial risks including HLA disparity, ABO mismatch, RIC regimens, infections, myelosuppressive drugs, low nucleated cell dose of the graft, TCD and allosensitization of the recipient.40 Progressive host chimerism has been identified as a risk factor for secondary graft failure in patients with bone marrow failure syndromes.41–43 In our cohort, graft failure was rare despite the presence of host immune cells in patients with MC post transplant. Although MC was more common in patients with non-malignant disorders this was mostly driven by MC in the T-cell lineage early post-HSCT and graft failure did not occur. It could be inferred that the transient presence of host T-cells early post-HSCT did not cause immune-mediated rejection and since all patients recovered CC for the T-cell lineage at 12 months post-HSCT, the risk for this adverse outcome was lower at this time. None of the patients with graft failure in our cohort had bone marrow failure as underlying diagnosis and they all developed progressive MC in all lineages leading to complete loss of donor chimerism. This suggests that donor PBL MC is not sufficient to predict graft failure, but progressive lineage-specific MC in all cell-lineages is suggestive of this complication and should lead to additional investigation and possible interventions.
MC in HSCT for malignant diseases can be concerning for the recurrence of host leukemic cells, although this remains controversial. In our cohort, post-HSCT myeloid lineage chimerism in patients with myeloid malignancies did not reveal MC in the time period leading to disease relapse, suggesting that myeloid-specific chimerism was unable to predict relapse. For the entire cohort (including all patients with known chimerism, regardless of relapse state), increases in host B-cell chimerism led to an increased risk of relapse, suggesting that mixed B-cell lineage chimerism could be an indicator of this complication. Increases in myeloid and T-cell host chimerism did not increase relapse risk. This differs from previously published data demonstrating an association between re-emergence of host-derived CD34+ and CD8+ cell subsets, but not CD19+ subsets and post-HSCT relapse in children with ALL.10 Other groups found no difference in 3-year cumulative relapse incidence between patients with hematologic malignancies with persistent MC or CC, concluding that MC is not predictive of relapse. 11, 12 Prospective studies of lineage-specific chimerism at specific post-HSCT time points are needed to clarify its relationship to hematologic malignancy relapse and to assess its application for early diagnosis of this complication.
In conclusion, lineage-specific chimerism analyses provide a more accurate representation of the post-HSCT donor-host chimerism dynamic than whole blood chimerism. Whole PBL MC, when caused by T-cell MC, is not associated with adverse outcomes such as graft failure or rejection, suggesting that decisions regarding interventions for MC should be made cautiously and with hesitation. Young age, underlying non-malignant disorder and use of RIC regimens were associated with PBL MC driven by the myeloid lineage, alluding to the need for close follow-up in these subgroups. We recommend monitoring for T, myeloid and PBL chimerism, at least 1, 3, and 12 months post-HSCT to determine the engraftment status and guide clinical decision making. Future prospective studies are needed to validate our proposed chimerism monitoring guidelines and increase our knowledge on the association of lineage-specific donor-host chimerism with pre-HSCT factors and post-HSCT adverse outcomes.
Supplementary Material
Highlights:
Young age, underlying non-malignant disorder diagnosis and the use of reduced intensity conditioning regimens are associated with whole blood mixed chimerism driven by the myeloid lineage, suggesting a need for closer follow-up in these subgroups.
Whole blood mixed chimerism driven by the T-cell lineage in the setting of T-cell depleted grafts is not related to adverse post-transplant and resolves without additional interventions such as changes in immunosuppression or donor lymphocyte infusions.
Lineage-specific chimerism analyses are a more accurate representation of the post-transplant donor-host chimerism status than whole blood chimerism and decision making related to interventions should be based in these results.
Acknowledgements
The authors thank the pediatric TCT attendings, advanced practice providers (APPs), nursing staff and patients and families; and Joseph Olechnowicz (Editor, Department of Pediatrics, Memorial Sloan-Kettering Cancer Center), for editorial assistance.
Financial Disclosure Statement:
G.L., K.F., A.M. and F.B report no financial disclosures. E.N. is employed by Rocket Pharmaceuticals, Inc. and has equity in the company. J.B. is a consultant for BlueRock, Advanced Clinical, Omeros, Avrobio and Race Oncology. S.P. receives support for the conduct of sponsored trials through MSK from Atara Biotherapeutics and Jasper and co-inventor on IP licensed to Atara with rights waived to MSK with no personal financial interests in Atara.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thomas ED. Karnofsky Memorial Lecture. Marrow transplantation for malignant diseases. J Clin Oncol. 1983;1:517–531. [DOI] [PubMed] [Google Scholar]
- 2.Khan F, Agarwal A, Agrawal S. Significance of chimerism in hematopoietic stem cell transplantation: new variations on an old theme. Bone Marrow Transplant. 2004;34:1–12. [DOI] [PubMed] [Google Scholar]
- 3.Haines HL, Bleesing JJ, Davies SM, et al. Outcomes of donor lymphocyte infusion for treatment of mixed donor chimerism after a reduced-intensity preparative regimen for pediatric patients with nonmalignant diseases. Biol Blood Marrow Transplant. 2015;21:288–292. [DOI] [PubMed] [Google Scholar]
- 4.Inagaki J, Fukano R, Nishikawa T, et al. Outcomes of immunological interventions for mixed chimerism following allogeneic stem cell transplantation in children with juvenile myelomonocytic leukemia. Pediatr Blood Cancer. 2013;60:116–120. [DOI] [PubMed] [Google Scholar]
- 5.Liou A, Wahlstrom JT, Dvorak CC, Horn BN. Safety of pre-emptive donor lymphocyte infusions (DLI) based on mixed chimerism (MC) in peripheral blood or bone marrow subsets in children undergoing hematopoietic stem cell transplant (HSCT) for hematologic malignancies. Bone Marrow Transplant. 2017;52:1057–1059. [DOI] [PubMed] [Google Scholar]
- 6.Kristt D, Stein J, Yaniv I, Klein T. Assessing quantitative chimerism longitudinally: technical considerations, clinical applications and routine feasibility. Bone Marrow Transplant. 2007;39:255–268. [DOI] [PubMed] [Google Scholar]
- 7.Shaw A, Passweg JR, De La Fuente J, et al. Relapse of Aplastic Anemia with Majority Donor Chimerism (Donor-Type Aplasia) Occurring Late after Bone Marrow Transplantation. Biol Blood Marrow Transplant. 2020;26:480–485. [DOI] [PubMed] [Google Scholar]
- 8.Stikvoort A, Sundin M, Uzunel M, et al. Long-Term Stable Mixed Chimerism after Hematopoietic Stem Cell Transplantation in Patients with Non-Malignant Disease, Shall We Be Tolerant? PLoS One. 2016;11:e0154737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Preuner S, Lion T. Post-transplant monitoring of chimerism by lineage-specific analysis. Methods Mol Biol. 2014;1109:271–291. [DOI] [PubMed] [Google Scholar]
- 10.Preuner S, Peters C, Pötschger U, et al. Risk assessment of relapse by lineage-specific monitoring of chimerism in children undergoing allogeneic stem cell transplantation for acute lymphoblastic leukemia. Haematologica. 2016;101:741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mellgren K, Arvidson J, Toporski J, Winiarski J. Chimerism analysis in clinical practice and its relevance for the detection of graft rejection and malignant relapse in pediatric hematopoietic stem cell transplant patients. Pediatr Transplant. 2015;19:758–766. [DOI] [PubMed] [Google Scholar]
- 12.Pichler H, Fritsch G, König M, et al. Peripheral blood late mixed chimerism in leucocyte subpopulations following allogeneic stem cell transplantation for childhood malignancies: does it matter? Br J Haematol. 2016;173:905–917. [DOI] [PubMed] [Google Scholar]
- 13.Devine SM, Carter S, Soiffer RJ, et al. Low risk of chronic graft-versus-host disease and relapse associated with T cell-depleted peripheral blood stem cell transplantation for acute myelogenous leukemia in first remission: results of the blood and marrow transplant clinical trials network protocol 0303. Biol Blood Marrow Transplant. 2011;17:1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jakubowski AA, Small TN, Young JW, et al. T cell depleted stem-cell transplantation for adults with hematologic malignancies: sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007;110:4552–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verdonck LF, Dekker AW, van Heugten H, van Kempen ML, Punt K, de Gast GC. Depletion of T cells from bone marrow grafts with soybean agglutinin and sheep red blood cells for prevention of graft-versus-host disease. Haematol Blood Transfus. 1987;30:563–566. [DOI] [PubMed] [Google Scholar]
- 16.Collins NH, Carabasi MH, Bleau S, et al. New technology for the depletion of T cells from soybean lectin agglutinated, HLA-matched bone marrow grafts for leukemia: initial laboratory and clinical results. Prog Clin Biol Res. 1992;377:427–439. [PubMed] [Google Scholar]
- 17.Antin JH, Childs R, Filipovich AH, et al. Establishment of complete and mixed donor chimerism after allogeneic lymphohematopoietic transplantation: recommendations from a workshop at the 2001 Tandem Meetings of the International Bone Marrow Transplant Registry and the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2001;7:473–485. [DOI] [PubMed] [Google Scholar]
- 18.Clark JR, Scott SD, Jack AL, et al. Monitoring of chimerism following allogeneic haematopoietic stem cell transplantation (HSCT): technical recommendations for the use of short tandem repeat (STR) based techniques, on behalf of the United Kingdom National External Quality Assessment Service for Leucocyte Immunophenotyping Chimerism Working Group. Br J Haematol. 2015;168:26–37. [DOI] [PubMed] [Google Scholar]
- 19.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855–864. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan KM, Agura E, Anasetti C, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28:250–259. [PubMed] [Google Scholar]
- 21.Lion T, Daxberger H, Dubovsky J, et al. Analysis of chimerism within specific leukocyte subsets for detection of residual or recurrent leukemia in pediatric patients after allogeneic stem cell transplantation. Leukemia. 2001;15:307–310. [DOI] [PubMed] [Google Scholar]
- 22.Lion T, Watzinger F, Preuner S, et al. The EuroChimerism concept for a standardized approach to chimerism analysis after allogeneic stem cell transplantation. Leukemia. 2012;26:1821–1828. [DOI] [PubMed] [Google Scholar]
- 23.Bartelink IH, Rademaker CM, Schobben AF, van den Anker JN. Guidelines on paediatric dosing on the basis of developmental physiology and pharmacokinetic considerations. Clin Pharmacokinet. 2006;45:1077–1097. [DOI] [PubMed] [Google Scholar]
- 24.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–1167. [DOI] [PubMed] [Google Scholar]
- 25.Burroughs L, Woolfrey A. Hematopoietic cell transplantation for treatment of primary immune deficiencies. Cell Ther Transplant. 2010;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamidieh AA, Behfar M, Pourpak Z, et al. Long-term outcomes of fludarabine, melphalan and antithymocyte globulin as reduced-intensity conditioning regimen for allogeneic hematopoietic stem cell transplantation in children with primary immunodeficiency disorders: a prospective single center study. Bone Marrow Transplant. 2016;51:219–226. [DOI] [PubMed] [Google Scholar]
- 27.Fitzhugh CD, Cordes S, Taylor T, et al. At least 20% donor myeloid chimerism is necessary to reverse the sickle phenotype after allogeneic HSCT. Blood. 2017;130:1946–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andreani M, Nesci S, Lucarelli G, et al. Long-term survival of ex-thalassemic patients with persistent mixed chimerism after bone marrow transplantation. Bone Marrow Transplant. 2000;25:401–404. [DOI] [PubMed] [Google Scholar]
- 29.Lucarelli G, Gaziev J. Advances in the allogeneic transplantation for thalassemia. Blood Rev. 2008;22:53–63. [DOI] [PubMed] [Google Scholar]
- 30.Gyurkocza B, Sandmaier BM. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood. 2014;124:344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sykes M. Mixed chimerism and transplant tolerance. Immunity. 2001;14:417–424. [DOI] [PubMed] [Google Scholar]
- 32.Chiesa R, Veys P. Reduced-intensity conditioning for allogeneic stem cell transplant in primary immune deficiencies. Expert Rev Clin Immunol. 2012;8:255–266; quiz 267. [DOI] [PubMed] [Google Scholar]
- 33.Satwani P, Cooper N, Rao K, Veys P, Amrolia P. Reduced intensity conditioning and allogeneic stem cell transplantation in childhood malignant and nonmalignant diseases. Bone Marrow Transplant. 2008;41:173–182. [DOI] [PubMed] [Google Scholar]
- 34.Rocha V, Cornish J, Sievers EL, et al. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood. 2001;97:2962–2971. [DOI] [PubMed] [Google Scholar]
- 35.Eapen M, Rubinstein P, Zhang MJ, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369:1947–1954. [DOI] [PubMed] [Google Scholar]
- 36.Esbenshade AJ, Zhao Z, Aftandilian C, et al. Multisite external validation of a risk prediction model for the diagnosis of blood stream infections in febrile pediatric oncology patients without severe neutropenia. Cancer. 2017;123:3781–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel SA, Allewelt HA, Troy JD, et al. Durable Chimerism and Long-Term Survival after Unrelated Umbilical Cord Blood Transplantation for Pediatric Hemophagocytic Lymphohistiocytosis: A Single-Center Experience. Biol Blood Marrow Transplant. 2017;23:1722–1728. [DOI] [PubMed] [Google Scholar]
- 38.Prasad VK, Mendizabal A, Parikh SH, et al. Unrelated donor umbilical cord blood transplantation for inherited metabolic disorders in 159 pediatric patients from a single center: influence of cellular composition of the graft on transplantation outcomes. Blood. 2008;112:2979–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elkaim E, Picard C, Galambrun C, et al. Peripheral blood cells chimerism after unrelated cord blood transplantation in children: kinetics, predictive factors and impact on post-transplant outcome. Br J Haematol. 2014;166:557–565. [DOI] [PubMed] [Google Scholar]
- 40.Locatelli F, Lucarelli B, Merli P. Current and future approaches to treat graft failure after allogeneic hematopoietic stem cell transplantation. Expert Opin Pharmacother. 2014;15:23–36. [DOI] [PubMed] [Google Scholar]
- 41.Lawler M, McCann SR, Marsh JC, et al. Serial chimerism analyses indicate that mixed haemopoietic chimerism influences the probability of graft rejection and disease recurrence following allogeneic stem cell transplantation (SCT) for severe aplastic anaemia (SAA): indication for routine assessment of chimerism post SCT for SAA. Br J Haematol. 2009;144:933–945. [DOI] [PubMed] [Google Scholar]
- 42.Kako S, Yamazaki H, Ohashi K, et al. Mixed Chimerism and Secondary Graft Failure in Allogeneic Hematopoietic Stem Cell Transplantation for Aplastic Anemia. Biol Blood Marrow Transplant. 2020;26:445–450. [DOI] [PubMed] [Google Scholar]
- 43.Gomez JR, Garcia MJ, Serrano J, et al. Chimerism analysis in long-term survivor patients after bone marrow transplantation for severe aplastic anemia. Haematologica. 1997;82:588–591. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.