Abstract
Psilocybe magic mushrooms are best known for their main natural product, psilocybin, and its dephosphorylated congener, the psychedelic metabolite psilocin. Beyond tryptamines, the secondary metabolome of these fungi is poorly understood. The genomes of five species (P. azurescens, P. cubensis, P. cyanescens, P. mexicana, and P. serbica) were browsed to understand more profoundly common and species‐specific metabolic capacities. The genomic analyses revealed a much greater and yet unexplored metabolic diversity than evident from parallel chemical analyses. P. cyanescens and P. mexicana were identified as aeruginascin producers. Lumichrome and verpacamide A were also detected as Psilocybe metabolites. The observations concerning the potential secondary metabolome of this fungal genus support pharmacological and toxicological efforts to find a rational basis for yet elusive phenomena, such as paralytic effects, attributed to consumption of some magic mushrooms.
Keywords: aeruginascin, kinases, metabolome, psilocybin, secondary metabolism
Beyond the magic: Psilocybe mushrooms are known for their psychotropic effects. Yet, some species are suspected to cause paralysis. A genomic survey revealed an unexpectedly diverse repertoire of natural product genes in these mushrooms, much more diverse than evident by chemical analyses. Knowledge on the realm of the Psilocybe secondary metabolome helps anticipate potential toxicities in this iconic fungal genus.
Introduction
The fungal genus Psilocybe is recognized for its metabolic capacity to produce psilocybin (Figure 1A). [1] This simple, yet unique l‐tryptophan‐derived phosphorylated tryptamine is the immediate and chemically stable precursor of psilocin which interferes with serotonergic neurotransmission and induces psychedelic effects. [2] Psilocybin‐producing so‐called magic mushrooms rank among the most well‐known fungi. Although illicit in many countries in the European Union and many states in the US, some species are grown or collected as recreational drugs. The pharmaceutical interest in psilocybin is rapidly increasing as successful clinical studies have demonstrated psilocybin's potential to treat patients who suffer from otherwise therapy‐refractory depression. [3]
Figure 1.
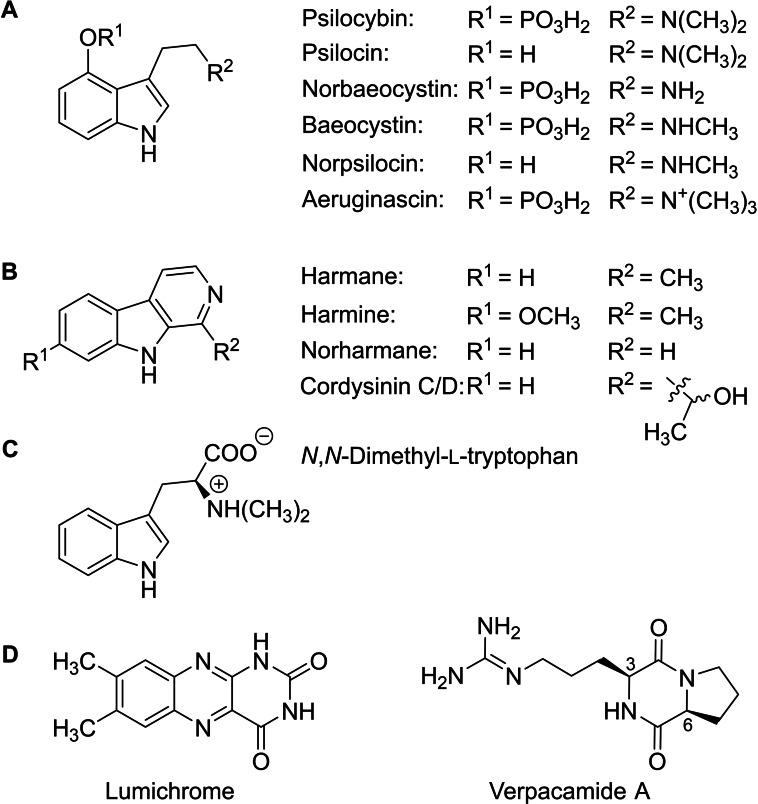
Chemical structures of Psilocybe natural products. A: tryptamines, B: β‐carbolines, C: N,N‐dimethyl‐l‐tryptophan, D: lumichrome and verpacamide A, i. e., the Psilocybe natural products identified by LC–MS in this study.
Research with magic mushrooms at the chemistry/mycology interface has traditionally emphasized their major natural product psilocybin. Subsequently, related tryptamines, e. g., aeruginascin and norpsilocin, were found and identified as other bioactive compounds (Figure 1A). [4] Recently, various β‐carbolines (Figure 1B) and N,N‐dimethyl‐l‐tryptophan (Figure 1C) were discovered as Psilocybe natural products, [5] which pointed to a more diverse metabolome than previously anticipated.
A greater metabolic diversity is also implied by reports on the controversially discussed entourage effect. It generally describes that two or more compounds contribute synergistically to a given pharmacological action, as observed in the context of cannabis research. [6] While clinical studies rely on pure psilocybin, some reports have questioned whether all psychotropic effects of magic mushrooms can be attributed solely to pure psilocybin. [7] Rather, these authors suggest that an effect existed that may be explained by varying relative amounts of structurally and potentially pharmacologically dissimilar tryptamines.
Another phenomenon has remained even more enigmatic, which is paralysis after uptake of magic mushrooms. It was first described in 1973 for P. subcaerulipes, a wood‐inhabiting species. [8] More recently, the colloquial term wood lover's paralysis (WLP) has been coined in anecdotal reports that (re‐) describe numbness, incoordinated or lost motoric control of limbs or facial muscles or even severe paralysis of individuals who had consumed other lignicolous species as recreational drugs, this is most frequently P. cyanescens and P. azurescens. The eponymous feature of either species is the intense bluing after injury or aging, which causes psilocin to oligomerize. [9] The former species is widely distributed in North America and Europe. Its carpophores often grow gregariously on lignin‐rich substrates, such as plant beds, covered with wood chips, in residential areas and parks. The other species, P. azurescens is native to Oregon and Washington. Like P. cyanescens, it prefers lignin‐rich material and woody debris as substrate. Interestingly, none of the reports on WLP pertains to P. cubensis, a dung‐inhabiting species. These peripheral paralytic effects reportedly last up to 24 h. This duration is clearly longer than psilocin's psychotropic action on the central nervous system, which typically subsides after 3–4 h due to renal psilocin clearance via O‐glucuronylation and due to formation of 4‐hydroxyindol‐3‐yl‐acetaldehyde by monoamine oxidase A activity.[ 2a , 10 ] Systematic clinical investigations on WLP and the compound(s) causing it have not been carried out yet. However, if these symptoms were the consequence of mushroom uptake, they appeared incompatible with the pharmacology of known Psilocybe tryptamines. This, in return, may point to undiscovered natural products interfering with peripheral neurotransmission.
To support pharmacological and toxicological research into the above‐mentioned phenomena, a systematic inventory of natural product genes is presented, based on five Psilocybe genomes, including both lignicolous and dung‐inhabiting species. This genetic approach identified a much more diverse repertoire of natural product biosynthetic capacities than evident from parallel chromatographic analyses.
Results and Discussion
Genome sequencing of P. mexicana and P. azurescens
P. mexicana, a Central American species inhabiting meadows and open grass‐covered areas, was the first species from which psilocybin was isolated. [1] It was included in this study for phylogenetic reasons. Psilocybe species fall in two evolutionary clades. [11] P. mexicana is a clade I species, as opposed to the other species analyzed in this study, which all belong to clade II. P. azurescens was chosen as it shows a high psilocybin content [12] and is notorious for causing WLP, according to unsubstantiated reports. Recently, draft genome sequences of these species were presented. [13] However, the sequences were highly fragmented and could potentially include incoherent biosynthetic gene clusters which may have unfavorably impacted our survey. Therefore, contiguous genomic sequences for P. mexicana and P. azurescens were produced during this study. Genomic sequencing resulted in assemblies of 274 and 1088 contigs, respectively (N50 values of 507,670 and 230,470 bp). The genome sizes were 65.2 and 71.4 Mb, the average GC contents were 47.4 % and 46 %, which is comparable to other Psilocybe genomes (Table S1). [14]
Inventory of Psilocybe natural product genes
Surprisingly, a systematic investigation on secondary metabolite genes of Psilocybe spp. has not been conducted yet. For a representative survey on the metabolic capacity of this genus, the genomes of the two above‐mentioned species were analyzed for natural product genes. Published genomic data of P. cyanescens, P. serbica and two genomes of P. cubensis were also included. [14]
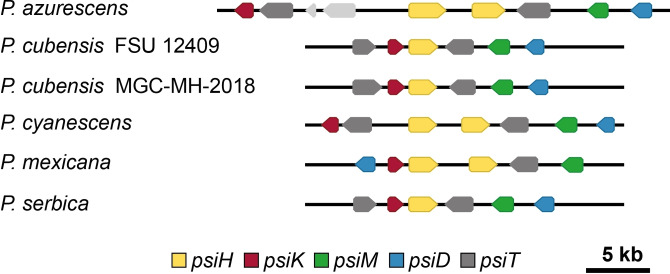
As expected, all five species encode the complete genetic locus for psilocybin biosynthesis, including P. azurescens, for which a recent report found only an incomplete locus. [13] The loci in this species and in P. mexicana are dissimilar to the previously known clusters both in the number and the arrangement of genes (Figure 2).
Figure 2.
Gene arrangement in loci for psilocybin biosynthesis. The psiH gene encodes the cytochrome P450 monooxygenase for tryptamine 4‐hydroxylation, psiK encodes the 4‐hydroxytryptamine kinase, psiM the N‐methyltransferase, psiD the gateway l‐tryptophan decarboxylase, psiT denotes a hypothetical transporter gene.[14a] Genes shown in light grey do not belong to the psilocybin biosynthesis.
All species encode only one non‐ribosomal peptide synthetase (NRPS), i. e., a type VI siderophore synthetase (predicted length and mass for P. cubensis: 2410 aa, 268.8 kDa), whose gene clusters with a monooxygenase gene (Table 1). [15] The sole biochemically characterized representative of this NRPS type, CsNPS2 of Ceriporiopsis subvermispora, was shown to catalyze the biosynthesis of the iron chelator basidioferrin, a linear N 5‐acetyl‐N 5‐hydroxy‐l‐ornithine trimer, (Figure S1). [15b] Furthermore, one gene (two in P. mexicana) for a non‐reducing polyketide synthase (NR‐PKS) was found. Its very close phylogenetic relationship to various ArmB‐like orsellinic acid synthases [16] (Figures S2 and S3) suggested the capacity of Psilocybe species to produce an as yet unidentified aromatic polyketide as well. Except P. mexicana, all investigated species encode a hybrid NRPS/PKS, whose domain setup (adenylation, thiolation, keto synthase, acyl transferase, thiolation) is strongly reminiscent of that of the hispidin synthase HispS. [17] A cytochrome P450 monooxygenase, similar to the hispidine‐3‐hydroxylase (H3H), is encoded next to the HispS‐type enzyme (Figure S4A).
Table 1.
Number of natural product genes per biosynthetic category in Psilocybe genomes, see also Table S2. NR‐PKS: non‐reducing polyketide synthase; NRPS: non‐ribosomal peptide synthetase; NRPS/PKS: hybrid non‐ribosomal peptide synthetase/polyketide synthase; TS: terpene synthase.
|
|
P. azurescens |
P. cubensis [a] |
P. cubensis [b] |
P. cyanescens |
P. mexicana |
P. serbica |
|---|---|---|---|---|---|---|
|
NR‐PKS |
1 |
1 |
1 |
1 |
2 |
1 |
|
NRPS[c] |
1 |
1 |
1 |
1 |
1 |
1 |
|
NRPS/PKS |
3 |
2 |
2 |
3 |
4 |
3 |
|
TS |
25 |
20 |
21 |
22 |
17 |
26 |
|
Halogenase |
1 |
1 |
1 |
1 |
1 |
1 |
|
NRPS‐like |
8 |
8 |
8 |
7 |
6 |
12 |
|
Psilocybin locus |
1 |
1 |
1 |
1 |
1 |
1 |
[a] P. cubensis FSU 12409. [b] P. cubensis MGC‐MH‐2018. [c] Type VI siderophore synthetase.
In another mushroom, Neonothopanus nambi, these two enzymes (plus a luciferase) catalyze the biosynthesis leading to fungal luciferin for mushroom bioluminescence (“foxfire”). [17] However, this phenomenon is not known from Psilocybe species. Consistently, fungal luciferase genes, which are prerequisite for bioluminescence, were not found in Psilocybe genomes. The function of this Psilocybe HispS‐like NRPS/PKS hybrid remains shrouded. Perhaps, the gene represents a relic of a lost capacity to bioluminesce. A second type of NRPS/PKS hybrid enzyme, also of unclear function, is encoded in all investigated genomes. It follows the HispS architecture with an N‐terminal adenylation‐thiolation didomain, but additionally includes a keto reductase and a dehydratase domain. In the phylogenetic tree (Figure S3), this type of enzyme is referred to as “reductive hybrids”. While P. cubensis encodes only one copy, all other species show two paralogs, separated by a putative transporter gene.
Apart from one canonical oxidosqualene (i. e., triterpene) synthase for ergosterol biosynthesis, all investigated genomes encode numerous terpene synthases (Table 1, Table S2), ranging from 17 (P. mexicana) to 26 (P. serbica), per species. Of those, between 17 (P. mexicana) and 24 (P. serbica) are putative sesquiterpene synthases. The majority thereof (six in P. mexicana, 12 both in P. serbica and P. azurescens) belongs to clade III sesquiterpene synthases. [18] These synthases catalyze the C1,11 cyclization of (2E,6E)‐farnesyl diphosphate, which yields, via the trans‐humulyl cation, scaffolds such as Δ6‐protoilludene or hirsutene. [19] However, such compounds or other (sesqui‐)terpenes have not yet been described from the genus Psilocybe. In all investigated species, another natural product‐related gene encodes a flavin‐dependent halogenase. It closely resembles characterized fungal halogenases that regioselectively chlorinate the aromatic moiety of melleolide antibiotics (ArmH1 of Armillaria mellea, 45–47 % identical amino acids) or that of radicicol (RadH of Chaetomium chiversii).[ 20 , 21 ] Finally, genes for NRPS‐like putative adenylating reductases and quinone synthetase enzymes were found (Figure S5, Table S2), for which precedence exists in basidiomycetes as well. [22]
We sought to identify natural product genes unique to P. azurescens and P. cyanescens, i. e., the two species which reportedly cause WLP. A biosynthetic gene cluster network analysis (Figure S6) identified three loci for sesquiterpene synthases that were not encoded in the other investigated species. Two loci encode type IV sesquiterpene cyclases, which mediate the C1,6 cyclization of (3R)‐nerolidyl‐diphosphate into, e. g., the α‐cuprenene scaffold, [19] while the third locus encodes putative type III sesquiterpene cyclases. However, further functional characterization is required to evaluate if these enzymes are active and if their products may be connected to WLP.
Chemical analysis
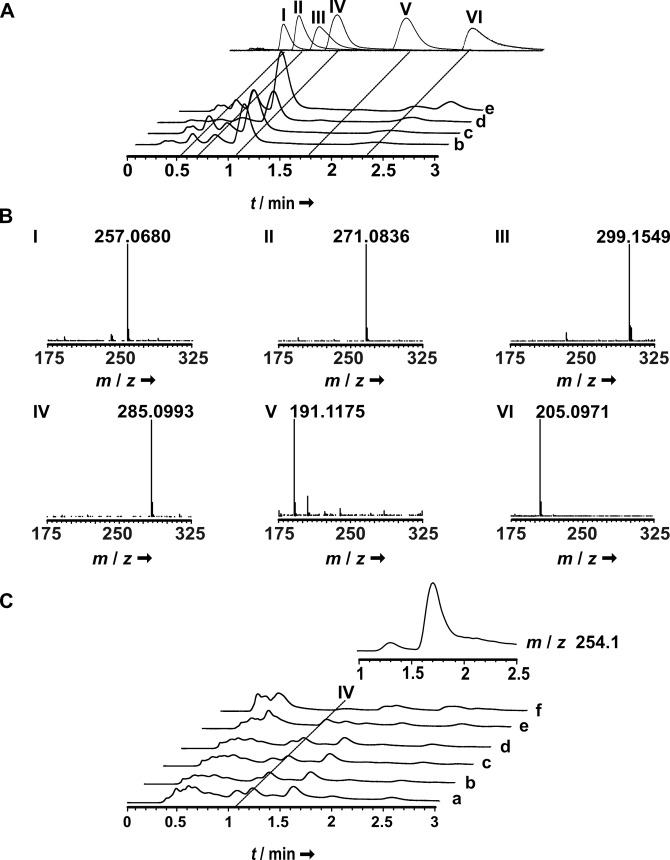
The potential natural product diversity, suggested by genomic data, prompted us to re‐investigate the five Psilocybe species chromatographically. The mycelia of all species as well as carpophores, which were available for two P. cubensis strains, for P. cyanescens, and for P. mexicana were subjected to LC–MS analyses. Both methanolic and ethyl acetate extracts were prepared to extract polar and more lipophilic compounds. Besides psilocybin as major tryptamine, the methanolic carpophore extracts contained norbaeocystin, baeocystin, along with minor amounts of psilocin and norpsilocin (Figure 3A). Subsequently, the extracts were re‐analyzed using single ion monitoring to improve the limit of detection. Both in P. mexicana and P. cyanescens carpophores, aeruginascin was unambiguously detected, yet in very minor amounts (<0.1 % of the psilocybin area under the curve). This is the first description of this quaternary ammonium compound from these species. Consistent with earlier results, [23] aeruginascin was found in P. cubensis carpophores as well.
Figure 3.
LC–MS analysis of methanolic carpophore and mycelial extracts to detect tryptamines. A: carpophore extracts. Top trace: authentic standards of norbaeocystin (I), baeocystin (II), aeruginascin (III), psilocybin (IV), norpsilocin (V), and psilocin (VI) are shown as overlay of separate chromatograms. B: mass spectra of tryptamines, detected in carpophores. C: mycelial extracts. The inset above the chromatograms shows an extracted ion chromatogram for m/z 254 [M+H]+, i. e., the mass of cyclo(Arg‐Pro). Assignment of chromatograms to species: a: P. azurescens; b: P. cubensis FSU12407; c: P. cubensis FSU12410; d: P. cyanescens; e: P. mexicana; f: P. serbica.
Psilocybin and other tryptamines were also detected in mycelial extracts of all species (Figure 3C), albeit at very low concentrations, which is consistent with previous results. [24] Interestingly, at t R =1.7 min, our LC–MS analyses identified a compound both in the mycelia of all species and in P. mexicana carpophores whose UV/Vis spectrum and exact mass did not match those of known tryptamines. Instead, the mass was compatible with that of the diketopiperazine cyclo(Arg‐Pro) (found: m/z 254.1607 [M+H]+, calcd: 254.1611, Figures 1D, 3 C and S7). Cyclo(l‐Arg‐l‐Pro), which is identical with verpacamide A, and its diastereomer cyclo(l‐Arg‐d‐Pro), also referred to as compound CI‐4, are sponge and bacterial 2,5‐diketopiperazine natural products, respectively, that possess strong chitinase inhibiting activity. [25] To determine the configurations of the amino acids, the diketopiperazine was chromatographically purified, hydrolyzed, and subjected to Marfey's analysis. Both the arginine and the proline moiety were l‐configured. The notion of Psilocybe as a verpacamide A‐producing genus was corroborated by LC–MS/MS data (Figure S7).
Biosynthesis of diketopiperazines is mediated by NRPSs, as shown for fungi, e. g., for the NRPSs GliP, forming the gliotoxin scaffold. [26] Besides NRPSs, bacteria also use tRNA‐dependent cyclodipeptide synthases, such as AlbC for albonoursein biosynthesis in Streptomyces noursei. [27] Curiously, neither class of enzymes is encoded in Psilocybe species, according to the genomic survey, and verpacamide A biosynthesis in Psilocybe remains unclear. We cannot rule out that this diketopiperazine had formed spontaneously. However, other diketopiperazines were not found, in particular not those whose spontaneous formation is likely under physiological conditions [28] which is why an enzymatically catalyzed origin was assumed.
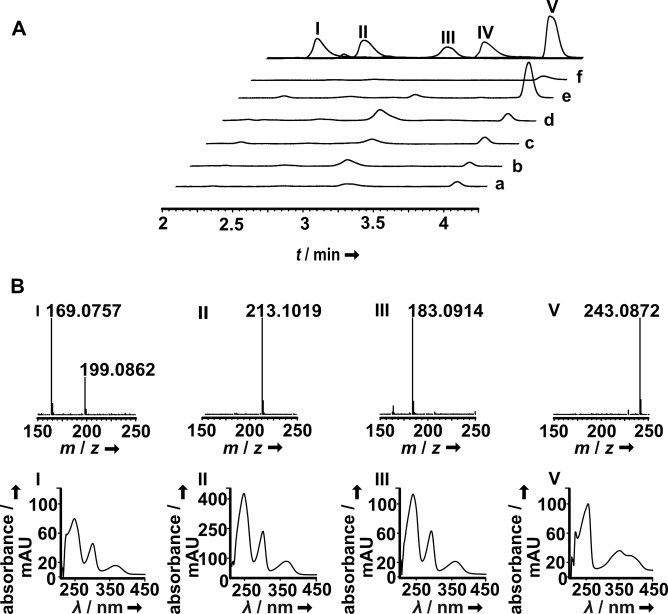
Chromatographic analysis of the mycelial ethyl acetate extracts and the comparison with reference compounds identified norharmane, harmane, traces of the enantiomeric cordysinins C and/or D, along with a previously identified isomer of harmol (m/z 199.0862 [M+H]+) that co‐elutes with norharmane (m/z 169.0757 [M+H]+, Figure 4). [5a]
Figure 4.
LC–MS analysis of ethyl acetate extracts of Psilocybe mycelia. A: top trace: authentic standards of norharmane (I, t R=2.2 min), racemate of enantiomeric cordysinins C and D (II, t R=2.8 min), harmane (III, t R=3.0 min), harmol (IV, t R=3.4 min) and lumichrome (V, t R=3.9 min) are shown as overlay of separate chromatograms. Below: chromatograms of fungal extracts, recorded at λ=340 nm. Trace a: P. azurescens; b: P. cubensis FSU12407; c: P. cubensis FSU12410; d: P. cyanescens; e: P. mexicana; f: P. serbica. B: Representative mass and UV/Vis spectra, extracted at the respective retention times of sample chromatograms.
Furthermore, in all investigated species a compound was detected at t R=3.9 min whose UV/Vis spectrum did not match that of simple β‐carbolines (Figure 4), but whose exact mass m/z 243.0872 [M+H]+ was consistent with that of lumichrome (Figure 1D), an isoalloxazine and follow‐up product of riboflavin. A comparison with an authentic standard confirmed the identity of this compound that has not been described yet from Psilocybe species. All investigated species encode a flavin‐dependent halogenase (Table 1). However, mass spectrometry did not point to chlorinated or brominated natural products in Psilocybe extracts, as a distinctive isotopic pattern for halogenated compounds was not found.
In vitro kinase assays
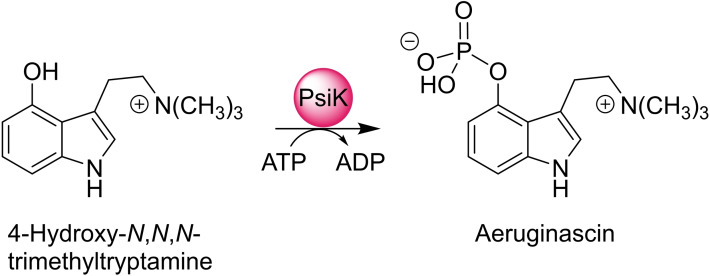
The analytical results unambiguously identified P. cyanescens and P. mexicana as producers of the quaternary amine aeruginascin, which was also found in extracts of P. cubensis, [23] while the dephosphorylated congener 4‐hydroxy‐N,N,N‐trimethyltryptamine (4‐OH‐TMT) was not detected in any of these species. Minor amounts of psilocin were present as well. PsiK, the kinase of psilocybin biosynthetic pathway, has a repair function to keep psilocin levels low by rephosphorylation to psilocybin. [29] We therefore hypothesized that PsiK may analogously O‐phosphorylate 4‐OH‐TMT to aeruginascin. To test the hypothesis (Scheme 1), heterologously produced hexahistidine‐tagged PsiK of P. cubensis and P. cyanescens was used for in vitro reactions.
Scheme 1.
PsiK‐catalyzed ATP‐dependent phosphorylation of 4‐hydroxy‐N,N,N‐trimethyltryptamine to aeruginascin.
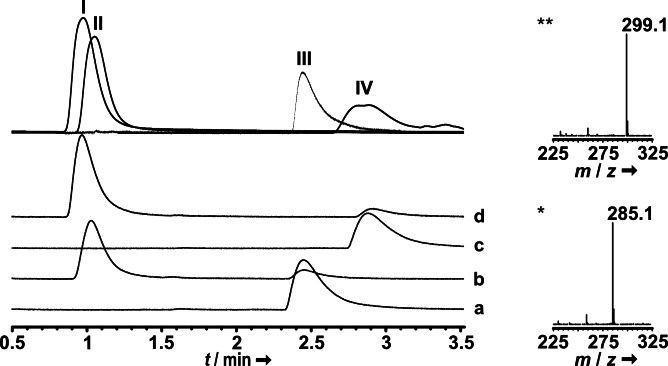
LC‐ESI‐MS analyses of reactions, sampled after the reaction had proceeded for 2 min, showed that PsiK accepts 4‐OH‐TMT as a phosphate acceptor substrate, and 37 % and 22 % (P. cubensis and P. cyanescens PsiK, respectively) of the 4‐OH‐TMT substrate (t R=2.9 min, Figure 5) were converted to aeruginascin (t R=1.05 min). Parallel reactions with psilocin (t R=2.7 min) as positive control indicated that 46 and 28 % were converted to psilocybin (t R=1.2 min). PsiK's ability to also phosphorylate a quaternary amine provides a plausible explanation why aeruginascin, but not 4‐OH‐TMT, was found in P. cubensis and P. cyanescens.
Figure 5.
LC–MS analysis of PsiK in vitro assays. Chromatograms were recorded at λ=280 nm. Top trace: authentic standards of aeruginascin (I), psilocybin (II), psilocin (III), and 4‐OH‐TMT (IV) are shown as an overlay of separate chromatograms. Trace a: negative control with psilocin and heat‐inactivated enzyme; trace b: reaction with psilocin and native PsiK; trace c: negative control with 4‐OH‐TMT and heat‐inactivated enzyme; trace d: reaction with 4‐OH‐TMT and native PsiK. Mass spectra of product peaks show aeruginascin (m/z 299.1 [M]+) and psilocybin (m/z 285.1 [M+H]+) formation, respectively.
Conclusion
The psychotropic magic mushrooms of the genus Psilocybe and their natural products are of dual relevance for pharmaceutical sciences. Current clinical studies demonstrated that psilocybin is an urgently needed, promising drug candidate against major depressive disorder. [30] Characterization of its biosynthetic enzymes enabled strategies for its biotechnological production in vitro and in vivo.[ 3c , 14a , 29 , 31 ] From a toxicological perspective, increasingly liberal policies in some states of the US have decriminalized the consumption of magic mushrooms as recreational drugs. Given anecdotal reports on WLP and (except for indoleethylamines) the yet little understood realm of natural products that Psilocybe mushrooms can potentially produce, emergency medicine needs to be prepared for an increasing number of mushroom‐related hospitalizations. The number of genetic loci presumably dedicated to secondary metabolism in Psilocybe species exceeds the number of known compounds by far. Comparing genetic and analytical data, we conclude that only a small portion of the true natural product metabolome of Psilocybe has been discovered. Terpenes have not yet been described from the investigated Psilocybe species. Solely one report describes two sesquiterpenes, the psilosamuiensins A and B, from another species, the East Asian P. samuiensis. [32] However, according to this study, the potential terpene diversity is notable. The non‐volatile representatives are potentially relevant, in analogy to research on Cannabis, as a starting point for research into the entourage effect of mushrooms, as minor Cannabis mono‐ and sesquiterpenes seem to modulate the activity of its major psychotropic metabolites. [6]
The genetic results dispel the earlier notion of little diversity in the Psilocybe small molecule metabolome, and further research at the intersection of natural product chemistry, pharmacy, and toxicology is warranted to elucidate the complete metabolic diversity of potentially bioactive, synergistic, and toxic compounds in one of the most iconic mushroom genera.
Experimental Section
Mycological methods: Psilocybe azurescens FSU 13761, P. cubensis strains FSU 12407 and FSU 12410, P. cyanescens FSU 12414, P. mexicana FSU 13617, and P. serbica FSU 12416 were maintained on MEP (15 g L−1 malt extract, 3 g L−1 peptone, 18 g L−1 agar) plates. To produce biomass for natural product analyses, a 200 mL seed culture (MEP liquid medium) was incubated for 7 d at 25 °C and 140 rpm) and subsequently homogenized. A 15 mL portion was then used to inoculate a 500 mL main liquid culture (MEP) shaken for 7 d at 25 °C and 140 rpm. For P. cubensis and P. mexicana, carpophore formation was induced as described, [4c] P. cyanescens carpophores were collected in the vicinity of Jena, Germany. Liquid MEP cultures (incubated for 4 d at 25 °C and 140 rpm) were also used to isolate genomic DNA for sequencing. Mycelia were collected, shock‐frozen in liquid nitrogen and lyophilized prior to RNA isolation or metabolite quantification. For genomic DNA sequencing, freshly ground mycelium from submerse cultures (<10 d) was used.
Genomic sequencing and sequence assembly: The biomass (50–100 mg) from submerse cultures of P. azurescens or P. mexicana was harvested, suspended in 800 μL CTAB buffer, and incubated at 65 °C for 2 h. Cell debris was removed by centrifugation. The cleared supernatant was mixed with 10 μL Monarch RNAse A (20 μg μL−1, NEB) and kept at 37 °C for 30 min. The samples were extracted with a mixture of phenol, chloroform and isoamyl alcohol (25 : 24 : 1). DNA was then precipitated with ice‐cold isopropanol, kept at −20 °C for 30 min and centrifuged at 20,800×g and 4 °C for 20 min. The pellet was washed 8–12 times with 70 % ethanol before drying and solving in 10 mm TRIS‐buffer (pH 8.5) overnight. 400 ng DNA were used per sequencing run. Libraries were generated using the Oxford Nanopore Rapid Sequencing kit and sequenced on a MinION flow cell, following the manufacturer's protocol. Genomes were assembled using CANU [33] v.1.9. assuming a genome size of 60 Mb. Assembled draft genomes were improved by comparison against signal level reads using nanopolish, [34] after sorting and mapping the reads with minimap [35] and samtools. [36] Genomic DNA sequences are accessible under JAJIRX000000000 (for P. azurescens) and JAJIRY000000000 (for P. mexicana).
Bioinformatic analysis: The genomes of P. cubensis FSU12409 and MGC‐MH‐2018 were annotated using Braker version 2.1.5 [37] with Augustus version 3.4.0 [38] and GeneMark‐ES version 4.62 [39] in “–fungus” mode. Published RNAseq data [14c] (NCBI accession #: PRJNA450675) was aligned to the genomes using HiSat2 (version 4.8.2). [40] The resulting bam file was used as input to Braker. Genomes for P. azurescens, P. cyanescens, P. mexicana and P. serbica were predicted with Augustus 3.4.0 with the newly generated species model for P. cubensis. Biosynthetic gene clusters were predicted using fungiSMASH version 6.0.0. [41] GenBank files from the fungiSMASH analysis were then further processed using the BiG‐SCAPE network prediction software. [42] Network data was adjusted manually and illustrated using Cytoscape 3.8.0. [43] Genome and corresponding protein sequences were further analyzed using Geneious Prime 2021.0.3 (Biomatters, Ltd.). Predictive phylogenetic frameworks were built from characterized fungal polyketide synthases (Table S3). Initial trees for the heuristic search were obtained automatically by applying Neighbor‐Join and BioNJ algorithms to a matrix of pairwise distances estimated using the JTT model, and then selecting the topology with superior log likelihood value. A discrete Gamma distribution was used to model evolutionary rate differences among sites. All positions containing gaps and missing data were eliminated, and the analyses were conducted in MEGA X. [44]
Extraction of natural products: Lyophilized fungal mycelium or carpophores were ground to a fine powder. For tryptamine analysis, it was extracted three times (5 min each) with methanol (MeOH, 20 mL per gram dry biomass) in an ultrasonic bath. After each extraction step, the tubes containing the extract were centrifuged for 10 min at 13,700×g. The methanolic extracts were pooled, and the solvent was removed under reduced pressure. The dry extract was dissolved in a final volume of 2 mL and subsequently analyzed using LC–MS. To detect β‐carbolines and other metabolites, the flask containing the MeOH‐insoluble residue was washed with alkaline water solution (pH 12 with 6 m NaOH), and the aqueous phase was extracted three times with 50 mL ethyl acetate per extraction. The organic layer was pooled and removed under reduced pressure, and the residue was dissolved in MeOH for LC–MS analysis.
Heterologous production of PsiK and in vitro kinase assays: P. cubensis and P. cyanescens PsiK were produced in E. coli as N‐terminally tagged hexahistidine fusion proteins and purified as described [14a] For production of P. cyanescens PsiK, pET28a‐based plasmid pTS19 was created, which harbored the psiK cDNA of P. cyanescens FSU12414, ligated between the BamHI and HindIII sites. Purified PsiK was desalted on a PD‐10 column (GE Healthcare), and eluted with sodium phosphate buffer (50 mm, pH 7). Protein concentrations were determined by Bradford's assay. [45] Enzyme assays were carried out in triplicate. Reactions (50 μL) were set up in 50 mm phosphate buffer (pH 7), and consisted of 1 μm PsiK, 2 mm ATP, 2 mm MgCl2, 3 mm β‐mercaptoethanol and 1 mm acceptor substrate (psilocin or 4‐hydroxy‐N,N,N‐trimethyltryptamine). Reactions were incubated for 2 min at 33 °C, before they were frozen in liquid nitrogen and lyophilized. Subsequently, the residue was dissolved in 100 μL MeOH, centrifuged, and used for chromatography. Heat inactivated enzyme served as negative control.
Chromatography: Fungal extracts were chromatographically analyzed on an Agilent Infinity II 1290 UHPLC‐MS instrument, equipped with a diode array and a 6130 quadrupole mass detector using electrospray ionization in positive mode, and a Phenomenex Luna Omega polar C18 column (50×2.1 mm, 1.6 μm). To detect tryptamines, the following method was used: Solvent A: 0.1 % (v/v) formic acid (FA) in water, solvent B: acetonitrile (ACN). The gradient was: 0 min, 1 % B; within 3 min to 5 % B; within another min to 100 % B; held at 100 % B for 2 min. The flow rate was 0.5 mL min−1 and the chromatograms were extracted at λ=280 nm. For control and to rule out that aeruginascin is an artefact that may have formed from psilocybin during work up, the psilocybin standard was processed like the samples. For Marfey's analysis, the same solvents and instruments and the following gradient were used: 0 min, 1 % B; within 9 min to 50 % B; within another min to 100 % B at a flow of 1 mL min−1, detecting with mass spectrometry. Retention times were: l‐Arg‐l‐FDLA: t R=5.3 min, d‐Arg‐l‐FDLA: t R=5.0 min, l‐Pro‐l‐FDLA: t R=6.1 min, d‐Pro‐l‐FDLA: t R=6.6 min (l‐FDLA: 1‐fluoro‐2,4‐dinitrophenyl‐5‐l‐leucinamide). To analyze β‐carbolines, the same equipment, but an Agilent EclipsePlus C18 (50×2.1 mm, 1.8 μm) column was used. Parameters were: flow: 1 mL min−1, diode array detection: λ=200–500 nm, the chromatograms were extracted at λ=340 nm. Solvent A was 0.1 % (v/v) FA in water, solvent B was ACN. The linear gradient was 5 % B over 0.5 min, then 5 % to 27 % B within 5.5 min, to 100 % B within further 2 min. PsiK kinase assays were analyzed with the method for tryptamines, described above. Purification of verpacamide A was carried out on an Agilent 1260 Infinity semipreparative chromatograph, equipped with a Thermo Scientific Hypercarb column (150×4.6 mm, 5 μm), using 0.1 % (v/v) trifluoroacetic acid (TFA) in water (solvent A) and ACN (solvent B) at 2 mL min−1 for isocratic chromatography (90 % A/10 % B). Final purification was accomplished on the same instrument using a Merck Ascentis Express F5 column (100×2.1 mm, 2.7 μm) under isocratic conditions: 0.1 % (v/v) TFA in water (solvent A) and MeOH (solvent B) at 0.55 mL min−1 (99 % A/1 % B). Chromatograms were extracted at λ=195 nm.
Diketopiperazine purification and Marfey's analysis: The methanolic extracts of the biomass from P. mexicana liquid cultures (2.5 L total volume) were evaporated under reduced pressure and the residue was dissolved in 10 mL of water. The aqueous phase was washed three times with ethyl acetate (10 mL each), followed by isocratic semipreparative HPLC (above). The organic layer was evaporated under reduced pressure, and the purified compound was dissolved in 100 μL 6 M HCl. The reaction tube was sealed and incubated at 100 °C for 18 h. Subsequently, the reaction was cooled to room temperature and the liquid was evaporated under reduced pressure. The residue was dissolved in 50 μL H2O. 20 μL thereof were mixed with 20 μL of 1 m NaHCO3 and 50 μL l‐FDLA in acetone, and incubated at 40 °C for 60 min. The reaction was stopped by adding 20 μL of 1 m HCl and diluted with an equal volume of MeOH. Standards for l‐ and d‐configured arginine and proline were prepared using 100 mm stock solutions. The amino acid solution (10 μL) was mixed with 10 μL of 1 m NaHCO3 and 50 μL l‐FDLA and incubated at 40 °C for 60 min, then stopped and prepared like the samples. The standards and the samples were analyzed as described above.
Chemical synthesis of 4‐hydroxy‐N , N , N ‐trimethyltryptamine: The PsiK substrate 4‐hydroxy‐N,N,N‐trimethyltryptamine was synthesized following a previously reported protocol. [46]
Conflict of interest
A.R.C. reports an ownership interest in CaaMTech, LLC.
1.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
J.F. was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy – EXC 2051, project ID 390713860. Paula Sophie Seibold (Friedrich Schiller University, Jena) and Felix Trottmann (Leibniz Institute for Natural Product Research and Infection Biology – Hans Knöll Institute, Jena) are acknowledged for providing Psilocybe RNA, additional cultivation, and assistance with analyses, respectively. We also gratefully acknowledge Alexander Sherwood, Ph.D. (Usona Institute, Madison, WI) for authentic standards. The authors are grateful to CaaMTech, LLC. (Issaquah, WA) for support, and to Simon Beck, MD and Caine Barlow, MSc (Australian Psychedelic Society) for valuable information on wood lover paralysis. Open Access funding enabled and organized by Projekt DEAL.
S. Dörner, K. Rogge, J. Fricke, T. Schäfer, J. M. Wurlitzer, M. Gressler, D. N. K. Pham, D. R. Manke, A. R. Chadeayne, D. Hoffmeister, ChemBioChem 2022, 23, e202200249.
Data Availability Statement
The data that support the findings of this study are openly available in GenBank at https://www.ncbi.nlm.nih.gov/genbank, reference number 0.
References
- 1.
- 1a. Hofmann A., Heim R., Brack A., Kobel H., Experientia 1958, 14, 107–109; [DOI] [PubMed] [Google Scholar]
- 1b. Hofmann A., Heim R., Brack A., Kobel H., Frey A., Ott H., Petrzilka T., Troxler F., Helv. Chim. Acta 1959, 42, 1557–1572. [Google Scholar]
- 2.
- 2a. Tyls F., Palenicek T., Horacek J., Eur. Neuropsychopharmacol. 2014, 24, 342–356; [DOI] [PubMed] [Google Scholar]
- 2b. Kargbo R. B., ACS Med. Chem. Lett. 2020, 11, 399–402; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2c. Nichols D. E., J. Antibiot. 2020, 73, 679–686. [DOI] [PubMed] [Google Scholar]
- 3.
- 3a. Geiger H. A., Wurst M. G., Daniels R. N., ACS Chem. Neurosci. 2018, 9, 2438–2447; [DOI] [PubMed] [Google Scholar]
- 3b. Lenz C., Sherwood A., Kargbo R., Hoffmeister D., ChemPlusChem 2021, 86, 28–35; [DOI] [PubMed] [Google Scholar]
- 3c. Blei F., Baldeweg F., Fricke J., Hoffmeister D., Chem. Eur. J. 2018, 24, 10028–10031; [DOI] [PubMed] [Google Scholar]
- 3d. Carhart-Harris R., Giribaldi B., Watts R., Baker-Jones M., Murphy-Beiner A., Murphy R., Martell J., Blemings A., Erritzoe D., Nutt D. J., N. Engl. J. Med. 2021, 384, 1402–1411. [DOI] [PubMed] [Google Scholar]
- 4.
- 4a. Leung A. Y., Paul A. G., J. Pharm. Sci. 1968, 57, 1667–1671; [DOI] [PubMed] [Google Scholar]
- 4b. Jensen N., Gartz J., Laatsch H., Planta Med. 2006, 72, 665–666; [DOI] [PubMed] [Google Scholar]
- 4c. Lenz C., Wick J., Hoffmeister D., J. Nat. Prod. 2017, 80, 2835–2838; [DOI] [PubMed] [Google Scholar]
- 4d. Sherwood A. M., Halberstadt A. L., Klein A. K., McCorvy J. D., Kaylo K. W., Kargbo R. B., Meisenheimer P., J. Nat. Prod. 2020, 83, 461–467. [DOI] [PubMed] [Google Scholar]
- 5.
- 5a. Blei F., Dörner S., Fricke J., Baldeweg F., Trottmann F., Komor A., Meyer F., Hertweck C., Hoffmeister D., Chem. Eur. J. 2020, 26, 729–734; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5b. Blei F., Fricke J., Wick J., Slot J. C., Hoffmeister D., ChemBioChem 2018, 19, 2160–2166. [DOI] [PubMed] [Google Scholar]
- 6.
- 6a. Russo E. B., Br. J. Pharmacol. 2011, 163, 1344–1364; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6b. Russo E. B., Front. Plant Sci. 2019, 9, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.
- 7a. Gartz J., Int. J. Crude Drug Res. 1989, 27, 141–144; [Google Scholar]
- 7b. Matsushima Y., Shirota O., Kikura-Hanajiri R., Goda Y., Eguchi F., Biosci. Biotechnol. Biochem. 2009, 73, 1866–1868; [DOI] [PubMed] [Google Scholar]
- 7c. Zhuk, Jasicka-Misiak I., Poliwoda A., Kazakova A., Godovan W., Halama M., Wieczorek P. P., Toxin Rev. 2015, 7, 1018–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yokoyama K., Trans. Mycol. Soc. Jpn. 1973, 14, 317–320. [Google Scholar]
- 9. Lenz C., Wick J., Braga D., García-Altares M., Lackner G., Hertweck C., Gressler M., Hoffmeister D., Angew. Chem. Int. Ed. 2020, 59, 1450–1454; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 1466–1470. [Google Scholar]
- 10. Hasler F., Bourquin D., Brenneisen R., Bär T., Vollenweider F. X., Pharm. Acta Helv. 1997, 72, 175–184. [DOI] [PubMed] [Google Scholar]
- 11. Ramírez-Cruz V., Guzmán G., Villalobos-Arámbula A. R., Rodríguez A., Matheny P. B., Sánchez-García M., Guzmán-Dávalos L., Botany 2013, 91, 573–591. [Google Scholar]
- 12.
- 12a. Gartz J., Wiedemann G., Drug Test Anal. 2015, 7, 853–857; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12b. Stamets P., Gartz J., Integration 1995, 6, 21. [Google Scholar]
- 13.K. J. McKernan, L. T. Kane, Y. Helbert, L. Zhang, N. Houde, S. McLaughlin, Zenodo 2021 (Version 1.0), 10.5281/zenodo.5062843. [DOI] [PMC free article] [PubMed]
- 14.
- 14a. Fricke J., Blei F., Hoffmeister D., Angew. Chem. Int. Ed. 2017, 56, 12352–12355; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 12524–12527; [Google Scholar]
- 14b. Reynolds H. T., Vijayakumar V., Gluck-Thaler E., Korotkin H. B., Matheny P. B., Slot J. C., Evol. Lett. 2018, 2, 88–101; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14c. Torrens-Spence M. P., Liu C. T., Pluskal T., Chung Y. K., Weng J. K., ACS Chem. Biol. 2018, 13, 3343–3353. [DOI] [PubMed] [Google Scholar]
- 15.
- 15a. Bushley K. E., Ripoll D. R., Turgeon B. G., BMC Evol. Biol. 2008, 8, 328; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15b. Brandenburger E., Gressler M., Leonhardt R., Lackner G., Habel A., Hertweck C., Brock M., Hoffmeister D., Appl. Environ. Microbiol. 2017, 83, e01478–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.
- 16a. Ishiuchi K., et al., ChemBioChem 2012, 13, 846–854; [DOI] [PubMed] [Google Scholar]
- 16b. Lackner G., Bohnert M., Wick J., Hoffmeister D., Chem. Biol. 2013, 20, 1101–1106; [DOI] [PubMed] [Google Scholar]
- 16c. Yu P.-W., Chang Y.-C., Liou R.-F., Lee T.-H., Tzean S.-S., J. Nat. Prod. 2016, 79, 1485–1491; [DOI] [PubMed] [Google Scholar]
- 16d. Braesel J., Fricke J., Schwenk D., Hoffmeister D., Fungal Genet. Biol. 2017, 98, 12–19. [DOI] [PubMed] [Google Scholar]
- 17. Kotlobay A. A., Sarkisyan K. S., Mokrushina Y. A., Marcet-Houben M., Serebrovskaya E. O., Markina N. M., Somermeyer L. G., Gorokhovatsky A. Y., Vvedensky A., Purtov K. V., Petushkov V. N., Rodionova N. S., Chepurnyh T. V., Fakhranurova L. I., Guglya E. B., Ziganshin R., Tsarkova A. S., Kaskova Z. M., Shender V., Abakumov M., Abakumova T. O., Povolotskaya I. S., Eroshkin F. M., Zaraisky A. G., Mishin A. S., Dolgov S. V., Mitiouchkina T. Y., Kopantzev E. P., Waldenmaier H. E., Oliveira A. G., Oba Y., Barsova E., Bogdanova E. A., Gabaldón T., Stevani C. V., Lukyanov S., Smirnov I. V., Gitelson J. I., Kondrashov F. A., Yampolsky I. V., Proc. Natl. Acad. Sci. USA 2018, 115, 12728–12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nagamine S., Liu C., Nishishita J., Kozaki T., Sogahata K., Sato Y., Minami A., Ozaki T., Schmidt-Dannert C., Maruyama J.-I., Oikawa H., Appl. Environ. Microbiol. 2019, 85, e00409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.
- 19a. Quin M. B., Flynn C. M., Schmidt-Dannert C., Nat. Prod. Rep. 2014, 31, 1449–1473; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19b. Gressler M., Löhr N. A., Schäfer T., Lawrinowitz S., Seibold P. S., Hoffmeister D., Nat. Prod. Rep. 2021, 38, 702–722. [DOI] [PubMed] [Google Scholar]
- 20. Wick J., Heine D., Lackner G., Misiek M., Tauber J., Jagusch H., Hertweck C., Hoffmeister D., Appl. Environ. Microbiol. 2015, 82, 1196–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang S., Xu Y., Maine E. A., Wijeratne E. M.K, Espinosa-Artiles P., Gunatilaka A. A. L., Molnár I., Chem. Biol. 2008, 15, 1328–1338. [DOI] [PubMed] [Google Scholar]
- 22.
- 22a. Brandenburger E., Braga D., Kombrink A., Lackner G., Gressler J., Künzler M., Hoffmeister D., Fungal Genet. Biol. 2018, 112, 55–63; [DOI] [PubMed] [Google Scholar]
- 22b. Wackler B., Lackner G., Chooi Y. H., Hoffmeister D., ChemBioChem 2012, 13, 798–804; [DOI] [PubMed] [Google Scholar]
- 22c. Schneider P., Bouhired S., Hoffmeister D., Fungal Genet. Biol. 2008, 45, 1487–1496. [DOI] [PubMed] [Google Scholar]
- 23. Gotvaldová K., Hájková K., Borovička J., Jurok R., Cihlářová P., Kuchař M., Drug Test Anal. 2021, 13, 439–446. [DOI] [PubMed] [Google Scholar]
- 24. Demmler R., Fricke J., Dörner S., Gressler M., Hoffmeister D., ChemBioChem 2020, 21,1364–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.
- 25a. Izumida H., Imamura N., Sano H., J. Antibiot. 1996, 49, 76–80; [DOI] [PubMed] [Google Scholar]
- 25b. Houston D. R., Eggleston I., Synstad B., Eijsink V. G. H., van Aalten D. M. F., Biochem. J. 2002, 368, 23–27; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25c. Vergne C., Boury-Esnault N., Perez T., Martin M.-T., Adeline M.-T., Dau E. T. H., Al-Mourabit Ali, Org. Lett. 2006, 8, 2421–2424. [DOI] [PubMed] [Google Scholar]
- 26.
- 26a. Kupfahl C., Heinekamp T., Geginat G., Ruppert T., Härtl A., Hof H., Brakhage A. A., Mol. Microbiol. 2006, 62, 292–302; [DOI] [PubMed] [Google Scholar]
- 26b. Balibar C. J., Walsh C. T., Biochemistry 2006, 45, 15029–15038; [DOI] [PubMed] [Google Scholar]
- 26c. R. A. Cramer, Jr. , Gamcsik M. P., Brooking R. M., Najvar L. K., Kirkpatrick W. R., Patterson T. F., Balibar C. J., Graybill J. R., Perfect J. R., Abraham S. N., Steinbach W. J., Eukaryotic Cell 2006, 5, 972–980; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26d. Baccile J. A., Le H. H., Pfannenstiel B. T., Bok J. W., Gomez C., Brandenburger E., Hoffmeister D., Keller N. P., Schroeder F. C., Angew. Chem. Int. Ed. 2019, 58, 14589–14593; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 14731–14735. [Google Scholar]
- 27. Lautru S., Gondry M., Genet R., Pernodet J. L., Chem. Biol. 2002, 9, 1355–1364. [DOI] [PubMed] [Google Scholar]
- 28. Lyons B., Kwan A. H., Truscott R., FEBS J. 2014, 281, 2945–2955. [DOI] [PubMed] [Google Scholar]
- 29. Fricke J., Kargbo R., Regestein L., Lenz C., Peschel G., Rosenbaum M., Sherwood A., Hoffmeister D., Chem. Eur. J. 2020, 26, 8281–8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Griffiths R. R., Johnson M. W., Carducci M. A., Umbricht A., Richards W. A., Richards B. D., Cosimano M. P., Klinedinst M. A., J. Psychopharmacol. 2016, 30, 1181–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.
- 31a. Hoefgen S., Lin J., Fricke J., Stroe M., Mattern D. J., Kufs J. E., Hortschansky P., Brakhage A. A., Hoffmeister D., Valiante V., Metab. Eng. 2018, 48, 44–51; [DOI] [PubMed] [Google Scholar]
- 31b. Adams A. M., Kaplan N. A., Wei Z., Brinton J. D., Monnier C. S., Enacopol A. L., Ramelot T. A., Jones J. A., Metab. Eng. 2019, 56,111–119; [DOI] [PubMed] [Google Scholar]
- 31c. Milne N., Thomsen P., Mølgaard Knudsen N., Rubaszka P., Kristensen M., Borodina I., Metab. Eng. 2020, 60, 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pornpakakul S., Suwancharoen S., Petsom A., Roengsumran S., Muangsin N., Chaichit N., Piapukiew J., Sihanonth P., Allen J. W., J. Asian Nat. Prod. Res. 2009, 11, 12–17. [DOI] [PubMed] [Google Scholar]
- 33.
- 33a. Koren S., Walenz B. P., Berlin K., Miller J. R., Bergman N. H., Phillippy A. M., Genome Res. 2017, 27, 722–736; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33b. Koren S., Rhie A., Walenz B. P., Dilthey A. T., Bickhart D. M., Kingan S. B., Hiendleder S., Williams J. L., Smith T. P. L., Phillippy A. M., Nat. Biotechnol. 2018, 36, 1174–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Loman N., Quick J., Simpson J. A., Nat. Methods 2015, 12, 733–735. [DOI] [PubMed] [Google Scholar]
- 35. Li H., Bioinformatics 2018, 34, 3094–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Danecek P., Bonfield J. K., Liddle J., Marshall J., Ohan V., Pollard M. O., Whitwham A., Keane T., McCarthy S. A., Davies R. M., Li H., Gigascience 2021, 10, giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brůna T., Hoff K. J., Lomsadze A., Stanke M., Borodovsky M., NAR Genom. Bioinform. 2021, 3, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stanke M., Steinkamp R., Waack S., Morgenstern B., Nucleic Acids Res. 2004, 32, 309–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brůna T., Lomsadze A., Borodovsky M., NAR Genom. Bioinform. 2020, 2, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim D., Paggi J. M., Park C., Bennett C., Salzberg S. L., Nat. Biotechnol. 2019, 37, 907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Blin K., Shaw S., Steinke K., Villebro R., Ziemert N., Lee S. Y., Medema M. H., Weber T., Nucleic Acids Res. 2019, 47, W81–W87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Navarro-Muñoz J. C., Selem-Mojica N., Mullowney M. W., Kautsar S. A., Tryon J. H., Parkinson E. I., De Los Santos E. L. C., Yeong M., Cruz-Morales P., Abubucker S., Roeters A., Lokhorst W., Fernandez-Guerra A., Cappelini L. T. D., Goering A. W., Thomson R. J., Metcalf W. W., Kelleher N. L., Barona-Gomez F., Medema M. H., Nat. Chem. Biol. 2020, 16, 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shannon P., Markiel A., Ozier O., Baliga N. S., Wang J. T., Ramage D., Amin N., Schwikowski B., Ideker T., Genome Res. 2003,13, 2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kumar S., Stecher G., Li M., Knyaz C., Tamura K., Mol. Biol. Evol. 2018, 35, 1547–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bradford M. M., Anal. Biochem. 1976, 72, 248–254. [DOI] [PubMed] [Google Scholar]
- 46. Chadeayne A. R., Pham D. N. K., Reid B. G., Golen J. A., Manke D. R., ACS Omega 2020, 5, 16940–16943. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Data Availability Statement
The data that support the findings of this study are openly available in GenBank at https://www.ncbi.nlm.nih.gov/genbank, reference number 0.