Abstract
Inactivation of the Escherichia coli gene ydeA, which encodes a member of the major facilitator superfamily, decreased the efflux of l-arabinose, thereby affecting the expression of AraC-regulated genes. In addition, overexpression of ydeA decreased the expression of genes regulated by isopropyl-β-d-thiogalactopyranoside.
The major facilitator superfamily (MFS) is the main class of proteins that utilize protons as symporters or antiporters to transport a variety of molecules across the cytoplasmic membrane of bacteria and eukaryotes. Some MSF members, such as TetA, responsible for resistance to tetracycline, have a narrow substrate specificity. Others, such as EmrB or Bmr, can exclude from the cells a wider range of organic molecules (10, 13). The complete sequencing of bacterial genomes has revealed a large number of genes belonging to the MFS. In Escherichia coli, 64 such genes can be identified on the basis of sequence similarities (14), and the exact number may be somewhat higher (13). A majority of these genes were identified in the course of sequence annotation, and their transport capabilities have not been established yet. Here we report that an MFS transporter encoded by a gene in the terminus region of the Escherichia coli chromosome, ydeA, excludes l-arabinose (l-Ara) and isopropyl-β-d-thiogalactopyranoside (IPTG).
The bacterial strains used in this study are listed in Table 1.
TABLE 1.
Bacterial strains
| Designation | Relevant genotype | Source |
|---|---|---|
| JS219 | araD Δ(ara-leu) Δ(lacIZYA)X74 malPp::lacIq | Our collection |
| JS1855 | JS219 ΔyihA::cat/p(araC+ PBAD-yihA) | Our collection |
| JS1908 | JS1855 ydeA::mini-tet no. 4 | This work |
| JS1909 | JS1855 ydeA::mini-tet no. 11 | This work |
| JS1910 | JS219 ydeA::mini-tet no. 4 | This work |
| JS1921 | JS219/pJPB274 | This work |
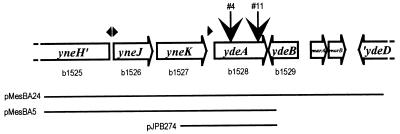
The yihA gene codes for an essential GTPase (1). We have constructed a strain, JS1855 (Table 1), which expresses yihA from the l-Ara-regulated PBAD promoter on a low-copy-number plasmid. Since JS1855 is deleted for the ara-leu region, yihA expression can be regulated by adjusting the extracellular l-Ara concentration. Strain JS1855 grew normally with 1 mg of l-Ara/ml or more but very slowly with 20 μg (133 μM) of l-Ara/ml. To isolate suppressors of this growth deficiency, we mutagenized JS1855 with mini-tet by infection with phage λ1098 (19) as described previously (12) and plated appropriate dilutions on Luria agar plates containing 20 μg of l-Ara/ml and 15 μg of tetracycline/ml. Fast-growing colonies were isolated, and this phenotype was ascertained by transducing the mini-tet-containing mutation into JS1855 and verifying the ability of the tetracycline-resistant transductants to grow rapidly with 20 μg of l-Ara/ml. DNA from appropriate clones was digested with the frequently cutting enzymes ApoI, BsaHI, or HaeII, ligated, and subjected to reverse PCR by using primers MTL (AATAATCCAAATCCAGCCATCCC) and MTR (GATAAAAGGCACCTTTGGTCAACC). Then, the chromosomal DNA sequence at the junction with the mini-tet was established by using amplified DNA and MTR as template and primer, respectively. Two mini-tet insertions were within the same gene, ydeA. Insertion no. 4 was located before nucleotide 580 of the ydeA coding sequence (1615632 of the E. coli K-12 sequence, version M52), with tetA in the same orientation as the disrupted gene. Insertion no. 11 was after nucleotide 1115 (1616167 of genome sequence) and had the opposite orientation (Fig. 1).
FIG. 1.
Map of the chromosomal region carried by suppressing plasmids and location of mini-tet insertions. Gene b numbers (1a) are indicated below four-letter designations (18). Relevant putative promoters are shown above intergenic spaces. Mini-tet insertions in ydeA are indicated by vertical arrows. The size of the pMESBA24 insert is 7,460 bp.
Gene ydeA codes for a 396-amino-acid protein with 12 predicted transmembrane-spanning segments and closely resembles known MFS proteins, such as the chloramphenicol exclusion protein CmlR of Streptomyces lividans. Return to fast growth in the presence of a low extracellular l-Ara concentration might be explained by a higher intracellular l-Ara content resulting from ydeA disruption. To test this possibility, we determined the effect of the mutation on the sensitivity of PBAD to added l-Ara. The ydeA::mini-tet no. 4 mutation was transduced into the Δlac strain JS219, yielding JS1910. Then JS219 and JS1910 were transformed with pDAG92, a pBAD18 (7) derivative carrying lacZ under PBAD control. Steady-state levels of β-galactosidase were measured in cultures grown in Luria broth containing high or low levels of l-Ara. In the presence of 2 mg of l-Ara/ml, β-galactosidase specific activity (in units/optical density at 600 nm [OD600]) was essentially the same for both strains (21,000 and 23,000 U of JS219 and JS1910, respectively). In sharp contrast, the disruption strain expressed PBAD-lacZ at a much higher rate (15,000 U) than the parent strain (2,400 U) in the presence of 5 μg (33 μM) of l-Ara/ml. This suggested that the disruption strain retained more intracellular arabinose than the wild-type parent.
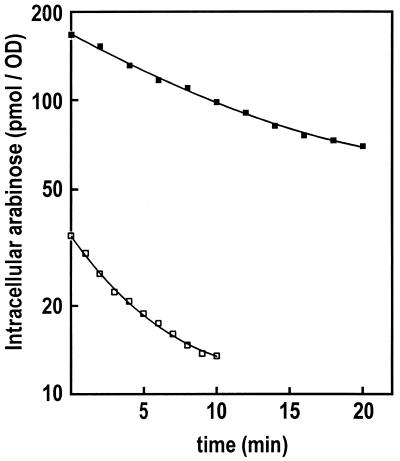
To establish directly that YdeA expels l-arabinose from the cells, accumulation and efflux of l-Ara were determined in strains JS219 (ydeA+) and JS1910 (ydeA::minitet) (Fig. 2). The concentration of l-Ara (5 μM) used in these experiments was such that only the ATP-dependent high-affinity AraFGH system could contribute significantly to l-Ara uptake (9). As expected, accumulation of l-Ara was higher in the ydeA mutant than in the wild-type parent. Since the strains used lack araC and are therefore not l-Ara inducible, an indirect interference of YdeA with l-Ara uptake capabilities can be ruled out. In addition, the initial rate of efflux, 14.3%/min at 25°C, was higher in the wild type than in the mutant (6.2%/min) (Fig. 2). The efflux data strongly favor the hypothesis that YdeA promotes l-Ara export.
FIG. 2.
Arabinose efflux in ydeA+ and ydeA::mini-tet strains. Strains JS219 (ydeA+) and JS1910 (ydeA::mini-tet) were grown in M9 medium supplemented with 1 μg of thiamine/ml, 0.2% Casamino Acids, and 1% glycerol until the OD600 was nearly 0.6. Cultures (5 ml) were transferred to 25°C, and 3H-labeled l-Ara (5 μM [0.2 Ci/mmol]; Moravek Radiochemicals) was added for 15 min. The cells were filtered through a 48-mm-diameter 0.45-μm HAWP Millipore filter and washed briefly with and resuspended into the same volume of Ara-free medium. Then, 0.2-ml samples were taken at 1-min (JS219) or 2-min (JS1910) intervals, filtered through 25-mm-diameter Millipore filters, washed, and counted. Open squares, JS219; closed squares, JS1910.
We have previously reported the inhibitory effect on cell division of a Φ(malE-minE) fusion gene overexpressed from the PTAC promoter (15) and the isolation of multicopy suppressors of this effect (15, 16). Two suppressor plasmids, pMesBA5 and pMesBA24, were localized with respect to the Kohara library of ordered phage recombinants (8), as described for pMesJE11 (16), and aligned to Kohara’s map by restriction analysis (Fig. 1). A PstI deletion derivative of pMesBA5, pJPB274 (Fig. 1), still suppressed the filamentation phenotype of overexpressed Φ(malE-minE). The only complete gene contained in the plasmid insert was ydeA. YdeA is located distally in an operon also containing yneJ (a member of the lysR family) and yneK, of unknown function. In the pMesBA plasmids, transcription of ydeA may occur from the 5′ end of the operon and from a weak promoter in the yneK-ydeA intergenic region (3). In pJPB274, transcription can take place from the ydeA promoter and from resistance gene aadA in the vector.
The results indicating that ydeA excludes l-Ara prompted us to determine whether the suppression of lac-dependent filamentation by Φ(malE-minE) could be due to inducer exclusion. To test this possibility, strain JS219 was transformed with pJPB274, resulting in JS1921. Then, strains JS219 and JS1921, which express ydeA at normal and elevated levels, respectively, as well as JS1910 (ydeA::mini-tet) were transformed with pMLB1115, a pBR322 derivative carrying lacIq, the lac regulatory region, and lacZ (5). The strains were compared for lacZ induction in the presence of different extracellular concentrations of IPTG (Table 2). The presence of the ydeA-overexpressing plasmid led to a significant reduction in lac activity at low or intermediate concentrations of inducer. These data suggest that ydeA is capable of also excluding IPTG and that this accounts for the MalE-MinE suppression phenotype. However, this activity seems to be weak compared to l-Ara exclusion, since no IPTG exclusion effect was observed with the wild-type strain compared to the ydeA deletion strain (Table 2).
TABLE 2.
Induction of the lac promoter in different ydeA contexts
| Strain and ydeA status | LacZ activity (U/OD600) with indicated IPTG concn (μM)a
|
||
|---|---|---|---|
| 10 | 50 | 2,000 | |
| JS1910/pMLB1115 ydeA::mini-tet | 30 | 439 | 7,854 |
| JS219/pMLB1115 ydeA+ | 37 | 602 | 9,325 |
| JS219/pJPB274/pMLB1115 ydeA+; p(ydeA+) | 8 | 70 | 5,772 |
Values are the averages of two independent experiments.
Comparisons of sequences (13) and of hydropathy profiles (11) have revealed that YdeA belongs to a defined subfamily among the 12-transmembrane segment (12-TMS) efflux proteins. Paulsen et al. (13) defined it as subgroup d, which includes S. lividans cml, Pseudomonas aeruginosa cmlB and opdE, Bacillus subtilis ywfA, and Synechocystis sp. open reading frame Slr0616 gene products. Lolkema and Slotboom (11) proposed a subfamily including the products of araJ, ydhP, ydeA, and yicM of E. coli and of ybcL, ydhL, ytbD, and yfhI of B. subtilis. A BLAST search with YdeA as the query sequence indicated that except for Slr0616, all these proteins have Expect values (number of expected matches by chance) of <10−20.
Only a few proteins of this group have a known function. cml and cmlA confer resistance to chloramphenicol. We compared the wild-type, deletion, and YdeA-overproducing strains for their resistance to chloramphenicol. No difference was observed. Another gene of the subfamily, araJ, is positively regulated by araC and arabinose. Reeder and Schleif proposed that AraJ might be a transporter for arabinose-containing oligosaccharides (17). The resemblance of the ydeA gene product to AraJ suggested that the actual substrates for YdeA might be arabinose-containing antibiotics. To examine this possibility, strains JS1910, JS219, and JS1921 were transformed with plasmid pUT-DCK, a pBR322 derivative containing the gene for human deoxycytidine kinase constitutively expressed from a strong synthetic promoter. This allowed phosphorylation of cytidine derivatives and made the cells sensitive to cytosine arabinoside (cytarabin, Ara-C) (4). Then the three derived strains were compared for susceptibility to Ara-C in Luria broth. In each case, the transition between normal cell morphology and a mixture of normal cells and filaments appeared between 10 and 20 μM Ara-C. Therefore, ydeA does not appear to confer resistance to arabinosyl nucleoside compounds.
Recently, Bohn and Bouloc reported that another MFS gene, cmlA/mdfA, was also capable of expelling IPTG from the cells (2). cmlA was first shown to confer resistance to chloramphenicol in E. coli and then to a variety of drugs, such as ethidium bromide, tetraphenylphosphonium, rhodamine, daunomycin, and puromycin (6). These findings suggest that the substrates most efficiently transported by YdeA may have only a remote relationship with the two compounds identified in this study.
ADDENDUM
After this article was submitted for publication, Bost et al. (3) reported that YdeA interferes with the accumulation of l-Ara and thus with the induction of the PBAD promoter.
Acknowledgments
We thank David Lane (Toulouse) for a gift of pDAG92 and Jean-Paul Reynes (St. Cayla, Toulouse) for providing plasmid pUT-DCK.
REFERENCES
- 1.Arigoni F, Talabot F, Peitsch M, Edgerton M D, Meldrum E, Allet E, Fish R, Jamotte T, Curchod M L, Loferer H. A genome-based approach for the identification of essential bacterial genes. Nat Biotech. 1998;16:851–856. doi: 10.1038/nbt0998-851. [DOI] [PubMed] [Google Scholar]
- 1a.Blattner, F. R. 2 September 1997, revision date. Escherichia coli K-12 sequence, version M52. Department of Genetics, University of Wisconsin-Madison. http://www.genetics. wisc. edu. [25 June 1999, last accessed.]
- 2.Bohn C, Bouloc P. The Escherichia coli clmA gene encodes the multidrug efflux pump Cmr/MdfA and is responsible for isopropyl-β-d-thiogalactopyranoside exclusion and spectinomycin sensitivity. J Bacteriol. 1998;180:6072–6075. doi: 10.1128/jb.180.22.6072-6075.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bost S, Silva F, Belin D. Transcriptional activation of ydeA, which encodes a member of the major facilitator superfamily, interferes with arabinose accumulation and induction of the Escherichia coli arabinose PBAD promoter. J Bacteriol. 1999;181:2185–2191. doi: 10.1128/jb.181.7.2185-2191.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouzon M, Marlière P. Human deoxycytidine kinase as a conditional mutator in Escherichia coli. C R Acad Sci III. 1997;320:427–434. doi: 10.1016/s0764-4469(97)81969-5. [DOI] [PubMed] [Google Scholar]
- 5.de Boer P A J, Crossley R E, Rothfield L I. A division inhibitor and a topological specificity factor coded by the minicell locus determine proper placement of the division septum in E. coli. Cell. 1989;56:641–649. doi: 10.1016/0092-8674(89)90586-2. [DOI] [PubMed] [Google Scholar]
- 6.Edgar R, Bibi E. MdfA, an Escherichia coli multidrug resistance protein with an extraordinary broad spectrum of drug recognition. J Bacteriol. 1997;179:2274–2280. doi: 10.1128/jb.179.7.2274-2280.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guzman L-M, Belin D, Carson M J, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohara Y, Akiyama K, Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987;50:495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- 9.Kolodrubetz D, Schleif R. l-arabinose transport systems in Escherichia coli. J Bacteriol. 1981;148:472–479. doi: 10.1128/jb.148.2.472-479.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis K. Multidrug resistance in bacteria: variations on a theme. Trends Biochem Sci. 1994;19:119–123. doi: 10.1016/0968-0004(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 11.Lolkema J S, Slotboom D-J. Hydropathy profile alignment: a tool to search for structural homologues of membrane proteins. FEMS Microbiol Rev. 1998;22:305–322. doi: 10.1111/j.1574-6976.1998.tb00372.x. [DOI] [PubMed] [Google Scholar]
- 12.Miller J. A short course in bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 13.Paulsen I T, Brown M H, Skurray R A. Proton-dependent multidrug efflux systems. Microbiol Rev. 1996;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paulsen I T, Sliwinski M K, Saier M H., Jr Microbial genome analyses: global comparisons of transport capabilities based on phylogenies, bioenergetics and substrate specificities. J Mol Biol. 1998;277:573–592. doi: 10.1006/jmbi.1998.1609. [DOI] [PubMed] [Google Scholar]
- 15.Pichoff S, Vollrath B, Bouché J-P. MinCD-independent inhibition of cell division by a protein that fuses MalE to the topological specificity factor MinE. J Bacteriol. 1997;179:4616–4619. doi: 10.1128/jb.179.14.4616-4619.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pichoff S, Alibaud L, Guédant A, Castagné M P, Bouché J P. An E. coli gene (yaeO) suppresses temperature-sensitive mutations in essential genes by modulating Rho-dependent transcription termination. Mol Microbiol. 1998;29:859–870. doi: 10.1046/j.1365-2958.1998.00981.x. [DOI] [PubMed] [Google Scholar]
- 17.Reeder T, Schleif R. Mapping, sequence, and apparent lack of function of araJ, a gene of the Escherichia coli arabinose regulon. J Bacteriol. 1991;173:7765–7771. doi: 10.1128/jb.173.24.7765-7771.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudd K E. Linkage map of Escherichia coli K-12, edition 10: the physical map. Microbiol Mol Biol Rev. 1998;62:985–1019. doi: 10.1128/mmbr.62.3.985-1019.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Way J C, Davis M A, Morisato D, Roberts D E, Kleckner N. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene. 1984;32:369–379. doi: 10.1016/0378-1119(84)90012-x. [DOI] [PubMed] [Google Scholar]