Abstract
Whilst allowing for easy access to synthetically versatile motifs and for modification of bioactive molecules, the chemoselective benzylic oxidation reactions of functionalized alkyl arenes remain challenging. Reported in this study is a new non‐heme Mn catalyst stabilized by a bipiperidine‐based tetradentate ligand, which enables methylene oxidation of benzylic compounds by H2O2, showing high activity and excellent chemoselectivity under mild conditions. The protocol tolerates an unprecedentedly wide range of functional groups, including carboxylic acid and derivatives, ketone, cyano, azide, acetate, sulfonate, alkyne, amino acid, and amine units, thus providing a low‐cost, more sustainable and robust pathway for the facile synthesis of ketones, increase of complexity of organic molecules, and late‐stage modification of drugs.
Keywords: Benzylic Oxidation, Cyclic Imines, Ketones, Manganese Catalysts, Selective Oxidation
A new bipiperidine‐based manganese catalyst is introduced, which catalyzes the chemoselective benzylic oxidation of a wide range of diverse functionalized alkyl arenes with H2O2, affording various functionalized aryl ketones, cyclic imines, and bioactive molecules under mild conditions in a short time.

Introduction
Catalytic oxidation of C−H bonds is a one‐step transformation to access highly value‐added hydroxyl and carbonyl compounds. [1] Great strides have been made in understanding, controlling and expanding the scope of the reaction in recent years, thanks to the contributions of a number of research groups. [2] Selective benzylic C−H oxidation would allow direct access to alkyl aryl ketones, such as those bearing functional groups on the alkyl unit, which are ubiquitous in fine chemicals, natural products and pharmaceuticals. They are also used in the synthesis of a wide range of essential organic building blocks, such as amines, amino acids, lactones, and heterocyclic compounds (see Figures S‐1, S‐2 and S‐3 in the Supporting Information). Indeed, various oxidation methods have been developed to enable benzylic oxidation.[ 1b , 3 ] However, there remains a significant issue, i.e. low compatibility with functional groups, with few methods known that tolerate some of the most common functional groups in organic synthesis (Figure 1a).[ 1g , 1h , 1i , 2 , 3 ] Benzylic oxidation is traditionally performed with stoichiometric strong oxidants, such as CrO3, Na2CrO4, KMnO4, 2‐iodoxybenzoic acid and t BuOOH.[ 1g , 1h , 1i ] Apart from generating at least stoichiometric amounts of waste, these reagents are tarnished by the low tolerance of functional groups and they are mainly restricted to nonfunctionalized alkyl arenes.[ 1g , 1h , 1i , 2 , 3 ] In response to these challenges, benzylic oxidation with transition metal catalysis,[ 2b , 2d , 2e , 2f , 2g , 3 ] and more recently, organocatalysis,[ 2c , 4a ] electrochemistry,[ 2j , 4b ] and photoredox chemistry[ 2a , 4c ] has been actively pursued. Whilst these catalytic systems have advanced selective benzylic oxidation considerably, few of them have been demonstrated to tolerate a wide variety of functionalities on the alkyl side chains in benzylic compounds. [4] Notably, the group of Oisaki and Kanai reported a N‐oxyl radical‐catalyzed benzylic oxidation of side chains bearing various functional groups, [4d] and Sun and co‐workers reported a manganese catalyst that promotes benzylic oxidation of phenethyl acetate (Figure 1b). [4h] However, no catalytic systems are known of capable of benzylic oxidation of side chains bearing such important functionalities as alkyne, alkene, azide, amino acid, or free amine moieties.
Figure 1.

Catalytic benzylic methylene oxidation of alkyl chains bearing functional groups.
Being the most widely seen functionality in natural products and pharmaceuticals, amines draw particular attention. However, the nitrogen lone pair lowers the bond dissociation energy (BDE) of the neighboring C−H bond (c.f. BDE 98 for MeCH2 Me and 90 for MeCH2 NH2 kcal mol−1) [5] and is prone to coordinate to metal centers, complicating the chemoselectivity of oxidation while poisoning metal‐based catalysts.[ 4j , 4k , 6 ] Indeed, very few examples have been reported of benzylic oxidation of aryl aliphatic amines. By virtue of Brönsted acid protonation of or Lewis acid coordination to nitrogen, Sanford and co‐workers achieved an iron‐catalyzed remote benzylic oxidation with t BuOOH of a range of aliphatic tertiary amines, in which CF3CO2H was used to protonate the amines (Figure 1b). [4j] In a similar fashion, the groups of Du Bois and Sigman disclosed a ruthenium‐catalyzed benzylic oxidation of aliphatic primary amines which led to cyclic imines, with H5IO6 being the oxidant and CF3SO3H as an acid additive (Figure 1b). [4k] However, the use of a strong acid such as CF3SO3H and a strong oxidant like H5IO6 may limit the application of these methods. For instance, functionalities, such as nitriles, alkynes, esters, amides and the common protecting groups for amines, alcohols and amino acids, could undergo acid‐promoted decomposition. Aiming to expand the boundary of functional groups in benzylic oxidation, we have become interested in developing a more versatile, enabling catalytic system that is desirably also cheaper and cleaner.
In biological systems, selective C−H bond oxidation is primarily performed by high‐valent metal‐oxo species formed by oxygenases reacting with O2. [7] In pioneering studies, Que and co‐workers reported the non‐heme biomimetic Fe complex [Fe(TPA)(CH3CN)2]2+ (TPA=tris(2‐pyridylmethyl)amine), which catalyzes selective oxidation by H2O2, with water as the only byproduct. [8] This is followed by the advent of a series of highly efficient oxygenation catalysts based on Fe and Mn complexes bearing tetradentate amino ligands,[ 2d , 2f , 7d , 9 ] culminated by the novel bipyrrolidine‐bipyridine (BPBP) ligands discovered by the White group and further developed by the groups of White, Costas, and Bryliakov. [10] In 2012, Rybak‐Akimova and co‐workers reported a similar Fe‐PYBP complex, with the PYBP ligand featuring two pyridines bridged by a bipiperidine. The complex was shown to be highly active and selective in catalyzing olefin epoxidation with H2O2. [11] In our search for competent catalysts for selective C−H oxidation, we have found that the Mn complex of a similar ligand allows for highly selective benzylic C−H oxidation of arene side chains with H2O2, tolerating an unprecedentedly wide variety of functional groups and thus providing a more general method for benzylic oxidation (Figure 1c).
Results and Discussion
Reaction Development
In continuing our study of selective oxidation with catalysts and oxidants that respond to the Green Chemistry agenda, [12] we concentrated on searching for an able catalyst based on the cheap, biocompatible Fe or Mn for the selective oxidation of functionalized alkylarenes with H2O2 as oxidant, which would generate water as the only byproduct. Considering the importance of alkyl aryl ketones functionalized with a carboxylate group in synthesis (see Figure S‐3 for examples), we set out to investigate the oxidation of 4‐phenylbutanoic acid (2) as the model substrate to identify a possible catalyst. The results of examining the effect of various metal salts and ligands on the oxidation are summarized Table 1 (for more details, see Table S‐1 in the Supporting Information). As can be seen, the simple manganese salt, Mn(OTf)2 (2 mol %), showed no activity for the catalytic oxidation. Following on from the study of Rybak‐Akimova, [11] we envisaged that bipiperidine, which is readily accessible from the hydrogenation of bipyridine, could provide a rigid ligand backbone, and therefore synthesized the PYBP type ligand 1,1′‐bis((3,5‐dimethylpyridin‐2‐yl)methyl)‐2,2′‐bipiperidine L1 . The ligand exists as racemic and meso isomers, rac‐L1 and meso‐L1 , which could be separated into analytically pure forms by flash column chromatography (see Supporting Information for details). Delightfully, combining rac‐L1 with Mn(OTf)2 (1 : 1 ratio) afforded the ketone 2 a in an excellent yield of 90 %. In stark contrast, the analogous meso‐L1 was much less effective, as can be seen from Table 1. This is reminiscent of the observations made with other meso‐PYBP and meso‐BPBP ligands in Fe and Mn catalyzed oxidation reactions. [13]
Table 1.
Identification of catalyst for benzylic oxidation.

Aimed at increasing the catalyst activity further, we prepared the electronically and sterically varied analogues of rac‐L1 , i.e. R,R‐L2 , R,R‐L3 , R,R‐L4 , and R,R‐L5 , and examined their effect on the model oxidation, in addition to that of the BPBP ligands L6 and L7 . [14] As can be seen, none of them outperformed rac‐L1 , and in particular, whilst the electron‐donating methoxy substituent reduces the catalytic activity (entry 4), the electron‐withdrawing nitro group renders the catalyst much less active (entry 5). The reason as to why both substituents deactivate the catalyst is not immediately clear. Further screening of other ligands as well as the combination of rac‐L1 with other manganese or iron salts demonstrated that rac‐L1 combined with Mn(OTf)2 exhibits the best catalytic activity in the oxidation of 2 with H2O2 (see Table S‐1 in the Supporting Information). The reaction time could be shortened from 5 h to 1 h by adding 5 equivalents of acetic acid without affecting the yield of 2 a (entry 11). Carboxylic acids are known to promote manganese‐catalyzed oxidation.[ 7d , 9 , 10 , 11 ]
Reacting rac‐L1 and meso‐L1 with Mn(OTf)2 led to the complexes rac‐1 and meso‐1, respectively (Figure 2a). The structures of rac‐1 and the aqua derivatives of rac‐1 and meso‐1 resulting from substitution of the triflate anion with water have been determined by X‐ray diffraction (see Supporting Information for details). [15] The latter two exhibit very similar M−L bond lengths and coordination geometries, and both show a distorted octahedral cis‐α geometry (Figure 2b). The complex [rac‐L1 Mn(H2O)2]2+ is approximately C 2 symmetric, in which both piperidine rings exhibit chair conformation, while in the meso‐analogue one piperidine ring has a chair and the other a boat conformation. The axial Mn−N distances are very similar in both aqua complexes measuring between 2.24 and 2.26 Å. The equatorial Mn−N distances in [rac‐L1 Mn(H2O)2]2+ are virtually identical, while in [meso‐L1 Mn(H2O)2]2+ they are slightly longer to the ligand N‐atom that is part of the boat‐configured piperidine ring. The boat conformation indicates that the meso‐ligand is under some strain. The metal coordination forces the C−N bond that is both part of the ligand backbone and the piperidine ring into an eclipsed conformation which imposes the boat shape.
Figure 2.

Formation of rac‐1 and meso‐1 and the X‐ray structures of rac‐1 and the cations [L1 Mn(H2O)2]2+ of rac‐1 and meso‐1. Selected bond lengths [Å] for rac‐1: Mn1−N1 2.237(2), Mn1−N3 2.260(2), Mn1−N2 2.284(2), Mn1−N4 2.299(2), Mn1−O1 2.131(2), Mn1−O4 2.140(2); for [rac‐L1 Mn(H2O)2]2+: Mn1−N1 2.2528(19), Mn1−N2 2.2979(18), Mn1−N3 2.2412(19), Mn1−N4 2.2999(18), Mn1−O1 2.1624(17), Mn1−O2 2.1644(18); for [meso‐L1 Mn(H2O)2]2+: Mn1−N1 2.244(12), Mn1−N2 2.281(15), Mn1−N3 2.243(12), Mn1−N4 2.309(12), Mn1−O1 2.13(2), Mn1−O2 2.222(18). Hydrogen atoms have been omitted for clarity. See the Supporting Information for more details.
The isolated rac‐1 and meso‐1 displayed a similar activity to that prepared in situ (Table 1, entries 2 vs 12, and entries 3 vs 13). Further screening led to the optimized oxidation conditions as: rac‐1 (2 mol %) being the catalyst, H2O2 (5 equiv) as the oxidant, acetic acid (5 equiv) as an additive in CH3CN at room temperature, and 1 h reaction time (see Table S‐2). Under the optimized conditions, 2 was oxidized to 2 a with a high yield of 96 % (entry 12).
The superior activity of rac‐1 to meso‐1 is further illustrated by the kinetic profiles observed in the oxidation of ethyl benzene under the optimized conditions (Figure 3). While rac‐1 catalyzed fast conversion of ethyl benzene to acetophenone with t 1/2<20 min, meso‐1 showed only insignificant activity throughout the course of the reaction. The color change is also indicative. The rac‐1 mediated reaction turned to reddish almost immediately upon introduction of H2O2, whereas the meso‐1 reaction remained almost colorless throughout, suggesting inability to activate H2O2. This activity difference is in stark contrast to the structural similarity seen in the two complexes. We also compared the activity of rac‐1 with two well‐known manganese oxidation catalysts[ 4h , 9b , 10 ] in the benzylic oxidation of alkyl chains with a range of functional groups. It is remarkable that in all the cases examined, rac‐1 afforded significantly higher product yields (see Table S‐6 in Supporting Information).
Figure 3.

The time course of rac‐1 and meso‐1 catalyzed benzylic oxidation of ethyl benzene under the standard conditions.
Scope of Reaction
Benzylic Oxidation to Access Functionalized Arene Ketones
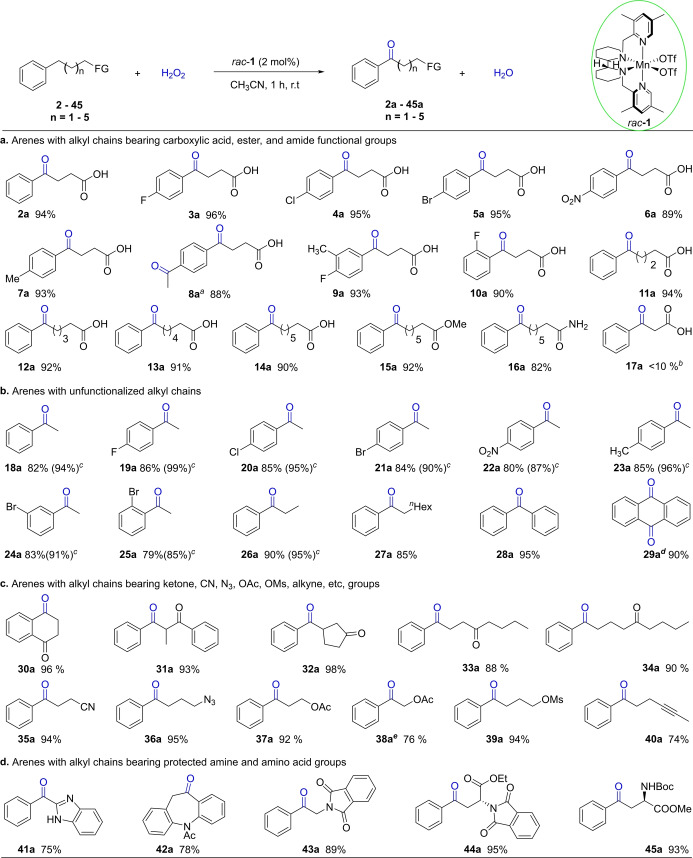
Under the optimized reaction conditions, the catalytic system proved to be generally effective for the selective benzylic oxidation of various aryl alkanoic acids to aryl ketone acids (Figure 4a). Using 4‐phenylbutanoic acid as a reference, the effect of substituent group at the phenyl ring was examined. As is clear, the protocol tolerates both electron‐donating (p‐Me) and electron‐withdrawing (p‐F, p‐Cl, p‐Br, p‐NO2) substituents of high Hammett constant (σp=0.78, NO2), affording the aryl ketone acids with excellent yields (2 a–9 a). Note that on replacing p‐Me with p‐Et on the phenyl ring, both benzylic sites of substrate 8 were oxidized to give the diketone 8 a with 88 % yield when more H2O2 was used (8 equiv). meta‐Substitution has no notable effect on the product yield (9 a), and the same is true with ortho‐fluoro substitution (10 a).
Figure 4.
Substrate scope of the rac‐1 catalyzed benzylic oxidation of alkylarenes. General reaction conditions: substrate (0.5 mmol), rac‐1 (2 mol %), and AcOH (2.5 mmol) were dissolved in MeCN (1.5 mL), and then H2O2 (2.5 mmol) in 1 mL of MeCN was introduced with a syringe pump over 1 h under stirring at room temperature without nitrogen protection. Isolated yield reported; [a] H2O2 (4 mmol); [b] 1H NMR yield; [c] GC yield in parentheses; [d] 2 mol % of rac‐1 at the beginning followed by another 2 mol % 20 minutes later; H2O2 (4 mmol) in 1 mL of MeCN delivered with a syringe pump over 1 h; [e] AcOH (7.5 mmol, 15 equiv).
Considering the electron‐withdrawing effect of the carboxylic acid unit and its potential coordination to the metal center, both of which could affect the benzylic oxidation, the impact of the alkyl chain length was examined. As demonstrated by the high yields of 11 a–14 a, separating the phenyl ring from the carboxylic acid with longer alkyl chains had little effect on the efficacy of the oxidation. Furthermore, the aryl acid derivatives, an aryl ketone ester 15 a and amide 16 a, were also obtained in good yield. These observations appear to indicate that the acid functionality has no directing or promoting effect on the oxidation under the conditions employed, where excess AcOH was present. [16] However, the shorter chain substrate 3‐phenylpropanoic acid 17 showed only a low activity (<10 % yield of 17 a). This could result from a deactivating effect of the electron‐withdrawing carboxyl group [2g] and/or product chelation to the metal center which inhibits the catalytic turnover. In support of the hypothesis on chelation, addition of 2‐oxo‐2‐phenylacetic acid to the model reaction reduced the yield of 2 a to 50 % (cf. entry 24, Table S‐2).
As maybe expected, alkylbenzenes without the acid functionality are viable (Figure 4b). Thus, ethyl benzene and its derivatives containing both electron‐donating and withdrawing substituents were oxidized, leading to the aryl ketones with excellent yields (18 a–25 a). Note that the large ortho‐bromo moiety does not appear to hinder the oxidation of 25. Subsequently, phenyl rings bearing longer alkyl chains (26, 27) and a benzyl group (28) were oxidized to phenyl ketones (26 a–28 a) in high yields under the standard conditions. Anthraquinone (29 a), an important industrial organic chemical, was obtained in 90 % yield, albeit with a higher catalyst loading of 4 mol %.
To probe further the functional group tolerance of the protocol enabled by rac‐1, we examined alkylarenes containing a range of diverse functionalities in the alkyl chain (Figure 4c). As can be seen, substrates bearing ketone (30–34), nitrile (35), azide (36), OAc (37, 38), OMs (39) and alkyne (40) units all underwent the benzylic oxidation, affording ketones in high yields in general. The lower yield of 38 a is probably again due to a deactivating and/or coordinating effect of the acetate unit. In the case of 40 a, the lower yield results partially from the oxidation of the alkyne moiety.
A still further demonstration of the applicability of the protocol is seen in the selective benzylic oxidation of compounds having protected amino groups (Figure 4d). Thus, under the optimized conditions, 2‐benzyl‐1H‐1,3‐benzodiazole (41) was converted to the corresponding ketone (41 a) in 75 % yield, and oxidation of 42 yielded an analogue of oxcarbazepine (42 a), a drug used to treat epilepsy and bipolar disorder, in 78 % yield. Notably, phthalimide 43 was oxidized to 43 a in 89 % yield, in which the relatively bulky, potentially coordinating phthalimide neighbors the newly formed ketone. In addition, the protected amino acids 44 and 45 were both oxyfunctionalized with excellent yield via benzylic oxidation. Further examination of the oxidation of 45 shows the reaction to be highly site selective, with the α−C−H bond of the amino ester unit remaining intact, as revealed by the full retention of its stereochemistry (Figure 5). With other methods, such bonds could be more prone to react. [17] Interestingly, the enantiomerically pure catalysts, (R,R)‐1 and (S,S)‐1 prepared from the enantiomerically pure L1 , showed little kinetic differentiation in oxidizing 45, as evidenced by the time‐yield data given in Figure 5.
Figure 5.

Kinetic follow of the oxidation of a chiral substrate with chiral catalysts. Reaction conditions were the same as the general conditions given in Figure 4; [a] (R,R)‐1 as catalyst, 1 h, with ee measured by chiral HPLC and isolated yield given. [b] 1H NMR yield.
The survival of the various functional groups in the oxidation above is remarkable, demonstrating the rac‐1 enabled protocol to be highly tolerate of functionalities. It is noted that these functionalities are characterized by widely differing electronic and steric properties and sensitivity to redox reactions, acids and aqueous conditions, as highlighted by the highly electron‐withdrawing ketone and nitrile moieties and highly reactive azide and alkyne groups. The presence of a second functionality in the ketone products makes the ketones synthetically much more useful; a number of bioactive molecules can be derived from these products (see Supporting Information, Figure S‐2). As far as we are aware, there appear to be only a few reports on the oxidation of alkylarenes with the alkyl chain functionalized with a nitrile, an acetoxy, or a mesylate moiety,[ 4d , 4f , 4h ] and no study is known of those bearing an azide or alkyne group. [18]
Benzylic Oxidation of Primary Amines to Access Cyclic Imines
Prompted by the importance of and challenges in amine oxidation, we went on to explore the benzylic oxidation of alkylarenes bearing an aliphatic amine side chain under the catalysis of rac‐1 (Figure 6). Delightedly, with 4‐phenylbutan‐1‐amine (46) as a model substrate, the cyclic imine 46 a, formed clearly via the ketone intermediate resulting from benzylic oxidation, was obtained in 23 % yield under the standard conditions. Increasing the amount of catalyst loading from 2 mol % to 3 mol %, the yield increased to 35 %. Bearing in mind the benefiting effect of acids demonstrated in previous studies of amine oxidation,[ 4j , 4k ] we varied the amount of the acid. Remarkably, when the oxidation was conducted in a mixed solvent of AcOH and MeCN (1 : 1 volume), which did not decompose the catalyst, the starting material was completely transformed to the product 46 a with an excellent isolated yield of 93 % (see Table S‐3 in the Supporting Information). Using an analogous Fe‐BPBP catalyst for a similar oxidation, Sanford and co‐workers noticed only substrate decomposition in the presence of CF3CO2H and no formation of the desired amino ketone product. [4j]
Figure 6.
Substrate scope of rac‐1 catalyzed benzylic oxidation of alkylarenes containing primary amines. General reaction conditions: substrate (0.5 mmol) and rac‐1 (3 mol %) were dissolved in AcOH (1.5 mL), and then H2O2 (2.5 mmol) in 1.5 mL of MeCN was introduced with a syringe pump over 1 h under stirring at room temperature without nitrogen protection. Isolated yield reported.
Under the newly optimized conditions, rac‐1 was shown to be effective for the selective benzylic oxidation of a wide range of aliphatic amines, affording, in one step, synthetically highly valuable five and six‐membered cyclic imines (Figure 6). Thus, starting with 4‐arylbutan‐1‐amines, the 5‐memebred dihydropyrroles were obtained in over 90 % yields, regardless of the 4‐substituent on the phenyl ring being electron‐withdrawing (p‐F, p‐Cl, p‐Br) or electron‐donating (p‐Me) (46 a–51 a). The utility of the protocol was further explored by installing substituents α or β to the amino unit; this would lead to value‐added multisubstituted pyrrolidines. Pleasingly, the protocol tolerates a range of sterically and electronically varied alkyl and aryl substituents, as showcased by the imine products 5‐phenyl‐2‐alkyl (52 a–60 a) and 5‐phenyl‐2‐aryl (61 a–66 a) dihydropyrroles. Of particular note is that amines bearing the sterically demanding 2‐ t Bu and 2‐(o‐tolyl) moieties are viable, although the product yields are lower. Equally significant is the synthesis of 2‐aryl‐4,4‐dimenthyl substituted five as well as six‐membered cyclic imines (67 a–71 a,72 a–75 a) in high yields. An easy one‐step reaction, i.e. hydrogenation, would convert these N‐heterocycles into highly sought‐after pyrrolidines and piperidines, the most important heterocycles seen in top‐selling drugs. [19]
Application in the Synthesis of Drug and Bioactive Molecules
The high reactivity and chemoselectivity displayed by rac‐1 for benzylic methylene oxidations in the presence of pharmaceutically relevant arenes and aminoalkanes provide an opportunity to effect late‐stage oxyfunctionalization of drug or other bioactive molecules. As an example, the drug molecule haloperidol (76 a), a nonselective D4 antagonist, [20] was obtained in 90 % yield from the readily available precursor 76, when HOAc was replaced with 10 equiv of a stronger acid, ClCH2CO2H (Figure 7). Under the standard conditions (see Figure 6), a much lower conversion of 76 (<10 %) was observed, however. The analogous dehydroxylated 77 a–83 a were obtained in high yields similarly. Note that in these tertiary amino substrates, there are multiple sites that are susceptible to oxidation, i.e. the tertiary benzylic C−H bond and the secondary C−H bonds α to the nitrogen. The oxidation of 81 with t BuOOH (18 equiv) catalyzed by FeCl3 has been reported by Sanford and co‐workers; but the yield was lower (41 %). [4j] The isolation of 77 a–83 a in high yields demonstrates the high chemoselectivity of rac‐1.
Figure 7.
Oxyfunctionalization of bioactive and drug molecules via rac‐1 catalyzed benzylic oxidation. General reaction conditions: substrate (0.25 mmol), rac‐1 (3 mol %), and ClCH2COOH (2.5 mmol) were dissolved in MeCN (1.0 mL), and then H2O2 (1.25 mmol) in 1 mL of MeCN was introduced with a syringe pump over 1 h under stirring at room temperature without nitrogen protection. Isolated yield reported. [a] 2 mol % rac‐1 at the beginning followed by another 2 mol % 20 minutes later; H2O2 (2 mmol) in 1 mL of MeCN delivered with a syringe pump over 1 h; [b] 85 (3.1 mmol, 1.0 g); [c] reaction conditions were the same as the general conditions given in Figure 6.
Where there are two benzylic sites, double oxidation takes place, as in the case of dihydroanthracene (29, Figure 4). This feature was taken advantage of in the synthesis of the drug lenperone (84 a), an antipsychotic, and an analogue 85 a, with both compounds obtained in high yields. Although 84 could be oxidized to 84 a with t BuOOH under FeCl3 catalysis [4j] or with free radical‐enabled molecular oxygen, [4i] the reaction was less efficient and featured harsher conditions (54 equiv oxidant, 5 days, 26 % yield in the former case; 90 °C, 36 h, 62 % yield in the latter). Oxidation of a secondary amine, tetrahydrobenzazepine (86), was also possible, affording in good yield the tetrahydrobenzazepinone 86 a, a building block for the synthesis of a respiratory syncytial virus fusion inhibitor (Figure 7c). [21] Note that the oxidation took place remotely to the amino moiety; this may stem from an acid‐induced deactivation of the benzylic C−H bond α to nitrogen via protonation. [6f] Furthermore, the protocol is shown to be highly efficient in the selective oxidation of a more complex steroid molecule, an estrone derivative 87, converting it to a ketone product 87 a with 88 % yield under the standard conditions (Figure 7d). In addition to the piperidine‐type tertiary amine substrates shown in Figure 7a, tertiary amines featuring pyrrolidine (88) and benzyl (89) units were also demonstrated to be viable. Oxidation of both substrates afforded the benzylic oxidation products in high yields (Figure 7e).
To further demonstrate the synthetic utility of rac‐1, the substrate 85 was subjected to a gram scale reaction. As shown in Figure 7b, the corresponding product 85 a was obtained in 79 % isolated yield within a 1 h reaction. The somewhat lowered yield is due to the oxidation being incomplete in 1 h, with some 85 remaining in the reaction mixture.
Conclusion
Green, selective oxidation of C−H bonds is one of the most significant challenges facing organic synthesis. The key to addressing the challenge lies in developing able catalysts. The rac‐1 complex described in this paper contributes to widening the scope of benzylic oxidation with a benign oxidant, H2O2, showing an unprecedented level of functional group tolerance. As such, functionalized aryl ketones, cyclic imines, and modified bioactive molecules can be reached in a single step of oxidation, with no toxic waste generated. Carboxylic acid‐assisted mechanisms of benzylic oxidation with PYBP type ligands have been well studied by several groups.[ 9a , 22 ] A similar reaction pathway involving active MnV=O species may well follow by the rac‐1 catalyst.[ 22c , 22d , 22e , 22f , 22g , 22h ] Although the full mechanistic details, including the cause of the remarkable difference between rac‐1 and meso‐1, remain to be delineated, we anticipate that the rac‐1/H2O2 system will find applications in selective oxidation of C−H bonds and in the synthesis of complex organic chemicals.
Conflict of interest
The authors declare no conflict of interest.
1.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Acknowledgements
The authors are grateful for the financial support of the Fundamental Research Funds for the National Natural Science Foundation of China (21971156), the Shaanxi Provincial Natural Science Foundation (2020JM267), the Central University (GK202002009), the start‐up funds from Shaanxi Normal University, and the 111 project (B14041). We also thank the EPSRC (EP/R009694/1) for funding.
J. Zhou, M. Jia, M. Song, Z. Huang, A. Steiner, Q. An, J. Ma, Z. Guo, Q. Zhang, H. Sun, C. Robertson, J. Bacsa, J. Xiao, C. Li, Angew. Chem. Int. Ed. 2022, 61, e202205983; Angew. Chem. 2022, 134, e202205983.
Contributor Information
Prof. Dr. Jianliang Xiao, Email: jxiao@liverpool.ac.uk.
Prof. Dr. Chaoqun Li, Email: lichaoqun@snnu.edu.cn.
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
References
- 1.
- 1a. Godula K., Sames D., Science 2006, 312, 67–72; [DOI] [PubMed] [Google Scholar]
- 1b. Bergman R. G., Nature 2007, 446, 391–393; [DOI] [PubMed] [Google Scholar]
- 1c. L. Que, Jr. , Tolman W. B., Nature 2008, 455, 333–340; [DOI] [PubMed] [Google Scholar]
- 1d. Newhouse T., Baran P. S., Angew. Chem. Int. Ed. 2011, 50, 3362–3374; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 3422–3435; [Google Scholar]
- 1e. Cernak T., Dykstra K. D., Tyagarajan S., Vachal P., Krska S. W., Chem. Soc. Rev. 2016, 45, 546–576; [DOI] [PubMed] [Google Scholar]
- 1f. Börgel J., Ritter T., Chem 2020, 6, 1877–1887; [Google Scholar]
- 1g. Bäckvall J. E., Modern Oxidation Methods, Wiley-VCH, Weinheim, 2010; [Google Scholar]
- 1h. Muñiz K., Catalytic Oxidation in Organic Synthesis, Thieme, Stuttgart, 2018; [Google Scholar]
- 1i. Jiao N., Stahl S. S., Green Oxidation in Organic Synthesis, Wiley-VCH, Weinheim, 2019. [Google Scholar]
- 2.For recent reviews, see:
- 2a. Romero N. A., Nicewicz D. A., Chem. Rev. 2016, 116, 10075–10166; [DOI] [PubMed] [Google Scholar]
- 2b. Liang Y. F., Jiao N., Acc. Chem. Res. 2017, 50, 1640–1653; [DOI] [PubMed] [Google Scholar]
- 2c. Qin Y., Zhu L., Luo S., Chem. Rev. 2017, 117, 9433–9520; [DOI] [PubMed] [Google Scholar]
- 2d. Bryliakov K. P., Chem. Rev. 2017, 117, 11406–11459; [DOI] [PubMed] [Google Scholar]
- 2e. Huang X., Groves J. T., Chem. Rev. 2018, 118, 2491–2553; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2f. White M. C., Zhao J., J. Am. Chem. Soc. 2018, 140, 13988–14009; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2g. Milan M., Salamone M., Costas M., Bietti M., Acc. Chem. Res. 2018, 51, 1984–1995; [DOI] [PubMed] [Google Scholar]
- 2h. Sterckx H., Morel B., Maes B. U. W., Angew. Chem. Int. Ed. 2019, 58, 7946–7970; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 8028–8055; [Google Scholar]
- 2i. Chakrabarty S., Wang Y., Perkins J. C., Narayan A. R. H., Chem. Soc. Rev. 2020, 49, 8137–8155; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2j. Wang F., Stahl S. S., Acc. Chem. Res. 2020, 53, 561–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.For recent reviews on benzylic oxidation, see:
- 3a. Chen K., Zhang P., Wang Y., Li H., Green Chem. 2014, 16, 2344–2374; [Google Scholar]
- 3b. Urgoitia G., SanMartin R., Herrero M. T., Dominguez E., Catalysts 2018, 8, 640–660; [Google Scholar]
- 3c. Shen H. M., Liu L., Qi B., Hu M. Y., Ye H. L., She Y. B., J. Mol. Catal. 2020, 493, 111102. [Google Scholar]
- 4.
- 4a. Yoshino Y., Hayashi Y., Iwahama T., Sakaguchi S., Ishii Y., J. Org. Chem. 1997, 62, 6810–6813; [Google Scholar]
- 4b. Kawamata Y., Yan M., Liu Z., Bao D. H., Chen J., Starr J. T., Baran P. S., J. Am. Chem. Soc. 2017, 139, 7448–7451; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4c. Mühldorf B., Wolf R., Angew. Chem. Int. Ed. 2016, 55, 427–430; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 437–441; [Google Scholar]
- 4d. Kadoh Y., Oisaki K., Kanai M., Chem. Pharm. Bull. 2016, 64, 737–753; [DOI] [PubMed] [Google Scholar]
- 4e. Zhu X., Liu Y., Liu C., Yang H., Fu H., Green Chem. 2020, 22, 4357–4363; [Google Scholar]
- 4f. Moriyama K., Takemura M., Togo H., Org. Lett. 2012, 14, 2414–2417; [DOI] [PubMed] [Google Scholar]
- 4g. Lesieur M., Genicot C., Pasau P., Org. Lett. 2018, 20, 1987–1990; [DOI] [PubMed] [Google Scholar]
- 4h. Shen D., Miao C., Wang S., Xia C., Sun W., Org. Lett. 2014, 16, 1108–1111; [DOI] [PubMed] [Google Scholar]
- 4i. Liu J., Hu K. F., Qu J. P., Kang Y. B., Org. Lett. 2017, 19, 5593–5596; [DOI] [PubMed] [Google Scholar]
- 4j. Mbofana C. T., Chong E., Lawniczak J., Sanford M. S., Org. Lett. 2016, 18, 4258–4261; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4k. Mack J. B. C., Gipson J. D., Du Bois J., Sigman M. S., J. Am. Chem. Soc. 2017, 139, 9503–9506; [DOI] [PubMed] [Google Scholar]
- 4l. Ottenbacher R. V., Talsi E. P., Rybalova T. V., Bryliakov K. P., ChemCatChem 2018, 10, 5323–5330; [Google Scholar]
- 4m. Wang B., Lin J., Sun Q., Xia C., Sun W., ACS Catal. 2021, 11, 10964–10973. [Google Scholar]
- 5. Luo Y. R., Comprehensive Handbook of Chemical Bond Energies, CRC, Boca Raton, 2007. [Google Scholar]
- 6.
- 6a. Asensio G., Gonzalez-Nunez M. E., Bernardini C. B., Mello R., Adam W., J. Am. Chem. Soc. 1993, 115, 7250–7253; [Google Scholar]
- 6b. Lee M., Sanford M. S., J. Am. Chem. Soc. 2015, 137, 12796–12799; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6c. Howell J. M., Feng K., Clark J. R., Trzepkowski L. J., White M. C., J. Am. Chem. Soc. 2015, 137, 14590–14593; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6d. Schultz D. M., Lévesque F., DiRocco D. A., Reibarkh M., Ji Y., Joyce L. A., Dropinski J. F., Sheng H., Sherry B. D., Davies I. W., Angew. Chem. Int. Ed. 2017, 56, 15274–15278; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 15476–15480; [Google Scholar]
- 6e. Olivo G., Farinelli G., Barbieri A., Lanzalunga O., Di Stefano S., Costas M., Angew. Chem. Int. Ed. 2017, 56, 16347–16351; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 16565–16569; [Google Scholar]
- 6f. Bietti M., Lanzalunga O., Lapi A., Martin T., Mazzonna M., Polin M., Salamone M., J. Org. Chem. 2017, 82, 5761–5768; [DOI] [PubMed] [Google Scholar]
- 6g. Bietti M., Angew. Chem. Int. Ed. 2018, 57, 16618–16637; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 16858–16878. [Google Scholar]
- 7.
- 7a. Costas M., Mehn M. P., Jensen M. P., L. Que, Jr. , Chem. Rev. 2004, 104, 939–986; [DOI] [PubMed] [Google Scholar]
- 7b. Ortiz de Montellano P. R., Chem. Rev. 2010, 110, 932–948; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7c. Solomon E. I., Heppner D. E., Johnston E. M., Ginsbach J. W., Cirera J., Qayyum M., Kieber-Emmons M. T., Kjaergaard C. H., Hadt R. G., Tian L., Chem. Rev. 2014, 114, 3659–3853; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7d. Guo M., Corona T., Ray K., Nam W., ACS Cent. Sci. 2019, 5, 13–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.
- 8a. Chen K., L. Que, Jr. , J. Am. Chem. Soc. 2001, 123, 6327–6337; [DOI] [PubMed] [Google Scholar]
- 8b. Chen K., Costas M., Kim J., Tipton A. K., L. Que, Jr. , J. Am. Chem. Soc. 2002, 124, 3026–3035. [DOI] [PubMed] [Google Scholar]
- 9.
- 9a. Olivo G., Cussó O., Borrell M., Costas M., JBIC J. Biol. Inorg. Chem. 2017, 22, 425–452; [DOI] [PubMed] [Google Scholar]
- 9b. Vicens L., Olivo G., Costas M., ACS Catal. 2020, 10, 8611–8631; [Google Scholar]
- 9c. Masferrer-Rius E., Borrell M., Lutz M., Costas M., Klein Gebbink R. J. M., Adv. Synth. Catal. 2021, 363, 3783–3795; [Google Scholar]
- 9d. Vicens L., Bietti M., Costas M., Angew. Chem. Int. Ed. 2021, 60, 4740–4746; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2021, 133, 4790–4796. [Google Scholar]
- 10.
- 10a. Chen M. S., White M. C., Science 2007, 318, 783–787; [DOI] [PubMed] [Google Scholar]
- 10b. Lyakin O. Y., Ottenbacher R. V., Bryliakov K. P., Talsi E. P., ACS Catal. 2012, 2, 1196–1202; [Google Scholar]
- 10c. Cussó O., Garcia-Bosch I., Font D., Ribas X., Lloret-Fillol J., Costas M., Org. Lett. 2013, 15, 6158–6161. [DOI] [PubMed] [Google Scholar]
- 11. Mikhalyova E. A., Makhlynets O. V., Palluccio T. D., Filatov A. S., Rybak-Akimova E. V., Chem. Commun. 2012, 48, 687–689. [DOI] [PubMed] [Google Scholar]
- 12.
- 12a. Gonzalez-de-Castro A., Robertson C. M., Xiao J., J. Am. Chem. Soc. 2014, 136, 8350–8360; [DOI] [PubMed] [Google Scholar]
- 12b. Gonzalez-de-Castro A., Xiao J., J. Am. Chem. Soc. 2015, 137, 8206–8218; [DOI] [PubMed] [Google Scholar]
- 12c. Liu Y., Wang C., Xue D., Xiao M., Li C., Xiao J., Chem. Eur. J. 2017, 23, 3051–3061; [DOI] [PubMed] [Google Scholar]
- 12d. Gonzalez-de-Castro A., Robertson C. M., Xiao J., Chem. Eur. J. 2019, 25, 4345–4357; [DOI] [PubMed] [Google Scholar]
- 12e. Wang K., Zhou J., Jiang Y., Zhang M., Wang C., Xue D., Tang W., Sun H., Xiao J., Li C., Angew. Chem. Int. Ed. 2019, 58, 6380–6384; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 6446–6450; [Google Scholar]
- 12f. Huang Z., Guan R., Shanmugam M., Bennett E. L., Robertson C. M., Brookfield A., McInnes E. J. L., Xiao J., J. Am. Chem. Soc. 2021, 143, 10005–10013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.
- 13a. Yazerski V. A., Spannring P., Gatineau D., Woerde C. H. M., Wieclawska S. M., Lutz M., Kleijn H., Klein Gebbink R. J. M., Org. Biomol. Chem. 2014, 12, 2062–2070; [DOI] [PubMed] [Google Scholar]
- 13b. Zhu F., Yang G., Zoll A. J., Rybak-Akimova E. V., Zhu X., Catalysts 2020, 10, 285. [Google Scholar]
- 14.The optically pure (R,R)-L1 and (S,S)-L1 were prepared similarly (see Supporting Information).
- 15.Deposition Numbers 2046042 (for rac-1), 2046041 (for [rac-L 1 Mn(H2O)2]2+), and 2046040 (for [meso-L 1 Mn(H2O)2]2+) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 16.
- 16a. Bigi M. A., Reed S. A., White M. C., J. Am. Chem. Soc. 2012, 134, 9721–9726; [DOI] [PubMed] [Google Scholar]
- 16b. Cianfanelli M., Olivo G., Milan M., Klein Gebbink R. J. M., Ribas X., Bietti M., Costas M., J. Am. Chem. Soc. 2020, 142, 1584–1593. [DOI] [PubMed] [Google Scholar]
- 17.
- 17a. Milan M., Carboni G., Salamone M., Costas M., Bietti M., ACS Catal. 2017, 7, 5903–5911; [Google Scholar]
- 17b. Noisier A. F. M., Brimble M. A., Chem. Rev. 2014, 114, 8775–8806. [DOI] [PubMed] [Google Scholar]
- 18. Zhao J. P., Nanjo T., E. C. de Lucca Jr. , White M. C., Nat. Chem. 2019, 11, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.
- 19a. Vitaku E., Smith D. T., Njardarson J. T., J. Med. Chem. 2014, 57, 10257–10274; [DOI] [PubMed] [Google Scholar]
- 19b. Heravi M. M., Zadsirjan V., RSC Adv. 2020, 10, 44247–44311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seeman P., Lee T., Chau-Wong M., Wong K., Nature 1976, 261, 717–719. [DOI] [PubMed] [Google Scholar]
- 21. Zheng X., Liang C., Wang L., Wang B., Liu Y., Feng S., Wu J. Z., Gao L., Feng L., Chen L., Guo T., Shen H. C., Yun H., J. Med. Chem. 2018, 61, 10228–10241. [DOI] [PubMed] [Google Scholar]
- 22.For examples of studies on carboxylic acid-assisted mechanisms of C−H oxidation, see:
- 22a. White M. C., Doyle A. G., Jacobsen E. N., J. Am. Chem. Soc. 2001, 123, 7194–7195; [DOI] [PubMed] [Google Scholar]
- 22b. Mas-Ballesté R., L. Que, Jr. , J. Am. Chem. Soc. 2007, 129, 15964–15972; [DOI] [PubMed] [Google Scholar]
- 22c. Ottenbacher R. V., Talsi E. P., Bryliakov K. P., ACS Catal. 2015, 5, 39–44; [Google Scholar]
- 22d. Du J., Miao C., Xia C., Lee Y. M., Nam W., Sun W., ACS Catal. 2018, 8, 4528–4538; [Google Scholar]
- 22e. Li X. X., Guo M., Qiu B., Cho K. B., Sun W., Nam W., Inorg. Chem. 2019, 58, 14842–14852. For reviews, see: [DOI] [PubMed] [Google Scholar]
- 22f. Oloo W. N., L. Que, Jr. , Acc. Chem. Res. 2015, 48, 2612–2621; [DOI] [PubMed] [Google Scholar]
- 22g. Lyakin O. Y., Bryliakov K. P., Talsi E. P., Coord. Chem. Rev. 2019, 384, 126–139; [Google Scholar]
- 22h. Kal S., Xu S., L. Que, Jr. , Angew. Chem. Int. Ed. 2020, 59, 7332–7349; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 7400–7419. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.