Abstract
The purpose of this study was to examine neurobehavioral symptom reporting in a large sample of military veterans (N=12,144) who completed the Comprehensive Traumatic Brain Injury Evaluation (CTBIE) and enrolled in the VA’s Million Veteran Program (MVP). The CTBIE is a clinician-administered interview that assesses for historical, deployment-related traumatic brain injury (TBI) and evaluates symptoms using the Neurobehavioral Symptom Inventory (NSI). Clinicians completing the CTBIE made clinical determinations about participants’ (1) TBI diagnostic status (i.e., CTBIE+ or CTBIE−) and (2) current symptom etiology (i.e., Symptom Resolution, TBI, Behavioral Health, Comorbid TBI + Behavioral Health [Comorbid], or Other). We evaluated the association of TBI diagnostic status and symptom etiology group with neurobehavioral symptoms. Results showed a significant association between TBI diagnostic status and all NSI variables, with CTBIE+ veterans endorsing greater symptoms than CTBIE− veterans. There was also a significant association between symptom etiology group and all NSI variables; specifically, the Comorbid and Behavioral Health groups generally endorsed significantly greater symptoms compared to the other groups. Follow-up analyses showed that relative to the Symptom Resolution group, the Comorbid and Behavioral Health groups had increased odds of severe/very severe cognitive and affective symptoms, whereas the TBI and Other groups did not. Finally, presence of psychiatric symptoms, pain, post-traumatic amnesia, loss of consciousness, and blast exposure significantly predicted Comorbid symptom etiology group membership. Findings from this large epidemiologic MVP study have relevant clinical implications and further highlight the importance of prioritizing integrated behavioral health interventions for this vulnerable population.
Keywords: traumatic brain injury, post-concussive symptoms, CTBIE, military veterans, behavioral health
Introduction
Accumulating evidence demonstrates that a remarkable subset of U.S. military service members and veterans with a history of traumatic brain injury (TBI) report debilitating symptoms and poor clinical and functional outcomes chronically following injury (i.e., for many months and even years post-injury; VA/DoD Clinical Practice Guidelines, 2016). Specifically, a wide range of neurobehavioral symptoms are often endorsed post-injury, including somatic (e.g., headache), vestibular (e.g., dizziness), cognitive (e.g., difficulties with memory and concentration), and affective-related symptoms (e.g., increased irritability and mood changes) (MacGregor et al., 2013; Schwab et al., 2017; Vanderploeg et al., 2015). Although these symptoms are generally expected to resolve within weeks to months post-injury (Boyle et al., 2014), these sequelae can persist for much longer (Schwab et al., 2017; Stein et al., 2016) and can interfere with daily functioning and overall quality of life (Haagsma et al., 2015; Lange, Lippa, et al., 2020). In fact, of the estimated 20% of Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans with a history of at least one TBI, approximately one-third of these individuals report symptoms several months to years following injury (Lindquist et al., 2017). Thus, it is critical to understand these clinical sequelae and to identify the etiology of such symptoms so that suitable care and evidence-based treatments can be provided to this vulnerable population.
In response to the increased prevalence of TBI and its associated sequelae, the Veterans Health Administration (VHA) implemented a nationwide screening system in 2007 to routinely assess for TBI in OEF/OIF veterans and to offer further evaluation of veterans who screened positive for TBI (VHA, 2007; VHA, 2010). According to VHA Directive 2007-013, “It is VHA policy that all OEF and OIF veterans receiving medical care, within VHA, must be screened for possible TBI; those who, on the basis of the screen, might have TBI must be offered further evaluation and treatment by clinicians with expertise in the area of TBI” (VHA, 2007). The initial screen (referred to hereafter as the “TBI Clinical Reminder Screen”) is administered to veterans upon enrollment in the VA, typically by a primary care provider, and includes four questions to ascertain TBI history (i.e., establishing possible TBI events/mechanisms of injury, presence of immediate signs or symptoms associated with the event/injury, symptom progression following the event/injury, and current symptoms). A positive TBI screen reflects endorsement of all four questions on the TBI Clinical Reminder Screen. Importantly, only OEF/OIF-era veterans who have not previously been diagnosed with a TBI are administered the TBI Clinical Reminder Screen.
Any veteran with a positive screen is then referred to a TBI specialist (i.e., a “licensed independent medical provider” with “experience and advanced training in TBI”) who completes the Comprehensive Traumatic Brain Injury Evaluation (CTBIE; VHA Directive 2010-012; VHA, 2010), a clinician-administered interview that assesses for historical, deployment-related TBIs. At the conclusion of the CTBIE, the clinician makes a determination about the veteran’s (1) TBI diagnostic status and (2) current symptom etiology. Regarding TBI diagnostic status, the clinician is asked, “Based on the history of the injury and course of clinical symptoms, did the Veteran sustain a TBI during OEF/OIF deployment?” The clinician must indicate (1) ‘Yes’ (i.e., CTBIE+) or (2) ‘No’ (i.e., CTBIE−). With regard to current symptom etiology, the clinician is asked, “In your clinical judgment the current clinical symptom presentation is most consistent with…” and must select one of the following options: (1) ‘Symptom resolution (patient is currently not reporting symptoms)’ [Symptom Resolution]; (2) ‘An OEF/OIF deployment-related TBI residual problems’ [TBI]; (3) ‘Behavioral health conditions (e.g., PTSD, depression)’ [Behavioral Health]; (4) ‘A combination of OEF/OIF deployment-related TBI and behavioral health condition(s)’ [Comorbid]; or (5) ‘Other condition not related to OEF/OIF deployment-related TBI or behavioral health condition(s) [Other].
Previous studies have evaluated the TBI Clinical Reminder Screen and CTBIE with respect to its reliability, validity, and other psychometric properties, and findings have been mixed (Belanger et al., 2016; Belanger et al., 2012; Donnelly et al., 2011; Fortier et al., 2015; Pape et al., 2018; Pogoda et al., 2014; Radigan et al., 2018; Van Dyke et al., 2010). For example, Belanger et al. (2012) showed that the TBI Clinical Reminder Screen has good sensitivity but poor specificity for detecting historical TBI, whereas Pape et al. (2018) showed moderate sensitivity and moderate-to-good specificity, noting that the psychometrics of the measure largely depend on the “diagnostic reference standard to which it is being compared.” As for the CTBIE, Radigan et al. (2018) showed it has moderate sensitivity, but poor specificity compared to the Boston Assessment of TBI-Lifetime. Although there is some variability in the psychometrics associated with the TBI Clinical Reminder Screen and CTBIE, these measures continue to be utilized across the VHA; thus, it is beneficial to evaluate these tools and understand how they inform clinical care.
In addition to psychometric studies, several other investigators have used CTBIE data to examine a wide range of clinical outcomes in the context of military and/or deployment related TBI (Carlson et al., 2010; Gray et al., 2020; Iverson et al., 2011; Pogoda et al., 2012; Pogoda et al., 2016; Scholten et al., 2012; Seal et al., 2016). Of relevance to the current study, Scholten et al. (2012) examined veterans who completed the CTBIE between 2007 and 2010 and found that regardless of CTBIE status (i.e., among veterans with and without a CTBIE-confirmed history of TBI), it was common for all veterans to experience moderate-to-severe neurobehavioral symptoms (Scholten et al., 2012). Notably, though, CTBIE+ veterans endorsed a significantly higher rate of symptoms compared to CTBIE− veterans. Scholten and colleagues (2012) additionally reported that among CTBIE+ veterans, clinicians most often attributed patients’ current symptom presentation to a combination of TBI and behavioral health. However, how clinicians made this determination was not evaluated. Two major takeaways from this study were (1) the high rates of neurobehavioral symptoms in veterans who screen positive for TBI (regardless of TBI history status on the CTBIE) and (2) the presumed role of behavioral health in the maintenance of neurobehavioral symptoms. Since then, several other studies have similarly highlighted the strong association between behavioral health comorbidities (e.g., posttraumatic stress disorder [PTSD] and depression) and neurobehavioral symptoms following TBI (Andrews et al., 2018; Lange, French, et al., 2020; Porter et al., 2018), emphasizing the negligible influence of TBI itself on neurobehavioral symptoms—especially symptoms endorsed chronically following injury.
To date, CTBIE outcome studies have largely focused on TBI diagnostic status and surprisingly few studies have examined clinician-rated symptom etiology data. In fact, no studies, to our knowledge, have evaluated associations between self-reported neurobehavioral symptoms and clinicians’ symptom etiology classifications on the CTBIE. In the present study, we examined neurobehavioral symptom reporting in a large, nationwide sample of military veterans who (1) completed the CTBIE between 2007 and 2019 and (2) enrolled in the VA’s Million Veteran Program (MVP; Gaziano et al., 2016). Our first objective was to compare neurobehavioral symptoms across clinician-rated TBI diagnostic groups (i.e., CTBIE+ vs. CTBIE−), similar to what was accomplished in Scholten et al.’s (2012) study. However, we evaluated CTBIE data spanning over a decade, but only among MVP-enrolled veterans. This aim was conducted to verify that our MVP sample was representative of the broader CTBIE cohort from which prior studies have been based. Our second objective was to evaluate neurobehavioral symptoms as a function of clinician-rated symptom etiology groups (i.e., Symptom Resolution vs. TBI vs. Behavioral Health vs. Comorbid vs. Other)—something that has not previously been examined. We hypothesized that in our sample of MVP-enrolled veterans who completed the CTBIE, that (1) veterans with a history of TBI (CTBIE+) would experience a greater symptom burden compared to those without a history of TBI (CTBIE−) and (2) veterans classified as either Comorbid or Behavioral Health would endorse a greater symptom burden compared to all other symptom etiology groups. Finally, we compared rates of severe/very symptoms in CTBIE+ veterans across symptom etiology groups.
Material and Methods
Procedures and Participants
The current study was conducted using data from the VA’s MVP, a national research program that seeks to evaluate how lifestyle factors, military exposure, and genes influence health and illness (Gaziano et al., 2016). Any veteran is eligible to participate in MVP (as long as they are able to provide consent); thus, the MVP cohort reflects a nationwide sample of veterans. As part of MVP enrollment, participants (1) consent to investigators accessing their electronic health record (EHR) data; (2) complete self-report questionnaires; and (3) provide a blood sample for genetic analysis. For the purpose of this study, only EHR data assembled from the VA’s Corporate Data Warehouse (Fihn et al., 2014) was utilized. Specifically, the CTBIE1 served as the primary data source (see below for details). The overarching MVP project was granted Institutional Review Board (IRB) approval in 2010 and enrollment into MVP began in 2011. IRB approval for the present study (project “MVP026”) was obtained in 2019.
Eligible participants for the current study included MVP-enrolled veterans who were administered the CTBIE (between 2007 and 2019) following a positive TBI Clinical Reminder Screen (VHA, 2007; VHA, 2010). Participants were excluded from the present study if (1) CTBIE TBI diagnostic data were unavailable, incomplete, or uncertain; (2) neurobehavioral symptom data were missing, and/or (3) they failed symptom validity testing (described in more detail below under “Measures”). See Figure 1 for a flow diagram representing the study’s inclusion and exclusion criteria. Applying these criteria resulted in a final sample of N=12,144.
Figure 1.
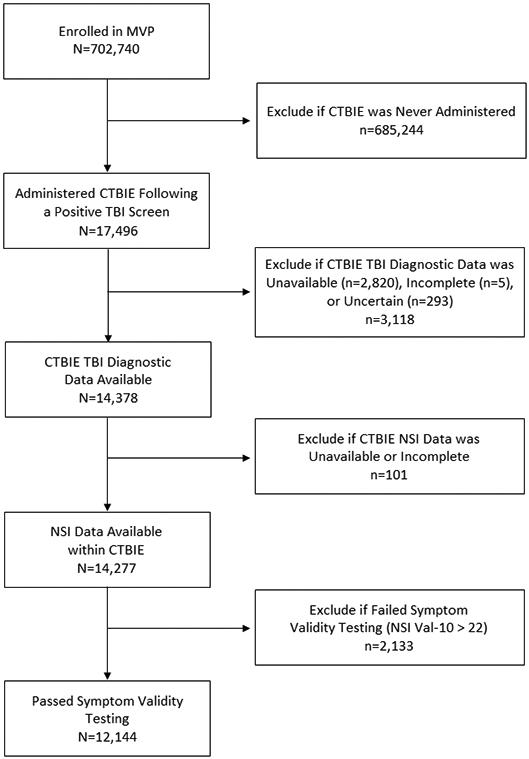
Flow diagram representing study inclusion/exclusion criteria.
Measures
Comprehensive Traumatic Brain Injury Evaluation (CTBIE)
The CTBIE is a tool that was designed for VA clinicians with expertise in TBI to assess for historical TBIs sustained during an OEF/OIF-related deployment (VHA, 2010). As part of the CTBIE interview template, clinicians gather basic sociodemographic data and inquire about deployment-related injuries sustained during OEF/OIF. Specifically, information is collected regarding mechanism of injury (categories include bullet, vehicular, fall, and blast) as well as other injury characteristics such as loss of consciousness (LOC), alteration of consciousness (AOC), and post-traumatic amnesia (PTA). Clinicians also ask about whether the veteran sustained any TBIs prior to or since deployment. Specifically, the CTBIE states, “Prior to your OEF/OIF deployment, did you experience a brain injury or concussion?” and “Since your OEF/OIF deployment, have you experienced a brain injury or concussion?”, with the following response options: ‘Yes’, ‘No’, ‘Uncertain’, and ‘Not Assessed’. Comorbid psychiatric symptoms (i.e., is the veteran currently experiencing psychiatric symptoms, with response options including ‘Yes’, ‘No’, ‘Suspected/Probable’, and ‘Not Assessed’) and pain (i.e., has the veteran had any problems with pain in the last 30 days, with response options including ‘Yes’ and ‘No’) are also assessed. Finally, neurobehavioral symptoms are evaluated as part of the CTBIE using the Neurobehavioral Symptom Inventory (NSI; described below).
As described previously, at the conclusion of the CTBIE, the clinician is instructed to render two diagnostic decisions, one pertaining to TBI diagnostic status (i.e., “Based on the history of the injury and the course of clinical symptoms, did the veteran sustain a TBI during OEF/IOF deployment?) and the other pertaining to current symptom etiology (i.e., “In your clinical judgment the current clinical symptom presentation is most consistent with…”). For the TBI diagnostic status question, response options are ‘Yes’ and ‘No’; for the symptom etiology question, the response options are: (1) ‘Symptom resolution (patient is currently not reporting symptoms)’ [Symptom Resolution]; (2) ‘An OEF/OIF deployment-related TBI residual problems’ [TBI]; (3) ‘Behavioral health conditions (e.g., PTSD, depression)’ [Behavioral Health]; (4) ‘A combination of OEF/OIF deployment-related TBI and behavioral health condition(s)’ [Comorbid]; or (5) ‘Other condition not related to OEF/OIF deployment-related TBI or behavioral health condition(s) [Other].
Neurobehavioral Symptom Inventory (NSI)
The NSI (Cicerone & Kalmar, 1995) is comprised of 22 unique “post-concussive” symptoms. The NSI is a self-report measure with excellent internal consistency, test-retest reliability, and concurrent and construct validity (King et al., 2012; Menatti et al., 2020; Soble et al., 2014; Vos et al., 2019). Respondents are asked to rate the extent to which they have experienced each symptom over the past 30 days using a 5-point scale ranging from 0-4, where 0=None, 1=Mild, 2=Moderate, 3=Severe, and 4=Very Severe. In addition to evaluating the individual items, several scores were generated from the NSI to capture both symptom severity and symptom breadth.
Total Score: The NSI total score was computed by summing the ratings across the 22 individual items (range: 0-88); this score reflects overall symptom severity, with higher scores indicative of more severe symptom endorsement.
Symptom Domain Scores: Based on the results of a previous factor analysis conducted on the NSI using a similar military sample (Vanderploeg et al., 2015), four symptom domain scores were computed reflecting vestibular symptoms (items 1-3; range: 0-12), somatic/sensory symptoms (items 4-7 and 9-11; range: 0-28), cognitive symptoms (items 13-16; range: 0-16); and affective symptoms (items 17-22; range: 0-24). As with the NSI total score, each symptom domain score was computed by summing the ratings of the individual items associated with each domain. The symptom domain scores reflect domain-specific symptom severity, with higher scores indicative of more severe symptom endorsement.
Positive Symptom Total (PST) Scores: A ‘PST-Mild’ score was calculated by counting how many of the 22 NSI items were endorsed at a mild or greater severity level (denoted by a rating of “1” or more on an individual item; range: 0-22); a ‘PST-Moderate’ score was calculated by counting how many of the 22 items were endorsed at a moderate or greater severity level (denoted by a rating of “2” or more on an individual item; range: 0-22); and a ‘PST-Severe’ score was calculated by counting how many of the 22 items were endorsed at a severe or greater severity level (denoted by a rating of “3” or more on an individual item; range: 0-22). The PST scores reflect symptom breadth, with higher scores indicative of greater symptom breadth (Derogatis, 1994; Merritt et al., 2015).
Symptom Interference Score: The ‘symptom interference’ score was derived from a single item on the CTBIE that asked participants to rate how much the NSI symptoms interfered with their life over the past 30 days, using a similar 0-4 rating scale where 0=Not at all, 1=Mildly, 2=Moderately, 3=Severely, and 4=Extremely.
Symptom Validity: The ‘symptom validity’ score was derived from the NSI using 10 infrequently endorsed items (Vanderploeg et al., 2014). The score from each of these items is added together to create the Validity-10 index; scores greater than 22 reflect symptom over-reporting whereas scores of 22 or less are considered valid (Vanderploeg et al., 2014). Given the high base rate of symptom exaggeration in military cohorts (Armistead-Jehle, 2010) and the possibility of secondary gain (i.e., disability pensions) in this cohort, anyone with a Validity-10 score greater than 22 was excluded to minimize the possible effects of symptom overreporting on our results.
Statistical Analyses
Stata (Stata/MP 15.1, StataCorp LLC, College Station, TX) was used to conduct all statistical analyses. Independent variables of interest from the CTBIE were clinician ratings on (1) TBI diagnostic status and (2) current symptom etiology, and dependent variables included NSI symptoms (i.e., summary scores, symptom domain scores, and individual items). Descriptive statistics were conducted on all variables of interest and analyses of covariance (ANCOVAs) adjusting for relevant sociodemographic characteristics (i.e., age, sex, race/ethnicity, premilitary education, employment status, and marital status) were used to evaluate the effect of (1) TBI diagnostic group and (2) symptom etiology group on neurobehavioral symptoms. Adjusted effect sizes are reported as partial eta-squared (ηp2 ) values, with the following interpretation: small = 0.01; medium = 0.06; and large = 0.14; unadjusted effect sizes are reported as Cohen’s d values, with the following interpretation: small = 0.20; medium = 0.50; and large = 0.80.
In follow-up analyses, logistic regression adjusting for sociodemographic variables (i.e., age, sex, race/ethnicity, education, employment status, and marital status) was used to (1) estimate the odds of having severe/very severe symptoms as a function of symptom etiology group and (2) evaluate the variables most associated with being classified in the ‘Comorbid’ symptom etiology group. Odds ratios and 95% confidence intervals were computed for all logistic regression models.
Results
Participant Characteristics
In total, 12,144 MVP-enrolled veterans were included in this study; all completed the CTBIE between 2007 and 2019. Participants were, on average, 34.9 years of age (SD=9.6) and the majority were male (91.0%). Participant characteristics (i.e., sociodemographic, injury-related variables, and CTBIE diagnostics) are presented in Table 1, both for the full sample (i.e., CTBIE+ and CTBIE− veterans) and for the CTBIE+ sample only. Of the 12,144 veterans included in the present study, 62.8% were classified as CTBIE+, meaning that a clinician determined that their history was consistent with a TBI, and the remaining 37.2% were classified as CTBIE−. With regard to symptom etiology, close to half of the participants from the full sample (i.e., CTBIE+ and CTBIE− veterans) were classified as Behavioral Health (47.8%), meaning that clinicians determined that veterans’ current symptoms were primarily due to behavioral health conditions. In contrast, when examining only CTBIE+ veterans (N=7,631), symptoms were most often attributed to Comorbid TBI + Behavioral Health (42.9%), followed by Behavioral Health (35.5%).
Table 1.
CTBIE Participant Characteristics for the Full Sample (CTBIE+ and CTBIE− Veterans) and CTBIE+ Sample.
| Variables | Full Sample† | CTBIE+ Sample‡ | ||
|---|---|---|---|---|
| Sociodemographics | N | % | N | % |
| Age at CTBIE | ||||
| 18-29 | 4,404 | 36.7 | 2,990 | 39.7 |
| 30-39 | 3,984 | 33.2 | 2,573 | 34.2 |
| 40-49 | 2,548 | 21.2 | 1,413 | 18.8 |
| 50+ | 1,060 | 8.8 | 559 | 7.4 |
| Sex | ||||
| Male | 11,049 | 91.0 | 7,009 | 91.9 |
| Female | 1,095 | 9.0 | 622 | 8.2 |
| Race/Ethnicity | ||||
| White | 6,602 | 54.4 | 4,199 | 55.0 |
| Hispanic | 1,945 | 16.0 | 1,320 | 17.3 |
| Black | 1,813 | 14.9 | 951 | 12.4 |
| Asian | 310 | 2.6 | 205 | 2.7 |
| Another Race | 613 | 5.0 | 372 | 4.9 |
| Unknown/Not Reported | 861 | 7.1 | 584 | 7.7 |
| Education Level (Pre-Military) | ||||
| High School or Less | 6,929 | 58.5 | 4,430 | 59.4 |
| Some College | 4,030 | 34.0 | 2,500 | 33.5 |
| College Degree or More | 888 | 7.5 | 525 | 7.0 |
| Employment Status | ||||
| Employed | 5,310 | 44.6 | 3,203 | 42.7 |
| Unemployed | 4,349 | 36.5 | 2,772 | 36.9 |
| Student | 2,106 | 17.7 | 1,435 | 19.1 |
| Volunteer/Homemaker | 154 | 1.3 | 96 | 1.3 |
| Marital Status | ||||
| Single/Never Married | 2,867 | 23.6 | 1,793 | 23.5 |
| Married or Partnered | 6,236 | 51.4 | 3,936 | 51.7 |
| Divorced or Separated | 2,969 | 24.5 | 1,857 | 24.4 |
| Widowed | 52 | 0.4 | 34 | 0.5 |
| Psychiatric Symptoms | ||||
| Yes | 6,237 | 64.2 | 4,126 | 67.2 |
| No | 1,274 | 13.1 | 735 | 12.0 |
| Suspected/Probable | 1,547 | 15.9 | 860 | 14.0 |
| Not Assessed | 660 | 6.8 | 415 | 6.8 |
| Problems with Pain | ||||
| Yes | 11,092 | 91.4 | 7,089 | 93.0 |
| No | 1,041 | 8.6 | 536 | 7.0 |
| Injury-Related Characteristics | N | % | N | % |
| Mechanism of Injury^ | ||||
| Bullet | 265 | 2.2 | 182 | 3.0 |
| Vehicular | 2,299 | 18.9 | 1,690 | 26.9 |
| Fall | 2,859 | 23.5 | 2,098 | 32.9 |
| Blast | 7,113 | 58.6 | 5,141 | 74.7 |
| LOC Present | ||||
| Yes | 4,170 | 45.5 | 3,814 | 55.9 |
| No | 4,285 | 46.7 | 2,434 | 35.6 |
| Uncertain | 718 | 7.8 | 579 | 8.5 |
| AOC Present | ||||
| Yes | 7,905 | 79.2 | 6,691 | 92.3 |
| No | 1,749 | 17.5 | 414 | 5.7 |
| Uncertain | 322 | 3.2 | 144 | 2.0 |
| PTA Present | ||||
| Yes | 2,320 | 28.8 | 2,195 | 37.0 |
| No | 4,932 | 61.2 | 3,075 | 51.8 |
| Uncertain | 811 | 10.1 | 663 | 11.2 |
| TBI Prior to Deployment | ||||
| Yes | 2,754 | 22.7 | 1,640 | 21.5 |
| No | 8,669 | 71.4 | 5,588 | 73.2 |
| Uncertain | 509 | 4.2 | 296 | 3.9 |
| Not Assessed | 204 | 1.7 | 105 | 1.4 |
| TBI Since Deployment | ||||
| Yes | 1,306 | 10.8 | 822 | 10.8 |
| No | 10,314 | 84.9 | 6,520 | 85.5 |
| Uncertain | 353 | 2.9 | 208 | 2.7 |
| Not Assessed | 164 | 1.4 | 79 | 1.0 |
| Diagnostics | N | % | N | % |
| CTBIE TBI Diagnosis | -- | -- | ||
| TBI (CTBIE+) | 7,631 | 62.8 | ||
| No TBI (CTBIE−) | 4,513 | 37.2 | ||
| CTBIE Current Symptom Etiology | ||||
| Symptom Resolution | 750 | 7.2 | 408 | 6.5 |
| TBI | 612 | 5.9 | 588 | 9.3 |
| Behavioral Health | 4,965 | 47.8 | 2,236 | 35.5 |
| Comorbid | 2,773 | 26.7 | 2,702 | 42.9 |
| Other | 1,282 | 12.3 | 371 | 5.9 |
Abbreviations: CTBIE = Comprehensive Traumatic Brain Injury Evaluation; LOC = loss of consciousness; AOC = alteration of consciousness; PTA = post-traumatic amnesia; TBI = traumatic brain injury.
Notes:
The “full sample” refers to veterans who completed the CTBIE, regardless of TBI diagnostic status (i.e., CTBIE+ and CTBIE− veterans make up this sample); N=12,144; however, n’s may not total 12,144 due to missing data.
The “CTBIE+ sample” refers to veterans who completed the CTBIE and were confirmed to have a history of TBI; N=7,631; however, n’s may not total 7,631 due to missing data.
Not mutually exclusive categories; thus, it is possible for a participant to endorse more than one mechanism of injury.
Neurobehavioral Symptoms: Summary Data
Table 2 presents descriptive statistics for the NSI variables (summary scores, symptom domain scores, and individual items) across the full sample (CTBIE+ and CTBIE− veterans) and Figure 2 displays the 22 individual neurobehavioral symptoms by severity level. As shown in Figure 2, the most commonly endorsed severe/very severe symptoms were difficulty falling or staying asleep (56.4%), irritability (50.5%), feeling anxious or tense (45.9%), and forgetfulness (42.3%). The least commonly endorsed symptoms (i.e., symptoms rate as “none”) were change in taste and/or smell (70.6%), nausea (51.6%), and loss of appetite or increased appetite (39.5%).
Table 2.
Descriptive Statistics (Full Sample†): Neurobehavioral Symptom Inventory (NSI) Variables.
| NSI Variables | M | SD | Mdn | Min | Max |
|---|---|---|---|---|---|
| NSI Summary Scores | |||||
| Total Score | 35.03 | 14.17 | 36 | 0 | 70 |
| PST-Mild | 16.58 | 4.54 | 18 | 0 | 22 |
| PST-Moderate | 11.58 | 5.24 | 12 | 0 | 22 |
| PST-Severe | 5.40 | 4.37 | 5 | 0 | 18 |
| Symptom Interference | 2.16 | 0.90 | 2 | 0 | 4 |
| NSI Symptom Domain Scores | |||||
| Vestibular | 3.15 | 2.19 | 3 | 0 | 12 |
| Somatic/Sensory | 8.73 | 4.51 | 9 | 0 | 25 |
| Cognitive | 7.36 | 3.84 | 8 | 0 | 16 |
| Affective | 13.06 | 5.47 | 13 | 0 | 24 |
| NSI Individual Items | |||||
| Feeling dizzy | 1.07 | 0.86 | 1 | 0 | 4 |
| Loss of balance | 1.04 | 0.88 | 1 | 0 | 4 |
| Poor coordination, clumsy | 1.03 | 0.92 | 1 | 0 | 4 |
| Headaches | 2.06 | 1.11 | 2 | 0 | 4 |
| Nausea | 0.74 | 0.91 | 0 | 0 | 4 |
| Vision problems | 1.16 | 1.01 | 1 | 0 | 4 |
| Sensitivity to light | 1.47 | 1.17 | 1 | 0 | 4 |
| Hearing difficulty | 1.57 | 1.10 | 2 | 0 | 4 |
| Sensitivity to noise | 1.45 | 1.16 | 1 | 0 | 4 |
| Numbness or tingling | 1.39 | 1.18 | 1 | 0 | 4 |
| Change in taste and/or smell | 0.46 | 0.82 | 0 | 0 | 4 |
| Loss of appetite or increased appetite | 1.15 | 1.14 | 1 | 0 | 4 |
| Poor concentration | 2.05 | 1.12 | 2 | 0 | 4 |
| Forgetfulness | 2.22 | 1.09 | 2 | 0 | 4 |
| Difficulty making decisions | 1.41 | 1.15 | 1 | 0 | 4 |
| Slowed thinking | 1.68 | 1.16 | 2 | 0 | 4 |
| Fatigue | 1.95 | 1.16 | 2 | 0 | 4 |
| Difficulty falling or staying asleep | 2.54 | 1.18 | 3 | 0 | 4 |
| Feeling anxious or tense | 2.28 | 1.15 | 2 | 0 | 4 |
| Feeling depressed or sad | 1.79 | 1.25 | 2 | 0 | 4 |
| Irritability, easily annoyed | 2.42 | 1.14 | 3 | 0 | 4 |
| Poor frustration tolerance | 2.09 | 1.21 | 2 | 0 | 4 |
Abbreviations: NSI = Neurobehavioral Symptom Inventory; PST = Positive Symptom Total; M = mean; SD = standard deviation; Mdn = median; Min = minimum; Max = maximum.
Notes:
The “full sample” refers to veterans who completed the CTBIE, regardless of TBI diagnostic status (i.e., CTBIE+ and CTBIE− veterans make up this sample); N=12,144.
Figure 2.
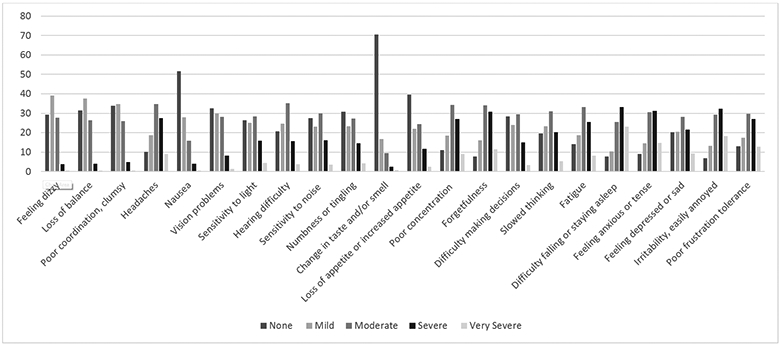
Neurobehavioral Symptom Inventory (NSI) Symptom Endorsement by Severity Level (Full Sample†).
Notes: †N=12,144.
Neurobehavioral Symptoms by CTBIE Diagnostic Group
Table 3 presents the ANCOVA results comparing CTBIE diagnostic groups (i.e., CTBIE+ and CTBIE− groups) across NSI variables. There was a significant association between CTBIE group and all NSI items (i.e., summary scores, symptom domain scores, and individual items; all p’s<.001, adjusted effect sizes: ηp2=.001-.027; unadjusted effect sizes: d=0.06-0.32), such that CTBIE+ veterans endorsed greater symptoms than CTBIE− veterans.
Table 3.
Comparison of Neurobehavioral Symptom Inventory (NSI) Variables by CTBIE Diagnostic Group: Results of ANCOVAs.
| NSI Variables | TBI (CTBIE+) | No TBI (CTBIE−) | Summary Statistics | Adjusted ES | Unadjusted ES | |
|---|---|---|---|---|---|---|
| M (SE) | M (SE) | F | p | ηp2 | d | |
| NSI Summary Scores | ||||||
| Total Score | 36.56 (0.16) | 32.49 (0.21) | 260.50 | <.001 | .022 | 0.29 |
| PST-Mild | 17.07 (0.05) | 15.74 (0.07) | 283.08 | <.001 | .024 | 0.30 |
| PST-Moderate | 12.20 (0.06) | 10.55 (0.08) | 314.15 | <.001 | .027 | 0.32 |
| PST-Severe | 5.72 (0.05) | 4.89 (0.07) | 109.26 | <.001 | .009 | 0.18 |
| Symptom Interference | 2.23 (0.01) | 2.04 (0.01) | 102.88 | <.001 | .009 | 0.21 |
| NSI Symptom Domain Scores | ||||||
| Vestibular | 3.33 (0.03) | 2.83 (0.03) | 177.36 | <.001 | .015 | 0.23 |
| Somatic/Sensory | 9.17 (0.05) | 8.02 (0.07) | 242.14 | <.001 | .021 | 0.26 |
| Cognitive | 7.75 (0.04) | 6.71 (0.06) | 203.12 | <.001 | .017 | 0.27 |
| Affective | 13.45 (0.06) | 12.45 (0.08) | 93.38 | <.001 | .008 | 0.18 |
| NSI Individual Items | ||||||
| Feeling dizzy | 1.13 (0.01) | 0.97 (0.01) | 111.47 | <.001 | .010 | 0.18 |
| Loss of balance | 1.10 (0.01) | 0.95 (0.01) | 114.10 | <.001 | .010 | 0.17 |
| Poor coordination, clumsy | 1.10 (0.01) | 0.91 (0.01) | 130.63 | <.001 | .011 | 0.21 |
| Headaches | 2.17 (0.01) | 1.90 (0.02) | 169.01 | <.001 | .015 | 0.24 |
| Nausea | 0.79 (0.01) | 0.67 (0.01) | 52.93 | <.001 | .005 | 0.13 |
| Vision problems | 1.20 (0.01) | 1.08 (0.02) | 81.29 | <.001 | .007 | 0.13 |
| Sensitivity to light | 1.58 (0.01) | 1.31 (0.02) | 153.02 | <.001 | .013 | 0.23 |
| Hearing difficulty | 1.67 (0.01) | 1.40 (0.02) | 143.47 | <.001 | .012 | 0.24 |
| Sensitivity to noise | 1.53 (0.01) | 1.33 (0.02) | 104.53 | <.001 | .009 | 0.17 |
| Numbness or tingling | 1.42 (0.01) | 1.33 (0.02) | 31.23 | <.001 | .003 | 0.07 |
| Change in taste and/or smell | 0.49 (0.01) | 0.40 (0.01) | 52.23 | <.001 | .005 | 0.11 |
| Loss of appetite or increased appetite | 1.20 (0.01) | 1.08 (0.02) | 34.46 | <.001 | .003 | 0.11 |
| Poor concentration | 2.15 (0.01) | 1.87 (0.02) | 157.51 | <.001 | .014 | 0.25 |
| Forgetfulness | 2.34 (0.01) | 2.02 (0.02) | 233.81 | <.001 | .020 | 0.29 |
| Difficulty making decisions | 1.49 (0.01) | 1.29 (0.02) | 90.61 | <.001 | .008 | 0.17 |
| Slowed thinking | 1.77 (0.01) | 1.53 (0.02) | 121.05 | <.001 | .010 | 0.20 |
| Fatigue | 2.01 (0.01) | 1.85 (0.02) | 68.31 | <.001 | .006 | 0.13 |
| Difficulty falling or staying asleep | 2.61 (0.01) | 2.42 (0.02) | 74.02 | <.001 | .006 | 0.16 |
| Feeling anxious or tense | 2.35 (0.01) | 2.18 (0.02) | 53.80 | <.001 | .005 | 0.15 |
| Feeling depressed or sad | 1.82 (0.01) | 1.74 (0.02) | 14.68 | <.001 | .001 | 0.06 |
| Irritability, easily annoyed | 2.49 (0.01) | 2.30 (0.02) | 65.22 | <.001 | .006 | 0.17 |
| Poor frustration tolerance | 2.17 (0.01) | 1.96 (0.02) | 72.24 | <.001 | .006 | 0.16 |
Abbreviations: NSI = Neurobehavioral Symptom Inventory; CTBIE = Comprehensive Traumatic Brain Injury Evaluation; TBI = traumatic brain injury; ES = effect size; PST = Positive Symptom Total.
Notes: N=12,144; TBI (CTBIE+): n=7,631; No TBI (CTBIE−): n=4,513; however, actual N for each outcome of interest may be less due to missing data for covariates. Adjusted means and standard errors are reported in the table, adjusting for age, sex, race/ethnicity, education, employment status, and marital status. Effect sizes are reported for both the adjusted and unadjusted models; partial eta-squared (ηp2) effect size interpretation = small (0.01), medium (0.06), large (0.14); Cohen’s d effect size interpretation = small (0.20), medium (0.50), large (0.80).
Neurobehavioral Symptoms by CTBIE Symptom Etiology Group
Participant characteristics (i.e., sociodemographic and injury-related variables) are presented in Table 4 by CTBIE symptom etiology group (i.e., Symptom Resolution, TBI, Behavioral Health, Comorbid, and Other groups) for the CTBIE+ sample, and Table 5 presents the ANCOVA results comparing symptom etiology groups across NSI variables for the CTBIE+ sample. There was a significant association between symptom etiology group and all NSI items (i.e., summary scores, symptom domain scores, and individual items; all p’s<.001, ηp2=.003-.080; see Table 5). Post-hoc analyses, displayed in Table 6, generally showed that the Comorbid and Behavioral Health groups endorsed significantly greater symptoms compared to the Symptom Resolution, TBI, and Other groups. Additionally, the Comorbid group endorsed significantly greater symptoms relative to the Behavioral Health group for many of the NSI summary and symptom domain scores. Finally, there were no significant differences between the TBI and Symptom Resolution groups for the majority of NSI summary and symptom domain scores; the only exception to this was the somatic/sensory and cognitive symptom domain scores, where the TBI group endorsed more severe symptoms than the Symptom Resolution group. Supplemental Table 1 provides summary statistics and ANCOVA results for the NSI variables by CTBIE symptom etiology group across the full sample (including both CTBIE+ and CTBIE− veterans).
Table 4.
CTBIE Participant Characteristics by CTBIE Symptom Etiology Group (CTBIE+ Sample†):
| Variables | 1. Symptom Resolution |
2. TBI | 3. Behavioral Health |
4. Comorbid | 5. Other | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sociodemographics | N | % | N | % | N | % | N | % | N | % |
| Age at CTBIE | ||||||||||
| 18-29 | 142 | 35.5 | 255 | 43.9 | 816 | 37.0 | 1,029 | 38.8 | 98 | 26.6 |
| 30-39 | 146 | 36.5 | 174 | 30.0 | 819 | 37.1 | 986 | 37.2 | 126 | 34.2 |
| 40-49 | 84 | 21.0 | 107 | 18.4 | 409 | 18.5 | 472 | 17.8 | 88 | 23.9 |
| 50+ | 28 | 7.0 | 45 | 7.7 | 164 | 7.4 | 165 | 6.2 | 56 | 15.2 |
| Sex | ||||||||||
| Male | 377 | 92.4 | 538 | 91.5 | 2,051 | 91.7 | 2,482 | 91.9 | 335 | 90.3 |
| Female | 31 | 7.6 | 50 | 8.5 | 185 | 8.3 | 220 | 8.1 | 36 | 9.7 |
| Race/Ethnicity | ||||||||||
| White | 209 | 51.2 | 321 | 54.6 | 1,273 | 56.9 | 1,397 | 51.7 | 194 | 52.3 |
| Hispanic | 63 | 15.4 | 112 | 19.1 | 357 | 16.0 | 509 | 18.8 | 53 | 14.3 |
| Black | 58 | 14.2 | 65 | 11.0 | 280 | 12.5 | 318 | 11.8 | 52 | 14.0 |
| Asian | 8 | 2.0 | 28 | 4.8 | 48 | 2.2 | 75 | 2.8 | 12 | 3.2 |
| Another Race | 20 | 4.9 | 29 | 4.9 | 92 | 4.1 | 135 | 5.0 | 29 | 7.8 |
| Unknown/Not Reported | 50 | 12.3 | 33 | 5.6 | 186 | 8.3 | 268 | 9.9 | 31 | 8.4 |
| Education Level (Pre-Military) | ||||||||||
| High School or Less | 216 | 53.9 | 314 | 54.8 | 1,400 | 63.2 | 1,547 | 58.4 | 214 | 59.1 |
| Some College | 139 | 34.6 | 204 | 35.6 | 661 | 29.9 | 904 | 33.5 | 108 | 29.8 |
| College Degree or More | 46 | 11.5 | 55 | 9.6 | 152 | 6.9 | 218 | 8.1 | 40 | 11.1 |
| Employment Status | ||||||||||
| Employed | 188 | 46.3 | 273 | 47.3 | 928 | 41.8 | 1,081 | 40.6 | 157 | 42.7 |
| Unemployed | 138 | 34.0 | 185 | 32.1 | 874 | 39.3 | 978 | 36.7 | 125 | 34.0 |
| Student | 76 | 18.7 | 116 | 20.1 | 401 | 18.0 | 564 | 21.2 | 80 | 21.7 |
| Volunteer/Homemaker | 4 | 1.0 | 3 | 0.5 | 20 | 0.9 | 41 | 1.5 | 6 | 1.6 |
| Marital Status | ||||||||||
| Single/Never Married | 96 | 23.5 | 178 | 30.3 | 444 | 19.9 | 645 | 23.9 | 72 | 19.4 |
| Married or Partnered | 209 | 51.2 | 285 | 48.6 | 1,197 | 53.6 | 1,379 | 51.1 | 217 | 58.5 |
| Divorced or Separated | 100 | 24.5 | 124 | 21.1 | 584 | 26.1 | 658 | 24.4 | 80 | 21.6 |
| Widowed | 3 | 0.7 | 0 | 0.0 | 8 | 0.4 | 17 | 0.6 | 2 | 0.5 |
| Psychiatric Symptoms | ||||||||||
| Yes | 70 | 64.4 | 109 | 22.0 | 1,434 | 82.0 | 1,699 | 76.2 | 121 | 41.8 |
| No | 210 | 21.5 | 242 | 48.9 | 28 | 1.6 | 88 | 4.0 | 100 | 34.6 |
| Suspected/Probable | 18 | 5.5 | 57 | 11.5 | 220 | 12.6 | 269 | 12.0 | 34 | 11.8 |
| Not Assessed | 28 | 8.6 | 87 | 17.6 | 67 | 3.8 | 174 | 7.8 | 34 | 11.8 |
| Problems with Pain | ||||||||||
| Yes | 342 | 83.8 | 544 | 92.5 | 2,044 | 91.5 | 2,556 | 94.7 | 348 | 94.0 |
| No | 66 | 16.2 | 44 | 7.5 | 191 | 8.5 | 143 | 5.3 | 22 | 6.0 |
| Injury-Related Characteristics | N | % | N | % | N | % | N | % | N | % |
| Mechanism of Injury^ | ||||||||||
| Bullet | 9 | 2.4 | 12 | 2.7 | 42 | 2.2 | 67 | 2.5 | 5 | 1.6 |
| Vehicular | 101 | 26.5 | 126 | 26.0 | 432 | 21.9 | 667 | 24.7 | 79 | 24.0 |
| Fall | 126 | 33.1 | 165 | 34.7 | 575 | 28.8 | 765 | 28.3 | 117 | 35.2 |
| Blast | 259 | 66.1 | 338 | 66.7 | 1,542 | 73.3 | 1,992 | 73.7 | 212 | 63.3 |
| LOC Present | ||||||||||
| Yes | 196 | 50.1 | 307 | 60.3 | 966 | 46.5 | 1,462 | 54.1 | 171 | 50.3 |
| No | 167 | 42.7 | 170 | 33.4 | 902 | 43.5 | 979 | 36.3 | 135 | 39.7 |
| Uncertain | 28 | 7.2 | 32 | 6.3 | 208 | 10.0 | 260 | 9.6 | 34 | 10.0 |
| AOC Present | ||||||||||
| Yes | 344 | 86.2 | 513 | 92.1 | 1,992 | 92.2 | 2,487 | 92.1 | 315 | 88.2 |
| No | 49 | 12.3 | 36 | 6.5 | 129 | 6.0 | 141 | 5.2 | 35 | 9.8 |
| Uncertain | 6 | 1.5 | 58 | 1.4 | 39 | 1.8 | 73 | 2.7 | 7 | 2.0 |
| PTA Present | ||||||||||
| Yes | 103 | 27.5 | 189 | 42.2 | 595 | 31.2 | 1,137 | 42.1 | 94 | 29.8 |
| No | 245 | 65.5 | 213 | 47.5 | 1,108 | 58.1 | 1,251 | 46.3 | 179 | 56.8 |
| Uncertain | 26 | 7.0 | 46 | 10.3 | 203 | 10.7 | 312 | 11.6 | 42 | 13.3 |
| TBI Prior to Deployment | ||||||||||
| Yes | 79 | 19.4 | 123 | 20.9 | 469 | 21.0 | 573 | 21.2 | 109 | 29.4 |
| No | 299 | 73.2 | 430 | 73.1 | 1,631 | 72.9 | 2,013 | 74.5 | 244 | 65.8 |
| Uncertain | 24 | 5.9 | 27 | 4.6 | 103 | 4.6 | 83 | 3.1 | 14 | 3.7 |
| Not Assessed | 6 | 1.5 | 8 | 1.4 | 33 | 1.5 | 32 | 1.2 | 4 | 1.1 |
| TBI Since Deployment | ||||||||||
| Yes | 38 | 9.3 | 63 | 10.7 | 222 | 9.9 | 343 | 12.7 | 47 | 12.7 |
| No | 344 | 81.9 | 504 | 85.7 | 1,917 | 85.7 | 2,287 | 84.6 | 308 | 83.0 |
| Uncertain | 30 | 7.3 | 15 | 2.6 | 74 | 3.3 | 53 | 2.0 | 11 | 3.0 |
| Not Assessed | 6 | 1.5 | 6 | 1.0 | 23 | 1.0 | 19 | 0.7 | 5 | 1.3 |
Abbreviations: CTBIE = Comprehensive Traumatic Brain Injury Evaluation; LOC = loss of consciousness; AOC = alteration of consciousness; PTA = post-traumatic amnesia; TBI = traumatic brain injury.
Notes:
The “CTBIE+ sample” refers to veterans who completed the CTBIE and were confirmed to have a history of TBI; N=6,305 (due to missing symptom etiology data); 1. Symptom Resolution: n=408; 2. TBI: n=588; 3. Behavioral Health: n=2,236; 4. Comorbid TBI + Behavioral Health: n=2,702; 5. Other: n=371; however, n’s may not total 6,305 due to missing data.
Not mutually exclusive categories; thus, it is possible for a participant to endorse more than one mechanism of injury.
Table 5.
Comparison of Neurobehavioral Symptom Inventory (NSI) Variables by CTBIE Symptom Etiology Group (CTBIE+ Sample†): Results of ANCOVAs.
| NSI Variables | 1. Symptom Resolution |
2. TBI | 3. Behavioral Health |
4. Comorbid | 5. Other | Summary Statistics | Adjusted ES |
|
|---|---|---|---|---|---|---|---|---|
| M (SE) | M (SE) | M (SE) | M (SE) | M (SE) | F | p | ηp2 | |
| NSI Summary Scores | ||||||||
| Total Score | 28.33 (0.66) | 29.8 (0.55) | 37.18 (0.28) | 38.45 (0.26) | 30.89 (0.69) | 98.18 | <.001 | .061 |
| PST-Mild | 14.50 (0.20) | 15.16 (0.17) | 17.15 (0.09) | 17.70 (0.08) | 15.75 (0.21) | 91.36 | <.001 | .057 |
| PST-Moderate | 9.28 (0.24) | 9.71 (0.21) | 12.30 (0.10) | 13.05 (0.09) | 10.13 (0.26) | 102.56 | <.001 | .063 |
| PST-Severe | 3.69 (0.21) | 4.00 (0.18) | 6.06 (0.09) | 6.03 (0.08) | 4.13 (0.22) | 55.15 | <.001 | .035 |
| Symptom Interference | 1.77 (0.44) | 1.91 (0.04) | 2.24 (0.02) | 2.33 (0.17) | 1.91 (0.05) | 60.42 | <.001 | .041 |
| NSI Symptom Domain Scores | ||||||||
| Vestibular | 2.74 (0.11) | 3.88 (0.09) | 3.22 (0.05) | 3.53 (0.04) | 2.99 (0.11) | 23.04 | <.001 | .015 |
| Somatic/Sensory | 7.27 (0.22) | 8.12 (0.18) | 8.98 (0.09) | 9.68 (0.08) | 8.56 (0.23) | 38.55 | <.001 | .025 |
| Cognitive | 5.49 (0.18) | 6.48 (0.15) | 7.86 (0.08) | 8.22 (0.70) | 6.22 (0.19) | 78.63 | <.001 | .049 |
| Affective | 10.54 (0.25) | 9.97 (0.21) | 14.18 (0.11) | 14.04 (0.10) | 10.75 (0.26) | 132.23 | <.001 | .080 |
| NSI Individual Items | ||||||||
| Feeling dizzy | 0.93 (0.43) | 1.01 (0.04) | 1.10 (0.02) | 1.18 (0.02) | 1.05 (0.45) | 11.57 | <.001 | .008 |
| Loss of balance | 0.94 (0.04) | 0.96 (0.04) | 1.05 (0.02) | 1.16 (0.02) | 0.99 (0.05) | 14.51 | <.001 | .009 |
| Poor coordination, clumsy | 0.87 (0.05) | 0.91 (0.04) | 1.07 (0.02) | 1.07 (0.02) | 1.19 (0.05) | 21.26 | <.001 | .014 |
| Headaches | 1.78 (0.05) | 2.12 (0.05) | 2.06 (0.22) | 2.27 (0.02) | 2.12 (0.06) | 25.26 | <.001 | .016 |
| Nausea | 0.54 (0.05) | 0.63 (0.04) | 0.75 (0.02) | 0.84 (0.02) | 0.72 (0.05) | 14.27 | <.001 | .009 |
| Vision problems | 0.95 (0.05) | 1.10 (0.04) | 1.15 (0.02) | 1.31 (0.02) | 1.06 (0.05) | 20.73 | <.001 | .013 |
| Sensitivity to light | 1.20 (0.06) | 1.47 (0.05) | 1.53 (0.02) | 1.70 (0.02) | 1.43 (0.06) | 22.42 | <.001 | .015 |
| Hearing difficulty | 1.44 (0.05) | 1.51 (0.05) | 1.68 (0.02) | 1.73 (0.02) | 1.38 (0.06) | 15.55 | <.001 | .010 |
| Sensitivity to noise | 1.17 (0.06) | 1.24 (0.05) | 1.58 (0.03) | 1.58 (0.02) | 1.31 (0.06) | 23.66 | <.001 | .015 |
| Numbness or tingling | 1.24 (0.06) | 1.21 (0.05) | 1.44 (0.03) | 1.46 (0.03) | 1.44 (0.06) | 6.55 | <.001 | .004 |
| Change in taste and/or smell | 0.41 (0.04) | 0.37 (0.03) | 0.47 (0.02) | 0.52 (0.02) | 0.48 (0.04) | 4.78 | <.001 | .003 |
| Loss of appetite or increased appetite | 0.85 (0.06) | 0.85 (0.05) | 1.23 (0.02) | 1.24 (0.02) | 0.98 (0.06) | 24.20 | <.001 | .016 |
| Poor concentration | 1.53 (0.05) | 1.85 (0.05) | 2.17 (0.02) | 2.28 (0.02) | 1.78 (0.06) | 60.49 | <.001 | .038 |
| Forgetfulness | 1.76 (0.05) | 2.12 (0.04) | 2.34 (0.02) | 2.46 (0.02) | 2.00 (0.05) | 54.74 | <.001 | .035 |
| Difficulty making decisions | 0.97 (0.06) | 1.07 (0.05) | 1.55 (0.02) | 1.60 (0.02) | 1.05 (0.06) | 59.32 | <.001 | .038 |
| Slowed thinking | 1.23 (0.06) | 1.44 (0.05) | 1.79 (0.02) | 1.88 (0.02) | 1.40 (0.06) | 47.48 | <.001 | .030 |
| Fatigue | 1.52 (0.06) | 1.66 (0.05) | 2.06 (0.02) | 2.08 (0.02) | 1.83 (0.06) | 36.79 | <.001 | .024 |
| Difficulty falling or staying asleep | 2.23 (0.06) | 1.19 (0.05) | 2.68 (0.02) | 2.68 (0.02) | 2.40 (0.06) | 33.90 | <.001 | .022 |
| Feeling anxious or tense | 1.84 (0.05) | 1.64 (0.05) | 2.52 (0.02) | 2.47 (0.02) | 1.77 (0.06) | 118.33 | <.001 | .072 |
| Feeling depressed or sad | 1.38 (0.06) | 1.07 (0.05) | 1.99 (0.03) | 1.92 (0.02) | 1.28 (0.06) | 91.90 | <.001 | .057 |
| Irritability, easily annoyed | 2.01 (0.05) | 1.89 (0.05) | 1.63 (0.02) | 2.60 (0.02) | 1.90 (0.06) | 99.17 | <.001 | .061 |
| Poor frustration tolerance | 1.56 (0.06) | 1.53 (0.05) | 2.30 (0.02) | 2.29 (0.02) | 1.56 (0.06) | 104.48 | <.001 | .065 |
Abbreviations: NSI = Neurobehavioral Symptom Inventory; CTBIE = Comprehensive Traumatic Brain Injury Evaluation; TBI = traumatic brain injury; ES = effect size; PST = Positive Symptom Total.
Notes:
Data reflect only veterans with a CTBIE-confirmed history of TBI (CTBIE+ veterans); N=6,305 (due to missing symptom etiology data); 1. Symptom Resolution: n=408; 2. TBI: n=588; 3. Behavioral Health: n=2,236; 4. Comorbid TBI + Behavioral Health: n=2,702; 5. Other: n=371; however, actual N for each outcome of interest may be less due to missing data for covariates. Adjusted means and standard errors are reported in the table, adjusting for age, sex, race/ethnicity, education, employment status, and marital status. Partial eta-squared (ηp2) effect size interpretation = small (0.01), medium (0.06), large (0.14).
Table 6.
CTBIE Symptom Etiology Groups (CTBIE+ Sample†): Results of Pairwise Comparisons.
| NSI Variables | 1 vs. 2 | 1 vs. 3 | 1 vs. 4 | 1 vs. 5 | 2 vs. 3 | 2 vs. 4 | 2 vs. 5 | 3 vs. 4 | 3 vs. 5 | 4 vs. 5 |
|---|---|---|---|---|---|---|---|---|---|---|
| NSI Summary Scores | ||||||||||
| Total Score | .019 | <.001 | <.001 | .038 | <.001 | <.001 | .779 | <.001 | <.001 | <.001 |
| PST-Mild | .008 | <.001 | <.001 | .011 | <.001 | <.001 | .564 | <.001 | <.001 | <.001 |
| PST-Moderate | .043 | <.001 | <.001 | .076 | <.001 | <.001 | .881 | <.001 | <.001 | <.001 |
| PST-Severe | .078 | <.001 | <.001 | .102 | <.001 | <.001 | .748 | .548 | <.001 | <.001 |
| Symptom Interference | .002 | <.001 | <.001 | .072 | <.001 | <.001 | .755 | <.001 | <.001 | <.001 |
| NSI Symptom Domain Scores | ||||||||||
| Vestibular | .087 | <.001 | <.001 | .264 | .021 | <.001 | .779 | <.001 | .044 | <.001 |
| Somatic/Sensory | <.001 | <.001 | <.001 | .001 | .007 | <.001 | .578 | <.001 | .064 | <.001 |
| Cognitive | <.001 | <.001 | <.001 | .010 | <.001 | <.001 | .100 | <.001 | <.001 | <.001 |
| Affective | .384 | <.001 | <.001 | .724 | <.001 | <.001 | .130 | .672 | <.001 | <.001 |
| NSI Individual Items | ||||||||||
| Feeling dizzy | .082 | <.001 | <.001 | .104 | .118 | <.001 | .920 | <.001 | .207 | .002 |
| Loss of balance | .256 | .034 | <.001 | .832 | .276 | <.001 | .513 | <.001 | .111 | <.001 |
| Poor coordination, clumsy | .176 | <.001 | <.001 | .346 | .003 | <.001 | .968 | <.001 | .034 | <.001 |
| Headaches | <.001 | <.001 | <.001 | <.001 | .084 | .010 | .989 | <.001 | .171 | .027 |
| Nausea | .073 | <.001 | <.001 | .005 | .019 | <.001 | .128 | <.001 | .558 | .016 |
| Vision problems | .009 | <.001 | <.001 | .390 | .708 | <.001 | .131 | <.001 | .044 | <.001 |
| Sensitivity to light | <.001 | <.001 | <.001 | .018 | .843 | <.001 | .451 | <.001 | .184 | <.001 |
| Hearing difficulty | .309 | <.001 | <.001 | .490 | .006 | <.001 | .064 | .020 | <.001 | <.001 |
| Sensitivity to noise | .258 | <.001 | <.001 | .269 | <.001 | <.001 | .939 | .589 | <.001 | <.001 |
| Numbness or tingling | .728 | .003 | <.001 | .060 | .005 | <.001 | .048 | .178 | .714 | .197 |
| Change in taste and/or smell | .771 | .158 | .009 | .322 | .054 | <.001 | .132 | .016 | .931 | .122 |
| Loss of appetite or increased appetite | .687 | <.001 | <.001 | .048 | <.001 | <.001 | .110 | .631 | <.001 | <.001 |
| Poor concentration | <.001 | <.001 | <.001 | .004 | <.001 | <.001 | .197 | <.001 | <.001 | <.001 |
| Forgetfulness | <.001 | <.001 | <.001 | .002 | <.001 | <.001 | .144 | <.001 | <.001 | <.001 |
| Difficulty making decisions | .037 | <.001 | <.001 | .419 | <.001 | <.001 | .152 | .042 | <.001 | <.001 |
| Slowed thinking | <.001 | <.001 | <.001 | .052 | <.001 | <.001 | .203 | .002 | <.001 | <.001 |
| Fatigue | .040 | <.001 | <.001 | .002 | <.001 | <.001 | .217 | .176 | <.001 | <.001 |
| Difficulty falling or staying asleep | .951 | <.001 | <.001 | .079 | <.001 | <.001 | .025 | .590 | <.001 | <.001 |
| Feeling anxious or tense | .077 | <.001 | <.001 | .306 | <.001 | <.001 | .286 | .296 | <.001 | <.001 |
| Feeling depressed or sad | .001 | <.001 | <.001 | .231 | <.001 | <.001 | .057 | .050 | <.001 | <.001 |
| Irritability, easily annoyed | .231 | <.001 | <.001 | .366 | <.001 | <.001 | .752 | .498 | <.001 | <.001 |
| Poor frustration tolerance | .996 | <.001 | <.001 | .918 | <.001 | <.001 | .825 | .973 | <.001 | <.001 |
Abbreviations: CTBIE = Comprehensive Traumatic Brain Injury Evaluation; NSI = Neurobehavioral Symptom Inventory; 1 = Symptom Resolution; 2 = TBI; 3 = Behavioral Health; 4 = Comorbid; 5 = Other; PST = Positive Symptom Total.
Notes:
Data reflect only veterans with a CTBIE-confirmed history of TBI (CTBIE+ veterans). N’s for each symptom etiology group are as follows: 1. Symptom Resolution: n=408; 2. TBI: n=588; 3. Behavioral Health: n=2,236; 4. Comorbid: n=2,702; 5. Other: n=371; however, actual N for each pairwise comparison may be less due to missing data for covariates. The adjusted p-value of each pairwise comparison is presented in the table (covariates include age, sex, race/ethnicity, education, employment status, and marital status).
Follow-up Analyses Examining Neurobehavioral Symptoms Across Symptom Etiology Groups
Given the significant associations between symptom etiology group and neurobehavioral symptoms, we conducted follow-up analyses to explore severe/very severe neurobehavioral symptoms as a function of symptom etiology group. For these analyses, we dichotomized symptom scores into high and low symptom groups (Bouldin et al., 2021; Iverson et al., 2011). High symptoms were defined as a rating of 3 (“severe”) or 4 (“very severe”) whereas low symptoms were defined as a rating of 0 (“none”), 1 (“mild”), or 2 (“moderate”).2 Across all symptom etiology groups, endorsement of severe/very severe vestibular and somatic/sensory symptoms was low (0.0%-1.2%), whereas endorsement of severe/very severe cognitive and affective symptoms was comparatively high (6.5%-27.9%). Table 7 displays the proportion of CTBIE+ veterans reporting severe/very severe symptoms by symptom etiology group, and Supplemental Table 2 displays similar data for the full sample (including both CTBIE+ and CTBIE− veterans).
Table 7.
Neurobehavioral Symptoms by CTBIE Symptom Etiology Group (CTBIE+ Sample†): Proportion of Veterans Reporting ‘Severe/Very Severe’ Symptoms.
| NSI Variables‡ | 1. Symptom Resolution |
2. TBI | 3. Behavioral Health |
4. Comorbid | 5. Other | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | |
| NSI Summary Score | ||||||||||
| Symptom Interference | 82 | 22.2 | 121 | 21.5 | 799 | 37.5 | 977 | 39.1 | 90 | 25.4 |
| NSI Symptom Domain Scores | ||||||||||
| Vestibular | 0 | 0 | 5 | 0.9 | 16 | 0.7 | 33 | 1.2 | 4 | 1.1 |
| Somatic/Sensory | 1 | 0.3 | 1 | 0.2 | 8 | 0.4 | 10 | 0.4 | 1 | 0.3 |
| Cognitive | 38 | 9.3 | 60 | 10.2 | 408 | 18.3 | 489 | 18.1 | 24 | 6.5 |
| Affective | 55 | 13.5 | 67 | 11.4 | 623 | 27.9 | 690 | 25.5 | 45 | 12.1 |
| NSI Individual Items | ||||||||||
| Feeling dizzy | 8 | 2.0 | 25 | 4.3 | 92 | 4.1 | 119 | 4.4 | 15 | 4.0 |
| Loss of balance | 12 | 2.9 | 22 | 3.7 | 104 | 4.7 | 128 | 4.7 | 13 | 3.5 |
| Poor coordination, clumsy | 18 | 4.4 | 21 | 3.6 | 124 | 5.6 | 179 | 6.6 | 18 | 4.9 |
| Headaches | 110 | 27.0 | 227 | 38.6 | 832 | 37.2 | 1,146 | 42.4 | 136 | 36.7 |
| Nausea | 10 | 2.5 | 23 | 3.9 | 95 | 4.3 | 142 | 5.3 | 18 | 4.9 |
| Vision problems | 20 | 4.9 | 69 | 11.7 | 204 | 9.1 | 288 | 10.7 | 30 | 8.1 |
| Sensitivity to light | 61 | 15.0 | 116 | 19.7 | 492 | 22.0 | 649 | 24.0 | 75 | 20.2 |
| Hearing difficulty | 51 | 12.5 | 97 | 16.5 | 504 | 22.5 | 613 | 22.7 | 58 | 15.6 |
| Sensitivity to noise | 53 | 13.0 | 94 | 16.0 | 505 | 22.6 | 571 | 21.1 | 57 | 15.4 |
| Numbness or tingling | 56 | 13.3 | 85 | 14.5 | 457 | 20.4 | 559 | 20.7 | 76 | 20.5 |
| Change in taste and/or smell | 14 | 3.4 | 15 | 2.6 | 73 | 3.3 | 95 | 3.5 | 10 | 2.7 |
| Loss of appetite or increased appetite | 32 | 7.8 | 46 | 7.8 | 370 | 16.6 | 425 | 15.7 | 40 | 10.8 |
| Poor concentration | 85 | 20.8 | 163 | 27.7 | 893 | 39.9 | 1,127 | 41.7 | 99 | 26.7 |
| Forgetfulness | 104 | 20.5 | 206 | 35.0 | 1,031 | 46.1 | 1,321 | 48.9 | 121 | 32.6 |
| Difficulty making decisions | 41 | 10.1 | 73 | 12.4 | 472 | 21.1 | 576 | 21.3 | 32 | 8.6 |
| Slowed thinking | 59 | 14.5 | 120 | 20.4 | 636 | 28.4 | 780 | 28.9 | 65 | 17.5 |
| Fatigue | 87 | 21.3 | 149 | 25.3 | 854 | 38.2 | 973 | 36.0 | 107 | 28.8 |
| Difficulty falling or staying asleep | 191 | 46.8 | 264 | 44.9 | 1,382 | 61.8 | 1,640 | 60.7 | 192 | 51.8 |
| Feeling anxious or tense | 131 | 32.1 | 154 | 26.2 | 1,217 | 54.4 | 1,331 | 49.3 | 102 | 27.5 |
| Feeling depressed or sad | 91 | 22.3 | 84 | 14.3 | 797 | 35.6 | 907 | 33.6 | 57 | 15.4 |
| Irritability, easily annoyed | 152 | 37.3 | 187 | 31.8 | 1,332 | 59.6 | 1,473 | 54.5 | 123 | 33.2 |
| Poor frustration tolerance | 101 | 24.8 | 136 | 23.1 | 1,046 | 46.8 | 1,177 | 43.6 | 87 | 23.5 |
Abbreviations: CTBIE = Comprehensive Traumatic Brain Injury Evaluation; NSI = Neurobehavioral Symptom Inventory; TBI = traumatic brain injury.
Notes:
Data reflect only veterans with a CTBIE-confirmed history of TBI (CTBIE+ veterans); N=6,305; 1. Symptom Resolution: n=408; 2. TBI: n=588; 3. Behavioral Health: n=2,236; 4. Comorbid: n=2,702; 5. Other: n=371; symptom interference data was missing for 390 participants.
NSI variables were dichotomized into high (severe/very severe) and low (none/mild/moderate) symptom groups using a cutoff of ≥3 on each NSI outcome of interest.
Logistic regression analyses adjusting for sociodemographic variables (i.e., age, sex, race/ethnicity, education, employment status, and marital status) were then used to estimate the odds of having severe/very severe symptoms as a function of symptom etiology group for the CTBIE+ veteran sample. Results showed that relative to the Symptom Resolution group (the reference group), both the Comorbid and Behavioral Health groups had increased odds of endorsing severe/very severe cognitive and affective symptoms, as well as symptom interference with daily life, whereas the TBI and Other groups did not (see Table 8). Supplemental Table 3 shows the logistic regression results when analyzing the full sample (including both CTBIE+ and CTBIE− veterans).
Table 8.
Associations Between Neurobehavioral Symptom Scores and CTBIE Symptom Etiology Groups (CTBIE+ Sample†): Logistic Regression Results.
| NSI Variables‡ | 1. Symptom Resolution | 2. TBI | 3. Behavioral Health | 4. Comorbid | 5. Other | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Symptom Interference | 1.00 | Ref | 0.99 | 0.71 – 1.37 | 2.03*** | 1.55 – 2.65 | 2.23*** | 1.71 – 2.90 | 1.17 | 0.82 – 1.67 |
| Vestibular | 1.00 | Ref | 0.49 | 0.11 – 2.20 | 0.63 | 0.21 – 1.92 | 1.08 | 0.37 – 3.10 | 1.00 | -- |
| Somatic/Sensory | 1.00 | Ref | 0.94 | 0.06 – 15.42 | 1.39 | 0.17 – 11.34 | 1.55 | 0.20 – 12.42 | 1.00 | -- |
| Cognitive | 1.00 | Ref | 1.11 | 0.72 – 1.72 | 2.07*** | 1.45 – 2.96 | 2.13*** | 1.49 – 3.03 | 0.66 | 0.38 – 1.14 |
| Affective | 1.00 | Ref | 0.79 | 0.53 – 1.18 | 2.34*** | 1.72 – 3.17 | 2.11*** | 1.56 – 2.86 | 0.89 | 0.58 – 1.38 |
Abbreviations: CTBIE = Comprehensive Traumatic Brain Injury Evaluation; NSI = Neurobehavioral Symptom Inventory; TBI = traumatic brain injury; OR = odds ratio; CI = confidence interval.
Notes: Logistic regression was used to estimate the odds of having ‘severe/very severe’ symptoms as a function of symptom etiology group. The Symptom Resolution group served as the reference group; thus, odds ratios compare the odds of having severe/very severe symptoms in a given symptom etiology group to the odds of having severe/very severe symptoms in the Symptom Resolution group. All models are adjusted for age, sex, race/ethnicity, education, employment status, and marital status.
Data reflect only veterans with a CTBIE-confirmed history of TBI (CTBIE+ veterans); N=6,305; 1. Symptom Resolution: n=408; 2. TBI: n=588; 3. Behavioral Health: n=2,236; 4. Comorbid: n=2,702; 5. Other: n=371; however, actual N for each outcome of interest may be less due to missing data for covariates.
NSI variables were dichotomized into high (severe/very severe) and low (none/mild/moderate) symptom groups using a cutoff of ≥3 on each NSI outcome of interest.
p<.001.
Finally, given the particularly high symptom burden within the Comorbid group, we conducted a final set of analyses focusing on this subgroup of vulnerable veterans. Logistic regression analyses evaluated several predictors associated with being classified in the Comorbid symptom etiology group within the CTBIE+ sample. Predictor variables (all categorical3) included age, sex, race/ethnicity, education, employment status, marital status, and presence of psychiatric symptoms, pain, blast exposure, LOC, and PTA; the most significant predictors of Comorbid group membership included endorsement of psychiatric symptoms (p < .001; OR = 5.92; 95% CI = 4.63-7.57) and pain (p < .001; OR = 1.90; 95% CI = 1.48-2.44) on the CTBIE, as well as several injury-related characteristics including presence of PTA (p < .001; OR = 1.44; 95% CI = 1.25-1.65), LOC (p < .001; OR = 1.40; 95% CI = 1.23-1.61), and blast exposure (p = .001; OR = 1.26; 95% CI = 1.10-1.45).
Discussion
Leveraging a large, nationwide sample of veterans enrolled in the VA’s MVP, we sought to evaluate neurobehavioral symptoms obtained from the CTBIE as a function of (1) clinician-rated TBI diagnostic status (i.e., CTBIE+ vs. CTBIE−) and (2) clinician-rated symptom etiology groups (i.e., Symptom Resolution vs. TBI vs. Behavioral Health vs. Comorbid vs. Other). Results were consistent with our expectations—those with a history of TBI (CTBIE+) experienced a greater symptom burden compared to those without a history of TBI (CTBIE−). Additionally, veterans classified as Comorbid and Behavioral Health experienced the greatest symptom burden compared to all other symptom etiology groups (i.e., Symptom Resolution, TBI, and Other). Our findings have relevant clinical implications and further highlight the importance of prioritizing behavioral health treatments in this veteran cohort.
Prior work by Scholten and colleagues (2012) evaluated a national sample of veterans who completed the CTBIE between 2007 and 2010 and found that while both CTBIE+ and CTBIE− veterans experienced moderate-to-severe neurobehavioral symptoms, CTBIE+ veterans endorsed a significantly higher rate of symptoms. Moreover, they showed that among CTBIE+ veterans, clinicians most often attributed patients’ current symptoms to Comorbid TBI + Behavioral Health conditions (61%), followed by Behavioral Health conditions alone (23%). In our cohort of MVP-enrolled veterans who completed the CTBIE between 2007 and 2019, we similarly showed high rates of neurobehavioral symptoms in both CTBIE+ and CTBIE− veterans, with CTBIE+ veterans endorsing significantly greater symptoms than CTBIE− veterans. We likewise found that among CTBIE+ veterans, clinicians most often attributed patients’ symptoms to Comorbid TBI + Behavioral Health conditions (43%), followed by Behavioral Health conditions alone (36%). Interestingly, when examining the full cohort of veterans in our sample (i.e., CTBIE+ and CTBIE− veterans), clinicians rated Behavioral Health conditions as the leading etiology of patients’ symptoms (48%). Taken together, these findings support the view that behavioral health plays a prominent role in the presentation and maintenance of neurobehavioral symptoms, both in veterans screening positive for TBI and in those with confirmed TBI histories. In fact, the vast majority of the CTBIE+ sample (78.4%) were classified as having a symptom presentation involving a behavioral health component. Although it should be acknowledged that CTBIE+ veterans endorsed significantly greater symptoms than CTBIE− veterans, effect sizes were small and findings highlight the high degree of symptom distress in all veterans completing the CTBIE, regardless of TBI diagnostic status. It is also notable that the most commonly endorsed severe/very severe symptoms (across the full sample of CTBIE+ and CTBIE− veterans) included difficulty falling or staying asleep, irritability, feeling anxious or tense, and forgetfulness—all of which are non-specific to TBI. Importantly, these findings were observed in veterans who passed symptom validity testing, further strengthening the validity of these results.
The more novel aspect of this study was the comparison of neurobehavioral symptoms across CTBIE clinician-rated symptom etiology groups. For these analyses, we focused our findings on the CTBIE+ group; however, the results for the full sample (CTBIE+ and CTBIE− veterans) are provided in the supplemental data. As expected, our results demonstrated that veterans classified as Comorbid and Behavioral Health endorsed the greatest symptom burden, whereas veterans classified as TBI had symptom profiles most similar to the Symptom Resolution group. These findings are consistent with prior research that prospectively recruited distinct groups of veterans (i.e., those with comorbid TBI + PTSD, PTSD-alone, TBI-alone, and controls [those without a history of TBI or PSTD]) and found that the highest rates of neurobehavioral symptoms occurred in veterans with comorbid TBI + PTSD and PTSD-alone as opposed to veterans with TBI-alone and controls (Balba et al., 2018; Combs et al., 2015; Merritt, Jurick, et al., 2019).
Moreover, when examining severe/very severe neurobehavioral symptoms among CTBIE+ veterans in follow-up analyses, the Comorbid and Behavioral Health groups were at least two times more likely than the Symptom Resolution group to endorse cognitive and affective symptoms, as well as significant symptom interference with daily life. In contrast, the symptoms reported by the TBI group were generally comparable to the symptoms reported by the Symptom Resolution group, which is a notable finding in and of itself. Altogether, these findings add further evidence to suggest that behavioral health comorbidities are a driving factor of chronic neurobehavioral symptoms in this population and results highlight the importance of referring distressed veterans for interdisciplinary, complementary treatments including psychoeducation, cognitive rehabilitation, and integrated behavioral health interventions such as cognitive behavioral therapy, cognitive processing therapy, or SMART-CPT (Cooper et al., 2015; Jak et al., 2019).
Finally, when examining CTBIE+ veterans, we found that those who endorsed psychiatric symptoms and pain on the CTBIE and who experienced PTA, LOC, and blast exposure were at greatest risk for being classified in the Comorbid symptom etiology group. No prior studies have examined how clinicians make their symptom etiology ratings, and these data offer insight into the factors that may contribute to, or influence, clinicians’ symptom etiology ratings on the CTBIE. Furthermore, these findings underscore the importance of offering psychoeducation to this group of veterans (Cooper et al., 2015; Venkatesan & Ramanathan-Elion, 2021). Although more research is needed to evaluate the efficacy of psychoeducation offered in the chronic phase of injury, it is possible that providing psychoeducation to patients at the time of CTBIE completion could help patients with managing expectations about symptom etiology, attribution, and recovery, as well as understanding the overlay between common neurobehavioral symptoms and behavioral health symptoms (Cooper et al., 2015; Merritt et al., 2020). Notably, a recent review highlighted the use of “personalized psychoeducation” when working with veterans with a history of TBI (Venkatesan & Ramanathan-Elion, 2021); widespread implementation of this approach could have significant benefits to veterans who undergo the TBI screen and CTBIE.
Although identifying the precise etiology of clinical sequelae observed following TBI is challenging, further elucidating the mechanisms that underly neurobehavioral symptoms in this vulnerable population could significantly aid in tailoring treatment interventions for patients with chronic symptoms. Studies have overwhelmingly shown that structural and brain alterations (e.g., cortical thinning, reduced cerebral blood flow), particularly in frontal and brainstem regions, likely underlie the cognitive and affective symptomatology often observed following neurotrauma (Clark et al., 2018; Delano-Wood et al., 2015; Ozturk & Tan, 2018; Sorg et al., 2014; Sorg et al., 2021; Sorg et al., 2016). Furthermore, it has been suggested that genetic polymorphisms such as apolipoprotein E ε4 (APOE-ε4) status may play a role in neurobehavioral symptom onset and maintenance (Merritt, Lapira, et al., 2019). Consideration of polygenic risk scores for at-risk individuals may also lead to promising insights regarding neurobehavioral symptom reporting in the chronic phase of injury (Polimanti et al., 2017). Future efforts by our laboratory will focus on expanding the results of the current study by exploring these possibilities using MVP clinical and genetic data.
It is important to note study limitations of the present research. Given that our sample included Iraq/Afghanistan-era veterans with a history of deployment who were enrolled in the VA’s MVP and completed the CTBIE, our results may not extend to veterans who served in other eras or who never experienced deployment. Furthermore, our sample was comprised of predominantly male veterans who were likely in the chronic phase of injury, limiting the generalizability of our findings to females, civilians, and those in the acute or post-acute phases of injury. It is also important to highlight that the CTBIE is based on clinicians’ evaluations of patients’ self-reported histories of events that likely took place years prior. Consequently, determining an exact “time since injury” (i.e., days between TBI event and date of CTBIE completion) is difficult, as is corroborating self-reported injury details.
Another study limitation is that our findings are based on retrospective, cross-sectional medical record data; this type of data is subject to potential inaccuracies related to the charting and documentation of TBI. Relatedly, although clinicians who administer the CTBIE are instructed to make their TBI diagnostic decisions based on LOC, AOC, and PTA status alone, we cannot be certain that patients’ self-reported NSI symptoms do not play into clinicians’ diagnostic decisions regarding TBI. Finally, the psychometrics of the TBI Clinical Reminder Screen and CTBIE are also somewhat variable and should be considered in the interpretation of these results (Belanger et al., 2016; Belanger et al., 2012; Donnelly et al., 2011; Fortier et al., 2015; Pape et al., 2018; Pogoda et al., 2014; Radigan et al., 2018; Van Dyke et al., 2010). Nevertheless, the utility of large datasets such as the one used in the present study is considerably valuable and findings from this large clinical epidemiologic study set the stage for future research within MVP to further elucidate the mechanisms that underly neurobehavioral symptoms.
Supplementary Material
Acknowledgments
The authors sincerely thank the Veterans who volunteered to participate in the Million Veteran Program. This research is based on data from the Million Veteran Program, Office of Research and Development, Veterans Health Administration, and was supported by award # IK2 CX001952. This publication does not represent the views of the Department of Veteran Affairs or the United States Government.
Financial Support
This work was supported by a Career Development Award awarded to Dr. Merritt from the VA Clinical Science Research & Development Service (IK2 CX001952). Erin D. Ozturk was funded by the National Science Foundation Graduate Research Fellowship Program.
Footnotes
Note that for this study, data access was limited to veterans enrolled in MVP who completed the CTBIE (in other words, we did not evaluate [or have access to] all veterans with completed CTBIE’s, only those enrolled in MVP with completed CTBIE’s).
For these analyses, symptom domain total scores were transformed to scaled scores by dividing the symptom domain total score by the number of items within that domain; these scaled scores were subsequently dichotomized into high and low groups.
References
- Andrews RJ, Fonda JR, Levin LK, McGlinchey RE, & Milberg WP (2018). Comprehensive analysis of the predictors of neurobehavioral symptom reporting in veterans. Neurology, 91(8), e732–e745. 10.1212/wnl.0000000000006034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armistead-Jehle P (2010). Symptom validity test performance in U.S. veterans referred for evaluation of mild TBI. Appl Neuropsychol, 17(1), 52–59. 10.1080/09084280903526182 [DOI] [PubMed] [Google Scholar]
- Balba NM, Elliott JE, Weymann KB, Opel RA, Duke JW, Oken BS, Morasco BJ, Heinricher MM, & Lim MM (2018). Increased Sleep Disturbances and Pain in Veterans With Comorbid Traumatic Brain Injury and Posttraumatic Stress Disorder. J Clin Sleep Med, 14(11), 1865–1878. 10.5664/jcsm.7482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger HG, Vanderploeg RD, & Sayer N (2016). Screening for Remote History of Mild Traumatic Brain Injury in VHA: A Critical Literature Review. J Head Trauma Rehabil, 31(3), 204–214. 10.1097/HTR.0000000000000168 [DOI] [PubMed] [Google Scholar]
- Belanger HG, Vanderploeg RD, Soble JR, Richardson M, & Groer S (2012). Validity of the Veterans Health Administration's traumatic brain injury screen. Arch Phys Med Rehabil, 93(7), 1234–1239. 10.1016/j.apmr.2012.03.003 [DOI] [PubMed] [Google Scholar]
- Bouldin ED, Swan AA, Norman RS, Tate DF, Tumminello C, Amuan ME, Eapen BC, Wang CP, Trevino A, & Pugh MJ (2021). Health Phenotypes and Neurobehavioral Symptom Severity Among Post-9/11 Veterans With Mild Traumatic Brain Injury: A Chronic Effects of Neurotrauma Consortium Study. J Head Trauma Rehabil, 36(1), 10–19. 10.1097/htr.0000000000000574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle E, Cancelliere C, Hartvigsen J, Carroll LJ, Holm LW, & Cassidy JD (2014). Systematic review of prognosis after mild traumatic brain injury in the military: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch Phys Med Rehabil, 95(3 Suppl), S230–237. 10.1016/j.apmr.2013.08.297 [DOI] [PubMed] [Google Scholar]
- Carlson KF, Nelson D, Orazem RJ, Nugent S, Cifu DX, & Sayer NA (2010). Psychiatric diagnoses among Iraq and Afghanistan war veterans screened for deployment-related traumatic brain injury. J Trauma Stress, 23(1), 17–24. 10.1002/jts.20483 [DOI] [PubMed] [Google Scholar]
- Cicerone K, & Kalmar K (1995). Persistent postconcussion syndrome: The structure of subjective complaints after mild traumatic brain injury. The Journal of Head Trauma Rehabilitation, 10(3), 1–17. 10.1097/00001199-199510030-00002 [DOI] [Google Scholar]
- Clark AL, Merritt VC, Bigler ED, Bangen KJ, Werhane M, Sorg SF, Bondi MW, Schiehser DM, & Delano-Wood L (2018). Blast-Exposed Veterans With Mild Traumatic Brain Injury Show Greater Frontal Cortical Thinning and Poorer Executive Functioning. Front Neurol, 9, 873. 10.3389/fneur.2018.00873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs HL, Berry DT, Pape T, Babcock-Parziale J, Smith B, Schleenbaker R, Shandera-Ochsner A, Harp JP, & High WM Jr. (2015). The Effects of Mild Traumatic Brain Injury, Post-Traumatic Stress Disorder, and Combined Mild Traumatic Brain Injury/Post-Traumatic Stress Disorder on Returning Veterans. J Neurotrauma, 32(13), 956–966. 10.1089/neu.2014.3585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DB, Bunner AE, Kennedy JE, Balldin V, Tate DF, Eapen BC, & Jaramillo CA (2015). Treatment of persistent post-concussive symptoms after mild traumatic brain injury: a systematic review of cognitive rehabilitation and behavioral health interventions in military service members and veterans. Brain Imaging Behav, 9(3), 403–420. 10.1007/s11682-015-9440-2 [DOI] [PubMed] [Google Scholar]
- Delano-Wood L, Bangen KJ, Sorg SF, Clark AL, Schiehser DM, Luc N, Bondi MW, Werhane M, Kim RT, & Bigler ED (2015). Brainstem white matter integrity is related to loss of consciousness and postconcussive symptomatology in veterans with chronic mild to moderate traumatic brain injury. Brain Imaging Behav, 9(3), 500–512. 10.1007/s11682-015-9432-2 [DOI] [PubMed] [Google Scholar]
- Derogatis LR (1994). SCL–90–R symptom checklist 90-R admin-istration, scoring and procedures manual. Minneapolis, MN:National Computer Systems. [Google Scholar]
- Donnelly KT, Donnelly JP, Dunnam M, Warner GC, Kittleson CJ, Constance JE, Bradshaw CB, & Alt M (2011). Reliability, sensitivity, and specificity of the VA traumatic brain injury screening tool. J Head Trauma Rehabil, 26(6), 439–453. 10.1097/HTR.0b013e3182005de3 [DOI] [PubMed] [Google Scholar]
- Fihn SD, Francis J, Clancy C, Nielson C, Nelson K, Rumsfeld J, Cullen T, Bates J, & Graham GL (2014). Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood), 33(7), 1203–1211. 10.1377/hlthaff.2014.0054 [DOI] [PubMed] [Google Scholar]
- Fortier CB, Amick MM, Kenna A, Milberg WP, & McGlinchey RE (2015). Correspondence of the Boston Assessment of Traumatic Brain Injury-Lifetime (BAT-L) clinical interview and the VA TBI screen. J Head Trauma Rehabil, 30(1), E1–7. 10.1097/htr.0000000000000008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaziano JM, Concato J, Brophy M, Fiore L, Pyarajan S, Breeling J, Whitbourne S, Deen J, Shannon C, Humphries D, Guarino P, Aslan M, Anderson D, LaFleur R, Hammond T, Schaa K, Moser J, Huang G, Muralidhar S, Przygodzki R, & O'Leary TJ (2016). Million Veteran Program: A mega-biobank to study genetic influences on health and disease. J Clin Epidemiol, 70, 214–223. 10.1016/j.jclinepi.2015.09.016 [DOI] [PubMed] [Google Scholar]
- Gray M, Adamson MM, Thompson RC, Kapphahn KI, Han S, Chung JS, & Harris OA (2020). Sex differences in symptom presentation and functional outcomes: a pilot study in a matched sample of veterans with mild TBI. Brain Inj, 34(4), 535–547. 10.1080/02699052.2020.1725979 [DOI] [PubMed] [Google Scholar]
- Haagsma JA, Scholten AC, Andriessen TM, Vos PE, Van Beeck EF, & Polinder S (2015). Impact of depression and post-traumatic stress disorder on functional outcome and health-related quality of life of patients with mild traumatic brain injury. J Neurotrauma, 32(11), 853–862. 10.1089/neu.2013.3283 [DOI] [PubMed] [Google Scholar]
- Iverson KM, Hendricks AM, Kimerling R, Krengel M, Meterko M, Stolzmann KL, Baker E, Pogoda TK, Vasterling JJ, & Lew HL (2011). Psychiatric diagnoses and neurobehavioral symptom severity among OEF/OIF VA patients with deployment-related traumatic brain injury: a gender comparison. Womens Health Issues, 21(4 Suppl), S210–217. 10.1016/j.whi.2011.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jak AJ, Jurick S, Crocker LD, Sanderson-Cimino M, Aupperle R, Rodgers CS, Thomas KR, Boyd B, Norman SB, Lang AJ, Keller AV, Schiehser DM, & Twamley EW (2019). SMART-CPT for veterans with comorbid post-traumatic stress disorder and history of traumatic brain injury: a randomised controlled trial. J Neurol Neurosurg Psychiatry, 90(3), 333–341. 10.1136/jnnp-2018-319315 [DOI] [PubMed] [Google Scholar]
- King PR, Donnelly KT, Donnelly JP, Dunnam M, Warner G, Kittleson CJ, Bradshaw CB, Alt M, & Meier ST (2012). Psychometric study of the Neurobehavioral Symptom Inventory. J Rehabil Res Dev, 49(6), 879–888. 10.1682/jrrd.2011.03.0051 [DOI] [PubMed] [Google Scholar]
- Lange RT, French LM, Lippa SM, Bailie JM, & Brickell TA (2020). Posttraumatic Stress Disorder is a Stronger Predictor of Long-Term Neurobehavioral Outcomes Than Traumatic Brain Injury Severity. J Trauma Stress, 33(3), 318–329. 10.1002/jts.22480 [DOI] [PubMed] [Google Scholar]
- Lange RT, Lippa SM, French LM, Bailie JM, Gartner RL, Driscoll AE, Wright MM, Sullivan JK, Varbedian NV, Barnhart EA, Holzinger JB, Schaper AL, Reese MA, Brandler BJ, Camelo-Lopez V, & Brickell TA (2020). Long-term neurobehavioural symptom reporting following mild, moderate, severe, and penetrating traumatic brain injury in U.S. military service members. Neuropsychol Rehabil, 30(9), 1762–1785. 10.1080/09602011.2019.1604385 [DOI] [PubMed] [Google Scholar]
- Lindquist LK, Love HC, & Elbogen EB (2017). Traumatic Brain Injury in Iraq and Afghanistan Veterans: New Results From a National Random Sample Study. J Neuropsychiatry Clin Neurosci, 29(3), 254–259. 10.1176/appi.neuropsych.16050100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor AJ, Dougherty AL, Tang JJ, & Galarneau MR (2013). Postconcussive symptom reporting among US combat veterans with mild traumatic brain injury from Operation Iraqi Freedom. J Head Trauma Rehabil, 28(1), 59–67. 10.1097/HTR.0b013e3182596382 [DOI] [PubMed] [Google Scholar]
- Menatti ARR, Melinder MRD, & Warren SL (2020). Limited Prediction of Performance Validity Using Embedded Validity Scales of the Neurobehavioral Symptom Inventory in an mTBI Veteran Sample. J Head Trauma Rehabil, 35(1), E36–e42. 10.1097/htr.0000000000000467 [DOI] [PubMed] [Google Scholar]
- Merritt VC, Jurick SM, Crocker LD, Hoffman SN, Keller AV, DeFord N, & Jak AJ (2019). Evaluation of objective and subjective clinical outcomes in combat veterans with and without mild TBI and PTSD: A four-group design. J Clin Exp Neuropsychol, 41(7), 665–679. 10.1080/13803395.2019.1610161 [DOI] [PubMed] [Google Scholar]
- Merritt VC, Jurick SM, Sakamoto MS, Crocker LD, Sullan MJ, Hoffman SN, Davey DK, & Jak AJ (2020). Post-concussive symptom endorsement and symptom attribution following remote mild traumatic brain injury in combat-exposed Veterans: An exploratory study. J Psychiatr Res, 130, 224–230. 10.1016/j.jpsychires.2020.08.006 [DOI] [PubMed] [Google Scholar]
- Merritt VC, Lange RT, & French LM (2015). Resilience and symptom reporting following mild traumatic brain injury in military service members. Brain Inj, 29(11), 1325–1336. 10.3109/02699052.2015.1043948 [DOI] [PubMed] [Google Scholar]
- Merritt VC, Lapira KM, Clark AL, Sorg SF, Werhane ML, Jak AJ, Bondi MW, Schiehser DM, & Delano-Wood L (2019). APOE-ε4 Genotype is Associated with Elevated Post-Concussion Symptoms in Military Veterans with a Remote History of Mild Traumatic Brain Injury. Arch Clin Neuropsychol, 34(5), 706–712. 10.1093/arclin/acy082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturk ED, & Tan CO (2018). Human cerebrovascular function in health and disease: insights from integrative approaches. J Physiol Anthropol, 37(1), 4. 10.1186/s40101-018-0164-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape TLB, Smith B, Babcock-Parziale J, Evans CT, Herrold AA, Phipps Maieritsch K, & High WM Jr. (2018). Diagnostic Accuracy of the Veteran Affairs' Traumatic Brain Injury Screen. Arch Phys Med Rehabil, 99(7), 1370–1382. 10.1016/j.apmr.2017.11.017 [DOI] [PubMed] [Google Scholar]
- Pogoda TK, Hendricks AM, Iverson KM, Stolzmann KL, Krengel MH, Baker E, Meterko M, & Lew HL (2012). Multisensory impairment reported by veterans with and without mild traumatic brain injury history. J Rehabil Res Dev, 49(7), 971–984. 10.1682/jrrd.2011.06.0099 [DOI] [PubMed] [Google Scholar]
- Pogoda TK, Iverson KM, Meterko M, Baker E, Hendricks AM, Stolzmann KL, Krengel M, Charns MP, Amara J, Kimerling R, & Lew HL (2014). Concordance of clinician judgment of mild traumatic brain injury history with a diagnostic standard. J Rehabil Res Dev, 51(3), 363–375. 10.1682/jrrd.2013.05.0115 [DOI] [PubMed] [Google Scholar]
- Pogoda TK, Stolzmann KL, Iverson KM, Baker E, Krengel M, Lew HL, Amara JH, & Meterko M (2016). Associations Between Traumatic Brain Injury, Suspected Psychiatric Conditions, and Unemployment in Operation Enduring Freedom/Operation Iraqi Freedom Veterans. J Head Trauma Rehabil, 31(3), 191–203. 10.1097/htr.0000000000000092 [DOI] [PubMed] [Google Scholar]
- Polimanti R, Chen CY, Ursano RJ, Heeringa SG, Jain S, Kessler RC, Nock MK, Smoller JW, Sun X, Gelernter J, & Stein MB (2017). Cross-Phenotype Polygenic Risk Score Analysis of Persistent Post-Concussive Symptoms in U.S. Army Soldiers with Deployment-Acquired Traumatic Brain Injury. J Neurotrauma, 34(4), 781–789. 10.1089/neu.2016.4550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter KE, Stein MB, Martis B, Avallone KM, McSweeney LB, Smith ER, Simon NM, Gargan S, Liberzon I, Hoge CW, & Rauch SAM (2018). Postconcussive symptoms (PCS) following combat-related traumatic brain injury (TBI) in Veterans with posttraumatic stress disorder (PTSD): Influence of TBI, PTSD, and depression on symptoms measured by the Neurobehavioral Symptom Inventory (NSI). J Psychiatr Res, 102, 8–13. 10.1016/j.jpsychires.2018.03.004 [DOI] [PubMed] [Google Scholar]
- Radigan LJ, McGlinchey RE, Milberg WP, & Fortier CB (2018). Correspondence of the Boston Assessment of Traumatic Brain Injury-Lifetime and the VA Comprehensive TBI Evaluation. J Head Trauma Rehabil, 33(5), E51–e55. 10.1097/htr.0000000000000361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholten JD, Sayer NA, Vanderploeg RD, Bidelspach DE, & Cifu DX (2012). Analysis of US Veterans Health Administration comprehensive evaluations for traumatic brain injury in Operation Enduring Freedom and Operation Iraqi Freedom Veterans. Brain Inj, 26(10), 1177–1184. 10.3109/02699052.2012.661914 [DOI] [PubMed] [Google Scholar]
- Schwab K, Terrio HP, Brenner LA, Pazdan RM, McMillan HP, MacDonald M, Hinds SR, & Scher AI (2017). Epidemiology and prognosis of mild traumatic brain injury in returning soldiers: A cohort study. Neurology, 88(16), 1571–1579. 10.1212/WNL.0000000000003839 [DOI] [PubMed] [Google Scholar]
- Seal KH, Bertenthal D, Samuelson K, Maguen S, Kumar S, & Vasterling JJ (2016). Association between mild traumatic brain injury and mental health problems and self-reported cognitive dysfunction in Iraq and Afghanistan Veterans. J Rehabil Res Dev, 53(2), 185–198. 10.1682/jrrd.2014.12.0301 [DOI] [PubMed] [Google Scholar]
- Soble JR, Silva MA, Vanderploeg RD, Curtiss G, Belanger HG, Donnell AJ, & Scott SG (2014). Normative Data for the Neurobehavioral Symptom Inventory (NSI) and post-concussion symptom profiles among TBI, PTSD, and nonclinical samples. Clin Neuropsychol, 28(4), 614–632. 10.1080/13854046.2014.894576 [DOI] [PubMed] [Google Scholar]
- Sorg SF, Delano-Wood L, Luc N, Schiehser DM, Hanson KL, Nation DA, Lanni E, Jak AJ, Lu K, Meloy MJ, Frank LR, Lohr JB, & Bondi MW (2014). White matter integrity in veterans with mild traumatic brain injury: associations with executive function and loss of consciousness. J Head Trauma Rehabil, 29(1), 21–32. 10.1097/HTR.0b013e31828a1aa4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg SF, Merritt VC, Clark AL, Werhane ML, Holiday KA, Schiehser DM, Bondi M, & Delano-Wood L (2021). Elevated Intraindividual Variability in Executive Functions and Associations with White Matter Microstructure in Veterans with Mild Traumatic Brain Injury. J Int Neuropsychol Soc, 27(4), 305–314. 10.1017/S1355617720000879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg SF, Schiehser DM, Bondi MW, Luc N, Clark AL, Jacobson MW, Frank LR, & Delano-Wood L (2016). White Matter Microstructural Compromise Is Associated With Cognition But Not Posttraumatic Stress Disorder Symptoms in Military Veterans With Traumatic Brain Injury. J Head Trauma Rehabil, 31(5), 297–308. 10.1097/HTR.0000000000000189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Ursano RJ, Campbell-Sills L, Colpe LJ, Fullerton CS, Heeringa SG, Nock MK, Sampson NA, Schoenbaum M, Sun X, Jain S, & Kessler RC (2016). Prognostic Indicators of Persistent Post-Concussive Symptoms after Deployment-Related Mild Traumatic Brain Injury: A Prospective Longitudinal Study in U.S. Army Soldiers. J Neurotrauma, 33(23), 2125–2132. 10.1089/neu.2015.4320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke SA, Axelrod BN, & Schutte C (2010). Test-retest reliability of the Traumatic Brain Injury Screening Instrument. Mil Med, 175(12), 947–949. 10.7205/milmed-d-10-00337 [DOI] [PubMed] [Google Scholar]
- Vanderploeg RD, Cooper DB, Belanger HG, Donnell AJ, Kennedy JE, Hopewell CA, & Scott SG (2014). Screening for postdeployment conditions: development and cross-validation of an embedded validity scale in the neurobehavioral symptom inventory. J Head Trauma Rehabil, 29(1), 1–10. 10.1097/HTR.0b013e318281966e [DOI] [PubMed] [Google Scholar]
- Vanderploeg RD, Silva MA, Soble JR, Curtiss G, Belanger HG, Donnell AJ, & Scott SG (2015). The structure of postconcussion symptoms on the Neurobehavioral Symptom Inventory: a comparison of alternative models. J Head Trauma Rehabil, 30(1), 1–11. 10.1097/HTR.0000000000000009 [DOI] [PubMed] [Google Scholar]
- Venkatesan UM, & Ramanathan-Elion DM (2021). Psychoeducation as Precision Health in Military-Related Mild Traumatic Brain Injury. Arch Phys Med Rehabil. 10.1016/j.apmr.2021.08.012 [DOI] [PubMed] [Google Scholar]
- Vos L, Whiteneck GG, Ngan E, Leon Novelo L, Harik LM, & Sherer M (2019). Comparison of the Neurobehavioral Symptom Inventory and the Rivermead Postconcussion Symptoms Questionnaire. Brain Inj, 33(9), 1165–1172. 10.1080/02699052.2019.1637024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


