Abstract
Purpose:
Sensitive methods for risk stratification, monitoring therapeutic efficacy, and early relapse detection may have a major impact on treatment decisions and patient management for stage III colorectal cancer patients. Beyond assessing the predictive power of postoperative ctDNA detection, we explored the added benefits of serial analysis: assessing adjuvant chemotherapy (ACT) efficacy, early relapse detection, and ctDNA growth rates.
Experimental Design:
We recruited 168 patients with stage III colorectal cancer treated with curative intent at Danish and Spanish hospitals between 2014 and 2019. To quantify ctDNA in plasma samples (n = 1,204), 16 patient-specific somatic single-nucleotide variants were profiled using multiplex-PCR, next-generation sequencing.
Results:
Detection of ctDNA was a strong recurrence predictor postoperatively [HR = 7.0; 95% confidence interval (CI), 3.7–13.5; P < 0.001] and directly after ACT (HR = 50.76; 95% CI, 15.4–167; P < 0.001). The recurrence rate of postoperative ctDNA-positive patients treated with ACT was 80% (16/20). Only patients who cleared ctDNA permanently during ACT did not relapse. Serial ctDNA assessment after the end of treatment was similarly predictive of recurrence (HR = 50.80; 95% CI, 14.9–172; P < 0.001), and revealed two distinct rates of exponential ctDNA growth, slow (25% ctDNA-increase/month) and fast (143% ctDNA-increase/month; P < 0.001). The ctDNA growth rate was prognostic of survival (HR = 2.7; 95% CI, 1.1–6.7; P = 0.039). Serial ctDNA analysis every 3 months detected recurrence with a median lead-time of 9.8 months compared with standard-of-care computed tomography.
Conclusions:
Serial postoperative ctDNA analysis has a strong prognostic value and enables tumor growth rate assessment. The novel combination of ctDNA detection and growth rate assessment provides unique opportunities for guiding decision-making.
Translational Relevance.
Sensitive methods for recurrence risk stratification, monitoring therapeutic efficacy, and early recurrence detection may have a major impact on treatment decisions and outcomes for patients with stage III colorectal cancer. Circulating tumor DNA assessments performed postoperative, postadjuvant, and serially during surveillance all allowed stratification of patients into high- and low-risk groups. CtDNA detected recurrence with a significant lead-time compared with CT imaging, and ctDNA growth rates were prognostic of survival. Treatment of ctDNA-positive patients with standard adjuvant therapy prevented recurrence in only 20% of patients. Accordingly, further studies exploring the optimal treatment for ctDNA-positive patients are needed, as well as interventional studies assessing the clinical utility of ctDNA-based risk stratification. A promising opportunity is risk-stratified allocation of surveillance resources, which may improve both the cost-effectiveness and the overall clinical outcome of surveillance. Finally, ctDNA growth rates may identify patients who could benefit from immediate therapeutic intervention compared with awaiting recurrence.
Introduction
Colorectal cancer is a major health burden worldwide (1). Patients with stage III disease have high risk of recurrence, indicating that a subset have residual disease (2). To eliminate potential residual disease, guidelines recommend selecting stage III patients for adjuvant chemotherapy (ACT; ref. 3). However, not all stage III patients have residual disease. It is estimated that 70% are cured by surgery alone (2, 4, 5). Thus, a more precise way to select patients for ACT would be to detect evidence of residual disease directly, thereby sparing the patients cured by surgery of the toxic side effects associated with ACT.
In addition, there are currently no biomarkers that can accurately monitor patients' response to ACT. Treatment failure is not recognized until clinical recurrence is diagnosed. Thus, the ability to determine which patients would recur despite completing ACT would potentially allow placing these patients on an accelerated path to receive additional therapy or intensified surveillance. Today, guidelines recommend radiological surveillance every 6 to 12 months for all patients (3, 6). The reported rate of recurrence in stage III patients is approximately 30% (3, 5, 7). Consequently, approximately 70% of patients who undergo routine posttreatment radiological surveillance do not recur. This indicates an unmet need to better allocate the available surveillance resources to high-risk patients.
Circulating tumor DNA (ctDNA) has emerged as a promising noninvasive biomarker for detection of cancer (8). Several studies have shown postoperative ctDNA detection to be associated with a high risk of recurrence (9–14). Detection of ctDNA can be interpreted as molecular confirmation of subclinical residual disease, and the level of ctDNA as a proxy of tumor burden. An advantage of ctDNA analysis is the ability to assess the ctDNA concentration serially, in principle enabling continuous assessment for molecular recurrence and changes in tumor burden, for example, reflecting treatment response.
Although randomized studies have shown a beneficial effect of ACT in unselected stage III colorectal cancer patients (15–17), it is currently unknown if those with postoperative ctDNA also benefit from ACT. While this ideally should be explored in randomized studies, serial ctDNA assessments before, during and after ACT could potentially give a first indication.
Here, we report results from a prospective, multicenter study exploring the clinical utility of serial ctDNA assessment in a homogenous cohort of patients with stage III colorectal cancer. Analysis included samples at diagnosis, postoperative, during adjuvant therapy and routine follow-up. Beyond confirming the association of postoperative ctDNA detection with high relapse risk, we also explored the added benefit of serial ctDNA analysis, which included the assessment of ACT efficacy, early relapse detection, and ctDNA growth rates and their association with the clinical behavior of relapsing disease.
Materials and Methods
Subjects and study design
This international, multicenter study recruited consecutive stage III colorectal cancer patients (N = 168) treated at six Danish hospitals between July 2014 and February 2019 and the Hospital Clínico Universitario de Valencia in Spain between June 2016 and December 2018. Patients were eligible if scheduled for curative intent treatment, and no metastatic disease was evident on CT of chest, abdomen, and pelvis before surgery. The patient and physician made the ACT treatment decision blinded to the ctDNA result. All patients were treated and monitored according to established National Guidelines. Standard ACT treatment was fluoropyrimidine ± oxaliplatin for 6 months. Twenty-one patients did not receive ACT either due to old age, postsurgical complications, being evaluated in too poor condition by the treating physician, or refusing the recommended ACT for personal reasons. For Danish patients, CT imaging was standardly conducted at 12 and 36 months after surgery. For Spanish patients CT imaging was standardly conducted every 6 months after surgery. For a subset of patients (n = 77), some ctDNA measurements have previously been reported in the context of stage II–III colorectal cancer (9). These results have been pooled with newly generated data on a homogeneous cohort of patients with stage III colorectal cancer. Analysis has been extended on longitudinal plasma samples (n = 374), and we present an additional >18 months of clinical follow-up, reaching 3 years for most patients. The Committees on Biomedical Research Ethics in the Central Region of Denmark and the Spanish ethics committee approved the study. The study was performed in accordance with the Declaration of Helsinki and all participants provided written informed consent.
Sample collection and extraction
For all 160 included patients (Fig. 1), 166 tumor biopsies were collected from the resected primary tumor, either as fresh frozen (n = 100) or as formalin-fixed and paraffin-embedded tissue (FFPE) (n = 66). In patients with synchronous colorectal cancer tumors (n = 5), tissue was collected from all primary tumors. Blood samples were collected in K2-EDTA 10 ml tubes (Becton Dickinson). Plasma was isolated within 2 hours of blood collection by double centrifugation. Buffy coat was collected after the first centrifugation. Plasma and buffy coat were stored at −80°C until use.
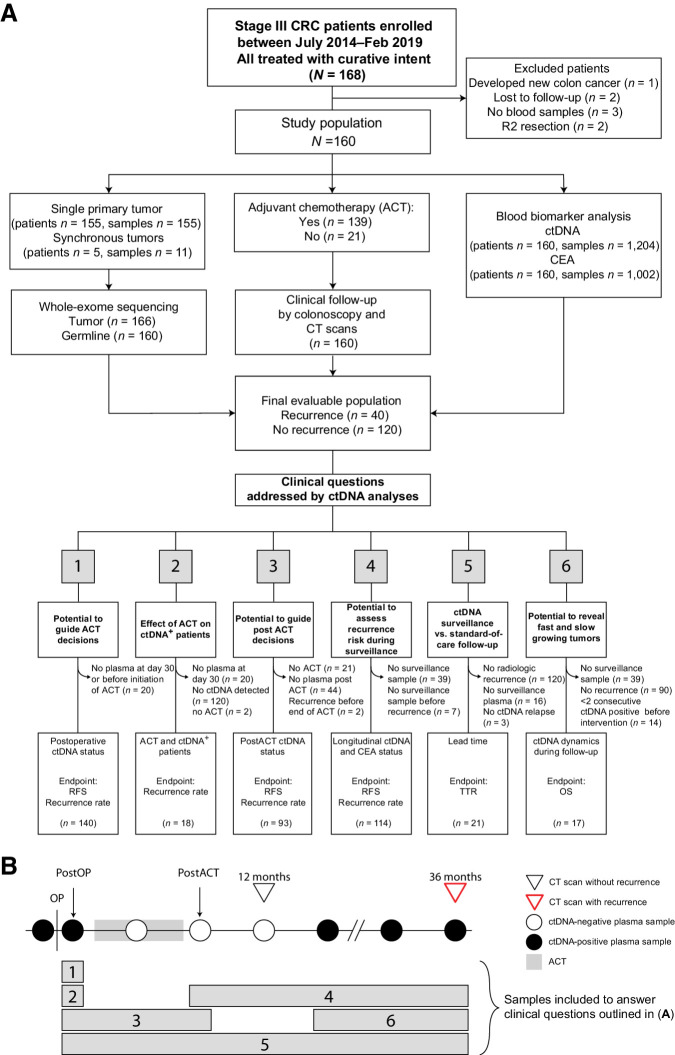
Figure 1.
Inclusion of patients in subanalyses. A, Flow diagram of patient inclusion in subanalyses with clinical questions answered by each analysis denoted. Clinical questions numbered from 1–6. B, Outline of plasma samples included in each subanalysis. Numbered bars correspond to numbered clinical questions denoted in A. CRC, colorectal cancer; OS, overall survival; postOP, postoperative blood sample; postACT, post adjuvant chemotherapy blood sample.
DNA was extracted using the Puregene DNA Purification Kit (Gentra Systems) on fresh-frozen tumor tissue samples, and from FFPE samples using the QiAamp DNA FFPE Tissue Kit (Qiagen).
In tissue and buffy coat, DNA was quantified by the Qubit dsDNA BR Assay Kit (Thermo Fisher Scientific). From plasma samples (median 8 mL; range, 1.3–10 mL) cfDNA was extracted using the QIAamp Circulating Nucleic Acid Kit (Qiagen) and eluted into 50 μL DNA Suspension Buffer (Sigma). Each cfDNA sample was quantified using the Quant-iT High Sensitivity dsDNA Assay Kit (Invitrogen).
Carcinoembryonic antigen analysis
Carcinoembryonic antigen (CEA) analysis was performed on a Cobas e601 platform (Roche), according to the manufacturer's recommendations using 500 μL serum from 1,002 serum samples covering the 160 included patients (median 6 samples/patient, IQR: 3–14 samples/patient; Fig. 1). The threshold levels were set according to national guidelines: in Denmark, 4.0 and 6.0 μg/L for nonsmokers and smokers, respectively; in Spain, 3.4 and 4.3 μg/L for nonsmokers and smokers, respectively. A person who had not smoked for 8 weeks before sample collection was considered a former smoker.
Whole-exome sequencing
A median of 500 ng (range: 181–500 ng) of genomic DNA from tumor and germline was subjected to Illumina-adapter based library preparation and subsequent whole exome sequencing (target size ∼40 Mb) using NovaSeq platform at 2 × 100 bp paired-end sequencing. Tumor and germline samples were sequenced at an average deduplicated on-target coverage of 180× and 50×, respectively. FastQ files were prepared using bcl2fastq2 (RRID:SCR_015058) and quality checked using FastQC (RRID:SCR_014583). Reads were mapped to the human reference genome hg19 using Burrows–Wheeler Alignment tool (v.0.7.12, RRID:SCR_010910) and quality checked using Picard (RRID:SCR_006525) and MultiQC (RRID:SCR_014982). Realignment QC and post-alignment QC metrics (including the total number of reads, deduplicate on-target coverage, uniformity of coverage) were examined to ensure the quality of whole exome sequencing data. SNP genotype concordance between tumor and matched germline DNA samples was examined to identify any sample swaps.
Somatic variant calling and Signatera ctDNA assay design
Somatic variant calling was performed using Natera's consensus variant calling method that uses sequencing input from both tumor tissue and germline. Variants previously reported to be germline in public datasets [1000 Genome project (RRID:SCR_008801), ExAC (RRID:SCR_004068), ESP (RRID:SCR_012761), dbSNP (RRID:SCR_002338)] were filtered out. The whole-exome sequencing (WES) data were then analyzed for quality metrics and sample concordance, prior to being processed through Natera's proprietary bioinformatics pipeline for identification of clonal somatic single-nucleotide variants (SNV). Of the candidate pool of clonal variants identified, a prioritized list of variants was used to design PCR amplicons based on optimized design parameters, ensuring uniqueness in the human genome, amplicon efficiency and primer interaction (9, 13, 18). Assays were successfully designed and applied to cfDNA for all patients.
Plasma DNA library preparation and plasma multiplex-PCR NGS workflow
Following plasma cfDNA extraction, cfDNA libraries were prepared using up to 66 ng (20,000 genome equivalents; Supplementary Fig. S1A) of cfDNA and was subjected to end-repairing, A-tailing and adapter ligation, followed by amplification and purification of the product using Ampure XP beads (Agencourt/Beckman Coulter). Following library preparation, a multiplex targeted PCR was conducted on an aliquot of each library and primers. Amplified, barcoded products were pooled and sequenced at an average depth per amplicon of >100,000× on an Illumina platform. A previously validated cutoff of ≥2 variants detected was used as criteria for ctDNA positivity (9, 13, 18). The cutoff was chosen based on a previously defined confidence threshold necessary to achieve high specificity of >99.8% while maintaining high sensitivity (19). The turnaround time for the first plasma cfDNA analysis including tumor WES and assay development was 2 to 3 weeks, and 1 week for all subsequent plasma ctDNA analyses.
Statistical analysis
Recurrence-free survival (RFS) was used as the primary outcome measure. RFS was assessed by standard radiologic criteria and measured from date of surgery to verified first radiologic recurrence (local or distant). Patients were censored at last follow-up (December 31, 2020) or death. Patients with no follow-up were excluded from the study. Comparison of unmatched groups was done using the Wilcoxon rank sum test for non-normal data checked for normality by Q-Q plot. Comparison of paired data was done using McNemar test on binary data. Cohen Kappa coefficient was used to estimate agreement between overlapping data. Graphical representation of survival was performed using the Kaplan–Meier method. Cox proportional hazards regression analysis was used to assess the impact of age, sex, MMR, resection, pT, pN, histology, tumor differentiation, venous invasion, ctDNA, and CEA on RFS. Multivariable analysis was carried out with backward stepwise Cox regression modeling based on Akaike Information Criterion (AIC) considering all variables from the univariable analysis. In analyses of serial ctDNA and CEA measurements, these were treated as time-varying independent variables. The proportional hazard assumption was tested by a global test of the Schoenfeld residuals.
To calculate the ctDNA growth rates a log-linear regression was fitted to each patient based on ctDNA level as a function of time before recurrence or intervention (Supplementary Table S1). The ctDNA growth rates were estimated from the slope of the regression lines. A histogram of slopes revealed a bimodal distribution. To identify the local minimum between two modes in the distribution, a real valued function was estimated using a kernel smoother with the smallest bandwidth to give a two-modal estimation. The local minimum was determined by applying the second derivative test for local extrema to the function. Cox proportional hazards regression analysis was used to assess the correlation between growth rates and overall survival (OS).
All P values were based on two-sided testing and differences were considered significant at P < 0.05. Statistical analysis was performed using R Statistical software (v.4.0, RRID:SCR_001905).
Data availability
The processed data generated in this study are available within the article and its supplementary data files. Due to privacy laws, access to raw data is restricted. The raw data can only be made available following approval from the ethics committees and data protection agencies in Spain and Denmark. Request for access should be directed to the corresponding author.
Results
Patient enrollment, sample collection and study overview is presented in Fig. 1. A total of 168 patients with stage III colorectal cancer were enrolled. Eight patients were excluded, as they developed metachronous cancer (n = 1), were lost to follow-up (n = 2), only had blood samples collected during ACT (n = 3) or received an R2 resection (n = 2); leaving 160 patients and 1,204 plasma samples (median 7 per patient, IQR 4–11 samples, Supplementary Table S1) for analysis. Table 1 and Supplementary Table S2 provides a summary of patient characteristics. Recurrence was diagnosed in 25% (40/160) of patients. The median follow-up for nonrecurrence patients was 35 months (IQR, 13–36 months). Plasma ctDNA levels were quantified using a previously validated ctDNA analysis pipeline, tracking tumor specific clonal variants in plasma (9, 13). For patients with synchronous primary tumors, clonal variants were tracked for each tumor. The importance of this approach is underlined in Supplementary Fig. S2, for a patient with three synchronous tumors, where only one of the primaries led to development of metastatic disease. Before surgery, ctDNA was detected in 139 of 153 (91%) of available plasma samples.
Table 1.
Patient characteristics, clinicopathologic parameters, and single time-point ctDNA and CEA detection correlated to recurrence-free survival.
| Patient characteristics | Postoperativea | Post-ACTb | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable (N = 140) | Multivariable (N = 120) | Univariable (N = 93) | Multivariable (N = 76) | |||||||||
| Nonrelapsed | Relapsed | All patients | HR (95% CI) | P valuec | HR (95% CI) | P valuec | HR (95% CI) | P valuec | HR (95% CI) | P valuec | ||
| Age | <70 | 73 (60.8) | 20 (50) | 93 (58.1) | — | — | — | — | — | — | — | — |
| ≥70 | 47 (39.2) | 20 (50) | 67 (41.9) | 1.32 (0.70–2.50) | 0.390 | 5.00 (1.81–13.74) | 0.002 | 1.09 (0.39–3.05) | 0.875 | 4.61 (0.76–27.8) | 0.096 | |
| Sex | Female | 46 (38.3) | 19 (47.5) | 65 (40.6) | — | — | — | — | — | — | — | — |
| Male | 74 (61.7) | 21 (52.5) | 95 (59.4) | 0.81 (0.43–1.54) | 0.522 | — | — | 0.59 (0.23–1.49) | 0.267 | — | — | |
| MMR | Deficient | 17 (14.2) | 3 (7.5) | 20 (12.5) | — | — | — | — | — | — | — | — |
| Proficient | 103 (85.8) | 37 (92.5) | 140 (87.5) | 1.72 (0.53–5.60) | 0.366 | 5.55 (1.41–21.75) | 0.019 | 2.20 (0.29–16.59) | 0.443 | — | — | |
| Resection | R0 | 105 (89.0) | 31 (79.5) | 136 (86.6) | — | — | — | — | — | — | — | — |
| R1-R2 | 13 (11.0) | 8 (20.5) | 21 (13.4) | 1.72 (0.75–3.91) | 0.198 | — | — | 0.96 (0.22–4.20) | 0.955 | — | — | |
| pT stage | pT1–2 | 10 (8.3) | 3 (7.5) | 13 (8.1) | — | — | — | — | — | — | — | — |
| pT3–4 | 110 (91.7) | 37 (92.5) | 147 (91.9) | 0.93 (0.75–3.91) | 0.919 | 0.25 (0.05–1.24) | 0.089 | 0.53 (0.12–2.44) | 0.417 | — | — | |
| pN stage | pN1 | 81 (67.5) | 21 (52.5) | 102 (63.8) | — | — | — | — | — | — | — | — |
| pN2 | 39 (32.5) | 19 (47.5) | 58 (36.2) | 1.52 (0.80–2.88) | 0.199 | — | — | 2.20 (0.87–5.59) | 0.096 | — | — | |
| Histology | Adenocarcinoma | 114 (95.0) | 35 (87.5) | 149 (93.1) | — | — | — | — | — | — | — | — |
| Other (medullar or mucinous) | 6 (5.0) | 5 (12.5) | 11 (6.9) | 1.66 (0.59–4.68) | 0.338 | — | — | 2.25 (0.65–7.77) | 0.202 | — | — | |
| Tumor differentiation | Well/moderate | 86 (81.1) | 26 (74.3) | 112 (79.4) | — | — | — | — | — | — | — | — |
| Poor | 20 (18.9) | 9 (25.7) | 29 (20.6) | 1.24 (0.58–2.65) | 0.588 | 2.28 (0.94–5.55) | 0.069 | 2.08 (0.67–6.45) | 0.205 | — | — | |
| Venous invasion | No | 77 (64.7) | 19 (47.5) | 96 (60.4) | — | — | — | — | — | — | — | — |
| Yes | 42 (35.3) | 21 (52.5) | 63 (39.6) | 1.82 (0.96–3.44) | 0.066 | — | — | 2.25 (0.89–5.70) | 0.088 | — | — | |
| ctDNA | Negative | 97 (95.1) | 22 (57.9) | 119 (85.0) | — | — | — | — | — | — | — | — |
| Positive | 5 (4.9) | 16 (42.1) | 21 (15.0) | 7.04 (3.68–13.48) | <0.001 | 30.97 (10.63–90.20) | <0.001 | 50.76 (15.43–167.03) | <0.001 | 94.25 (15.74–564.30) | <0.001 | |
| CEA | No | 85 (85.0) | 31 (81.6) | 116 (84.1) | — | — | — | - | — | — | — | — |
| Yes | 15 (15.0) | 7 (18.4) | 22 (15.9) | 1.49 (0.65–3.39) | 0.342 | 3.43 (1.39–8.42) | 0.009 | 2.70 (0.95–7.69) | 0.063 | — | — | |
| ACT | No | 12 (10.0 | 9 (22.5) | 21 (13.1) | — | — | — | — | — | — | — | — |
| Yes | 108 (90.0) | 31 (77.5) | 139 (86.9) | — | — | — | — | — | — | — | — | |
| AIC | 254.91 | 78.79 | ||||||||||
| Global goodness-of fit test for Cox proportional hazards models | 0.04 | 0.11 | ||||||||||
aPostoperative = Within 8 weeks of surgery, before start of adjuvant chemotherapy.
bPost-ACT = Within 3 months after end of adjuvant chemotherapy.
cStatistically significant P values (P < 0.05) are marked in bold.
Postoperative ctDNA status: sample timing effect and risk of recurrence
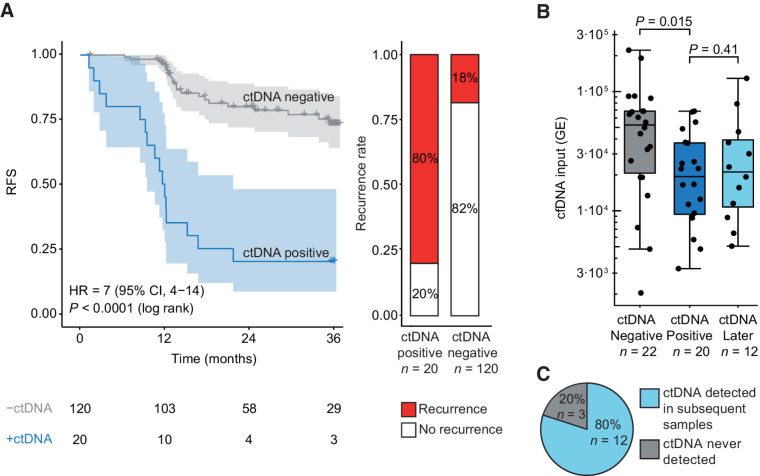
Postoperative plasma samples collected before initiation of ACT were available for 140 patients. The median sampling time point was 2 weeks after surgery (IQR, 2–4 weeks). ctDNA was detected in 14% (20/140) of samples. The recurrence rate was 80% for the ctDNA positive patients (16/20), in contrast to 18% (22/120) for the negative patients. RFS for the 20 ctDNA-positive patients was significantly shorter than those ctDNA-negative [HR = 7.0; 95% confidence interval (CI), 3.7–13.5; P < 0.001; Fig. 2A]. In a multivariable cox regression with stepwise backward variable removal, age ≥70, T4, tumor differentiation, CEA, and postoperative ctDNA status remained predictors of RFS. ctDNA was the strongest predictor (HR = 30.97; 95% CI, 10.63–90.20; P < 0.001; Table 1).
Figure 2.
Detection of ctDNA after surgery. A, Kaplan–Meier plot of RFS stratified for ctDNA detection in blood samples collected within 2 months after surgery. Recurrence rates in ctDNA-positive and ctDNA-negative patients are shown. B, Levels of cell-free DNA (cfDNA, in genome equivalents) in samples that were ctDNA negative immediately after surgery in recurrence patients; ctDNA positive immediately after surgery; or ctDNA positive >2 months after surgery in initially ctDNA-negative recurrence patients. Log-transformed cfDNA levels were compared by a Student t test. C, Recurrence patients without detectable ctDNA immediately after surgery and with samples collected >2 months after surgery were included in this analysis (n = 15). Proportion of patients, initially ctDNA negative, with ctDNA detected in subsequent samples, is shown.
We further sought to explore whether the postoperative sample timing affected the ctDNA detectability. Towards this, we noticed higher cfDNA levels in the 22 ctDNA-negative recurrence patients, than in the 20 ctDNA-positive patients (P = 0.015; Fig. 2B). High levels of wild-type cfDNA could effectively dilute the ctDNA, potentially below the detection level. Recently, increased release of wild-type DNA was reported to be a frequent consequence of surgical trauma (20). The trauma effect is only temporary, lasting up to four weeks (20). Hence, lower cfDNA levels and increased ctDNA detection rates would be expected if samples were collected later. Indeed, when samples collected >2 months after surgery were analyzed, cfDNA levels had decreased (Fig. 2B), and the ctDNA detection rate increased from 0% to 80% (Fig. 2C).
Postadjuvant chemotherapy ctDNA status: clearance and risk of recurrence
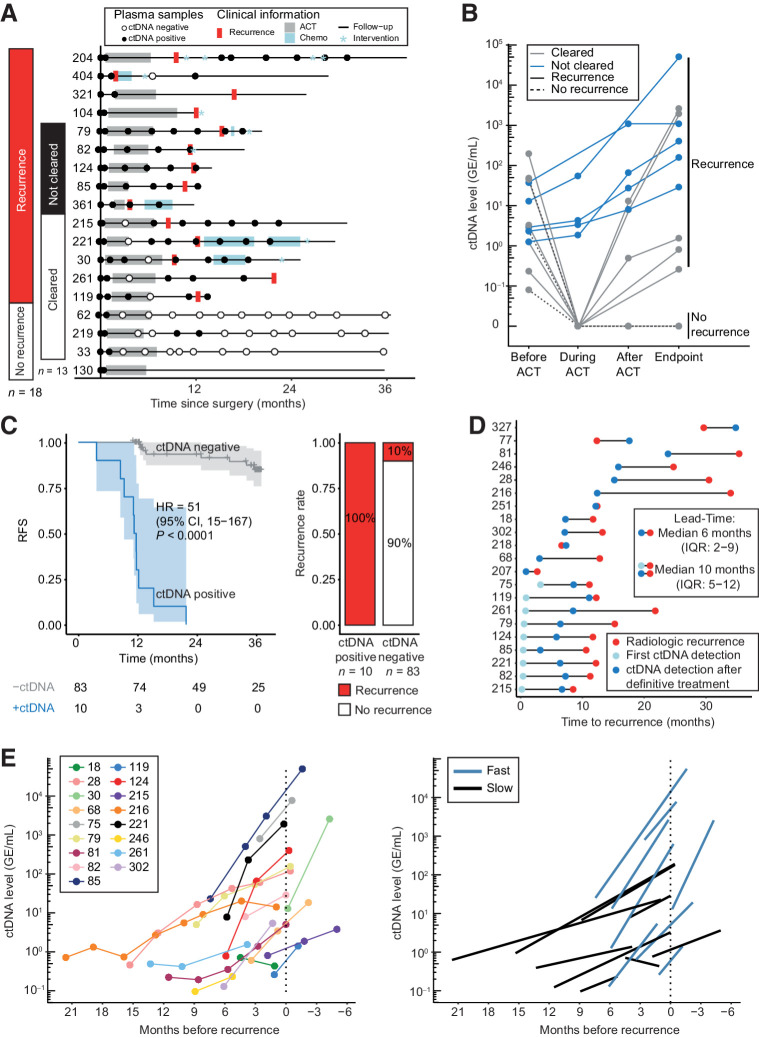
Adjuvant chemotherapy was given to 18 of 20 (90%) patients who were ctDNA positive postoperatively. For 13 of them, samples were collected during and after ACT, for up to 3 years, allowing us to assess the impact of ACT on ctDNA levels (Fig. 3A). Only 3 of 13 (23%; 95% CI, 8.2–50) patients presented a complete and permanent clearance of plasma ctDNA at the end of ACT and in further follow up. These three patients have not relapsed so far, at 36 months of follow-up. However, 10 of 10 (100%) patients, who presented a transient clearance or did not at all clear their plasma ctDNA, relapsed (Fig. 3B). When considering all ACT treated patients with a post-ACT sample available (n = 93), persistence of ctDNA after completing ACT was associated with a significantly shorter RFS (HR = 50.76; 95% CI, 15.4–167; P < 0.001; Fig. 3C). In a multivariable analysis, age ≥70 and post-ACT ctDNA status remained predictors of RFS. ctDNA had the highest effect size (HR = 94.25; 95% CI, 15.74–564.30; P < 0.001; Table 1).
Figure 3.
Using ctDNA for assessment of ACT effect and recurrence risk after end of treatment. A, Overview of blood samples analyzed for ctDNA in patients who were ctDNA positive within 2 months after surgery and received ACT. Patients are grouped according to recurrence status and whether the patient was cleared for ctDNA by ACT. B, ctDNA level before ACT, during ACT, immediately after ACT, and at time of recurrence or end of follow-up (endpoint). C, Kaplan–Meier plot of recurrence-free survival stratified for ctDNA detection in blood samples drawn within three months after the end of ACT. Recurrence rates in ctDNA-positive and ctDNA-negative patients are shown. D, Time to recurrence detection for ctDNA and CT imaging in ctDNA-positive recurrence patients with serially collected plasma samples after end of definitive therapy (n = 21). Lead-time calculated for (i) ctDNA detection after end of definitive therapy (dark blue dot) versus radiologic recurrence (red dot) and (ii) for ctDNA detection at any time (light and dark blue dot) versus radiologic recurrence (red dot). Recurrence was detected by ctDNA before or at the same time as CT imaging in 19 of 24 (79%) recurrence patients. E, An exponential increase in ctDNA levels was observed for recurrence patients after end of definitive treatment. Raw ctDNA measurements for each patient are shown in a unique color (left). Regression line of slow and fast growing ctDNA levels are shown (right).
Longitudinal ctDNA monitoring in posttreatment follow-up
We investigated whether serial ctDNA analysis improved relapse prediction compared with a single ctDNA analysis. To this end, we examined 114 patients with surveillance samples collected after ending definitive treatment (surgery or surgery/ACT). Of these, 24 relapsed, and 88% (21/24) of them tested ctDNA positive during surveillance. For comparison, of the 140 patients in the postoperative cohort 38 relapsed, and of these 42% (16/38) tested ctDNA positive in the postoperative sample.
Next, we explored the potential benefits of including longitudinal ctDNA analysis in posttreatment surveillance programs. Overall, 22 patients tested ctDNA-positive during surveillance and their recurrence rate (96%; 21/22) was significantly higher than in patients who were ctDNA-negative throughout surveillance (3%; 3/92, P < 0.001, Fisher exact test). In total, only 2 of 602 surveillance samples (0.3%) from the 92 nonrecurrence patients, tested ctDNA positive. These samples both belonged to one patient (pt219; Fig. 3A), and were followed by six ctDNA-negative samples. When including all 114 patients in a Cox regression analysis with serial ctDNA as a time-dependent variable, ctDNA detection was associated with a significantly shorter RFS (HR = 50.80; 95% CI, 14.9–172; P < 0.001). After multivariable adjustment, longitudinal ctDNA status was the only significant predictor of RFS (HR = 40.7; 95% CI, 11.6–143; P < 0.001; Table 2).
Table 2.
Patient characteristics, clinicopathologic parameters, and longitudinal ctDNA and CEA detection correlated to recurrence-free survival.
| Longitudinala | |||||
|---|---|---|---|---|---|
| Univariable (N = 114) | Multivariable (N = 114) | ||||
| HR (95% CI) | P valueb | HR (95% CI) | P valueb | ||
| Age | <70 | — | — | — | — |
| ≥70 | 1.43 (0.62–3.26) | 0.400 | — | — | |
| Sex | Female | — | — | — | — |
| Male | 0.52 (0.23–1.16) | 0.111 | — | — | |
| MMR | Deficient | — | — | — | — |
| Proficient | Not applicable | Not applicable | — | — | |
| Resection | R0 | — | — | — | — |
| R1—R2 | 0.70 (0.16–2.97) | 0.625 | — | — | |
| pT stage | pT1–2 | — | — | — | — |
| pT3–4 | 0.38 (0.11–1.33) | 0.131 | — | — | |
| pN stage | pN1 | — | — | — | — |
| pN2 | 1.69 (0.76–3.77) | 0.201 | — | — | |
| Histology | Adenocarcinoma | — | — | — | — |
| Other (medullar or mucinous) | 2.00 (0.60–6.72) | 0.261 | — | — | |
| Tumor differentiation | Well/moderate | — | — | — | — |
| Poor | 1.63 (0.60–3.28) | 0.342 | — | — | |
| Venous invasion | No | — | — | — | — |
| Yes | 1.47 (0.66–3.28) | 0.342 | — | — | |
| ctDNA | Negative | — | — | — | — |
| Positve | 50.80 (14.93–172.92) | <0.001 | 40.7 (11.6 -143) | <0.001 | |
| CEA | No | — | — | — | — |
| Yes | 8.19 (3.24–20.69) | <0.001 | 0.82 (0.27–2.52) | 0.73 | |
| Global goodness-of fit test for Cox proportional hazards model | 0.86 | ||||
aSamples collected serially after end of definitive treatment. ctDNA and CEA were treated as time-varying independent variables.
bStatistically significant P values (P < 0.05) are marked in bold.
ctDNA was detected before radiological recurrence for the majority of the 21 ctDNA-positive recurrence patients. During surveillance after end of definitive treatment, the median lead-time of ctDNA was 6 months (IQR: 2–9; P < 0.001, Wilcoxon signed rank test; Fig. 3D). Notably, ctDNA was detected already prior to conclusion of ACT in 43% (9/21) of these patients (Fig. 3D). Including these samples increased the median lead-time to 10 months (IQR: 5–12). In our cohort, ctDNA sampling was conducted with higher frequency (every 3 months) compared with CT imaging (standard-of-care at 12 and 36 months after surgery). To account for this, another lead-time analysis was conducted on 18 of 21 recurrence patients, where ctDNA was measured at the same time as CT imaging. Interestingly, ctDNA was still detected before recurrence in 33% (6/18) of these patients, with a median lead-time of 10 months (IQR: 8–14 months).
Changes in ctDNA levels indicate clinical behavior of relapses and predict survival
In this cohort, 17 relapsing patients had ≥2 consecutive ctDNA-positive samples (median: 3, range: 2–8) collected during follow up and before clinical recurrence. We investigated ctDNA change as a proxy for tumor growth. Exponential rise in ctDNA levels was observed for all patients (Fig. 3G). Log-linear regression models were fitted to the data, and for each patient, the pace of the increase/decrease in ctDNA was estimated by the slope of the regression line. Using this slope as a continuous variable in a Cox proportional hazard model revealed an association between ctDNA increase and poorer overall survival (OS; HR = 3; 95% CI, 1.1–7; P = 0.039). The distribution of the slopes was bimodal (Supplementary Fig. S3) indicating presence of two distinct growth patterns: fast (47%, 8/17, mean slope = 2.43 ± 0.6 SD, 143% increase/month) or slow (53%, 9/17, mean slope = 1.25 ± 0.17 SD, 25% increase/month; P < 0.001, Wilcoxon rank sum test; Fig. 3E). We compared the survival of the slow and fast groups to the survival of the 89 nonrecurrence patients from the longitudinal analysis. This revealed a similar OS for the nonrelapsing patients and the relapsing patients with slow growth (P = 0.18). Conversely, OS was significantly reduced for relapsing patients with fast growth (HR = 42; 95% CI, 8–221; P < 0.001; Supplementary Fig. S3). The clinical relevance of the fast and slow growth is indicated by the ctDNA fold changes observed from first ctDNA detection to radiological recurrence (fast: median fold change 117; range, 2–609; slow: median fold change 9; range, 0.5–358). We explored if the growth pattern could be robustly assessed using only the first two samples. A good agreement was observed, with 88% (15/17) of patients being classified to the same group as when using all available samples (P = 0.48, McNemar test; Cohen Kappa = 0.77, Supplementary Fig. S3). Similar agreements were reached when using any two consecutive time points, illustrating the robustness of the fast/slow calls (Supplementary Table S3).
Discussion
A validated and a sensitive biomarker could potentially improve outcomes in patients with stage III colorectal cancer by better: (i) defining risk of recurrence; (ii) predicting the outcome of ACT; (iii) identifying patients that may need additional treatment post-ACT; (iv) detecting recurrence during surveillance; and (v) predicting the growth rate of tumor burden, and thereby informing on the urgency of intervention.
The current study emphasizes on serial ctDNA measurements in patients with stage III colorectal cancer and demonstrates ctDNA as a prognostic marker after surgery with a potential to guide ACT decision-making. Furthermore, ctDNA was the strongest prognostic marker in multivariable analysis with conventionally used risk markers. The findings are consistent with and extend on previous colorectal cancer studies (9, 11, 12, 21–23). Together, these results have prompted planning and initiation of a range of prospective trials, investigating the benefit of ctDNA-guided ACT administration for patients with stage III colorectal cancer (24–27), many with an overarching aim to deescalate treatment for ctDNA-negative patients. For these studies, a high NPV of the ctDNA analysis is paramount. Of importance, our study showed how timing of postoperative blood sample collection could affect the NPV. We observed a surprisingly high recurrence rate (18%) for the postoperative ctDNA-negative patients, and our subsequent analyses suggested these false negatives were rooted in the blood sample timing. The majority of our postoperative blood samples (84%) were collected 2 to 4 weeks postsurgery. Incidentally, this interval overlapped with the recently identified 4-week surge in cfDNA caused by surgical trauma (20). Consistent with the wild-type cfDNA surge, the ctDNA-negative recurrence patients had high cfDNA levels, indicating that trauma-induced cfDNA may have diluted the ctDNA below our detection limit. In agreement, analysis of later samples, with normalized cfDNA levels, revealed ctDNA detection in 80% of the initially negative recurrence patients. Accordingly, in studies investigating treatment deescalation, it may be beneficial to collect an additional sample after week 4, as sensitivity of a single time point measurement close to surgery may be limited. This would allow normalization of high cfDNA before concluding on the ctDNA assessment, thereby improving the overall NPV.
The best treatment approach for ctDNA-positive patients remains an open question; for example, it is unclear to what extent these patients may benefit from standard ACT (28). Although limited by small numbers, our data showed 23% (95% CI, 8–50; 3/13) of the ACT-treated ctDNA-positive patients with plasma samples collected during/after ACT did not recur during three years of follow-up. This result was corroborated by our post-ACT serial ctDNA analysis, where these 23% showed ctDNA clearance. Hence, our results provide initial evidence that standard ACT may benefit a minor fraction of ctDNA-positive patients. The observed risk-reduction is consistent with the approximately 30% reported when standard ACT is administered to unselected stage III colon cancer patients (15–17, 29). However, the benefit of treating ctDNA positive patients should ultimately be assessed in randomized studies, such as the ongoing IMPROVE-IT trial (NCT03748680). Potentially, ctDNA-positive patients will benefit more from future adjuvant regimens (28). To determine the optimal regimen and associated benefit, prospective randomized studies are needed (25, 30).
Serial ctDNA analyses during and after ACT may further inform on ACT effectiveness. We observed a proportion of patients, who never cleared ctDNA during ACT, and these patients all recurred. Consequently, without clearance recurrence appears inevitable. Clearance, however, was temporal in 50% of cases and was not a guarantee for no-recurrence. Therefore, deescalation of therapy based on a single ctDNA measurement may not be warranted, and ctDNA status during and after end of therapy should be monitored. For this purpose, our study demonstrated ctDNA as a strong prognostic marker immediately post-ACT. This is consistent with previous studies in smaller and more heterogeneous cohorts of patients with colorectal cancer (9, 22).
Uniquely, we collected samples longitudinally during posttreatment surveillance, allowing us to demonstrate that serial ctDNA assessment, in comparison with single time point analysis, improves the sensitivity of the overall analysis, while retaining a high specificity. This high accuracy of serial ctDNA assessment opens new opportunities for ctDNA analysis, beyond postoperative assessment for residual disease. Such opportunities could be longitudinal risk-stratified allocation of imaging resources for recurrence surveillance. Our results suggest that radiological surveillance may be deescalated in low-risk (ctDNA-negative) patients with no/minimal effect on the outcome. Expectedly, this would lower surveillance costs, as this subgroup constitute the vast majority of patients. For high-risk (ctDNA-positive) patients, there is an opportunity for intensifying imaging immediately upon ctDNA detection. In our study, patients were standard surveilled by CT imaging at 12 and 36 months after surgery. Compared with this, we report a median lead-time of 6 months for ctDNA. This implies that by ctDNA-guided imaging allocation, imaging would be initiated a median 6 months earlier for ctDNA positive patients than by standard-of-care surveillance in Denmark and Spain. Accordingly, it could enable earlier recurrence detection, when tumor burden is lower, potentially making recurrence treatment more effective. Ideally, the clinical and health economic benefits of ctDNA-guided surveillance should be demonstrated in randomized studies.
The importance of early recurrence detection and intervention is emphasized by our ctDNA growth rate assessments, showing that 47% of recurrence patients have a fast ctDNA growth pattern, that is, a median 143% monthly increase. Assumedly, this increase in ctDNA reflects increased tumor burden. Hence, even a few months of prolonged surveillance may have insurmountable consequences, for example, a 14-fold increase in tumor burden in just 3 months, indicating that the size and/or number of metastatic lesions may quickly reach a level where curative intervention is no longer an option, and where palliative treatment will be less effective. Consistent with these assumptions, we found that patients with fast growth had a significantly poorer OS than those with slow growth. The growth pattern may thus inform clinicians on the urgency of intervention. In our study, growth patterns could be robustly assessed based on the first two consecutive blood samples with a 3-month interval. If growth patterns could be determined even quicker, for example, from samples collected with a few weeks apart, it would further increase the clinical utility. Further studies are needed to address this.
Our analysis comparing concurrent ctDNA and CT imaging assessments showed that in 33% of patients, who later recurred, ctDNA was detected at a time where no recurrence was visible by CT imaging. This indicates that ctDNA measurements in some cases may be more sensitive for recurrence detection than standard CT imaging. Consequently, it can be foreseen that ctDNA-guided surveillance could lead to a clinical dilemma, where no recurrence is visible on CT imaging after a positive ctDNA analysis. In this situation, waiting until the disease becomes visible by imaging could mean the advent of bulky disease. Potentially, a quick assessment of ctDNA growth pattern could help inform the decision, whether to initiate systemic therapy immediately or to continue imaging surveillance. Another possibility would be to consider a transition from CT imaging to alternative modalities with potential for a higher sensitivity, for example, PET/CT and liver MRI. These possibilities should optimally be explored in randomized studies.
To the best of our knowledge, we here report the largest study on stage III colorectal cancer; nevertheless, our study is limited by the modest number of patients included in the reported subset analyses. In addition, the relatively small number of patients with detectable ctDNA makes the study susceptible to inherent biases, which may limit the generalizability of the reported findings. Notwithstanding this, our finding that postoperative detection of ctDNA is a robust predictor of disease recurrence is consistent with several recent reports. We also note that in one patient we observed two consecutively positive ctDNA measurements, followed by multiple ctDNA negative measurements in the serial analysis. Whether these samples were true false positives or the signal disappeared due to other factors (such as immune activation; ref. 31) is unclear. Furthermore, we observed a few cases of no or late ctDNA detection in recurrence patients with serial sampling. Hence, studies aimed at using ctDNA to guide the intensity of radiological surveillance should consider a control CT-scan, for example, at 12 months to identify the few patients that might not shed enough ctDNA for detection. Finally, it should be noted that the standard-of-care imaging frequency differed between the Spanish (every 6 months) and Danish (at month 12 and 36) patients. This might have affected the ctDNA lead-time estimates, which should be viewed in this context.
Conclusion
This study represents one of the most comprehensive studies on ctDNA detection in stage III colorectal cancer and expands on previous studies conducted on smaller cohorts. We highlight several clinical utilities of ctDNA, some of which are currently being investigated in randomized trials, such as the IMPROVE-IT (27), DYNAMIC-III (26), VEGA (25), TRACC trials (24) for ctDNA-stratified administration of ACT, and the IMPROVE-IT2 trial (32) for using serial ctDNA measurements as risk stratification to allocate radiological surveillance resources.
Authors' Disclosures
S. Sharma reports other support from Natera Inc. during the conduct of the study and outside the submitted work. D. Renner reports other support from Natera Inc. during the conduct of the study and outside the submitted work. D. Hafez reports other support from Natera, Inc during the conduct of the study and outside the submitted work. H. Sethi reports other support from Natera, Inc during the conduct of the study. A. Aleshin reports other support from Natera, Inc. during the conduct of the study, Natera, Inc. outside the submitted work, and is an employee with stock/options. A. Cervantes reports grants from Natera and Instituto de Salud Carlos III during the conduct of the study. C.L. Andersen reports grants from Natera during the conduct of the study as well as grants from Danish Cancer Society, The Novo Nordisk Foundation, Innovation Fund Denmark, The Mark Foundation for Cancer Research, and C2i Genomics outside the submitted work. No disclosures were reported by the other authors.
Supplementary Material
Acknowledgments
We extend our thanks to the patients and their families and acknowledge the Danish Cancer Biobank for providing access to blood and tissue materials. Editorial input was provided by Meenakshi Malhotra from Natera Inc. This study was supported by the Novo Nordisk Foundation [grant number NNF17OC0025052 (C.L. Andersen)]; the Danish Cancer Society [grant numbers R133-A8520-00-S41 (C.L. Andersen), R146-A9466-16-S2 (C.L. Andersen), R231-A13845 (C.L. Andersen), and R257-A14700 (C.L. Andersen)]; Dansk Kræftforskningsfond [grant number FID1839672 (T.V. Henriksen)]; Krista and Viggo Petersen Foundation [grant number 6030/67 (T.V. Henriksen)]; Aarhus University; Instituto de Salud Carlos III [grant numbers PI18/01909 (A. Cervantes and D. Roda) and PT17/0015/0049 (INCLIVA BioBank)]; Juan Rodés [grant numbers JR16/00040 (D. Roda) and JR20/00005 (N. Tarazona)]; and Rio Hortega [grant number CM15/246 (N. Tarazona)]. ctDNA analysis was provided by Natera without cost. The opinions, results, and conclusions reported in this article are those of the authors, and are independent from these funding sources.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Contributions
T.V. Henriksen: Data curation, formal analysis, funding acquisition, investigation, visualization, writing–original draft, writing–review and editing. N. Tarazona: Data curation, funding acquisition, investigation, writing–original draft, writing–review and editing. A. Frydendahl: Data curation, formal analysis, investigation, visualization, writing–original draft, writing–review and editing. T. Reinert: Data curation, investigation, writing–review and editing. F. Gimeno-Valiente: Investigation, writing–review and editing. J.A. Carbonell-Asins: Formal analysis, investigation, writing–review and editing. S. Sharma: Data curation, investigation, methodology, writing–review and editing. D. Renner: Data curation, methodology, writing–review and editing. D. Hafez: Data curation, methodology, writing–review and editing. D. Roda: Resources, data curation, funding acquisition, writing–review and editing. M. Huerta: Resources, data curation, writing–review and editing. S. Roselló: Resources, data curation, writing–review and editing. A.H. Madsen: Resources, data curation, writing–review and editing. U.S. Løve: Resources, data curation, writing–review and editing. P.V. Andersen: Resources, data curation, writing–review and editing. O. Thorlacius-Ussing: Resources, data curation, writing–review and editing. L.H. Iversen: Resources, data curation, writing–review and editing. K.A. Gotschalck: Resources, data curation, writing–review and editing. H. Sethi: Resources, data curation, methodology, writing–review and editing. A. Aleshin: Resources, data curation, supervision, funding acquisition, investigation, methodology, project administration, writing–review and editing. A. Cervantes: Conceptualization, resources, data curation, supervision, funding acquisition, investigation, writing–original draft, project administration, writing–review and editing. C.L. Andersen: Conceptualization, resources, data curation, supervision, funding acquisition, investigation, writing–original draft, project administration, writing–review and editing.
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2020;71:209–49. [DOI] [PubMed] [Google Scholar]
- 2. Osterman E, Hammarström K, Imam I, Osterlund E, Sjöblom T, Glimelius B. Recurrence risk after radical colorectal cancer surgery-less than before, but how high is it? Cancers 2020;12:3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Argilés G, Tabernero J, Labianca R, Hochhauser D, Salazar R, Iveson T, et al. Localised colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up y on behalf of the ESMO Guidelines Committee. Ann Oncol 2020;31:1291–305. [DOI] [PubMed] [Google Scholar]
- 4. Pahlman LA, Hohenberger WM, Matzel K, Sugihara K, Quirke P, Glimelius B. Should the benefit of adjuvant chemotherapy in colon cancer be re-evaluated? J Clin Oncol 2016;34:1297–9. [DOI] [PubMed] [Google Scholar]
- 5. Osterman E, Glimelius B. Recurrence risk after up-to-date colon cancer staging, surgery, and pathology. Dis Colon Rectum 2018;61:1016–25. [DOI] [PubMed] [Google Scholar]
- 6. Meyerhardt JA, Mangu PB, Flynn PJ, Korde L, Loprinzi CL, Minsky BD, et al. Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol 2013;31:4465–70. [DOI] [PubMed] [Google Scholar]
- 7. Lash TL, Riis AH, Ostenfeld EB, Erichsen R, Vyberg M, Ahern TP, et al. Associations of statin use with colorectal cancer recurrence and mortality in a danish cohort. Am J Epidemiol 2017;186:679–87. [DOI] [PubMed] [Google Scholar]
- 8. Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med 2014;6:224ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reinert T, Henriksen TV, Christensen E, Sharma S, Salari R, Sethi H, et al. Analysis of plasma cell-free DNA by ultradeep sequencing in patients with stages i to iii colorectal Cancer. JAMA Oncol 2019;5:1124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Christensen E, Birkenkamp-Demtröder K, Sethi H, Shchegrova S, Salari R, Nordentoft I, et al. Early detection of metastatic relapse and monitoring of therapeutic efficacy by ultra-deep sequencing of plasma cell-free DNA in patients with urothelial bladder carcinoma. J Clin Oncol 2019;37:1547–57. [DOI] [PubMed] [Google Scholar]
- 11. Schøler LV, Reinert T, Ørntoft M-BW, Kassentoft CG, Árnadóttir SS, Vang S, et al. Clinical implications of monitoring circulating tumor DNA in patients with colorectal cancer. Clin Cancer Res 2017;23:5437–45. [DOI] [PubMed] [Google Scholar]
- 12. Tarazona N, Gimeno-Valiente F, Gambardella V, Zuñiga S, Rentero-Garrido P, Huerta M, et al. Targeted next-generation sequencing of circulating-tumor DNA for tracking minimal residual disease in localized colon cancer. Ann Oncol 2019;30:1804–12. [DOI] [PubMed] [Google Scholar]
- 13. Abbosh C, Birkbak NJ, Wilson GA, Jamal-Hanjani M, Constantin T, Salari R, et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature 2017;545:446–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parikh AR, Van Seventer EE, Siravegna G. Minimal residual disease detection using a plasma-only circulating tumor DNA assay in colorectal cancer patients. Clin Cancer Res 2021; Available from: https://clincancerres.aacrjournals.org/content/early/2021/04/28/1078-0432.CCR-21-0410.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109–16. [DOI] [PubMed] [Google Scholar]
- 16. Gill S, Loprinzi CL, Sargent DJ, Thomé SD, Alberts SR, Haller DG, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: Who benefits and by how much? J Clin Oncol 2004;22:1797–806. [DOI] [PubMed] [Google Scholar]
- 17. Haller DG, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011;29:1465–71. [DOI] [PubMed] [Google Scholar]
- 18. Magbanua MJM, Swigart LB, Wu H-T, Hirst GL, Yau C, Wolf DM, et al. Circulating tumor DNA in neoadjuvant-treated breast cancer reflects response and survival. Ann Oncol 2021;32:229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coombes RC, Page K, Salari R, Hastings RK, Armstrong A, Ahmed S, et al. Personalized detection of circulating tumor DNA antedates breast cancer metastatic recurrence. Clin Cancer Res 2019;25:4255–63. [DOI] [PubMed] [Google Scholar]
- 20. Henriksen TV, Reinert T, Christensen E, Sethi H, Birkenkamp-Demtröder K, Gögenur M, et al. The effect of surgical trauma on circulating free DNA levels in cancer patients—implications for studies of circulating tumor DNA. Mol Oncol. Wiley; 2020;14:1670–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reinert T, Schøler LV, Thomsen R, Tobiasen H, Vang S, Nordentoft I, et al. Analysis of circulating tumour DNA to monitor disease burden following colorectal cancer surgery. Gut 2016;65:625–34. [DOI] [PubMed] [Google Scholar]
- 22. Tie J, Cohen JD, Wang Y, Christie M, Simons K, Lee M, et al. Circulating tumor DNA analyses as markers of recurrence risk and benefit of adjuvant therapy for stage iii colon cancer. JAMA Oncol 2019;5:1719–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tie J, Wang Y, Tomasetti C, Li L, Springer S, Kinde I, et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med 2016;8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anandappa G, Starling N, Peckitt C, Bryant A, Begum R, Carter P, et al. TRACC: Tracking mutations in cell-free DNA to predict relapse in early colorectal cancer—A randomized study of circulating tumour DNA (ctDNA) guided adjuvant chemotherapy versus standard of care chemotherapy after curative surgery in patients with high risk stage II or stage III colorectal cancer (CRC). J Clin Orthod 2020;38:TPS4120. [Google Scholar]
- 25. Yukami H, Mishima S, Kotani D, Oki E, Taniguchi H, Nakamura Y, et al. P-120 Prospective observational study monitoring circulating tumor DNA in resectable colorectal cancer patients undergoing radical surgery: GALAXY study in CIRCULATE-Japan (trial in progress). Ann Oncol 2020;31:S128–9. [Google Scholar]
- 26. DYNAMIC-III: ANZCTR - Registration [Internet]. [cited 2021 Mar 5]. Available from: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=373948.
- 27. IMPROVE intervention trial implementing non-invasive circulating tumor dna analysis to optimize the operative and postoperative treatment for patients with colorectal cancer - full text view - clinicalTrials.gov [Internet]. [cited 2021 Mar 5]. Available from:https://clinicaltrials.gov/ct2/show/NCT03748680. [DOI] [PubMed]
- 28. Dasari A, Morris VK, Allegra CJ, Atreya C, Benson AB 3rd, Boland P, et al. ctDNA applications and integration in colorectal cancer: an NCI colon and rectal-anal task forces whitepaper. Nat Rev Clin Oncol 2020;17:757–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Upadhyay S, Dahal S, Bhatt VR, Khanal N, Silberstein PT. Chemotherapy use in stage III colon cancer: a national cancer database analysis. Ther Adv Med Oncol 2015;7:244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lonardi S, Montagut C, Pietrantonio F, Elez E, Sartore-Bianchi A, Tarazona N, et al. The PEGASUS trial: Post-surgical liquid biopsy-guided treatment of stage III and high-risk stage II colon cancer patients. J Clin Orthod 2020;38:TPS4124–. [Google Scholar]
- 31. Tie J, Cohen JD, Lo SN, Wang Y, Li L, Christie M, et al. Prognostic significance of postsurgery circulating tumor DNA in nonmetastatic colorectal cancer: Individual patient pooled analysis of three cohort studies. Int J Cancer 2021;148:1014–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nors J, Henriksen TV, Gotschalck KA, Juul T, Søgaard J, Iversen LH, et al. IMPROVE-IT2: implementing noninvasive circulating tumor DNA analysis to optimize the operative and postoperative treatment for patients with colorectal cancer–intervention trial 2. Study protocol. Acta Oncol 2020:59:336–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The processed data generated in this study are available within the article and its supplementary data files. Due to privacy laws, access to raw data is restricted. The raw data can only be made available following approval from the ethics committees and data protection agencies in Spain and Denmark. Request for access should be directed to the corresponding author.