Abstract
Purpose:
Standard-dose pembrolizumab plus alternative-dose ipilimumab (1 mg/kg Q3W for 4 doses) were tolerable and had robust antitumor activity in advanced melanoma in cohort B of the phase 1 KEYNOTE-029 study. Cohort C evaluated standard-dose pembrolizumab with two other alternative ipilimumab regimens.
Patients and Methods:
Patients with treatment-naive unresectable stage III/IV melanoma were randomly assigned 1:1 to pembrolizumab 200 mg Q3W for ≤24 months plus ipilimumab 50 mg Q6W for 4 doses (PEM200+IPI50), or the same pembrolizumab regimen plus ipilimumab 100 mg Q12W for 4 doses (PEM200+IPI100). Primary end points were incidence of grade 3–5 treatment-related adverse events (TRAE) and objective response rate (ORR) per RECIST v1.1 by independent central review. Per protocol-defined thresholds, grade 3–5 TRAE incidence ≤26% indicated meaningful toxicity reduction and ORR ≥48% indicated no decrease in efficacy versus data reported for other PD-1 inhibitor/ipilimumab combinations.
Results:
Median follow-up on February 18, 2019, was 16.3 months in PEM200+IPI50 (N = 51) and 16.4 months in PEM200+IPI100 (N = 51). Grade 3–5 TRAEs occurred in 12 (24%) patients in PEM200+IPI50 and 20 (39%) in PEM200+IPI100. One patient in PEM200+IPI50 died from treatment-related autoimmune myocarditis. Immune-mediated AEs or infusion reactions occurred in 21 (42%) patients in PEM200+IPI50 and 28 (55%) in PEM200+IPI100. ORR was 55% in PEM200+IPI50; 61% in PEM200+IPI100.
Conclusions:
Pembrolizumab 200 mg Q3W plus ipilimumab 50 mg Q6W or 100 mg Q12W demonstrated antitumor activity above the predefined threshold; pembrolizumab plus ipilimumab 50 mg Q6W had lower incidence of grade 3–5 TRAEs than the predefined threshold, suggesting a reduction in toxicity.
See related commentary by Jameson-Lee and Luke, p. 5153
Translational Relevance.
Combining programmed death 1 (PD-1) and cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) inhibitors provide substantial long-term benefit albeit with considerable toxicity in advanced melanoma. CTLA-4 inhibitors (e.g., ipilimumab) are associated with dose-dependent toxicity. Consequently, PD-1 inhibitors plus alternative ipilimumab dosing regimens have been tested to reduce toxicity while maintaining antitumor activity. We report results from cohort C of the phase I KEYNOTE-029 study involving standard-dose pembrolizumab plus alternative ipilimumab dosing regimens in patients with advanced melanoma. Patients received pembrolizumab 200 mg every 3 weeks for ≤24 months plus ipilimumab 50 mg every 6 weeks for 4 doses, or the same pembrolizumab regimen plus ipilimumab 100 mg every 12 weeks for 4 doses. Both regimens showed antitumor activity above the protocol-defined threshold, and pembrolizumab plus ipilimumab 50 mg met the threshold for meaningful reduction in toxicity. Further exploration of PD-1 inhibitors with alternative ipilimumab dosing is warranted.
Introduction
Programmed death 1 (PD-1) inhibitors are a standard treatment option for patients with advanced melanoma (1), and when given in combination with the cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) inhibitor ipilimumab, can provide substantial long-term benefit (2). This was initially demonstrated in the phase 2 CheckMate 069 and phase 3 CheckMate 067 studies (2–4). The latter investigated the PD-1 inhibitor nivolumab at 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks (Q3W) for 4 doses followed by nivolumab maintenance. The 5-year overall survival (OS) rate in CheckMate 067 was numerically higher (52%) for nivolumab plus ipilimumab compared with nivolumab (44%) or ipilimumab monotherapy (26%; ref. 2). However, the combination was associated with a higher incidence of grade 3/4 treatment-related adverse events (TRAE; 59%) compared with nivolumab (23%) or ipilimumab monotherapy (28%; ref. 2).
CTLA-4 inhibitors are known to be associated with dose-dependent toxicity and are associated with a higher incidence of fatal adverse events (AE; refs. 5, 6). Consequently, several studies have investigated alternative dosing combinations of PD-1 inhibitors and ipilimumab with the aim of reducing toxicity while retaining antitumor activity (7, 8).
The phase IIIb/IV CheckMate 511 study compared 2 dosing regimens of nivolumab with ipilimumab; the approved regimen of nivolumab 1 mg/kg plus ipilimumab 3 mg/kg Q3W for 4 doses followed by nivolumab maintenance (NIVO1+IPI3) versus nivolumab 3 mg/kg plus ipilimumab 1 mg/kg for 4 doses followed by nivolumab maintenance (NIVO3+IPI1). NIVO3+IPI1 was associated with a lower incidence of grade 3–5 TRAEs compared with NIVO1+IPI3 (34% vs. 48%; P = 0.006), and similar objective response rates (ORR; 45.6% vs. 50.6%, respectively), although the study was not powered to demonstrate noninferiority for efficacy (8).
Manageable toxicity was also observed in cohort B of the single-arm KEYNOTE-029 study (N = 153), which investigated pembrolizumab 2 mg/kg plus ipilimumab 1 mg/kg Q3W for 4 doses followed by pembrolizumab maintenance (7, 9). At a median follow-up of 36.8 months, grade 3/4 TRAEs occurred in 47.1% of patients; the ORR was 62.1%; the median duration of response (DOR), progression-free survival (PFS), and OS were not reached (9). This incidence of grade 3/4 TRAEs was lower than that reported for standard-dose nivolumab plus ipilimumab in CheckMate-069 and CheckMate-067 (54% and 59%, respectively) with a similar ORR (59% and 58%, respectively; refs. 2, 3). Although the results from cohort B of the KEYNOTE-029 study indicated that standard-dose pembrolizumab with reduced-dose ipilimumab had a manageable toxicity profile and robust antitumor activity, it remains unknown whether dose frequency has an impact on safety and efficacy of the combination.
The objective of this analysis was to establish the safety and antitumor activity of standard-dose pembrolizumab with 2 alternative flat-dosing regimens of ipilimumab [50 mg every 6 weeks (Q6W) for 4 doses or 100 mg every 12 weeks (Q12W) for 4 doses] in patients with advanced melanoma.
Patients and Methods
Study design and participants
Cohort C of the open-label phase I KEYNOTE-029 study recruited patients from 20 sites in Australia, Canada, France, New Zealand, and the United States. Eligible patients were 18 years or older, had previously untreated, histologically confirmed unresectable stage III or IV melanoma (not uveal or ocular), measurable disease per RECIST v1.1, Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and adequate organ function. Patients could have received prior adjuvant or neoadjuvant therapy on the condition that (1) treatment did not target PD-1, programmed death ligand 1 (PD-L1), BRAF, or MEK (2) they did not discontinue adjuvant/neoadjuvant treatment because of TRAEs and all TRAEs had resolved, and (3) if anti–CTLA-4 therapy was received, relapse did not occur during treatment or within the following 6 months. Patients were excluded if they had brain metastases or carcinomatous meningitis (patients with previously treated, stable brain metastases were eligible). Additional eligibility criteria are listed in the Supplementary Methods (study protocol available online).
The study protocol and amendments were approved by the appropriate institutional review boards and ethics committees for each center. The study was conducted in accordance with the protocol and subsequent amendments, Good Clinical Practice Guidelines, and the Declaration of Helsinki. All patients provided written informed consent.
Procedures
Patients were randomly assigned (1:1) to ipilimumab 50 mg Q6W intravenously (i.v.) for 4 doses plus pembrolizumab 200 mg Q3W i.v. for up to 24 months (PEM200+IPI50) or ipilimumab 100 mg Q12W i.v. for 4 doses plus pembrolizumab 200 mg Q3W i.v. for up to 24 months (PEM200+IPI100). Randomization was performed centrally using an interactive voice response/integrated web response system. Treatment with pembrolizumab was discontinued if patients had documented disease progression, unacceptable AEs, or withdrew from the study. Patients with radiologic progressive disease who were continuing to derive clinical benefit from therapy and were clinically stable were permitted to continue treatment at the discretion of the investigator and with sponsor approval. After at least 24 weeks of treatment with pembrolizumab, patients who attained an investigator-determined complete response (CR) could stop pembrolizumab treatment if at least 2 doses were received after CR was first documented.
Assessments
Tumor radiographic imaging was performed Q6W until week 24 and Q12W thereafter. Tumor response was assessed per RECIST v1.1 by independent central review. Investigator-assessed modified RECIST v1.1 was used for informing treatment decisions. Safety was assessed throughout the study and for 30 days thereafter (90 days for serious AEs and immune-mediated AEs), and AEs were graded per the National Cancer Institute Common Terminology Criteria for AEs, version 4.0. PD-L1 expression in tumor samples was assessed at a central laboratory using the PD-L1 IHC 22C3 pharmDx assay (Agilent Technologies). PD-L1 positivity was defined as staining on at least 1% of tumor cells or adjacent immune cells.
Primary end points were safety and tolerability, incidence of grade 3–5 TRAEs, and ORR. Secondary end points included PFS, DOR, and OS. Additional details regarding end points are included in the Supplementary Methods.
Statistical analysis
Fifty participants per arm were planned for enrollment in cohort C to provide adequate precision for estimating the primary end points. Given this sample size, an incidence of grade 3–5 TRAEs of ≤26% would suggest a meaningful reduction in toxicity compared with other combination regimens of PD-1 inhibitors and ipilimumab as the upper bound of a 90% confidence interval (CI) for the true incidence of grade 3–5 TRAEs excludes 40%, given rates for combinations of nivolumab and ipilimumab typically exceed 40% (3, 10). An ORR ≥48% would suggest efficacy similar to that of other combination regimens of PD-1 inhibitors and ipilimumab, as the 90% CI excludes 35%, which is a rate consistent with that observed in phase III studies of pembrolizumab monotherapy (11, 12). The efficacy population included all patients with measurable disease; the safety population included all patients who received at least 1 dose of study treatment. The Kaplan–Meier method was used for estimation of PFS, OS, and DOR. Exact 95% CIs were calculated for ORR. Exploratory subgroup analysis of ORR by patient baseline characteristics was also performed. Statistical analyses were performed using SAS, version 9.4. This multicohort trial is registered with ClinicalTrials.gov, number NCT02089685.
Results
Between June 15, 2017 and March 2, 2018, 102 patients were enrolled into cohort 1C (51 to PEM200+IPI50, 51 to PEM200+IPI100; Supplementary Fig. S1). At baseline, most patients had an ECOG performance status of 0 (88% PEM200+IPI50, 82% PEM200+IPI100), normal lactate dehydrogenase (LDH) level (59% and 71%), and PD-L1–positive tumors (63% and 61%; Table 1). For several baseline characteristics, there was a ≥10% difference between the treatment arms. Notably, there was a higher proportion of patients with poor prognostic factors in the PEM200+IPI50 arm; M1c stage (67% and 51%), elevated LDH levels (35% and 25%), and brain metastasis (10% and 0%). A higher proportion of patients in the PEM200+IPI100 arm had BRAF-mutant disease (29% and 39%).
Table 1.
Baseline characteristics.
| Pembrolizumab 200 mg Q3W | Pembrolizumab 200 mg Q3W | |
|---|---|---|
| + ipilimumab 50 mg Q6W | + ipilimumab 100 mg Q12W | |
| (n = 51) | (n = 51) | |
| Age, median (range; y) | 64 (27–78) | 63 (33–82) |
| Sex, n (%) | ||
| Male | 38 (75) | 33 (65) |
| Female | 13 (25) | 18 (35) |
| ECOG performance status, n (%) | ||
| 0 | 45 (88) | 42 (82) |
| 1 | 6 (12) | 9 (18) |
| Lactate dehydrogenase concentration, n (%) | ||
| Normal | 30 (59) | 36 (71) |
| >ULN | 18 (35) | 13 (25) |
| Unknown | 3 (6) | 2 (4) |
| PD-L1 statusa n (%) | ||
| Positive | 32 (63) | 31 (61) |
| Negative | 14 (28) | 13 (25) |
| Unknown | 5 (10) | 7 (14) |
| BRAF V600 mutation, n (%) | ||
| Present | 15 (29) | 20 (39) |
| Absent | 34 (67) | 31 (61) |
| Unknown | 2 (4) | 0 (0) |
| Disease stageb n (%) | ||
| IIIC | 2 (4) | 0 (0) |
| IVc | 49 (96) | 51 (100) |
| M1a | 5 (10) | 7 (14) |
| M1b | 9 (18) | 18 (35) |
| M1c | 34 (67) | 26 (51) |
| Melanoma subtype, n (%) | ||
| Cutaneous | 49 (96) | 50 (98) |
| Mucosal | 2 (4) | 1 (2) |
| Prior adjuvant therapyd n (%) | 1 (2) | 2 (4) |
Abbreviations: BRAF, B-Raf proto-oncogene, serine/threonine kinase; ECOG, Eastern Cooperative Oncology Group; M, metastasis; PD-L1, programmed death ligand 1; Q3W, every 3 weeks; Q6W, every 6 weeks; Q12W, every 12 weeks; ULN, upper limit of normal.
aPD-L1 positivity was defined as staining on at least 1% of tumor cells or mononuclear inflammatory cells intercalated within or contiguous to tumor nests.
bAmerican Joint Committee on Cancer Staging Manual, 7th edition (16).
cThe distant metastasis stage of 1 patient with stage IV melanoma receiving pembrolizumab 200 mg Q3W + ipilimumab 50 mg Q6W arm could not be determined [staged as T4b (thickness >4.0 mm with ulceration), N0 (no regional lymph node metastases detected), M1 (distant metastasis)].
dOne patient received 2-MpP (pBCAR3-phosphopeptide + pIRS2-phosphopeptide); PolyICLC, tetanus peptide (Peptide-tet), and montanide. Two patients received interferon.
Patient disposition
At the February 18, 2019, data cutoff, the median follow-up was 16.3 months (range, 0.8 to 20 months) for PEM200+IPI50 and 16.4 months (range, 0.4 to 20.2 months) for PEM200+IPI100, and 30 (59%) and 24 (47%) patients, respectively, were continuing study treatment (Supplementary Table S1). The most common reasons for discontinuation of study treatment were progressive disease [22% (n = 11), PEM200+IPI50; 12% (n = 6), PEM200+IPI100] and AEs [16% (n = 8), PEM200+IPI50; 20% (n = 10), PEM200+IPI100; Supplementary Fig. S1]. Five patients discontinued treatment after achieving CR (1 patient in the PEM200+IPI50 arm had received 11.8 months of study treatment; 4 patients in the PEM200+IPI100 arm who had received 8.5, 15.7, 16.7, and 18.5 months of study treatment, respectively).
Safety
Pembrolizumab and ipilimumab exposures were similar in both arms (Supplementary Table S2). Patients in PEM200+IPI50 received a median of 19 doses (range, 1 to 29 doses) of pembrolizumab and 4 doses (range, 1 to 6 doses) of ipilimumab; patients in PEM200+IPI100 received a median of 19 doses (range, 1 to 30 doses) of pembrolizumab and 4 doses (range, 1 to 4 doses) of ipilimumab. Most patients in both arms received 4 doses of ipilimumab [38 (75%) PEM200+IPI50; 31 (61%) PEM200+IPI100; Supplementary Table S2]. All 51 (100%) patients in PEM200+IPI50 and 49 (96%) patients in PEM200+IPI100 experienced ≥1 TRAE. Of 51 patients in each arm, 12 (24%) and 20 (39%) experienced ≥1 grade 3–5 TRAE (Table 2). In the PEM200+IPI50 arm, a 74-year-old male patient died on day 24 from first dose of study drug because of treatment-related autoimmune myocarditis; this patient had a prior medical history of grade 2 hypertension, treated with losartan. Eight (16%) patients in PEM200+IPI50 and 12 (24%) patients in PEM200+IPI100 discontinued one or both study drugs because of a TRAE (Supplementary Table S1). Discontinuation of both pembrolizumab and ipilimumab due to the same TRAE occurred in 6 (12%) patients in PEM200+IPI50 and 5 (10%) patients in PEM200+IPI100. No (0%) patients in PEM200+IPI50 and 2 (4%) patients in PEM200+IPI100 discontinued ipilimumab only because of a TRAE. After completion of ipilimumab, pembrolizumab was discontinued because of a TRAE in 1 (2%) patient in each arm. One (2%) patient in each arm discontinued ipilimumab for 1 TRAE and later discontinued pembrolizumab for another TRAE (Supplementary Table S1).
Table 2.
Treatment-related AEs of grade 1–4 severity that occurred in ≥10% of patients; presented by frequency at any grade and by maximum toxicity grade.
| Pembrolizumab 200 mg Q3W | Pembrolizumab 200 mg Q3W | |||||||
|---|---|---|---|---|---|---|---|---|
| + ipilimumab 50 mg Q6W | + ipilimumab 100 mg Q12W | |||||||
| (n = 51) | (n = 51) | |||||||
| Treatment-related adverse event, n (%) | Any grade | Grade 1/2 | Grade 3 | Grade 4 | Any grade | Grade 1/2 | Grade 3 | Grade 4 |
| Any | 51 (100) | 39 (77) | 6 (12) | 5 (10) | 49 (96) | 29 (57) | 18 (35) | 2 (4) |
| Fatigue | 29 (57) | 29 (57) | 0 (0) | 0 (0) | 26 (51) | 26 (51) | 0 (0) | 0 (0) |
| Pruritus | 16 (31) | 16 (31) | 0 (0) | 0 (0) | 27 (53) | 27 (53) | 0 (0) | 0 (0) |
| Rash | 20 (39) | 20 (39) | 0 (0) | 0 (0) | 21 (41) | 21 (41) | 0 (0) | 0 (0) |
| Diarrhea | 13 (25) | 12 (24) | 1 (2) | 0 (0) | 18 (35) | 18 (35) | 0 (0) | 0 (0) |
| Arthralgia | 12 (24) | 12 (24) | 0 (0) | 0 (0) | 11 (22) | 10 (20) | 1 (2) | 0 (0) |
| Nausea | 8 (16) | 8 (16) | 0 (0) | 0 (0) | 12 (24) | 12 (24) | 0 (0) | 0 (0) |
| Lipase increased | 5 (10) | 0 (0) | 2 (4) | 3 (6) | 10 (20) | 2 (4) | 6 (12) | 2 (4) |
| Hypothyroidism | 7 (14) | 7 (14) | 0 (0) | 0 (0) | 9 (18) | 9 (18) | 0 (0) | 0 (0) |
| Rash pruritic | 5 (10) | 5 (10) | 0 (0) | 0 (0) | 9 (18) | 9 (18) | 0 (0) | 0 (0) |
| Aspartate aminotransferase increased | 7 (14) | 7 (14) | 0 (0) | 0 (0) | 4 (8) | 4 (8) | 0 (0) | 0 (0) |
| Alanine aminotransferase increased | 7 (14) | 7 (14) | 0 (0) | 0 (0) | 2 (4) | 2 (4) | 0 (0) | 0 (0) |
| Vitiligo | 6 (12) | 6 (12) | 0 (0) | 0 (0) | 7 (14) | 7 (14) | 0 (0) | 0 (0) |
| Dry mouth | 5 (10) | 5 (10) | 0 (0) | 0 (0) | 7 (14) | 7 (14) | 0 (0) | 0 (0) |
| Amylase increased | 2 (4) | 1 (2) | 1 (2) | 0 (0) | 7 (14) | 5 (10) | 2 (4) | 0 (0) |
| Decreased appetite | 6 (12) | 6 (12) | 0 (0) | 0 (0) | 6 (12) | 5 (10) | 1 (2) | 0 (0) |
| Myalgia | 4 (8) | 4 (8) | 0 (0) | 0 (0) | 6 (12) | 5 (10) | 1 (2) | 0 (0) |
| Asthenia | 5 (10) | 5 (10) | 0 (0) | 0 (0) | 3 (6) | 3 (6) | 0 (0) | 0 (0) |
| Rash macular | 5 (10) | 5 (10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Note: One patient died from a treatment-related adverse event (autoimmune myocarditis, grade 5). Data are presented in order of descending total frequency.
Abbreviations: Q3W, every 3 weeks; Q6W, every 6 weeks; Q12W, every 12 weeks.
The most common TRAEs of any grade in the PEM200+IPI50 and PEM200+IPI100 arms were fatigue (57% and 51%), pruritus (31% and 53%), rash (39% and 41%), and diarrhea (25% and 35%; Table 2). The most common grade 3/4 TRAEs were increased lipase (10% PEM200+IPI50 and 16% PEM200+IPI100), colitis (4% and 6%), and increased amylase (2% and 4%; Supplementary Table S3).
Immune-mediated AEs (derived from a predefined, sponsor-specified list of AEs with immunologic mechanisms of action) and infusion reactions occurred in 21 (41%) patients in PEM200+IPI50 and 28 (55%) patients in PEM200+IPI100 and were predominantly grade 1 or 2 in severity (Supplementary Table S4). The most common immune-mediated AEs (≥10% of patients in either arm) were hypothyroidism (14% PEM200+IPI50 and 20% PEM200+IPI100) and colitis (10% and 12%). Grade 3 immune-mediated AEs that occurred in more than 1 patient in either arm were colitis (6% PEM200+IPI50, 8% PEM200+IPI100) and hepatitis (2% and 4%). One (2%) patient in the PEM200+IPI50 arm had grade 4 myositis and 1 (2%) patient in the PEM200+IPI50 arm died because of immune-mediated myocarditis. Ten (48%) patients in the PEM200+IPI50 arm and 19 (68%) patients in the PEM200+IPI100 arm with immune-mediated AEs or infusion reactions were treated with systemic corticosteroids (Supplementary Table S5).
Efficacy
The ORR was 55% (28 of 51 patients; 95% CI, 40%–69%) in PEM200+IPI50 and 61% (31 of 51 patients; 95% CI, 46%–74%) in PEM200+IPI100, including 8 CRs (16%) in PEM200+IPI50 and 13 CRs (25%) in PEM200+IPI100 (Table 3). Median time to response was 1.4 months (range, 1.3 to 9.3 months) in PEM200+IPI50 and 1.5 months (range, 1.3 to 10.9 months) in PEM200+IPI100. In PEM200+IPI50, 38 of 43 (88%) evaluable patients experienced a reduction in target lesion size from baseline (Fig. 1A). In PEM200+IPI100, 40 of 44 (91%) evaluable patients experienced a reduction in target lesion size from baseline (Fig. 1B). Four of 28 (14%) responders in PEM200+IPI50 had progressed at data cutoff (Fig. 2A), and 2 of 31 (6%) in PEM200+IPI10 (Fig. 2B); the median DOR was not reached in PEM200+IPI50 (range, 1.4+ to 17.9+ months) or PEM200+IPI100 (range, 2.8+ to 18.3+ months); the percentage of patients with ongoing response at 12 months was estimated to be 88% in PEM200+IPI50 and 93% in PEM200+IPI100 (Fig. 3A). Subgroup analysis of ORR by patient baseline characteristics showed treatment benefit from both regimens regardless of baseline clinical or demographic characteristics, although patient numbers were small in some subgroups (Supplementary Fig. S2). Patients with BRAF-mutant versus BRAF wild-type melanoma, normal versus elevated baseline LDH level, and PD-L1–positive versus negative melanoma had higher response rates in both arms.
Table 3.
Best overall response by independent central review per RECIST v1.1.
| Pembrolizumab 200 mg Q3W | Pembrolizumab 200 mg Q3W | |
|---|---|---|
| + ipilimumab 50 mg Q6W | + ipilimumab 100 mg Q12W | |
| (n = 51) | (n = 51) | |
| Objective response rate | ||
| N | 28 | 31 |
| % (95% CIa) | 55 (40–69) | 61 (46–74) |
| Best overall response, n (%) | ||
| Complete response | 8 (16) | 13 (25) |
| Partial response | 20 (39) | 18 (35) |
| Stable disease | 10 (20) | 8 (16) |
| Progressive disease | 8 (16) | 5 (10) |
| Disease not measurable per central review at baseline, that did not completely resolve or progress | 2 (4) | 5 (10) |
| Non-evaluable | 1 (2) | 1 (2) |
| No assessment done | 2 (4) | 1 (2) |
| Time to response in months, median (range) | 1.4 (1.3–8.3) | 1.5 (1.3–10.9) |
| Duration of response in months, median (range) | Not reached (1.4+ to 17.9+) | Not reached (2.8+ to 18.3+) |
Abbreviations: Q3W, every 3 weeks; Q6W, every 6 weeks; Q12W, every 12 weeks; RECIST v1.1, Response Evaluation Criteria in Solid Tumors, version 1.1.
aBased on binomial exact confidence interval method.
Figure 1.
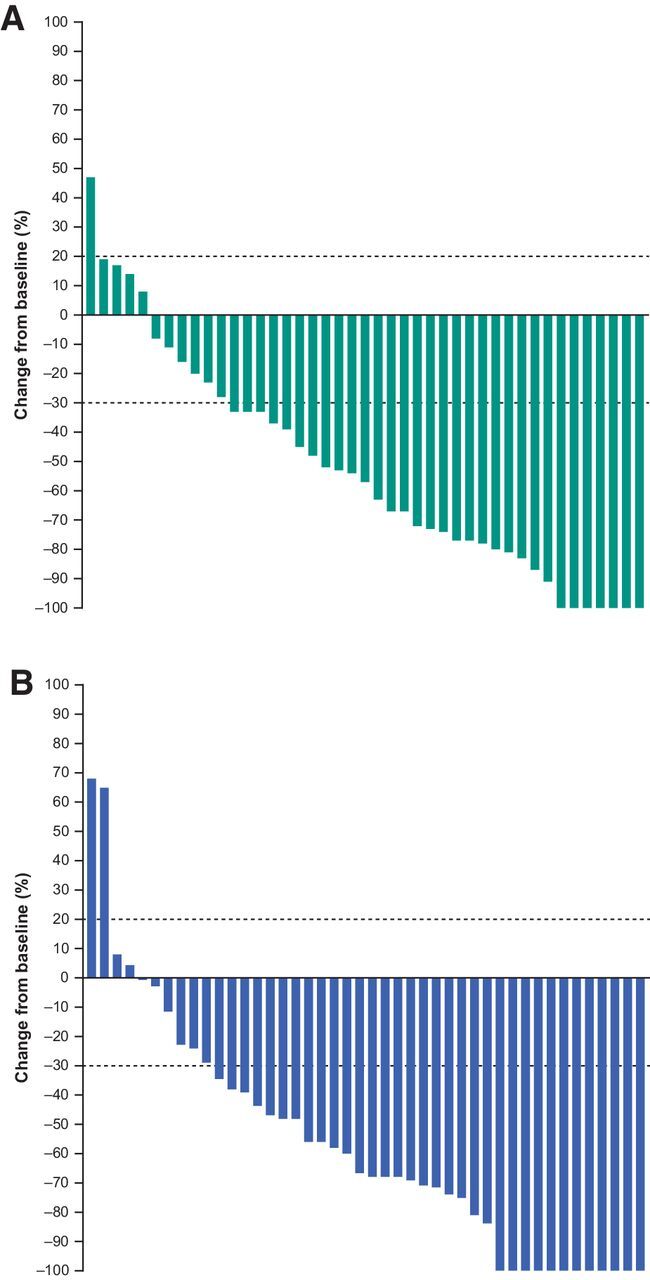
Best percentage change from baseline in target lesion size (RECIST, version 1.1, by central review) in patients in PEM200+IPI50 (pembrolizumab 200 mg Q3W + ipilimumab 50 mg Q6W; A) and PEM200+IPI100 (pembrolizumab 200 mg Q3W + ipilimumab 100 mg Q12W; B). Abbreviations: Q3W, every 3 weeks; Q6W, every 6 weeks; Q12W, every 12 weeks.
Figure 2.
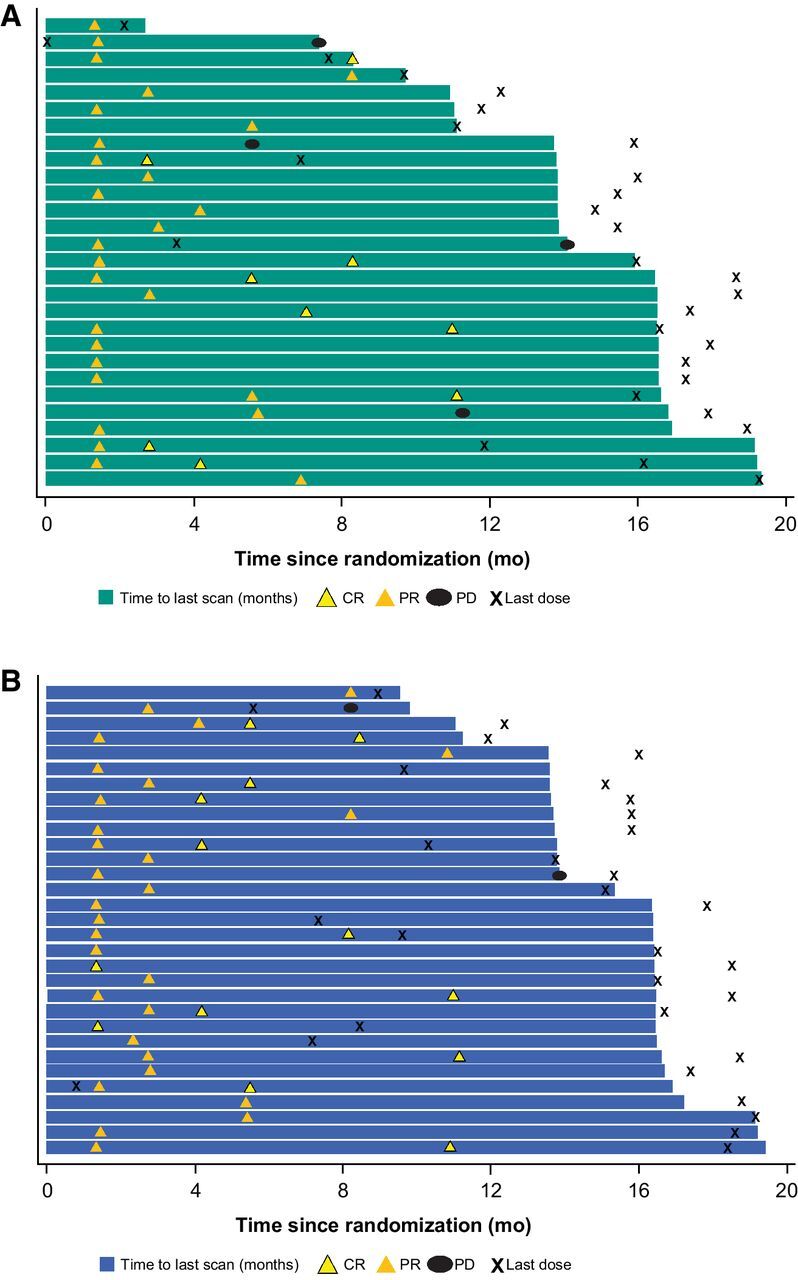
Duration of treatment and response in patients in PEM200+IPI50 (pembrolizumab 200 mg Q3W + ipilimumab 50 mg Q6W; A) and PEM200+IPI100 (pembrolizumab 200 mg Q3W + ipilimumab 100 mg Q12W; B). Abbreviations: CR, complete response; PD, progressive disease; PR, partial response; Q3W, every 3 weeks; Q6W, every 6 weeks; Q12W, every 12 weeks.
Figure 3.
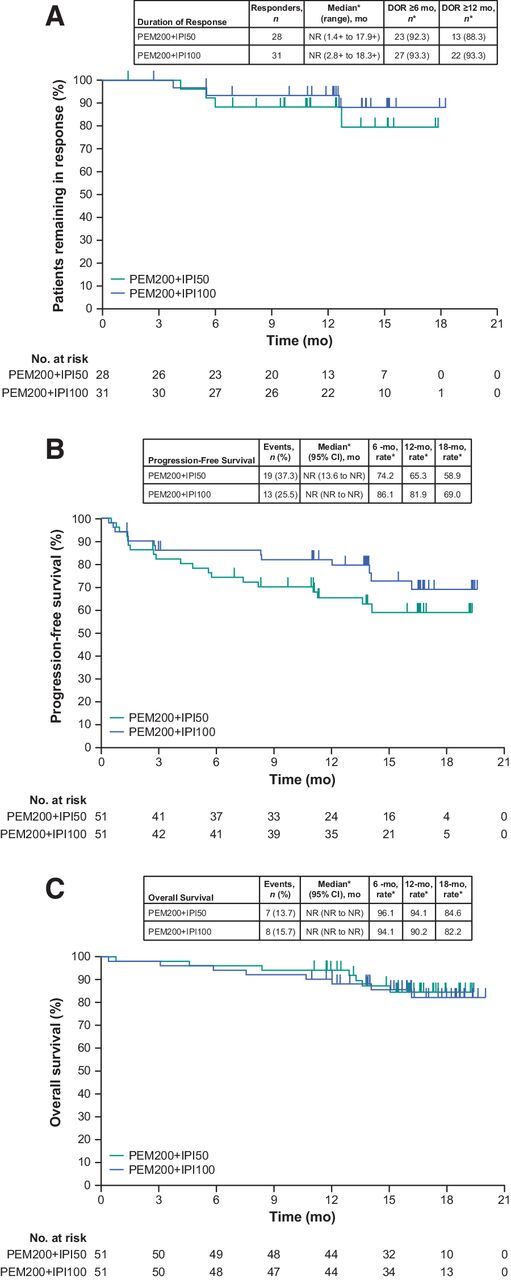
Kaplan–Meier estimates of (A) duration of response, (B) progression-free survival, and (C) overall survival in PEM200+IPI50 (pembrolizumab 200 mg Q3W + ipilimumab 50 mg Q6W) and PEM200+IPI100 (pembrolizumab 200 mg Q3W + ipilimumab 100 mg Q12W) arms. *From Kaplan–Meier method. Abbreviations: DOR, duration of response; NR, not reached; Q3W, every 3 weeks; Q6W, every 6 weeks; Q12W, every 12 weeks.
At data cutoff, 19 (37%) of 51 patients in PEM200+IPI50 and 13 (25%) of 51 in PEM200+IPI100 had a progression event; median PFS was not reached in either arm. The 6-month PFS rate was 74% (95% CI, 60%–84%) in PEM200+IPI50 and 86% (95% CI, 73%–93%) in PEM200+IPI100; the 12-month PFS rate was 65% (95% CI, 50%–77%) in PEM200+IPI50 and 82% (95% CI, 68%–90%) in PEM200+IPI100; the 18-month PFS rate was 59% (95% CI, 43%–72%) in PEM200+IPI50 and 69% (95% CI, 52%–81%) in PEM200+IPI100 (Fig. 3B).
Fourteen (27%) patients in PEM200+IPI50 arm and 12 (24%) patients in PEM200+IPI100 received subsequent anticancer therapy after discontinuation of study treatment. Of these, 10 (20%) in the PEM200+IPI50 arm and 4 (8%) in the PEM200+IPI100 received therapy after discontinuing the study because of progressive disease (Supplementary Table S6). Eleven (22%) and 6 (12%) patients received subsequent immunotherapy in the PEM200+IPI50 and PEM200+IPI100 arms, respectively (Supplementary Table S6). After discontinuing study treatment, most patients received a checkpoint inhibitor alone or combined with an experimental therapy, and all patients with BRAF-mutant disease who experienced disease progression received a BRAF+ MEK inhibitor (1 patient in the PEM200+IPI50 arm and 3 in the PEM200+IPI100 arm).
At data cutoff, 5 (10%) patients in PEM200+IPI50 and 6 (12%) in PEM200+IPI100 had died because of progression of melanoma, and 1 patient in the PEM200+IPI50 arm died because of a TRAE. Median OS was not reached in either arm; the 12-month OS rate was 94% (95% CI, 83%–98%) in PEM200+IPI50 and 90% (95% CI, 78%–96%) in PEM200+IPI100; 18-month OS rate was 85% (95% CI, 70%–92%) in PEM200+IPI50 and 82% (95% CI, 67%–91%) in PEM200+IPI100 (Fig. 3C).
Discussion
Cohort 1C of the KEYNOTE 029 study was designed to investigate standard-dose pembrolizumab with alternative-dose ipilimumab to determine whether the efficacy of combined PD-1 and CTLA-4 inhibitor therapy could be maintained while reducing toxicity. With an incidence of grade 3–5 TRAEs of 24%, PEM200+IPI50 met the predefined threshold (≤26%) for a meaningful reduction in toxicity compared with the incidence reported in other studies investigating combined PD-1 and CTLA-4 inhibitor regimens (7, 8). This threshold was not met with PEM200+IPI100 (grade 3–5 TRAEs 39%). Notably, the ORR in both PEM200+IPI50 (55%) and PEM200+IPI100 (61%) met the predefined threshold of ≥48% for equivalent efficacy with other PD-1 and CTLA-4 inhibitor combinations.
The ORR and CR results reported in the current study (PEM200+IPI50: ORR, 55%, and CR, 16%; PEM200+IPI100: ORR, 61%, and CR, 25%) are within the ranges reported in previous studies of PD-1 plus CTLA-4 inhibitors in melanoma, although cross-trial comparisons should be made cautiously because of differences in patient populations, study procedures, and length of follow-up. In CheckMate 067 (2) and CheckMate 511 (8), standard or alternate nivolumab plus ipilimumab dosing resulted in ORRs of 51% to 58% for standard dosing and 46% for alternate dosing, and CRs of 14% to 22% and 15%, respectively. In cohort B of the KEYNOTE-029 study, ipilimumab 1 mg/kg plus standard-dose pembrolizumab resulted in an ORR of 62% and CR of 27% (9). Similarly, the 12-month PFS rates in the current study (65%, PEM200+IPI50; 82%, PEM200+IPI100) were favorable compared with the 12-month PFS rates reported with other PD-1 plus CTLA-4 inhibitor regimens: 46% to 53% with standard ipilimumab plus nivolumab dosing, 47% with ipilimumab 1 mg/kg plus nivolumab 3 mg/kg, and 68% with ipilimumab 1 mg/kg plus standard-dose pembrolizumab (3, 8, 9). Similar findings are observed when comparing 12-month OS rates in the current study (≥90% in each arm) with 12-month OS rates for other CTLA-4 plus PD-1 inhibitor regimens (73%–89%; refs. 3, 7).
The results of this study suggest that further exploration of alternative ipilimumab dosing in combination with PD-1 inhibitors is warranted. Randomized controlled trials comparing alternative dosing regimens to standard dosing regimens are needed, as there may be a dose–response with CTLA-4 inhibitors that has not been observed with PD-1 inhibitors. For example, in a randomized phase 3 trial, ipilimumab administered at 10 mg/kg Q3W for 4 doses improved the OS in patients with advanced melanoma compared with 3 mg/kg (13). In contrast, pembrolizumab has a similar efficacy whether administered at 10 mg/kg every 2 or 3 weeks, or 2 mg/kg Q3W (12, 14, 15). In addition to dose–response, the effect of varying CTLA-4 inhibitor duration in the treatment schedule also needs to be explored. In this study, 75% of patients in the PEM200+IPI50 arm and 61% of patients in the PEM200+IPI100 arm received 4 doses of ipilimumab. In contrast, patients in CheckMate 067 receiving nivolumab 1 mg/kg + ipilimumab 3 mg/kg every 3 weeks for 4 doses, followed by nivolumab 3 mg/kg every 2 weeks, had lower ipilimumab exposure, with only 57% of patients receiving 4 doses (2). Factors that may have contributed to this difference include the dose of PD-1 and CTLA-4 inhibitor received and the treatment schedule. Although not powered to make comparisons, our current study showed a numerically higher ORR and a higher proportion of CRs in PEM200+IPI100 versus PEM200+IPI50; this should be interpreted with caution because a higher proportion of patients had poorer baseline prognostic factors in PEM200+IPI50 versus PEM200+IPI100 [e.g., elevated LDH (35% vs. 26%) and M1c (67% vs. 51%)].
Ongoing studies of pembrolizumab plus CTLA-4 inhibitors in patients with advanced melanoma include a phase 2 study of pembrolizumab plus low-dose ipilimumab in patients with brain metastases (ClinicalTrials.gov, NCT03873818) and a phase 2 study of pembrolizumab plus ipilimumab in patients pretreated with an anti–PD-1/PD-L1 antibody (ClinicalTrials.gov, NCT02743819). In addition, an ongoing phase 1/2 study (ClinicalTrials.gov, NCT03179436) is assessing the safety, pharmacokinetics, and efficacy of pembrolizumab plus the anti–CTLA-4 antibody MK-1308 in patients with advanced solid tumors, including PD-1/PD-L1 refractory melanoma. Results from these studies may provide further evidence for the benefit-risk profile of various dosing regimens of pembrolizumab with CTLA-4 inhibitors.
This study demonstrated robust antitumor activity in patients with treatment-naive advanced melanoma who received standard-dose pembrolizumab 200 mg Q3W combined with either ipilimumab 50 mg Q6W or 100 mg Q12W. Although the ipilimumab 100 mg Q12W arm did not meet the predefined threshold for a reduction in toxicity compared with other anti–PD-1 plus anti–CTLA-4 combination regimens, the ipilimumab 50 mg Q6W arm did meet this threshold, warranting further investigation of this combination. Longer follow-up and appropriately powered randomized controlled trials are required to confirm that these alternative dosing regimens reduce toxicity without compromising efficacy in patients with advanced melanoma.
Authors' Disclosures
G.V. Long reports personal fees from Aduro Biotech Inc., Amgen Inc., Array Biopharma Inc., Boehringer Ingelheim International, Bristol–Myers Squibb, Hexel AG, Highlight Therapeutics S.L., Merck Sharpe & Dohme, Novartis Pharma AG, OncoSec, Pierre Fabre, Q Biotics Group Limited, Regeneron Pharmaceuticals, SkylineDX B.V., and Specialized Therapeutics Australia Pty Limited outside the submitted work. C. Robert reports personal fees from BMS, MSD, Roche, Novartis, Pierre Fabre, and Sanofi outside the submitted work. M.O. Butler reports grants from BMS during the conduct of the study; as well as personal fees from BMS, and grants and personal fees from Merck, and personal fees from Novartis, Pfizer, Sanofi, Adaptimmune, GSK, Immunocore, and grants from Takara outside the submitted work. M.S. Carlino reports personal fees from MSD, BMS, Novertis, Sanofi, Merck, Pierre Fabre, Amgen, Regeneron, Eisai, Ideaya, Nektar, Oncosec, Q Biotics, and Roche outside the submitted work. S. O'Day reports consulting or advisory role for Merck, Bristol Myers Squibb, Agenus, Immunosync, Biothera, and Exicure; speaker fee from Bristol Myers Squibb; and research funding from Merck, Bristol Myers Squibb, Exicure, Agenus, Biothera, ImaginAb, Ultimovacs, Genocea, Vyraid, and Shattuck Labs. V. Atkinson reports personal fees and non-financial support from BMS, as well as personal fees from MSD, Merck, Novartis, Nektar therapeutics, Pierre Fabre, Roche, and Q Biotics outside the submitted work. J.S. Cebon reports grants from MSD during the conduct of the study. M.P. Brown reports grants and personal fees from MSD during the conduct of the study; as well as other grants from MSD and BMS outside the submitted work. S. Dalle reports grants from BMS and MSD outside the submitted work. G.T. Gibney reports personal fees from Merck, Bristol Myers Squibb, Novartis, Genentech, Regeneron, Sapience Therapeutics, Exicure, as well as grants from Exelixis and Lucerno Dynamics outside the submitted work. S. McCune reports other support from Merck during the conduct of the study. A.M. Menzies reports personal fees from BMS, MSD, Novartis, Roche, Pierre-Fabre, and Q Biotics outside the submitted work. N. Ibrahim reports Merck stock. B. Homet Moreno reports personal fees from Merck during the conduct of the study; as well as personal fees from Merck outside the submitted work. A. Diab reports grants from Merck during the conduct of the study; as well as grants from Pfizer, grants and personal fees from Nektar therapeutics, apexigen, Bristol-Myers and Squibb, and Idera outside the submitted work. No disclosures were reported by the other authors.
Supplementary Material
Clean version
Figure S1
Figure S2
Acknowledgments
The authors thank James R. Anderson, of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, for critical review of the article. Medical writing and editorial assistance was provided by Doyel Mitra, CMPP, and Jemimah Walker, of ApotheCom, Yardley, PA, and was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ. This work was supported by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ. The sponsor collaborated with academic advisers to design the study and gather, analyze, and interpret the results. All authors had full access to all study data and approved the decision to submit the article for publication.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
This article is featured in Highlights of This Issue, p. 5151
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Contributions
G.V. Long: Conceptualization, data curation, formal analysis, investigation, writing–original draft, writing–review and editing. C. Robert: Conceptualization, formal analysis, investigation, writing–review and editing. M.O. Butler: Formal analysis, investigation, writing–review and editing. F. Couture: Investigation, writing–review and editing. M.S. Carlino: Conceptualization, investigation, writing–review and editing. S. O'Day: Formal analysis, investigation, writing–review and editing. V. Atkinson: Formal analysis, investigation, writing–original draft, writing–review and editing. J.S. Cebon: Investigation, writing–review and editing. M.P. Brown: Investigation, writing–review and editing. S. Dalle: Investigation, writing–review and editing. A.G. Hill: Investigation, writing–review and editing. G.T. Gibney: Formal analysis, investigation, writing–review and editing. S. McCune: Formal analysis, investigation, writing–review and editing. A.M. Menzies: Investigation, writing–review and editing. C. Niu: Formal analysis, writing–review and editing. N. Ibrahim: Conceptualization, formal analysis, writing–original draft, writing–review and editing. B.H. Moreno: Conceptualization, formal analysis, investigation, writing–original draft, writing–review and editing. A. Diab: Conceptualization, formal analysis, investigation, writing–original draft, writing–review and editing.
References
- 1. Dummer R, Hauschild A, Lindenblatt N, Pentheroudakis G, Keilholz U, Committee EG. Cutaneous melanoma: ESMO clinical practice guidelines for diagnosis, treatment, and follow-up. Ann Oncol 2015;26:v126–132. [DOI] [PubMed] [Google Scholar]
- 2. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2019;381:1535–46. [DOI] [PubMed] [Google Scholar]
- 3. Hodi FS, Chesney J, Pavlick AC, Robert C, Grossmann KF, McDermott DF, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 2016;17:1558–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jiang Y, Zhang N, Pang H, Gao X, Zhang H. Risk and incidence of fatal adverse events associated with immune checkpoint inhibitors: a systematic review and meta-analysis. Ther Clin Risk Manag 2019;15:293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti–CTLA-4 antibodies: systematic review and meta-analysis. BMC Med 2015;13:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Long GV, Atkinson V, Cebon JS, Jameson MB, Fitzharris BM, McNeil CM, et al. Standard-dose pembrolizumab in combination with reduced-dose ipilimumab for patients with advanced melanoma (KEYNOTE-029): an open-label, phase 1b trial. Lancet Oncol 2017;18:1202–10. [DOI] [PubMed] [Google Scholar]
- 8. Lebbe C, Meyer N, Mortier L, Marquez-Rodas I, Robert C, Rutkowski P, et al. Evaluation of two dosing regimens for nivolumab in combination with ipilimumab in patients with advanced melanoma: results from the phase IIIb/IV CheckMate 511 trial. J Clin Oncol 2019;37:867–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carlino MS, Menzies AM, Atkinson V, Cebon JS, Jameson MB, Fitzharris BM, et al. Long-term follow-up of standard-dose pembrolizumab plus reduced-dose ipilimumab in patients with advanced melanoma: KEYNOTE-029 part 1B. Clin Cancer Res 2020;26:5086–91. [DOI] [PubMed] [Google Scholar]
- 10. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–32. [DOI] [PubMed] [Google Scholar]
- 12. Schachter J, Ribas A, Long GV, Arance A, Grob J-J, Mortier L, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 2017;390:1853–62. [DOI] [PubMed] [Google Scholar]
- 13. Ascierto PA, Del Vecchio M, Robert C, Mackiewicz A, Chiarion-Sileni V, Arance A, et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 2017;18:611–22. [DOI] [PubMed] [Google Scholar]
- 14. Robert C, Ribas A, Hamid O, Daud A, Wolchok JD, Joshua AM, et al. Three-year overall survival for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. J Clin Oncol 2016;34:9503. [Google Scholar]
- 15. Robert C, Ribas A, Schachter J, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol 2019;20:1239–51. [DOI] [PubMed] [Google Scholar]
- 16. Edge SB, Byrd DR, Carducci MA. AJCC cancer staging manual. New York, NY: Springer-Verlag; 2010. p. 325–340. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clean version
Figure S1
Figure S2


