Although bacterial DNA methyltransferases are generally associated with restriction-modification systems, DNA methylation also regulates chromosome replication, transcription, repair, and most likely other fundamental processes. The two best-studied DNA methyltransferases without apparent cognate restriction enzymes are the Escherichia coli Dam and Caulobacter crescentus CcrM enzymes. Dam methylation is required for the control of chromosome replication (5, 18), the direction of strand-specific mismatch repair (22, 23), and the regulation of transcription of certain genes (3, 6, 24). Exciting new findings show that Dam is required for the expression of virulence genes in Salmonella typhimurium and that Dam− mutants are avirulent (15). Thus, the pathogenicity of this enteric bacterium is dependent on DNA adenine methylation.
Dam is found primarily in members of the gamma subdivision of Proteobacteria (2, 8), although it has also been identified in the spirochete Treponema pallidum (31). CcrM, on the other hand, is widely distributed among bacteria in the alpha subdivision of Proteobacteria (32). We consider here the properties of the CcrM DNA methyltransferase and the emerging evidence that differential DNA methylation controls multiple aspects of the cell cycle in Caulobacter, Rhizobium meliloti, Brucella abortus, and other alpha subdivision bacteria. Of particular relevance to cell cycle control is the observation that, in Caulobacter, the DNA methylation state reflects the progression of chromosome replication, providing a biochemical signal that could link the timing of replication of specific regions of the chromosome to other cell cycle events.
CCRM DNA METHYLTRANSFERASE
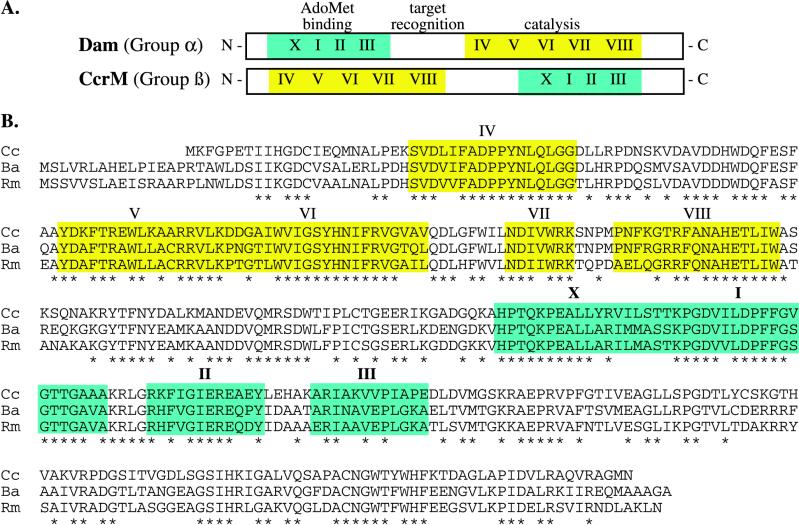
CcrM (for cell cycle-regulated methyltransferase), originally identified as the enzyme responsible for methylating GANTC sites in the Caulobacter genome, is a 39-kDa protein with 49% identity to the Haemophilus influenzae HinfI.M adenine DNA methyltransferase (37). However, unlike HinfI.M, CcrM appears to lack a cognate restriction enzyme. Plasmid DNA containing unmethylated GANTC sites is readily detected in Caulobacter, indicating that it is not digested (37). Furthermore, there is no gene encoding a restriction enzyme adjacent to ccrM on the chromosome, as would be expected from the organization of most restriction-modification pairs. Like Dam, CcrM is an adenine methyltransferase that catalyzes the transfer of the methyl group from S-adenosylmethionine (AdoMet) to the adenine in its target DNA sequence (4, 10). CcrM binds preferentially to hemimethylated DNA and appears to act processively (4). The amino acid sequences of CcrM and Dam have only limited regions of homology within motifs conserved among all adenine methyltransferases. As shown in Fig. 1, both enzymes contain catalytic and AdoMet-binding domains. However, the order of these domains is reversed, placing the two enzymes in different subgroups of DNA methyltransferases (19).
FIG. 1.
Domain organization of Dam and CcrM. (A) Diagram comparing the order of the conserved AdoMet-binding (green) and catalytic (yellow) domains in Dam and CcrM. As shown, the order of these domains is reversed in the α and β groups of methyltransferases. The putative target DNA recognition region is located between these domains. Conserved sequence motifs within the domains are indicated by Roman numerals (19). (B) Alignment of the C. crescentus (Cc), B. abortus (Ba), and R. meliloti (Rm) CcrM homologs. The three proteins are highly conserved (76 to 87% similarity). The green and yellow boxes highlight the proposed AdoMet-binding and catalytic domains, respectively. The asterisks below the sequence mark identical amino acids in all three proteins.
CcrM is essential for viability in Caulobacter. The chromosomal ccrM gene cannot be disrupted under normal growth conditions unless a functional copy of the gene is present on a plasmid (32). To our knowledge, CcrM is the first example of an essential DNA methyltransferase that is not part of a restriction-modification system. Dam, in contrast, is dispensable for growth, and isolated dam mutants are viable (22). While it is not known why CcrM is required for growth, inappropriate expression of CcrM throughout the cell cycle results in abnormal morphology and the disruption of the normal control of chromosome replication (36, 37), suggesting that CcrM methylation helps to regulate these processes.
CCRM IS WIDESPREAD IN THE ALPHA SUBDIVISION OF PROTEOBACTERIA
At least 20 members of the alpha subdivision of Proteobacteria methylate the CcrM recognition sequence (GANTC), as assayed by the resistance of chromosomal DNA to HinfI digestion. Furthermore, the ccrM gene hybridizes with DNA from these bacteria, but not from other groups, on Southern blots (32). The broad distribution of CcrM homologs suggests that the physiological functions of CcrM may also be conserved. ccrM homologs have now been cloned from two other members of the alpha subdivision: the nitrogen-fixing soil bacterium R. meliloti and the animal pathogen B. abortus (Fig. 1B). Both genes are also essential, since ccrM cannot be deleted in these bacteria (28, 35). In addition, the R. meliloti and C. crescentus ccrM homologs are functionally interchangeable (35). When ccrM is overexpressed, each of these three species develops abnormal morphology, aberrant chromosome replication, and disruption of cell division (28, 35, 37). While the functions of CcrM methylation are unknown, these early studies in Rhizobium and Brucella suggest common roles in the cell cycle of the bacteria in the alpha subdivision.
DNA METHYLATION DURING THE CAULOBACTER CELL CYCLE
During the cell cycle, Caulobacter undergoes a series of changes in cell morphology and competence for chromosome replication. The swarmer cell is unable to initiate DNA replication until it sheds its flagellum and differentiates into a stalked cell at the beginning of S phase (Fig. 2B). Chromosome replication is initiated only once per cell cycle and only in the stalked cell (20). As DNA replication proceeds, the stalked cell elongates and differentiates into a predivisional cell with a flagellum at the pole opposite the stalk. After completing DNA replication, the predivisional cell divides into a new swarmer cell and a stalked cell that immediately reinitiates DNA replication (21).
FIG. 2.
Changes in DNA methylation reflect the progression of chromosome replication during the Caulobacter cell cycle. (A) Diagram of the C. crescentus chromosome showing the locations of the origin of replication (Cori), the replication terminus, and three hypothetical methylation sites (labeled A, B, and C). (B) Methylation state of GANTC sites located at different distances from the origin at different stages in the cell cycle. Sites A, B, and C are fully methylated in the swarmer cell. After DNA replication initiates in the stalked cell, the time when each site becomes hemimethylated depends on its distance from Cori. The origin and surrounding sequences, such as site A, become hemimethylated earlier in the cell cycle and remain hemimethylated for a longer period of time compared to sites that are further from the origin (sites B and C). DNA near the replication terminus is hemimethylated very briefly. Just before cell division, a burst of CcrM methylation (shown by the yellow CcrM bar) restores the chromosome to the fully methylated state (32). The black oval and green theta structures within the cells represent quiescent and replicating chromosomes, respectively. (C) Diagram of fully methylated and hemimethylated DNA.
In Caulobacter, the methylation of GANTC sites is strictly regulated during the cell cycle. Chromosome replication initiates only on fully methylated DNA (37). As replication proceeds bidirectionally from the origin, there is an ordered appearance of hemimethylated DNA in the chromosome (12, 20, 32) (Fig. 2). (In hemimethylated DNA, the parental strand is methylated, while the daughter strand remains unmethylated.) Initial studies using a small number of chromosomal loci containing methylation-sensitive restriction sites showed that the appearance of hemimethylated DNA at these sites reflected their position on the chromosome (32, 37). A recent study using a transposon-based methylation probe placed at 11 sites around the chromosome confirmed that GANTC sites located near the replication origin (Cori) become hemimethylated earlier and remain hemimethylated longer than more-distant sites (20). The chromosome, which is fully methylated in the swarmer cell during G1, becomes progressively hemimethylated from the origin to the terminus in the course of DNA replication (Fig. 2B). Thus, the methylation state of the chromosome serves as an index of DNA replication. Remethylation of the chromosome is confined to a short period late in the cell cycle because CcrM is present in the predivisional cell only near the end of S phase (Fig. 3A) (32, 36). At this time, CcrM catalyzes the methylation of approximately 30,000 GANTC sites in the two newly replicated chromosomes.
FIG. 3.
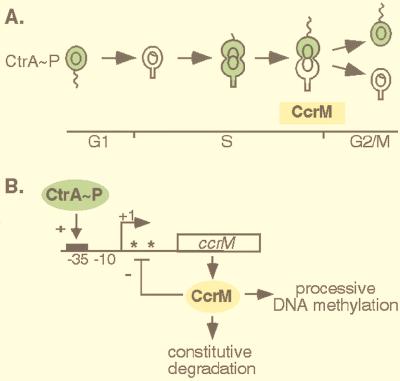
Control of CcrM methylation in Caulobacter. (A) Temporal and spatial control of CcrM and CtrA expression. The stage of the cell cycle when the CcrM DNA methyltransferase is present is indicated by the yellow CcrM bar beneath the cells. The presence of phosphorylated CtrA (CtrA∼P) during the cell cycle is shown in green. (B) Transcriptional regulation and constitutive proteolysis restrict CcrM expression to a narrow window of the cell cycle. CtrA∼P activates ccrM transcription by binding to its recognition sequence (black box) in the ccrM promoter. CcrM methylation of GANTC sites in the mRNA leader region (asterisks) represses transcription.
REGULATION OF CCRM EXPRESSION
Dual control mechanisms of transcriptional regulation and Lon-dependent proteolysis restrict the CcrM protein to a brief period before cell division. The global regulator CtrA (for cell cycle transcriptional regulator) plays a central role in controlling CcrM expression (Fig. 3B). Like other members of the response regulator family, CtrA is activated by phosphorylation (13, 25). Phosphorylated CtrA controls multiple cell cycle events in Caulobacter, including the transcription of ccrM as well as genes required for flagellar biogenesis and cell division (17, 25). CtrA is also crucial to the regulation of chromosome replication. The CtrA protein is present in swarmer cells, where it binds to Cori and prevents the initiation of DNA replication (26). At the G1-S (swarmer-stalked cell) transition, CtrA is rapidly degraded so that it is absent in stalked cells, which then initiate replication (13). CtrA gradually accumulates during S phase when it first activates transcription of the early flagellar genes and later ccrM transcription (27). Shortly before cell division, CtrA is cleared from the stalked compartment of the late predivisional cell (Fig. 3A) (13). The CcrM methyltransferase, in contrast, is cleared from both incipient progeny cells; it is present only in late S phase when it methylates the newly replicated chromosomes (Fig. 3A).
Transcription of ccrM is under strict temporal control and is maximal in late S phase. The promoter (Fig. 3B) contains a consensus CtrA binding site in the −35 region as well as an inverted repeat in the mRNA leader region which bears tandem CcrM methylation sites (25, 27, 33). Phosphorylated CtrA binds to the ccrM promoter and activates transcription of this gene (27). However, it binds with relatively low affinity, ensuring that ccrM transcription will be maximal late in S phase when CtrA levels are elevated. Although the mechanism of inactivation of ccrM transcription is not well understood, there is evidence that CcrM methylation itself plays a role in inhibiting ccrM transcription (33). When the methylation sites in its promoter are mutated, ccrM continues to be transcribed after cell division. Thus, it is possible that methylation of the ccrM promoter provides a means of ccrM autoregulation. Transcription of ccrM shuts off in swarmer cells while CtrA is still abundant, suggesting that additional factors are necessary for its attenuation.
Restriction of CcrM to a narrow window of the cell cycle also requires selective proteolysis. Rapid clearance of CcrM from the cell just before cell division is dependent on the Lon protease (36). In a lon null mutant, the CcrM protein is stable and present throughout the cell cycle. Consequently, the replicating chromosome becomes fully methylated at all times in the cell cycle. Because Lon can act as both a chaperone and a protease (14), its direct role in CcrM degradation is unclear. Transcription of ccrM from a constitutive promoter can override proteolysis, resulting in the presence of CcrM throughout the cell cycle and consequent defects in cell division, morphology, and chromosome replication (36, 37).
ROLE OF METHYLATION IN DNA REPLICATION
Dam methylation contributes to the control of the initiation of DNA replication in E. coli (22, 29), and it appears that CcrM methylation plays a comparable role in Caulobacter. Flow cytometry analysis of wild-type Caulobacter cultures reveals two distinct DNA peaks, indicative of cell populations with either one or two chromosomes (36, 37). However, when ccrM is transcribed constitutively and the replicating chromosomes are fully methylated throughout the cell cycle, a significant proportion of cells contain three chromosomes. In addition, the cells become elongated and divide aberrantly. Similarly, when CcrM is overexpressed in R. meliloti or B. abortus, the cells develop abnormal morphology and accumulate multiple chromosomes (28, 35). The aneuploidy seen with constitutive CcrM methylation in these species raises the possibility that CcrM, like Dam, participates in regulating chromosome replication.
In E. coli, the initiation of chromosome replication is coupled to the cell cycle by delayed remethylation of GATC sites in the origin (oriC) (1). Normally, Dam is present throughout the cell cycle and rapidly methylates the GATC sequences of newly synthesized DNA. However, the methylation of oriC is significantly delayed compared to that of other chromosomal loci (9). The SeqA protein binds hemimethylated GATC sites in the origin and sequesters the newly replicated oriC from remethylation, making it inaccessible to replication proteins until later in the cell cycle (7, 30). If the multiple Dam methylation sites in oriC are not sequestered from remethylation, the cells contain increased numbers of chromosomes, suggesting that methylation is involved in controlling primary initiation as well as the proper timing of initiation (1, 5).
Both CcrM methylation and binding of the CtrA response regulator to Cori contribute to the temporal control of DNA replication initiation in Caulobacter. Replication initiation is restricted to stalked cells where the chromosome is fully methylated (11, 21), implying that methylation of the origin is necessary for replication competence in Caulobacter as it is in E. coli (29). While CcrM methylation sites are not clustered in the Caulobacter Cori with the frequency that Dam methylation sites are found in the E. coli oriC, they are nonetheless found at a higher than expected frequency and in close proximity to putative DnaA boxes. Therefore, the ability to methylate DNA only in late S phase may impose a temporal sequestration of Cori. As described above, physical sequestration of the E. coli origin by SeqA delays replication initiation. Similarly, Cori appears to be protected by CtrA throughout most of the cell cycle. The different replicative capacities of the swarmer and stalked cells, which both contain fully methylated DNA, are controlled by CtrA, which binds to Cori and prevents replisome formation in the swarmer cell but is absent in the stalked cell (13, 26). Consequently, only the stalked cell is able to initiate replication after the chromosome is fully methylated. Additional mechanisms may prevent premature reinitiation in early S phase before CtrA is resynthesized (16).
OTHER FUNCTIONS OF METHYLATION
Another function of Dam is its role in DNA mismatch repair. Dam methylation marks the template (parent) strand and directs repair enzymes to correct the new unmethylated strand (reviewed in reference 22). CcrM and its homologs may play comparable roles in mismatch repair, although this possibility has not been tested. While the prolonged period of hemimethylation of the Caulobacter chromosome may well provide extra time for surveillance mechanisms, the rapidity with which DNA mismatch repair is known to occur in other organisms and with which it must occur near the terminus, suggest that this is not the principal reason why CcrM methylation is so highly regulated.
However, a function for CcrM methylation that could contribute to the control of cell cycle progression is the regulation of gene expression. In E. coli, methylation of GATC sites in 5′ noncoding sequences alters gene transcription by modifying the binding of regulatory proteins to promoter DNA. Perhaps the best-studied example of transcriptional regulation by methylation is Pap pilus gene expression (6, 24). Alternate methylation of the two Dam sites in the pap promoter influences the binding of the Lrp global regulatory protein to the pap promoter and acts as an on/off switch for expressing the pap operon. In addition, there are a small number of GATC sites in the E. coli chromosome that remain unmethylated throughout the cell cycle (34). Because many of these sites contain binding motifs for known regulatory proteins, it has been proposed that proteins occupy these sites continuously. Dam methylation also alters the transcription of genes with Dam sites in the −10 and −35 hexamers, implying that methylation directly affects the access of RNA polymerase to these promoters (3). As described above, attenuation of ccrM transcription in the Caulobacter swarmer cell may be in part autoregulatory, controlled by methylation of the ccrM promoter itself (33). Methylation sites are also found in the DNA encoding the mRNA leader region of the fliL and fliQ flagellar genes and in the promoters of several other genes, including the ctrA response regulator and the ftsZ gene which encodes a tubulin-like protein required for cell division. The effect of methylation on the activity of these promoters has not yet been tested but may eventually provide another reason for the existence of a temporal gradient of chromosome methylation. In this context, changes in the methylation state of regulatory DNA during chromosome replication could link gene expression to cell cycle progression, thus providing an additional control mechanism in the highly regulated differentiation established by this bacterium. Perhaps the switch from a fully methylated promoter to a hemimethylated promoter controls the temporal expression of a cell cycle signal transduction gene. Another possibility is that a DNA binding protein which functions in chromosome separation recognizes its DNA binding motif only when it is hemimethylated. Thus, the DNA methylation state could communicate the status of chromosome replication to factors that control subsequent cell cycle events.
SUMMARY
In bacteria, DNA methylation functions primarily in restriction-modification systems. The discovery of the Dam methyltransferase in E. coli, however, showed that methylation also plays regulatory roles in the cell, including control of DNA replication and transcription. Recently, Dam methylation was found to be required for pathogenicity in the enteric bacterium S. typhimurium and to regulate the expression of virulence genes (15). Now, studies of the CcrM methyltransferase in Caulobacter, Rhizobium, and Brucella suggest that bacteria of the alpha subdivision also use adenine methylation as a regulatory mechanism. Because of the ease of studying the cell cycle in Caulobacter, this organism provides a unique opportunity to explore the relationships among DNA methylation, chromosome replication, and cell cycle progression. CcrM, like Dam, may also play a role in the virulence of pathogenic alpha subdivision bacteria. It is likely that as yet undiscovered DNA adenine methyltransferases in other groups of bacteria play comparable roles in regulating cell cycle events and pathogenicity.
ACKNOWLEDGMENTS
We thank Greg Marczynski for critically reading this manuscript, and Greg Robertson, Rachel Wright, and R. M. Roop for sharing unpublished results.
This work was supported in part by NIH grants GM32506/5120MZ and GM51426 and by DARPA MDA 972-97-1-0008.
REFERENCES
- 1.Bakker A, Smith D W. Methylation of GATC sites is required for precise timing between rounds of DNA replication in Escherichia coli. J Bacteriol. 1989;171:5738–5742. doi: 10.1128/jb.171.10.5738-5742.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbeyron T, Kean K, Forterre P. DNA adenine methylation of GATC sequences appeared recently in the Escherichia coli lineage. J Bacteriol. 1984;160:586–590. doi: 10.1128/jb.160.2.586-590.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barras F, Marinus M G. The great GATC: DNA methylation in E. coli. Trends Genet. 1989;5:139–143. doi: 10.1016/0168-9525(89)90054-1. [DOI] [PubMed] [Google Scholar]
- 4.Berdis A J, Lee I, Coward J K, Stephens C, Wright R, Shapiro L, Benkovic S J. A cell cycle-regulated adenine DNA methyltransferase from Caulobacter crescentus processively methylates GANTC sites on hemimethylated DNA. Proc Natl Acad Sci USA. 1998;95:2874–2879. doi: 10.1073/pnas.95.6.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boye E, Lobner-Olesen A. The role of dam methyltransferase in the control of DNA replication in E. coli. Cell. 1990;62:981–989. doi: 10.1016/0092-8674(90)90272-g. [DOI] [PubMed] [Google Scholar]
- 6.Braaten B A, Nou X, Kaltenbach L S, Low D A. Methylation patterns in pap regulatory DNA control pyelonephritis-associated pili phase variation in E. coli. Cell. 1994;76:577–588. doi: 10.1016/0092-8674(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 7.Brendler T, Abeles A, Austin S. A protein that binds to the P1 origin core and the oriC 13mer region in a methylation-specific fashion is the product of the host seqA gene. EMBO J. 1995;14:4083–4089. doi: 10.1002/j.1460-2075.1995.tb00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks J E, Blumenthal R M, Gingeras T R. The isolation and characterization of the Escherichia coli DNA adenine methylase (dam) gene. Nucleic Acids Res. 1983;11:837–851. doi: 10.1093/nar/11.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell J L, Kleckner N. E. coli oriC and the dnaA gene promoter are sequestered from the dam methyltransferase following passage of the chromosomal replication fork. Cell. 1990;62:967–979. doi: 10.1016/0092-8674(90)90271-f. [DOI] [PubMed] [Google Scholar]
- 10.Cheng X. Structure and function of DNA methyltransferases. Annu Rev Biophys Biomol Struct. 1995;24:293–318. doi: 10.1146/annurev.bb.24.060195.001453. [DOI] [PubMed] [Google Scholar]
- 11.Degnen S T, Newton A. Chromosome replication during development in Caulobacter crescentus. J Mol Biol. 1972;64:671–680. doi: 10.1016/0022-2836(72)90090-3. [DOI] [PubMed] [Google Scholar]
- 12.Dingwall A, Shapiro L. Rate, origin, and bidirectionality of Caulobacter chromosome replication as determined by pulsed-field gel electrophoresis. Proc Natl Acad Sci USA. 1989;86:119–123. doi: 10.1073/pnas.86.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domian I J, Quon K C, Shapiro L. Cell type-specific phosphorylation and proteolysis of a transcriptional regulator controls the G1-to-S transition in a bacterial cell cycle. Cell. 1997;90:415–424. doi: 10.1016/s0092-8674(00)80502-4. [DOI] [PubMed] [Google Scholar]
- 14.Gottesman S, Wickner S, Maurizi M R. Protein quality control: triage by chaperones and proteases. Genes Dev. 1997;11:815–823. doi: 10.1101/gad.11.7.815. [DOI] [PubMed] [Google Scholar]
- 15.Heithoff D M, Sinsheimer R L, Low D A, Mahan M J. An essential role for DNA adenine methylation in bacterial virulence. Science. 1999;284:967–970. doi: 10.1126/science.284.5416.967. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs C, Domian I J, Maddock J R, Shapiro L. Cell cycle-dependent polar localization of an essential bacterial histidine kinase that controls DNA replication and cell division. Cell. 1999;97:111–120. doi: 10.1016/s0092-8674(00)80719-9. [DOI] [PubMed] [Google Scholar]
- 17.Kelly A J, Sackett M J, Din N, Quardokus E, Brun Y V. Cell cycle-dependent transcriptional and proteolytic regulation of FtsZ in Caulobacter. Genes Dev. 1998;12:880–893. doi: 10.1101/gad.12.6.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lobner-Olesen A, Boye E, Marinus M G. Expression of the Escherichia coli dam gene. Mol Microbiol. 1992;6:1841–1851. doi: 10.1111/j.1365-2958.1992.tb01356.x. [DOI] [PubMed] [Google Scholar]
- 19.Malone T, Blumenthal R M, Cheng X. Structure-guided analysis reveals nine sequence motifs conserved among DNA amino-methyltransferases, and suggests a catalytic mechanism for these enzymes. J Mol Biol. 1995;253:618–632. doi: 10.1006/jmbi.1995.0577. [DOI] [PubMed] [Google Scholar]
- 20.Marczynski G T. Chromosome methylation and measurement of faithful, once and only once per cell cycle chromosome replication in Caulobacter crescentus. J Bacteriol. 1999;181:1984–1993. doi: 10.1128/jb.181.7.1984-1993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marczynski G T, Shapiro L. Cell-cycle control of a cloned chromosomal origin of replication from Caulobacter crescentus. J Mol Biol. 1992;226:959–977. doi: 10.1016/0022-2836(92)91045-q. [DOI] [PubMed] [Google Scholar]
- 22.Marinus M G. Methylation of DNA. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger M E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C: ASM Press; 1996. pp. 782–791. [Google Scholar]
- 23.Marinus M G, Poteete A, Arraj J A. Correlation of DNA adenine methylase activity with spontaneous mutability in Escherichia coli K-12. Gene. 1984;28:123–125. doi: 10.1016/0378-1119(84)90095-7. [DOI] [PubMed] [Google Scholar]
- 24.Nou X, Skinner B, Braaten B, Blyn L, Hirsch D, Low D. Regulation of pyelonephritis-associated pili phase-variation in Escherichia coli: binding of the PapI and the Lrp regulatory proteins is controlled by DNA methylation. Mol Microbiol. 1993;7:545–553. doi: 10.1111/j.1365-2958.1993.tb01145.x. [DOI] [PubMed] [Google Scholar]
- 25.Quon K, Marczynski G T, Shapiro L. Cell cycle control by an essential bacterial two-component signal transduction protein. Cell. 1996;84:83–93. doi: 10.1016/s0092-8674(00)80995-2. [DOI] [PubMed] [Google Scholar]
- 26.Quon K C, Yang B, Domian I J, Shapiro L, Marczynski G T. Negative control of bacterial DNA replication by a cell cycle regulatory protein that binds at the chromosome origin. Proc Natl Acad Sci USA. 1998;95:120–125. doi: 10.1073/pnas.95.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reisenauer A, Quon K, Shapiro L. The CtrA response regulator mediates temporal control of gene expression during the Caulobacter cell cycle. J Bacteriol. 1999;181:2430–2439. doi: 10.1128/jb.181.8.2430-2439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robertson, G., A. Reisenauer, R. Wright, A. E. Jensen, L. Shapiro, and R. M. Roop. Unpublished results.
- 29.Russell D W, Zinder N D. Hemimethylation prevents DNA replication in E. coli. Cell. 1987;50:1071–1079. doi: 10.1016/0092-8674(87)90173-5. [DOI] [PubMed] [Google Scholar]
- 30.Slater S, Wold S, Lu M, Boye E, Skarstad K, Kleckner N. E. coli SeqA protein binds oriC in two different methyl-modulated reactions appropriate to its roles in DNA replication initiation and origin sequestration. Cell. 1995;82:927–936. doi: 10.1016/0092-8674(95)90272-4. [DOI] [PubMed] [Google Scholar]
- 31.Stamm L V, Greene S R, Barnes N Y, Bergen H L, Hardham J M. Identification and characterization of a Treponema pallidum subsp. pallidum gene encoding a DNA adenine methyltransferase. FEMS Microbiol Lett. 1997;155:115–119. doi: 10.1111/j.1574-6968.1997.tb12694.x. [DOI] [PubMed] [Google Scholar]
- 32.Stephens C, Reisenauer A, Wright R, Shapiro L. A cell cycle-regulated bacterial DNA methyltransferase is essential for viability. Proc Natl Acad Sci USA. 1996;93:1210–1214. doi: 10.1073/pnas.93.3.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stephens C M, Zweiger G, Shapiro L. Coordinate cell cycle control of a Caulobacter DNA methyltransferase and the flagellar genetic hierarchy. J Bacteriol. 1995;177:1662–1669. doi: 10.1128/jb.177.7.1662-1669.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tavazoie S, Church G M. Quantitative whole-genome analysis of DNA-protein interactions by in vivo methylase protection in E. coli. Nature Biotechnol. 1998;16:566–571. doi: 10.1038/nbt0698-566. [DOI] [PubMed] [Google Scholar]
- 35.Wright R, Stephens C, Shapiro L. The CcrM DNA methyltransferase is widespread in the alpha subdivision of proteobacteria, and its essential functions are conserved in Rhizobium meliloti and Caulobacter crescentus. J Bacteriol. 1997;179:5869–5877. doi: 10.1128/jb.179.18.5869-5877.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright R, Stephens C, Zweiger G, Shapiro L, Alley M R K. Caulobacter Lon protease has a critical role in cell-cycle control of DNA methylation. Genes Dev. 1996;10:1532–1542. doi: 10.1101/gad.10.12.1532. [DOI] [PubMed] [Google Scholar]
- 37.Zweiger G, Marczynski G, Shapiro L. A Caulobacter DNA methyltransferase that functions only in the predivisional cell. J Mol Biol. 1994;235:472–485. doi: 10.1006/jmbi.1994.1007. [DOI] [PubMed] [Google Scholar]