Abstract
Purpose:
Marginal zone lymphoma (MZL) is an uncommon non–Hodgkin lymphoma with malignant cells that exhibit a consistent dependency on B-cell receptor signaling. We evaluated the efficacy and safety of zanubrutinib, a next-generation selective Bruton tyrosine kinase inhibitor, in patients with relapsed/refractory (R/R) MZL.
Patients and Methods:
Patients with R/R MZL were enrolled in the phase II MAGNOLIA (BGB-3111–214) study. The primary endpoint was overall response rate (ORR) as determined by an independent review committee (IRC) based on the Lugano 2014 classification.
Results:
Sixty-eight patients were enrolled. After a median follow-up of 15.7 months (range, 1.6 to 21.9 months), the IRC-assessed ORR was 68.2% and complete response (CR) was 25.8%. The ORR by investigator assessment was 74.2%, and the CR rate was 25.8%. The median duration of response (DOR) and median progression-free survival (PFS) by independent review was not reached. The IRC-assessed DOR rate at 12 months was 93.0%, and IRC-assessed PFS rate was 82.5% at both 12 and 15 months. Treatment was well tolerated with the majority of adverse events (AE) being grade 1 or 2. The most common AEs were diarrhea (22.1%), contusion (20.6%), and constipation (14.7%). Atrial fibrillation/flutter was reported in 2 patients; 1 patient had grade 3 hypertension. No patient experienced major hemorrhage. In total, 4 patients discontinued treatment due to AEs, none of which were considered treatment-related by the investigators.
Conclusions:
Zanubrutinib demonstrated high ORR and CR rate with durable disease control and a favorable safety profile in patients with R/R MZL.
Translational Relevance.
Marginal zone lymphoma (MZL) is an uncommon non–Hodgkin lymphoma (NHL) with malignant cells exhibiting consistent dependency on B-cell receptor (BCR) signaling. Its rarity has hindered the identification of optimal treatment strategies with therapeutic approaches historically based on studies of follicular lymphoma. Like other indolent NHLs, advanced-stage disease is generally considered incurable, with most patients experiencing a continuing pattern of relapse and remission. Patients with relapsed/refractory (R/R) MZL often respond poorly to chemoimmunotherapy, and there is an unmet need for novel, more effective, and tolerable therapies. Here we present safety and efficacy data for 68 patients with R/R MZL from the phase II MAGNOLIA (BGB-3111–214) study who received the Bruton tyrosine kinase (BTK) inhibitor, zanubrutinib. In this study, zanubrutinib exhibited a favorable safety profile and demonstrated high response rates including in patients aged ≥ 75 years, which, if sustained, could represent a significant therapeutic advancement in patients with R/R MZL.
Introduction
Marginal zone lymphoma (MZL) accounts for approximately 8% to 12% of all non–Hodgkin lymphomas (NHL). The disease arises from memory B lymphocytes, which are normally present in the marginal zone of secondary lymphoid follicles within the spleen, lymph nodes, and mucosal lymphoid tissues (1). There are three subtypes of MZL: (i) extranodal MZL, mostly represented by mucosa-associated lymphoid tissue (MALT) lymphoma; (ii) nodal MZL (NMZL); and (iii) splenic MZL (SMZL; ref. 2).
Like other indolent lymphomas, patients with advanced-stage disease are incurable, and MZL is managed as a chronic disease in most patients (3). Systemic treatment is often based on regimens employed in follicular lymphoma and includes an anti-CD20 monoclonal antibody (mAb) alone or in combination with chemotherapy. However, many patients with advanced disease experience serial relapses, supporting the need for new agents and novel combinations to improve outcomes for these patients (3, 4).
Improved understanding of disease biology has altered the therapeutic landscape of MZL. Targeted therapies focusing on intracellular signaling pathways, such as the B-cell receptor (BCR) signaling pathway, have resulted in improved efficacy and tolerable toxicity profiles over chemoimmunotherapy-based approaches (3). The FDA recently approved ibrutinib [a first-in-class Bruton tyrosine kinase (BTK) inhibitor; ref. 4], the combination of lenalidomide plus rituximab (5), and umbralisib (6) for the treatment of patients with relapsed/refractory (R/R) MZL.
The pivotal study of ibrutinib in 63 patients with R/R MZL previously treated with at least one anti-CD20–based therapy demonstrated an overall response rate (ORR) of 48%. The most common grade 3 or higher adverse events (AE) were anemia (14%), pneumonia (8%), and fatigue (6%). Atrial fibrillation occurred in 6% of patients, 17% of patients discontinued treatment due to AEs, and 10% of patients had dose reduction due to AEs (4).
The combination of lenalidomide plus rituximab in patients with indolent lymphoma including R/R MZL was evaluated in two phase III studies, AUGMENT and MAGNIFY. The ORR was 65% in AUGMENT (n = 31 R/R MZL) and 51% (n = 45 R/R MZL) in the MAGNIFY study. In the AUGMENT study, infections (63% vs. 49%) and cutaneous reactions (32% vs. 12%) were more common in the lenalidomide plus rituximab arm compared with the control arm (placebo plus rituximab), as was grade 3 or higher neutropenia (50% vs. 13%, respectively; ref. 5). AEs that led to treatment discontinuation occurred in 14.6% of patients with R/R MZL who received lenalidomide plus rituximab (5, 7).
The study of umbralisib in patients with indolent lymphoma included 69 patients with R/R MZL previously treated with ≥ 1 anti-CD20–based therapy, and demonstrated an ORR of 49%. The most common grade ≥ 3 AEs in ≥ 10% of patients were neutropenia (11.5%) and diarrhea (10.1%). Other grade ≥ 3 AEs of interest included alanine aminotransferase (ALT) and aspartate aminotransferase (AST) elevations (6.7% and 7.2%, respectively), pneumonitis (1%), and noninfectious colitis (0.5%). Treatment-related AEs led to discontinuation in 14.9% of patients (6).
In addition, phosphoinositide 3-kinase (PI3K) inhibitors copanlisib (8) and parsaclisib (9) have also shown activity in patients with R/R MZL. In the CHRONOS-3 study, 66 patients with R/R MZL received the copanlisib plus rituximab combination, demonstrating an ORR of 76%. Grade ≥ 3 AEs included hyperglycemia (56%), hypertension (40%), pneumonitis (7%), and colitis (1%). AEs led to treatment discontinuation in 34% of all patients treated (8). In the CITADEL-204 study, parsaclisib demonstrated an ORR of 54% in 94 patients with R/R MZL. Neutropenia and diarrhea (8.1% each) were the most common grade ≥3 AEs. Treatment discontinuation due to AEs was reported in 15.2% of patients (9).
While these new therapies provide additional options, they are not without toxicity; therefore, there remains an unmet need for more effective and better-tolerated therapies to improve long-term outcomes. In particular, the AEs observed with the first-generation BTK inhibitor ibrutinib, are believed to be caused by the inhibition of off-target tyrosine kinases (10). To address this gap, zanubrutinib was designed with significantly higher BTK selectivity, which may confer tolerability advantages over ibrutinib, particularly for treatment-limiting toxicities such as atrial fibrillation/flutter, diarrhea, and hypertension.
Zanubrutinib is a potent, highly specific, and irreversible next-generation BTK inhibitor. It achieves deep and durable responses in patients with Waldenström macroglobulinemia (11), chronic lymphocytic leukemia/small lymphocytic lymphoma (12), and mantle cell lymphoma (MCL; ref. 13). Zanubrutinib has been approved by the FDA under the accelerated approval mechanism for the treatment of patients with MCL who have received at least one prior therapy (14).
Here, we present the efficacy and safety outcomes of zanubrutinib in 68 patients with R/R MZL enrolled in the phase II MAGNOLIA (BGB-3111–214) study.
Patients and Methods
Study design and treatment
The MAGNOLIA study (BGB-3111–214; NCT03846427) is a phase II study of zanubrutinib in patients with R/R MZL who had previously received one or more lines of therapy, including at least one CD20-directed regimen.
This is a single arm, open-label study (no randomization or blinding) that is being conducted at 31 sites in 9 countries. All patients were treated with zanubrutinib 160 mg twice daily until disease progression or unacceptable toxicity.
Ethics
This study is being conducted according to the principles of the Declaration of Helsinki and International Conference on Harmonisation guidelines, and the protocol was approved by the institutional review boards/independent ethics committees at each site. All 68 patients provided written informed consent, and study conduct was approved by local human investigation committees or institutional review boards in accordance with assurances filed with and approved by the US Department of Health and Human Services.
Patients
Eligible patients were ≥ 18 years old; had a histologically confirmed diagnosis of MZL, including SMZL, NMZL, and extranodal (MALT) subtypes; and required systemic therapy (e.g., because of compromised organ function, presence of symptoms, cytopenias, or increase in disease tempo). Patients had previously received one or more lines of therapy, including prior treatment with an anti-CD20 therapy, and had an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 to 2, with adequate organ function as demonstrated by the following: neutrophil count ≥ 1.0 × 109/L, platelet count ≥75 × 109/L, creatinine clearance ≥ 30 mL/minute, AST and ALT levels ≤ 2.5 times the upper limit of normal (ULN), and total bilirubin < 2.0 times ULN. Key exclusion criteria included previous exposure to a BTK inhibitor, central nervous system (CNS) involvement by MZL, known transformation to aggressive lymphoma, clinically relevant cardiovascular disease, and active infection. Patients requiring concurrent strong CYP3A inhibitors/inducers were excluded, but antiplatelet therapy and anticoagulants, including warfarin, were permitted.
Assessments
Disease response was assessed by investigators and by an independent review committee (IRC) in accordance with the Lugano classification for NHL (15). Imaging studies (contrast-enhanced CT or MRI) were conducted at screening, every 12 weeks during the first year, then every 24 weeks thereafter until disease progression. Pretreatment PET was required and repeated every 12 weeks during the first year, at week 72, and for complete response (CR) or progressive disease (PD) confirmation among patients with IRC-confirmed fluorodeoxyglucose (FDG)–avid disease. Only patients with both pretreatment and at least one postbaseline PET scan were evaluated using the PET-based Lugano criteria. Baseline target and nontarget lesions selected by the IRC were FDG-positive in patients with FDG-avid disease. A follow-up bone marrow test and/or endoscopy were required (regardless of PET status) to confirm CR in patients with histologic evidence of bone marrow and/or gastrointestinal involvement at baseline.
Safety assessments included evaluation of AEs (collected up to 30 days after the last dose of zanubrutinib) and clinically significant laboratory and vital sign abnormalities. AE severity was graded according to the NCI Common Terminology Criteria for Adverse Events (version 4.03). The incidence and severity of AEs of interest were evaluated on the basis of the known and theoretical toxicity for the BTK inhibitor class; these include bleeding (including major hemorrhage), hypertension, atrial fibrillation/flutter, second primary malignancies, infections, and peripheral blood cytopenias.
Statistical analysis
Approximately 65 patients were planned for enrollment which would provide 82% power for an alternative ORR of 48% versus the prespecified null hypothesis of 30%. All patients with R/R MZL who received at least one dose of zanubrutinib were included in the safety analysis. The efficacy analysis set included patients who received at least one dose of zanubrutinib and have centrally confirmed diagnosis of MZL.
The primary efficacy endpoint for this study was ORR, defined as the proportion of patients who achieved a best overall response of partial response (PR) or CR. A binomial exact test was performed to test against the null hypothesized ORR of 30% using the significance level of 0.025 (one sided). Secondary efficacy endpoints included ORR by investigator assessment, progression-free survival (PFS), duration of response (DOR), and overall survival (OS).
Subgroup analyses were performed using prespecified variables. Median PFS, DOR, OS, and event-free rates at landmark time points were estimated using the Kaplan–Meier method with corresponding 95% confidence intervals (CI; ref. 16). The reverse Kaplan–Meier method was utilized to estimate follow-up times for PFS, DOR, and OS. Standard descriptive statistics were used to summarize AE data.
Results
Patients and baseline characteristics
Between February 19, 2019 and January 6, 2020, 68 patients were enrolled in the MAGNOLIA study and received zanubrutinib 160 mg twice a day. The median age was 70 years (range, 37 to 95 years) and 27.9% of patients were ≥ 75 years old. Most had a baseline ECOG performance status (PS) of 0 or 1 (57.4% and 35.3%, respectively). The majority of patients were Caucasian (60.3%) with slight male predominance (52.9%). Twenty-six patients had NMZL (38.2%), 26 had extranodal MZL (38.2%), and 12 had SMZL (17.6%). Four (5.9%) patients presented with concurrent nodal and extranodal disease, and the investigators were unable to classify the primary MZL subtype. All 68 patients received at least one prior line of CD20-directed treatment per study eligibility. The median number of lines of prior systemic anticancer therapies was two (range, 1 to 6), and 32.4% of patients had refractory disease at study entry. The most common prior regimens were rituximab plus cyclophosphamide, vincristine, and prednisolone (36.8%); bendamustine plus rituximab (32.4%); and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (25%). Seven (10.3%) patients received rituximab as their only prior line of therapy (Table 1).
Table 1.
Demographics and baseline disease characteristics.
| BGB-3111–214 (MAGNOLIA study) | |
|---|---|
| Characteristics | R/R MZL (N = 68) |
| Age, years | |
| Median (range) | 70 (37–95) |
| Age category, n (%) | |
| ≥65 years and <75 years | 22 (32.4) |
| ≥75 years | 19 (27.9) |
| Sex, n (%) | |
| Male | 36 (52.9) |
| Female | 32 (47.1) |
| Baseline ECOG PS score, n (%) | |
| 0 | 39 (57.4) |
| 1 | 24 (35.3) |
| 2 | 5 (7.4) |
| Bulky disease, n (%) | |
| LDi >5 cm | 25 (36.8) |
| Bone marrow involvement, n (%)a | 29 (42.6) |
| Extranodal disease, n (%)b | 53 (77.9) |
| Refractory diseasec | 22 (32.4) |
| Evidence of FDG-avid disease by IRC, n (%) | |
| FDG-avid | 61 (89.7) |
| Non–FDG-avid | 7 (10.3) |
| MZL subtype, n (%) | |
| Extranodal (MALT) | 26 (38.2) |
| Nodal | 26 (38.2) |
| Splenic | 12 (17.6) |
| Unknownd | 4 (5.9) |
| Site of disease for MALT subtype, n (%) | |
| Gastric | 2 (7.7) |
| Cutaneous | 4 (15.4) |
| Nongastric/noncutaneous | 19 (73.1) |
| Unknown | 1 (3.8) |
| Baseline cytopeniae | 20 (29.4) |
| LDH (U/L) | |
| Median (range) | 204 (128.5–1,405) |
| LDH, n (%) | |
| Above normal | 16 (23.5) |
| Number of previous therapies | |
| Median (range) | 2 (1–6) |
| Time from end of last therapy to study entry (months) | |
| Median (range) | 20.6 (1–176.6) |
| Previous therapyf, n (%) | |
| Rituximab-based chemotherapy | 60 (88.2) |
| R-CVP | 25 (36.8) |
| BR | 22 (32.4) |
| R-CHOP | 17 (25.0) |
| Rituximab monotherapy | 15 (22.1)g |
| Rituximab + lenalidomide | 2 (2.9) |
| Radiation therapy | 15 (22.1) |
| Splenectomy | 7 (10.3) |
| Autologous hematopoietic stem cell transplant | 4 (5.9) |
Abbreviations: BR, bendamustine/rituximab; LDi, longest diameter; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; R-CVP, rituximab plus cyclophosphamide, vincristine sulfate, and prednisone.
aDerived from baseline bone marrow biopsy/aspiration per investigator assessment.
bExtranodal disease is defined as patients with extranodal baseline target or nontarget lesions, or bone marrow involvement, as per investigator assessment.
cRefractory disease is defined as best overall response of stable disease or PD from last prior anticancer regimen.
dFour patients presented with both nodal and extranodal lesions; investigators were unable to classify the primary MZL subtype.
eCytopenia is defined as baseline neutrophils ≤1.5 × 109/L or baseline platelet ≤100 × 109/L or baseline hemoglobin ≤110 g/L.
fCategories are not mutually exclusive. A patient could have been included under multiple regimens.
gOf the 15 patients who received prior rituximab monotherapy, rituximab was the only prior treatment in 7 patients.
Median duration of treatment was 60.1 weeks (range, 3.7 to 85.1) with a median of 15 treatment cycles (range, 1 to 21). At a median study follow up of 15.7 months (range 1.6 to 21.9), 28 (41.2%) patients discontinued study treatment [PD per investigator assessment (n = 20), AE (n = 4), required prohibited medication (n = 3), withdrew consent (n = 1)]. Forty (58.8%) patients were continuing on zanubrutinib and 59 (86.8%) patients remain in the study.
Efficacy
Sixty-six patients were evaluable for efficacy (2 patients were excluded as central pathology showed transformation of MZL to diffuse large B-cell lymphoma). The IRC-assessed ORR was 68.2% (95% CI, 55.56–79.11). The ORR for the extranodal (n = 25), nodal (n = 25), splenic (n = 12), and unknown (n = 4) subtypes was 64%, 76%, 66.7%, and 50%, respectively. The median time to response was 2.8 months (range, 1.7 to 11.1).
Twenty-eight (42.4%) patients achieved PR while 17 (25.8%) patients achieved CR. The IRC-assessed CR rates for each MZL subtype were 40% (extranodal), 20% (nodal), 8.3% (splenic), and 25% (unknown subtype). Among the 17 patients who achieved CR, only 1 patient had disease progression and 13 patients remain on study treatment. Of the 13 (19.7%) patients with a best response of stable disease, 5 (7.6%) patients were continuing on zanubrutinib.
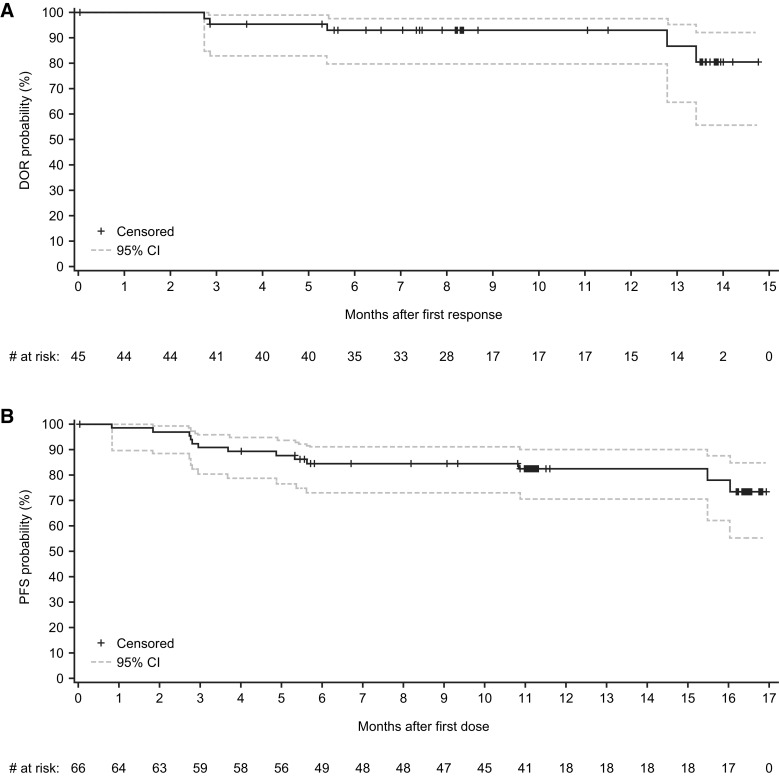
The IRC-assessed median DOR was not reached. The estimated DOR rate at 12 months after first response was 93% (Fig. 1A). At a median study follow-up of 15.7 months, 40 (89%) of the 45 responders were free from progression or death with 34 (76%) patients continuing on zanubrutinib. The IRC-assessed median PFS was not reached. The estimated PFS rates were 82.5% at 12 and 15 months (Fig. 1B).
Figure 1.
A, Kaplan–Meier plot of duration of response with zanubrutinib per IRC assessment based on Lugano classification (15) for patients with MZL in the MAGNOLIA study (BGB-3111–214). Only patients with either PR or CR were included. B, Kaplan–Meier plot of PFS with zanubrutinib per IRC assessment based on Lugano classification (15) for patients with MZL in the MAGNOLIA study (BGB-3111–214). CIs were calculated using a generalized Brookmeyer and Crowley method.
The median DOR was not reached in any of the subgroups analyzed. The median PFS was not reached in all subgroups except in the subgroup of patients with above normal lactate dehydrogenase (LDH; n = 15) who had a median PFS of 15.5 months.
A total of 7 patients died: 4 following disease progression and 3 due to AEs [COVID-19 pneumonia (n = 2) and myocardial infarction (n = 1)]. The median OS was not reached. The 12- and 15-month OS rates were 95.3% and 92.9%, respectively (Table 2).
Table 2.
Analysis of response by IRC based on Lugano classification (15).
| BGB-3111–214 (MAGNOLIA study) | |
|---|---|
| Response | R/R MZL (N = 66) |
| Median study follow-up, (range), months | 15.7 (1.6–21.9) |
| Response rates | |
| ORR, n (%; 95% CI)a | 68.2 (55.56–79.11) |
| CR, n (%) | 17 (25.8) |
| PR, n (%) | 28 (42.4) |
| Stable disease, n (%) | 13 (19.7) |
| Non-PD, n (%)b | 1 (1.5) |
| PD, n (%) | 6 (9.1) |
| Discontinued study prior to first assessment | 1 (1.5) |
| Median time to response (≥PR; range), months | 2.8 (1.7–11.1) |
| Responses by subtype | |
| Extranodal (MALT; n = 25) | |
| ORR, n (%) | 16 (64.0) |
| CR, n (%) | 10 (40.0) |
| Nodal (n = 25) | |
| ORR, n (%) | 19 (76.0) |
| CR, n (%) | 5 (20.0) |
| Splenic (n = 12) | |
| ORR, n (%) | 8 (66.7) |
| CR, n (%) | 1 (8.3) |
| Unknown (n = 4) | |
| ORR, n (%) | 2 (50.0) |
| CR, n (%) | 1 (25.0) |
| Median and event-free rate | |
| Median DOR, months (range)c,d | NE (NE–NE) |
| 12-month DOR, % (95% CI) | 93.0 (79.8–97.7) |
| 15-month DOR, % (95% CI) | NE (NE–NE) |
| Median PFS, months (range)c,d | NE (NE–NE) |
| 12-month PFS, % (95% CI) | 82.5 (70.55–89.93) |
| 15-month PFS, % (95% CI) | 82.5 (70.55–89.93) |
| Median OS, months (range)c,d | NE (NE–NE) |
| 12-month OS, % (95% CI) | 95.3 (86.0–98.4) |
| 15-month OS, % (95% CI) | 92.9 (81.9–97.3) |
Note: Percentages based on number of patients who received at least one dose of zanubrutinib.
Abbreviations: NE, not estimable; PD, progressive disease; +, censored.
aTwo-sided Clopper–Pearson, 95% CI.
bOne patient with FDG-avid disease missed the PET scan at Cycle 3 and was assessed as having non-PD by independent review due to missing PET scan. CT scan results showed stable disease at Cycle 3.
cMedians were estimated by Kaplan–Meier method with 95% CIs estimated using the Brookmeyer and Crowley method.
dEvent-free rates were estimated by Kaplan–Meier method with 95% CIs estimated using the Greenwood formula.
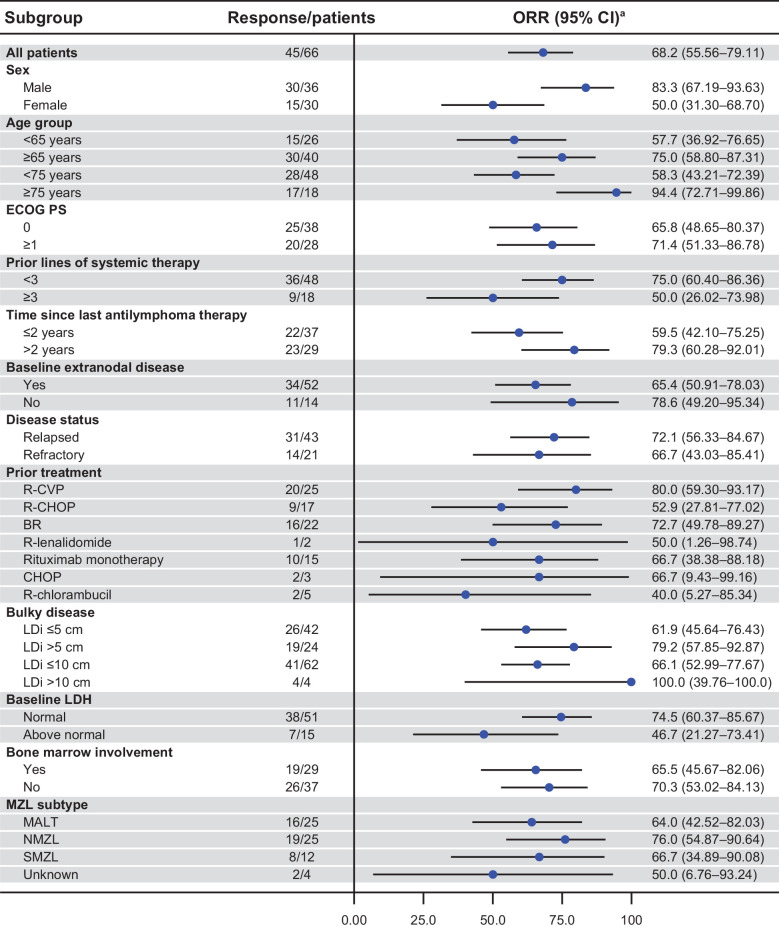
In a subgroup of 18 patients ≥ 75 years of age (4 of the 18 patients were previously treated with rituximab as their only prior line of therapy), the IRC-assessed response rate was 94.4%. ORR of 79.2%, 66.7%, and 76.0% were observed in patients with at least one target lesion of more than 5 cm, refractory disease, and NMZL subtype, respectively (Fig. 2). IRC- and investigator-assessed ORRs are shown in Fig. 3 and Supplementary Fig. S1. The concordance rate for IRC- and investigator-assessed ORR was 87.9%. A waterfall plot for reduction in sum of products of perpendicular diameters of target lesions by best overall response per IRC assessment is shown in Supplementary Fig. S2.
Figure 2.
Forest plot representing subgroup analysis of ORR per IRC assessment based on Lugano classification (15) for patients with MZL in the MAGNOLIA study (BGB-3111–214). CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone. aTwo-sided Clopper–Pearson 95% CIs for ORR.
Figure 3.
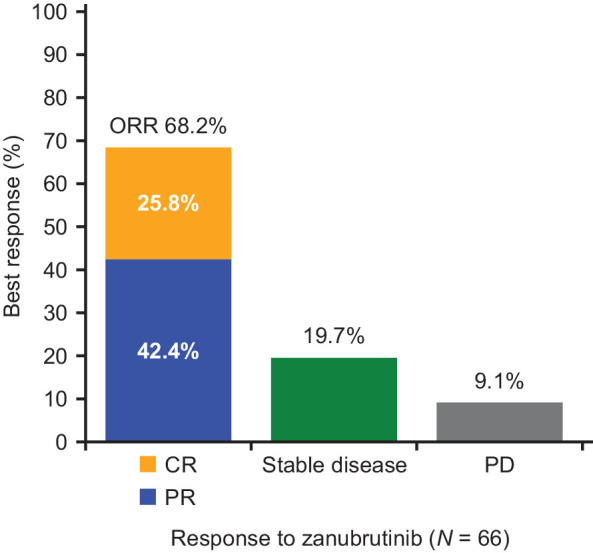
ORR per IRC assessment based on Lugano classification (15) for MZL patients in the MAGNOLIA study (BGB-3111–214).
Safety
Sixty-five (95.6%) patients reported at least one AE of any grade, and 27 (39.7%) patients had at least one grade ≥ 3 AE (Table 3). The most common AEs were diarrhea (22.1%), contusion (20.6%), constipation (14.7%), pyrexia (13.2%), abdominal pain (11.8%), upper respiratory tract infection (11.8%), back pain (10.3%), and nausea (10.3%). Grade ≥ 3 AEs reported in at least 2 patients were neutropenia (10.3%), thrombocytopenia (4.4%), COVID-19 pneumonia (4.4%), diarrhea (2.9%), anemia (2.9%), pyrexia (2.9%), and pneumonia (2.9%). Serious AEs occurred in 26 (38.2%) patients. Serious AEs reported in more than 1 patient included COVID-19 pneumonia (n = 3), pyrexia (n = 3), and fall (n = 2). Infections were reported in 31 (45.6%) patients. The most frequently reported infection was upper respiratory tract infection (11.8%). Grade ≥ 3 infections were reported in 11 (16.2%) patients. Grade ≥ 3 infections occurring in more than 1 patient included COVID-19 pneumonia (n = 3), and pneumonia (n = 2). A grade 3 opportunistic infection (herpes ophthalmic) occurred in 1 patient. Ten (14.7%) patients had infections that met the criteria for seriousness.
Table 3.
Treatment-emergent AEs in >10% of patients, grade ≥3 adverse events in ≥2 patients, and AEs of interest.
| BGB-3111–214 (MAGNOLIA study) | ||
|---|---|---|
| (N = 68) | ||
| AE, n (%) | Any-grade AE | Grade ≥3 AE |
| Patients with ≥1 AE | 65 (95.6) | 27 (39.7) |
| Diarrhea | 15 (22.1) | 2 (2.9) |
| Contusion | 14 (20.6) | 0 |
| Constipation | 10 (14.7) | 0 |
| Pyrexia | 9 (13.2) | 2 (2.9) |
| Abdominal pain | 8 (11.8) | |
| Upper respiratory tract infection | 8 (11.8) | 1 (1.5) |
| Back pain | 7 (10.3) | 0 |
| Nausea | 7 (10.3) | 0 |
| COVID-19 pneumonia | 4 (5.9) | 3 (4.4) |
| Pneumonia | 2 (2.9) | 2 (2.9) |
| AE of interest | ||
| Bleeding | 25 (36.8) | 0 |
| Major hemorrhagea | 0 | 0 |
| Atrial fibrillation/flutter | 2 (2.9) | 1 (1.5) |
| Hypertensionb | 2 (2.9) | 1 (1.5) |
| Second primary malignanciesc | 5 (7.4) | 3 (4.4) |
| Skin cancers | 2 (2.9) | 0 |
| Infections | 31 (45.6) | 11 (16.2)f |
| Opportunistic infections | 2 (2.9) | 1 (1.5) |
| Tumor lysis syndrome | 0 | 0 |
| Anemia | 4 (5.9) | 2 (2.9) |
| Neutropeniad | 9 (13.2) | 7 (10.3) |
| Thrombocytopeniae | 10 (14.7) | 3 (4.4) |
Abbreviation: MedDRA, Medical Dictionary for Regulatory Activities.
aDefined as any serious or grade ≥3 bleed at any site, or central nervous system bleed of any grade.
bIncludes the MedDRA-preferred terms “hypertension” and “prehypertension.”
cIncludes BCC and SCC of skin (in 2 patients with prior history of skin cancer), acute myeloid leukemia (in a patient with prior exposure to an alkylating agent), bladder cancer recurrent, and papillary thyroid cancer (in a patient with pre-existing thyroid nodule).
dIncludes the MedDRA-preferred terms “neutropenia,” and “neutrophil count decreased.”
eIncludes the MedDRA-preferred terms “thrombocytopenia” and “platelet count decreased.”
fCOVID-19 pneumonia and pneumonia are the only grade ≥3 infections occurring in more than one patient.
Four patients were diagnosed with COVID-19 pneumonia. Two patients enrolled from Italy recovered from the event. Two patients (1 from the United States, and 1 from France) died from complications of COVID-19 pneumonia. One patient with bone marrow involvement had a preexisting neutropenia at baseline while the 3 other patients were non-neutropenic at the time of the event. These cases were assessed by the investigators as not related to zanubrutinib.
Any-grade neutropenia occurred in 9 (13.2%) patients, 3 of whom had concurrent infections (upper respiratory tract infection, pneumonia, and COVID-19 pneumonia). Two patients required treatment interruption due to neutropenia and 4 patients received growth factor support. Febrile neutropenia was not observed. Bleeding events were reported in 25 (36.8%) patients; all were grade 1 or 2. The most common were contusion (20.6%), epistaxis (4.4%), and petechiae (4.4%). There were no events of major hemorrhages. Grade 3 atrial fibrillation (in a patient with a history of atrial fibrillation) and grade 2 atrial flutter (in a patient with no known cardiac risk factors) were reported in 2 patients. The event of atrial fibrillation occurred 21 days after the last dose of zanubrutinib. The event of atrial flutter resolved spontaneously and the patient continued with study treatment with no dose modification. Grade 3 hypertension occurred in 1 patient. Second primary malignancies were reported in 5 patients: basal cell carcinoma (BCC) and squamous cell carcinoma (SCC; in 2 patients with prior history of skin cancer), recurrent bladder cancer (in 1 patient), papillary thyroid cancer (in 1 patient with preexisting thyroid mass), and acute myeloid leukemia (in 1 patient with prior exposure to alkylating agents). None of these events led to treatment discontinuation.
Twenty (29.4%) patients had dose interruption due to AEs. The most common AEs leading to treatment interruption were COVID-19 pneumonia (n = 3), neutropenia (n = 2), and pyrexia (n = 2). No AEs led to dose reduction. Four patients permanently discontinued zanubrutinib: fatal COVID-19 pneumonia (n = 2), pyrexia (n = 1; attributed to disease progression), and fatal myocardial infarction (in a patient with a preexisting cardiovascular disease). All four events were assessed by the investigators as unrelated to study treatment.
Discussion
The optimal therapeutic strategy for patients with R/R MZL remains uncertain with systemic therapy based historically on strategies employed in follicular lymphoma, including rituximab alone or in combination with chemotherapy (17). However, many patients with MZL are elderly, and may not be candidates for intensive chemoimmunotherapy. While new therapeutic regimens such as ibrutinib, lenalidomide plus rituximab, and umbralisib have provided treatment alternatives, these therapies also carry important treatment-limiting toxicities. Hence, additional targeted treatment options with fewer toxicities, better tolerability, and disease control are needed.
Zanubrutinib was shown to be highly active in patients with R/R MZL as demonstrated by high objective response and CR rates (68.2% and 25.8% by IRC, respectively). The ORR of 68.2% by independent review was significantly higher than the prespecified null hypothesized ORR of 30% with a P value < 0.0001.
Of note, 5 (7.6%) patients with a best response of stable disease remain on study treatment. Objective responses to BTK inhibitors can occur slowly in some patients as supported by the observation that the initial responses to ibrutinib were observed as late as 16.4 months after treatment onset (4), supporting the premise that the ORR to zanubrutinib may continue to increase with additional follow up.
Furthermore, response rates were high across all MZL subtypes, and responses were durable with 40 (89%) of the 45 responders being free from progression or death at a median study follow-up of 15.7 months. Among the 45 responders, 34 (76%) patients were continuing on zanubrutinib.
Despite differences in some subgroups with small sample sizes, a general trend to high response rates was observed across the subgroups analyzed. Responses did not appear to be impacted by prior therapies, except for R-CVP (80%) and BR (72.7%) where responses were higher than the overall population (68.2%). Similar ORRs were observed in patients receiving prior rituximab either alone or in combination therapy. High response rates were also observed in patients that have traditionally responded poorly, namely patients ≥ 75 years of age, presence of a target lesion of more than 5 cm, refractory disease, and NMZL subtype. The median DOR was not reached in any of the subgroups analyzed. The median PFS was not reached in all subgroups except in patients with above-normal LDH (n = 15), which showed a median PFS of 15.5 months.
Zanubrutinib was well tolerated as demonstrated by a low incidence of treatment discontinuation (n = 4; 5.9%) due to AEs (including 2 patients who died from COVID-19 pneumonia). Seven (10.3%) patients had dose interruption due to treatment-related AEs. No patient required a dose reduction due to an AE. The majority of the AEs were low-grade in severity. AEs associated with zanubrutinib were manageable and reversible with temporary dose modification and supportive care. Infection was the most common category of AEs of clinical interest which occurred in 31 (45.6%) patients, however the majority were low-grade in severity and were nonserious. The most common infection was upper respiratory tract infection (n = 8). Grade 3 or higher infections occurred in 11 (16.2%) patients while 10 (14.7%) patients had infections that met the criteria for seriousness. Two patients had fatal COVID-19 pneumonia.
Other AEs of clinical interest were infrequent: atrial fibrillation/flutter (n = 2), hypertension (n = 2), grade ≥3 diarrhea (n = 2), grade ≥3 neutropenia (n = 7), grade ≥3 thrombocytopenia (n = 3), and grade ≥3 anemia (n = 2). Twenty-three (33.8%) patients received concurrent antithrombotic agents, with no observed major hemorrhage. The cytopenias observed in the current study were mostly asymptomatic laboratory findings. Grade 3/4 neutropenia (10.3%, n = 7) was managed with growth factor support (n = 4) or temporary drug hold (n = 2), or resolved without need for management (n = 1). No febrile neutropenia was reported. The safety data observed in patients with R/R MZL were consistent with the known safety profile of zanubrutinib.
Recognizing the differences in the methods of efficacy evaluation among these studies and the limitations of cross-trial comparisons, the IRC-assessed ORR (68.2%) observed in this study was higher than those reported for ibrutinib (48% by IRC; ref. 4), umbralisib (49% by IRC; ref. 6), and parsaclisib (54% by IRC; ref. 8). The ORR for this trial was comparable with lenalidomide plus rituximab (65% by IRC; ref. 5), and copanlisib (76% by IRC; ref. 7). The CR rate (25.8%) demonstrated in this trial was higher than ibrutinib (3% by IRC; ref. 4), umbralisib (16% by IRC; ref. 6), and parsaclisib (6% by IRC; ref. 8). The CR rate was similar to lenalidomide plus rituximab (29% by IRC; ref. 5).
Zanubrutinib demonstrated a favorable safety and tolerability profile. AEs that were commonly reported with ibrutinib were reported less frequently with zanubrutinib treatment. The incidences of treatment discontinuations due to AEs (5.9%) were low compared to other FDA-approved therapies 17% for ibrutinib, 14.6% for lenalidomide plus rituximab, and 15.4% for umbralisib; refs. 4–6, 18). Adverse events with fatal outcome were uncommon and none were attributed to zanubrutinib. None of the patients required dose reduction due to AE while 10% dose reduction was reported for ibrutinib, 26% for lenalidomide plus rituximab, and 9.6% for umbralisib.
The safety findings observed in this study are consistent with those observed in the phase III ASPEN (BGB-3111–302) study in which 201 patients with R/R Waldenström macroglobulinemia were randomized in a 1:1 ratio to zanubrutinib (n = 102) versus ibrutinib (n = 99). Clinically meaningful safety and tolerability advantages were observed in the patients randomized to zanubrutinib versus ibrutinib, specifically a reduction in the risk of atrial fibrillation/flutter (2.0% vs. 15.3%), lower rates of major bleeding events (5.9% vs. 9.2%), diarrhea (20.8% vs. 32.7%), and hypertension (10.9% vs. 17.3%). Further, treatment with zanubrutinib was associated with fewer dose reductions in comparison with ibrutinib (13.9% vs. 23.5%) and treatment discontinuations due to AEs (4.0% vs. 9.2%; ref. 18).
In conclusion, the MAGNOLIA study met its primary endpoint. Zanubrutinib was effective in patients with R/R MZL as demonstrated by high ORR and CR rates, which compares favorably with the currently marketed therapies (ibrutinib, lenalidomide plus rituximab, and umbralisib) and investigational (copanlisib and parsaclisib) agents for MZL. Responses were durable and generally consistent across MZL subtypes and difficult-to-treat subgroups. As a selective BTK inhibitor, zanubrutinib also appears to demonstrate an improved safety and tolerability profile over existing treatment options. Taken together, the safety and efficacy results of this study represent a potentially clinically meaningful addition to available therapies in patients with R/R MZL.
Authors' Disclosures
S. Opat reports other support from BeiGene during the conduct of the study. K. Linton reports other support from BeiGene during the conduct of the study; K. Linton also reports grants from Roche Products Ltd, as well as personal fees from Roche Products Ltd, Genmab, Aptitude Health, Hartley, Bristol Myers Squibb Pharmaceuticals Ltd, Gilead Sciences Ltd, Simon Kucher & Partners Strategy & Marketing Consultants, Celgene Corporation, and Karyopharm Therapeutics Inc. outside the submitted work. In addition, K. Linton reports travel grants in 2019 from Janssen and Celgene. P. McKay reports personal fees from BeiGene advisory board outside the submitted work. In addition, P. McKay reports honoraria from Roche, Takeda, Recordati Rare Diseases, AstraZeneca, and AbbVie; lecture fees from Gilead and Janssen; and advisory board fees from Roche, Takeda, Gilead, Celgene, BeiGene, KITE, and BMS. B. Hu reports other support from BeiGene, Cellectar, Gilead, Astrellas, and Bayer outside the submitted work. H. Chan reports personal fees and non-financial support from Janssen; personal fees from EUSA and Abbvie; and non-financial support from Amgen and Celgene outside the submitted work. P.L. Zinzani reports other support from Janssen, Merck, EUSA Pharma, Takeda, Roche, AstraZeneca, Kyowa Kirin, Novartis, Gilead, and Sanofi outside the submitted work. C. Thieblemont reports personal fees from Roche, Janssen, Novartis, Gilead, BMS, and Incyte outside the submitted work. P. Browett reports grants from BeiGene during the conduct of the study, as well as personal fees from MSD, AbbVie, and Janssen-Cilag outside the submitted work. C.A. Portell reports grants and personal fees from BeiGene during the conduct of the study. C.A. Portell also reports grants from AbbVie and Acerta/AstraZeneca; personal fees from Janssen/Pharmacyclics; and grants and personal fees from TG Therapeutics outside the submitted work. K. Ardeshna reports personal fees and other support from Gilead, Celgene, Roche, and Novartis, as well as personal fees from BeiGene outside the submitted work. F. Bijou reports other support from BMS during the conduct of the study. E.A. Hawkes reports grants, personal fees, and other support from Roche, Bristol Myers Squibb, Celgene, and AstraZeneca; grants from Merck KgA; other support from Merck Sharpe & Dohme, Gilead, Antigene, and Novartis; personal fees and other support from Janssen; and personal fees from Specialised Therapeutics and Regeneron outside the submitted work. D. Talaulikar reports grants and personal fees from Janssen; grants, personal fees, and non-financial support from Amgen and Roche; grants from Takeda; and personal fees from EUSA and CSL during the conduct of the study. M. Co reports personal fees from BeiGene during the conduct of the study. X. Li reports other support from BeiGene outside the submitted work. W. Zhou reports other support from BeiGene during the conduct of the study, as well as other support from BeiGene outside the submitted work. M. Cappellini reports personal fees from BeiGene outside the submitted work; in addition, M. Cappellini is employed by BeiGene and holds BeiGene (BGNE) shares. C. Tankersley is employed by BeiGene. J. Huang reports other support from BeiGene during the conduct of the study, as well as other support from BeiGene outside the submitted work. J. Trotman reports grants from BeiGene during the conduct of the study, as well as grants from Roche, Celgene/BMS, PCYC, Janssen, and Takeda outside the submitted work. No disclosures were reported by the other authors.
Acknowledgments
The authors would like to thank the patients who participated in the study, their supporters, and the investigators and clinical research staffs from the study centers. This study was supported by research funding from BeiGene (Beijing) Co., Ltd., Beijing, China. Editorial assistance was funded by BeiGene and provided by Tracy McNally and Ify Sargeant of Twist Medical.
The study was supported by BeiGene (Beijing) Co., Ltd. The sponsor was involved in the study design, data collection, data analysis, interpretation, and manuscript development.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
This article is featured in Highlights of This Issue, p. 6279
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Contributions
S. Opat: Conceptualization, data curation, formal analysis, writing–review and editing. A. Tedeschi: Data curation, formal analysis, writing–review and editing. K. Linton: Data curation, formal analysis, writing–review and editing. P. McKay: Data curation, formal analysis, writing–review and editing. B. Hu: Data curation, formal analysis, writing–review and editing. H. Chan: Data curation, formal analysis, writing–review and editing. J. Jin: Data curation, formal analysis, writing–review and editing. M. Sobieraj-Teague: Data curation, formal analysis, writing–review and editing. P.L. Zinzani: Data curation, formal analysis, writing–review and editing. M. Coleman: Data curation, formal analysis, writing–review and editing. C. Thieblemont: Data curation, formal analysis, writing–review and editing. P. Browett: Data curation, formal analysis, writing–review and editing. X. Ke: Data curation, formal analysis, writing–review and editing. M. Sun: Data curation, formal analysis, writing–review and editing. R. Marcus: Data curation, formal analysis, writing–review and editing. C.A. Portell: Data curation, formal analysis, writing–review and editing. K. Ardeshna: Data curation, formal analysis, writing–review and editing. F. Bijou: Data curation, formal analysis, writing–review and editing. P. Walker: Data curation, formal analysis, writing–review and editing. E.A. Hawkes: Data curation, formal analysis, writing–review and editing. S. Mapp: Data curation, formal analysis, writing–review and editing. S.-J. Ho: Data curation, formal analysis, writing–review and editing. D. Talaulikar: Data curation, formal analysis, writing–review and editing. K.-S. Zhou: Data curation, formal analysis, writing–review and editing. M. Co: Data curation, formal analysis, writing–review and editing. X. Li: Data curation, formal analysis, writing–review and editing. W. Zhou: Data curation, formal analysis, writing–review and editing. M. Cappellini: Data curation, formal analysis, writing–review and editing. C. Tankersley: Data curation, formal analysis, writing–review and editing. J. Huang: Conceptualization, data curation, formal analysis, writing–review and editing. J. Trotman: Conceptualization, data curation, formal analysis, writing–review and editing.
References
- 1. Kahl B, Yang D. Marginal zone lymphomas: management of nodal, splenic, and MALT NHL. Hematology Am Soc Hematol Educ Program 2008;359–64. [DOI] [PubMed] [Google Scholar]
- 2. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Denlinger NM, Epperla N, William BM. Management of relapsed/refractory marginal zone lymphoma: focus on ibrutinib. Cancer Manag Res 2018;10:615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Noy A, de Vos S, Thieblemont C, Martin P, Flowers CR, Morschhauser F, et al. Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood 2017;129:2224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leonard JP, Trneny M, Izutsu K, Fowler NH, Hong X, Zhu J, et al. AUGMENT: a phase III study of lenalidomide plus rituximab versus placebo plus rituximab in relapsed or refractory indolent lymphoma. J Clin Oncol 2019;37:1188–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fowler NH, Samaniego F, Jurczak W, Ghosh N, Derenzini E, Reeves JA, et al. Umbralisib, a dual PI3Kδ/CK1ε inhibitor in patients with relapsed or refractory indolent lymphoma. J Clin Oncol 2021:JCO2003433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Revlimid (lenalidomide) [US prescribing information]. Summit, NJ: Celgene Corp; 2019. [Google Scholar]
- 8. Matasar MJ, Capra M, Özcan M, Lv F, Li W, Yañez E, et al. Copanlisib plus rituximab versus placebo plus rituximab in patients with relapsed indolent non-Hodgkin lymphoma (CHRONOS-3): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2021;22:678–89. [DOI] [PubMed] [Google Scholar]
- 9. Phillips TJ, Corradini P, Gurion R, Patti C, Tani M, Avigdor A, et al. Phase 2 study evaluating the efficacy and safety of parsaclisib in patients with relapsed or refractory marginal zone lymphoma (CITADEL-204). Blood 2020;136:27–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu J, Liu C, Tsui ST, Liu D. Second-generation inhibitors of Bruton tyrosine kinase. J Hematol Oncol 2016;9:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tam CS, Opat S, D'Sa S, Jurczak W, Lee H-P, Cull G, et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: the ASPEN study. Blood 2020;136:2038–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tam CS, Robak T, Ghia P, Kahl BS, Walker P, Janowski W, et al. Efficacy and safety of zanubrutinib in patients with treatment-naive chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) with Del(17p): initial results from arm C of the Sequoia (BGB-3111–304) trial. Blood 2019;134:499. [Google Scholar]
- 13. Song Y, Zhou K, Zou D, Zhou J, Hu J, Yang H, et al. Treatment of patients with relapsed or refractory mantle-cell lymphoma with zanubrutinib, a selective inhibitor of Bruton's tyrosine kinase. Clin Cancer Res 2020;26:4216–24. [DOI] [PubMed] [Google Scholar]
- 14. Brukinsa (zanubrutinib) [US prescribing information]. San Mateo, CA: BeiGene USA, Inc; 2019. [Google Scholar]
- 15. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014;32:3059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics 1982;38:29–41. [Google Scholar]
- 17. Sindel A, Al-Juhaishi T, Yazbeck V. Marginal zone lymphoma: state-of-the-art treatment. Curr Treat Options in Oncol 2019;20:90. [DOI] [PubMed] [Google Scholar]
- 18. Tam CSL, Opat S, D'Sa S, Jurczak W, Lee H-P, Cull G, et al. ASPEN: results of a phase III randomized trial of zanubrutinib versus ibrutinib for patients with Waldenström macroglobulinemia (WM). J Clin Oncol 2020;38:(abstr 8007). [Google Scholar]