Abstract
Purpose:
We previously reported a 44% overall response rate (ORR) with the oral BCL-2 inhibitor venetoclax in a phase I study of relapsed/refractory non–Hodgkin lymphoma (NHL). Complete response (CR) was observed in patients with mantle cell lymphoma [(MCL), 21%, n = 6/28] and follicular lymphoma [(FL), 17%, n = 5/29], and partial response (PR) noted in several patients with Waldenström macroglobulinemia (WM), and marginal zone lymphoma (MZL). Here, we report the long-term outcomes of these four cohorts.
Patients and Methods:
All patients (n = 106) received venetoclax monotherapy in dose cohorts of 200 to 1,200 mg daily until disease progression or unacceptable toxicity. ORR, progression-free survival (PFS), duration of response (DoR), and adverse events (AEs) were evaluated.
Results:
At a median follow-up of 38.5 months (range, 30.0–46.5), the median PFS for all 106 patients was 5.4 [95% confidence interval (CI), 3.5–8.4] months (FL, 10.8; MCL, 11.3; MZL, 21.2; and WM, 30.4). The median DoR was 14.9 (95% CI, 9.7–27.6) months (FL, 26.6; MCL, 15.7; MZL, 20.1; and WM, 25.3). Achievement of CR versus PR predicted longer DoR in both MCL (31.5 vs. 10.1 months) and FL (37.6 vs. 9.7 months). All grade hematologic AEs were infrequent: neutropenia (19%), anemia (19%), and thrombocytopenia (17%), with no new cytopenias after 2 years on therapy. Nonhematologic AEs included nausea (49%), diarrhea (46%), fatigue (44%), with decreased incidence after 1 year.
Conclusions:
Venetoclax monotherapy has a manageable safety profile and achieves durable responses in a subset of patients with FL, MCL, WM, and MZL, particularly in those who achieve CR. Further research is warranted on combination strategies to enhance the durability of response to venetoclax.
Translational Relevance.
Non–Hodgkin lymphoma (NHL) is a heterogeneous group of lymphoid malignancies with a spectrum of clinical behaviors. In the first-in-human phase I study of venetoclax monotherapy in patients with relapsed/refractory NHL, we observed an overall response rate of 44% among all NHL cohorts, with responses observed in both aggressive and indolent subtypes. Here, with a longer-term follow-up (median of more than 3 years), we report durable responses in several patients with indolent NHL who achieved complete response. The durability of response with venetoclax monotherapy in patients with NHL who achieve complete response reinforces the importance of BCL-2 as a target in indolent lymphomas. However, our data also suggest that further exploration of venetoclax combination approaches will be essential.
Introduction
Overexpression of the anti-apoptotic protein B-cell lymphoma/leukemia-2 (BCL-2) provides a survival advantage for malignant B cells and is involved in tumorigenesis, disease progression, and resistance to chemotherapy in B-cell malignancies (1–3). Thus, BCL-2 is a potentially important therapeutic target in B-cell non–Hodgkin lymphoma (NHL). Venetoclax is an orally bioavailable, selective BCL-2 inhibitor that has demonstrated efficacy in NHL both in vitro and in vivo patient-derived cancer models (4–6) and is approved by the FDA for the treatment of patients with chronic lymphocytic leukemia (CLL) and acute myeloid leukemia (7).
We have previously reported that targeting BCL-2 with venetoclax in relapsed/refractory (R/R) NHL was well tolerated as monotherapy, leading to an overall response rate (ORR) of 44%, but with variability among disease cohorts (8). Promising response rates were seen among patients with mantle cell lymphoma (MCL) (75%) and follicular lymphoma (FL; 38%), with responses also noted in all four patients with Waldenström macroglobulinemia (WM) and two of three patients with marginal zone lymphoma (MZL; ref. 8). Here, we report the long-term follow-up of these four cohorts from the phase I study, now with a median follow-up greater than 3 years, focusing on long-term safety and efficacy.
Patients and Methods
The trial (NCT01328626) was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization (ICH) for Good Clinical Practice Guidelines. The clinical study protocol and related documents were approved by the applicable regional review boards or ethic committees. All patients provided written informed consent. All authors had access to the primary clinical data.
Patients
This first-in-human study enrolled 106 patients with R/R NHL that included patients with FL (n = 29), MCL (n = 28), MZL (n = 3), WM (n = 4), diffuse large B-cell lymphoma [DLBCL (n = 41)], and multiple myeloma (n = 1). Details of patient eligibility and enrollment have been described previously (8). Of note, the cohort of patients with DLBCL did not show a significant difference in response outcome from that reported previously (8), so we chose to focus this new analysis on these other four cohorts.
Treatment
Patients were treated with daily oral venetoclax doses ranging between 200 and 1,200 mg per day, depending on the dose cohort. All patients continued treatment until they developed progressive disease (PD) or unacceptable toxicity. Intrapatient dose escalation was allowed. Patients who had PD during treatment were allowed to continue venetoclax at the same dose when progression occurred for the duration of clinical benefit (9). Patients with MCL could increase their venetoclax dose up to 1,200 mg. Per protocol, if progression occurred while on venetoclax, the patients could continue to receive venetoclax at the same dose or were allowed to increase the dose to 1,200 mg and could be treated with rituximab at 375 mg/m2 weekly for 3 consecutive weeks, followed by a maintenance dosing schedule at the same dose for up to 1 year. Some responses to this strategy were seen in CLL (10). The patients treated with rituximab were monitored for tumor lysis syndrome per protocol.
Assessments
Disease responses were assessed using the 2007 International Working Group response criteria and the Fourth International Workshop on Waldenström macroglobulinemia criteria (11, 12). Long-term efficacy endpoints included progression-free survival (PFS) and duration of response (DoR), defined as the number of months from the date of first response as partial response (PR) or better until PD or death.
Safety was measured by the incidence and severity of treatment-emergent adverse events (TEAEs) defined according to ICH guidelines. The severity of AEs was based on the NCI Common Terminology Criteria for Adverse Events, v4.0. Long-term safety was assessed by the frequency and severity of TEAEs among patients who received treatment with venetoclax beyond 1 year.
Statistical analyses
The final data cutoff for this study was June 30, 2020. All patients who received at least one dose of venetoclax were included in both efficacy and safety analyses. Descriptive statistics, including means, medians, SDs, and ranges, were calculated. The Kaplan–Meier methodology was used for time-to-event analyses.
Data sharing statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis datasets), as well as other information (e.g., protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Results
Patient characteristics and disposition
Patient characteristics are presented in Supplementary Table S1 and were described previously (8). Of the 64 patients enrolled within the four cohorts analyzed, 15 (23%) patients received venetoclax treatment beyond 2 years (FL 5/29, MCL 7/28, MZL 1/3, WM 2/4).
Efficacy
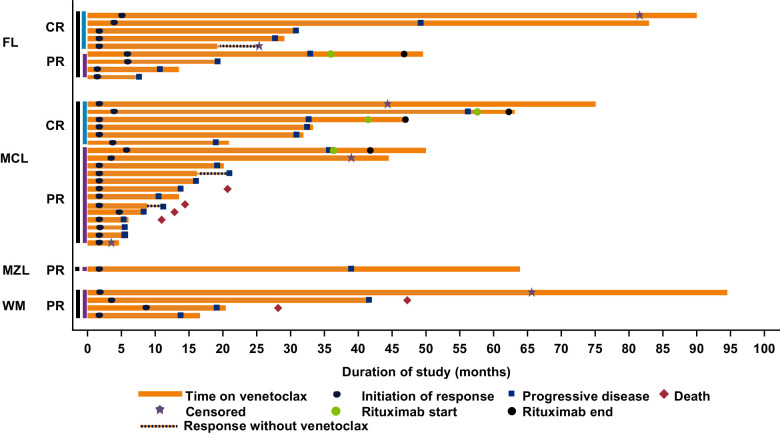
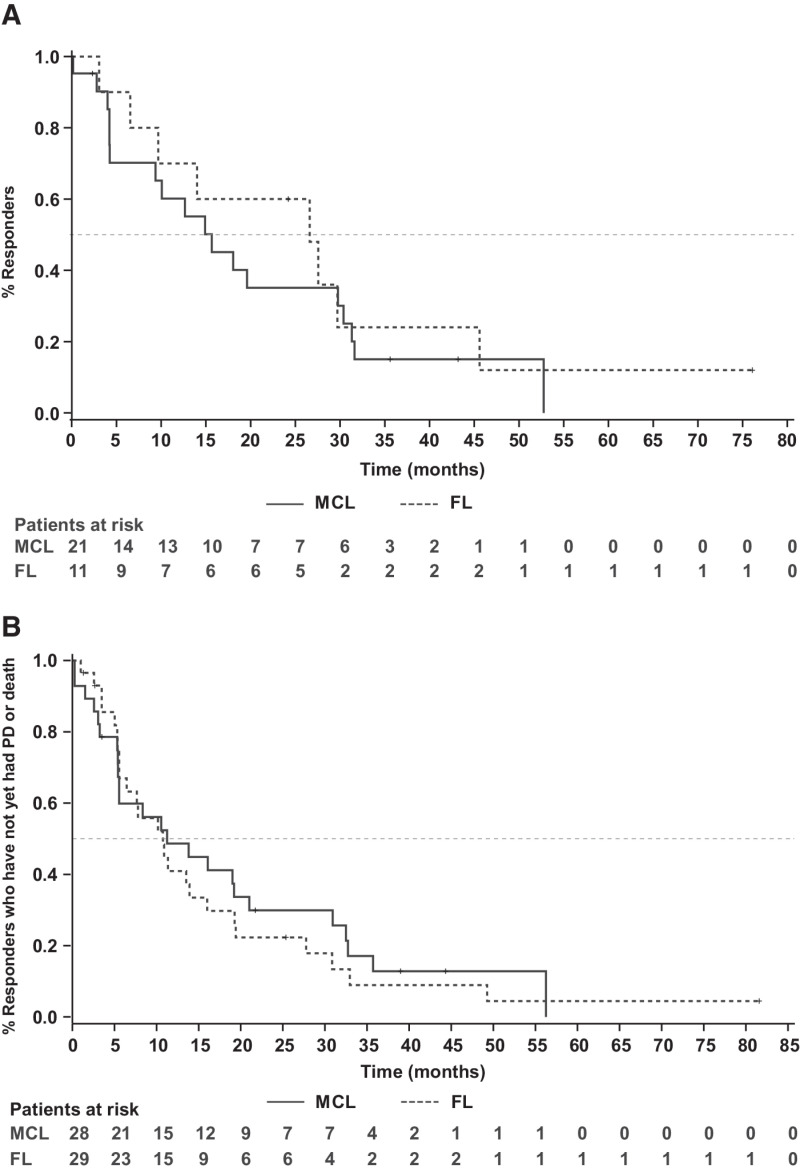
The median duration of venetoclax treatment for all treated patients was 5.3 (range, 0.2–94.5) months and 19.1 (range, 3.7–94.5) months among responders (≥PR). Overall responses were attained in 38% (11/29) FL, 75% (21/28) MCL, 67% (2/3) MZL, and 100% (4/4) patients with WM. Complete remission (CR) was achieved by 17% (5/29) in FL and 21% (6/28) in patients with MCL. CR was not achieved by patients in the MZL and WM cohorts. The DoR among patients who achieved a response of PR or better is presented in Fig. 1. Patients with FL had a median follow-up of 41.4 months (range, 7.7–90.5). The median PFS and DoR for all treated patients with FL were 10.8 months (95% CI, 5.6–16.0) and 26.6 months (95% CI, 3.1–45.6) respectively (Fig. 2A and B). For patients with FL who achieved CR (n = 5), the median DoR was 37.6 months [95% CI, 26.6–not reachable (NR)], and for those who achieved PR (n = 6), the median DoR was 9.7 months (95% CI, 3.1–NR; Supplementary Fig. S1A).
Figure 1.
Swimmer plot describing overall response and DoR in patients. CR, complete remission; PR, partial remission; FL, follicular lymphoma; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; WM, Waldenström macroglobulinemia. Patients were censored at their last disease assessment if they did not have confirmed disease progression. Rituximab was administered to patients at doses of 375 mg/m2.
Figure 2.
Kaplan–Meier curves duration of response (A) PFS (B) in patients with MCL and FL.
The median follow-up time for patients in the MCL cohort was 45.3 months (range, 0.3–77.0). The median PFS and DoR for all patients with MCL treated with venetoclax were 11.3 months (95% CI, 5.4–21.0) and 15.7 months (95% CI, 4.2–30.4). Among patients who achieved CR (n = 6), the median DoR was 31.5 months (95% CI, 15.7–NR), and among those who achieved PR (n = 15), the median DoR was 10.1 months (95% CI, 4.0–18.1; Supplementary Fig. S1B).
Patients with MZL had a median follow-up of 65.0 months (range, 4.9–65.0). The median PFS and DoR for all treated patients with MZL were 21.2 months (95% CI, 3.5–39.0) and 20.1 months (95% CI, 2.3–NR), respectively. The median follow-up time was 75.3 months (range, 28.2–94.5) in the WM cohort, and the median PFS and DoR for all treated patients with WM were 30.4 months (95% CI, 13.8–NR) and 25.3 months (95% CI, 11.1–NR), respectively.
Of four patients who received rituximab, three attained stable disease, and one had PR, followed by PD.
Safety
Eighty-four of 106 (79%) patients were enrolled to venetoclax doses >400 mg. All dose levels, including the 1,200 mg dose, were tolerated in all NHL cohorts. The recommended phase II dose was determined to be 1,200 mg based on data from all patients with NHL in the dose-escalation cohorts and was the recommended dose for the NHL safety expansion cohort.
During the first year of treatment with venetoclax, the majority of patients experienced at least one TEAE, and the severity of AEs was independent of dose. (8) The rates of all grade hematologic AEs occurring within the first year were neutropenia (19%), anemia (17%), and thrombocytopenia (15%; Table 1). No new cytopenias were observed in patients on therapy for over 2 years. The most common nonhematologic AEs with onset in the first year were nausea (53%), diarrhea (47%), and fatigue (34%). The most commonly occurring infections were upper respiratory tract infections (23%). Common grade ≥3 AEs reported in 35/64 NHL subjects (55%) were neutropenia (13%) and anemia (11%; Supplementary Table S2).
Table 1.
Treatment-emergent adverse events.
| First events with onset ≤12 months | New events with onset 12–24 months | New events with onset >24 months | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (N = 64) | (N = 33) | (N = 15) | ||||||||||
| n/N | % | Person-yearsc | Incidence rateb | n/N | % | Person-years | Incidence rate | n/N | % | Person-years | Incidence rate | |
| Any adverse event | 62/64 | (96.9) | 3.1 | 1984.7 | 26/33 | (78.8) | 7.6 | 340.7 | 13/15 | (86.7) | 9.3 | 139.6 |
| Hematologica | 18/64 | (28.1) | 37.1 | 48.5 | 5/33 | (15.2) | 21.8 | 22.9 | 0/15 | 0 | 40.1 | 0 |
| Neutropenia | 13/64 | (20.3) | 39.1 | 33.3 | 1/25 | (4.0) | 17.7 | 5.6 | 0/13 | 0 | 38.0 | 0 |
| Thrombocytopenia | 9/64 | (14.1) | 44.1 | 20.4 | 1/29 | (3.4) | 19.9 | 5.0 | 0/14 | 0 | 34.5 | 0 |
| Anemia | 7/64 | (10.9) | 45.3 | 15.4 | 2/31 | (6.5) | 21.0 | 9.5 | 0/13 | 0 | 36.6 | 0 |
| Nonhematologic | ||||||||||||
| Nausea | 34/64 | (53.1) | 21.4 | 158.5 | 1/11 | (9.1) | 6.1 | 16.3 | 1/2 | (50.0) | 2.9 | 34.3 |
| Diarrhea | 30/64 | (46.9) | 27.0 | 111.3 | 3/13 | (23.1) | 5.3 | 56.6 | 1/1 | (100) | 1.7 | 58.1 |
| Fatigue | 22/64 | (34.4) | 35.2 | 62.5 | 3/21 | (14.3) | 12.5 | 24.0 | 2/7 | (28.6) | 18.5 | 10.8 |
| Upper respiratory tract infection | 15/64 | (23.4) | 39.7 | 37.8 | 1/20 | (5.0) | 13.9 | 7.2 | 3/8 | (37.5) | 11.2 | 26.7 |
| Constipation | 12/64 | (18.8) | 41.3 | 29.1 | 0/29 | 0 | 20.8 | 0 | 2/13 | (15.4) | 32.8 | 6.1 |
| Headache | 12/64 | (18.8) | 41.1 | 29.2 | 2/25 | (8.0) | 15.0 | 13.3 | 0/8 | 0 | 14.6 | 0 |
| Vomiting | 11/64 | (17.2) | 40.0 | 27.5 | 1/24 | (4.2) | 15.3 | 6.5 | 3/10 | (30.0) | 19.1 | 15.7 |
| Decreased appetite | 10/64 | (15.6) | 42.1 | 23.7 | 1/25 | (4.0) | 17.1 | 5.8 | 0/10 | 0 | 28.0 | 0 |
| Cough | 10/64 | (15.6) | 42.0 | 23.8 | 2/26 | (7.7) | 17.1 | 11.7 | 2/9 | (22.2) | 16.1 | 12.5 |
aHematologic and nonhematologic adverse events ≥15% occurrence by incidence rate sorted by first events with onset ≤12 months.
bIncidence rate = number of patients with an event per 100 person-years at risk.
cPerson-years for the calculation of the incidence rate is the total time at risk of an event across all patients. Only new events not reported before this time period were counted in the summary of events with onset in one time period.
Beyond the first year of venetoclax treatment, no new types of TEAEs were identified, and the incidence rates for all AEs decreased over time.
Discussion
Venetoclax monotherapy was well-tolerated and produced durable responses in a subset of patients with FL, MCL, MZL, or WM cohorts. A small number of these patients remained on venetoclax treatment for more than 5 years.
Venetoclax doses up to 1,200 mg were well tolerated in all the dose-escalation cohorts, and 1,200 mg was the recommended phase II dose. Neutropenia was the most notable AE, but the proportion of patients with NHL with neutropenia (19%) was lower than what has previously been reported in patients with CLL (13), and the incidence of new-onset neutropenia decreased significantly during year 2 (0%), with no new occurrences of neutropenia observed after 2 years on therapy. The majority of patients with neutropenia also had preexisting risk factors for neutropenia. Neutropenia events did not result in a fatal outcome, and the patients were managed with dose modifications and standard support. There were no new risks or increased severity of identified risks during this long-term follow-up period. Most AEs reported were mild and low-grade gastrointestinal toxicities. Infections are common in patients with NHL due to cumulative disease and treatment-related immunosuppression; however, the infection rate observed in patients undergoing treatment with venetoclax did not appear excessive, and most infections were low grade and predominantly upper respiratory tract infections. Grade 3 or 4 infections were uncommon and did not lead to discontinuation of venetoclax. All infections were managed effectively with standard antimicrobial therapy, and no unusual infections were observed with long-term follow-up.
The results of this long-term follow-up analysis suggest that for patients with certain types of NHL, venetoclax monotherapy may be an effective treatment strategy. For example, the effectiveness of the single-agent venetoclax in patients with WM has been confirmed in a subsequent phase II study, where the objective response rate was 87%, with a major response rate (PR or better, at least a 50% decreased in serum IgM level from baseline) of 80%, including patients previously treated with inhibitors to Bruton tyrosine kinase (14). In other NHL subtypes, the activity of venetoclax monotherapy was more variable. Depth of response appears to be an important factor predicting durability, as patients who achieved CR tended to have longer remissions. It would be informative to evaluate predictive biomarkers to help assess the likelihood of complete response. We previously reported that the intensity of BCL-2 protein staining by IHC was not associated with response or PFS in this study (8). Unfortunately, a limitation of this phase I study was that pretreatment tissue samples were not uniformly collected, and as such, we were unable to report on whether additional factors such as protein expression of other BCL-2 family members or somatic mutational profiles were predictive of response. Similarly, tissue samples were not routinely collected in this study at the time of disease progression, so we are unable to comment on potential mechanisms of emergent resistance, although, in an individual patient with FL, an acquired BCL-2 mutation was found at progression (15). Moving forward, incorporating such routine tissue collection into trials of venetoclax in NHL will be critical to understanding the biological underpinnings of primary and secondary resistance.
Although our data suggest single-agent activity in certain indolent NHL subtypes, it is likely that the future role of venetoclax in NHL will be as a combination partner with other active therapies. For example, the combination of venetoclax with ibrutinib led to a 42% CR rate in patients with relapsed MCL by CT scan. This CR rate was twice that reported in this study with venetoclax monotherapy (16), translated into longer PFS, with a median of 29 months, which is favorable in light of the 11-month PFS of venetoclax monotherapy in this study (17). In patients with MZL, the same combination of venetoclax and ibrutinib resulted in an ORR of 58% (25% CR, ref. 17).
Collectively, our results have several potential implications for the use of venetoclax in the therapy of patients with NHL. With respect to venetoclax monotherapy, our long-term data do suggest that this may be an effective treatment approach for a subset of patients with various types of NHL who have exhausted other standard options. Venetoclax can affect durable responses in such patients, particularly those who achieve CR; however, our data also suggest that for the majority of patients with NHL, the responses to venetoclax monotherapy were not durable. For patients who are candidates for allogeneic transplantation, our data support a potential role for venetoclax monotherapy as a bridge to transplant.
For the majority of patients, our data suggest that the emphasis of clinical investigation with venetoclax in NHL going forward should be on further exploration of optimal combination partners to enhance the durability of response. A phase Ib study (NCT01594229) is evaluating the safety and efficacy of venetoclax in combination with bendamustine and rituximab in patients with R/R NHL. Initial results found that the safety profile of the combination was acceptable, and the ORR was 65% (18). The phase III study SYMPATICO (NCT03112174) is investigating the safety and efficacy of venetoclax and ibrutinib in patients with MCL, while the phase I/II study OAsIs (NCT02558816) is evaluating the combination of venetoclax with ibrutinib and obinutuzumab in patients with R/R MCL (19), and if positive, would extend the initial benefits seen in this study with venetoclax monotherapy to a much larger proportion of patients with NHL.
Authors' Disclosures
M.S. Davids reports grants from Genentech, Novartis, Pharmacyclics, TG Therapeutics, AstraZeneca, Surface Oncology, MEI Pharma, Ascentage, and Verastem during the conduct of the study, as well as personal fees from AbbVie, BeiGene, BMS, Celgene, Genentech, Pharmacyclics, Janssen, Takeda, TG Therapeutics, Gilead, AstraZeneca, Merck, Novartis, Eli Lilly, MEI Pharma, Syros, Verastem, Adaptive Biosciences, Ascentage, and Zentalis outside the submitted work. A.W. Roberts reports grants from Servier, AbbVie, and Janssen outside the submitted work; in addition, A.W. Roberts has a patent for 16/827,650 pending and is an employee of Walter and Eliza Hall Institute, which has received milestone and royalty payments related to venetoclax, and A.W. Roberts receives a share of these incomes from the Institute. T.J. Kipps reports that cirmtuzumab was developed by Thomas J. Kipps in the Thomas J. Kipps laboratory and is licensed by the University of California to Oncternal Therapeutics, Inc., which provided stock options and research funding to the Thomas J. Kipps laboratory. A.H. Salem reports other support from AbbVie during the conduct of the study and other support from AbbVie outside the submitted work. J.A. Arzt reports other support from AbbVie, Inc. outside the submitted work. S.Y. Kim reports other support from AbbVie during the conduct of the study; other support from AbbVie outside the submitted work; and is an employee of AbbVie and owns AbbVie stock. J.F. Seymour reports grants and personal fees from AbbVie and Celgene; grants from Janssen; personal fees from Acerta, Genentech, Sunesis, and Takeda; and grants, personal fees, and other support from Roche outside the submitted work. No disclosures were reported by the other authors.
Supplementary Material
Acknowledgments
The authors, AbbVie, and Genentech would like to thank the patients, their families and caregivers, as well as the M12-175 study investigators, research, and supporting staff.
Medical writing support was provided by Dalia Majumdar of AbbVie.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Contributions
M.S. Davids: Conceptualization, investigation, writing–original draft, writing–review and editing. A.W. Roberts: Conceptualization, investigation, writing–original draft, writing–review and editing. V.P. Kenkre: Investigation, writing–original draft, writing–review and editing. W.G. Wierda: Writing–original draft, writing–review and editing. A. Kumar: Writing–original draft, writing–review and editing. T.J. Kipps: Investigation, writing–review and editing. M. Boyer: Writing–review and editing. A.H. Salem: Methodology, writing–review and editing. J.C. Pesko: Methodology, writing–review and editing. J.A. Arzt: Data curation, methodology, writing–review and editing. M. Mantas: Data curation, writing–review and editing. S.Y. Kim: Conceptualization, data curation, supervision, methodology, writing–original draft, writing–review and editing. J.F. Seymour: Conceptualization, data curation, supervision, investigation, methodology, writing–original draft, writing–review and editing.
References
- 1. Davids MS, Letai A. ABT-199: taking dead aim at BCL-2. Cancer Cell 2013;23:139–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Iqbal J, Neppalli VT, Wright G, Dave BJ, Horsman DE, Rosenwald A, et al. BCL2 expression is a prognostic marker for the activated B-cell–like type of diffuse large B-cell lymphoma. J Clin Oncol 2006;24:961–8. [DOI] [PubMed] [Google Scholar]
- 3. Davids MS, Letai A. Targeting the B-cell lymphoma/leukemia 2 family in cancer. J Clin Oncol 2012;30:3127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pham LV, Huang S, Zhang H, Zhang J, Bell T, Zhou S, et al. Strategic therapeutic targeting to overcome venetoclax resistance in aggressive B-cell lymphomas. Clin Cancer Res 2018;24:3967–80. [DOI] [PubMed] [Google Scholar]
- 5. Choe H, Ruan J. Next generation of targeted molecules for non-Hodgkin lymphomas: small-molecule inhibitors of intracellular targets and signaling pathways. Oncology 2016;30:847–58. [PubMed] [Google Scholar]
- 6. Zhang L, Nomie K, Zhang H, Bell T, Pham L, Kadri S, et al. B-cell lymphoma patient-derived xenograft models enable drug discovery and are a platform for personalized therapy. Clin Cancer Res 2017;23:4212–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. VENCLEXTA. Prescribing information. North Chicago, IL: AbbVie Inc.; South San Francisco, CA: Genentech USA Inc; 2020. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208573s009lbl.pdf. [Google Scholar]
- 8. Davids MS, Roberts AW, Seymour JF, Pagel JM, Kahl BS, Wierda WG, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-hodgkin lymphoma. J Clin Oncol 2017;35:826–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Younes A, Hilden P, Coiffier B, Hagenbeek A, Salles G, Wilson W, et al. International Working Group consensus response evaluation criteria in lymphoma (RECIL 2017). Ann Oncol 2017;28:1436–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Handunnetti S, Anderson MA, Roberts AW, Davids MS, Ma S, Boyer M, et al. Addition of rituximab in relapsed/refractory chronic lymphocytic leukemia after progression on venetoclax monotherapy. eJHaem 2021;2:266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579–86. [DOI] [PubMed] [Google Scholar]
- 12. Dimopoulos MA, Gertz MA, Kastritis E, Garcia-Sanz R, Kimby EK, Leblond V, et al. Update on treatment recommendations from The Fourth International Workshop on Waldenstrom's Macroglobulinemia. J Clin Oncol 2009;27:120–6. [DOI] [PubMed] [Google Scholar]
- 13. Davids MS, Hallek M, Wierda W, Roberts AW, Stilgenbauer S, Jones JA, et al. Comprehensive safety analysis of venetoclax monotherapy for patients with relapsed/refractory chronic lymphocytic leukemia. Clin Cancer Res 2018;24:4371–9. [DOI] [PubMed] [Google Scholar]
- 14. Castillo JJ, Gustine J, Meid K, Dubeau T, Keezer A, Allan JN, et al. Multicenter prospective phase II study of venetoclax in patients with previously treated waldenstrom macroglobulinemia. Blood 2018;132:2888. [Google Scholar]
- 15. Blombery P, Birkinshaw RW, Nguyen T, Gong JN, Thompson ER, Xu Z, et al. Characterization of a novel venetoclax resistance mutation (BCL2 Phe104Ile) observed in follicular lymphoma. Br J Haematol 2019;186:e188–e91. [DOI] [PubMed] [Google Scholar]
- 16. Tam CS, Anderson MA, Pott C, Agarwal R, Handunnetti S, Hicks RJ, et al. Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N Engl J Med 2018;378:1211–23. [DOI] [PubMed] [Google Scholar]
- 17. Handunnetti SM, Khot A, Anderson MA, Blombery P, Burbury K, Ritchie D, et al. Safety and efficacy of ibrutinib in combination with venetoclax in patients with marginal zone lymphoma: preliminary results from an open label, phase II study. Blood 2019;134:3999. [Google Scholar]
- 18. de Vos S, Swinnen LJ, Wang D, Reid E, Fowler N, Cordero J, et al. Venetoclax, bendamustine, and rituximab in patients with relapsed or refractory NHL: a phase Ib dose-finding study. Ann Oncol 2018;29:1932–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Le Gouill S, Morschhauser F, Chiron D, Bouabdallah K, Cartron G, Casasnovas O, et al. Ibrutinib, obinutuzumab, and venetoclax in relapsed and untreated patients with mantle cell lymphoma: a phase 1/2 trial. Blood 2021;137:877–87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.