Abstract
Purpose:
Safety, efficacy, and exploratory biomarker analyses were evaluated in patients with advanced HER2-negative germline breast cancer susceptibility gene (gBRCA)-associated breast cancer enrolled in the BROCADE3 trial who received crossover veliparib monotherapy after disease progression on placebo plus carboplatin/paclitaxel.
Patients and Methods:
Eligible patients (N = 513) were randomized 2:1 to veliparib plus carboplatin/paclitaxel or placebo plus carboplatin/paclitaxel; patients had variable platinum-free intervals (PFI) at progression. In the placebo arm, patients were eligible to receive crossover veliparib monotherapy (300–400 mg twice daily continuous). Antitumor activity and adverse events were assessed during crossover veliparib treatment. BRCA reversion mutations at crossover were analyzed retrospectively using next-generation sequencing on plasma circulating tumor DNA (ctDNA).
Results:
Seventy-five patients in the placebo plus carboplatin/paclitaxel arm received ≥1 dose of crossover veliparib postprogression (mean treatment duration: 154 days). Eight of 50 (16%) patients with measurable disease had a RECIST v1.1 response. Activity was greater in patients with PFI ≥180 days compared with <180 days [responses in 23.1% (3/13) vs. 13.5% (5/37) of patients]. BRCA reversion mutations that restored protein function were detected in ctDNA from 4 of 28 patients tested, and the mean duration of crossover veliparib monotherapy was <1 month in these 4 patients versus 7.49 months in patients lacking reversion mutations. The most frequent adverse events were nausea (61%), vomiting (29%), and fatigue (24%).
Conclusions:
Crossover veliparib monotherapy demonstrated limited antitumor activity in patients who experienced disease progression on placebo plus carboplatin/paclitaxel. PFI appeared to affect veliparib activity. BRCA reversion mutations may promote cross-resistance and limit veliparib activity following progression on platinum.
Translational Relevance.
Advanced breast cancers (ABC) associated with germline breast cancer susceptibility gene (gBRCA) mutations are sensitive to platinum chemotherapy and PARP inhibitors (PARPi). However, shared mechanisms of acquired resistance to these therapies may exist, including BRCA reversion mutations. The phase III study BROCADE3 demonstrated that veliparib added to carboplatin/paclitaxel improved progression-free survival in patients with HER2-negative gBRCA-associated ABC. Patients who progressed on placebo plus carboplatin/paclitaxel were eligible for crossover veliparib monotherapy. Antitumor activity was observed in 16% of evaluable patients receiving veliparib monotherapy. Platinum-free interval varied among patients; intervals <180 days correlated with lower veliparib activity. BRCA reversion mutations were detected in 14% of patients prior to crossover veliparib initiation and were associated with poor outcomes. Time from prior platinum exposure may also be associated with cross-resistance to PARPi in patients with gBRCA-associated ABC.
Introduction
Approximately 10% of unselected patients with breast cancer harbor germline (g) mutations in either the breast cancer susceptibility (BRCA)1 or BRCA2 genes (1). Such mutations have been shown to put patients at approximately 60% greater lifetime risk of developing breast cancer (2, 3). Recent studies have shown that breast cancers with germline or somatic mutations in either BRCA1 or BRCA2 are particularly sensitive to platinum chemotherapy (4, 5), as well as to inhibitors of PARP (PARPi; refs. 6, 7), due to a deficiency in homologous recombination repair (8, 9). While high response rates have been observed in patients with BRCA mutations receiving these therapies, the development of acquired resistance remains a challenge. Multiple mechanisms conferring resistance to both platinum chemotherapy and PARPi have been described, including the acquisition of somatic BRCA reversion mutations that potentially restore BRCA protein function (10). These BRCA reversion mutations have been detected both in tumors and circulating tumor DNA (ctDNA) and are reported to occur at varying frequencies in patients with breast or ovarian cancer treated with platinum-based chemotherapy or PARPi. However, evidence for their frequency and presence in advanced breast cancer is limited (11–15). Given the potential for cross-resistance, phase III trials of PARPi for the treatment of advanced breast cancer have generally excluded patients who previously progressed on platinum-based therapy (6, 7, 16).
Veliparib (previously ABT-888) is a potent PARP1 and PARP2 inhibitor (17) that has a manageable safety profile as well as antitumor activity when given as monotherapy and in combination with platinum-based agents in BRCA1/2-mutated metastatic breast cancer (18, 19). While phase III trials have evaluated the efficacy of the PARPis olaparib and talazoparib as monotherapy in patients with advanced BRCA mutation-associated breast cancer (6, 7), veliparib has been evaluated in a phase III trial in combination with platinum-based chemotherapy.
The phase III BROCADE3 study (NCT02163694) evaluated the efficacy and safety of veliparib versus placebo in combination with carboplatin and paclitaxel in patients with HER2-negative metastatic or locally advanced unresectable breast cancer and a gBRCA mutation. The addition of veliparib to carboplatin/paclitaxel resulted in a significant improvement in progression-free survival [PFS; 14.5 months compared with 12.6 months in the placebo plus carboplatin/paclitaxel group; HR (95% confidence interval, CI) = 0.71 (0.57–0.88); P = 0.002], with 25.7% (95% CI, 20.3–31.4) of patients randomized to veliparib alive and progression free at 3 years compared with 10.7% (95% CI, 5.8–17.3) of patients randomized to placebo. The treatment was generally well tolerated (16).
Patients enrolled in BROCADE3 who were randomized to the placebo plus carboplatin/paclitaxel arm were eligible to cross over to veliparib monotherapy after progression. Because each component of the treatment regimen studied (veliparib/placebo, carboplatin, and paclitaxel) could be discontinued independently prior to progression, patients had varying platinum-free intervals (PFI) when crossover treatment was initiated. Herein we report efficacy, safety, and BRCA reversion mutation analyses for patients who received crossover veliparib monotherapy after progression on platinum-based therapy.
Patients and Methods
Study design, participants, and treatment
BROCADE3 is a randomized, double-blind, placebo-controlled, phase III study conducted at 147 study sites worldwide (Supplementary Fig. S1); detailed descriptions of study design, eligibility criteria, and treatment schedules have been published previously (16). In brief, eligible patients at the time of randomization were adult (≥18 years of age) women and men [Eastern Cooperative Oncology Group (ECOG) performance status 0–2] who had histologically or cytologically confirmed metastatic or locally advanced unresectable HER2-negative breast cancer and deleterious or suspected deleterious gBRCA1 or gBRCA2 mutations. Patients had received ≤2 prior lines of cytotoxic chemotherapy for metastatic breast cancer and ≤1 prior line of platinum therapy without progression within 12 months of completing treatment. Prior therapy with PARPi was not allowed.
Patients were randomized 2:1 to veliparib (120 mg orally twice daily on days −2 to 5) or matched placebo, and carboplatin (AUC 6 mg/mL/minute intravenously) on day 1 and paclitaxel (80 mg/m2 i.v.) on days 1, 8, and 15 of 21-day cycles. Veliparib/placebo, carboplatin, and paclitaxel could be discontinued independently before disease progression at the discretion of the treating investigator, resulting in variable PFIs at the time of progression. The primary endpoint of the blinded study was PFS.
After documentation of disease progression per RECIST version 1.1 (RECIST v1.1) on blinded therapy, a patient's treatment arm could be unblinded to determine eligibility for open-label crossover to veliparib monotherapy. Patients randomized to placebo plus carboplatin/paclitaxel who were eligible for crossover therapy initiated treatment within 60 days of progression. The starting dose for unblinded oral veliparib monotherapy was 300 mg twice a day continuously administered; after 2 weeks, the dose could be increased to 400 mg at the investigator's discretion if tolerated. Patients were treated until a second progression event or unacceptable toxicity occurred.
This study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and are consistent with International Conference on Harmonization Good Clinical Practice guidelines and applicable regulatory requirements. Institutional review boards and independent ethics committees at each of the participating study sites reviewed and approved the protocol and all relevant study forms before study initiation. Written informed consent was obtained from each patient before the study. An independent data monitoring committee monitored patient safety. This study is registered with ClinicalTrials.gov: NCT02163694.
Assessments
A new baseline tumor burden was established for each patient at the start of crossover veliparib monotherapy treatment. Tumor assessments were conducted every 9 weeks from cycle 1, day 1 of crossover treatment. Tumor response [complete response (CR) or partial response (PR)] was assessed by the investigator using RECIST v1.1 in patients with measurable disease. PFS (time from first dose of crossover treatment to disease progression per investigator or death from any cause within 63 days of last tumor assessment) and clinical benefit rate (CBR; progression-free rate at 24 weeks estimated using Kaplan–Meier methodology) were also assessed. PFI was defined as the time from last dose of carboplatin in the blinded study to first dose of crossover veliparib monotherapy. Duration of treatment exposure was the number of days a patient was exposed to study treatment.
Retrospective analysis for BRCA reversion mutations restoring BRCA1/2 protein function was accomplished by next-generation sequencing (NGS) of plasma ctDNA using a custom-targeted amplicon multiplex assay that surveys 67 cancer-related genes. Twenty-eight of 75 patients were consented for plasma collection; plasma was not available for ctDNA analysis from the remaining 47 patients. Plasma ctDNA (3 mL each) was harvested during the blinded portion of the study at pretreatment (cycle 1), cycle 3 (∼63 days posttreatment) of placebo plus carboplatin/paclitaxel, and at the time of disease progression on placebo plus carboplatin/paclitaxel (final visit). NGS libraries were prepared using anchored-multiplex ArcherDX chemistry (LiquidPlex). All NGS was performed on the Illumina HiSeq 4000 instrument. Bioinformatic analysis of ctDNA NGS was conducted with the Archer Analysis v6.0.4 variant-calling pipeline by ArcherDX Inc with annotation support from Golden Helix VarSeq v2.1.0. Visual inspection of reversion was conducted in Golden Helix GenomeBrowse v3.0.0 using human genome build GRCh37 (hg19). Variant allele fraction (VAF) of distinct TP53 and MLL3 (KMT2C) somatic mutations were used to determine tumor fraction in cell-free DNA (cfDNA), given that these genes are frequently mutated in metastatic breast cancer (20, 21).
Treatment-emergent adverse events (TEAE) were assessed during crossover veliparib monotherapy until 30 days after the last dose. AE severity was graded by the investigator according to the NCI Common Terminology Criteria for Adverse Events v4.03.
Statistical analyses
The cut-off date for efficacy data included in this article was April 5, 2019. This analysis was conducted on patients who received ≥1 dose of crossover veliparib monotherapy. Descriptive statistics are provided for demographic and baseline characteristics, exposure, activity, and safety variables. Categorical variables are summarized with frequency and percentage, and continuous variables are summarized with median and range. The Kaplan–Meier method was used to analyze PFS and calculate landmark values including medians and CBR. Analyses were done with SAS version 9.4. Activity and PFS were also evaluated in subgroups defined by BRCA1 mutation, BRCA2 mutation, estrogen and/or progesterone receptor positivity (hormone receptor–positive disease), or estrogen and progesterone receptor negativity [triple-negative breast cancer (TNBC)]. In the BRCA1 mutation and BRCA2 mutation subgroups, patients who had mutations in both BRCA1 and BRCA2 were excluded.
Biomarker analyses were evaluated in patients who had available samples at each of the following visits: pretreatment, cycle 3, and progression. Biomarker statistical analyses were conducted using TIBCO Spotfire v7.8 or 10.3.3 (RRID:SCR_008858) and GraphPad Prism v8.0 (RRID:SCR_002798).
Data sharing statement
AbbVie is committed to responsible data sharing regarding the clinical trials they sponsor. This includes access to anonymized, individual and trial-level data (analysis datasets), as well as other information (e.g., protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and statistical analysis plan and execution of a data sharing agreement. Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Results
Patients
At the time of the primary analysis, 132 of 174 patients (76%) on the placebo plus carboplatin/paclitaxel arm of BROCADE3 had a progression event, and 75 of these patients had received ≥1 dose of open-label crossover veliparib monotherapy after progression (Supplementary Fig. S2). Demographic and baseline clinical characteristics for patients in the crossover veliparib monotherapy cohort are summarized in Table 1. Among patients who started crossover therapy, 47% had documented gBRCA1 mutations and 56% had documented gBRCA2 mutations at the time of randomization to the blinded study. Of the patients who started crossover therapy, 63% (n = 47) had tumors that were estrogen receptor (ER) positive or progesterone receptor (PgR) positive, and 37% (n = 28) had tumors that were ER and PgR negative (TNBC) at the time of randomization to the blinded study. Eight patients (10.7%) received platinum prior to randomization in the blinded study. Crossover patients received a mean (range) of 11.7 (3–53) cycles of carboplatin before progression on placebo plus carboplatin/paclitaxel in the blinded study. At the start of crossover veliparib monotherapy, the mean PFI for these patients was 5.6 months, with a wide range of 0.4–37.4 months. While 37 (49.3%) patients continued to receive carboplatin until progression on the blinded study, 38 (50.7%) discontinued carboplatin prior to progression.
Table 1.
Patient demographics and clinical characteristics.
| Crossover veliparib monotherapy | |
|---|---|
| Characteristic | (N = 75) |
| Sexa, n (%) | |
| Female | 74 (99) |
| Male | 1 (1) |
| Median agea, years (range) | 44 (28–71) |
| Racea, n (%) | |
| White | 68 (91) |
| Black or African American | 1 (1) |
| Asian | 6 (8) |
| Geographic regiona, n (%) | |
| USA | 11 (15) |
| Other | 64 (85) |
| gBRCA1 or gBRCA2 mutation status by core laboratorya, n (%) | |
| gBRCA1 mutation positive | 35 (47) |
| gBRCA2 mutation positive | 42 (56) |
| Hormone receptor expressiona, n (%) | |
| ER and/or PgR positive | 47 (63) |
| ER and PgR negative (TNBC) | 28 (37) |
| Platinum exposure in the blinded study | |
| Number of carboplatin cycles received, mean (range) | 11.7 (3–53) |
| Platinum-free interval, mean, months (range) | 5.6 (0.4–37.4) |
| Continued carboplatin until progression in the blinded study | |
| Yes | 37 (49.3) |
| No | 38 (50.7) |
Abbreviations: BRCA, breast cancer susceptibility gene; ER, estrogen receptor; g, germline; PgR, progesterone receptor; TNBC, triple-negative breast cancer.
aAs reported at the time of randomization.
Veliparib monotherapy exposure
Patients who received crossover veliparib monotherapy had a mean treatment duration of 154 days (range, 2–966; Table 2). Patients who continued receiving carboplatin until the time of progression in the blinded study had a numerically shorter duration of treatment with veliparib monotherapy compared with those who discontinued carboplatin prior to progression [median: 121.9 days (range, 2–539) vs 196.3 (range, 5–967), respectively]. Treatment duration was also shorter for patients with a PFI <180 days [median: 133.7 days (range, 2–680)] versus those with a PFI ≥180 days [median: 222.1 days (range, 5–967)] at the start of crossover veliparib monotherapy (Table 2).
Table 2.
Exposure to and activity of crossover veliparib monotherapy.
| Progression on carboplatin in the blinded studya | Platinum-free interval at crossover veliparib monotherapy start | ||||
|---|---|---|---|---|---|
| Crossover veliparib monotherapy | Yes | No | <180 days | ≥180 days | |
| (N = 75) | (n = 37) | (n = 38) | (n = 53) | (n = 22) | |
| Mean exposure to crossover veliparib monotherapy, days (range) | 154 (2–966) | 121.9 (2–539) | 196.3 (5–967) | 133.7 (2–680) | 222.1 (5–967) |
| Best responseb, n/N (%) | |||||
| Complete response | 0 | 0 | 0 | 0 | 0 |
| Partial response | 8/50 (16) | 3/27 (11.1) | 5/23 (21.7) | 5/37 (13.5) | 3/13 (23.1) |
| Clinical benefit rate at 24 weeksc, % (95% CI) | 30.5 (21.9–39.5) | 27.3 (13.6–43.0) | 46.9 (29.0–62.9) | 30.0 (17.7–43.3) | 55.0 (29.8–74.5) |
| Median progression-free survivald, months (95% CI) | 2.1 (2.1–4.4) | 2.1 (1.9–3.7) | 4.4 (2.1–8.2) | 2.1 (2.0–4.1) | 8.2 (1.9–NR) |
Abbreviation: NR, not reached.
aProgressive disease per protocol indicated as reason for carboplatin discontinuation during placebo plus carboplatin/paclitaxel treatment. All patients receiving crossover veliparib monotherapy were required to have progressed on placebo plus carboplatin/paclitaxel; however, placebo, carboplatin, and paclitaxel could be discontinued independently prior to progression.
bIncludes patients with at least one measurable lesion at baseline.
cFrom Kaplan–Meier estimates. Clinical benefit rate is defined as the progression-free rate at 24 weeks (168 days) estimated using Kaplan–Meier methodology.
dMedian progression-free survival is evaluated from first day of crossover treatment.
Efficacy
In total, 50 patients had measurable disease and were evaluable for best response; no patients achieved a CR, but 8 (16%) patients had a PR on crossover veliparib monotherapy. At the time of data cutoff, 5 of these patients had PR confirmed on a subsequent assessment. Durations of response in these 5 patients were 151 days in 1 patient who had progressed, and 47, 178, 458, and 501 days in 4 patients with responses ongoing. Among all patients receiving crossover veliparib monotherapy, the CBR at 24 weeks was 30.5% (95% CI, 21.9–39.5) and the median PFS (investigator-assessed) was 2.1 months (95% CI, 2.1–4.4).
Among the 8 patients who achieved PR, 3 had progressed on carboplatin in the blinded study (PFI range, 20–70 days) and 5 discontinued carboplatin prior to progression (PFI range, 119–1,063 days; Table 2). The mean PFI for patients who achieved PR was 9.3 months (range, 0.7–34.9).
Patients who progressed on carboplatin versus those who discontinued prior to progression had numerically lower rates of PR, lower CBR, and shorter median PFS. Similarly, patients who had a PFI <180 days had numerically lower rates of PR, lower CBR, and shorter median PFS (Table 2). The PFI was numerically shorter among patients who had progressed by 24 weeks after starting crossover veliparib monotherapy, compared with those who remained progression free at 24 weeks [3.1 months (range, 0.4–10.9) vs. 8.1 months (1.0–34.9)].
Among the 8 patients who had a PR, 1 had a BRCA1 mutation and 7 had a BRCA2 mutation at the time of randomization in the blinded study (Supplementary Table S1). Two had TNBC and 6 had hormone receptor–positive disease. Median PFS was numerically longer for the BRCA2 [4.2 months (95% CI, 2.1–7.3)] and hormone receptor–positive [2.7 months (95% CI, 2.1–6.0)] subgroups compared with the BRCA1 [2.1 months (95% CI, 1.8–2.7)] and TNBC [2.1 months (95% CI, 1.8–9.7)] subgroups. However, within each subgroup, there were patients who were progression free for 12 months or longer (BRCA1, 2 patients; BRCA2, 3 patients; TNBC, 3 patients; hormone receptor positive, 2 patients; Supplementary Fig. S3).
BRCA reversion analysis
Plasma cfDNA was obtained from 28 of 75 (37%) patients in the blinded portion of the crossover study, and BRCA mutational status was successfully assessed at cycle 1, cycle 3, and at the time of disease progression for these 28 patients only (all female). Longitudinal plasma biopsies were not available for cfDNA analysis from the remaining 47 of 75 patients. All 28 patients had measurable cfDNA levels [median: 20.8 ng (range, 4.3–635.9 ng)]. BRCA1/2 reversion status by gBRCA mutational status and hormone receptor expression at the time of randomization is summarized in Supplementary Table S2. BRCA reversion mutations that restored BRCA protein function were detected in 4 patients, although only in their postprogression plasma cfDNA sample, with a VAF ranging from 0.011 to 0.062 (Table 3). Mean duration of veliparib monotherapy was 0.78 months in patients with tumors that acquired BRCA reversion mutations during the blinded portion of the study, compared with 7.49 months in patients with tumors that did not acquire these mutations (Supplementary Table S3). The molecular characteristics of the reversion mutations observed in 2 different patients are shown in Supplementary Fig. S4. Several patients developed multiple reversion mutations with varying VAF levels, suggesting multiclonal heterogeneity. A BRCA1 reversion mutation (ER/PgR-negative breast cancer) was identified in 1 patient, while BRCA2 reversion mutations (2 with ER-positive and PgR-negative and 1 with ER/PgR-positive breast cancer) were identified in 3 patients (Supplementary Table S2).
Table 3.
Molecular characteristics of patients with BRCA reversion mutations.
| Pt | Symbol | Primary BRCA1/2 mutation (confirmed in pretreatment cfDNA) | Reversion mutation detected (protein) | Clinical status at time of reversion | Reversion mutation (coding) | VAFb | Type | Consequence | Exon | Fraction ctDNA in cfDNA (VAF range) | Mean duration of veliparib monotherapy, months |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | BRCA2 | p.Gln84* | p.Gln84His | Progression on blinded portion | c.252A>T | 0.011 | SNP | Missense | 3|27 | 0.36a | 0.62 |
| 2 | BRCA2 | p.Gln2957* | p.Met2952_Lys2958del | Progression on blinded portion | c.8856_8873del | 0.018 | DEL | Frameshift | 22|27 | 0.05 (0.012–0.071) | 0.07 |
| 2 | BRCA2 | p.Gln2957* | p.Glu2956_Lys2958del | Progression on blinded portion | c.8866_8874del | 0.040 | DEL | Frameshift | 22|27 | 0.05 (0.012–0.071) | 0.07 |
| 3 | BRCA2 | p.Ser1982Argfs* | p.Gln1998* | Progression on blinded portion | c.5992_6014del | 0.026 | DEL | Frameshift | 11|27 | 0.064 (0.064–0.214) | 0.72 |
| 3 | BRCA2 | p.Ser1982Argfs* | p.Gln1998Cysfs* | Progression on blinded portion | c.5991_5992insTGCATTA | 0.041 | INS | Frameshift | 11|27 | 0.064 (0.064–0.214) | 0.72 |
| 3 | BRCA2 | p.Ser1982Argfs* | p.Val1999_ Phe2000delinsAspAla | Progression on blinded portion | c.5996_5999delinsATGC | 0.039 | MNP | Missense | 11|27 | 0.064 (0.064–0.214) | 0.72 |
| 4 | BRCA1 | p.Tyr130* | p.Iso124_Asn132del | Progression on blinded portion | c.386-409del | 0.062 | DEL | Frameshift | 6|27 | 0.084a | 1.71 |
Abbreviations: BRCA, breast cancer susceptibility gene; cfDNA, cell-free DNA; ctDNA, circulating tumor DNA; DEL, deletion; INS, insertion; MNP, multiple nucleotide polymorphism; NGS, next-generation sequencing; Pt, patient; SNP, single nucleotide polymorphism; VAF, variant allele fraction.
*Amino acid change resulting in STOP codon.
aIndicates patients with only one mutation.
bVAF indicates fraction of NGS reads supporting mutation over total reads. Fraction ctDNA in cfDNA denotes quantification of tumor-derived DNA fraction using distinct TP53 or MLL3 VAF in same sample. TP53/MLL3 VAF range denotes the VAF range for distinct clonal mutations used to determine fraction ctDNA in cfDNA.
To determine the proportion of ctDNA in cfDNA (Table 3), the VAF of TP53 and MLL3 (KMT2C) somatic alterations postprogression were assessed; they were detectable in all 28 patients of the biomarker subgroup presented herein. All BRCA reversions were detected at VAFs lower than those of TP53 and/or MLL3 (VAF ranges, 1.2%–36%), suggesting that they represent a clonal fraction of neoplastic cfDNA.
A swimmer plot detailing the treatment received, BRCA reversion, and survival outcomes for each patient is displayed in Fig. 1. Of the 28 patients analyzed, 3 achieved PR while on crossover veliparib monotherapy; none of them had BRCA reversion mutations. The 4 patients with BRCA reversion mutations received eight (2 patients), nine, and 23 cycles of carboplatin prior to progression on placebo plus carboplatin/paclitaxel (Fig. 1B). Similar to the overall trial population in which a high proportion of patients had no prior platinum exposure, none of these patients had received prior platinum therapy in the neoadjuvant/adjuvant or metastatic setting prior to enrollment in the trial.
Figure 1.
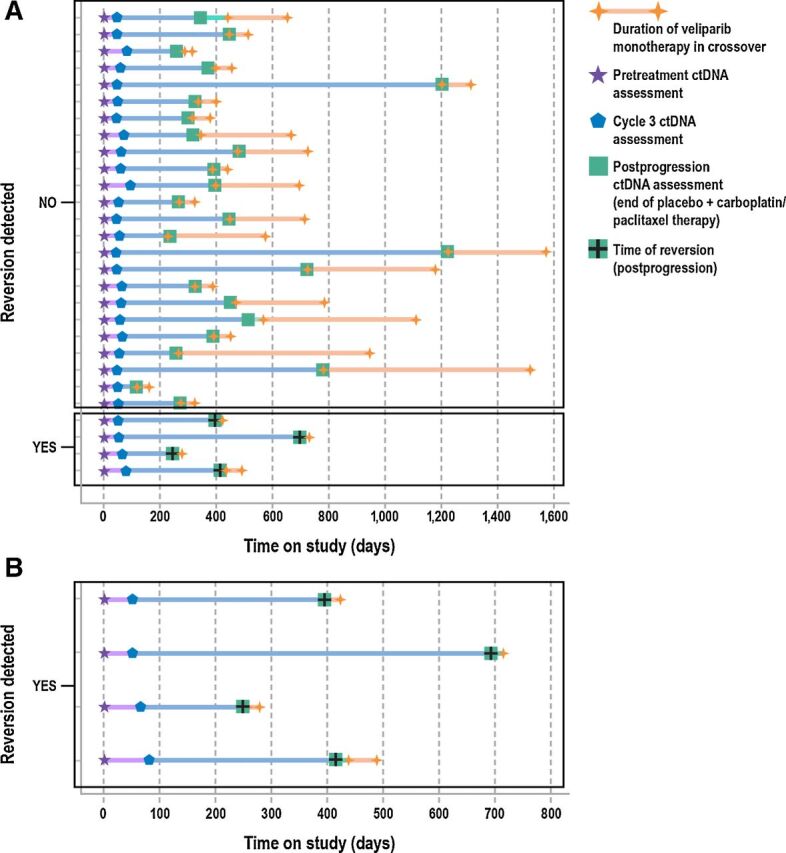
Swimmer plot of treatment overview and time of reversion in the biomarker subgroup (n = 28) for all patients (A) and patients with BRCA reversion (B). Each line represents an individual patient and each symbol indicates a key event (treatment, reversion). Left axis indicates patients who reverted versus those who did not. Colored line segments correspond to different periods in the treatment overview as indicated in the legend, with orange lines showing time on veliparib monotherapy after crossover. Timing of ctDNA assessments is indicated by pretreatment (star), cycle 3 (pentagon), and postprogression (square colored symbols). Green squares also denote completion of placebo plus carboplatin/paclitaxel treatment period. Time of detection of BRCA1/2 reversion mutations is denoted by a black cross.
The mean duration of crossover veliparib monotherapy was shorter in patients with BRCA reversion mutations [n = 4; 0.78 months (range, 0.07–1.71)] than in those who had no reversion mutations [n = 24; 7.49 months (range, 0.92–24.4)]. The mean PFI was slightly shorter in patients who had BRCA reversions [4.36 months (range, 0.95–7.5)] than in those who did not [5.81 months (range, 0.39–34.9)].
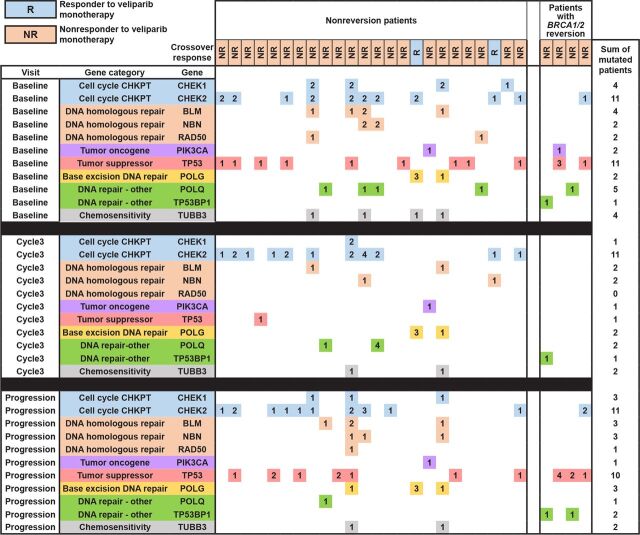
Several DNA repair genes, PARPi resistance–associated genes, tumor driver genes, and tumor suppressor genes were surveyed for single-nucleotide variants (snv) and genomic insertions or deletions (indels) in the 28 crossover patients. Among those, the prevalence and co-occurrence of deleterious non-BRCA1/2 alterations in patients from the biomarker subgroup who had no BRCA1/2 reversions are shown in Supplementary Table S4 and Fig. 2. The duration of veliparib monotherapy was also calculated for each alteration-positive group. The most frequent alterations were CHEK2, which was observed in 13 patients (54%), and TP53, which was found in 12 patients (50%). TP53 variants were prevalent across patients with or without BRCA1/2 reversions and changed longitudinally during the blinded period. In baseline ctDNA, 11 of 24 patients carried a TP53 mutation, which diminished to 1 of 24 patients in cycle 3 ctDNA and reappeared in postprogression ctDNA (10/24 patients). Notably, in 9 of those patients, TP53 mutations that were undetectable at cycle 3 were reacquired by postprogression (Fig. 2). Duration of veliparib monotherapy in patients with TP53 mutations was 7.3 months (range, 0.9–24.4) versus 7.7 months in nonmutated TP53 patients (Supplementary Table S4). Detection of alterations in three genes involved in homologous DNA repair (HR; RAD50, BLM, and NBN) were observed only in nonreversion patients (Supplementary Table S4 and Fig. 2). Three (13%), 4 (17%), and 5 (21%) patients had mutated RAD50, NBN, and BLM, respectively, and at differing visits (Fig. 2). Duration of veliparib monotherapy in patients with RAD50 and BLM mutations was 4.1 and 4.7 months, respectively, versus 8.0 and 8.2 months, respectively, in those without RAD50 or BLM mutations (Supplementary Table S4).
Figure 2.
A heatmap representing co-occurring and deleterious non-BRCA1/2 alterations in all 28 patients in the ctDNA analysis cohort. Patients are organized in columns and grouped by reversion status, while genes are organized into rows and grouped by timepoint and mutation category (color coded). Response status to veliparib monotherapy in crossover period is indicated in orange or blue boxes per column and in legend. Each colored square in the heatmap corresponds to a potentially deleterious alteration in that gene for each patient per visit. The number in each colored square indicates the number of alterations detected in that gene per patient. Per gene row, total patients with alterations in that gene are enumerated in the rightmost column.
Safety
Of the 75 patients who received crossover veliparib monotherapy, 41 (54.7%) escalated the dose from 300 to 400 mg twice daily. The most common TEAEs reported were gastrointestinal [nausea, n = 46 (61%); vomiting, n = 22 (29%); Table 4]. These events were predominantly grade 1–2. Hematologic toxicities were reported at lower frequencies [neutropenia, n = 11 (15%); anemia, n = 5 (7%); thrombocytopenia, n = 5 (7%)]. Three patients (4%) had a convulsion event. Serious adverse events (SAE) were reported in 17/75 patients (23%; Supplementary Table S5). No SAE occurred in more than 1 patient, except for malignant neoplasm progression and metastases to the central nervous system [4 (5%) and 2 (3%) patients, respectively); these were not considered related to veliparib. One patient had a TEAE of respiratory failure that was fatal and was attributed to disease progression and unrelated to veliparib by the investigator's assessment.
Table 4.
Summary of TEAEs and select TEAEs of special interest during crossover veliparib monotherapy.
| TEAE | Crossover veliparib monotherapy (N = 75), n (%) | |
|---|---|---|
| Any TEAE | 68 (91) | |
| Any TEAE related to study druga | 57 (76) | |
| Grade ≥3 TEAE | 29 (39) | |
| Serious AE | 17 (23) | |
| Any TEAE leading to study discontinuation not due to PD | 1 (1) | |
| Most frequent nonhematologic TEAEs (in >10% of patients) | Any grade | Grade 3 or 4 |
| Nausea | 46 (61) | 1 (1) |
| Vomiting | 22 (29) | 0 |
| Fatigue | 18 (24) | 3 (4) |
| Diarrhea | 16 (21) | 1 (1) |
| Headache | 13 (17) | 1 (1) |
| Decreased appetite | 11 (15) | 0 |
| Pain in extremity | 11 (15) | 0 |
| Arthralgia | 10 (13) | 0 |
| Insomnia | 9 (12) | 1 (1) |
| Most frequent hematologic TEAEs (in >5% of patients) | Any grade | Grade 3 or 4 |
| Neutropenia | 11 (15) | 4 (5) |
| Anemia | 5 (7) | 3 (4) |
| Thrombocytopenia | 5 (7) | 1 (1) |
| Select TEAEs of special interest b | Any grade | Grade 3 or 4 |
| Infections within 14 days of neutropeniac | 1 (1) | 0 |
| Hemorrhages within 14 days of thrombocytopenia | 0 | 0 |
| Myelodysplastic syndromes | 0 | 0 |
| Acute myeloid leukemia | 0 | 0 |
| Convulsionsd | 3 (4) | 2 (3) |
| Fertility disorders | 0 | 0 |
| Secondary malignanciese | 2 (3) | 2 (3) |
Abbreviations: PD, progressive disease; TEAE, treatment-emergent adverse event.
aAs assessed by the investigator.
bAdditional prespecified adverse events of special interest (nausea/vomiting, anemia, thrombocytopenia, and neutropenia) are reflected above and not included among the TEAEs of special interest.
cPreferred term of reported event was “gingivitis”.
dTwo patients were reported to have an event of seizure (n = 1, grade 2; n = 1, grade 3); one patient had a grade 3 event of partial seizure and a grade 1 event of petit mal epilepsy.
ePreferred term of reported event was “metastases to central nervous system” in both patients.
Discussion
In recent years, two PARPis have been approved for the treatment of advanced HER2-negative breast cancer in patients with gBRCA mutations. Median PFS of 7.0–8.6 months has been reported with the PARPis olaparib and talazoparib as monotherapy in this population in phase III trials (6, 7). It is notable, however, that these studies did not allow enrollment of patients with progression on prior platinum therapy. There is evidence that platinum-based therapies are active in patients with BRCA mutation-associated advanced TNBC, with a reported objective response rate of 68% to carboplatin alone and a median PFS of 6.8 months in a subgroup analysis of a phase III trial (4). Patients with advanced breast cancer often receive multiple lines of treatment including platinum, particularly patients with gBRCA mutations. Data are limited on the outcomes of PARPi in patients with germline BRCA mutations who previously received and progressed on platinum therapy due to exclusion of these patients from phase III PARPi trials.
The phase III BROCADE3 study of veliparib in patients with advanced BRCA mutation-associated breast cancer evaluated the efficacy and tolerability of a PARPi used in combination with platinum-based chemotherapy (16). Whereas previous phase III studies of the PARPis olaparib and talazoparib used the agents as monotherapy (6, 7), in BROCADE3 veliparib was combined with carboplatin/paclitaxel and compared with carboplatin/paclitaxel alone. The addition of veliparib was found to significantly improve PFS when combined with carboplatin and paclitaxel. Addition of veliparib to carboplatin/paclitaxel was also generally well tolerated, with <10% of patients discontinuing study drug due to AEs not related to progression. BROCADE3 also uniquely allowed crossover to veliparib monotherapy for patients randomized to placebo plus carboplatin/paclitaxel after disease progression.
In the current analysis, activity of crossover veliparib monotherapy was demonstrated in patients treated after progression on placebo plus carboplatin/paclitaxel, with 16% of patients having a PR and 30.5% of crossover patients experiencing clinical benefit, defined as the progression-free rate at 24 weeks. In comparison, previously reported response rates for veliparib monotherapy treatment in a phase II trial that included 44 patients with BRCA-associated metastatic breast cancer, who had a median of 1 (0–5) prior line of chemotherapy for metastatic breast cancer but had not received prior platinum-based therapy for metastatic disease, were 14% and 36% in patients with BRCA1 and BRCA2 mutations, respectively (18).
The flexibility to discontinue veliparib/placebo, carboplatin, and paclitaxel independently during blinded treatment in BROCADE3 led to a heterogeneous veliparib monotherapy crossover population. The population included both patients who received carboplatin until progression and patients who discontinued carboplatin prior to progressing on the blinded study regimen. The PFI of patients starting crossover veliparib monotherapy was highly variable.
Given the eligibility criteria for previous PARPi trials, there is limited available information on the response of platinum-resistant tumors to PARPi. It is therefore notable that among the 27 patients who were receiving carboplatin at the time of progression in the blinded study, 3 patients (11.1%) had a PR to veliparib as next line of therapy. However, the data suggest that the use of carboplatin treatment until disease progression and a PFI of <180 days correlated with lower activity of veliparib monotherapy. Platinum retreatment is common practice for patients with ovarian cancer relapsing >6 months after their last dose of platinum therapy, as they are considered potentially platinum sensitive. This definition of platinum sensitivity has also been used to identify patients with recurrent disease who may benefit from PARPi therapy, with phase III trials of PARPi maintenance in recurrent ovarian cancer restricted to patients with platinum-sensitive disease (22–24). The data from patients receiving crossover veliparib monotherapy presented here demonstrate that the 6-month PFI may also be predictive of response to PARPi after platinum therapy in patients with advanced breast cancer.
In this analysis, a higher proportion of patients with BRCA2 mutations compared with BRCA1 mutations and with hormone receptor–positive disease compared with TNBC responded to crossover veliparib monotherapy. While a lower level of activity was observed with PARPi as the next line of therapy after platinum in the BRCA1 and TNBC subgroups, there were patients with durable benefit regardless of the mutated BRCA gene or hormone receptor status.
BRCA reversion mutations have been documented following treatment with single-agent platinum or PARPi in BRCA-related breast cancers (14, 25, 26) and have been associated with subsequent cross-resistance to PARPi (10). The exploratory biomarker analysis reported herein confirms, albeit in small numbers of patients, that the presence of BRCA reversion mutations at the time of disease progression on or following carboplatin/paclitaxel is associated with poor clinical outcomes and low response rates to veliparib monotherapy. These data suggest that cross-resistance may limit PARPi efficacy after a platinum-based regimen in a subset of patients, particularly in those with a PFI of <24 weeks. However, it is noteworthy that in our study only a minority (4/28, 14.3%) of crossover patients were found to have BRCA reversion mutations. This is similar to observations in high-grade serous ovarian carcinoma (HGSOC) where 8 of 97 (8.2%) patients with HGSOC had either germline or somatic BRCA reversion mutations detected in plasma prior to rucaparib treatment; an additional 8 of 78 (10.3%) patients had BRCA reversion mutations identified in plasma samples collected at disease progression (15). Interestingly, the majority of unique reversion mutations (8/9) were detected in BRCA2 as opposed to BRCA1, an observation made previously (26, 27). We speculate that differences in the chromatin landscape and length of protein-coding domains are influential in this difference. The relatively small proportions of patients with BRCA reversion mutations at the time of progression indicate that there are likely many other potential mechanisms of resistance.
Indeed, we observed dynamic changes in the clonal fractions of TP53 somatic mutations that resulted in reemergence of clonal TP53 mutations, hypothetically driving disease progression on subsequent veliparib treatment. However, it is worth noting that duration of veliparib monotherapy in patients with TP53 mutations versus those who had no TP53 mutations was not significantly different. In addition, while we did not detect TP53BP1 loss-of-function alterations in the nonreversion subgroup (a recognized PARPi resistance mechanism), we uncovered, at postprogression, the presence of somatic, protein-altering mutations (snvs and indels) in RAD50, NBN, and BLM, genes that are involved in HR. While most of these mutations are categorized as protein altering, it remains unclear whether they reactivate HR function. Previous studies have suggested a role for genomic lesions in HR pathway genes as conferring resistance or sensitivity to PARPi (25, 28–32).
Intriguingly, HR gene alterations were only observed in the subgroup of nonreversion patients (Fig. 2). Four patients presented with one or more potentially pathogenic alterations in RAD50, NBN, and BLM prior to crossover to veliparib. Hypothetically, these loss-of-function alterations in RAD50 and NBN would disrupt the MRN complex and disrupt DNA double-strand break recognition, potentially sensitizing patients to a DNA-damaging agent. Meanwhile, mutations in BLM would disrupt RAD51/52-mediated DNA end resection and repair, thus potentially also predicting a favorable response to a PARPi. The average duration of veliparib monotherapy in the 4 patients with HR mutations was 5.7 months (range, 2.0–9.9 months) versus 7.6 months (range, 0.92–24.3 months) in the other 20 non–HR-mutated patients. Therefore, it remains unclear whether these specific lesions confer additional sensitivity to subsequent veliparib treatment or represent alternative genetic susceptibilities that evade pressure from veliparib.
In conclusion, crossover veliparib monotherapy was active in a subset of patients with advanced HER2-negative gBRCA mutation-associated breast cancer after disease progression on a regimen of carboplatin/paclitaxel. PFI and progression on platinum treatment versus progression >6 months after the end of platinum treatment appeared to affect activity. Although BRCA reversion mutations were observed infrequently, their presence may promote cross-resistance, thereby limiting PARPi efficacy after platinum failure, and warrants further evaluation. The specific role of additional molecular mechanisms in the response to veliparib treatment merits additional investigation. The data suggest that prior platinum treatment does not preclude use of a PARPi in patients with gBRCA-associated advanced breast cancer, particularly if the platinum usage occurred more distantly in the patient's history and the patient is considered platinum sensitive. If BRCA reversion testing is available, the results may help to further refine which platinum-treated patients could be considered candidates for PARPi therapy.
Statement on originality of the work
The manuscript represents original work and has not been submitted for publication elsewhere nor previously published.
Authors’ Disclosures
S.L. Puhalla reports grants and personal fees from AbbVie during the conduct of the study. S.L. Puhalla also reports personal fees from Pfizer, MedImmune, Celldex, Puma, AstraZeneca, Eisai, and Nanostring, as well as grants from Pfizer, Lilly, Novartis, Incyte, Covance-Bayer, AstraZeneca, Genentech, and Medivation outside the submitted work. V. Diéras reports personal fees from AbbVie during the conduct of the study, as well as personal fees from Roche Genentech, Novartis, Lilly, MSD, Pfizer, Daiichi Sankyo, AstraZeneca, and Gilead outside the submitted work. B.K. Arun reports non-financial support from AbbVie, Invitae, and AstraZeneca during the conduct of the study. H. Wildiers reports other support from Immutep Pty, MSD, AstraZeneca Ireland, Daiichi, AbbVie, Lilly, PSI CRO AG, KCE, Eisai, Roche, Congres care, Pfizer, ARIEZ, Sirtex, Pfizer, TRM Oncology, ORION Corporation, Th Planning Shop, Novartis, Biocartes, and Puma Biotech, as well as grants from Roche outside the submitted work. H.S. Han reports grants from Department of Defense, personal fees from Lilly, and other support from AbbVie, Arvinas, BMS, GSK, G1 Therapeutics, Marker Therapeutics, Horizon Pharma, Seattle Genetics, Quantum Leap Healthcare Collaborative, Zymeworks, Pfizer, and Novartis outside the submitted work. J.-P. Ayoub reports grants from AbbVie during the conduct of the study, as well as personal fees from Pfizer, Roche, AstraZeneca, and Knight Therapeutics outside the submitted work. V. Stearns reports grants from AbbVie during the conduct of the study. V. Stearns also reports grants from Biocept, Pfizer, Novartis, Medimmune, and Puma Biotechnology outside the submitted work, and is a member of the Data Safety Monitoring Board at Immunomedics, Inc. Y. Yuan reports grant/research support from Eisai, Genentech, Merck, Novartis, Pfizer, and Puma; is a consultant for Eisai, Immunomedics, Gilead, Novartis, and Pfizer; and has been a speaker for Eisai, Genentech, AstraZeneca, Daiichi Sankyo, Immunomedics, Gilead, Novartis, and Pfizer. T. Helsten reports other support from Eli Lilly and Syndax/Byondis outside the submitted work. B. Riley-Gillis reports other support from AbbVie (employment) outside the submitted work. D. Maag reports personal fees from AbbVie during the conduct of the study. C.K. Ratajczak reports personal fees and other support from AbbVie outside the submitted work. C.Y. Ramathal reports personal fees and other support from AbbVie outside the submitted work. M. Friedlander reports other support from AbbVie during the conduct of the study. M. Friedlander also reports grants, personal fees, and other support from AstraZeneca; grants and personal fees from Novartis; personal fees from GSK, Lilly, Takeda, MSD, and ACT Genomics; and other support from AbbVie outside the submitted work. No disclosures were reported by the other authors.
Author Contributions
S.L. Puhalla: Conceptualization, data curation, investigation, writing–review and editing. V. Diéras: Conceptualization, data curation, investigation, writing–review and editing. B.K. Arun: Conceptualization, data curation, investigation, writing–review and editing. B. Kaufman: Conceptualization, data curation, investigation, writing–review and editing. H. Wildiers: Conceptualization, data curation, investigation, writing–review and editing. H.S. Han: Conceptualization, data curation, investigation, writing–review and editing. J.-P. Ayoub: Conceptualization, data curation, investigation, writing–review and editing. V. Stearns: Conceptualization, data curation, investigation, writing–review and editing. Y. Yuan: Conceptualization, data curation, investigation, writing–review and editing. T. Helsten: Conceptualization, data curation, investigation, writing–review and editing. B. Riley-Gillis: Conceptualization, data curation, writing–review and editing. E. Murphy: Data curation, writing–review and editing. M.G. Kundu: Conceptualization, data curation, formal analysis, methodology, writing–review and editing. M. Wu: Conceptualization, data curation, formal analysis, methodology, writing–review and editing. D. Maag: Conceptualization, data curation, writing–review and editing. C.K. Ratajczak: Conceptualization, data curation, writing–review and editing. C.Y. Ramathal: Conceptualization, data curation, writing–review and editing. M. Friedlander: Conceptualization, data curation, investigation, writing–review and editing.
Supplementary Material
Supplementary Tables S1-S5
Supplementary Figure Legends for Fig. S1-S4
BROCADE3 study design
CONSORT diagram
PFS KM curves
Molecular characteristics of BRCA reversion mutations
Acknowledgments
The authors thank all the trial investigators and the patients who participated in this clinical trial. Medical writing support was provided by Thayer Darling from Aptitude Health, Atlanta, Georgia, and funded by AbbVie.
AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the article. All authors had access to relevant data and participated in the drafting, review, and approval of this article. No honoraria or payments were made for authorship.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
This article is featured in Highlights of This Issue, p. 4945
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
References
- 1. Tung N, Lin NU, Kidd J, Allen BA, Singh N, Wenstrup RJ, et al. Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J Clin Oncol 2016;34:1460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peto J, Collins N, Barfoot R, Seal S, Warren W, Rahman N, et al. Prevalence of BRCA1 and BRCA2 gene mutations in patients with early-onset breast cancer. J Natl Cancer Inst 1999;91:943–9. [DOI] [PubMed] [Google Scholar]
- 3. Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 2017;317:2402–16. [DOI] [PubMed] [Google Scholar]
- 4. Tutt A, Tovey H, Cheang MCU, Kernaghan S, Kilburn L, Gazinska P, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT trial. Nat Med 2018;24:628–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Isakoff SJ, Mayer EL, He L, Traina TA, Carey LA, Krag KJ, et al. TBCRC009: a multicenter phase II clinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative breast cancer. J Clin Oncol 2015;33:1902–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 2017;377:523–33. [DOI] [PubMed] [Google Scholar]
- 7. Litton JK, Rugo HS, Ettl J, Hurvitz SA, Gonçalves A, Lee KH, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med 2018;379:753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Farmer H, McCabe N, Lord CJ, Tutt ANJ, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005;434:917–21. [DOI] [PubMed] [Google Scholar]
- 9. Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005;434:913–7. [DOI] [PubMed] [Google Scholar]
- 10. Lord CJ, Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science 2017;355:1152–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Quigley D, Alumkal JJ, Wyatt AW, Kothari V, Foye A, Lloyd P, et al. Analysis of circulating cell-free DNA identifies multiclonal heterogeneity of BRCA2 reversion mutations associated with resistance to PARP inhibitors. Cancer Discov 2017;7:999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ganesan S. Tumor suppressor tolerance: reversion mutations in BRCA1 and BRCA2 and resistance to PARP inhibitors and platinum. JCO Precis Oncol 2018;2:1–4. [DOI] [PubMed] [Google Scholar]
- 13. Christie EL, Fereday S, Doig K, Pattnaik S, Dawson SJ, Bowtell DDL. Reversion of BRCA1/2 germline mutations detected in circulating tumor DNA from patients with high-grade serous ovarian cancer. J Clin Oncol 2017;35:1274–80. [DOI] [PubMed] [Google Scholar]
- 14. Weigelt B, Comino-Méndez I, de Bruijn I, Tian L, Meisel JL, García-Murillas I, et al. Diverse BRCA1 and BRCA2 reversion mutations in circulating cell-free DNA of therapy-resistant breast or ovarian cancer. Clin Cancer Res 2017;23:6708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin KK, Harrell MI, Oza AM, Oakin A, Ray-Coquard I, Tinker AV, et al. BRCA reversion mutations in circulating tumor DNA predict primary and acquired resistance to the PARP inhibitor rucaparib in high-grade ovarian carcinoma. Cancer Discov 2019;9:210–9. [DOI] [PubMed] [Google Scholar]
- 16. Diéras V, Han HS, Kaufman B, Wildiers H, Friedlander M, Ayoub JP, et al. Veliparib with carboplatin and paclitaxel in BRCA-mutated advanced breast cancer (BROCADE3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2020;21:1269–82. [DOI] [PubMed] [Google Scholar]
- 17. Donawho CK, Luo Y, Luo Y, Penning TD, Bauch JL, Bouska JJ, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res 2007;13:2728–37. [DOI] [PubMed] [Google Scholar]
- 18. Somlo G, Frankel PH, Arun BK, Ma CX, Garcia AA, Cigler T, et al. Efficacy of the PARP inhibitor veliparib with carboplatin or as a single agent in patients with germline BRCA1- or BRCA2-associated metastatic breast cancer: California Cancer Consortium trial NCT01149083. Clin Cancer Res 2017;23:4066–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Han HS, Diéras V, Robson M, Palácová M, Marcom PK, Jager A, et al. Veliparib with temozolomide or carboplatin/paclitaxel versus placebo with carboplatin/paclitaxel in patients with BRCA1/2 locally recurrent/metastatic breast cancer: randomized phase II study. Ann Oncol 2018;29:154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yates LR, Knappskog S, Wedge D, Farmery JHR, Gonzalez S, Martincorena I, et al. Genomic evolution of breast cancer metastasis and relapse. Cancer Cell 2017;32:169–184.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pereira B, Chin SF, Rueda OM, Moen Vollan HK, Provenzano E, Bardwell HA, et al. The somatic mutation profiles of 2,433 breast cancers refines their genomic and transcriptomic landscapes. Nat Commun 2016;7:11479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med 2016;375:2154–64. [DOI] [PubMed] [Google Scholar]
- 23. Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:1274–84. [DOI] [PubMed] [Google Scholar]
- 24. Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:1949–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Waks AG, Cohen O, Kochupurakkal B, Kim D, Dunn CE, Buendia J, et al. Reversion and non-reversion mechanisms of resistance to PARP inhibitor or platinum chemotherapy in BRCA1/2-mutant metastatic breast cancer. Ann Oncol 2020;31:590–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vidula N, Rich TA, Sartor O, Yen J, Hardin A, Nance T, et al. Routine plasma-based genotyping to comprehensively detect germline, somatic, and reversion BRCA mutations among patients with advanced solid tumors. Clin Cancer Res 2020;26:2546–55. [DOI] [PubMed] [Google Scholar]
- 27. Tobalina L, Armenia J, Irving E, O'Connor MJ, Forment JV. A meta-analysis of reversion mutations in BRCA genes identifies signatures of DNA end-joining repair mechanisms driving therapy resistance. Ann Oncol 2021;32:103–12. [DOI] [PubMed] [Google Scholar]
- 28. Zhang M, Liu G, Xue F, Edwards R, Sood AK, Zhang W, et al. Copy number deletion of RAD50 as predictive marker of BRCAness and PARP inhibitor response in BRCA wild type ovarian cancer. Gynecol Oncol 2016;141:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sullivan-Reed K, Bolton-Gillespie E, Dasgupta Y, Langer S, Siciliano M, Nieborowska-Skorska M, et al. Simultaneous targeting of PARP1 and RAD52 triggers dual synthetic lethality in BRCA-deficient tumor cells. Cell Rep 2018;23:3127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bian L, Meng Y, Zhang M, Li D. MRE11-RAD50-NBS1 complex alterations and DNA damage response: implications for cancer treatment. Mol Cancer 2019;18:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stover EH, Konstantinopoulos PA, Matulonis UA, Swisher EM. Biomarkers of response and resistance to DNA repair targeted therapies. Clin Cancer Res 2016;22:5651–60. [DOI] [PubMed] [Google Scholar]
- 32. Patel DS, Misenko SM, Her J, Bunting SF. BLM helicase regulates DNA repair by counteracting RAD51 loading at DNA double-strand break sites. J Cell Biol 2017;216:3521–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables S1-S5
Supplementary Figure Legends for Fig. S1-S4
BROCADE3 study design
CONSORT diagram
PFS KM curves
Molecular characteristics of BRCA reversion mutations