Abstract
The design of distinctive chemical synthesis strategies aims for the most efficient routes towards versatile compounds in drug target studies. Here, we establish a powerful hybrid synthetic approach of total chemical and chemoenzymatic synthesis to efficiently obtain various 7‐deoxy‐sedoheptulose (7dSh, 1) analogues, unique C7 sugars, for structure‐activity relationship studies. 7dSh (1) is a rare microbial sugar with in planta herbicidal activity. As natural antimetabolite of 3‐dehydroquinate synthase (DHQS), 7dSh (1) inhibits the shikimate pathway, which is essential for the synthesis of aromatic amino acids in bacteria, fungi, and plants, but absent in mammals. As glyphosate, the most used chemical herbicide faces restrictions worldwide, DHQS has gained more attention as valid target of herbicides and antimicrobial agents. In vitro and in vivo analyses of the C7‐deoxysugars confirm DHQS as enzymatic target, highlight the crucial role of uptake for inhibition and add molecular aspects to target mechanism studies of C7‐sugars as our contribution to global efforts for alternative weed‐control strategies.
Keywords: carbohydrates, chemoenzymatic synthesis, herbicides, total synthesis, shikimate pathway inhibition
7‐Deoxy‐sedoheptulose is a cyanobacterial antimetabolite of the 3‐dehydroquinate synthase, second enzyme of the shikimate pathway. Design of a hybrid synthesis of chemical and chemoenzymatic steps using heterologously expressed transketolase efficiently yielded the heptulose family. In vivo and in vitro studies on Anabaena variabilis and Arabidopsis thaliana gave a profound understanding of the mode of action.
Introduction
Antimetabolites are small molecules which efficiently inhibit enzymes by mimicking the architecture of physiological substrates and targeting the active site. Whereas most commonly used antimetabolites are synthetic compounds, microbial bioactive antimetabolites are rare. [1] The C7‐sugar 7‐deoxy‐sedoheptulose (7dSh, 1) which is biosynthesized in the cyanobacterium Synechococcus elongatus, was isolated, structure‐elucidated and shown to inhibit the growth of photoautotrophic organisms like various cyanobacteria, plants and additionally auxotrophic yeast.[ 2 , 3 ] Its herbicidal activity turned out to be as potent as that of glyphosate on seedling growth and germination while no or little cytotoxic effects were observed on mammalian cells and fish, respectively.[ 2 , 4 ] On the molecular level, 7dSh (1) was suggested to inhibit dehydroquinate synthase (DHQS) within the shikimate pathway (Figure 1A).
Total chemical syntheses of unmodified heptuloses normally comprise multiple steps with selective protection regimes and redoxchemistry steps, prior to C−C‐bond elongation of the carbon skeleton. Two synthetic routes are common but mainly restricted to manno‐ and glucoheptulose syntheses: one is a rearrangement e. g., by the Lobry de Bruyn‐Alberda van Ekenstein transformation, and the other includes chain elongation at C‐1, e. g., by oxidation and subsequent Wittig reaction.[ 5 , 6 ] Our use of the enzyme transketolase simplifies the synthesis, based on the transfer of a C2‐unit onto an C5‐aldehyde in only one step from the modified C5‐aldehyde to the C7‐carbohydrate. However, the enzyme reaction is restricted by two essential prerequisites, namely the preferred (R)‐configuration at C‐2 of the C5‐aldehyde and the (S)‐configuration of the newly formed chiral center, leading to an (3S, 4R)‐ketose. [7] As C2‐unit donor β‐hydroxypyruvate is favored because of efficient CO2‐release, which prevents back‐reaction and shifts the equilibrium to the ketose products (Figure 2B).[ 2 , 8 ] Sedoheptulose derivatives with their stereochemical architecture are underexplored.
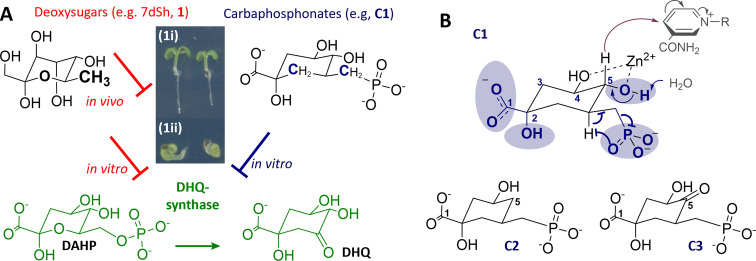
Because of the huge potential of DHQS as valid herbicidal and antimicrobial target, intensive research in the 1990s led to the development of the synthetic carbaphosphonates, with analogue C1 being the founding member of this large class. As strong antimetabolites, these carbaphosphonates inhibit the activity of DHQS at nanomolar concentrations in in vitro enzyme assays (Tab. S1, e. g., C1−C3).[ 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 ] The inhibitory effect depends on the phosphonate group which impairs the β‐elimination and the formation of the carbocycle which enhances the redox potential at C‐5 (Figure 1B) compared to the oxacyclic ring. [12] However, a serious issue of carbaphophonates is the lack of in vivo activity as evidenced by the absence of any herbicidal effects. [18] In contrast to carbaphosphonates, 7‐deoxy‐sedoheptulose (7dSh, 1) exhibits a fundamentally different and new inhibitory activity towards DHQS. Mechanistically, the inhibitory effect of 7dSh (1) may be ascribed to the 7‐methyl moiety mimicking the natural 7‐O‐phosphate substrate (DAHP) of DHQS.
Figure 1.
A) Basis of chemical synthetic study: The conversion of DAHP to 3‐dehydroquinate (DHQ) by DHQS and inhibitors 1 and C1: 7dSh (1) as representative of C7‐deoxy sugars, and C1 for the class of carbaphosphonates ((1i) Arabidopsis thaliana, control; (1ii) 7dSh applied). B) Synthetic carbaphosphonates (C1−C3). DHQS is inhibited since β‐elimination is impaired. [19]
Remarkably, although 7dSh (1) was confirmed to be a competitive inhibitor, it shows bactericidal effects. [3] This is due to a fast and effective uptake of 7dSh (1) via promiscuous transporters, leading to intracellular enrichment and metabolic perturbations. [3] The shikimate pathway is indispensable for the synthesis of aromatic amino acids and for other aromatic metabolites in plants, lower eukaryotes, and bacteria, but it is absent in mammals. This makes 7dSh (1) and its analogues very attractive to us for a structure‐activity‐relationship (SAR) study of selected functional group variations of C7‐sugars in a knowledge‐based attempt towards a yet unknown putative herbicide class.
The shikimate pathway hosts seven enzymes, which convert erythrose 4‐phosphate (C4) and phosphoenolpyruvate (C3) via shikimate to chorismate (C7).[ 20 , 21 ] The second enzyme, DHQS, catalyzes the reaction of DAHP to 3‐dehydroquinate (DHQ; Figure 1A). This C−C bond‐forming reaction includes five cascade steps. Starting with the coenzyme NAD+‐dependent oxidation of the C‐5 hydroxyl group followed by β‐elimination of inorganic phosphate, a reduction at C‐5 reusing NADH precedes ring opening of the hemiketal and intramolecular aldol condensation to achieve the C7‐carbocycle (Scheme S1).[ 19 , 22 ] Cofactors are NAD+, and the divalent cations Zn2+ or Co2+. [16]
We designed a unique synthetic route towards the new C7‐carbon skeletons by combining the asymmetric multi‐step chemical total synthesis of pentoses (C5) and the distinct chemoenzymatic step to enable a fast and straightforward expansion of the family of new 7dSh herbicide compounds (Figure 2A). The transketolase (EC 2.2.1.1) for the stereospecific formation of the C7‐carbohydrates [7] was prepared as recombinant enzyme, derived from the Synechococcus elongatus genome and heterologously produced in Escherichia coli. [2]
Figure 2.
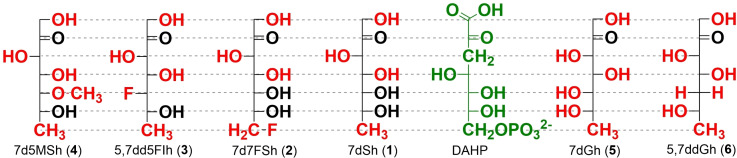
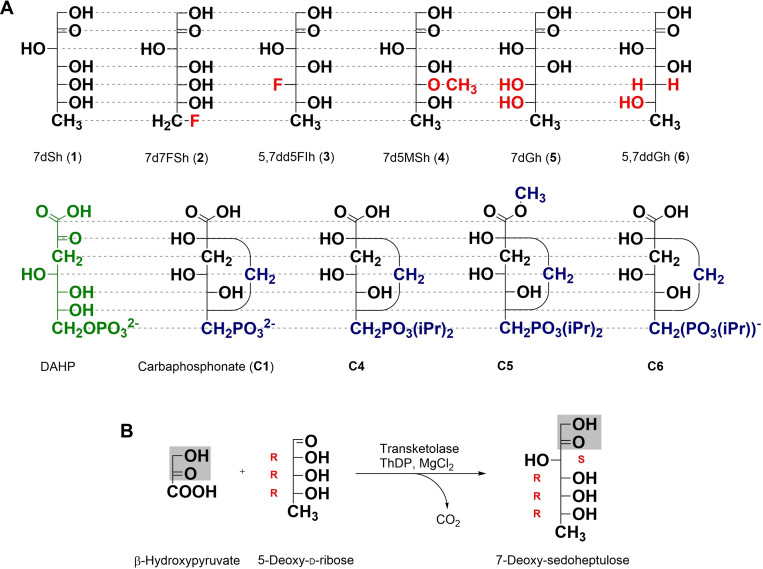
A) Structural comparison of DAHP, carbaphosphonates (C1, C4−C6), 7dSh (1) and C7‐sugar analogues (2–6). Compared to 7dSh (1), all structural differences are highlighted in red. Compared to the natural DHQS substrate DAHP (green), all structural differences are highlighted in blue. B) Chemoenzymatic reaction of β‐hydroxypyruvate with 5‐deoxy‐d‐ribose to 7‐deoxy‐sedoheptulose (7dSh, 1) with the enzyme transketolase.
The view of the structural architecture for the syntheses, first, focusses on the natural DHQS substrate DAHP which exists entirely as pyranose, as verified by our NMR analyses in D2O (Figures S94–95) and in acetic acid. [23] The same applies to its crystal structures in complex with DHQS. [19] As 7dSh (1) adopts three isomeric states (Figure S1) with only 21 % in the pyranosyl form in water, similarly to sedoheptulose, [24] we followed the SAR strategy of maintaining 7dSh analogues fixed in the pyranose architecture assuming this to be the active isomer of 7dSh (1). Based on the carbaphosphonate structures, our rationale for the synthesis of derivatives of this natural herbicide comprised three different approaches. First, alteration of the stereo configuration favoring the pyranose form. [25] Second, introduction of different residues at C‐5 to prevent furanose formation. Third, derivatization of the terminal 7‐methyl moiety to analyze the importance of this unique methyl‐group. Additionally, a comparison to a subset of carbaphosphonates was consequently performed.
Results
Hybrid chemical total and chemoenzymatic synthesis of versatile C7‐deoxysugars requires the C5‐aldehydes with the stereochemistry of interest. Starting from the readily available chiral pool of pentoses and hexoses, distinct proficient organic syntheses can efficiently yield the C5‐substrates for the final chemoenzymatic step. The two different synthetic strategies we used, comprised the following routes:
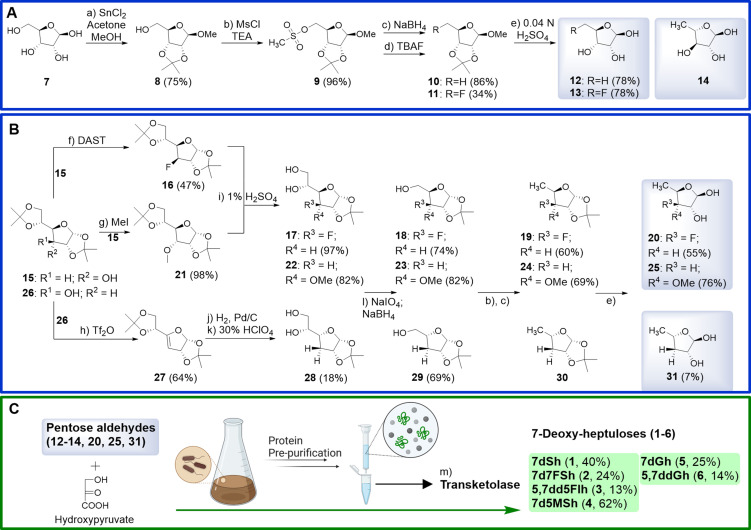
The first strategy (Scheme 1A, C) aimed to efficiently synthesize the natural product 7dSh (1) itself and the new fluorinated 7d7FSh (2) from their pentoses 5‐deoxy‐ribose (5dR, 12) and 5‐deoxy‐5‐fluoro‐ribose (13). Here, d‐ribose served as the starting carbohydrate. Protected as acetonide, the unique deoxy group of 5dR (12) was formed via methanesulfonate and sodium borohydride following the synthetic approach leading to the chemotherapeutic agent capecitabine.[ 26 , 27 , 28 ] In contrast, the pentose 13 received its fluorine group from TBAF (tetrabutylammonium fluoride), for targeted C‐7 alteration of 7d7FSh (2). [29] Final deprotection gave the deoxy sugars 12 and 13, both, ready to use for chemoenzymatic synthesis of 1 and 2.
Scheme 1.
Hybrid approach synthesis of the unique C7‐sugars (1–6) from synthetic pentoses (A, B) and with enzymatic transketolase C2‐coupling (C).
The second route (Scheme 1B, C) for the synthesis of the pyranosyl 6‐membered ring isomers of 7‐deoxy sugars derivatized in the C‐5‐position started from the double acetonide protected hexoses allose (15) or glucose (26) as reported in the literature.[ 30 , 31 , 32 ] Due to selective acetonide protection, the furanose‐C‐3 position could easily be modified. Finally, the transketolase reaction turned the C‐3 carbon position of the pentose into the corresponding C‐5 of the target heptuloses, which are then forced into their pyranose forms (Figure 2B). In the case of the fluorinated idoheptulose 5,7dd5FIh (3), the hydroxyl group had to be replaced with fluorine. Among the methods to exchange this group, we opted for direct addition of the reagent DAST. [33] To obtain the same stereochemistry of 7dSh (1), glucose seems to be the obvious educt, however, this reaction is known to yield dehydration instead of nucleophilic substitution, which would result in olefin 27. [34] Therefore, our synthesis started from protected allose (15) to yield the pentose substrate 20 with the opposite (S)‐configuration (20, 3‐deoxy‐3‐fluoro‐d‐xylose) for final transketolase transformation. The 7d5MSh (4) sugar congener should undergo pyranose formation only, due to methylation of its C‐5‐hydroxyl group and yet retaining the oxygen, e. g., for hydrogen bonding. Derived from classical synthesis with allose diacetonide 15, direct treatment with methyl iodide provided the methylated 3‐methoxy‐allose 21. [31] In contrast, 5,7ddGh (6) was synthesized from double acetonide protected glucose by insertion of triflate and immediate elimination with DBU, which gave olefin 27. Subsequent hydrogenation with H2, Pd/C led to the double acetonide protected 3‐deoxy‐gulose.[ 32 , 35 ] After replacing the hydroxyl group substituents at C‐3, a selective deprotection of the 5,6‐O‐isopropylidene group under mild conditions (1 % H2SO4, MeOH, 24 h) selectively gave access to free vicinal diols at C‐5/C‐6 (17, 22). Unfortunately, these conditions resulted in a complete deprotection of 27 to 3‐deoxy‐gulose. Use of 30 % perchloric acid at 0 °C solved this problem. [32] With the arranged hexoses 17, 22 and 28, the still indispensable pentose chemistry was initiated via oxidative cleavage of the vicinal diols with sodium periodate, and the following reduction of the aldehyde with sodium borohydride yielded the C5‐sugar series of intermediates 18, 23 and 29. [30] Introducing methanesulfonate and subsequent reduction with sodium borohydride generated the unique deoxy group, a characteristic attribute of the parent natural herbicide 7dSh (1). [26] In case of 3‐deoxy‐pentose 29, again, standard conditions (85 °C) for the reduction were unsuitable. Only harsher conditions (100 °C) successfully yielded the protected deoxy sugar. Facile full deprotection delivered all desired novel deoxy pentoses (20, 25, 31).
As a saccharide that favors the pyranose form, 7dGh (5) was directly synthesized through the terminal transketolase reaction with the commercially available 5‐deoxy‐l‐arabinose (14, Scheme 1A). 7dGh (5) and 5,7ddGh (6) are ideal candidates to prove the impact of a hydroxyl group with a converted stereoconfiguration or a missing hydroxyl group on the in vitro activity.
The transketolase from S. elongatus was heterologously expressed and purified from E. coli as described by Brilisauer et al. [2] and we now optimized the reaction conditions. [36] Chemoenzymatic synthesis efficiently generated all the 7‐deoxy‐heptuloses 1–6, confirming this approach of the hybrid synthesis as an efficient access to new heptuloses.
In vitro activity of 7dSh and carbaphosphonate derivatives
To evaluate the inhibitory potential of 7dSh (1) and derivatives (2–6), we analyzed their effect on dehydroquinate synthase of Arabidopsis thaliana (AtDHQS). Therefore, the DHQS gene of A. thaliana (AT5G66120) was cloned into an E. coli overexpression vector, and the protein was expressed and purified. The DHQS‐mediated conversion of DAHP into 3‐dehydroquinate yielded an apparent KM value of 2.4±0.3 μM and a vmax value of 170.4±6.1 μM (Figure S2). These values are in the same range as those reported for the only DHQS of plant origin known so far. [37]
In the prospect of employing them as future herbicides, we decided to evaluate the inhibitory potential of 7dSh and carbaphosphonate derivatives towards the plant AtDHQS by the determination of IC50‐values (Figure S3, Table 1) to prioritize particular compounds for herbicide development.
Table 1.
Overview of IC50 values of DHQS inhibitors.
|
Target DHQS isolated from |
Inhibitor |
IC50 [μM] |
|---|---|---|
|
Anabaena variabilis |
|
|
|
AvDHQS |
7dSh (1) |
23.3[a] |
|
Arabidopsis thaliana |
|
|
|
AtDHQS |
7dSh (1) |
143.5[a]/85.5[b] |
|
AtDHQS |
7d7FSh (2) |
200.4[a] |
|
AtDHQS |
5,7dd5FIh (3) |
250.1[a] |
|
AtDHQS |
7d5MSh (4) |
∞[a] |
|
AtDHQS |
7dGh (5) |
464.7[a] |
|
AtDHQS |
Sedoheptulose |
∞[a] |
|
AtDHQS |
Carbaphosphonate C1 |
0.5×10−3[a] |
|
AtDHQS |
C4 |
929.8[a] |
|
AtDHQS |
C5 |
147.6[a] |
|
AtDHQS |
C6 |
0.6×10−3[a] |
|
AtDHQS |
5,7ddGh (6) |
118.3[b] |
[a, b] Values of different series of experiments with two different preparations of the A. thaliana enzyme expressed in and purified from E. coli. ∞: no inhibition observed even with high concentration (1000 μM) of applied compound.
Only 7d5MSh (4) and sedoheptulose showed no inhibitory effect on AtDHQS. The other 7dSh and carbaphosphonate derivatives turned out to be inhibitors of the DHQS‐mediated reaction with IC50‐values ranging from low nM to high μM concentrations (Table 1). In order to utilize Anabaena variabilis as in vivo test system, we additionally tested 7dSh (1) against the DHQS from A. variabilis (AvDHQS). With an IC50‐value of 23.3 μM the inhibitory potential was even higher than against the Arabidopsis homolog (Table 1).
Inhibitory activity of 7dSh derivatives on cyanobacterial growth
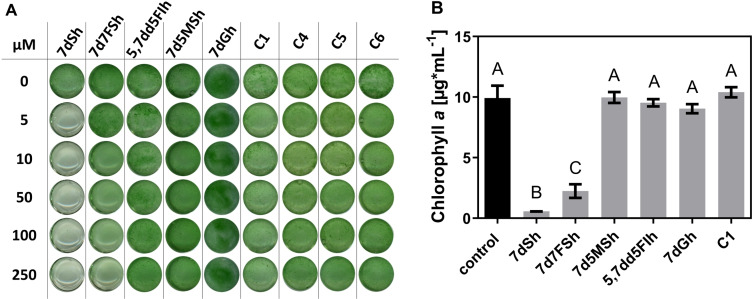
In the field of herbicide discovery, the first screenings are commonly conducted with easy cultivable phototrophic organisms in cell suspensions.[ 38 , 39 ] In our previous work we established a straightforward bioactivity‐guided assay to monitor the activity of 7dSh (1) using the 7dSh‐sensitive multicellular cyanobacterium A. variabilis as target. [2] Here we used this model system to study the in vivo activity of the 7dSh derivatives. A. variabilis was cultivated in the presence of different concentrations of the respective inhibitors (Figure 3A). After 4 days of cultivation only 7dSh (1) showed a significant effect on the growth of A. variabilis even at 5 μM. 7d7FSh (2) also inhibited the growth of A. variabilis, however, a first effect was only visible at a concentration of 100 μM. All other derivatives showed no bioactivity towards A. variabilis. To further quantify the inhibitory effect of the derivatives, the chlorophyll a content as indicator of the cell density was determined after four‐day long cultivation of A. variabilis in the presence of 250 μM of the respective compound (Figure 3B). The cell density was significantly reduced in 7dSh (1) and 7d7FSh (2) treated cells compared to the control but also compared to the other derivatives. However, the growth inhibitory effect of 7dSh (1) was stronger than that of 7d7FSh (2).
Figure 3.
Effects of compounds on the growth of A. variabilis. A) A. variabilis cultivated in the presence of respective compound. B) Cell density (expressed as Chl a) of A. variabilis cells treated with the indicated compound. Means that were significantly different (p‐value<0.05) are marked with different capital letters.
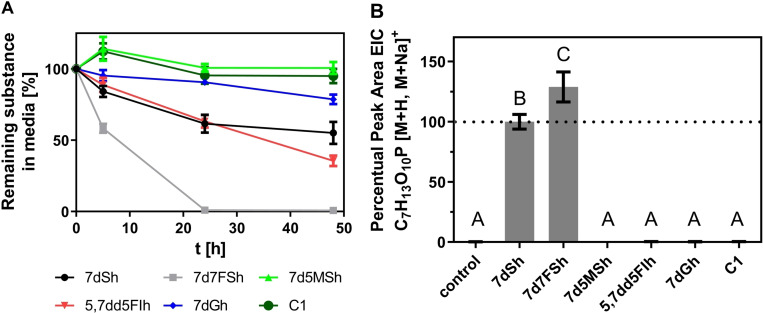
One known reason for the lack of in vivo activity of the carbocyclic inhibitors with greatest potency in vitro is their highly charged residue at C‐7 (Figure S4) which hinders penetration into the cells. [15] Therefore, uptake experiments with the derivatives were performed with A. variabilis. Figure 4A shows the percent amount of the initially applied substance in the supernatant of A. variabilis cultures over 48 h. After this time point only about 50 % of the initially applied 7dSh (1) could be detected in the supernatant by ESI‐MS. 5,7dd5FIh (3) showed a similar decrease. In the case of 7d5MSh (4) and carbaphosphonate C1 no changes in the amount of the compound were observed. Only a slight decrease of 7dGh (5) was detected. Interestingly, after 24 h none of the initially applied 7d7FSh (2) was retrieved in the supernatant.
Figure 4.
Effect of the indicated compounds on A. variabilis. A) Decrease of the indicated compounds in the supernatant of A. variabilis. 250 μM of the compounds were applied. B) Accumulation of DAHP in A. variabilis cells treated with 250 μM of the indicated compound. Significantly different means (p‐value<0.05) are marked with capital letters.
To further examine the in vivo activity of the derivatives, treated cells were examined for DAHP accumulation (Figure 4B). Previously, A. variabilis was shown to speedily and strongly accumulate DAHP when treated with 7dSh (1). [2] In contrast, only very small amounts of DAHP were found in the untreated control as well as in the cells treated with 5,7dd5FIh (3), 7dGh (5) and carbaphosphonate after 5 h of cultivation. While in 7d5MSh (4) treated cells no DAHP was found, significantly higher amounts of DAHP accumulated in 7dSh (1) and 7d7FSh (2) treated cells. Interestingly, the DAHP accumulation in 7d7FSh (2) was significantly elevated compared to 7dSh (1) treatment.
Inhibitory activity of 7dSh derivatives on plant growth
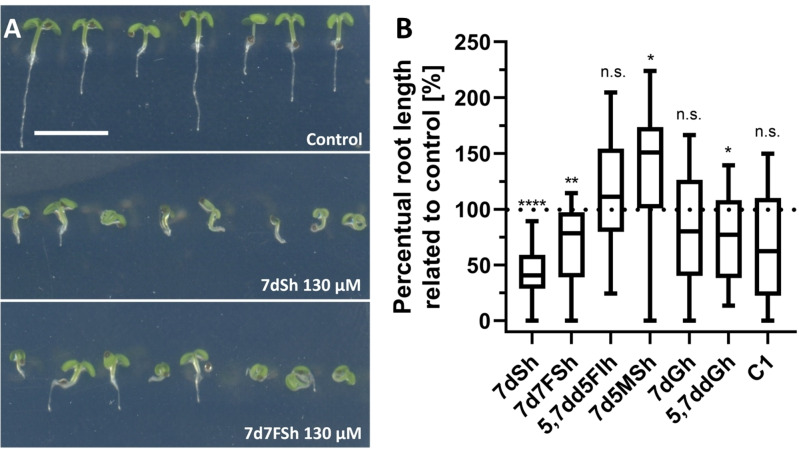
In order to answer the question whether DHQS inhibition correlates with herbicidal activity of 7dSh (1) and its derivatives, we tailored our assays to A. thaliana. After 7 days of germination, seedlings on untreated control plates formed distinct roots and green cotyledons, and exhibited regular gravitropism (Figure 5A). 7dSh (1), 7d7FSh (2) and 5,7ddGh (6) significantly affected the growth and morphology of the seedlings, apparent through shorter roots, smaller cotyledons and partially impaired gravitropism (Figure 5A, B). The effects of 7dSh (1) were comparable to previous studies. [2] In case of 5,7dd5FIh (3), 7dGh (5), and C1 no statistical significant differences between control and treatment were detected. Treatment with 7d5MSh (4) even led to enhanced root elongation.
Figure 5.
Effects of different compounds on A. thaliana seedling growth. A) Morphological appearance of A. thaliana seedlings 7 days after germination. Scale bar=5 mm. B) Box‐and‐whisker plots of root length 7 days after germination; relation to the control was analyzed in unpaired t‐tests (n≥18 seedlings; *p<0.05; **p<0.01; ****p<0.0001; n.s., not significant).
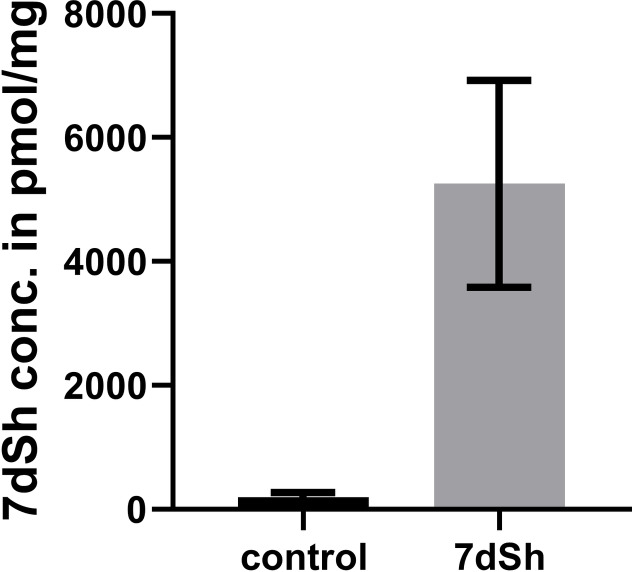
At this point the question arose if the 7dSh (1) concentration in planta is sufficient to block the shikimate pathway by DHQS inhibition within the plant cells. We developed a protocol to extract 7dSh (1) out of seedlings grown on medium with 100 μM 7dSh (1), and could show that washing the seedlings up to five times did not lead to a decrease of the measured 7dSh (1) in the extracts (Figure S5). Therefore, we could exclude contamination of residual, extracellular 7dSh (1). Further measurements revealed an intracellular accumulation of 7dSh (1) to more than 500 pmole/mg or 500 μmole/kg fresh seedlings (Figure 6).
Figure 6.
Intracellular accumulation of 7dSh (1). Shown is the mean concentration of 7dSh (1) in 20 d old Arabidopsis thaliana seedlings (dry weight) that were treated or not with 100 μM 7dSh (1) and afterwards, extensively washed (n=3; p<0.05). Significance between samples was calculated with a Wilcox‐Test (non‐parametric). Error bars: SD.
As mentioned above, 7d7FSh (2) was the only tested compound with significant herbicidal effects on Arabidopsis beside 7dSh (1, Figure 5), which raises the question if 7d7FSh (2) is taken up like 7dSh (1). When seedlings were germinated on media with 7d7FSh (2) they accumulated this compound to a degree similar to 7dSh (1). Furthermore, doubling the 7d7FSh (2) content in the medium did not increase its cellular content significantly (Figure S6) indicating maximal uptake and saturated cellular level of this compound in the seedlings.
Discussion
The here presented hybrid strategy of chemical total carbohydrate synthesis and chemoenzymatic approach to produce new C7‐sugars led to a tremendous reduction of synthetic complexity. The selection of the C7‐products was based on the inhibition mechanism in the DHQS enzyme pocket. The set of the six chemoenzymatically synthesized 7dSh‐inhibitors displays a comprehensive diversity. Applied assays are isolated DHQS from i) Anabaena as well as ii) Arabidopsis, iii) cellular transport, and iv) Arabidopsis plants in the green house for this structure‐activity relationship study. Our analysis of their in vivo and in planta activity sheds lights on the mechanism of action of potent shikimate pathway inhibitors. Additionally, we performed a careful comparison to the potent DHQS inhibitor class of the carbaphosphonates in terms of both in vivo and in vitro bioactivities. In combination with metabolite and uptake measurements the results allow us to draw the following conclusions with respect to a potential new herbicide class.
With an IC50‐value of 85.5 μM to 143.5 μM against isolated AtDHQS enzyme of A. thaliana 7dSh (1) is more potent towards the purified cyanobacterial enzyme AvDHQS (23.3 μM). [3] Nevertheless, both enzymes are inhibited by 7dSh (1) in a similar range.
Carbaphosphonate (C1) and three additional new analogues (C4−C6) were analyzed as promising DHQS inhibitors and herbicide candidates. Accordingly, some carbaphosphonates were found to inhibit DHQS at a low nanomolar range (Table 1), with the highly ionizable residues as well as the carbocyclic scaffold presumably being the reason for the superior in vitro activity. The lack of ionizable residues in carbaphosphonate analogues frequently results in a reduced in vitro activity, as evaluated by Chandran et al. [40] C4, compared to C1, is a isopropanol‐diester at the phosphonate group and shows a loss of activity with an IC50‐value of 929.8 μM (AtDHQS). We propose that the voluminous residues deplete polar interactions of the phosphonate group and reduce ionizable interactions in structure C4. Tian et al. postulated that carbaphosphonates with an ability to form a trianionic ionization state are more potent inhibitors. [15] Compared to C4, C5 has a methyl esterified carboxylic acid and improved in vitro activity (IC50=147.6 μM). Carbaphosphonate analogues with esterified carboxylic acids have not been biologically analyzed before. Notably, the activity improvement shown here for C5 argues against the trianionic ionization state hypothesis.
C6 as a carbaphosphonate isopropyl monoester showed an IC50‐value of 0.6 nM (AtDHQS). This improvement compared to C4 and C5 could be due to the smaller size of one isopropyl group or additional ionizable groups. Astonishingly, carbaphosphonate (C1) displayed a similar IC50‐value of 0.5 nM (AtDHQS), in the same range as previously reported.[ 9 , 14 , 16 ]
Although our studies confirmed the high potential of DHQS inhibition of particular carbaphosphonates, neither cyanobacterial nor plant growth could be inhibited by this class of compounds in accordance with the literature. [18] Additionally, neither decrease of C1 in the supernatant of cyanobacterial growth medium nor accumulation of DAHP in the cells could be observed. Altogether, these data indicate that uptake of carbaphosphonates seems to be blocked efficiently, which awaits to be confirmed experimentally. Nevertheless, the discrepancy between in vitro inhibition of DHQS by carbaphosphonates and their lack of plant growth inhibition still represents a major hurdle to the development of these compounds as potential herbicides.
The ability of 7dSh (1) to inhibit DHQS and to display herbicidal activity made this compound an interesting starting molecule for further structure related inhibition studies as well as for testing the herbicidal activity of its derivatives. In the present study evidence is provided that 7dSh (1) directly inhibits the plant DHQS, AtDHQS. Additionally, cellular uptake of this compound could be shown either indirectly by depletion in cyanobacterial growth medium (Figure 4A) or directly by its detection in plant extracts (Figure 6, Figure S5). Combined with the accumulation of DAHP (Figure 4B), these data confirm the previous findings that a fast and strong uptake leads to a strong accumulation, essential for the activity. [3] It is noteworthy in this regard, that the 7dSh (1) concentration in plant seedlings was over 500 μmole/kg and the IC50 of 7dSh (1) of AtDHQS was found in the range of 100 μmole/L. As plant tissue consists to 90 % of water, sufficient 7dSh (1) was found to accumulate in plant extracts to explain the herbicidal effect by intracellular DHQS inhibition.
With respect to 7dSh (1) derivatives, 7dGh (5) and 5,7ddGh (6) are structurally most different from DAHP and 7dSh (1) among the tested derivatives. Our choice to synthesize them, aimed to investigate the pyranose forms as well as the impact of the opposite stereo configuration at C‐5 (7dGh, 5) compared to a missing hydroxyl group (5,7ddGh, 6) at this position.
In the case of 7dGh (5) a small decrease of its content in the supernatant of treated cells could be measured, but neither DAHP accumulation nor inhibitory impact on cell growth was observed. Despite the significant structural differences of 7dGh (5) compared to compound 1, we surprisingly measured an in vitro activity of 464.7 μM. Conformational changes from the 5C2 conformation to the 2C5 conformation (Scheme S2) could explain the measured activity, because the hydroxyl groups at C‐4 and C‐5 might be able to interact with the Zn2+ ion. Furthermore, the 2C5 conformation is preferred in aqueous solution due to the more favored ratio of axial and equatorial residues, proven by chemical NMR analysis with 3 J 5,6‐NMR coupling constants of 9.6 Hz.
Comparison of the relative activity of 7dGh (5) and 5,7ddGh (6), of ≈0.3 (143.5 : 464.7, IC50) and ≈0.7 (85.5 : 118.3, IC50), respectively, in relation to 7dSh (1) indicated that the opposite stereo chemistry at C‐5 has an adverse impact on the inhibitory activity. Diametral spatial charge distribution or insufficient space within the active pocket (as for 7d5MSh, 4) could be responsible for the lower activity of 7dGh (5). In accordance with the good in vitro activity of 5,7ddGh (6), a decreased root length could be detected.
5,7dd5FIh (3) lacks the C‐5 hydroxyl group compared to 7dSh (1), and features fluorine with opposite stereo configuration instead. This prevents formation of the furanose form. Notably, besides the enforced pyranose structure, the deoxy‐idose 3 shows an open chain constitution with a ratio of 20 %. The compound has an in vitro activity of 250.1 μM, remarkable for further SAR‐studies. This inhibition caused by heptulose 3 is in contradiction with the tremendous loss in activity described for carbaphosphonates lacking the hydroxyl group at C‐5.[ 9 , 13 ] Here, the lack of the 5‐OH‐group in compound 3 resulted in only a minor decrease in activity, i. e., 0.6‐fold. We learn that these two aspects, the amount of pyranose form and the presence of the hydroxyl group at C‐5, are equal antagonists. Nonetheless, it's not clear whether the fluorine atom takes part in the stabilization of the compound within the active pocket. Furthermore, choosing a distinct new substituent could outclass the original hydroxyl group. Despite the significant in vitro inhibition of AtDHQS and its depletion in cyanobacterial growth medium, 5,7dd5FIh (3) treatment did neither result in DAHP accumulation nor in growth inhibition in A. variabilis. This contradiction could originate from the fact that the import of compound 3, DAHP accumulation and growth effects were studied in A. variabilis, and substrate specificity of AvDHQS may differ from that of AtDHQS. Instead, the lack of inhibitory activity in planta could originate from poor uptake of the compound through the roots or transport across the plants. Also, enhanced intracellular inactivation of 5,7dd5FIh (3) should be considered.
Evaluation of the inhibitory activities of the synthesized and tested 7dSh derivatives pinpoints cellular uptake as crucial, but it does not explain all observed phenomena. 7d5MSh (4) with C‐5‐methoxide support that both compounds in their full pyranose conformation should exhibit the same known interactions with DHQS. The obtained in vitro data showed no activity for compound 4 in our assays and its concentration in the supernatant of Anabaena cultures did not change over time. Therefore we conclude that import through the fructose transporter of A. variabilis is inhibited due to the methoxy group. It is noteworthy, that this compound even stimulated Arabidopsis seedling root growth (Figure 5B). It is well documented, that herbicides like glyphosate in sublethal concentrations can affect plant growth in a positive way (for a summary see Jalal et al.). [41] Such a hormesis effect may also explain the root growth stimulation by compound 4, which will be investigated in the future.
7d7FSh (2) revealed to be the most promising candidate among all tested 7dSh derivatives. As most similar 7dSh‐analogue with its C‐7‐fluorine group, 2 shows nearly the same isomeric ratio (Figure S1), and its IC50‐value of 200.4 μM is close to that of 7dSh (1). Additionally, it inhibited growth of A. variabilis and A. thaliana in a comparable range to 7dSh (1). Most interestingly, import of 7d7FSh (2) in cyanobacterial cells was the best among all tested compounds. Its import into Arabidopsis seedlings was in a comparable range to 7dSh (1), whereupon addition of 130 μM led to a saturation of intracellular 7d7FSh (2) accumulation (Figure S6). It is tempting to speculate, if this limitation is caused by transport or by chemical transformation processes. Nevertheless, the discovery of a 7dSh derivative with comparable activity properties to its origin represents a good starting point for further characterization and development of this novel herbicide by derivatization: On the one hand such derivatives can be applied for the characterization of the molecular mode of action. On the other hand they can be useful to develop variants with enhanced or modified properties e. g. in regard of import, enzyme inhibition or accumulation. Although all these processes await to be analyzed for 7dSh (1), it is a very promising candidate to substitute glyphosate and will be intensively investigated in the future.
Conclusion
In this comprehensive study we developed a new hybrid synthesis strategy combining chemical total and chemoenzymatic synthesis to obtain distinct C7‐sugars. This straightforward approach enabled us to expand the novel compound family of 7dSh analogues, a potential herbicide class which demands a chemical structure‐activity relationship study with careful transport and plant assays. The recombinant transketolase from S. elongatus was used as robust catalyst, and especially stereoselective sugar synthesis benefits from chemoenzymatic strategies. [42] Thus, five new 7dSh analogues (2–6) originated from this approach, whereby four turned out to be in vitro inhibitors of the A. thaliana DHQS in a micromolar range. Modification of the C‐5 residue confirmed that the amount of pyranosyl form of the inhibitor and the presence of the hydroxyl group at C‐5 with the correct stereo configuration are equal antagonists in this SAR study. Future studies might truly disprove that the proposed C‐5 hydroxyl group is essential for strong inhibition. Comparison of in vitro enzyme inhibition with in vivo growth inhibition studies revealed 7d7FSh (2) as the only in vivo‐active synthetic derivative highlighting the important differences between in vitro and in vivo data. Our analysis further shows that the uptake of compounds may be a major obstacle to herbicidal activity. The in vivo activity towards A. variabilis was comparable to that observed in planta, once again implying the uptake as of major relevance. Nonetheless, further uptake studies are needed, which have to be corroborated by additional intracellular inactivation analyses, to confirm the crucial role of import in the in vivo activity of 7dSh derivatives. Furthermore, we analyzed three new carbaphosphonate derivatives, which mirror the in vitro‐only activity in the range of carbaphosphonate C1 and 7dSh (1). As numerous states or individual cities start to ban the worldwide successfully applied, but controversial, herbicide glyphosate, we feel that our completely new concept of C7‐sugars with herbicidal activity needs to be further explored.
Conflict of interest
Eberhard Karls University Tübingen holds a patent (WO 2019/101937 A2/A3) covering the compound 7dSh (1) and derivatives.
1.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
P.R., J.R., K.B., K.F. and S.G. were supported by the “Glycobiotechnology” initiative (Ministry for Science, Research and Arts Baden‐Württemberg) and the RTG 1708 “Molecular principles of bacterial survival strategies”. M.B. and Ü.K. were supported by the Federal Ministry of Education and Research within the joint research project “7dSherbizid” (03VP09361). K.B. received a grant from the Institutional Strategy of the University of Tübingen (Deutsche Forschungsgemeinschaft, ZUK 63). The work was further supported by infrastructural funding from the DFG Cluster of Excellence EXC 2124 Controlling Microbes to Fight Infections (CMFI), ID 390838134. We especially thank the BASF SE company for valuable discussions and providing carbaphosphonates C1 and C4−C6. Also, we thank Michaela Schuppe for the seedling growth test support and Libera Lo Presto for critical reading of the article. Open Access funding enabled and organized by Project DEAL. Open Access funding enabled and organized by Projekt DEAL.
P. Rath, J. Rapp, K. Brilisauer, M. Braun, Ü. Kolukisaoglu, K. Forchhammer, S. Grond, ChemBioChem 2022, 23, e202200241.
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
References
- 1. Cole P. D., Zebala J. A., Kamen B. A., Drug Discovery Today Ther. Strategies 2005, 2, 337–342. [Google Scholar]
- 2. Brilisauer K., Rapp J., Rath P., Schöllhorn A., Bleul L., Weiß E., Stahl M., Grond S., Forchhammer K., Nat. Commun. 2019, 10, 545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rapp J., Wagner B., Brilisauer K., Forchhammer K., Front. Microbiol. 2021, 12, 692986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schweizer M., Brilisauer K., Triebskorn R., Forchhammer K., Kohler H. R., PeerJ 2019, 7, e7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jacobsen A., Thiem J., Curr. Org. Chem. 2014, 18, 2833–2841. [Google Scholar]
- 6. Waschke D., Thimm J., Thiem J., Org. Lett. 2011, 13, 3628–3631. [DOI] [PubMed] [Google Scholar]
- 7. Turner N. J., Curr. Opin. Biotechnol. 2000, 11, 527–531. [DOI] [PubMed] [Google Scholar]
- 8. Charmantray F., Helaine V., Legeret B., Hecquet L., J. Mol. Catal. B 2009, 57, 6–9. [Google Scholar]
- 9. Montchamp J. L., Piehler L. T., Tolbert T. J., Frost J. W., Bioorg. Med. Chem. Lett. 1992, 2, 1349–1352. [Google Scholar]
- 10. Lemarechal P., Froussios C., Level M., Azerad R., Biochem. Biophys. Res. Commun. 1980, 92, 1104–1109. [DOI] [PubMed] [Google Scholar]
- 11. Myrvold S., Reimer L. M., Pompliano D. L., Frost J. W., J. Am. Chem. Soc. 1989, 111, 1861–1866. [Google Scholar]
- 12. Bender S. L., Widlanski T., Knowles J. R., Biochemistry 1989, 28, 7560–7572. [DOI] [PubMed] [Google Scholar]
- 13. Montchamp J. L., Piehler L. T., Tolbert T. J., Frost J. W., Bioorg. Med. Chem. Lett. 1993, 3, 1403–1408. [Google Scholar]
- 14. Montchamp J. L., Peng J. R., Frost J. W., J. Org. Chem. 1994, 59, 6999–7007. [Google Scholar]
- 15. Tian F., Montchamp J. L., Frost J. W., J. Org. Chem. 1996, 61, 7373–7381. [DOI] [PubMed] [Google Scholar]
- 16. Montchamp J. L., Frost J. W., J. Am. Chem. Soc. 1997, 119, 7645–7653. [Google Scholar]
- 17. Widlanski T., Bender S. L., Knowles J. R., J. Am. Chem. Soc. 1989, 111, 2299–2300. [Google Scholar]
- 18. Montchamp J. L., Piehler L. T., Frost J. W., J. Am. Chem. Soc. 1992, 114, 4453–4459. [Google Scholar]
- 19. Carpenter E. P., Hawkins A. R., Frost J. W., Brown K. A., Nature 1998, 394, 299–302. [DOI] [PubMed] [Google Scholar]
- 20. Herrmann K. M., Weaver L. M., Annu. Rev. Plant Physiol. 1999, 50, 473–503. [DOI] [PubMed] [Google Scholar]
- 21. Nikolaides N., Ganem B., Tetrahedron Lett. 1989, 30, 1461–1464. [Google Scholar]
- 22. Veraszto H. A., Logotheti M., Albrecht R., Leitner A., Zhu H. B., Hartmann M. D., Nat. Chem. Biol. 2020, 16, 973–978. [DOI] [PubMed] [Google Scholar]
- 23. Garner C. C., Herrmann K. M., Carbohydr. Res. 1984, 132, 317–322. [DOI] [PubMed] [Google Scholar]
- 24. Ceusters J., Godts C., Peshev D., Vergauwen R., Dyubankova N., Lescrinier E., De Proft M. P., Van den Ende W., J. Exp. Bot. 2013, 64, 1497–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.P. M. Collins, Dictionary of Carbohydrates, 2 ed., Routledge, 2006.
- 26. Sairam P., Puranik R., Sreenivasa Rao B., Veerabhadra Swamy P., Chandra S., Carbohydr. Res. 2003, 338, 303–306. [DOI] [PubMed] [Google Scholar]
- 27.R. J. Herr, in Modern Drug Synthesis, Wiley, 2010, pp. 57–71.
- 28. Mekala N., Moturu M. V. R. K., Dammalapati R. V. L. N., Parimit A. R., Org. Process Res. Dev. 2016, 20, 609–614. [Google Scholar]
- 29. Downey A. M., Pohl R., Roithova J., Hocek M., Chem. Eur. J. 2017, 23, 3910–3917. [DOI] [PubMed] [Google Scholar]
- 30. Liu Z. L., Jenkinson S. F., Vermaas T., Adachi I., Wormald M. R., Hata Y., Kurashima Y., Kaji A., Yu C. Y., Kato A., Fleet G. W. J., J. Org. Chem. 2015, 80, 4244–4258. [DOI] [PubMed] [Google Scholar]
- 31. Sharma G. V. M., Yadav T. A., Choudhary M., Kunwar A. C., J. Org. Chem. 2012, 77, 6834–6848. [DOI] [PubMed] [Google Scholar]
- 32. Pawar V. U., Ghosh S., Chopade B. A., Shinde V. S., Bioorg. Med. Chem. Lett. 2010, 20, 7243–7245. [DOI] [PubMed] [Google Scholar]
- 33. Haga M., Takano M., Tejima S., Carbohydr. Res. 1972, 21, 440–446. [Google Scholar]
- 34. Foster A. B., Hems R., Webber J. M., Carbohydr. Res. 1967, 5, 292–301. [Google Scholar]
- 35. Hussain N., Tatina M. B., Mukherjee D., Org. Biomol. Chem. 2018, 16, 2666–2677. [DOI] [PubMed] [Google Scholar]
- 36. Rapp J., Rath P., Kilian J., Brilisauer K., Grond S., Forchhammer K., J. Biol. Chem. 2021, 296, 100621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mittelstadt G., Negron L., Schofield L. R., Marsh K., Parker E. J., Arch. Biochem. Biophys. 2013, 537, 185–191. [DOI] [PubMed] [Google Scholar]
- 38. Grossmann K., Berghaus R., Retzlaff G., Pestic. Sci. 1992, 35, 283–289. [Google Scholar]
- 39. Grossmann K., Pest Manage. Sci. 2005, 61, 423–431. [DOI] [PubMed] [Google Scholar]
- 40. Chandran S. S., Frost J. W., Bioorg. Med. Chem. Lett. 2001, 11, 1493–1496. [DOI] [PubMed] [Google Scholar]
- 41. Jalal A., de Oliveira J. C., Ribeiro J. S., Fernandes G. C., Mariano G. G., Trindade V. D. R., dos Reis A. R., Ecotoxicol. Environ. Saf. 2021, 207. [DOI] [PubMed] [Google Scholar]
- 42. Kalera K., Stothard A. I., Woodruff P. J., Swarts B. M., Chem. Commun. 2020, 56, 11528–11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.