Abstract
After more than 20 years of trials, a practical scalable approach to fluoro‐substituted bicyclo[1.1.1]pentanes (F‐BCPs) has been developed. The physicochemical properties of the F‐BCPs have been studied, and the core was incorporated into the structure of the anti‐inflammatory drug Flurbiprofen in place of the fluorophenyl ring.
Keywords: Bicyclo[1.1.0]butane, Bicyclo[1.1.1]pentane, Bioisosteres, Fluorine
After more than 20 years of trials, a practical scalable approach to fluoro‐substituted bicyclo[1.1.1]pentanes (F‐BCPs) has been developed. The physicochemical properties of the F‐BCPs have been studied, and the core was incorporated into the structure of the anti‐inflammatory drug Flurbiprofen in place of the fluorophenyl ring.
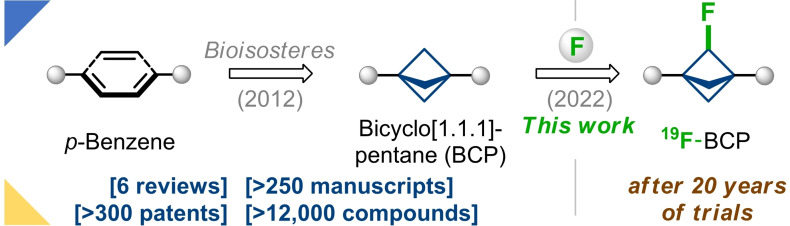
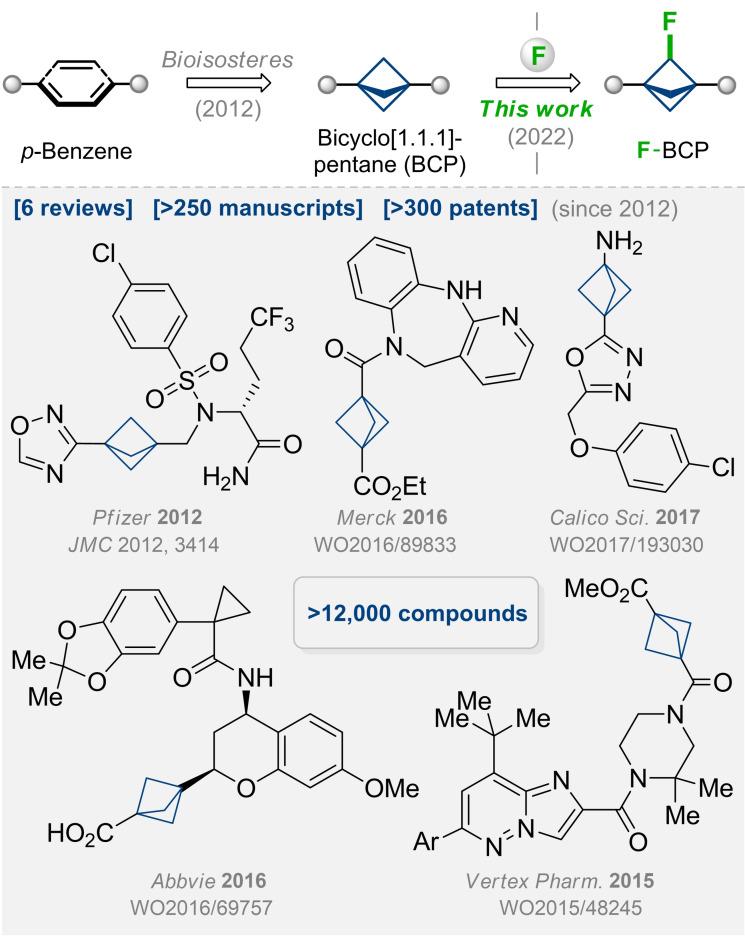
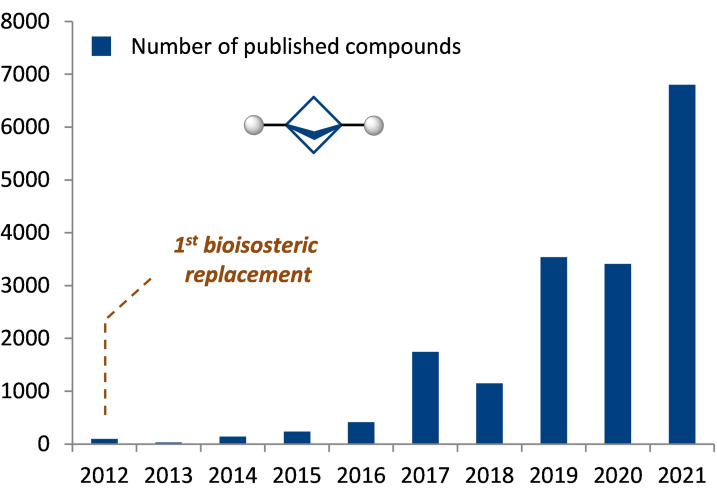
The phenyl ring is one of the most popular structural motifs in natural compounds and synthetic drugs. [1] Ten years ago, Stepan and co‐workers replaced the phenyl ring in a γ‐secretase inhibitor with the bicyclo[1.1.1]pentyl (BCP) skeleton. [2] The obtained analogue showed higher activity and improved physicochemical properties—that was a starting point for the sunrise of bicyclo[1.1.1]pentanes in various aspects of chemistry: from medicinal chemistry to supramolecular chemistry. Today, bicyclo[1.1.1]pentanes are highly popular in academic and industrial research.[ 3 , 4 , 5 ] In fact, at least 6 reviews,[ 6 , 7 ] 250 research manuscripts, 300 patents, and 12 000 BCP‐containing compounds appeared during the past decade (Figure 1). [8] Moreover, the number of BCP‐containing molecules is growing dramatically every year (Figure 2).
Figure 1.
Bicyclo[1.1.1]pentanes (BCPs): state‐of‐the‐art. Aim of this work: fluoro‐bicyclo[1.1.1]pentanes (F‐BCPs).
Figure 2.
Number of bicyclo[1.1.1]pentanes (BCPs) published every year.
Chemists often incorporate a fluorine atom into organic molecules to fine‐tune their physicochemical properties, [9] adjust the acidity/basicity of the neighboring functional groups, [10] and control the conformation. [11] Given the high popularity of bicyclo[1.1.1]pentanes, it is not surprising that chemists also tried to selectively decorate them with a fluorine atom. Indeed, previously, synthetic approaches to the corresponding bridgehead‐,[ 12 , 13 ] gem‐ [14] and polyfluorinated [15] derivatives had appeared, and these molecules possess significant value for medicinal chemistry purposes.[ 14a , 14b , 14c ] At the same time, bicyclo[1.1.1]pentanes with a single fluorine atom in the bridge position have remained elusive to this day.
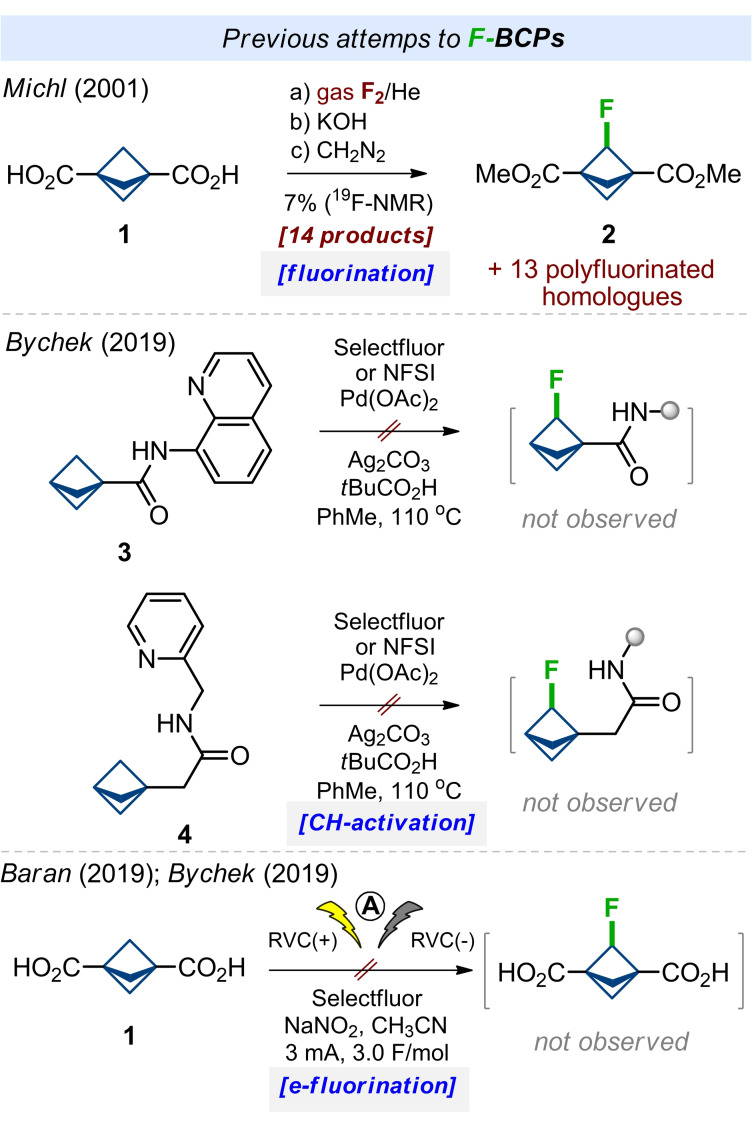
Previously, several attempts to synthesize monofluorinated bicyclo[1.1.1]pentanes (19F‐BCPs) were reported in the literature. In 2001, Michl and colleagues performed fluorination of diacid 1 by a mixture of fluorine gas with helium. Saponification and the subsequent esterification with diazomethane gave a mixture of 14 isomeric/homologous polyfluorinated compounds (Scheme 1). The needed diester 2 was present in 7 % according to 19F NMR. It was isolated by a silica gel column and additional preparative GC. [15b] In 2019, our group also tried to synthesize 19F‐BCPs by directed‐group mediated CH‐activation of amides 3 and 4. In both cases, the formation of the needed products was not observed. [14b] In 2019, Baran and colleagues developed a practical electrochemical C(sp3)‐H fluorination. [16] Being involved in the project at that time, we also tried to achieve electrochemical fluorination of diacid 1. [14b] Again, the formation of the needed product was not detected.
Scheme 1.
Previous attempts to fluoro‐bicyclo[1.1.1]pentanes (19F‐BCPs).
In this work, we finally solved this long‐standing problem and developed a practical scalable approach to fluoro‐bicyclo[1.1.1]pentanes (19F‐BCPs).
After unsuccessful attempts to access 19F‐BCPs by either protecting‐group directed CH‐activation of BCP‐scaffold or its electrochemical fluorination (Scheme 1), we decided to challenge a strain‐release addition of carbenes to bicyclo[1.1.0]butanes. [17]
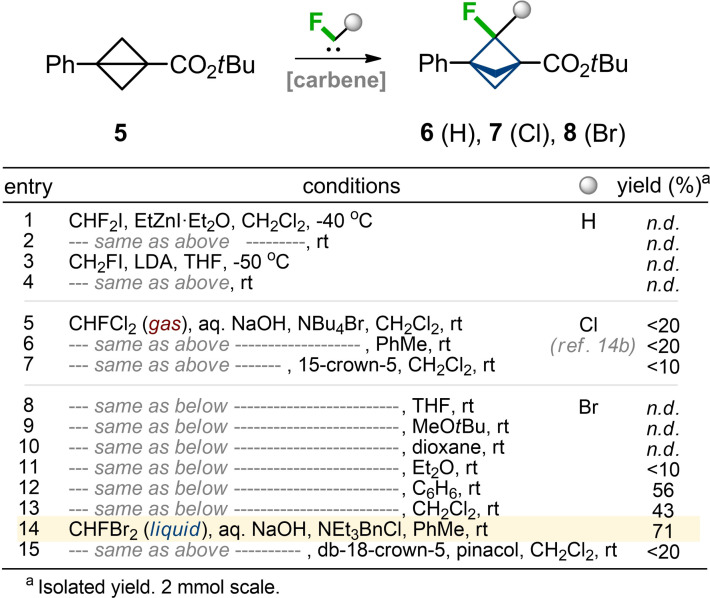
The direct addition of fluorocarbene (:CHF) to bicyclo[1.1.0]butanes is unknown. However, previously practical protocols for the addition of fluorocarbene to activated alkenes were developed.[ 18 , 19 ] These involved the use of freons CHF2I [18] and CH2FI. [19] We tried both literature protocols on the model substrate 5, but the formation of the needed product 6 was not observed (Table 1, entries 1–4). Next, we tried an addition of chlorofluorocarbene (:CClF). Previously, we demonstrated one example of such a reaction between gaseous CHFCl2 and methyl analog of compound 5 in a low yield. [14b] We did observe the formation of the needed product 7, but despite all efforts, we could not increase the reaction yield significantly (entries 5–7). The addition of bromofluorocarbene (:CBrF) to bicyclo[1.1.0]butanes was unknown in the literature, but we also tried it. First, we did not observe the formation of the product (entries 8–10), but screening of solvents and phase‐transfer catalysts (entries 8–15) allowed us to identify the suitable conditions for an efficient process (entry 14). The reaction of substrate 5 with commercially available CHFBr2 and aq. NaOH in the presence of NEt3BnCl in toluene at room temperature gave the needed product 8 in 71 % yield. Formation of the undesired 1,4‐diene and its cyclopropanated derivatives was also observed, but we could isolate the pure product 8 by column chromatography. From the practical standpoint, the protocol was convenient, because CHFBr2 is a liquid at room temperature (b.p. 65 °C), in contrast to the previously attempted gaseous freons (CHF2I, CHFCl2).
Table 1.
Optimization of synthesis of F‐BCP 8.
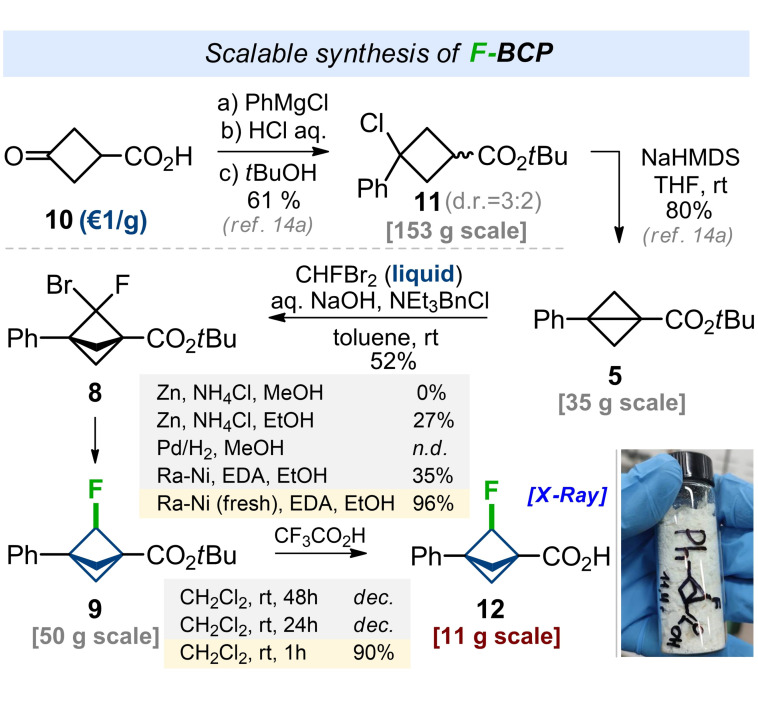
Having an optimized procedure in hand, we studied its scalability. The synthesis commenced from the commercially available ketoacid 10 (ca. 1 €/g, Scheme 2). Chloride 11 was obtained from acid 10 in three steps following the literature protocol. [14a] Cyclization of 11 was performed with NaHMDS in THF at room temperature to provide bicyclo[1.1.0]pentane 5 in 80 % yield. The scaled‐up reaction of 5 with CHFBr2 under the previously developed conditions gave bicyclo[1.1.1]pentane 8 in a lower yield of 52 %. However, 21 g of product 8 was obtained in one run. Cleavage of C−Br bond was developed next (Scheme 2). Reduction with either Zn/NH4Cl or Pd/H2 gave only traces of the product. However, the reaction of bromide 9 with the freshly prepared Raney nickel in the presence of ethylenediamine (EDA) in ethanol smoothly afforded the desired monofluoro‐bicyclo[1.1.1]pentane 9 in 96 % yield. Finally, cleavage of the tert‐butyl ester was performed with a catalytic amount of trifluoroacetic acid in dichloromethane at room temperature for one hour. The needed acid 12 was obtained as a crystalline solid in 90 % yield in an 11 g amount. The structure of product 12 was confirmed by X‐ray analysis. [20]
Scheme 2.
Scalable synthesis of F‐BCP carboxylic acid 12.
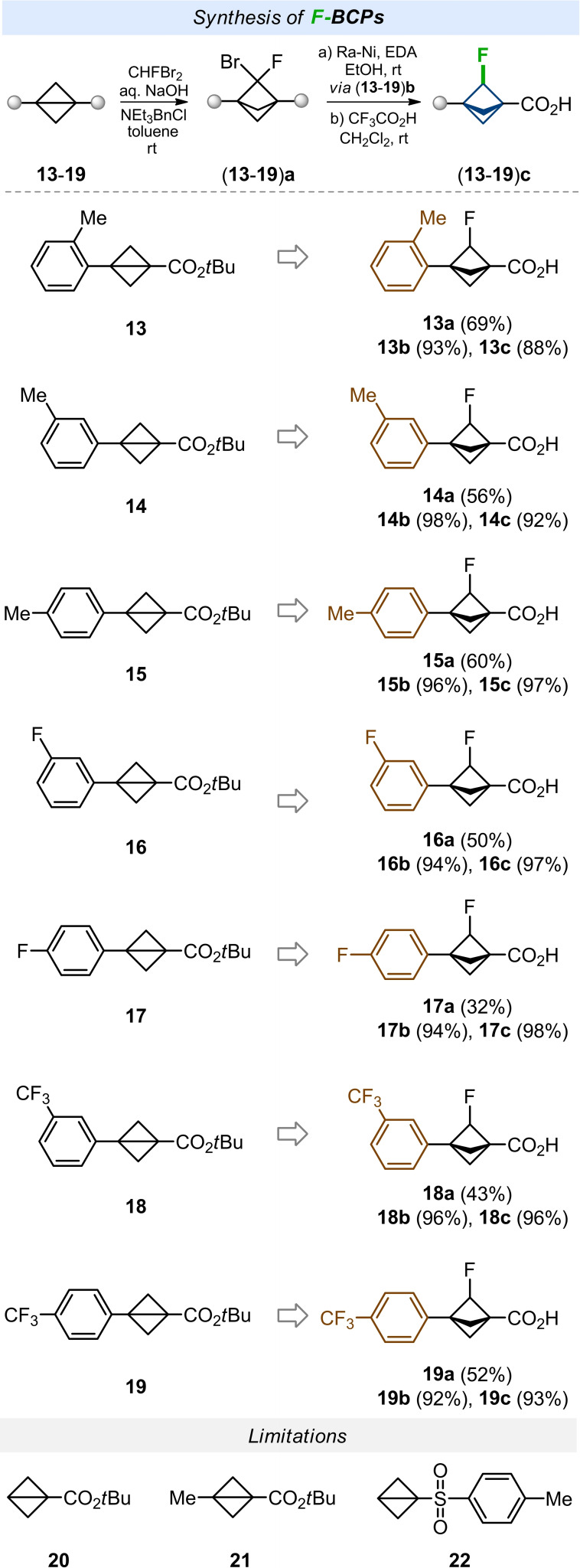
Next, we studied the generality of the developed protocol. First, we synthesized representative starting compounds bearing simple methyl (13–15), fluorine (16, 17), and trifluoromethyl (18, 19) substituents on the phenyl ring (Scheme 3). All bicyclo[1.1.0]butanes were prepared from ketoacid 10 analogously to the initial substrate 5 (Scheme 2, see Supporting Information). Bicyclo[1.1.0]butanes 13–15 with methyl groups reacted with CHFBr2 in 56–69 % yield. Interestingly to note, the ortho‐substituted substrate 13 gave the highest yield of 69 %. Fluorine‐substituted substrates 16, 17 gave the corresponding derivatives 16 a and 17 a in 50 % and 32 % yield, correspondingly. Trifluoromethyl‐substituted bicyclo[1.1.0]butanes 18, 19 gave products 18 a and 19 a in 43 % and 52 % yield. All compounds 13 a–19 a were next converted in two steps (via esters 13 b‐‐19 b) into the desired acids 13 c–19 c in high yields. Most of the final acids were obtained on a gram scale.
Scheme 3.
Synthesis of various F‐BCPs.
The developed approach was not without limitations, however. Substrates 20–22 having no phenyl substituents failed to react with CHFBr2 (Scheme 3).
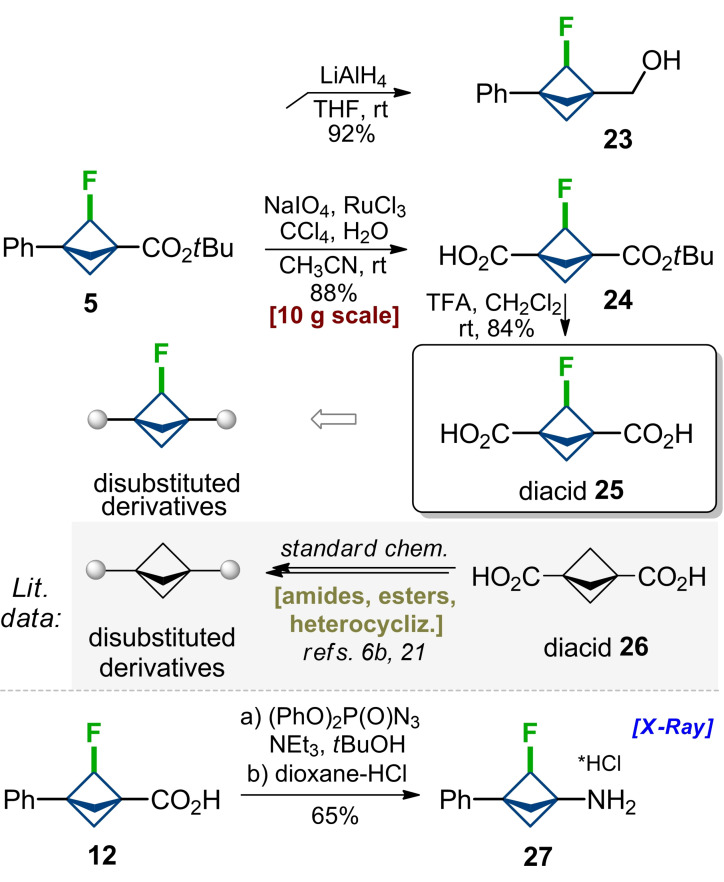
Some key modifications of fluoro‐bicyclo[1.1.1]pentanes 5 and 12 were undertaken to show their high synthetic value (Scheme 4). Reduction of the ester group with LiAlH4 in tetrahydrofuran at room temperature gave alcohol 23 in 92 % yield. Reduction of C−F bond was not observed. Oxidation of the phenyl ring in 5 with NaIO4/RuCl3 (cat.) in acetonitrile‐water‐tetrachloromethane mixture gave monoacid 24 in 88 % yield. The product was obtained in 10 g amount in one run. Cleavage of the tert‐butyl ester with a catalytic amount of trifluoracetic acid in dichloromethane at room temperature gave diacid 25 in 84 % yield. Both compounds 24 and 25 open a way to synthesize various mono‐ and bifunctional derivatives fluoro‐bicyclo[1.1.1]pentanes by standard stepwise modifications of carboxylic groups: synthesis of amides, esters, heterocyclizations etc. This approach, for example, is commonly used to prepare bis‐substituted derivatives of bicyclo[1.1.1]pentanes from diacid 26 and its monoesters (Scheme 4).[ 6b , 21 ] Curtius reaction of acid 12 with (PhO)2P(O)N3/NEt3 in tert‐butanol gave, after acidic cleavage of N‐Boc group in the intermediate, the final fluoroamine 27 as a hydrochloride salt in 65 % yield. The structure of amine 27 was confirmed by X‐ray analysis. [20]
Scheme 4.
Chemical modifications of F‐BCPs.
All acids 12, 13 c–19 c, 25, alcohol 23, and amine 27 were obtained as crystalline solids. We stored them at room temperature in closed vials on the shelf and did not observe any detectable decomposition according to 1H NMR for at least three months.
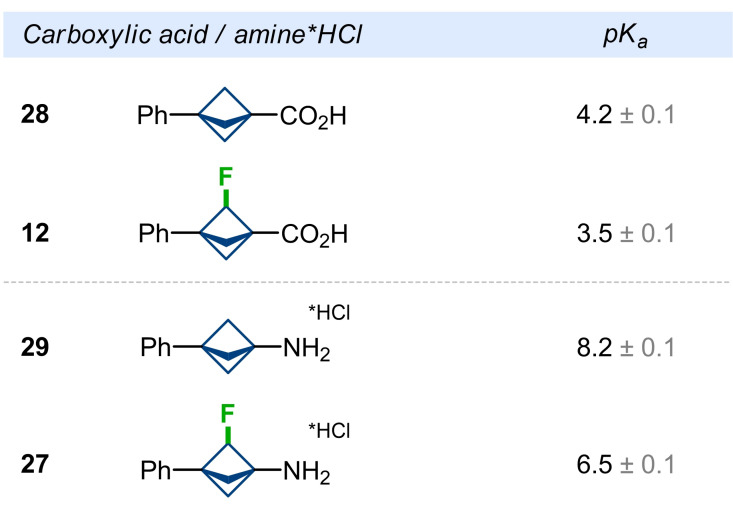
The incorporation of a fluorine atom into organic compounds could dramatically alter the acidity/basicity of the neighboring functional groups. [10] Therefore, to study the influence of the fluorine atom on the electronic properties of the bicyclo[1.1.1]pentane skeleton, we experimentally measured pK a values of acids 28 and 12 and amine hydrochlorides 29 and 27 (Figure 3). Bridge‐fluorination of acid 28 significantly increased its acidity from pK a (28)=4.2 to pK a (12)=3.5. On the other hand, bridge‐fluorination of amine 29 reduced its basicity even more ‐ by more than one order of magnitude: pK a (29*HCl)=8.2 vs. pK a (27*HCl)=6.5. Because basic nitrogen atoms could cause toxicity of bioactive compounds, [22] the incorporation of a fluorine atom at BCP‐containing amines could be a solution here.
Figure 3.
Experimental pK a values of F‐BCP carboxylic acids and amines.
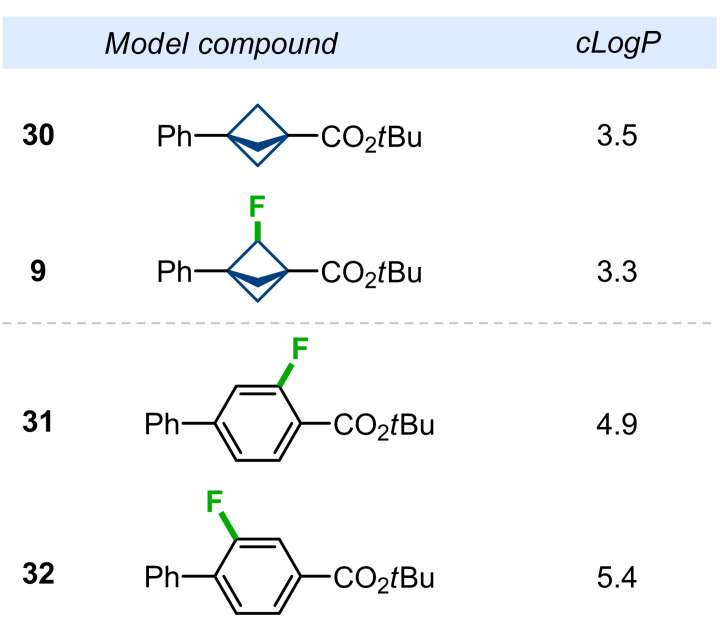
Fluorination is known also to affect the physicochemical properties of organic compounds, such as lipophilicity. [9] Therefore, we next calculated lipophilicity as measured by logP index of model BCP‐compounds 9, 30, and isomeric aromatic compounds 31, 32 (Figure 4). [23] Incorporation of a fluorine atom into the bicyclo[1.1.1]pentane slightly decreased lipophilicity, clogP: 3.5 (30) vs. 3.3 (9) (Figure 4). On the other hand, the lipophilicity of F‐BCP scaffold was almost 2 orders of magnitude lower than that of F−Ph, clogP: 3.3 (9) vs 4.9 (31) vs 5.4 (32).
Figure 4.
Calculated lipophilicity (clogP) for compounds 9, 30–32.
In a short summary, bridge‐fluorination of bicyclo[1.1.1]‐pentane seems to slightly decrease its lipophilicity (clogP). While the replacement of the fluorophenyl ring (31, 32) with fluoro‐bicyclo[1.1.1]pentane (9) dramatically reduced lipophilicity (clogP) by almost two orders of magnitude, which might be beneficially used in medicinal chemistry programs.
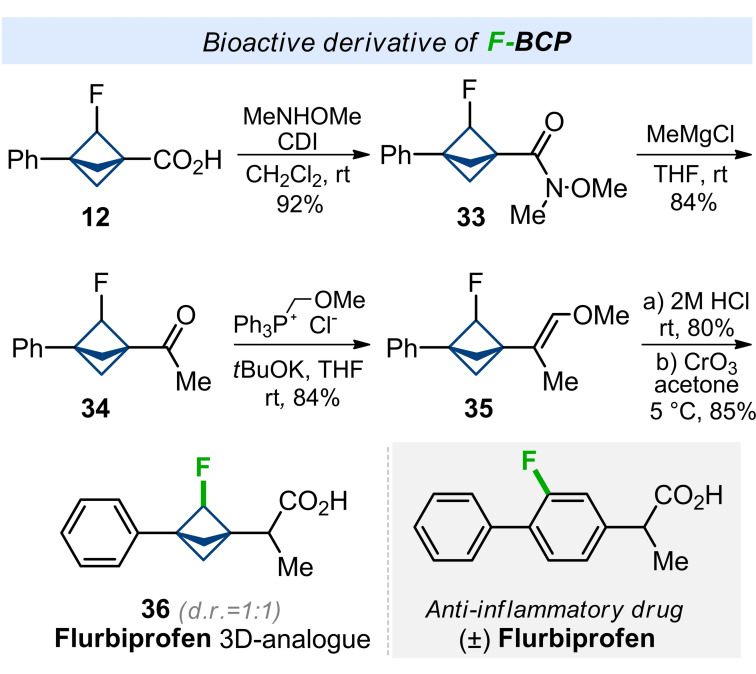
To demonstrate the practical utility of the F‐BCP scaffold, we incorporated it into the structure of Flurbiprofen, a commercialized nonsteroidal anti‐inflammatory drug, instead of fluorophenyl core (Scheme 5). Worth noting, that Flurbiprofen is used in practice as a racemic mixture. The synthesis started from carboxylic acid 12. Weinreb amide 33 was easily obtained in one step in a 92 % yield. Treatment of the latter with MeMgCl gave ketone 34 in 84 % yield. Wittig reaction of the keto group with (Ph3PCH2OMe)Cl in tetrahydrofuran in the presence of KOtBu gave alkene 35. Acidic hydrolysis of the vinyl ether moiety followed by oxidation of the intermediate aldehyde with CrO3 gave the needed acid 36 as a non‐separable mixture of two diastereomers. Compounds 36 can be viewed as a 3D‐shaped saturated analogue of Flurbiprofen.
Scheme 5.
Synthesis of F‐BCP 36—a saturated analogue of a nonsteroidal anti‐inflammatory drug Flurbiprofen.
Since the pioneering work of Stepan and colleagues ten years ago, [2] bicyclo[1.1.1]pentanes (BCPs) have become extremely popular in chemistry (Figure 2). At least 6 reviews, 250 research manuscripts, and 300 patents are devoted to them.[ 3 , 4 , 5 , 6 , 7 , 8 ] Different research groups, including ours, tried to selectively incorporate a single fluorine atom into the bridge‐position of bicyclo[1.1.1]pentane for a long time. Here, we finally solved this long‐standing problem: a practical scalable method to fluoro‐bicyclo[1.1.1]pentanes (19F‐BCP) has been developed. Crystallographic analysis of the obtained compounds was performed, and their physicochemical properties have been studied. Finally, 19F‐BCP core was incorporated into the structure of the nonsteroidal anti‐inflammatory drug Flurbiprofen, instead of the fluorophenyl ring.
We believe that with a practical scalable protocol, 19F‐BCPs will soon find a solid practical application in medicinal chemistry [7a] and supramolecular chemistry. [7b]
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
The authors are grateful to Prof. A. A. Tolmachev for the support of this work. This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement No. 101000893—BENOVELTY). P.M. is also grateful to Mrs. I. Sadkova for the help with the preparation of the manuscript, Dr. E. Rusanov (IOC, Kyiv) for X‐ray studies, Dr. Oleg Shablykin for the synthesis of model compounds 30–32, Dr. A. Konovets and Mrs. M. Bolhova for pK a measurements, and Mrs. A. Grynyova for proofreading the text.
Dedicated to the people of Ukraine
R. Bychek, P. K. Mykhailiuk, Angew. Chem. Int. Ed. 2022, 61, e202205103; Angew. Chem. 2022, 134, e202205103.
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
References
- 1. Taylor R. D., MacCoss M., Lawson A. D. G., J. Med. Chem. 2014, 57, 5845–5859. [DOI] [PubMed] [Google Scholar]
- 2. Stepan A. F., Subramanyam C., Efremov I. V., Dutra J. K., O'Sullivan T. J., DiRico K. J., McDonald W. S., Won A., Dorff P. H., Nolan C. E., Becker S. L., Pustilnik L. R., Riddell D. R., Kauffman G. W., Kormos B. L., Zhang L., Lu Y., Capetta S. H., Green M. E., Karki K., Sibley E., Atchison K. P., Hallgren A. J., Oborski C. E., Robshaw A. E., Sneed B., O'Donnell C. J., J. Med. Chem. 2012, 55, 3414–3424. [DOI] [PubMed] [Google Scholar]
- 3.Some recent examples:
- 3a. Gianatassio R., Lopchuk J. M., Wang J., Pan C.-M., Malins L. R., Prieto L., Brandt T. A., Collins M. R., Gallego G. M., Sach N. W., Spangler J. E., Zhu H., Zhu J., Baran P. S., Science 2016, 351, 241–246; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3b. Kanazawa J., Maeda K., Uchiyama M., J. Am. Chem. Soc. 2017, 139, 17791–17794; [DOI] [PubMed] [Google Scholar]
- 3c. Makarov I. S., Brocklehurst C. E., Karaghiosoff K., Koch G., Knochel P., Angew. Chem. Int. Ed. 2017, 56, 12774–12777; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 12949–12953; [Google Scholar]
- 3d. Lopchuk J. M., Fjelbye K., Kawamata Y., Malins L. R., Pan C.-M., Gianatassio R., Wang J., Prieto L., Bradow J., Brandt T. A., Collins M. R., Elleraas J., Ewanicki J., Farrell W., Fadeyi O. O., Gallego G. M., Mousseau J. J., Oliver R., Sach N. W., Smith J. K., Spangler J. E., Zhu H., Zhu J., Baran P. S., J. Am. Chem. Soc. 2017, 139, 3209–3226; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3e. Caputo D. F. J., Arroniz C., Dürr A. B., Mousseau J. J., Stepan A. F., Mansfield S. J., Anderson E. A., Chem. Sci. 2018, 9, 5295–5390; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3f. Shelp R. A., Walsh P. J., Angew. Chem. Int. Ed. 2018, 57, 15857–15861; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 16083–16087; [Google Scholar]
- 3g. Hughes J. M. E., Scarlata D. A., Chen A. C.-Y., Burch J. D., Gleason J. L., Org. Lett. 2019, 21, 6800–6804; [DOI] [PubMed] [Google Scholar]
- 3h. Wong M. L. J., Mousseau J. J., Mansfield S. J., Anderson E. A., Org. Lett. 2019, 21, 2408–2411; [DOI] [PubMed] [Google Scholar]
- 3i. Nugent J., Arroniz C., Shire B. R., Sterling A. J., Pickford H. D., Wong M. L. J., Mansfield S. J., Caputo D. F. J., Owen B., Mousseau J. J., Duarte F., Anderson E. A., ACS Catal. 2019, 9, 9568–9574; [Google Scholar]
- 3j. Nugent J., Shire B. R., Caputo D. F. J., Pickford H. D., Nightingale F., Houlsby I. T. T., Mousseau J. J., Anderson E. A., Angew. Chem. Int. Ed. 2020, 59, 11866–11870; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 11964–11968; [Google Scholar]
- 3k. Zhang X., Smith R. T., Le C., McCarver S. J., Shireman B. T., Carruthers N. I., MacMillan D. W. C., Nature 2020, 580, 220–226; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3l. Garlets Z. J., Sanders J. N., Malik H., Gampe C., Houk K. N., Davies H. M. L., Nat. Catal. 2020, 3, 351–357; [Google Scholar]
- 3m. Schwärzer K., Zipse H., Karaghiosoff K., Knochel P., Angew. Chem. Int. Ed. 2020, 59, 20235–20241; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 20412–20418; [Google Scholar]
- 3n. Kim J. H., Ruffoni A., Al-Faiyz Y. S. S., Sheikh N. S., Leonori D., Angew. Chem. Int. Ed. 2020, 59, 8225–8231; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 8302–8308; [Google Scholar]
- 3o. Yu S., Jing C., Noble A., Aggarwal V. K., Angew. Chem. Int. Ed. 2020, 59, 3917–3921; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 3945–3949; [Google Scholar]
- 3p. Yu S., Jing C., Noble A., Aggarwal V. K., Org. Lett. 2020, 22, 5650–5655; [DOI] [PubMed] [Google Scholar]
- 3q. Shelp R. A., Ciro A., Pu Y., Merchant R. R., Hughes J. M. E., Walsh P. J., Chem. Sci. 2021, 12, 7066–7072; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3r. Wong M. L. J., Sterling A. J., Mousseau J. J., Duarte F., Anderson E. A., Nat. Commun. 2021, 12, 1644; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3s. Shin S., Lee S., Choi W., Kim N., Hong S., Angew. Chem. Int. Ed. 2021, 60, 7873–7879; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2021, 133, 7952–7958; [Google Scholar]
- 3t. Pickford H. D., Nugent J., Owen B., Mousseau J. J., Smith R. C., Anderson E. A., J. Am. Chem. Soc. 2021, 143, 9729–9736; [DOI] [PubMed] [Google Scholar]
- 3u. Nugent J., Sterling A. J., Frank N., Mousseau J. J., Anderson E. A., Org. Lett. 2021, 23, 8628–8633; [DOI] [PubMed] [Google Scholar]
- 3v. Shelp R., Merchant R. R., Hughes J. M. E., Walsh P. J., Org. Lett. 2022, 24, 110–114; [DOI] [PubMed] [Google Scholar]
- 3w. Mousseau J. J., Perry M. A., Bundesmann M. W., Chinigo G. M., Choi C., Gallego G., Hicklin R. W., Hoy S., Limburg D. C., Sach N. W., Zhang Y., ACS Catal. 2022, 12, 600–606; [Google Scholar]
- 3x. Livesley S., Sterling A. J., Robertson C. M., Goundry W. R. F., Morris J. A., Duarte F., Aïssa C., Angew. Chem. Int. Ed. 2022, 61, e202111291; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2022, 134, e202111291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.1,2-Disubstituted BCPs:
- 4a. Ma X., Han Y., Bennett D. J., Org. Lett. 2020, 22, 9133–9138; [DOI] [PubMed] [Google Scholar]
- 4b. Zhao J.-X., Chang Y.-X., He C., Burke B. J., Collins M. R., Del Bel M., Elleraas J., Gallego G. M., Montgomery T. P., Mousseau J. J., Nair S. K., Perry M. A., Spangler J. E., Vantourout J. C., Baran P. S., Proc. Natl. Acad. Sci. USA 2021, 118, e2108881118; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4c. Yang Y., Tsien J., Hughes J. M. E., Peters B. K., Merchant R. R., Qin T., Nat. Chem. 2021, 13, 950–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Our contributions on bicyclo[1.1.1]pentanes, and their N/O-homologues:
- 5a. Mikhailiuk P. K., Afonin S., Chernega A. N., Rusanov E. B., Platonov M. O., Dubinina G. G., Berditsch M., Ulrich A. S., Komarov I. V., Angew. Chem. Int. Ed. 2006, 45, 5659–5661; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2006, 118, 5787–5789; [Google Scholar]
- 5b. Tkachenko A. N., Radchenko D. S., Mykhailiuk P. K., Grygorenko O. O., Komarov I. V., Org. Lett. 2009, 11, 5674–5676; [DOI] [PubMed] [Google Scholar]
- 5c. Mykhailiuk P. K., Voievoda N. M., Afonin S., Ulrich A. S., Komarov I. V., J. Fluorine Chem. 2010, 131, 217–202; [Google Scholar]
- 5d. Mykhailiuk P. K., Kubyshkin V., Bach T., Budisa N., J. Org. Chem. 2017, 82, 8831–8841; [DOI] [PubMed] [Google Scholar]
- 5e. Levterov V. V., Michurin O., Borysko P. O., Zozulya S., Sadkova I. V., Tolmachev A. A., Mykhailiuk P. K., J. Org. Chem. 2018, 83, 14350–14361; [DOI] [PubMed] [Google Scholar]
- 5f. Levterov V. V., Panasyuk Y., Pivnytska V. O., Mykhailiuk P. K., Angew. Chem. Int. Ed. 2020, 59, 7161–7167; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 7228–7234; [Google Scholar]
- 5g. Denisenko A., Garbuz P., Shishkina S. V., Voloshchuk N. M., Mykhailiuk P. K., Angew. Chem. Int. Ed. 2020, 59, 20515–20521; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 20696–20702. [Google Scholar]
- 6.Reviews on the synthesis of bicyclo[1.1.1]pentanes:
- 6a. Kanazawa J., Uchiyama M., Synlett 2019, 30, 1–11; [Google Scholar]
- 6b. Ma X., Pham L. N., Asian J. Org. Chem. 2020, 9, 8–22; [Google Scholar]
- 6c. He F.-S., Xie S., Yao Y., Wu J., Chin. Chem. Lett. 2020, 31, 3065–3072; [Google Scholar]
- 6d. Anderson J. M., Measom N. D., Murphy J. A., Poole D. L., Angew. Chem. Int. Ed. 2021, 60, 24754–24769; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2021, 133, 24958–24973. [Google Scholar]
- 7.Reviews on applications of bicyclo[1.1.1]pentanes:
- 7a. Mykhailiuk P. K., Org. Biomol. Chem. 2019, 17, 2839–2849; [DOI] [PubMed] [Google Scholar]
- 7b. Locke G. M., Bernhard S. S. R., Senge M. O., Chem. Eur. J. 2019, 25, 4590–4647. [DOI] [PubMed] [Google Scholar]
- 8.The search was performed at reaxys.db on 1 February 2022.
- 9.
- 9a. Müller K., Faeh C., Diederich F., Science 2007, 317, 1881–1886; [DOI] [PubMed] [Google Scholar]
- 9b. Hagmann W. K., J. Med. Chem. 2008, 51, 4359–4369; [DOI] [PubMed] [Google Scholar]
- 9c. O'Hagan D., Chem. Soc. Rev. 2008, 37, 308–319; [DOI] [PubMed] [Google Scholar]
- 9d. Gillis E. P., Eastman K. J., Hill M. D., Donnelly D. J., Meanwell N. A., J. Med. Chem. 2015, 58, 8315–8359; [DOI] [PubMed] [Google Scholar]
- 9e. Meanwell N. A., J. Med. Chem. 2018, 61, 5822–5880; [DOI] [PubMed] [Google Scholar]
- 9f. Mykhailiuk P. K., Chem. Rev. 2021, 121, 1670–1715. [DOI] [PubMed] [Google Scholar]
- 10. Morgenthaler M., Schweizer E., Hoffmann-Roeder A., Benini F., Martin R. E., Jaeschke G., Wagner B., Fischer H., Bendels S., Zimmerli D., Schneider J., Diederich F., Kansy M., Müller K., ChemMedChem 2007, 2, 1100–1115. [DOI] [PubMed] [Google Scholar]
- 11.
- 11a. Thiehoff C., Rey Y. P., Gilmour R., Isr. J. Chem. 2017, 57, 92–100; [Google Scholar]
- 11b. Hunter L., Beilstein J. Org. Chem. 2010, 6, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bridgehead-fluorinated BCPs:
- 12a. Adcock W., Binmore G. T., Krstic A. R., Walton J. C., Wilkie J., J. Am. Chem. Soc. 1995, 117, 2758–2766; [Google Scholar]
- 12b. Goh Y. L., Adsool V. A., Org. Biomol. Chem. 2015, 13, 11597–11601; [DOI] [PubMed] [Google Scholar]
- 12c. Kokhan S. O., Tymtsunik A. V., Grage S. L., Afonin S., Babii O., Berditsch M., Strizhak A. V., Bandak D., Platonov M. O., Komarov I. V., Ulrich A. S., Mykhailiuk P. K., Angew. Chem. Int. Ed. 2016, 55, 14788–14792; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 15008–15012. [Google Scholar]
- 13.Application of bridgehead-fluorinated BCPs in organic synthesis and medicinal chemistry has been described in 20 additional research manuscripts (see Supporting Information for the full list of references) and 40 patents.
- 14. gem-Fluorinated BCPs:
- 14a. Ma X., Sloman D. L., Han Y., Bennett D. J., Org. Lett. 2019, 21, 7199–7203; [DOI] [PubMed] [Google Scholar]
- 14b. Bychek R. M., Hutskalova V., Bas Y. P., Zaporozhets O. A., Zozulya S., Levterov V. V., Mykhailiuk P. K., J. Org. Chem. 2019, 84, 15106–15117; [DOI] [PubMed] [Google Scholar]
- 14c. Ma X., Pinto W., Pham L. N., Sloman D. L., Han Y., Eur. J. Org. Chem. 2020, 4581–4605; [Google Scholar]
- 14d. Le T. P., Rončević I., Dračínský M., Císařová I., Šolínová V., Kašička V., Kaleta J., J. Org. Chem. 2021, 86, 10303–10319; [DOI] [PubMed] [Google Scholar]
- 14e. Ma X., Yeung C. S., J. Org. Chem. 2021, 86, 10672–10698; [DOI] [PubMed] [Google Scholar]
- 14f. McNamee R. E., Haugland M. M., Nugent J., Chan R., Christensen K. E., Anderson E. A., Chem. Sci. 2021, 12, 7480–7485; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14g. McNamee R. E., Thompson A. L., Anderson E. A., J. Am. Chem. Soc. 2021, 143, 21246–21251. [DOI] [PubMed] [Google Scholar]
- 15.Poly-fluorinated BCPs:
- 15a. Levin M. D., Hamrock S. J., Kaszynski P., Shtarev A. B., Levina G. A., Noll B. C., Ashley M. E., Newmark R., Moore G. G. I., Michl J., J. Am. Chem. Soc. 1997, 119, 12750–12761; [Google Scholar]
- 15b. Shtarev A. B., Pinkhassik E., Levin M. D., Stibor I., Michl J., J. Am. Chem. Soc. 2001, 123, 3484–3492. [DOI] [PubMed] [Google Scholar]
- 16. Takahira Y., Chen M., Kawamata Y., Mykhailiuk P., Nakamura H., Peters B. K., Reisberg S. H., Li C., Chen L., Hoshikawa T., Shibuguchi T., Baran P. S., Synlett 2019, 30, 1178–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.
- 17a. Hall H. K., Smith C. D., Blanchard E. P., Cherkofsky C. S., Sieja J. B., J. Am. Chem. Soc. 1971, 93, 121–130; [Google Scholar]
- 17b. Applequist D. E., Renken T. L., J. Org. Chem. 1982, 47, 4985–4995; [Google Scholar]
- 17c. Measom N. D., Down K. D., Hirst D. J., Jamieson C., Manas E. S., Patel V. K., Somers D. O., ACS Med. Chem. Lett. 2017, 8, 43–48; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17d. Fawcett A., Pure Appl. Chem. 2020, 92, 751–765; [Google Scholar]
- 17e. Applequist D. E., Wheeler J. W., Tetrahedron Lett. 1977, 18, 3411–3412; [Google Scholar]
- 17f. Rablen P. R., Paiz A. A., Thuronyi B. W., Jones M., J. Org. Chem. 2009, 74, 4252–4261. [DOI] [PubMed] [Google Scholar]
- 18. Navuluri C., Charette A. B., Org. Lett. 2015, 17, 4288–4291. [DOI] [PubMed] [Google Scholar]
- 19. Colella M., Tota A., Großjohann A., Carlucci C., Aramini A., Sheikh N. S., Degennaro L., Luisi R., Chem. Commun. 2019, 55, 8430–8433. [DOI] [PubMed] [Google Scholar]
- 20.Deposition Numbers 2161708 (for 12) and 2161709 (for 27) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 21. Ripenko V., Vysochyn D., Klymov I., Zhersh S., Mykhailiuk P. K., J. Org. Chem. 2021, 86, 14061–14068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Doerwald F. Z., Lead optimizations for medicinal chemists, Wiley-VCH, Weinheim, 2012, p. 107. [Google Scholar]
- 23.Calculations have been made with “Cxcalc” (ChemAxon).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.