Abstract
Background and Aims
Coronavirus disease 2019 (COVID‐19) is a highly contagious infection, and new variants of its causative virus continue to emerge all around the world. Meanwhile, mass vaccination represents a highly effective measure to reduce the disease burden. Not only do vaccines immunize individuals, but they also protect the entire population through achieving herd immunity. They are composed of various ingredients, some of which may induce hypersensitivity reactions, namely anaphylaxis and cutaneous allergic reactions. This review aims to provide an explicit overview of the pathophysiology, suspected responsible components, and management of COVID‐19 vaccine‐induced allergic reactions, and their effect on acquiring herd immunity.
Methods
To perform this narrative review, a comprehensive literature search based on our selected terms was conducted in online databases of PubMed/Medline and Google Scholar for finding the relevant studies published from 2019 to 2022.
Results
COVID‐19 vaccines introduce several advantages that outweigh their potential risks, such as allergic reactions. Allergic reactions are mainly attributed to polyethylene glycol and polysorbate excipients that can provoke IgE‐mediated reactions and hypersensitivity reactions. These reactions should be managed properly to avoid having serious sequelae.
Conclusion
It is of great importance to immediately recognize and manage vaccine hypersensitivity reactions, especially anaphylaxis, to avoid allergic patients being excluded from the vaccination program, and more importantly, to stop the spreading of unfounded vaccine hesitancy leading to delayed herd immunity.
Keywords: allergic reaction, COVID‐19 vaccine, herd immunity, hypersensitivity, PEG, skin
1. INTRODUCTION
The ongoing coronavirus disease 2019 (COVID‐19) pandemic induced by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has thus far infected nearly 220 million people and caused over 4.5 million deaths worldwide. Being highly contagious, COVID‐19 has led to substantial changes in our lives, so many countries have struggled to find the optimal strategy for combating it. None of these approaches that range from precautionary measures to searching for efficacious treatment options have been as promising as the vaccination strategy. Mass vaccination is an encouraging cost‐effective intervention to reduce morbidity and mortality, achieve herd immunity, and perhaps the most practical strategy to stop this pandemic.
Vaccination against COVID‐19 represents an opportunity to retrieve normal conditions of life. Vaccines help develop immunity by imitating an infection and training lymphocytic cells to fight that disease in case of future invasion. 1 Not only do they immunize individuals, but they also protect the entire population by acquiring herd immunity. Vaccines have been applied as an effective public health intervention and have helped us dramatically improve human health and reduce the burden of infectious diseases since the mid‐20th century. 2 However, as the body builds immunity, we can expect typical minor symptoms after vaccination, such as fever or fatigue.
Furthermore, allergic reactions to vaccine components are possible, albeit rare and without serious dangers. COVID‐19 vaccines are not an exception, as several allergic reactions to their injection have been reported. They are mainly attributed to polyethylene glycol (PEG) and polysorbate excipients that provoke immunoglobulin E (IgE)‐mediated reactions. Their clinical manifestations range from skin disorders to life‐threatening reactions like anaphylaxis, mainly in those with a history of allergies. 3 Despite being rare and uncommon, these reactions have given rise to hesitation in receiving the COVID‐19 vaccine, which may delay achieving herd immunity towards this infection.
In this review, we provide an explicit overview of the pathophysiology of vaccine‐induced allergic reactions and the so‐far‐reported allergic reactions to available COVID‐19 vaccines. We also discuss their etiology, suspected responsible components, management, effect on vaccine hesitancy and aim to create a referable source for future studies. However, there is a limitation in comparing subcategories and different types of allergic reactions of each discussed vaccine in detail, as well as discussing allergic reactions to other available vaccines, both of which are due to the lack of sufficient clinical studies.
2. METHODS
To perform this narrative review, we conducted a comprehensive literature search based on the selected terms including “COVID‐19 vaccine,” “allergic reaction,” “hypersensitivity,” “herd immunity,” “anaphylaxis,” “cutaneous reaction,” “PEG,” and “polysorbate.” We explored online databases of PubMed/Medline and Google Scholar to find the relevant studies published from the emergence of COVID‐19 (2019) to 2022. We included the most appropriate studies without limiting them based on the article type. Preclinical studies were excluded.
3. BODY
3.1. Allergic reactions to vaccines
In recent years, there has been a surge in reports of possible allergic reactions to vaccination which stems from the increased use of vaccines in national health programs to avoid encountering newly emerged viral epidemics. However, the number of such confirmed cases is still relatively low, with an estimate of 1.31 cases of allergic reaction per million doses and less than 1 per 100,000 cases of anaphylaxis by different studies. 4 , 5 Yet, it is important to meticulously manage them to avoid severe complications. Influenza, measles‐mumps‐rubella (MMR), polio, and yellow fever vaccines are examples of immunization programs to which allergic reactions have been reported.
Vaccine‐induced reactions can be categorized in different ways. According to the World Health Organization (WHO), they can be categorized into systemic and local reactions. 6 Systemic reactions mainly occur in the first hour after the injection and are infrequent, though it is important to recognize and manage them quickly. An anaphylactic hypersensitivity reaction is a prime example that can be either IgE‐mediated or non‐IgE‐mediated. Other examples include fever, skin rashes, delayed urticaria, malaise, muscle pain, headache, and diarrhea. 4 However, local reactions appear as a result of postinjection inflammation and include pain, redness, and swelling at the injection site. Local reactions involve type IV hypersensitivity reactions such as subcutaneous nodules accompanied by itching and eczema and type III hypersensitivity (Arthus reactions). 4
From another point of view, these reactions can be classified into acute and delayed categories. Acute or immediate‐type reactions are primarily type I hypersensitivity reactions that start within minutes up to 4 h of exposure to the relevant allergens, including some vaccine components. 5 These reactions occur through the pathway of IgE‐mediated mast cell activation via Fcε receptor‐1, resulting in mast cell degranulation and secretion of histamine and other inflammatory markers. The events of this pathway have been confirmed by the specific IgEs detection and increased serum tryptase level. 7 Vaccine excipients are considered more likely to cause these allergic reactions than the vaccine antigen and the residual nonhuman protein. 8 It is of great importance to carefully evaluate acute‐onset IgE‐mediated hypersensitivity reactions since some of its symptoms, like anaphylaxis, may involve multiple organs rapidly and cause life‐threatening consequences. Such consequences can happen by means of Mas‐Related G Protein‐Coupled Receptor‐X2 (MRGPRX2), a receptor capable of inducing direct mast cell degranulation and anaphylactic reactions. This pathway is apparently more rapid, but more transient in comparison to IgE‐triggered events. 9 , 10 Other common symptoms of acute allergic reactions include: (1) urticarial rashes with itching and burning sensation, stemming from immunologic and nonimmunologic mast cell activation that usually resolve within 24 h and (2) angioedema with subcutaneous involvement that is often accompanied by pain and tenderness and generally resolves within 24–48 h. 11
Delayed or type IV hypersensitivity reactions generally occur within 48 h after vaccination and reach their peak between 72 and 96 h. 5 However, it has been stated that the initiation of symptoms may be delayed up to 2–3 weeks. 11 These reactions are cell‐mediated and antibody‐independent. They occur as a result of T cell, macrophage, or monocyte overstimulation and cytokine release, leading to inflammation and tissue damage. Delayed reactions are usually self‐limiting and do not contraindicate the administration of future doses of the same vaccine. Although the aforementioned urticaria and angioedema generally happen in the context of acute reactions, they can also happen as delayed‐type reactions. In this case, they are a result of non‐IgE mediated processes such as complement system activation, leading to the generation of anaphylatoxins C1q, C3a C4, and C5a and Factor B, which consequently activate mast cells and trigger their degranulation. 12 , 13 Some delayed reactions, such as persistent hard nodules, may not have underlying immunologic pathogenesis. These nodules involve nonspecific inflammation or irritant reactions induced by adjuvants and are not necessarily due to immunologic hypersensitivity to vaccine constituents. 11 , 14 , 15
3.2. COVID‐19 vaccines and allergic reactions
Exploration for finding an effective vaccine to arrest the COVID‐19 pandemic has resulted in the production of various vaccines with different modes of action that are mainly categorized into three groups. These include (1) vaccines using the whole disease‐causing virion, such as the Sinopharm and Covaxin vaccines; (2) adenoviral vector vaccines, such as Astra Zeneca, Johnson & Johnson, and Sputnik V COVID‐19 vaccines; and (3) messenger RNA (mRNA)‐based vaccines, prime examples of which are the Pfizer‐BioNTech and Moderna vaccines that use lipid nanoparticle (LNP) delivery system to prevent rapid enzymatic degradation of mRNA molecules. 16
Thus far, allergic reaction reports to COVID‐19 vaccines include anaphylactic and cutaneous reactions.
3.2.1. Anaphylaxis as a life‐threatening adverse reaction
Since the introduction of active vaccination against SARS‐CoV2 and the beginning of vaccination campaigns, early safety monitoring has detected anaphylactic reactions that emerge after the injection of the first dose vaccine and resolve after treatment. 17 Anaphylaxis is a severe life‐threatening systemic hypersensitivity reaction that occurs due to mast cell degranulation and the widespread release of mediators such as histamine. With a rapid onset, it usually occurs within minutes after the exposure to a specific allergen and is accompanied by airway constriction, circulatory problems, low level of consciousness, and may be associated with skin and mucosal changes. 18 Although anaphylactic reactions after vaccination are rare, they may be followed by serious complications. Thus, it is a matter of the utmost importance to identify and manage them quickly.
Most cases of anaphylactic reactions to COVID‐19 vaccines have occurred in less than 30 min after vaccination via immediate IgE‐mediated pathway in people with a history of allergic reactions, including anaphylaxis. 19 However, as is the case with any other medication, it is possible that anaphylactic reactions resulting from vaccination happen in the absence of a history of allergic diseases. 20
Regarding the epidemiology of these adverse events, reports imply that the overall occurrence of anaphylactic reactions due to COVID‐19 vaccination, estimated at around 4.5 in a million, is higher than the expected rate of severe allergic reactions with an incidence of one in a million. 13 , 19 , 21 , 22 Furthermore, studies demonstrate that the rate of these reactions in women is higher than men, one reason of which could be the greater number of women who have been vaccinated during these observations. 19 Of note is that the incidence of adverse effects is lower with the Pfizer‐BioNTech than with the Moderna vaccine. 23 A complete understanding of the underlying pathophysiology and the potential culprit of anaphylactic reactions to COVID‐19 vaccines is yet to be determined. 24
3.2.2. Cutaneous allergic reactions after administration of COVID‐19 vaccines
Another reported allergic reaction to COVID‐19 mRNA vaccines is dermatologic reactions, 25 , 26 some of which mimic the SARS‐CoV2 infection itself, suggesting that the skin eruptions may be caused by immune activation rather than directly caused by the virus. 27 These reactions happen scarcely as a study reported their incidence 0.22% in vaccinated individuals and 16.54% of the whole vaccination adverse effects. 28 Their wide spectrum involves injection site reactions and more extensive reactions. They include the more common delayed large local reactions, localized redness and swelling, urticaria, maculopapular rashes, and the less common erythromelalgia, chilblains, cosmetic filler reactions, and pityriasis‐rosea‐like eruptions. A study showed that delayed large local reactions and urticaria were the most common cutaneous reactions following vaccination with Moderna and Pfizer‐BioNTech, respectively. 29 Post‐COVID vaccination facial swelling in people with previous use of cosmetic fillers was reported as a consequence of both mRNA vaccines. 29 , 30 It can be attributed to either immediate or delayed hypersensitivity reactions to filler ensuing from an immunogenic stimulus. 30 , 31 , 32
Delayed localized cutaneous reactions to COVID‐19 in the injection site, called COVID arm or COVID vaccine arm, with a self‐limiting course have been observed in vaccinated individuals. These reactions emerge on average 7 days after the first dose injection. They can also appear after the second dose, though with a probability of less than 50%, faster development occurring in 2 days, and less severity. 29 These reactions happening after 4 h of the injection do not inhibit receiving a second vaccine dose. Although immediate hypersensitivity reactions that occur less than 4 h after the injection, such as pruritus, flushing, angioedema, and urticaria, are contraindicated for second dose injection of the vaccine. Thus the importance of distinguishing immediate and delayed reactions lies in the clinical decision for the administration of the second dose. 33
3.3. The culprit behind hypersensitivity reactions to COVID‐19 vaccines
Vaccines are comprised of two main kinds of components, namely the active component or the antigen and the additional components. The former can be the whole pathogen organism, parts of the organism, or inactivated toxins, and the latter can be conjugating agents, residual animal proteins, preservatives, metals, latex from sealing the vaccine ampoules, stabilizers, antimicrobial agents, adjuvants, contaminants, and culture medium used in the preparation process of the vaccines. Some of the most common individual vaccine components that may trigger anaphylaxis include egg protein, gelatin, milk protein, formaldehyde, thimerosal, and neomycin. Almost all components of the vaccine formulation can be considered as potential triggers for allergic reactions. 34 However, active components seldom are the culprit, and hypersensitivity reactions, especially immediate and IgE‐mediated reactions, are usually traced back to the additives or excipients. Excipients are used to induce a stronger immune response, stabilize the potency of the vaccine during transportation or storage, prevent vaccine contamination, and for some other purposes. 12 Being present in small amounts, these components generally do not induce allergic reactions. Nevertheless, In patients with unusually high levels of IgE antibody and those who have a genetic predisposition to produce significantly high amounts of antibodies during exposure to several allergens, severe reactions, including anaphylaxis, can originate from very small amounts of antigens and develop. 11 , 35 In spite of the fact that mRNA vaccines are new, most of their components were used in other medications and cosmetic products previously, which multiplies the odds of causing sensitization in genetically predisposed patients. 36 Table 1 summarizes the main ingredients of mRNA‐ and vector‐based COVID‐19 vaccines as well as the incidence of anaphylactic reactions to each vaccine. 37 Since currently, our understanding of the exact underlying pathogenesis is unclear, an investigation has been launched into the inciting agents to improve our knowledge about these reactions and their culprit.
Table 1.
Vector‐ and mRNA‐based COVID‐19 vaccines’ ingredients and similar vaccines containing the same suspected allergen component
| COVID‐19 vaccine | Active ingredient | Inactive ingredients | Storage | Incidence of anaphylactic reaction 37 | Suspected allergen excipient | Other vaccine types with the same excipient | Predictive factors |
|---|---|---|---|---|---|---|---|
| Pfizer‐BioNTech | Nucleoside‐modified mRNA encoding the viral spike (S) glycoprotein of SARS‐CoV‐2 | (4‐Hydroxybutyl)azanediyl)bis(hexane‐6,1‐diyl)bis(2‐hexyldecanoate) | −90 to −60°C | 12.36/million | PEG 2000 | N/A | previous anaphylactic reaction to drugs and vaccines; multiple allergies including drug allergies; mast cell disorders |
| 2[(PEG)‐2000]‐N,N‐ditetradecylacetamide | |||||||
| 1,2‐Distearoyl‐sn‐glycero‐3‐phosphocholine | |||||||
| Cholesterol | |||||||
| Salts, sugars, and buffers | |||||||
| Moderna | Nucleoside‐modified mRNA encoding the viral spike (S) glycoprotein of SARS‐CoV‐2 | 1,2‐Distearoyl‐sn‐glycero‐3‐phosphocholine | −20°C | 20.39/million | PEG 2000 | ||
| PEG 2000 dimyristoyl glycerol (DMG) | |||||||
| SM‐102 | |||||||
| Cholesterol | |||||||
| Salts, sugars, and buffers | |||||||
| AstraZeneca | Replication‐incompetent adenovirus vector, encoding a stabilized variant of the SARS‐CoV‐2 spike (S) protein | l‐histidine | 2–8°C | 17.64/million | Polysorbate 80 | Influenza, HPV, Hepatitis B, DTaP, Rotavirus, zoster, meningococcal group B, Japanese encephalitis | |
| l‐histidine hydrochloride monohydrate | |||||||
| Polysorbate 80 | |||||||
| Salts, sugars, and buffers | |||||||
| Johnson & Johnson | Replication‐incompetent adenovirus vector, encoding a stabilized variant of the SARS‐CoV‐2 spike (S) protein | 2‐hydroxypropyl‐β‐cyclodextrin (HBCD) | −20°C | 6.53/million | Polysorbate 80 | ||
| Polysorbate 80 | |||||||
| Salts, sugars, and buffers |
Abbreviations: N/A, not applicable; PEG, polyethylene glycol.
Polyethylene glycol (PEG), also known as macrogol, is a hydrophilic polyether polymer compound with variable chain length and a molecular weight that ranges from 20 to 10,000,000 g/mol. PEGylation, the process in which PEGs bind to the systemic drugs, not only increases molecular weight and prolongs circulation time but also prevents the opsonization of the drug by shielding it from the immune system. 38 As PEGylation has been introduced as a technology that improves drug delivery, PEG is widely used as an excipient in various medications, cosmetics, and food products. Some of the pharmaceuticals that use this polymer‐based drug delivery system are laxatives, penicillin, about 30% of tablets, chemotherapy drugs, and many injectable formulations. 39
In both Pfizer‐BioNTech and Moderna mRNA vaccines, one of the excipients is PEG 2000, a PEG with a molecular weight of 2000 g/mol. The modified mRNA used in these vaccines is easily taken up by mononuclear phagocytes, leading to rapid degradation by ribonucleases. Also, it has a poor penetration through the cell membrane originating from negative electric charge and high molecular weight. Thus, needing a protective shield for being delivered to cells, it is formulated into LNPs that contain low levels of PEGylated lipids, for stabilizing the nanoparticle and increasing their solubility by enabling the assembly of a hydrate shell. 40 Consequently, the function of LNPs as nonviral vectors minimizes nanoparticle aggregation at the intramuscular and intradermal injection sites, improves nanoparticle spreading and drainage into the initial lymphatic vessels, facilitates cellular uptake by coating the mRNA with cationic lipids and lipids that mimic the phospholipids of the cell membrane. 20 , 41 After entering the cell, the mRNA encodes the viral spike protein and instigates the immune response against it.
It has been speculated that PEGylated lipids are the potential allergic components and the culprit agent for the aforementioned hypersensitivity reactions to COVID‐19 vaccines especially IgE‐mediated anaphylaxis. Although PEG‐containing products are generally considered safe, reports of IgE‐mediated allergic reactions and anaphylaxis to PEGs of different molecular weights have been described in the literature. 42 , 43 , 44 There is difficulty in the assessment of anti‐PEG IgE via skin testing as wheal and flare are not always produced in patients with true PEG allergies. 24 , 45 Because of this, hypersensitivity to PEG has been described as rare previously, yet there has been a surge in reports of allergic reaction to PEG in recent years, which is attributed to the increased administration of certain drugs or certain personal hygiene products. 38 , 46 , 47 A study has demonstrated that up to 70% of patients undergoing treatment with PEGylated products will develop anti‐PEG IgG antibodies. 48 However, as mRNA vaccines use a novel technology and so far PEG has not been a common excipient in manufacturing vaccines, no reactions to the PEG‐containing vaccines have been described previously. An explanation for how a patient may be sensitized to PEG before COVID‐19 vaccination is their wide use in oral and injectable medications, cosmetics, and foods. 36
Allergic reactions to LNPs happen in case of previous exposure and antibody formation against a component of the LNP. The only component toward which anti‐LNP antibodies have been detected in animal models and humans is the PEG polymer. 48 The exact molecular mechanism of these reactions in humans is not clearly understood and may involve IgE‐ and non‐IgE‐mediated immediate hypersensitivity reactions. 49 It has been proposed that although the existence of antibodies before COVID‐19 vaccination may lead to allergic reactions, they may also produce stronger immune responses and increase the vaccine efficacy. This can occur by increased dendritic cell uptake of the LNP, enhanced delivery of the mRNA to the cytoplasm, more viral spike protein expression, and improved capacity for presentation to T cells. 50 There is a possibility that cross‐reactivity happens between PEG and polysorbate80, another excipient in some vaccines, due to containing chemically common structures. 43
Polysorbate 80, also known as Tween 80, is another excipient that is contained in two vector‐based COVID‐19 vaccines, AstraZeneca and Johnson & Johnson. Polysorbate 80 is a PEG derivative and synthetic non‐ionic surfactant that is used in the food industry and medications, including 70% of injectable biological agents and monoclonal antibodies. 44 , 51 It constitutes a risk for allergic reactions to steroids and chemotherapeutics. Also, unlike PEG, polysorbate has been used in vaccines previously, and represents a rare cause of IgE‐mediated hypersensitivity reactions to vaccines. Thus, polysorbate 80 may cause allergic reactions after the injection of COVID‐19 first dose vaccine due to previous exposure and sensitization to this excipient.
3.4. Management and vaccination contraindications
Acute anaphylactic reactions occur without any strong correlation with age, sex, asthma, atopic status, or previous nonsevere reactions 52 and, in case of not being resolved, may cause severe sequels. Thus, it is necessary for all vaccination facilities to be equipped enough to immediately recognize and manage anaphylaxis.
It is of great importance to take the background allergic history of individuals thoroughly to determine whether COVID‐19 vaccination is contraindicated in them or not. One of the points to be aware of is a history of mild or severe allergic reactions, especially to PEG‐ or polysorbate‐containing products. 53 It has been suggested that individuals with a history of allergic reactions due to any cause should be monitored for 30 min after COVID‐19 vaccination. 12 Patients suffering from allergic skin diseases do not have an increased risk of anaphylactic reaction to the covid‐19 vaccine, and thus, vaccination should not be deferred in individuals with atopic dermatitis, urticaria, and other skin allergic reactions. 21 Also, previous anaphylaxis to other vaccines, foods, and medical products are not contraindications of COVID‐19 vaccination. Instead, an immunoallergy workup should take place before the vaccination of these individuals. 21 , 54
All in all, according to the centers for disease control and prevention (CDC), COVID‐19 mRNA vaccines are contraindicated in case of a known history of a severe allergic reaction to any component of the vaccine. 33 Thus, patients with a history of a severe allergy to COVID‐19 vaccine components, namely PEG or polysorbate, should avoid getting a COVID‐19 jab. Meanwhile, the second dose injection is contraindicated in the vaccine recipients who experienced a severe or immediate allergic reaction after the injection of the first dose. 33 The former mainly includes anaphylactic reactions, while the latter includes allergic reactions that happen within 4 h after vaccination. 29
When encountering the first signs of anaphylaxis, without any contraindication, 55 it is urgent to immediately administer intramuscular epinephrine as the prime and life‐saving drug of choice to decreases mast cell degranulation. Subsequent actions include volume replacement, assessing vital signs, clearing airways, giving oxygen, administering short‐acting beta‐agonists in case of severe dyspnea, and nebulization with epinephrine in case of severe upper airway obstruction. 20 For treating patients with cutaneous allergic reactions, symptomatic therapies, including topical corticosteroids, oral antihistamines, and pain‐relieving medications, are of use to resolve these reactions after a median of 3–4 days. 29
3.5. The effect of allergic reactions to COVID‐19 vaccines on our journey to reach herd immunity
Vaccines are one of the most effective measures that curb the spread of viruses and strengthen our ability to combat epidemics. Identical to all medications, they may cause adverse events in some recipients. However, the advantages represented by the vaccines outweigh their potential risks, including hypersensitivity and ultimately anaphylactic reactions.
Mass immunization strategy by vaccination establishes vaccine‐induced herd immunity that, in addition to the particular vaccinated individuals, protects unvaccinated, immunocompromised, and vulnerable groups who are unable to develop immunity. This effect is exerted by the resistance of the society against a disease which itself stems from the immunity of a large proportion of the members and the consequent lower probability of an infected individual coming into contact with a susceptible person. 56 Although achieving herd immunity through vaccination would considerably help control the disease, it will not end the pandemic. 57 Eradication of the smallpox virus by global mass vaccination is a good showcase for vaccination‐induced herd immunity. In this respect, immunization of more than 80% of the global population against the virus decreased the number of susceptible hosts to lower than the threshold needed for transmission. 58 The World Health Organization estimates that global vaccination programs save 2–3 million lives per year. 59 It is assumed that for the induction of herd immunity against COVID‐19, a protective immunity rate of approximately 60% of the total population is required. 60
There are several factors that form the compliance of citizens with vaccination strategy and their confidence in vaccines. One of these factors is the public knowledge about the mechanism and benefits of achieving herd immunity. 61 Other factors range from awareness in society about vaccines and their possible associated risks to religious, political, social, and economic status. Figure 1 shows the main factors that increase or decrease vaccine acceptability which has a strong association with herd immunity. Meanwhile, one of the most pivotal factors is the fear of hypersensitivity reactions peculiarly among those with a medical history of pre‐existing allergic diseases. Although allergic patients should not generally be excluded from the vaccination program, this may lead to a deep distrust of vaccines and delay deploying counter‐pandemic measures fast enough to balance out devastating economic, social, and public health consequences of the pandemic.
Figure 1.
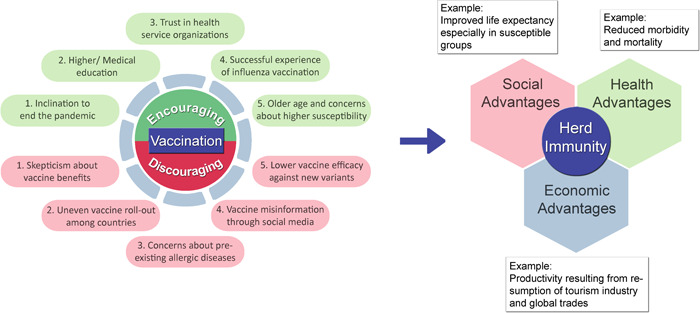
Key factors affecting vaccine acceptability and the journey to reach herd immunity. There are several positive and negative contributors to vaccine acceptability among people, an important factor of which is the history of pre‐existing allergic diseases and fear of severe reactions to vaccine components. The resultant vaccine acceptability rate determines the time of achieving herd immunity, the advantages of which involve social, health, and economic aspects
To avoid the detrimental repercussions of this unfounded vaccine hesitancy, health care workers should aim to dispel the misconceptions by providing accurate information for people about the potential benefits of being COVID‐19 vaccinated. Also, ensuring validity of vaccine‐related information through social networks is of great importance in eliminating conspiracy beliefs about vaccination. 62 Moreover, to safely vaccinate susceptible individuals, they should be aware of the possible COVID‐19 vaccine hypersensitivity reactions and explicit protocols for managing them.
4. CONCLUSION
In summary, COVID‐19 vaccines introduce several advantages that outweigh their potential risks, such as allergic reactions that occur through different pathways and whose proper management can arrest severe outcomes. These allergic reactions include anaphylaxis, a severe life‐threatening systemic reaction due to mast cell degranulation and mediators' release, and cutaneous reactions including delayed large local reactions, urticaria, maculopapular rashes, chilblains, cosmetic filler reactions, pityriasis‐rosea‐like eruptions, etc. PEG and polysorbate 80, excipients that stabilize vaccine potency and help it induce a stronger immune response, can provoke IgE‐mediated reactions and are the main identified culprits for these hypersensitivity reactions.
COVID‐19 vaccines are not the first pharmaceutics to which allergic reactions have been reported. Previous allergic reactions to medications, vaccines, and cosmetic products with different excipients have been reported. Moreover, vaccine hypersensitivity reactions are rare and, if managed properly, do not result in serious sequelae. Thus, the pivotal point is to provide well‐founded information for the public to quash vaccine hesitancy and tackle one of the problems that decrease vaccine acceptability and disrupt achieving herd immunity.
AUTHOR CONTRIBUTIONS
Conceptualization, Data Curation, Writing–Original Draft Preparation, Writing–Review and Editing: Sara Mahdiabadi. Conceptualization, Writing–Review and Editing, Supervision: Nima Rezaei. All authors have read and approved the final version of the manuscript. The corresponding author had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
TRANSPARENCY STATEMENT
The lead author (Nima Rezaei) affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
ACKNOWLEDGMENTS
No funding was received to assist with the preparation of this manuscript.
Mahdiabadi S, Rezaei N. Anaphylaxis and allergic reactions to COVID‐19 vaccines: a narrative review of characteristics and potential obstacles on achieving herd immunity. Health Sci. Rep. 2022;5:e787. 10.1002/hsr2.787
REFERENCES
- 1. CDC . Understanding How Vaccines Work; 2018.
- 2. Fritsche PJ, Helbling A, Ballmer‐Weber BK. Vaccine hypersensitivity—update and overview. Swiss Med Wkly. 2010;140:238‐246. [DOI] [PubMed] [Google Scholar]
- 3. Moghimi SM. Allergic reactions and anaphylaxis to LNP‐based COVID‐19 vaccines. Molecular Therapy. 2021;29:898‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nilsson L, Brockow K, Alm J, et al. Vaccination and allergy: EAACI position paper, practical aspects. Pediatr Allergy Immunol. 2017;28:628‐640. [DOI] [PubMed] [Google Scholar]
- 5. Chung EH. Vaccine allergies. Clin Exp Vaccine Res. 2014;3:50‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization . Regional Office for the Western P. Immunization safety surveillance: guidelines for immunization programme managers on surveillance of adverse events following immunization. 2nd ed. WHO Regional Office for the Western Pacific; 2013. [Google Scholar]
- 7. Khan S. Mast cell tryptase level should be checked in all patients with suspected Kounis syndrome. Eur Heart J. 2020;41:3018. [DOI] [PubMed] [Google Scholar]
- 8. Stone CA Jr., Rukasin CRF, Beachkofsky TM, Phillips EJ. Immune‐mediated adverse reactions to vaccines. Br J Clin Pharmacol. 2019;85:2694‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gaudenzio N, Sibilano R, Marichal T, et al. Different activation signals induce distinct mast cell degranulation strategies. J Clin Invest. 2016;126:3981‐3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grimbaldeston MA. Mast cell‐MrgprB2: sensing secretagogues or a means to overreact? Immunol Cell Biol. 2015;93:221‐223. [DOI] [PubMed] [Google Scholar]
- 11. McNeil MM, DeStefano F. Vaccine‐associated hypersensitivity. J Allergy Clin Immunol. 2018;141:463‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kounis NG, Koniari I, de Gregorio C, et al. Allergic reactions to current available COVID‐19 vaccinations: pathophysiology, causality, and therapeutic considerations. Vaccines. 2021;9:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dreskin SC, Halsey NA, Kelso JM, et al. International Consensus (ICON): allergic reactions to vaccines. World Allergy Organ J. 2016;9:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miliauskas JR, Mukherjee T, Dixon B. Postimmunization (vaccination) injection‐site reactions. A report of four cases and review of the literature. Am J Surg Pathol. 1993;17:516‐524. [PubMed] [Google Scholar]
- 15. Lauren CT, Belsito DV, Morel KD, LaRussa P. Case report of subcutaneous nodules and sterile abscesses due to delayed type hypersensitivity to aluminum‐containing vaccines. Pediatrics. 2016;138:e20141690. [DOI] [PubMed] [Google Scholar]
- 16. Dolgin E. COVID‐19 vaccines poised for launch, but impact on pandemic unclear. Nature Biotechnol . 2020. Published online November 25, 2020. [DOI] [PubMed]
- 17. Shimabukuro T. Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID‐19 vaccine ‐ United States, December 21, 2020‐January 10, 2021. Am J Transplant. 2021;21:1326‐1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cardona V, Ansotegui IJ, Ebisawa M, et al. World allergy organization anaphylaxis guidance 2020. World Allergy Organ J. 2020;13:100472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shimabukuro T. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer‐BioNTech COVID‐19 vaccine—United States, December 14‐23, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:46‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sokolowska M, Eiwegger T, Ollert M, et al. EAACI statement on the diagnosis, management and prevention of severe allergic reactions to COVID‐19 vaccines. Allergy. 2021;76:1629‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klimek L, Jutel M, Akdis CA, et al. ARIA‐EAACI statement on severe allergic reactions to COVID‐19 vaccines—an EAACI‐ARIA position paper. Allergy. 2021;76:1624‐1628. [DOI] [PubMed] [Google Scholar]
- 22. Silberman E. Serious COVID‐19 Vaccine Reactions are Rare, Says New CDC Report; 2021.
- 23. Meo SA, Bukhari IA, Akram J, Meo AS, Klonoff DC. COVID‐19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and moderna vaccines. Eur Rev Med Pharmacol Sci. 2021;25:1663‐1669. [DOI] [PubMed] [Google Scholar]
- 24. Greenhawt M, Abrams EM, Oppenheimer J, et al. The COVID‐19 pandemic in 2021: avoiding overdiagnosis of anaphylaxis risk while safely vaccinating the world. J Allergy Clin Immunol Pract. 2021;9:1438‐1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sun Q, Fathy R, McMahon DE, Freeman EE. COVID‐19 vaccines and the skin: the landscape of cutaneous vaccine reactions worldwide. Dermatol Clin. 2021;39:653‐673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McMahon DE, Kovarik CL, Damsky W, et al. Clinical and pathologic correlation of cutaneous COVID‐19 vaccine reactions including V‐REPP: a registry‐based study. J Am Acad Dermatol. 2022;86:113‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fattori A, Cribier B, Chenard MP, Mitcov M, Mayeur S, Weingertner N. Cutaneous manifestations in patients with coronavirus disease 2019: clinical and histological findings. Hum Pathol. 2021;107:39‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Farinazzo E, Ponis G, Zelin E, et al. Cutaneous adverse reactions after m‐RNA COVID‐19 vaccine: early reports from Northeast Italy. J Eur Acad Dermatol Venereol. 2021;35:e548‐e551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: A registry‐based study of 414 cases. J Am Acad Dermatol. 2021;85:46‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rice SM, Ferree SD, Mesinkovska NA, Kourosh AS. The art of prevention: COVID‐19 vaccine preparedness for the dermatologist. Int J Women's Dermatol. 2021;7:209‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384:403‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Munavalli GG, Guthridge R, Knutsen‐Larson S, et al. COVID‐19/SARS‐CoV‐2 virus spike protein‐related delayed inflammatory reaction to hyaluronic acid dermal fillers: a challenging clinical conundrum in diagnosis and treatment. Arch Dermatol Res. 2022;314:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. CDC . Interim Clinical Considerations for Use of COVID‐19 Vaccines Currently Approved or Authorized in the United States 2021. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html
- 34. Kounis NG, Mazarakis A, Tsigkas G, Giannopoulos S, Goudevenos J. Kounis syndrome: a new twist on an old disease. Future Cardiol. 2011;7:805‐824. [DOI] [PubMed] [Google Scholar]
- 35. Palmiere C, Tettamanti C, Scarpelli MP. Vaccination and anaphylaxis: a forensic perspective. Croat Med J. 2017;58:14‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kelso JM. Anaphylactic reactions to novel mRNA SARS‐CoV‐2/COVID‐19 vaccines. Vaccine. 2021;39:865‐867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hatziantoniou S, Anastassopoulou C, Lampropoulou V, et al. Comparative assessment of allergic reactions to COVID‐19 vaccines in Europe and the United States. Allergy. 2022;77:1630‐1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wenande E, Garvey LH. Immediate‐type hypersensitivity to polyethylene glycols: a review. Clin Exp Allergy. 2016;46:907‐922. [DOI] [PubMed] [Google Scholar]
- 39. Garvey LH, Nasser S. Anaphylaxis to the first COVID‐19 vaccine: is polyethylene glycol (PEG) the culprit? Br J Anaesth. 2021;126:e106‐e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Klimek L, Novak N, Hamelmann E, et al. Severe allergic reactions after COVID‐19 vaccination with the Pfizer/BioNTech vaccine in Great Britain and USA: position statement of the German allergy societies: medical association of German Allergologists (AeDA), German Society for Allergology and Clinical Immunology (DGAKI) and Society for Pediatric Allergology and Environmental Medicine (GPA). Allergo J Int. 2021;30:51‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moghimi SM. Modulation of lymphatic distribution of subcutaneously injected poloxamer 407‐coated nanospheres: the effect of the ethylene oxide chain configuration. FEBS Lett. 2003;540:241‐244. [DOI] [PubMed] [Google Scholar]
- 42. Boffito M, Sirianni P, Di Rienzo AM, Chiono V. Thermosensitive block copolymer hydrogels based on poly(ɛ‐caprolactone) and polyethylene glycol for biomedical applications: state of the art and future perspectives. J Biomed Mater Res A. 2015;103:1276‐1290. [DOI] [PubMed] [Google Scholar]
- 43. Stone CA, Jr. , Liu Y, Relling MV, et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. the journal of allergy and clinical immunology. In Pract. 2019;7:1533‐1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schwartzberg LS, Navari RM. Safety of polysorbate 80 in the oncology setting. Adv Ther. 2018;35:754‐767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bruusgaard‐Mouritsen MA, Jensen BM, Poulsen LK, Duus Johansen J, Garvey LH. Optimizing investigation of suspected allergy to polyethylene glycols. J Allergy Clin Immunol. 2022;149:168‐175. [DOI] [PubMed] [Google Scholar]
- 46. Borderé A, Stockman A, Boone B, et al. A case of anaphylaxis caused by macrogol 3350 after injection of a corticosteroid. Contact Dermatitis. 2012;67:376‐378. [DOI] [PubMed] [Google Scholar]
- 47. Wylon K, Dölle S, Worm M. Polyethylene glycol as a cause of anaphylaxis. Allergy Asthma Clin Immunol. 2016;12:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang Q, Lai SK. Anti‐PEG immunity: emergence, characteristics, and unaddressed questions. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7:655‐677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Giavina‐Bianchi P, Kalil J. May polyethylene glycol be the cause of anaphylaxis to mRNA COVID‐19 vaccines? World Allergy Organ J. 2021;14:100532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Risma KA, Edwards KM, Hummell DS, et al. Potential mechanisms of anaphylaxis to COVID‐19 mRNA vaccines. J Allergy Clin Immunol. 2021;147:2075‐2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hawe A, Filipe V, Jiskoot W. Fluorescent molecular rotors as dyes to characterize polysorbate‐containing IgG formulations. Pharm Res. 2010;27:314‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Anagnostou K, Turner PJ. Myths, facts and controversies in the diagnosis and management of anaphylaxis. Arch Dis Child. 2019;104:83‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Banerji A, Wickner PG, Saff R, et al. mRNA vaccines to prevent COVID‐19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2021;9:1423‐1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Worm M, Ring J, Klimek L, et al. [Covid‐19 vaccination and risk of anaphylaxis‐recommendations for practical management]. MMW Fortschr Med. 2021;163:48‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. de Silva D, Singh C, Muraro A, et al. Diagnosing, managing and preventing anaphylaxis: systematic review. Allergy. 2021;76:1493‐1506. [DOI] [PubMed] [Google Scholar]
- 56. Gonçalves G. Herd immunity: recent uses in vaccine assessment. Expert Rev Vaccines. 2008;7:1493‐1506. [DOI] [PubMed] [Google Scholar]
- 57. Giurgea LT, Morens DM. Great expectations of COVID‐19 herd immunity. mBio. 2022;13:e0349521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Brilliant LB, Hodakevic LN. Certification of smallpox eradication. Bull World Health Organ. 1978;56:722‐733. [PMC free article] [PubMed] [Google Scholar]
- 59. WHO . Immunization Coverage; 2017. https://www.who.int/en/news-room/fact-sheets/detail/immunization-coverage
- 60. Scarbrough Lefebvre CD, Terlinden A, Standaert B. Dissecting the indirect effects caused by vaccines into the basic elements. Hum Vaccines Immunother. 2015;11:2142‐2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pfattheicher S, Petersen MB, Böhm R. Information about herd immunity through vaccination and empathy promote COVID‐19 vaccination intentions. Health Psychol. 41, 2022:85‐93. [DOI] [PubMed] [Google Scholar]
- 62. Dadras O, SeyedAlinaghi S, Karimi A, et al. Public acceptability of COVID‐19 vaccines and its predictors in Middle Eastern/North African (MENA) countries: a systematic review. Hum Vaccines Immunother. 2022;18:2043719. [DOI] [PMC free article] [PubMed] [Google Scholar]


