Abstract
An 83-year-old woman presented with a new onset of dyspnea and dysphonia. Physical examination revealed no abnormalities. Computerized tomography, bidimensional echocardiography, and cardiac magnetic resonance confirmed the presence of a cardiac mass in the left atrium. Surgical resection was uneventful and showed the origin of the mass in the ostium of the left inferior pulmonary vein. Histological evaluation revealed undifferentiated pleomorphic sarcoma with myxoid features. This case highlights the importance of considering cardiac neoplasms as a rare differential diagnosis, including rare and misleading clinical presentations.
Keywords: cardiopulmonary bypass, magnetic resonance imaging, sarcoma
Introduction
Primary cardiac tumors are rare, with an incidence ranging from 0.001 to 0.03. 1 Approximately 25% of them are malignant, sarcomas being the most prevalent in Hudzik et al. 2 Although very rare, undifferentiated pleomorphic sarcoma is the major type of primary cardiac malignancies, typically found in the left atrium and tends to involve the mitral valve. 3 Undifferentiated pleomorphic sarcoma is locally aggressive invasive tumors, frequently making complete surgical excision infeasible, implying a dismal prognosis and low survival rate. 4 Initial clinical and radiologic features of undifferentiated pleomorphic sarcoma might be mistaken for other sarcomas or even benign cardiac masses, making the diagnosis particularly challenging.
Case Presentation
We present an 83-year-old White non-smoker woman (1.70 m, 67 kg, BMI 23) with a history of systemic arterial hypertension, diabetes mellitus type II, and a positive family history of sudden cardiac death “SCD.” Her past medical history also included a hysterectomy for endometrial adenocarcinoma 14 years ago. She underwent a cardiological evaluation due to dyspnea under normal effort, which occurred in the last 2 weeks, and new onset of voice hoarseness. She denied any chest pain, fever, abdominal pain, nausea, or weight loss. The patient was normotensive during the physical examination, with a non-rhythmic heartbeat and normophonetic heart sounds, without any exciting heart murmurs. There were no signs of heart failure and no focal neurological deficit. ECG shows a new onset of atrial fibrillation. The chest CT scan showed a large left atrial mass with a tumor plug extending into the left inferior pulmonary vein, which was new in comparison to the computerized tomography scan from 2016 “5 years ago.” She underwent trans-esophageal echocardiography that showed a large mass in the left atrium approximately 5 × 4 × 3.8 cm, blocking a significant portion of the left atrium ( Fig. 1 ), a contrast medium examination with SonoVue was utilized, and the color-Doppler suspected the presence of small vessels within the mass.
Fig. 1.
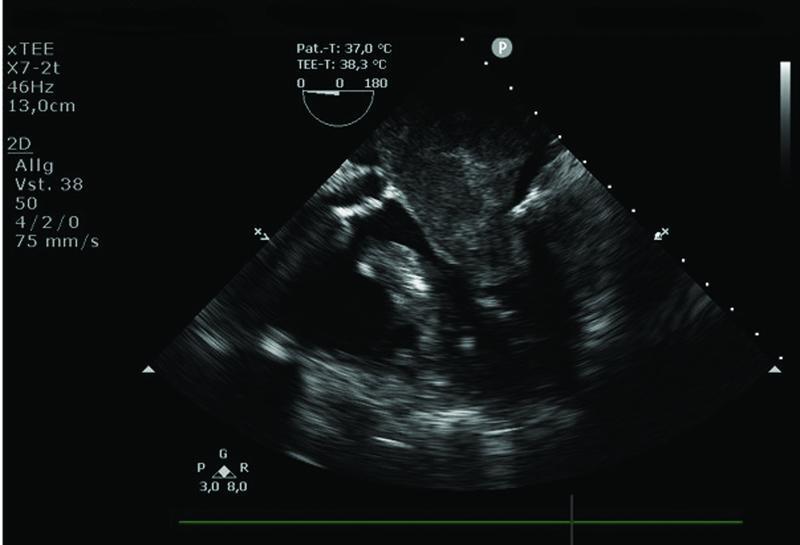
Transesophageal echocardiography mid-esophageal 5-chamber view showing the left atrial mass.
Cardiac magnetic resonance imaging “CMRI” using native as well as contrast medium enhanced sequences showed 55 × 40 × 48 mm rounded pedunculated mass in the left atrium ( Fig. 2 ), with tumor plug in the left inferior pulmonary vein, left ventricular ejection fraction, and heart dimensions were normal ( Fig. 3 ). The patient's complaint of dysphonia raised our suspicion for malignancy; for that reason, we performed a computerized tomography scan to screen for metastasis, which turned out to be unremarkable.
Fig. 2.
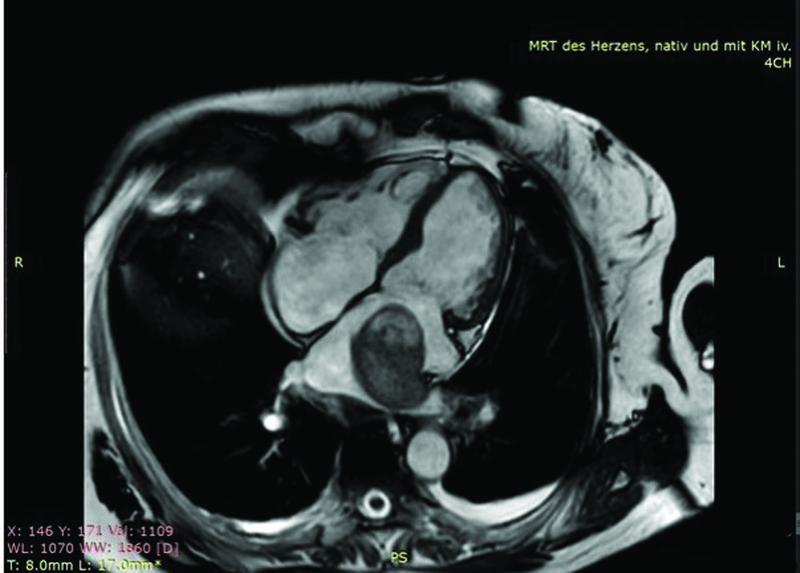
Cardiac magnetic resonance imaging “axial view” revealed the left atrial mass, which pedunculated with tumor plug in the left inferior pulmonary vein.
Fig. 3.
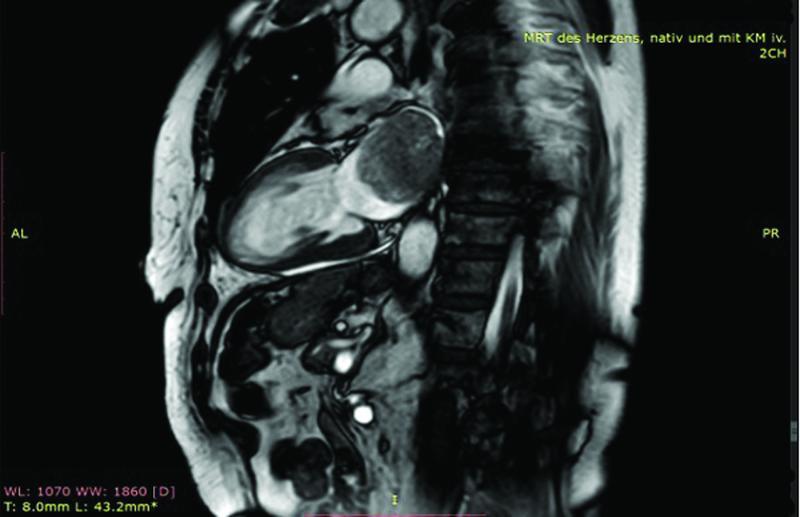
Cardiac magnetic resonance imaging “sagittal view” revealed the huge left atrial mass.
The cardiac catheterization showed normal coronary arteries and preserved the left ventricle's contractility.
The patient underwent surgical excision of the left atrial mass using cardiopulmonary bypass “CPB” ( Fig. 4 ). After median sternotomy, she was heparinized with checking of activated clotting time. Aortic-bicaval cannulation was installed, and CPB was established for 80 minutes. An aortic cross-clamp was applied for 48 minutes; the heart was arrested with an antegrade infusion of normothermic blood cardioplegic solution in the aortic root. After that, a left atriotomy was performed, revealing a large mobile vascularized tumor (5 cm × 6 cm) with pedicled origin in the left inferior pulmonary vein. The tumor was then resected ( Fig. 5 ). Special care was taken to minimize the risk of embolic complications to prevent its fragmentation. The left atrium was then closed in layers, and the root was vented. The heart was de-aired, and the aortic cross-clamp was removed.
Fig. 4.
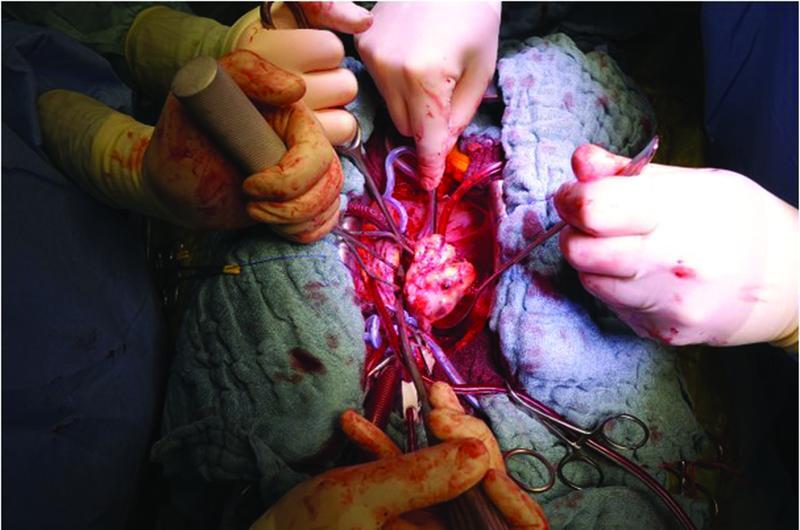
Intraoperative picture demonstrating the resection of tumor. The head of the patient is at 6:00, the legs at 12:00.
Fig. 5.
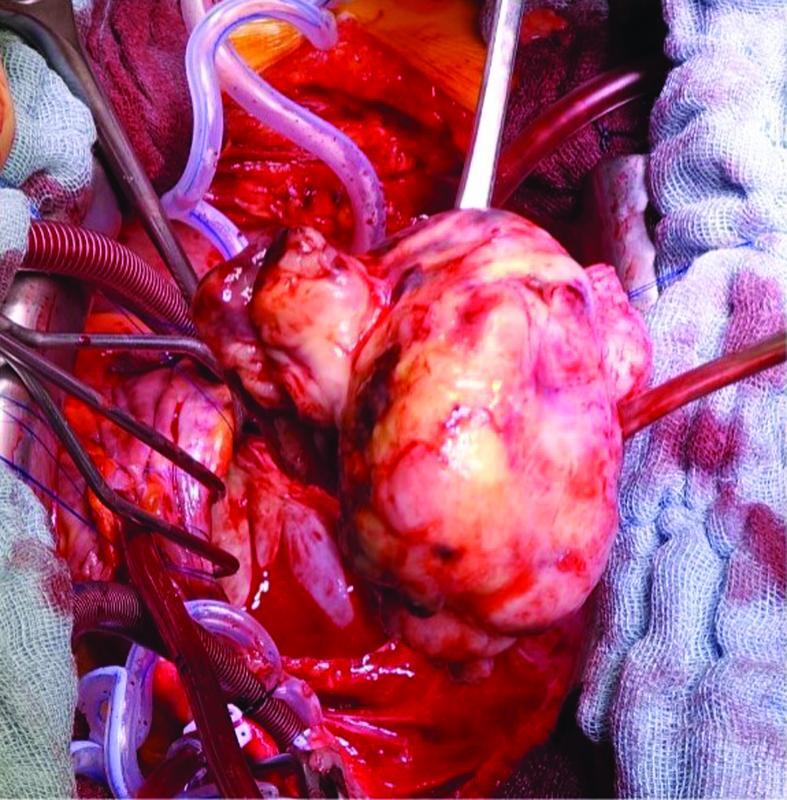
Intraoperative close-up photography of the extracted tumor.
The weaning off cardiopulmonary bypass was routinely performed after the spontaneous return of the heartbeats. Protamine decannulation was done, and hemostasis was secured. The chest was closed in layers. The patient tolerated all the following procedures well and was monitored in an intensive care unit for 24 hours and shifted to the surgical ward later for further postoperative care, which was uneventful.
Gross pathological examination of the resected mass revealed a partly smooth-surfaced tumor, measuring 6.0 × 5.2 × 4.5 cm with focal hemorrhages, foci of necrosis, and cystic degeneration on the cut surface. Histological examination revealed myxoid undifferentiated pleomorphic malignancy with variable myxoid areas, high cellularity with brisk mitotic activity in excess of 30 mitoses in 10 high power fields, and scattered immunoreactivity with MDM2 and CDK4 ( Fig. 6 ). These findings are consistent with myxoid undifferentiated pleomorphic sarcoma (Grade 3 according to FNCLCC).
Fig. 6.
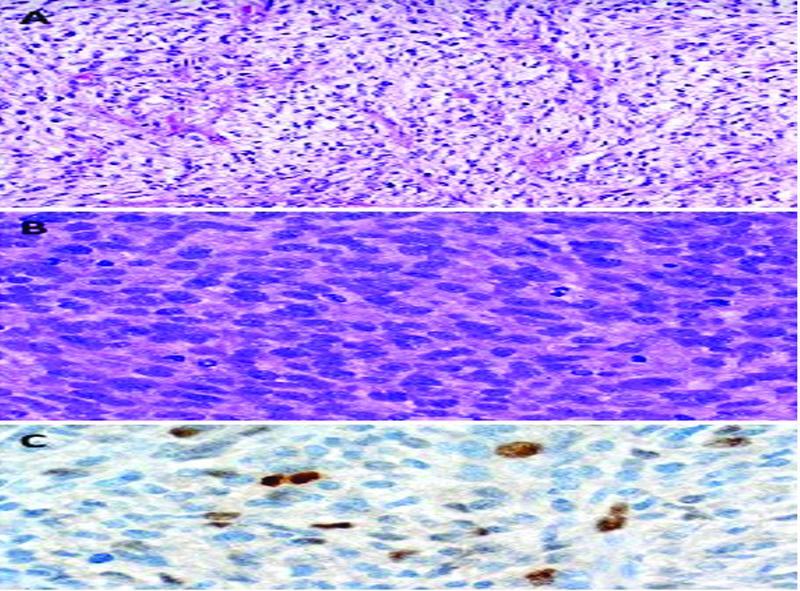
( A ) High myxoid areas with prominent vascular network (can be easily mistaken for myxoma). ( B ) High cellularity with pleomorphism and brisk mitotic activity. ( C ) Scattered MDM2 positive tumor cells.
The patient denied any aggressive adjuvant therapy because of her advanced age. The tumor board decision was cardiac and chest CT in 3 months, and the patient was then discharged free of symptoms.
Discussion
This case of undifferentiated sarcoma was misdiagnosed as a myxoma. The patient was oligosymptomatic, with recent onset of dyspnea during normal efforts without weight loss or embolic events. However, the new onset of dysphonia and the atypical origin of the tumor aroused suspicion toward malignancy; the metastasis screen came back negative. Cardiac tumors are rare, with only 25% of them being malignant. 5 Their frequency is similar in males and females, being higher in the right atrium, later left atrium, and both ventricles. 5 Differentiation between begin and malignant heart tumors is particularly challenging because of the similarity of presenting symptoms; however, the differences reside particularly in the clinical course and the histology. 6
Metastatic tumors are 20 to 40 times more common than primary malignant cardiac neoplasms. 7 Patients with malignant heart tumors of the left side have more frequent distant metastases, while the malignant heart tumors of the right side have more locally advanced disease. 8
In the largest study performed on cardiac masses, it was found that the average age at the appearance of the disease was 47.1 ± 16.1 years. The median follow-up was 51.2 months; until the analysis, 69.7% had died, 43% had metastasis, 44.9% of the patients had pulmonary metastasis and 20.9% had brain metastasis. 9 Optimal treatment is to obtain a complete surgical resection, which is possible in less than 50% of patients. Still, resection may be incomplete or even impossible because of the local extension; neoadjuvant chemotherapy may reduce the tumor burden improving resectability for large tumors. In a prospective analysis of right-sided tumors, this approach doubled the negative margin resection rate and survival. 10 Intensity-modulated radiotherapy “IMRT” as neoadjuvant radiotherapy seems promising in selected patients. It reduces the risk by focusing the radiation burden on the target neoplasm and limiting the involvement of the cardiac structures. Nevertheless, radiotherapy is rarely used in primary cardiac sarcomas because the target lesion is inside the heart. The constant movement of the heart makes it difficult to avoid the radiation of the surrounding structure. 11 The prognosis is very poor with a mean survival of 3 months to 1 year, 8 due to diagnostic delay, therapeutic difficulty, and high metastatic potential. For patients who underwent complete surgical resection, life expectancy is twice as long as for patients who underwent an incomplete surgical resection. 4 12 Local recurrence and metastases occur frequently and usually within 1 year. 13 This complex disease, in our opinion, should be treated by a multidisciplinary team that includes experienced cardio-oncologists, cardiac surgeons, imaging specialists, and sarcoma oncologists to get the best outcome. 14
Conclusion
This case highlights the surgical possibilities even in the elderly, as well as the importance of having a broad differential diagnosis, including rare entities.
Abbreviation
- CT
computerized tomography
- CMRI
cardiac magnetic resonance imaging
- CPB
cardiopulmonary bypass
- FNCLCC
Fédération Nationale des Centres de Lutte Contre Le Cancer
- CDK4
cyclin-dependent kinase 4
- MDM2
mouse double minute 2 homolog
Acknowledgments
The authors would like to acknowledge all who contributed to this case diagnosis, therapy, and decision-making. Acknowledgment is given to the Department of Cardiology in hospital and university hospital, especially for the heart surgery, oncology, and pathology department.
Funding Statement
Funding This publication was supported by Deutsche Forschungsgemeinschaft and University of Erlangen Foundation within the funding programme Open Access Publishing.
Conflict of Interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation to any qualified researcher.
Ethics Statement
The patient signed informed consent related to the clinical course, therefore and due to its retrospective nature of the educational case report, the Institutional Review Board was waived.
References
- 1.Amano J, Nakayama J, Yoshimura Y, Ikeda U. Clinical classification of cardiovascular tumors and tumor-like lesions, and its incidences. Gen Thorac Cardiovasc Surg. 2013;61(08):435–447. doi: 10.1007/s11748-013-0214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hudzik B, Miszalski-Jamka K, Glowacki J. Malignant tumors of the heart. Cancer Epidemiol. 2015;39(05):665–672. doi: 10.1016/j.canep.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Barreiro M, Renilla A, Jimenez J M. Primary cardiac tumors: 32 years of experience from a Spanish tertiary surgical center. Cardiovasc Pathol. 2013;22(06):424–427. doi: 10.1016/j.carpath.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Burke A P, Cowan D, Virmani R. Primary sarcomas of the heart. Cancer. 1992;69(02):387–395. doi: 10.1002/1097-0142(19920115)69:2<387::aid-cncr2820690219>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 5.Corradi D, Contini G A, Gherli T, Nicolini F. A left atrial myxomalike rhabdomyosarcoma. J Thorac Cardiovasc Surg. 2012;144(01):e7–e10. doi: 10.1016/j.jtcvs.2012.03.073. [DOI] [PubMed] [Google Scholar]
- 6.Strecker T, Rösch J, Weyand M, Agaimy A. Primary and metastatic cardiac tumors: imaging characteristics, surgical treatment, and histopathological spectrum: a 10-year-experience at a German heart center. Cardiovasc Pathol. 2012;21(05):436–443. doi: 10.1016/j.carpath.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Agaimy A, Rösch J, Weyand M, Strecker T. Primary and metastatic cardiac sarcomas: a 12-year experience at a German heart center. Int J Clin Exp Pathol. 2012;5(09):928–938. [PMC free article] [PubMed] [Google Scholar]
- 8.Ibrahim A, Luk A, Singhal P. Primary intimal (spindle cell) sarcoma of the heart: a case report and review of the literature. Case Rep Med. 2013;2013:461815. doi: 10.1155/2013/461815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isambert N, Ray-Coquard I, Italiano A. Primary cardiac sarcomas: a retrospective study of the French Sarcoma Group. Eur J Cancer. 2014;50(01):128–136. doi: 10.1016/j.ejca.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Abu Saleh W K, Ramlawi B, Shapira O M. Improved outcomes with the evolution of a neoadjuvant chemotherapy approach to right heart sarcoma. Ann Thorac Surg. 2017;104(01):90–96. doi: 10.1016/j.athoracsur.2016.10.054. [DOI] [PubMed] [Google Scholar]
- 11.Lestuzzi C, Cosei I, Ravasel A.P4656Short- and long-term evaluation of safety of cardiac sarcomas radiotherapy Eur Heart J 201940(suppl_1):ehz745.1038.https://doi.org/10.1093/eurheartj/ehz745.1038 [Google Scholar]
- 12.Devbhandari M P, Meraj S, Jones M T, Kadir I, Bridgewater B. Primary cardiac sarcoma: reports of two cases and a review of current literature. J Cardiothorac Surg. 2007;2(01):34. doi: 10.1186/1749-8090-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta A. Primary cardiac sarcomas. Expert Rev Cardiovasc Ther. 2008;6(10):1295–1297. doi: 10.1586/14779072.6.10.1295. [DOI] [PubMed] [Google Scholar]
- 14.Lestuzzi C, Reardon M J. Primary cardiac malignancies: the need for a multidisciplinary approach and the role of the cardio-oncologist. J Am Coll Cardiol. 2020;75(18):2348–2351. doi: 10.1016/j.jacc.2020.03.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation to any qualified researcher.


