Abstract
In light of the growing emphasis on classifying stroke patients for different levels of monitoring intensity and emergency treatments, we conducted a systematic review of a wide range of clinical studies, according to the preferred reporting items for systematic review and meta-analysis (PRISMA) guidelines, with no restrictions on the language or publication date, to analyze the potential of the neutrophil-to-lymphocyte ratio (NLR) as an early neurological deterioration (END) risk predictor. A comprehensive search was carried out in PubMed, Scopus, and Web of Science databases from the inception to March 13, 2022. Nine articles were included in our study. Stroke patients with END had significantly higher NLR levels than the those without END (SMD = 0.73; CI 95% = 0.42-1.05, P value < 0.001). In the subgroup analysis, according to ethnicity, East Asian patients with END had elevated levels of NLR compared to those without END (SMD = 0.79; CI 95% = 0.52-1.06, P value < 0.001). However, the difference in the Caucasian group was not significant (SMD = 0.60; CI 95% = −0.50-1.70, P value = 0.28). In the subgroup analysis according to the type of stroke, the NLR levels in patients with hemorrhagic stroke who developed END were similar to those without END (SMD = 0.84, CI 95% = −0.10-1.77, P value = 0.07). Vice versa, in the ischemic stroke group, patients with END had elevated levels of NLR compared to those without END (SMD = 0.67, CI 95% = 0.38-0.96, P value < 0.001). NLR is a unique inflammatory biomarker whose increase in END suggests an immune system dysfunction in the pathogenesis of the disease.
1. Introduction
Early neurological deterioration (END) refers to the worsening of symptoms days and weeks after a stroke [1]. Differences in case-mix and END definitions have resulted in a broad range of incidence estimates, with a prior review estimating frequencies ranging from 2.2 to 37.5% at 24 hours after onset [2]. A standard criterion is a two-point increase in the National Institutes of Health Stroke Score (NIHSS) within 24–48 hours after admission [2]. By any definition, the issue of predicting END risk at the time of admission has received considerable critical attention because END is associated with poorer outcomes since it reflects local hemodynamic disruptions, the extension of thrombosis, inflammation, excitotoxicity, and accelerated malfunction of penumbral tissue that covers a compressive hematoma or ischemic core [3]. Radiological and clinical associations with END have already been adequately reported [4]. END has been linked to inflammatory biomarkers such as high-sensitivity C-reactive protein (CRP), leucocyte count, homocysteine, CSF glutamate, and plasma glutamate, according to a recent meta-analysis on stroke biomarkers [5]. However, the neutrophil-to-lymphocyte ratio (NLR) was not taken into account in that meta-analysis.
The NLR is a biomarker that represents the balance between these two elements of the inflammatory response: adaptive immunity (as indicated by the lymphocyte count) and innate immunity (as indicated by the neutrophil count) [6]. It is determined as a simple ratio between the neutrophil and lymphocyte counts assessed in blood samples. NLR has been studied extensively and found to be linked to patient outcomes and disease progression in a range of medical illnesses, including infectious diseases [7], sepsis [8], major cardiac events [9], ischemic stroke [10], and cerebral hemorrhage [10]. Furthermore, greater NLR has been linked to a poor prognosis in cancer patients [11]. These negative connections could be due to the role of severe inflammation and a compromised immune system in the development of these disorders. In the context of stroke, it has been shown that NLR could predict poststroke infection [12], poststroke depression [13], mortality, and functional outcome [14]. By extension, NLR may be a predictor of END in stroke patients. Several studies have found that an elevated NLR is related to the development of END [15–23]. In light of the growing emphasis on classifying stroke patients for different levels of monitoring intensity and emergency treatments, we conducted a systematic review of a wide range of clinical studies, with no restrictions on the language or publication date, to analyze the potential of NLR as an END risk predictor. Although extensive research has been carried out on the relationship between NLR and poor outcome in stroke patients, no single study exists which review the published articles on the potential of NLR as an END risk predictor. This is, to our knowledge, the first meta-analysis on this context. A better knowledge of the link between NLR and END will assist in elucidating the role of inflammation and immunology in the progression and prognosis of this condition, as well as identify patients who require early intervention and further monitoring and imaging.
2. Methods
2.1. Search Strategy
These systematic review and meta-analysis were performed according to the preferred reporting items for systematic review and meta-analysis (PRISMA) guidelines. A comprehensive search was carried out in PubMed, Scopus, and Web of Science databases from the inception to March 13, 2022. The following keywords were used: (Early neurological deterioration) AND (stroke) AND (neutrophil to lymphocyte ratio OR NLR). References listed in the original reports were searched manually to find any missing articles and further potentially relevant articles.
2.2. Study Selection
The inclusion criteria were as follows: (1) case-control or cross-sectional studies, (2) a population including adult patients with stroke, including ischemic and hemorrhagic ones, (3) compared stroke patients with END with those without END, (4) reported the quantitative blood NLR level as mean ± standard deviation (SD) or median (range or interquartile range), and (5) full text being available. The exclusion criteria were as follows: (1) animal studies, review, case reports, comments, or letters; (2) studies that enrolled subjects with any concomitant disorders such as cancer; and (3) duplicate publication.
2.3. Data Extraction
The titles and abstracts of the identified studies were evaluated by two authors independently. Potentially, relevant articles identified in the initial assessment were further screened in full text. Data concerning the first author's name, country, year of publication, stroke type, sample size, and NLR levels were extracted independently by two authors. Any discrepancies were resolved by a third reviewer or by discussion.
2.4. Quality Assessment
The Newcastle-Ottawa scale (NOS) method with scores ranging from 0 to 9 was adopted to evaluate the quality of the studies [24]. The NOS has three sections: (1) selection of study populations, (2) comparability of populations, and (3) ascertainment of exposure. In the case of any disagreements, the authors would reach a consensus through discussion.
2.5. Statistical Analysis
The statistical analysis was conducted using Stata 11.2 software (Stata Corp, College Station, TX). The standard mean difference (SMD) and corresponding 95% confidence interval (CI) were reported. The inconsistency index (I2) test and Q-statistic were used to assess the heterogeneity across studies. I2 values of 25%, 50%, and 75% represented small, moderate, and high levels of heterogeneity, respectively. A random effects model would be adopted if significant heterogeneity was found. The method introduced by Wan et al. was adopted to estimate the mean and SD values using the median, range, or IQR values [25]. Subgroup meta-analyses were conducted according to ethnicity (Caucasian and East Asian) and stroke type (ischemic and hemorrhagic). Publication bias was assessed using the funnel plot and Egger test.
3. Results
3.1. Eligible Studies
Of the 101 related studies identified in the initial search, 12 duplicate publications were excluded and 89 remaining articles were screened in title and abstract. Then, 72 studies unrelated to NLR or END were excluded. After screening of the remaining 17 studies in full text, three studies without any data on NLR and END, two studies with missing SD data, one study including stroke patients suffering from cancer, one study without a control group, and one review article were excluded. Finally, nine articles were included in our study [15–23] (Figure 1).
Figure 1.
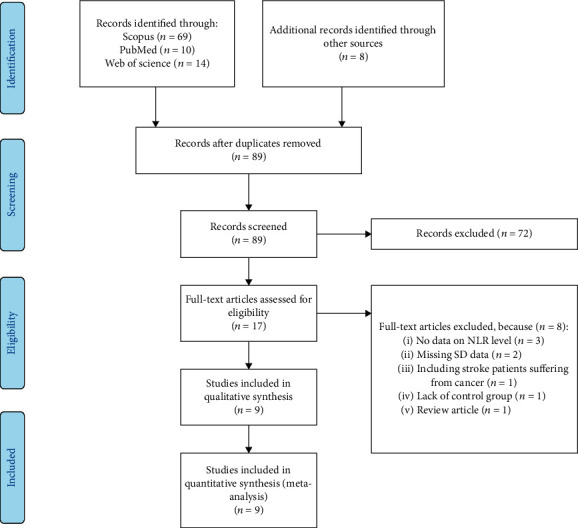
Flow chart of search and study selection.
3.2. Study Characteristics
All those publications were single-center studies that included adult patients with stroke. The quality score of the study ranged from 5 to 8 according to the NOS. Eight studies were in English and one in Chinese. Three studies were on ischemic stroke and six studies on hemorrhagic stroke. Three studies investigated Caucasian patients, and six studies investigated East Asian patients. One study was prospective, and eight studies were retrospective. Table 1 shows the characteristics of the included studies.
Table 1.
General characteristics of included studies.
| First author | Year | Country | Type of stroke | Ethnicity | Design | END definition | END group | Non-END group | NOS score | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | NLR | N | NLR | ||||||||
| Lattanzi et al. [19] | 2017 | Italy | Hemorrhagic | Caucasian | R | (1) An increase ≥ 4 on the NIHSS score, (2) a decrease ≥ 2 on GCS, or (3) death occurred from the time of admission to 7 days posthemorrhage | 54 | 9.46 ± 5.80 | 138 | 3.28 ± 1.98 | 8 |
| Qi et al. [22] | 2018 | China | Hemorrhagic | East Asian | R | (1) An increase ≥ 4 on the NIHSS score, (2) a decrease ≥ 2 on GCS, or (3) death occurred from the time of admission to 7 days posthemorrhage | 166 | 15.98 ± 8.83 | 392 | 8.03 ± 6.44 | 7 |
| Nam et al. [21] | 2019 | Korea | Ischemic | East Asian | R | An increase ≥ 2 on the total NIHSS score or ≥1 on the motor NIHSS score within the first 72 hours of admission | 63 | 3.49 ± 2.73 | 286 | 2.56 ± 1.72 | 7 |
| Chen et al. [15] | 2020 | China | Ischemic | East Asian | R | (1) An increase in NIHSS ≥ 4 from baseline or (2) an increase in Ia of NIHSS ≥ 1 within 72 h | 77 | 8.06 ± 8.36 | 180 | 5.24 ± 3.28 | 7 |
| Cong and Ma [16] | 2021 | China | Ischemic | East Asian | R | (1) An increase > 2 points in the total NIHSS score compared to the score at admission, (2) an increase > 1 point in the NIHSS specificity subitems, namely, level of consciousness (1a–1c) or motor capacity (5a–6b), or (3) new neurological deficits despite no change in the NIHSS score | 55 | 6.06 ± 8.36 | 74 | 4.16 ± 3.73 | 8 |
| Ferro et al. [17] | 2021 | Portugal | Ischemic | Caucasian | R | An increase in NIHSS at 24 hours from the baseline | 85 | 6.83 ± 5.81 | 240 | 6.05 ± 4.95 | 8 |
| Gong et al. [18] | 2021 | China | Ischemic | East Asian | R | An increase in the NIHSS score by ≥4 points in the total score within 24 h after thrombolysis | 193 | 8.42 ± 6.50 | 469 | 4.18 ± 1.57 | 7 |
| Mohamed et al. [20] | 2021 | Egypt | Hemorrhagic | Caucasian | P | (1) An increase ≥ 4 on the NIHSS score, (2) a decrease ≥ 2 on GCS, or (3) death occurred from the time of admission to 7 days posthemorrhage | 17 | 15.92 ± 6.86 | 63 | 23.25 ± 18.39 | 8 |
| Zhi and Xiaopeng [23] | 2021 | China | Ischemic | East Asian | R | An increase ≥ 2 on the total NIHSS score or ≥1 on the motor NIHSS score within the first 72 hours of admission | 32 | 3.30 ± 1.70 | 140 | 2.40 ± 0.97 | 5 |
R: retrospective; P: prospective; N: number; SD: standard deviation; NLR: neutrophil to lymphocyte ratio; NOS: Newcastle-Ottawa scale.
3.3. Overall Meta-Analysis
The nine studies [15–23] reported data belonging to 2724 stroke patients, 742 of whom developed END. The analysis was conducted using the random effects model because of the statistically significant heterogeneity (I2 = 91.1%, P value < 0.0001) (Figure 2). The END group had significantly higher NLR levels than the non-END group (SMD = 0.73; CI 95% = 0.42-1.05, P value < 0.001) (Figure 2).
Figure 2.
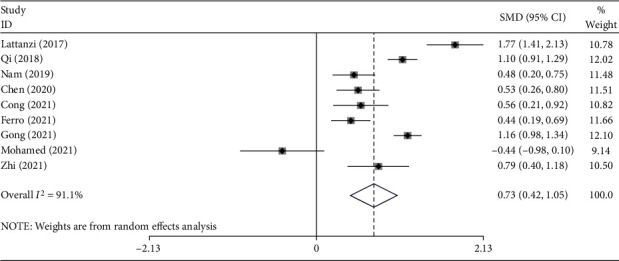
Meta-analysis of differences in NLR level between END and non-END patients.
In the subgroup analysis, according to ethnicity, three studies included Caucasian subjects [17, 19, 20], with 597 stroke patients, 156 of whom developed END, and six studies included East Asian subjects [15, 16, 18, 21–23] with 2127 stroke patients, 586 of whom developed END. East Asian patients with END had elevated levels of NLR compared to those without END (SMD = 0.79; CI 95% = 0.52-1.06, P value < 0.001). However, the difference in the Caucasian group was not significant (SMD = 0.60; CI 95% = −0.50-1.70, P value = 0.28) (Figure 3).
Figure 3.
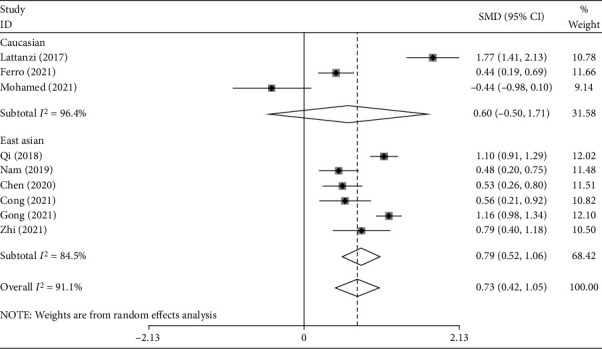
Subgroup analysis of differences in NLR level between END and non-END patients according to ethnicity.
In the subgroup analysis according to the type of stroke, there were three studies on hemorrhagic stroke [19, 20, 22], including 830 patients, of whom 237 developed END, and six studies on ischemic stroke [15–18, 21, 23], including 1894 patients, of whom 505 developed END. The NLR levels in patients with hemorrhagic stroke who developed END were similar to those without END (SMD = 0.84, CI 95% = −0.10-1.77, P value = 0.07). Vice versa, in the ischemic stroke group, patients with END had elevated levels of NLR compared to those without END (SMD = 0.67, CI 95% = 0.38-0.96, P value < 0.001) (Figure 4).
Figure 4.
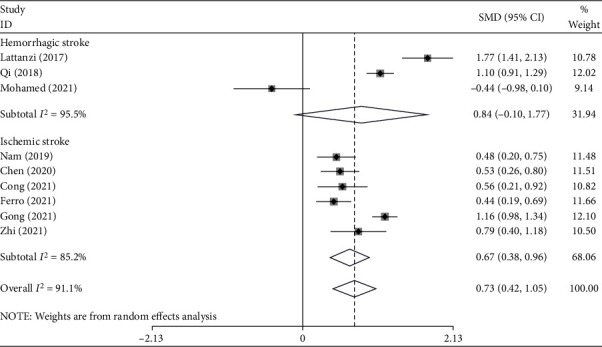
Subgroup analysis of differences in NLR level between END and non-END patients according to type of stroke.
3.4. Publication Bias
The results of studies on the role of NLR in END showed no publication bias (Egger's test P value = 0.13) (Figure 5).
Figure 5.
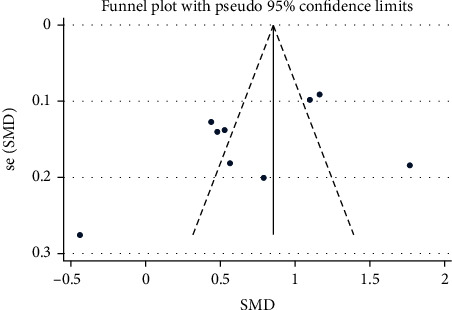
Funnel plot assessing publication bias.
4. Discussion
Inflammation is a hallmark of stroke progression and etiology [26]. Inflammation after a stroke is thought to be an essential pathogenic process. The blood-brain barrier (BBB), which was disrupted after the acute brain injury, is passed by stimulated peripheral immune cells such as neutrophils and monocytes, resulting in a variety of negative consequences. Furthermore, the poststroke immune reaction is linked to the severity of the stroke at the time of admission as assessed by the NIHSS. However, the link between poststroke inflammation and END remains unclear. As a result, we conducted this systematic review and meta-analysis to investigate further the impact of inflammatory mediators on END following stroke [26, 27].
The findings of our study show that there was a significant difference in NLR values between stroke patients who developed END and those who did not. NLR values were found to be higher in those who had END. Our findings imply that NLR is a distinct inflammatory marker that may reveal crucial physiologic abnormalities in the development of END.
Severe diseases such as stroke stimulate the generation of neutrophils in the bone marrow and can lead to lymphopenia in a variety of ways [28]. So, relative lymphopenia and neutrophilia can develop, resulting in an increased NLR.
Neutrophils are essential components of innate immunity that help to increase proinflammatory responses [28]. On the other hand, lymphocytes are adaptive immune system components that modulate immunological responses [29]. In the presence of a high NLR, the proinflammatory activity of neutrophils may exceed lymphocytes' regulatory function, creating an environment for uncontrolled peripheral inflammation to spread to a vulnerable brain [30].
In the case of END, a neuroinflammatory pathogenesis hypothesis has gotten a lot of attention [5, 31]. To date, there is strong evidence that neuroinflammation occurs as a result of systemic inflammation. It is postulated that a “crosstalk” occurs between the peripheral inflammatory response and the once “immune-privileged” central nervous system through transportation across the blood-brain barrier (BBB), afferent nerve stimulation, and transmission across circumventricular organs [32]. Uncontrolled peripheral inflammation, assessed by NLR, can take advantage of these pathways to trigger neuroinflammatory processes that lead to END-causing changes in brain systems. For example, new evidence suggests that neutrophils play a significant role in the production of cytokine and chemokine [33], which are commonly raised in END patients and are hypothesized to contribute to neuroinflammation, an essential mechanism in END development and progression [5]. Despite the fact that neutrophils release fewer inflammatory mediators in vitro than their leukocyte counterparts, the neutrophil's cumulative magnitude of secretion, due to their sheer number in circulation, is expected to contribute significantly to cytokine and chemokine pools [33].
CRP, TNF-alpha, IL-4, IL-6, IL-8, and IL-10 are among inflammatory mediators that have been linked to END [5, 34]. IL-6 is currently one of the most researched cytokines in END [35]. It has been shown in numerous research that it is elevated in patients with END [35]. Neutrophils are the main source of IL-6 production in vitro and a primary source of IL-6 in stroke models in rats [33]. Because cytokine secretion may be the result of an unrestrained neutrophilic reaction, normalizing NLR or targeting neutrophils may be an effective therapeutic target.
NLR is a simple biomarker that may be derived during admission from a white blood cell differential and requires no additional time and work. According to recent research, NLR has high sensitivity and specificity for predicting END [15–23]. However, it is worth noting that these figures differ between research, possibly due to differences in the study population and medical circumstances. As a result, in some cases, NLR may be best used in conjunction with other diagnostic markers. Notably, several researchers have constructed prediction models that dramatically boost areas under the receiver operating curves when NLR values are included.
5. Limitations
There are a few limitations in our research that must be addressed. To begin with, the study sample size for determining the NLR value in some subgroups, such as Caucasians and patients with hemorrhagic stroke, was limited, and hence, our findings may not be properly powered to draw conclusions about such values. It is also worth noting that NLR values differ by race, which could explain why geographic analysis for NLR had no significant results among Caucasians. It is probable that some populations do not undergo typical hematopoiesis changes after a stroke, and hence, NLR is not useful in those areas. A meta-analysis also carries the risk of study heterogeneity. More than one approach was used to diagnose END among included studies, and, among those used, there is a possibility of user variability due to their subjective nature. As a result, these studies may have varied rates of missed diagnoses, which could compromise their validity.
6. Conclusion
The findings of our investigation support a link between NLR levels and the development of END in stroke patients. NLR is a unique inflammatory biomarker whose increase in END suggests an immune system dysfunction in the pathogenesis of the disease. Furthermore, our data suggest that NLR is a viable biomarker that may be easily introduced into clinical settings to help predict and prevent END. Finally, the development of new therapeutic modalities and biomarkers will help us better prevent and treat END, lowering long-term mortality and morbidity.
Data Availability
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
References
- 1.Thanvi B., Treadwell S., Robinson T. Early neurological deterioration in acute ischaemic stroke: predictors, mechanisms and management. Postgraduate Medical Journal . 2008;84(994):412–417. doi: 10.1136/pgmj.2007.066118. [DOI] [PubMed] [Google Scholar]
- 2.Siegler J. E., Martin-Schild S. Early neurological deterioration (END) after stroke: the END depends on the definition. International Journal of Stroke . 2011;6(3):211–212. doi: 10.1111/j.1747-4949.2011.00596.x. [DOI] [PubMed] [Google Scholar]
- 3.Kwan J., Hand P. Early neurological deterioration in acute stroke: clinical characteristics and impact on outcome. Journal of the Association of Physicians . 2006;99(9):625–633. doi: 10.1093/qjmed/hcl082. [DOI] [PubMed] [Google Scholar]
- 4.Simonsen C. Z., Schmitz M. L., Madsen M. H., et al. Early neurological deterioration after thrombolysis: clinical and imaging predictors. International Journal of Stroke . 2016;11(7):776–782. doi: 10.1177/1747493016650454. [DOI] [PubMed] [Google Scholar]
- 5.Martin A. J., Price C. I. A systematic review and meta-analysis of molecular biomarkers associated with early neurological deterioration following acute stroke. Cerebrovascular Diseases . 2019;46(5-6):230–241. doi: 10.1159/000495572. [DOI] [PubMed] [Google Scholar]
- 6.Faria S. S., Fernandes P. C., Jr., Silva M. J. B., et al. The neutrophil-to-lymphocyte ratio: a narrative review. Ecancermedicalscience . 2016;10 doi: 10.3332/ecancer.2016.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naess A., Nilssen S. S., Mo R., Eide G. E., Sjursen H. Role of neutrophil to lymphocyte and monocyte to lymphocyte ratios in the diagnosis of bacterial infection in patients with fever. Infection . 2017;45(3):299–307. doi: 10.1007/s15010-016-0972-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Z., Fu Z., Huang W., Huang K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: a meta-analysis. The American Journal of Emergency Medicine . 2020;38(3):641–647. doi: 10.1016/j.ajem.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 9.Dong C.-H., Wang Z.-M., Chen S.-Y. Neutrophil to lymphocyte ratio predict mortality and major adverse cardiac events in acute coronary syndrome: a systematic review and meta-analysis. Clinical Biochemistry . 2018;52:131–136. doi: 10.1016/j.clinbiochem.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Song S.-Y., Zhao X.-X., Rajah G., et al. Clinical significance of baseline neutrophil-to-lymphocyte ratio in patients with ischemic stroke or hemorrhagic stroke: an updated meta-analysis. Frontiers in Neurology . 2019;10 doi: 10.3389/fneur.2019.01032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin Y., Wang J., Wang X., et al. Prognostic value of the neutrophil to lymphocyte ratio in lung cancer: a meta-analysis. Clinics . 2015;70(7):524–530. doi: 10.6061/clinics/2015(07)10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He L., Wang J., Wang F., Zhang L., Zhang L., Zhao W. Increased neutrophil-to-lymphocyte ratio predicts the development of post-stroke infections in patients with acute ischemic stroke. BMC Neurology . 2020;20(1):1–7. doi: 10.1186/s12883-020-01914-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H., Luan X., Zhao K., et al. The association between neutrophil-to-lymphocyte ratio and post-stroke depression. Clinica Chimica Acta . 2018;486:298–302. doi: 10.1016/j.cca.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 14.Yu S., Arima H., Bertmar C., Clarke S., Herkes G., Krause M. Neutrophil to lymphocyte ratio and early clinical outcomes in patients with acute ischemic stroke. Journal of the Neurological Sciences . 2018;387:115–118. doi: 10.1016/j.jns.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Chen Z., He Y., Su Y., Sun Y., Zhang Y., Chen H. Association of inflammatory and platelet volume markers with clinical outcome in patients with anterior circulation ischaemic stroke after endovascular thrombectomy. Neurological Research . 2021;43(6):503–510. doi: 10.1080/01616412.2020.1870359. [DOI] [PubMed] [Google Scholar]
- 16.Cong L., Ma W. Early neurological deterioration in cardiogenic cerebral embolism due to nonvalvular atrial fibrillation: predisposing factors and clinical implications. Brain and Behavior . 2021;11(2, article e01985) doi: 10.1002/brb3.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferro D., Matias M., Neto J., et al. Neutrophil-to-lymphocyte ratio predicts cerebral edema and clinical worsening early after reperfusion therapy in stroke. Stroke . 2021;52(3):859–867. doi: 10.1161/STROKEAHA.120.032130. [DOI] [PubMed] [Google Scholar]
- 18.Gong P., Liu Y., Gong Y., et al. The association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and lymphocyte to monocyte ratio with post-thrombolysis early neurological outcomes in patients with acute ischemic stroke. Journal of Neuroinflammation . 2021;18(1):1–11. doi: 10.1186/s12974-021-02090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lattanzi S., Cagnetti C., Provinciali L., Silvestrini M. Neutrophil-to-lymphocyte ratio and neurological deterioration following acute cerebral hemorrhage. Oncotarget . 2017;8(34):57489–57494. doi: 10.18632/oncotarget.15423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohamed W. S., Kamel A. E., Abdelwahab A. H., Mahdy M. E. Short-term outcome in ischemic stroke patients after thrombolytic therapy. The Egyptian Journal of Neurology, Psychiatry and Neurosurgery . 2021;57(1):1–6. doi: 10.1186/s41983-020-00251-7. [DOI] [Google Scholar]
- 21.Nam K.-W., Kim T. J., Lee J. S., et al. Neutrophil-to-lymphocyte ratio predicts early worsening in stroke due to large vessel disease. PLoS One . 2019;14(8, article e0221597) doi: 10.1371/journal.pone.0221597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi H., Wang D., Deng X., Pang X. Lymphocyte-to-monocyte ratio is an independent predictor for neurological deterioration and 90-day mortality in spontaneous intracerebral hemorrhage. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research . 2018;24:9282–9291. doi: 10.12659/MSM.911645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhi X., Xiaopeng Y. Value of neutrophil-lymphocyte ratio and mean platelet volume in predicting early neurological function deterioration in patients with acute ischemic stroke. Journal of Practical Clinical Medicine . 2021;25(7):42–46. [Google Scholar]
- 24.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European Journal of Epidemiology . 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 25.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology . 2014;14(1):1–13. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anrather J., Iadecola C. Inflammation and stroke: an overview. Neurotherapeutics . 2016;13(4):661–670. doi: 10.1007/s13311-016-0483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lambertsen K. L., Finsen B., Clausen B. H. Post-stroke inflammation—target or tool for therapy? Acta Neuropathologica . 2019;137(5):693–714. doi: 10.1007/s00401-018-1930-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jickling G. C., Liu D., Ander B. P., Stamova B., Zhan X., Sharp F. R. Targeting neutrophils in ischemic stroke: translational insights from experimental studies. Journal of Cerebral Blood Flow & Metabolism . 2015;35(6):888–901. doi: 10.1038/jcbfm.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doyle K. P., Quach L. N., Solé M., et al. B-lymphocyte-mediated delayed cognitive impairment following stroke. Journal of Neuroscience . 2015;35(5):2133–2145. doi: 10.1523/JNEUROSCI.4098-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tokgoz S., Kayrak M., Akpinar Z., Seyithanoğlu A., Güney F., Yürüten B. Neutrophil lymphocyte ratio as a predictor of stroke. Journal of Stroke and Cerebrovascular Diseases . 2013;22(7):1169–1174. doi: 10.1016/j.jstrokecerebrovasdis.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Alawneh J. A., Moustafa R. R., Baron J.-C. Hemodynamic factors and perfusion abnormalities in early neurological deterioration. Stroke . 2009;40(6):e443–e450. doi: 10.1161/STROKEAHA.108.532465. [DOI] [PubMed] [Google Scholar]
- 32.Tian L., Ma L., Kaarela T., Li Z. Neuroimmune crosstalk in the central nervous system and its significance for neurological diseases. Journal of Neuroinflammation . 2012;9(1):1–10. doi: 10.1186/1742-2094-9-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tecchio C., Micheletti A., Cassatella M. A. Neutrophil-derived cytokines: facts beyond expression. Frontiers in Immunology . 2014;5:p. 508. doi: 10.3389/fimmu.2014.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng Q.-W., Huang S., Li S., et al. Inflammatory factors as potential markers of early neurological deterioration in acute ischemic stroke patients receiving endovascular therapy – the AISRNA study. Journal of Inflammation Research . 2021;14:4399–4407. doi: 10.2147/JIR.S317147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vila N., Castillo J., Dávalos A., Chamorro A. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke . 2000;31(10):2325–2329. doi: 10.1161/01.STR.31.10.2325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


