Abstract
Background
Obesity, especially severe obesity, is associated with a higher risk of atherosclerotic cardiovascular disease (ASCVD) morbidity and mortality. Bariatric surgery is a durable and effective weight loss therapy for patients with severe obesity and weight-related comorbidities. Elevated plasma levels of lipoprotein (a) (Lp(a)) are causally associated with ASCVD. The aim of this meta-analysis was to analyze whether bariatric surgery is associated with Lp(a) concentrations.
Methods
A literature search in PubMed, Scopus, Embase, and Web of Science was performed from inception to May 1st, 2021. A random-effects model and the generic inverse variance weighting method were used to compensate for the heterogeneity of studies in terms of study design, treatment duration, and the characteristics of the studied populations. A random-effects metaregression model was used to explore the association with an estimated effect size. Evaluation of funnel plot, Begg's rank correlation, and Egger's weighted regression tests were used to assess the presence of publication bias in the meta-analysis.
Results
Meta-analysis of 13 studies including 1551 patients showed a significant decrease of circulating Lp(a) after bariatric surgery (SMD: -0.438, 95% CI: -0.702, -0.174, p < 0.001, I2: 94.05%). The results of the metaregression did not indicate any significant association between the changes in Lp(a) and duration of follow-up after surgery, reduction in body mass index, or baseline Lp(a) concentration. The reduction in circulating Lp(a) was robust in the leave-one-out sensitivity analysis.
Conclusion
Bariatric surgery significantly decreases circulating Lp(a) concentrations. This decrease may have a positive effect on ASCVD in obese patients.
1. Introduction
It is well known that obesity, especially severe obesity, is associated with a higher risk of atherosclerotic cardiovascular disease (ASCVD) morbidity and mortality [1]. Obesity is a widespread disease on a global scale and a major public health issue [2]. The use of bariatric surgical procedures has increased steadily over the past decades because these procedures result in significant and long-term weight loss, more than the one achieved by diet and lifestyle modifications alone. It is important to stress that bariatric surgery prolongs the lifespan in high-risk individuals for ASCVD [3, 4]. However, less than 2% of eligible patients have bariatric surgery despite the fact that bariatric therapies today are well established and have good safety and efficacy.
The most widely performed bariatric procedures are sleeve gastrectomy (SG), Roux-en-Y gastric bypass (RYGB), laparoscopic adjustable gastric band (LAGB), biliopancreatic diversion/duodenal switch (BPD/DS), and one anastomosis gastric bypass/minigastric bypass (OAGB/MGB) [5].
Weight loss, no matter how achieved, decreases the risk of ASCVD, cardiovascular events, and total mortality. Weight loss has beneficial effects on the main risk factors for ASCVD including elevated total and LDL-cholesterol (LDL-C), triglycerides, and decreased HDL-cholesterol (HDL-C) [6]. Bariatric surgery has beneficial effects on cardiovascular indices [7–9]. For instance, it has been shown that gastric bypass surgery improved all lipid profile parameters, although sleeve gastrectomy only improved HDL-C and triglyceride levels [10, 11]. It has also been recently demonstrated that sleeve gastrectomy causes an increase in HDL-C and that biliopancreatic diversion causes a significant decrease in total cholesterol, LDL-C, non-HDL-C, and LDL-C/non-HDL-C [12]. It has also been shown that bariatric surgery might prevent or slow atherogenesis in the early stages by breaking the vicious circle between inflammation and endothelial dysfunction [13]. Bariatric surgery also results in a decrease in pulse wave velocity (PWV), which might be used as an independent surrogate marker of ASCVD improvement [14].
Lipoprotein (a) (Lp(a)) is a cholesterol-rich LDL moiety that is covalently linked to a glycoprotein-apolipoprotein (a) ((apo (a)) by a disulfide bond [15]. Elevated plasma Lp(a) is widely accepted as a causal risk factor for myocardial infarction, atherothrombotic stroke, and calcified aortic stenosis [16–22]. Genetic findings strongly suggest that elevated plasma Lp(a), similarly to elevated LDL-C, is causally related to premature ASCVD and cardiovascular events, as well as mortality [23–26]. The accumulation of the LDL component in atherosclerotic plaque is regarded to be an important component of the atherogenic processes. Also, prothrombotic effects due to homology of apo (a) and plasminogen, as well as induction of a multilevel proinflammatory response mediated by oxidized phospholipid (OxPL), are supposed to be additional atherogenic mechanisms [27, 28]. The prothrombotic and proinflammatory effects of Lp(a) have been proposed to promote plaque instability, resulting in plaque rupture and atherothrombotic events [29]. Overall, Lp(a) plasma levels are stable, although variants in the LPA gene determine 30-40% of the variance [30]. However, LPA gene expression may be increased by inflammation, while diseases like hypothyroidism and chronic kidney disease may affect Lp(a) removal.
Considering the profound metabolic changes that occur after bariatric surgery and its effect on proatherogenic lipoproteins [12], the aim of this meta-analysis was to evaluate whether bariatric surgery could change Lp(a) concentrations. So far, no meta-analysis has been performed to analyze this issue.
2. Methods
2.1. Search Strategy
This meta-analysis was performed based on the 2009 preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines [31]. From inception to May 1st, 2021, PubMed, Scopus, Embase, and Web of Science were searched using the following keywords in titles and abstracts (also in combination with MESH terms): (“bariatric surgery” OR gastroplast∗ OR “gastric bypass” OR “Roux-en-Y” OR “gastric band” OR “biliopancreatic diversion” OR gastrectom∗ OR “duodenal switch” OR “gastrointestinal diversion” OR gastroenterostom∗ OR “jejunoileal bypass” OR “obesity surgery” OR “weight loss surgery” OR “weight-loss surgery” OR “bariatric procedure” OR “sleeve surgery” OR “metabolic surgery”) AND (“lipoprotein (a)” OR “lipoprotein (a)” OR “Lp(a)”).
2.2. Study Selection
Only original peer-reviewed studies written in English were considered. All forms of bariatric surgery procedures (with or without supplemental medical therapies) which reported circulating Lp(a) levels before and after surgery were studied. The exclusion criteria were abstracts only, letters, case reports, comments, meta-analyses, duplicate studies, animal studies, reviews, non-English language papers, studies with no surgical intervention, and studies without outcomes.
2.3. Data Extraction
After deleting duplicate studies, two independent authors examined the titles and abstracts of the remaining papers for eligibility. The full texts of the eligible studies were collected. If two (or more) papers on the same research topic were published by the same organization and/or authors, the more recent study with a larger sample size was included. Any disagreements were resolved by authors' discussion and consensus. The following information was extracted: (1) first author's name, (2) year of publication, (3) type of surgery, (4) study design, (5) characteristics of the patients, (6) levels of Lp(a), and (7) duration of follow-up.
2.4. Quality Assessment
The Newcastle-Ottawa Scale (NOS) was applied to evaluate study quality in this meta-analysis [32]. Three features of each study were taken into account for this scale: (1) the selection of studied patients (4 items), (2) the comparability of studied populations (1 item), and (3) the ascertainment of exposure (3 items) in case-control studies or outcome of interest in cohort studies.
2.5. Quantitative Data Synthesis
This meta-analysis was performed using Comprehensive Meta-Analysis (CMA) V2 software (Biostat, NJ) [33]. Information regarding sample size, means, and standard deviations from each group was extracted to calculate the standardized mean differences (SMDs). SMDs were applied because several different types of assays were used to determine plasma Lp(a) levels. Random effects meta-analysis was used to estimate the effect size. The heterogeneity of studies regarding treatment duration, study design, and the characteristics of the studied populations was determined using a random-effects model (owing to interstudy heterogeneity) and the generic inverse variance weighting approach [31]. When the outcome measures were reported as median and range (or 95% confidence interval (CI)), mean and SD values were computed by the approach described by Hozo et al. [34]. When only standard error of the mean (SEM) was reported, SD was computed using the following formula: SD = SEM × sqrt (n), where “n” represents the number of participants. To analyze the influence of each study on the overall effect size, a sensitivity analysis was done using the leave-one-out approach (i.e., deleting one study each time and repeating the analysis) [35, 36]. Statistical heterogeneity between the trials was evaluated using Cochran's Q test and I2 statistic as a measure of variability.
2.6. Metaregression
A metaregression analysis was performed to investigate the impact of changes in body mass index (BMI) and postsurgery follow-up duration with the estimated effect size of surgery on Lp(a) concentrations.
2.7. Publication Bias
To investigate the presence of publication bias, the funnel plot, Begg's rank correlation, and Egger's weighted regression tests were used. When funnel plot asymmetry was detected, potentially missing studies were inserted using the “trim and fill” approach. In case of a significant result, the number of potentially missing studies needed to render the p value nonsignificant was calculated by the “fail-safe N” approach as another indicator of publication bias [37].
3. Results
A thorough database search identified 99 published papers, 49 of which were directly related to the topic of this meta-analysis. After careful consideration, 36 studies were excluded: 10 studies were reviews, 10 studies did not meet the inclusion criteria, 15 studies did not report sufficient data, and one was a study protocol only. Therefore, 13 studies which evaluated the levels of Lp(a) before and after bariatric surgery were included (Table 1). The study selection process is shown in Figure 1. Assessment of risk of bias in the included studies is summarized in Table 2. Risk assessments for all studies were deemed to have high risk of bias.
Table 1.
Characteristics of studies measuring Lp(a).
| Study year | Study design | Baseline Lp(a) | Follow-up | Treatment | Control | Clinical outcome | Patients | No. of patients |
|---|---|---|---|---|---|---|---|---|
| Lp(a) | ||||||||
| Ram et al., 2007 [38] | Prospective study | 30.30 ± 3.65 | 3 months 12 months |
SRVG | — | Significant decrease in Lp(a) levels in both groups | Women with obesity Men with obesity |
14 11 |
|
| ||||||||
| Williams et al., 2007 [39] | Prospective study | 137.61 ± 45.90 | 3 months 6 months 12 months |
RYGB | — | Significant decrease in Lp(a) levels | Patients with obesity | 121 103 85 |
|
| ||||||||
| Morton and Boussard, 2009 [40] | Prospective study | 14.00 ± 3.65 | 12 months | LRYGB | — | Significant decrease in Lp(a) levels | Adolescents with obesity | 32 |
|
| ||||||||
| Woodard et al., 2010 [41] | Prospective study | 32.20 ± 2.40 35.40 ± 8.20 |
12 months | RYGB LAGB |
— | Unchanged | Patients with obesity | 765 73 |
|
| ||||||||
| To et al., 2012 [42] | Retrospective study | 35.00 ± 36.00 | 6 months 12 months 24 months |
LSG | — | Significant decrease in Lp(a) levels at 12 months | Patients with obesity | 52 39 5 |
|
| ||||||||
| Berk et al., 2017 [43] | Prospective study | 88.88 ± 189.62 | 3 months | RYGB or gastric banding | Obese individuals without type 2 diabetes (dietary intervention) | Unchanged | Patients with obesity without T2DM | 26 |
|
| ||||||||
| Gómez-Martin et al., 2017 [44] | Prospective study | 40.00 ± 39.00 43.00 ± 64.00 |
6 months 12 months |
LRYGB SG |
Women matched for age and cardiovascular risk (diet and lifestyle modification) | Unchanged | Women with obesity | 20 20 |
|
| ||||||||
| Lin et al., 2018 [45] | Prospective study | 34.25 ± 59.03 34.25 ± 26.67 |
1 month 6 months |
RYGB SG |
— | Significant decrease in Lp(a) levels Significant decrease in Lp(a) levels at 1 month |
Premenopausal women | 27 35 |
|
| ||||||||
| Carmona-Maurici et al., 2020 [46] | Prospective study | 258.17 ± 377.96 420.77 ± 462.56 |
6 months 12 months |
LRYGB or SG | — | Significant decrease in Lp(a) levels at 12 months in both groups | Patients with obesity without plaque Patients with obesity with plaque |
34 32 |
|
| ||||||||
| Després et al., 2020 [47] | Prospective study | 69.50 ± 411.16 | 24 hours 5 days 6 months 12 months |
Biliopancreatic diversion with duodenal switch (BPD-DS) | — | Significant increase in Lp(a) levels at 5 days Significant decrease in Lp(a) levels at 6 months |
Patients with obesity | 69 |
|
| ||||||||
| Kruschitz et al., 2020 [48] | Clinical trial | 56.40 ± 91.60 | 1 month 6 months 12 months |
Laparoscopic one anastomosis gastric bypass | — | Significant decrease in Lp(a) levels | Patients with obesity, serum 25(OH)D concentrations of <75 nmol/L | 50 43 37 |
|
| ||||||||
| Paredes et al., 2020 [49] | Retrospective study | 42.76 ± 126.82 | 12 months | SG | — | Significant decrease in Lp(a) levels Unchanged |
Patients without metabolic syndrome Patients without metabolic syndrome |
114 94 |
|
| ||||||||
| Ho et al., 2021 [50] | Prospective study | 40.97 ± 155.40 | 6 months 12 months |
RYGB or SG or omega loop bypass | Medical weight management | Significant increase in Lp(a) levels | Patients with obesity | 59 |
LRYGB: laparoscopic Roux-en-Y gastric bypass; LAGB: laparoscopic adjustable gastric banding; LSG: laparoscopic sleeve gastrectomy; SRVG: silastic ring vertical gastroplasty; RYGB: Roux-en-Y gastric bypass; SG: sleeve gastrectomy.
Figure 1.
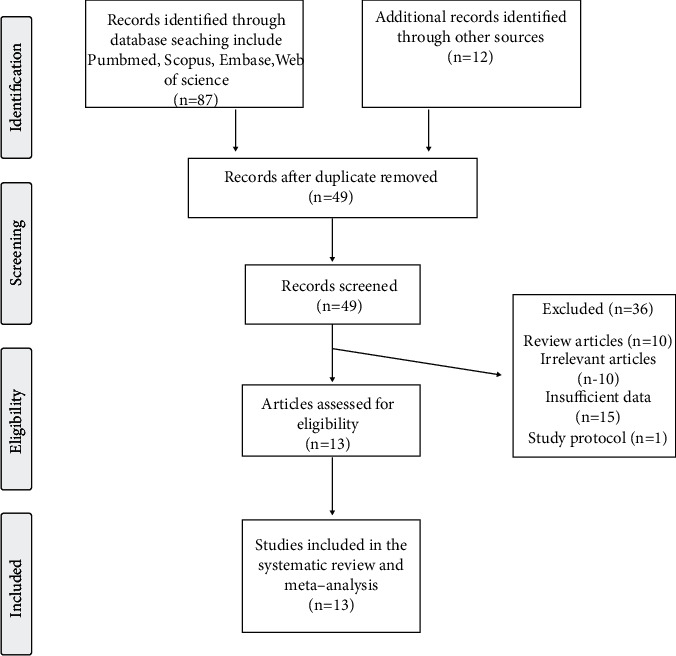
Flow chart of studies identified and included in the meta-analysis.
Table 2.
Quality of bias assessment of the included studies according to the Newcastle-Ottawa scale.
| Study | Selection | Comparability† | Exposure | |||||
|---|---|---|---|---|---|---|---|---|
| Case definition | Representativeness of the cases | Selection of controls | Definition of controls | Comparability of cases and controls | Ascertainment of exposure | Same method of ascertainment | Nonresponse rate | |
| Berk et al., 2017 | — | — | — | ∗ | ∗ | ∗ | ∗ | — |
| Després et al., 2020 | — | — | — | — | — | ∗ | — | — |
| Ram et al., 2007 | — | — | — | — | — | ∗ | — | — |
| Gómez-Martin et al., 2018 | ∗ | — | — | — | ∗ | ∗ | ∗ | ∗ |
| Ho et al., 2021 | — | — | — | — | — | ∗ | ∗ | — |
| Carmona-Maurici et al., 2020 | ∗ | — | — | ∗ | ∗ | ∗ | ∗ | — |
| Kruschitz et al., 2020 | — | — | — | — | ∗ | ∗ | ∗ | — |
| Lin et al., 2018 | — | — | — | — | ∗ | ∗ | ∗ | — |
| Paredes et al., 2020 | — | — | — | ∗ | ∗ | ∗ | ∗ | — |
| To et al., 2012 | — | — | — | — | — | ∗ | — | — |
| Williams et al., 2007 | — | — | — | — | — | ∗ | — | — |
| Woodard et al., 2010 | — | ∗ | — | — | — | ∗ | — | — |
| Morton et al., 2009 | ∗ | — | — | — | — | ∗ | ∗ | — |
†Only for comparability, a maximum of two stars can be given.
3.1. Quality Assessment of the Included Studies
Because most of the studies did not have a control group, they were not evaluated for selection of controls, definition of controls, comparability, the same method of ascertainment, and nonresponse rate. However, all studies which were included met the ascertainment of exposure criteria. Table 2 shows the details of quality assessment.
3.2. Methods for Measuring Lp(a)
In most of the included studies, serum Lp(a) was assessed using the enzyme-linked immunosorbent assay (ELISA) [46]. One study used standard colorimetric methods using the Architect ci8200 analyzer (Abbot Diagnostics, Berkshire, UK [44]. One study used particle-enhanced immunoturbidimetry (Diagnostic System, GmbH, Holzheim, Germany) [43] while another used the turbidimetric assay using the Tina-quant Lipoprotein (a) Gen.2 system (Cobas Integra 400/800, Roche Diagnostics, Mannheim, Germany) [47]. Another study assessed Lp(a) by chemiluminescent immunoassays [50], and one study used the Cobas Mira Plus (Roche Diagnostics) analyzer [38]. In seven studies, the method was not specifically mentioned [39–42, 45, 48, 49].
3.3. Effects of Bariatric Surgery on Circulating Concentrations of Lp(a)
Meta-analysis of 13 studies including 1551 subjects showed a significant decrease of circulating Lp(a) after bariatric surgery (SMD: -0.438, 95% CI: -0.702, -0.174, p < 0.001, I2: 94.05%) (Figure 2(a)). The reduction in circulating Lp(a) was robust in the leave-one-out sensitivity analysis (Figure 2(b)).
Figure 2.
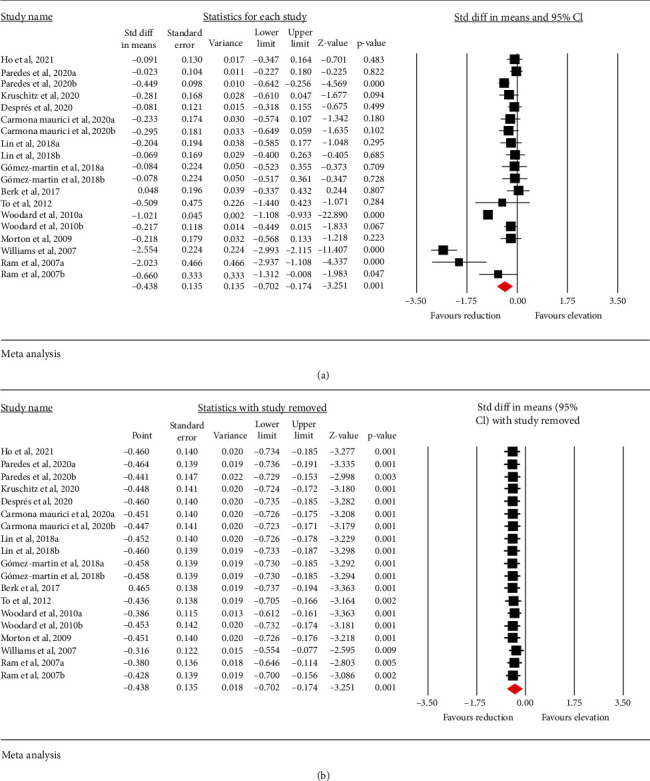
(a) Forest plot displaying weighted mean difference (SMD) and 95% confidence intervals (CI) for the effect of bariatric surgery on Lp(a). (b) Leave-one-out sensitivity analyses for the effect of bariatric surgery on Lp(a).
3.4. Effects of Bariatric Surgery on BMI and Circulating Concentrations of LDL-C, HDL-C, and oxLDL
BMI in 12 studies including 1597 subjects significantly decreased after bariatric surgery (WMD: -14.101 kg/m2, 95% CI: -16.308, -11.895, p < 0.001, I2: 99.69%) (Figure 3(a)). Also, 3 studies including 99 subjects showed a significant decrease of circulating oxLDL after bariatric surgery (WMD: -6.717 mg/dL, 95% CI: -12.413, -1.021, p = 0.021, I2: 93.90%) (Figure 3(b)). Meta-analysis of 12 studies including 1530 subjects showed a significant increase of HDL-C after bariatric surgery (WMD: 7.390 mg/dL, 95% CI: 5.733, 9.046, p < 0.001, I2: 94.86%) as well as significant reduction in LDL-C levels (WMD: -14.166 mg/dL, 95% CI: -21.831, -6.502, p < 0.001, I2: 92.96%) (Figures 3(c) and 3(d)).
Figure 3.
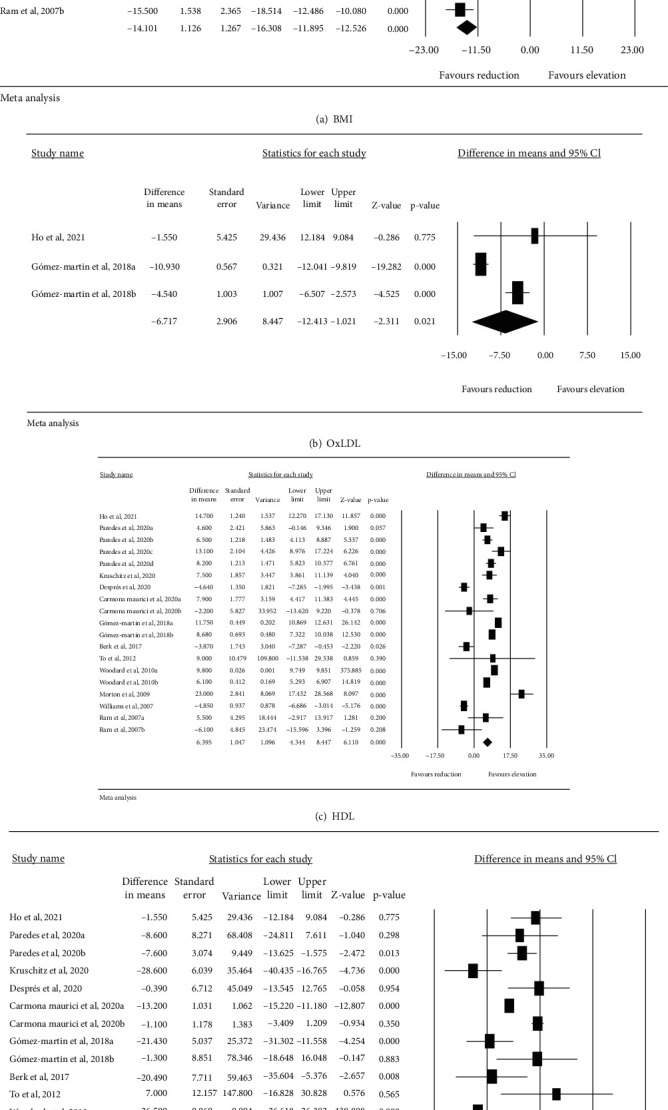
Effects of bariatric surgery on BMI and circulating concentrations of LDL-C, HDL-C, and oxLDL.
3.5. Metaregression
To investigate the impact of potential confounders on the Lp(a) lowering effect of bariatric surgery, random-effects metaregression was used. The results did not indicate any significant association between the changes in Lp(a) and percentage of BMI change (slope: 0.019; 95% CI: -0.037, 0.076; p = 0.507) or duration of follow-up (slope: -0.036; 95% CI: -0.112, 0.040; p = 0.355). Figure 4 is shown.
Figure 4.
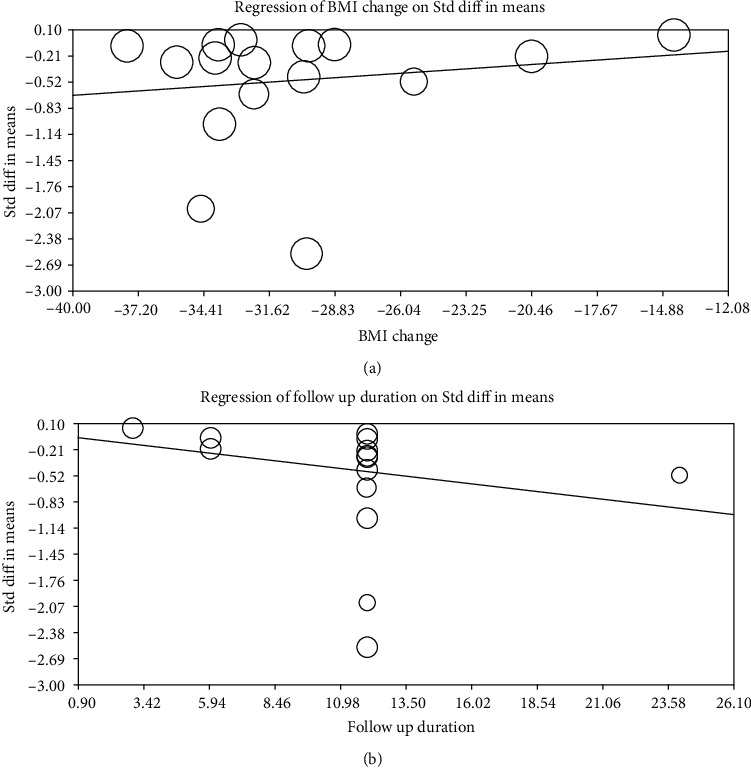
Random-effects metaregression for assessing the effect of % BMI change (a) and follow-up duration (b).
3.6. Publication Bias
Figure 5shows a funnel plot to evaluate publication bias across studies included in the meta-analysis. Egger's linear regression test (intercept = 2.935, standard error = 1.62; 95% CI = −0.498, 6.370, t = 1.803, df = 17, two-tailed p = 0.089) and Begg's rank correlation test (Kendall's Tau with continuity correction = −0.257, z = 1.57, two-tailed p = 0.115) did not indicate the presence of publication bias in this meta-analysis of bariatric surgery effects on circulating Lp(a). Trim-and-fill analysis indicated that among all papers included in this meta-analysis, there could be five missing studies. The “fail-safe N” test showed that 842 missing studies were required to reduce the effect size to a nonsignificant (p < 0.001) value. Statistical heterogeneity was observed as Cochran's Q-test and I2 (p < 0.05 and I2 > 50%, respectively).
Figure 5.
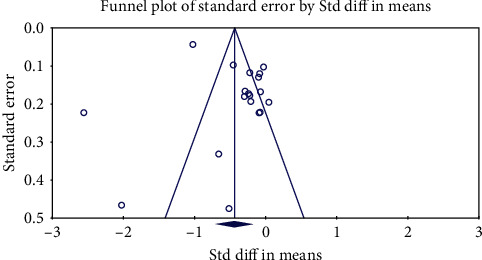
Funnel plot detailing publication bias in the studies reporting the effect of bariatric surgery on Lp(a).
4. Discussion
This meta-analysis showed a significant reduction in circulating Lp(a) levels after bariatric surgery. Several meta-analyses have analyzed these effects particularly in patients with type 2 diabetes. Favourable effects have been shown on serum triglycerides, total cholesterol, LDL-C, and HDL-C concentrations following bariatric surgery depending on its type and the anatomic alterations unique to each procedure [51–54].
Although an elevated Lp(a) level is independently associated with the incidence of cardiovascular events in the general population and it is an established predictor of cardiovascular events in patients with ASCVD [55, 56], the possibilities of decreasing elevated Lp(a) are still quite limited [57–63]. Unlike LDL-C and triglycerides, Lp(a) is relatively refractory to diet [64], lifestyle changes [65], and most drug interventions. Among medications, statins could increase plasma Lp(a) levels [66]. However, a decrease in Lp(a) concentrations can be achieved to a certain extent with niacin [67] which is not widely available. Mipomersen, an antisense oligonucleotide against apolipoprotein B [68–71], had the potential benefit to reduce Lp(a) by 20%, but this drug is approved by the FDA only as an orphan drug for homozygous familial hypercholesterolemia [72]. Currently, the only drugs on the market which can decrease Lp(a) significantly (about 25%) are proprotein subtilisin/kexin type 9 (PCSK9) inhibitors—alirocumab and evolocumab [73, 74]. A post hoc analysis of the Odyssey outcomes study suggested that a part of the benefits of alirocumab in reducing ASCVD events could be ascribed to its Lp(a) lowering effects, mainly on a subgroup of patients with high Lp(a) levels that had a recent myocardial infarction [75]. However, decreasing Lp(a) is not commonly accepted as an indication for use of PCSK9 inhibitors. Recently, data from a phase 2b trial with pelacarsen (an apo (a) antisense oligonucleotide) have attracted significant interest [76].
In this meta-analysis, bariatric surgery was shown to reduce Lp(a) levels despite the heterogeneity of the included studies. It has to be stressed that there was no association between changes in Lp(a) levels and weight loss or follow-up duration. An important question is: what mechanisms may cause these findings? One possibility is the consistent reduction in the obesity proinflammatory state indicated by lower levels of C reactive protein [77] and interleukin-6 (IL-6) after bariatric surgery [78] . Also, the LPA gene promoter contains IL-6-responsive elements consistent with Lp(a) acute phase response of apo (a) [79]. However, further studies are necessary to prove this hypothesis.
Previous findings on the reduction of LDL-C and atherogenic dyslipidemia [10–12] and Lp(a) as shown in this meta-analysis may explain the beneficial effects of bariatric surgery on individuals at high risk of ASCVD and mortality. However, this needs to be verified [3]. Moreover, it has been estimated that the magnitude of reduction required to achieve an ASCVD benefit is roughly 55 mg/dL [80]. Therefore, it has to be further explored whether Lp(a) reduction with bariatric surgery is of clinical relevance.
One of the limitations of this study was that the methods for measuring Lp(a) concentrations in some studies were different, and this might explain the heterogeneity in our findings. However, using SMD as the summary statistic in this meta-analysis could reduce this error. Indeed, because of the structural properties of Lp(a), none of the available commercial assays for Lp(a) quantification is 100% inherently isoform-sensitive [81]. We were also not able to establish the contribution of either apo (a) isoform size or variants in LPA gene [82]. Besides, some studies had no control group; some had small groups of patients or were not randomized. However, the results were still robust after the leave-one-out sensitivity analysis. Lp(a) has very little variability in different measures in individuals with stable health conditions [83]. We were also not able to evaluate the absolute reductions in Lp(a) and whether the effects were greater in patients with elevated Lp(a) levels. Finally, we were also unable to determine the specific impact of different bariatric surgery techniques, which may produce significantly stronger or weaker responses.
5. Conclusion
Obesity is associated with an increased ASCVD risk, and this association is consistent between sexes and across different parts of the world. This meta-analysis suggests that bariatric surgery significantly decreases circulating Lp(a) concentrations. Since elevated Lp(a) is independently associated with ASCVD, the results of this study may have clinical implications for severely obese individuals with high cardiovascular risk. However, it is worth mentioning that the estimates of the magnitude reduction required to achieve an ASCVD benefit are roughly 55 mg/dL [80].
Acknowledgments
Tannaz Jamialahmadi was supported by the Wael-Almahmeed & IAS Research Training Grant. RDS is a recipient of a research scholarship from Conselho Nacional de Pesquisa e Desenvolvimento Tecnológico, Brazil (CNPq) #303734/2018-3.
Data Availability
There is no primary data associated with this study.
Conflicts of Interest
RDS reports receiving the following: consulting fees and lecture fees from Abbott, Amgen, Astra Zeneca, Aché, EMS, Getz Pharma, Libbs, Merck, Merck Sharp & Dohme, PTC Therapeutics, Novo Nordisk, Novartis, and Sanofi-Regeneron Pharmaceuticals and grant support from Amgen, Kowa, Esperion, Kowa, Novartis, and Sanofi-Regeneron Pharmaceuticals outside this work. RDS has received honoraria related to consulting, speaker activities, and/or research from Abbott, Amgen, Astra Zeneca, Aché, Esperion, EMS, Hypera, Getz Pharma, Kowa, Libbs, PTC Pharmaceuticals, Pfizer, Medley, Merck, MSD, Novo-Nordisk, Novartis, and Sanofi.
References
- 1.Kim S., Lee M. W., Kim T. S., et al. Intracoronary dual-modal optical coherence tomography-near-infrared fluorescence structural-molecular imaging with a clinical dose of indocyanine green for the assessment of high-risk plaques and stent-associated inflammation in a beating coronary artery. European Heart Journal . 2016;37(37):2833–2844. doi: 10.1093/eurheartj/ehv726. [DOI] [PubMed] [Google Scholar]
- 2.Dai H., Alsalhe T. A., Chalghaf N., Riccò M., Bragazzi N. L., Wu J. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990–2017: an analysis of the Global Burden of Disease Study. PLoS Medicine . 2020;17(7, article e1003198) doi: 10.1371/journal.pmed.1003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Syn N. L., Cummings D. E., Wang L. Z., et al. Association of metabolic-bariatric surgery with long-term survival in adults with and without diabetes: a one-stage meta-analysis of matched cohort and prospective controlled studies with 174 772 participants. Lancet . 2021;397(10287):1830–1841. doi: 10.1016/S0140-6736(21)00591-2. [DOI] [PubMed] [Google Scholar]
- 4.Després J. P., Carpentier A. C., Tchernof A., Neeland I. J., Poirier P. Management of obesity in cardiovascular practice: Journal of the American College of Cardiology . 2021;78(5):513–531. doi: 10.1016/j.jacc.2021.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coelho C., Crane J., Agius R., McGowan B. The bariatric-metabolic physician’s role in managing clinically severe obesity. Current Obesity Reports . 2021;10(3):263–273. doi: 10.1007/s13679-021-00435-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Visseren F. L., Mach F., Smulders Y. M., et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. European Heart Journal . 2021;42(34):3227–3337. doi: 10.1093/eurheartj/ehab484. [DOI] [PubMed] [Google Scholar]
- 7.Jamialahmadi T., Reiner Ž., Alidadi M., et al. The effect of bariatric surgery on circulating levels of oxidized low-density lipoproteins is apparently independent of changes in body mass index: a systematic review and meta-analysis. Oxidative Medicine and Cellular Longevity . 2021;2021:13. doi: 10.1155/2021/4136071.4136071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jamialahmadi T., Alidadi M., Atkin S. L., et al. Effect of Bariatric Surgery on Flow-Mediated Vasodilation as a Measure of Endothelial Function: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine . 2022;11(14):p. 4054. doi: 10.3390/jcm11144054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nabavi N., Ghodsi A., Rostami R., et al. Impact of bariatric surgery on carotid intima-media thickness in patients with morbid obesity: a prospective study and review of the literature. Obesity Surgery . 2022;32(5):1563–1569. doi: 10.1007/s11695-022-05976-3. [DOI] [PubMed] [Google Scholar]
- 10.Carswell K. A., Belgaumkar A. P., Amiel S. A., Patel A. G. A systematic review and meta-analysis of the effect of gastric bypass surgery on plasma lipid levels. Obesity Surgery . 2016;26(4):843–855. doi: 10.1007/s11695-015-1829-x. [DOI] [PubMed] [Google Scholar]
- 11.Garay L. A., García M. I. N., Martínez R. G. C., Pérez N. M. T., Rojas J. L. V. Medium/long term evaluation of lipid profile after bariatric surgery (gastric bypass versus sleeve gastrectomy) Endocrinología, Diabetes y Nutrición . 2021;68(6):372–380. doi: 10.1016/j.endien.2021.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Arnáiz E. G., Ballesteros Pomar M. D., Roza L. G., et al. Evaluation of lipoprotein profile and residual risk three years after bariatric surgery. Obesity Surgery . 2021;31(9):4033–4044. doi: 10.1007/s11695-021-05543-2. [DOI] [PubMed] [Google Scholar]
- 13.Carmona-Maurici J., Cuello E., Ricart-Jané D., et al. Effect of bariatric surgery on inflammation and endothelial dysfunction as processes underlying subclinical atherosclerosis in morbid obesity. Surgery for Obesity and Related Diseases . 2020;16(12):1961–1970. doi: 10.1016/j.soard.2020.07.036. [DOI] [PubMed] [Google Scholar]
- 14.Jamialahmadi T., Reiner Ž., Alidadi M., et al. Impact of bariatric surgery on pulse wave velocity as a measure of arterial stiffness: a systematic review and meta-analysis. Obesity Surgery . 2021;31(10):4461–4469. doi: 10.1007/s11695-021-05611-7. [DOI] [PubMed] [Google Scholar]
- 15.Reiner Ž. Can Lp (A) Lowering Against Background Statin Therapy Really Reduce Cardiovascular Risk? Current Atherosclerosis Reports . 2019;21(4):1–8. doi: 10.1007/s11883-019-0773-y. [DOI] [PubMed] [Google Scholar]
- 16.Kronenberg F., Kronenberg M. F., Kiechl S., et al. Role of lipoprotein(a) and apolipoprotein(a) phenotype in atherogenesis. Circulation . 1999;100(11):1154–1160. doi: 10.1161/01.CIR.100.11.1154. [DOI] [PubMed] [Google Scholar]
- 17.Nordestgaard B. G., Chapman M. J., Ray K., et al. Lipoprotein (a) as a cardiovascular risk factor: current status. European Heart Journal . 2010;31(23):2844–2853. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boras J., Ljubic S., Car N., et al. Lipoprotein (a) predicts progression of carotid artery intima-media thickening in patients with type 2 diabetes: a four-year follow-up. Wiener klinische Wochenschrift . 2010;122(5-6):159–164. doi: 10.1007/s00508-010-1318-0. [DOI] [PubMed] [Google Scholar]
- 19.Reiner Ž., Catapano A. L., De Backer G., et al. ESC/EAS guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) European Heart Journal . 2011;32(14):1769–1818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 20.Thanassoulis G. Lipoprotein (a) in calcific aortic valve disease: from genomics to novel drug target for aortic stenosis. Journal of Lipid Research . 2016;57(6):917–924. doi: 10.1194/jlr.R051870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellis K. L., Boffa M. B., Sahebkar A., Koschinsky M. L., Watts G. F. The renaissance of lipoprotein(a): brave new world for preventive cardiology? Progress in Lipid Research . 2017;68:57–82. doi: 10.1016/j.plipres.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Ferretti G., Bacchetti T., Johnston T. P., Banach M., Pirro M., Sahebkar A. Lipoprotein(a): a missing culprit in the management of athero-thrombosis? Journal of Cellular Physiology . 2018;233(4):2966–2981. doi: 10.1002/jcp.26050. [DOI] [PubMed] [Google Scholar]
- 23.Kamstrup P. R., Tybjaerg-Hansen A., Steffensen R., Nordestgaard B. G. Genetically elevated lipoprotein (a) and increased risk of myocardial infarction. Journal of the American Medical Association . 2009;301(22):2331–2339. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 24.Zekavat S. M., Ruotsalainen S., Handsaker R. E., et al. Deep coverage whole genome sequences and plasma lipoprotein (a) in individuals of European and African ancestries. Nature Communications . 2018;9(1):1–14. doi: 10.1038/s41467-018-04668-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langsted A., Nordestgaard B. G., Kamstrup P. R. Low lipoprotein (a) levels and risk of disease in a large, contemporary, general population study. European Heart Journal . 2021;42(12):1147–1156. doi: 10.1093/eurheartj/ehaa1085. [DOI] [PubMed] [Google Scholar]
- 26.Gudbjartsson D. F., Thorgeirsson G., Sulem P., et al. Lipoprotein(a) concentration and risks of cardiovascular disease and diabetes. Journal of the American College of Cardiology . 2019;74(24):2982–2994. doi: 10.1016/j.jacc.2019.10.019. [DOI] [PubMed] [Google Scholar]
- 27.Kaiser Y., Daghem M., Tzolos E., et al. Association of lipoprotein(a) with atherosclerotic plaque progression. Journal of the American College of Cardiology . 2022;79(3):223–233. doi: 10.1016/j.jacc.2021.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gencer B., Kronenberg F., Stroes E. S., Mach F. Lipoprotein (a): the revenant. European Heart Journal . 2017;38(20):1553–1560. doi: 10.1093/eurheartj/ehx033. [DOI] [PubMed] [Google Scholar]
- 29.Nestel P. J., Barnes E. H., Tonkin A. M., et al. Plasma lipoprotein (a) concentration predicts future coronary and cardiovascular events in patients with stable coronary heart disease. Arteriosclerosis, Thrombosis, and Vascular Biology . 2013;33(12):2902–2908. doi: 10.1161/ATVBAHA.113.302479. [DOI] [PubMed] [Google Scholar]
- 30.Kronenberg F., Utermann G. Lipoprotein(a): resurrected by genetics. Journal of Internal Medicine . 2013;273(1):6–30. doi: 10.1111/j.1365-2796.2012.02592.x. [DOI] [PubMed] [Google Scholar]
- 31.Sutton A. J., Abrams K. R., Jones D. R., Jones D. R., Sheldon T. A., Song F. Methods for meta-analysis in medical research. Wiley Chichester . 2000;348 [Google Scholar]
- 32.Wells G. A., Shea B., O’Connell D., et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000.
- 33.Borenstein M., Hedges L., Higgins J., Rothstein H. Comprehensive Meta-Analysis, Version 2 Biostat . Englewood NJ: 2005. [Google Scholar]
- 34.Hozo S. P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology . 2005;5(1):1–10. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banach M., Serban C., Sahebkar A., et al. Impact of statin therapy on coronary plaque composition: a systematic review and meta-analysis of virtual histology intravascular ultrasound studies. BMC Medicine . 2015;13(1):1–21. doi: 10.1186/s12916-015-0459-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banach M., Serban C., Ursoniu S., et al. Statin therapy and plasma coenzyme Q10 concentrations--a systematic review and meta-analysis of placebo-controlled trials. Pharmacological Research . 2015;99:329–336. doi: 10.1016/j.phrs.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Duval S., Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics . 2000;56(2):455–463. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 38.Ram E., Vishne T., Magazanik A., et al. Changes in blood lipid levels following silastic ring vertical gastroplasty. Obesity Surgery . 2007;17(10):1292–1296. doi: 10.1007/s11695-007-9231-y. [DOI] [PubMed] [Google Scholar]
- 39.Williams D. B., Hagedorn J. C., Lawson E. H., et al. Gastric bypass reduces biochemical cardiac risk factors. Surgery for Obesity and Related Diseases . 2007;3(1):8–13. doi: 10.1016/j.soard.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Morton J., Boussard T. Gastric bypass reduces cardiac risk factors in the adolescent morbidly obese. Obesity Surgery . 2009;19(8, article 984) [Google Scholar]
- 41.Woodard G. A., Peraza J., Bravo S., Toplosky L., Hernandez-Boussard T., Morton J. M. One year improvements in cardiovascular risk factors: a comparative trial of laparoscopic Roux-en-Y gastric bypass vs. adjustable gastric banding. Obesity Surgery . 2010;20(5):578–582. doi: 10.1007/s11695-010-0088-0. [DOI] [PubMed] [Google Scholar]
- 42.To V. T., Huttl T. P., Lang R., Piotrowski K., Parhofer K. G. Changes in body weight, glucose homeostasis, lipid profiles, and metabolic syndrome after restrictive bariatric surgery. Experimental and Clinical Endocrinology & Diabetes . 2012;120(9):547–552. doi: 10.1055/s-0032-1323738. [DOI] [PubMed] [Google Scholar]
- 43.Berk K. A., Yahya R., Verhoeven A. J. M., et al. Effect of diet-induced weight loss on lipoprotein (a) levels in obese individuals with and without type 2 diabetes. Diabetologia . 2017;60(6):989–997. doi: 10.1007/s00125-017-4246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gómez-Martin J. M., Balsa J. A., Aracil E., et al. Beneficial changes on plasma apolipoproteins A and B, high density lipoproteins and oxidized low density lipoproteins in obese women after bariatric surgery: comparison between gastric bypass and sleeve gastrectomy. Lipids in Health and Disease . 2018;17(1):1–9. doi: 10.1186/s12944-018-0794-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin B. X., Weiss M. C., Parikh M., Berger J. S., Fisher E. A., Heffron S. P. Changes in lipoprotein(a) following bariatric surgery. American Heart Journal . 2018;197:175–176. doi: 10.1016/j.ahj.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carmona-Maurici J., Amigó N., Cuello E., et al. Bariatric surgery decreases oxidative stress and protein glycosylation in patients with morbid obesity. European Journal of Clinical Investigation . 2020;50(11, article e13320) doi: 10.1111/eci.13320. [DOI] [PubMed] [Google Scholar]
- 47.Després A. A., Piché M. E., Auclair A., et al. Acute and chronic impact of biliopancreatic diversion with duodenal switch surgery on plasma lipoprotein (a) levels in patients with severe obesity. Obesity Surgery . 2020;30(10):3714–3720. doi: 10.1007/s11695-020-04450-2. [DOI] [PubMed] [Google Scholar]
- 48.Kruschitz R., Wakolbinger M., Schindler K., et al. Effect of one-anastomosis gastric bypass on cardiovascular risk factors in patients with vitamin D deficiency and morbid obesity: a secondary analysis. Nutrition, Metabolism and Cardiovascular Diseases. . 2020;30(12):2379–2388. doi: 10.1016/j.numecd.2020.08.011. [DOI] [PubMed] [Google Scholar]
- 49.Paredes S., Alves M., Pereira M. L., Marques O., Ribeiro L. Lipoprotein (a) change after sleeve gastrectomy is affected by the presence of metabolic syndrome. Obesity Surgery . 2020;30(2):545–552. doi: 10.1007/s11695-019-04212-9. [DOI] [PubMed] [Google Scholar]
- 50.Ho J. H., Adam S., Liu Y., et al. Effect of bariatric surgery on plasma levels of oxidised phospholipids, biomarkers of oxidised LDL and lipoprotein(a) Journal of Clinical Lipidology . 2021;15(2):320–331. doi: 10.1016/j.jacl.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 51.Liu D.-f., Ma Z.-y., Zhang C.-s., et al. The effects of bariatric surgery on dyslipidemia and insulin resistance in overweight patients with or without type 2 diabetes: a systematic review and network meta-analysis. Surgery for Obesity and Related Diseases. . 2021;17(9):1655–1672. doi: 10.1016/j.soard.2021.04.005. [DOI] [PubMed] [Google Scholar]
- 52.Hasan B., Nayfeh T., Alzuabi M., et al. Weight loss and serum lipids in overweight and obese adults: a systematic review and meta-analysis. The Journal of Clinical Endocrinology & Metabolism . 2020;105(12):3695–3703. doi: 10.1210/clinem/dgaa673. [DOI] [PubMed] [Google Scholar]
- 53.Ding L., Fan Y., Li H., et al. Comparative effectiveness of bariatric surgeries in patients with obesity and type 2 diabetes mellitus: a network meta-analysis of randomized controlled trials. Obesity Reviews . 2020;21(8, article e13030) doi: 10.1111/obr.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heffron S. P., Parikh A., Volodarskiy A., et al. Changes in lipid profile of obese patients following contemporary bariatric surgery: a meta-analysis. The American Journal of Medicine . 2016;129(9):952–959. doi: 10.1016/j.amjmed.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willeit P., Ridker P. M., Nestel P. J., et al. Baseline and on-statin treatment lipoprotein(a) levels for prediction of cardiovascular events: individual patient-data meta-analysis of statin outcome trials. The Lancet . 2018;392(10155):1311–1320. doi: 10.1016/S0140-6736(18)31652-0. [DOI] [PubMed] [Google Scholar]
- 56.Wang Z., Zhai X., Xue M., Cheng W., Hu H. Effects of fat-to-sugar ratio in excess dietary energy on lipid abnormalities: a 7-month prospective feeding study in adult cynomolgus monkeys. Lipids in Health and Disease . 2019;18(1):1–9. doi: 10.1186/s12944-018-0950-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferretti G., Bacchetti T., Simental-Mendía L. E., Reiner Ž., Banach M., Sahebkar A. Raloxifene lowers plasma lipoprotein (a) concentrations: a systematic review and meta-analysis of randomized placebo-controlled trials. Cardiovascular Drugs and Therapy. . 2017;31(2):197–208. doi: 10.1007/s10557-017-6721-6. [DOI] [PubMed] [Google Scholar]
- 58.Kotani K., Sahebkar A., Serban C., et al. Tibolone decreases lipoprotein(a) levels in postmenopausal women: a systematic review and meta-analysis of 12 studies with 1009 patients. Atherosclerosis . 2015;242(1):87–96. doi: 10.1016/j.atherosclerosis.2015.06.056. [DOI] [PubMed] [Google Scholar]
- 59.Momtazi-Borojeni A. A., Katsiki N., Pirro M., Banach M., Rasadi K. A., Sahebkar A. Dietary natural products as emerging lipoprotein(a)-lowering agents. Journal of Cellular Physiology . 2019;234(8):12581–12594. doi: 10.1002/jcp.28134. [DOI] [PubMed] [Google Scholar]
- 60.Sahebkar A., Katsiki N., Ward N., Reiner Ž. Flaxseed supplementation reduces plasma lipoprotein (A) levels: a meta-analysis. Alternative Therapies in Health and Medicine . 2021;27(3):50–53. [PubMed] [Google Scholar]
- 61.Sahebkar A., Serban M. C., Penson P., et al. The effects of tamoxifen on plasma lipoprotein (a) concentrations: systematic review and meta-analysis. Drugs . 2017;77(11):1187–1197. doi: 10.1007/s40265-017-0767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sahebkar A., Simental-Mendía L. E., Stefanutti C., Pirro M. Supplementation with coenzyme Q10 reduces plasma lipoprotein(a) concentrations but not other lipid indices: a systematic review and meta-analysis. Pharmacological Research . 2016;105:198–209. doi: 10.1016/j.phrs.2016.01.030. [DOI] [PubMed] [Google Scholar]
- 63.Serban M. C., Sahebkar A., Mikhailidis D. P., et al. Impact of L-carnitine on plasma lipoprotein(a) concentrations: a systematic review and meta-analysis of randomized controlled trials. Scientific Reports . 2016;6(1) doi: 10.1038/srep19188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grundler F., Plonné D., Mesnage R., et al. Long-term fasting improves lipoprotein-associated atherogenic risk in humans. European Journal of Nutrition . 2021;60(7):4031–4044. doi: 10.1007/s00394-021-02578-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Enkhmaa B., Petersen K. S., Kris-Etherton P. M., Berglund L. Diet and Lp (a): does dietary change modify residual cardiovascular risk conferred by Lp (a)? Nutrients . 2020;12(7):p. 2024. doi: 10.3390/nu12072024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsimikas S., Gordts P. L., Nora C., Yeang C., Witztum J. L. Statin therapy increases lipoprotein (a) levels. European Heart Journal . 2020;41(24):2275–2284. doi: 10.1093/eurheartj/ehz310. [DOI] [PubMed] [Google Scholar]
- 67.Sahebkar A., Reiner Ž., Simental-Mendia L. E., Ferretti G., Cicero A. F. Effect of extended-release niacin on plasma lipoprotein(a) levels: a systematic review and meta-analysis of randomized placebo-controlled trials. Metabolism . 2016;65(11):1664–1678. doi: 10.1016/j.metabol.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 68.Santos R. D., Raal F. J., Catapano A. L., Witztum J. L., Steinhagen-Thiessen E., Tsimikas S. Mipomersen, an antisense oligonucleotide to apolipoprotein B-100, reduces lipoprotein (a) in various populations with hypercholesterolemia: results of 4 phase III trials. Arteriosclerosis, Thrombosis, and Vascular Biology . 2015;35(3):689–699. doi: 10.1161/ATVBAHA.114.304549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sahebkar A., Watts G. F. New therapies targeting apoB metabolism for high-risk patients with inherited dyslipidaemias: what can the clinician expect? Cardiovascular Drugs and Therapy . 2013;27(6):559–567. doi: 10.1007/s10557-013-6479-4. [DOI] [PubMed] [Google Scholar]
- 70.Sahebkar A., Watts G. F. New LDL-cholesterol lowering therapies: pharmacology, clinical trials, and relevance to acute coronary syndromes. Clinical Therapeutics . 2013;35(8):1082–1098. doi: 10.1016/j.clinthera.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 71.Macchi C., Sirtori C., Corsini A., Santos R., Watts G., Ruscica M. A new dawn for managing dyslipidemias: the era of RNA-based therapies. Pharmacological Research . 2019;150, article 104413 doi: 10.1016/j.phrs.2019.104413. [DOI] [PubMed] [Google Scholar]
- 72.Fogacci F., Ferri N., Toth P. P., Ruscica M., Corsini A., Cicero A. F. Efficacy and safety of mipomersen: a systematic review and meta-analysis of randomized clinical trials. Drugs . 2019;79(7):751–766. doi: 10.1007/s40265-019-01114-z. [DOI] [PubMed] [Google Scholar]
- 73.Schwartz G. G., Szarek M., Bittner V. A., et al. Lipoprotein(a) and benefit of PCSK9 inhibition in patients with nominally controlled LDL cholesterol. Journal of the American College of Cardiology . 2021;78(5):421–433. doi: 10.1016/j.jacc.2021.04.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Toth P. P., Jones S. R., Monsalvo M. L., Elliott-Davey M., López J. A. G., Banach M. Effect of evolocumab on non-high-density lipoprotein cholesterol, apolipoprotein B, and lipoprotein (a): a pooled analysis of phase 2 and phase 3 studies. Journal of the American Heart Association . 2020;9(5, article e014129) doi: 10.1161/JAHA.119.014129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Szarek M., Bittner V. A., Aylward P., et al. Lipoprotein (a) lowering by alirocumab reduces the total burden of cardiovascular events independent of low-density lipoprotein cholesterol lowering: ODYSSEY OUTCOMES trial. European Heart Journal . 2020;41(44):4245–4255. doi: 10.1093/eurheartj/ehaa649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsimikas S., Karwatowska-Prokopczuk E., Gouni-Berthold I., et al. Lipoprotein (a) reduction in persons with cardiovascular disease. New England Journal of Medicine . 2020;382(3):244–255. doi: 10.1056/NEJMoa1905239. [DOI] [PubMed] [Google Scholar]
- 77.Reyes-Soffer G., Westerterp M. Beyond lipoprotein(a) plasma measurements: lipoprotein(a) and inflammation. Pharmacological Research . 2021;169, article 105689 doi: 10.1016/j.phrs.2021.105689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rao S. R. Inflammatory markers and bariatric surgery: a meta-analysis. Inflammation Research . 2012;61(8):789–807. doi: 10.1007/s00011-012-0473-3. [DOI] [PubMed] [Google Scholar]
- 79.Wade D. P., Clarke J. G., Lindahl G. E., et al. 5' control regions of the apolipoprotein (a) gene and members of the related plasminogen gene family. Proceedings of the National Academy of Sciences of the United States of America . 1993;90(4):1369–1373. doi: 10.1073/pnas.90.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Madsen C. M., Kamstrup P. R., Langsted A., Varbo A., Nordestgaard B. G. Lipoprotein (a)-lowering by 50 mg/dL (105 nmol/L) may be needed to reduce cardiovascular disease 20% in secondary prevention: a population-based study. Arteriosclerosis, Thrombosis, and Vascular Biology . 2020;40(1):255–266. doi: 10.1161/ATVBAHA.119.312951. [DOI] [PubMed] [Google Scholar]
- 81.Marcovina S. M., Albers J. J. Lipoprotein (a) measurements for clinical application. Journal of Lipid Research . 2016;57(4):526–537. doi: 10.1194/jlr.R061648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Clarke R., Peden J. F., Hopewell J. C., et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. The New England Journal of Medicine . 2009;361(26):2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 83.Emerging Risk Factors Collaboration. Lipoprotein (a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. Journal of the American Medical Association . 2009;302(4):412–423. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There is no primary data associated with this study.


