Abstract
A novel quartz crystal microbalance (QCM) technique was used to study the adhesion of nonfimbriated and fimbriated Escherichia coli mutant strains to hydrophilic and hydrophobic surfaces at different ionic strengths. This technique enabled us to measure both frequency shifts (Δf), i.e., the increase in mass on the surface, and dissipation shifts (ΔD), i.e., the viscoelastic energy losses on the surface. Changes in the parameters measured by the extended QCM technique reflect the dynamic character of the adhesion process. We were able to show clear differences in the viscoelastic behavior of fimbriated and nonfimbriated cells attached to surfaces. The interactions between bacterial cells and quartz crystal surfaces at various ionic strengths followed different trends, depending on the cell surface structures in direct contact with the surface. While Δf and ΔD per attached cell increased for nonfimbriated cells with increasing ionic strengths (particularly on hydrophobic surfaces), the adhesion of the fimbriated strain caused only low-level frequency and dissipation shifts on both kinds of surfaces at all ionic strengths tested. We propose that nonfimbriated cells may get better contact with increasing ionic strengths due to an increased area of contact between the cell and the surface, whereas fimbriated cells seem to have a flexible contact with the surface at all ionic strengths tested. The area of contact between fimbriated cells and the surface does not increase with increasing ionic strengths, but on hydrophobic surfaces each contact point seems to contribute relatively more to the total energy loss. Independent of ionic strength, attached cells undergo time-dependent interactions with the surface leading to increased contact area and viscoelastic losses per cell, which may be due to the establishment of a more intimate contact between the cell and the surface. Hence, the extended QCM technique provides new qualitative information about the direct contact of bacterial cells to surfaces and the adhesion mechanisms involved.
Microorganisms occur in all natural and industrial aquatic systems, and bacterial adhesion to exposed surfaces is inevitable. The growth of attached bacteria may lead to the development of a complex biofilm which may cause health problems or economic problems in industrial settings (12). On the other hand, the immobilization of bacteria on surfaces may also be advantageous in biotechnological applications (10). To be able to understand and possibly control biofilm formation on solid surfaces, a comprehensive model is needed that explains and predicts the initial adhesion and subsequent interactions of bacteria with surfaces. Theories generally used to describe bacterial adhesion phenomena are the DLVO theory (named after its inventors Derjaguin, Landau, Verwey, and Overbeek) (14, 48) and the thermodynamic approach (1). According to the DLVO theory, adhesion is a function of separation distance between the cell and the surface and the balance between attractive van der Waals forces and generally repulsive electrostatic interactions. An additional energy term accounting for Lewis acid-base interactions, related to hydrophobic interactions and hydration effects, was included in an extended DLVO approach (47). The thermodynamic approach describes the forces involved in adhesion on the basis of a balance between the interfacial free energies of the surfaces and the medium involved (1). However, experimental results are not always in accordance with theoretical predictions made by these models (5, 34, 44, 49).
To study the dynamic character of bacterial adhesion processes, methods must be applied that measure adhesion in situ without interfering with the system, such as microscopic techniques (21, 31) combined with time-lapse video techniques (50), infrared absorption spectrometry (29), and fluorometry (3). The quartz crystal microbalance (QCM) has been applied in only a few studies on bacterial adhesion. The principle of this method is based on the converse piezoelectric effect (7). An alternating electric field causes a back-and-forth deformation in the bulk material of a quartz crystal at its resonant frequency, and mass loading on the quartz surface will cause a decrease in frequency (42). So far, the QCM has been applied to address the specific binding of bacteria to antibody-coated surfaces (e.g., reference 9) and has been used as a bacterial growth sensor (4, 11, 13, 15). Nivens et al. (29) were the first to recognize the potential of this technique to study biofilm formation. However, in these studies only frequency shifts were measured. These are proportional to the attached mass only when the mass is rigid and tightly coupled to the surface (24), which is not the case for bacterial cells. Nonrigid binding, in turn, involves energy dissipation due to internal friction or trapping of water by the cells, which causes damping of the oscillation of the crystal (39). To be able to reveal the dissipative properties of a viscoelastic overlayer, Rodahl et al. extended the QCM technique so that mass increase and energy dissipation of the oscillating system can be measured simultaneously (39–41). This method provided new qualitative information about the direct binding of biomolecules to surfaces in studies on eucaryotic cell adhesion (28) and protein interactions on surfaces (24).
Some of the key parameters in bacterial adhesion described by the main theories are the surface energy of the substratum, the ionic strength of the medium, the hydrophobicity and charge of the cell surface, and the presence of cell surface structures (22). In the present study, the influence of these factors on bacterial adhesion was studied in a new approach by the use of wettability gradient surfaces and the extended QCM technique.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The strains were selected on the basis of their cell surface properties and are listed in Table 1. In strain PC31Δfim (referred to in the text as MS7fim−) the fim gene cluster has been replaced by an npt cassette (43). The MS7fim+ strain contained the fim gene cluster on a high-copy-number plasmid (45). Strains were cultivated at 37°C in Luria broth (27). When required, media were supplemented with either 50 μg of ampicillin ml−1 or 25 μg of kanamycin ml−1. For adhesion experiments on gradient surfaces, bacterial cultures were labeled with a 35S-protein labeling mix (NEN Life Science Products, Inc., Boston, Mass.) at a final concentration of 0.011 μCi ml−1. Cells were grown overnight, harvested by centrifugation (12,100 × g for 10 min), washed in phosphate-buffered saline (PBS; pH 7.1), and incubated in the same buffer at 24°C for 24 h. Prior to experiments, these cell suspensions were centrifuged (12,100 × g for 10 min) and resuspended in PBS at the appropriate cell concentration (approximately 3 × 108 CFU ml−1). Aliquots were centrifuged (13,000 × g for 10 min) and resuspended in the same volume of PBS supplemented with different amounts of NaCl to produce various ionic strengths. The final ionic strengths of the solutions were as follows: 0.06 M (which corresponds to PBS with no NaCl added), 0.1225 M, 0.185 M, 0.31 M, and 0.56 M (which corresponds to PBS with 0.5 M NaCl added). The pH of all solutions was fixed at 7.1.
TABLE 1.
E. coli strains used in this study
| Strain | Relevant phenotype | Expression of type 1 fimbriae | CSH ± SD (%)a | Reference |
|---|---|---|---|---|
| MS7fim− | PC31Δfim; Kanr | − | 1.2 ± 0.5 | 45 |
| MS7fim+ | PC31Δfim(pPKL4); Apr | + | 6.0 ± 1.9 | 43 |
Data are presented as percentages of cells in a population with three or more attached hydrophobic microspheres (32). The adsorption of a high number of microspheres per cell is related to a high cell surface hydrophobicity (CSH), determined by microsphere adhesion to cells.
The viability of the cells was determined after 30 or 90 min of incubation at the different ionic strengths as the number of CFU after overnight incubation on Luria-Bertani plates.
Particle microelectrophoresis.
Washed bacterial cells were resuspended to a concentration of 107 to 108 cells ml−1 in PBS at various ionic strengths (modified by either dilution or addition of NaCl). The electrophoretic mobility of the different strains was measured in a Mark II microelectrophoresis meter (Rank Brothers Ltd., Cambridge, United Kingdom) at the following final ionic strengths: 0.012, 0.0245, 0.037, 0.0412, 0.06, and 0.1225 M. At electrolyte concentrations above these values, the voltage of the measuring apparatus was depressed, which caused a slower mobility and sedimentation of bacterial cells. Exact measurements at higher ionic strengths were therefore not possible. The zeta potentials (ζ) were calculated as a measure of the cell surface charge from the velocities by applying the Smoluchowski equation (23).
Preparation of wettability gradient surfaces.
In order to obtain different substratum wettabilities on the same surface, silicon oxide surfaces (10 by 25 mm) were prepared as described by Jönsson et al. (25). The surfaces were cleaned in a mixture of H2O:H2O2:NH4OH (ratio, 5:1:1 [vol/vol/vol]) and heated to about 80°C. After 10 min, the slides were thoroughly rinsed with distilled water. Thereafter, a mixture of H2O:H2O2:HCl (ratio, 6:1:1 [vol/vol/vol] was added. The solution was heated to about 80°C for 10 min and thoroughly rinsed with distilled water. Subsequently, the surfaces were rinsed with ethanol, trichloroethylene (TCE), and xylene and placed vertically in a tub filled with xylene. A 0.05% solution of dimethyldichlorosilane in TCE was slowly bedded under the xylene phase, thus allowing methylsilane to diffuse into the xylene phase and to bind to the silicon oxide surface. After 90 min of diffusion at 24°C, the solution was sucked out from the lower part of the tub. Subsequently, the surfaces were rinsed with ethanol and TCE and left in ethanol until use. The treatment resulted in a wettability gradient on the surface, with water contact angles of <11° and >85° at the hydrophilic and hydrophobic ends, respectively.
Adhesion to gradient surfaces.
Gradient surfaces were submerged in petri dishes filled with bacterial suspensions. 35S-labeled bacteria were suspended in sterile PBS at various ionic strengths (0.06, 0.1225, 0.185, 0.31, or 0.56 M) and allowed to attach to the surfaces for 30 min. The surfaces were removed from the suspensions, dried in nitrogen, and exposed to phosphorimager plates for 1 week. The plates were subsequently scanned in a phosphorimager (Molecular Dynamics) with a pixel size of 176 by 176 μm. Scanned pictures were analyzed by creating line graphs, where each measured point on the gradient corresponds to the sum of positively stained pixels across the width of the surface.
Preparation of quartz crystal surfaces.
AT-cut 5-MHz crystals from Maxtek Inc. (Torrance, Calif.), coated with an evaporated gold film, were used as sensors in QCM experiments. In AT-cut crystals, the orientation of the plate to the crystal structure is parallel to the transducer surface. The crystals were cleaned prior to the experiment as described previously (26) by treating them with a mixture of H2O:H2O2:NH4OH (ratio 6:1:0.8 [vol/vol/vol]) and heated to about 80°C. After 5 min, the crystals were rinsed with distilled water and dried in nitrogen. Subsequently, surfaces were oxidized for 10 min in a UV-ozone chamber, resulting in a hydrophilic surface. Hydrophobic, methyl-terminated surfaces were obtained as described previously (35) by immersing clean crystals in a saturated solution of octadecylmercaptan (Aldrich Chemical Co. Ltd.) in hexane for at least 12 h at 24°C. Immediately prior to the experiment, hydrophobic crystals were rinsed in pure hexane, ethanol, and distilled water and dried in nitrogen. Water contact angles on hydrophilic surfaces were <11° and on hydrophobic surfaces were >85°.
QCM analysis.
We used a QCM technique measuring both changes in frequency (Δf) and energy dissipation (ΔD) as described previously (41). The quartz crystal was mounted in a detection cell with electrodes connected to the driving unit via a relay to a signal generator. An alternating potential field across the crystal induces oscillation in the shear mode at the resonant frequency. Changes in the resonant frequency and the dissipation factor were measured with a time resolution of approximately 1 s, amplified, and recorded on a digitizing oscilloscope. The generated data were transferred to a personal computer and analyzed by a program from Q-Sense AB (Gotëborg, Sweden). The temperature of the detection cell was stabilized at 24°C.
QCM experiments.
To prevent the formation of air bubbles on the electrode, all sterile solutions were degassed prior to QCM measurements by sonication in vacuum for 5 min. The temperature of all solutions and bacterial suspensions was stabilized at 24°C. The chamber was rinsed several times with PBS. Under these conditions, the baselines for both frequency and dissipation were constant, indicating that no adsorption of contaminating particles occurred. Subsequently, the bacterial suspension was added to a final concentration of approximately 3 × 108 cells ml−1. The frequency and dissipation shifts caused by adhesion of bacteria to the crystal surface were measured continuously for 30 min in PBS at various final ionic strengths (0.06, 0.1225, 0.185, 0.31, and 0.56 M).
To study the continuous interactions of attached bacteria with the QCM surfaces at an ionic strength of 0.1225 or 0.31 M, the measuring chamber was rinsed with sterile PBS at the respective ionic strength after 30-min measurements to avoid further attachment of bacteria. The subsequent frequency and dissipation shifts were measured for an additional 60 min.
To check whether the bacterial cells release organic material into the surrounding solution that may adsorb to the crystal surfaces, bacterial cells were removed from suspensions by centrifugation (14,926 × g for 10 min) and QCM experiments were run with filter-sterilized supernatants at an ionic strength of 0.06 or 0.56 M NaCl, respectively. Additionally, all supernatants were analyzed with one-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12%) to reveal the presence of extracellular proteins.
Microscopy.
The cell concentration in the experiments was determined by acridine orange (AO) direct counts. Aliquots of bacterial suspensions were diluted in PBS (pH 7.1), and 1-ml samples were stained with an equal volume of filter-sterilized AO solution (100 μg of AO ml−1, 2% [vol/vol] formaldehyde in PBS) for 5 min and passed through a 0.2-μm-pore-size Nucleopore filter (prestained with Sudan black). Filters were viewed at a magnification of ×1,250 in a Zeiss epifluorescence microscope, and at least 30 fields of view were counted.
Attached bacteria were counted directly on the crystal surfaces. Crystals were removed from the QCM chamber and stained for 5 min in a filter-sterilized AO solution (100 μg of AO ml−1, 2% [vol/vol] formaldehyde in PBS). The surfaces were briefly dipped into filter-sterilized deionized water to get rid of excess stain and then air dried. Surfaces were examined by epifluorescence microscopy at a magnification of ×500. For every sample a minimum of 20 fields of view were counted. All cells were counted regardless of color.
RESULTS
Viability of bacterial cells at various ionic strengths.
No significant differences in the number of CFU of the strains were detected between the ionic strengths tested (data not shown). This indicates that the viability of cells was not affected by the change to any of the ionic strengths tested.
Cell surface hydrophobicity and zeta potentials.
The cell surface hydrophobicities of the MS7fim− and MS7fim+ strains were measured previously by microsphere adhesion to cells (32) and are shown in Table 1.
Both strains displayed negative overall cell charges at all ionic strengths tested. With increasing ionic strength, the measured electrophoretic mobilities and the zeta potentials were less negative except at 0.06 M. The zeta potentials of the nonfimbriated mutant were significantly more negative than those displayed by the fimbriated strain at all ionic strengths above 0.012 M.
Distribution of bacterial cells on wettability gradient surfaces at different ionic strengths.
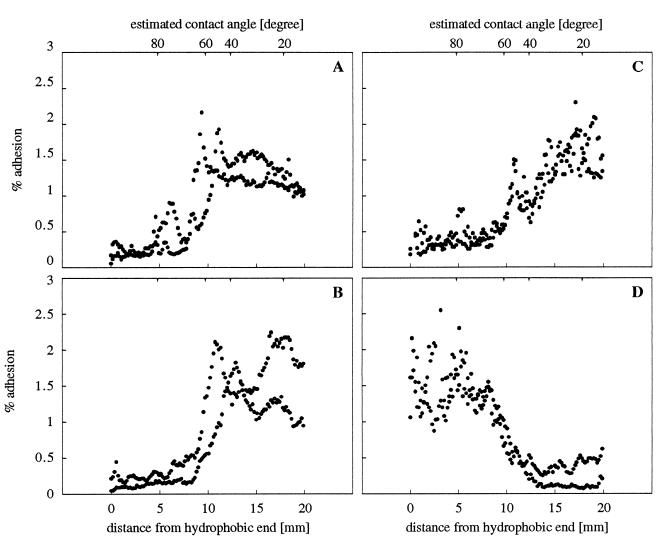
The total number of attached cells on the whole gradient increased for both strains between 0.06 and 0.1225 M but decreased at higher ionic strengths (data not shown). At all electrolyte concentrations tested, the nonfimbriated strain MS7fim− preferentially bound to the hydrophilic part of the surface, and only minor changes in the distribution pattern of attached cells were observed at different ionic strengths, as exemplified for ionic strengths of 0.1225 and 0.31 M in Fig. 1. Similarly, at low ionic strengths (0.06 and 0.1225 M) the fimbriated strain MS7fim+ showed a higher preference of binding to the hydrophilic part of the gradient surface. However, with increasing ionic strengths a higher number of fimbriated cells bound to the hydrophobic part of the gradient. This inversion of the distribution pattern is exemplified for the ionic strengths of 0.1225 and 0.31 M (Fig. 1).
FIG. 1.
Distribution of attached bacterial cells on wettability gradient surfaces as a function of ionic strength: E. coli MS7fim− at 0.1225 M (A) and 0.31 M (B) and MS7fim+ at 0.1225 M (C) and 0.31 M (D). Pixel values correspond to the relative number of 35S-labeled cells attached to the surface. A value of 100% corresponds to the total number of attached cells on the whole gradient. Intermediate contact angles were estimated from the surface concentration of adsorbed fibrinogen as described in reference 16. All data points from two independent experiments are shown.
Quantification of attached cells on hydrophilic and hydrophobic crystal surfaces at different ionic strengths.
In QCM experiments, for both strains the number of attached cells was generally higher on hydrophilic surfaces than hydrophobic surfaces (Table 2). This difference was more pronounced for the nonfimbriated strain than for the fimbriated strain. On hydrophilic surfaces, the level of adhesion of MS7fim− cells was slightly higher than that of MS7fim+ cells, whereas the latter attached in higher numbers to hydrophobic surfaces (except at the lowest ionic strength, 0.06 M).
TABLE 2.
Bacterial counts on hydrophilic and hydrophobic QCM crystal surfaces after 30 min of attachment at various ionic strengthsa
| Ionic strength (M) | No. of attached cells (106) per cm2 on crystal surfaces
|
|||
|---|---|---|---|---|
| Hydrophilic
|
Hydrophobic
|
|||
| MS7fim− | MS7fim+ | MS7fim− | MS7fim+ | |
| 0.06 | 1.8 ± 0.7 | 0.8 ± 0.6 | 1.0 ± 0.3 | 0.4 ± 0.3 |
| 0.1225 | 3.0 ± 0.9 | 2.4 ± 2.1 | 0.6 ± 0.3 | 1.1 ± 0.4 |
| 0.185 | 3.7 ± 0.5 | 2.0 ± 0.7 | 0.5 ± 0.4 | 1.2 ± 0.8 |
| 0.31 | 3.1 ± 0.4 | 1.6 ± 0.7 | 0.4 ± 0.2 | 1.2 ± 0.4 |
| 0.56 | 2.2 ± 0.4 | 1.7 ± 0.9 | 0.3 ± 0.2 | 0.9 ± 0.4 |
The applied cell concentration in the experiment was approximately 3 × 108 cells ml−1. Data are means ± standard deviations from three to four experiments.
The effect of increasing ionic strength on the number of attached bacteria was strongest between 0.06 and 0.1225 M on both surfaces. The numbers of attached MS7fim− cells increased on hydrophilic surfaces with increasing salt concentrations up to an ionic strength of 0.185 M, above which adhesion decreased. On hydrophobic surfaces, numbers of attached MS7fim− cells decreased slightly with increasing electrolyte concentration. The numbers of attached MS7fim+ cells increased from 0.06 to 0.1225 M on both surfaces. Above this ionic strength the number of attached cells declined on hydrophilic surfaces while it stayed approximately constant on hydrophobic surfaces.
Changes in mass and viscoelastic properties of the contact area between cells and surfaces at different ionic strengths.
When the measuring chamber was incubated with sterile PBS, no changes in frequency or dissipation were observed. Thus, negative frequency shifts (Δf) and positive dissipation shifts (ΔD) result from the attachment of bacteria. Supernatants taken from bacterial suspensions at the lowest ionic strength (0.06 M) contained several proteins, as revealed by one-dimensional SDS-polyacrylamide gel electrophoresis (data not shown). However, such supernatants gave rise to frequency and dissipation shifts that were minor compared to the adhesion response of bacterial cell suspensions.
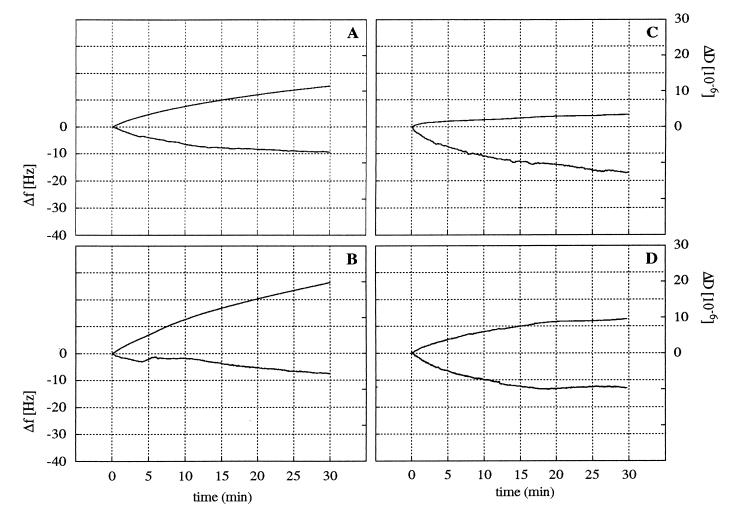
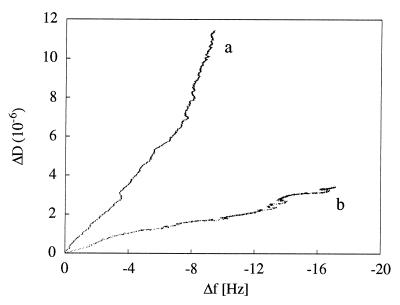
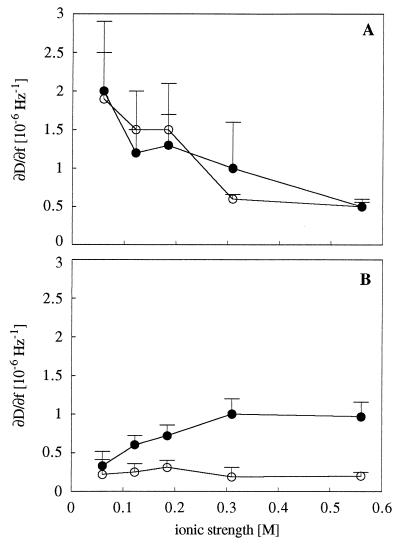
Frequency and dissipation shifts for strains MS7fim− and MS7fim+ were measured simultaneously as a function of time on hydrophilic and hydrophobic crystal surfaces as shown for one ionic strength, 0.1225 M (Fig. 2). These signals are caused both by cells attaching to the surface and by attached cells undergoing changes in contact with the surface. The QCM response can be interpreted from the ratio of dissipation shift to frequency shift (ΔD/Δf) for each time point during the experiment, as exemplified for adhesion of strains MS7fim− and MS7fim+ to hydrophilic surfaces at an ionic strength of 0.1225 M (Fig. 3). As the slopes of the resulting ΔD/Δf graphs (referred to as ∂D/∂f) are independent of both time and the number of attached cells, they distinguish qualitative characteristics of the adhesion responses. ∂D/∂f values for adhesion of MS7fim− and MS7fim+ cells to hydrophilic and hydrophobic surfaces were calculated for different ionic strengths (Fig. 4). For the nonfimbriated strain MS7fim−, on both hydrophilic and hydrophobic surfaces ∂D/∂f values were similar in size and decreased with increasing ionic strength. For the fimbriated strain MS7fim+, ∂D/∂f was very low and did not change significantly on hydrophilic surfaces over the whole range of ionic strengths tested. However, on hydrophobic surfaces there was an increase up to an ionic strength of 0.31 M.
FIG. 2.
Frequency shifts (lower line) and dissipation shifts (upper line) measured at an ionic strength of 0.1225 M for E. coli MS7fim− on hydrophilic (A) and hydrophobic (B) surfaces and MS7fim+ on hydrophilic (C) and hydrophobic (D) surfaces. Experiments have been repeated two to four times, and representative results are shown.
FIG. 3.
ΔD/Δf (dissipation per frequency shift) for adhesion of E. coli MS7fim− (a) and MS7fim+ (b) on hydrophilic surfaces at an ionic strength of 0.1225 M, based on the same data shown in Fig. 2.
FIG. 4.
∂D/∂f (slope of ΔD/Δf plots) for adhesion of E. coli MS7fim− (A) and MS7fim+ (B) to hydrophilic (open circles) and hydrophobic surfaces (filled circles) as a function of ionic strength. Data presented are means ± standard deviations (error bars) from two to four experiments.
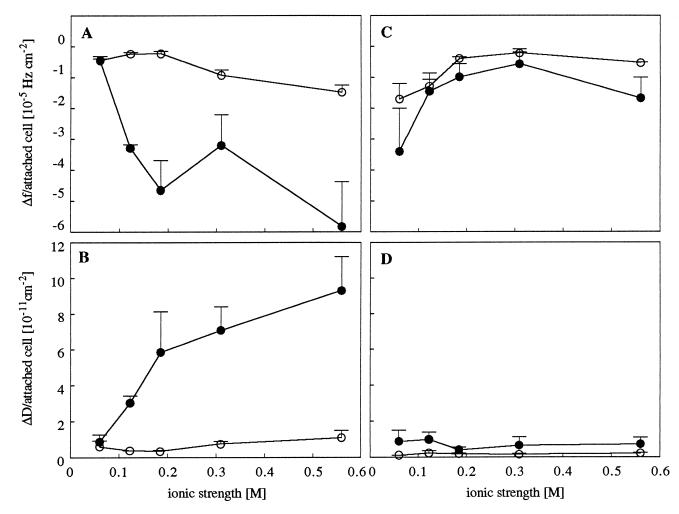
Changes in the final Δf and ΔD, measured after 30 min of incubation, at various ionic strengths were related to the respective number of attached bacteria on each surface (Fig. 5). The adhesion of strain MS7fim− caused larger frequency shifts per attached cell with increasing ionic strength, on hydrophilic surfaces and particularly on hydrophobic surfaces. In contrast, the magnitude of frequency shifts per attached cell for strain MS7fim+ declined with increasing ionic strengths up to a salt concentration of 0.31 M on both surfaces. Dissipation shifts per attached cell were low for strain MS7fim+ on both surfaces and for MS7fim− on hydrophilic surfaces. On hydrophobic surfaces, however, the adhesion of strain MS7fim− induced dissipation shifts per attached cell that were highly increasing with electrolyte concentration. For strain MS7fim+, the addition of d-mannose caused reduced frequency and dissipation shifts per cell on hydrophilic and hydrophobic surfaces (data not shown).
FIG. 5.
Frequency shifts (Δf) per attached cell for E. coli MS7fim− (A) and MS7fim+ (C) and dissipation shifts (ΔD) per attached cell for MS7fim− (B) and MS7fim+ (D) as a function of ionic strength. Open circles represent results for hydrophilic surfaces, and filled circles represent results for hydrophobic surfaces. Data presented are means ± standard deviations (error bars) from two to four experiments.
Changes in mass and viscoelastic properties of the contact area between cells and surfaces as a function of time.
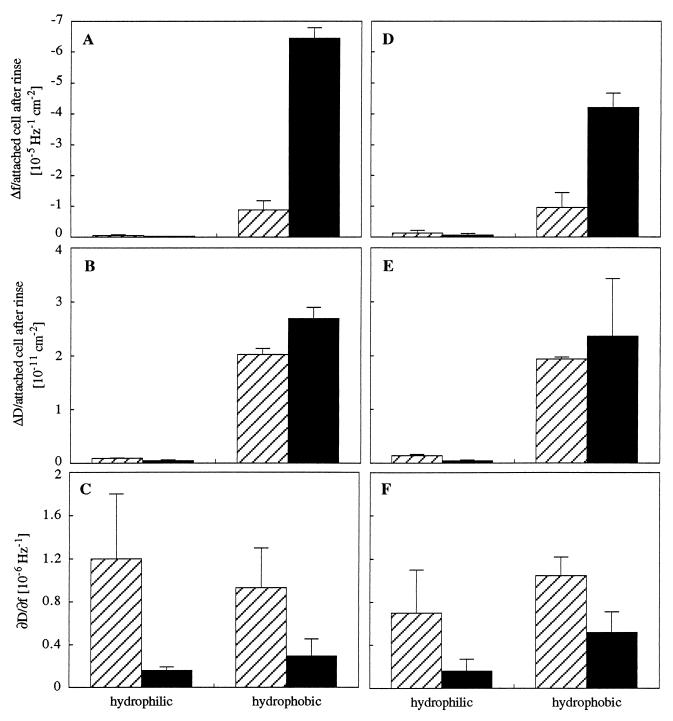
After 30 min of incubation, the measuring chamber was rinsed and subsequent frequency and dissipation shifts, solely caused by changes of attached cells in contact with the surface, were measured for 60 min (Fig. 6). The QCM response for this period of adhesion did not differ significantly between ionic strengths of 0.1225 and 0.31 M. For both strains, ongoing frequency and dissipation shifts per attached cell were higher on hydrophobic surfaces than on hydrophilic surfaces, and MS7fim+ cells caused a relatively higher magnitude of frequency shifts per cell than strain MS7fim−. The magnitude of dissipation shifts per contact point (∂D/∂f) was higher for strain MS7fim− even after prolonged incubation and corresponded proportionally to those during initial adhesion.
FIG. 6.
Subsequent interactions of attached cells with hydrophilic and hydrophobic surfaces upon rinse during 60 min of adhesion. Frequency shifts (Δf) per attached cell after a rinse at an ionic strength of 0.1225 M (A) and 0.31 M (D) and dissipation shifts (ΔD) per attached cell after a rinse at 0.1225 M (B) and 0.31 M (E). Also shown is the ∂D/∂f for adhesion at an ionic strength of 0.1225 M (C) and 0.31 M (F). Hatched bars represent the results for E. coli MS7fim−, and black bars represent the results for strain MS7fim+. Data presented are means ± standard deviations (error bars) from two to four experiments.
DISCUSSION
In this study, we examined how ionic strength influences the initial interactions of bacteria with surfaces by monitoring the adhesion process continuously with an extended QCM technique. To our knowledge, this method has not previously been used to study bacterial adhesion mechanisms. We found that the effect of ionic strength on mass increase (Δf) and on energy dissipation (ΔD) upon binding clearly differed between fimbriated and nonfimbriated Escherichia coli mutant strains. It is well documented that adsorption processes are influenced by ionic strength. For dilute solutions (0.0 to 0.2 M), bacterial adhesion to surfaces is often explained by the principles of the DLVO theory, by which the interaction between the cell and the surface is described in terms of attractive van der Waals forces and electrostatic interactions. The latter interactions are generally repulsive because most cells and surfaces have a negative net charge. E. coli is mainly found in body fluids with ionic strengths usually in the range of 150 mM or higher (26). However, this species is often shed into aquatic environments where it can survive for prolonged periods, probably in biofilms (2, 8, 17). The ionic strength of solutions in environmental systems varies from below 0.01 M in groundwater to approximately 0.3 M in brackish water and 0.57 M in oceanic seawater (22). Therefore, we chose to investigate the adhesion response within a broad range of ecologically relevant ionic strengths, even though the effects of higher ionic strengths (>0.2 M) cannot be explained by the DLVO theory.
The E. coli strains used in this study are negatively charged and, compared to other E. coli strains (51), are rather hydrophilic even though the fimbriated strain is clearly more hydrophobic than the nonfimbriated strain (Table 1). Zeta potentials of both strains were less negative with increasing ionic strengths, which is in agreement with previous results (26, 49). The presence of fimbriae may affect the exact location of the shear plane (46), and ionic groups on fimbriae may contribute with additional surface charge to the bacterial cells (38). As a result, the fimbriated strain generally exhibited a higher resistance in the electrical field and had zeta potentials less negative than those displayed by the nonfimbriated strain, which is in accordance with previous results (6).
Previously, we have shown that isogenic mutants differing in their fimbriation exhibit different adhesion patterns on wettability gradient surfaces (32). Here, we show that differences in the distribution pattern of attached fimbriated and nonfimbriated cells are most pronounced at high ionic strengths (Fig. 1). While the nonfimbriated strain preferentially attached to the hydrophilic part of the surface, the fimbriated strain showed a similar distribution pattern only at low ionic strengths but adhered better to the hydrophobic part of the surface at high ionic strengths. This effect is consistent with previously reported results (37) and may be due to a general shielding of cell surface charges and dipole interactions at high ionic strengths which may increase the tendency for hydrophobic interactions (30). Quantitative differences in adhesion may be a result of the different initial contact of fimbriated and nonfimbriated cells with the surface directly influenced by the ionic strength applied. To investigate such initial interactions continuously, we used the extended QCM method.
Although silicon oxide and gold surfaces differ in chemical aspects, after surface treatment the end points of gradient surfaces exhibit the same wettabilities as hydrophilic and hydrophobic crystal surfaces. In fact, relative adhesion to the gradient end points and to crystal surfaces generally followed the same trend (Fig. 1 and Table 2). On QCM surfaces, the number of attached cells generally increased up to an ionic strength of 0.1225 or 0.185 M, above which adhesion either leveled off or decreased. Similar findings have been reported previously (18, 19, 33) and are consistent with the DLVO theory. As the diffuse layer of counterions is compressed with increasing ionic strengths up to 0.2 M, repulsive interactions and the separation distance between the approaching surfaces decrease. On the other hand, for the nonfimbriated strain adhesion to hydrophobic surfaces decreased continuously with increasing ionic strength.
The number of attached bacteria on surfaces is only a quantitative measure of the adhesion process and cannot account for dynamic properties of the attached bacterial film. By combined measurements of frequency shifts (Δf), i.e., the increase in biomass attached to the surface (40), and dissipation shifts (ΔD), i.e., the loss of energy (damping) induced by the contact of the attached biomass to the surface (39), we were able to show clear differences in the viscoelastic behavior of fimbriated and nonfimbriated cells attached to surfaces (Fig. 2 to 5). Moreover, the interactions between bacterial cells and quartz crystal surfaces at various ionic strengths followed different trends depending on the cell surface structures in direct contact with the surface. The signals measured on-line were interpreted by calculations of ∂D/∂f, i.e., the energy dissipation per contact point, for different ionic strengths. For cells, the relationship between frequency shifts and the attached biomass is not linear (36), as the direct contact area between the cell and the surface is very small compared to whole bacterial cells and may vary depending on the surfaces, the adhesion mechanism involved, and the ionic strength applied (28). Therefore, we also calculated the final Δf per cell, i.e., the sum of contact points between the cell and the surface, and ΔD per cell, i.e., damping caused by each cell. It may also be considered that changes in frequency and dissipation at different ionic strengths may result from different interactions. The ionic strength of the surrounding medium may have a direct effect on the cell, thus modifying its rigidity, which in turn influences its damping effect. Furthermore, Δf and ΔD may originate from alterations in the cell surface molecules that mediate the attachment or from changes in the binding of these molecules to the surface (39).
Keeping these possible interpretations of the QCM signals in mind, we suggest the following explanation for the results in this study. For the nonfimbriated strain, the contact area of attached cells (Fig. 5A) and the energy loss per attached cell (Fig. 5B) increased with increasing ionic strengths, on hydrophilic and particularly on hydrophobic surfaces. This effect is consistent with predictions made by the DLVO theory. Due to increasing ionic strengths and a shorter separation distance between the cells and the surface, the cell may be coupled closer to the surface. However, ∂D/∂f decreased with increasing ionic strengths (Fig. 4). We suggest that a better coupling of nonfimbriated cells to the surface was due to an increase in the number of contact points but that each single contact point contributed less to the total energy loss measured.
Interestingly, changes in frequency and dissipation caused by the adhesion of the fimbriated strain followed an opposite trend. The adhesion of strain MS7fim+ was reduced by the addition of 10 mM d-mannose, the specific ligand for type 1 fimbriae (data not shown), which supports the idea that the contact between cells and surfaces is mediated by fimbriae. As the decay curve from the oscillating crystal reached about 250 nm from the surface (20), the contact area per cell was small at all ionic strengths tested and Δf even decreased up to an ionic strength of 0.31 M (Fig. 5C). This may be explained by a higher number of binding sites due to a more destabilized organization of fimbriae at low ionic strengths. With increasing ionic strengths, a tighter structural organization of fimbriae may result in a smaller contact area. On the other hand, ΔD per attached cell was low at all ionic strengths tested and changed only negligibly with increasing ionic strengths (Fig. 5D). This suggests a very flexible binding of attached fimbriated cells to the surface, where the cell is not coupled so closely to the surface, as its mass may contribute to the dissipation shift. On hydrophilic surfaces, the dissipation caused by each contact point (∂D/∂f) is low at all ionic strengths tested, whereas on hydrophobic surfaces ∂D/∂f increased up to 0.31 M (Fig. 4B). This may indicate that the coupling of fimbriated cells to the surface increases in each contact point with increasing ionic strengths. A more stable contact of the fimbriated strain to hydrophobic surfaces at high ionic strengths may, in turn, contribute to the observed inversion of the distribution pattern on gradient surfaces.
Prolonged measurements of frequency and dissipation shifts per attached cell, during which no further attachment of cells occurred, revealed that the contact area of attached cells of both strains increased as a function of time, particularly in contact with hydrophobic surfaces (Fig. 6). Ongoing interactions were higher for the fimbriated strain than the nonfimbriated strain, which may be understood as follows. The initial adhesion of strain MS7fim− already involves direct contact with a larger part of the cell, whereas the first contact of strain MS7fim+ is mediated by fimbriae. A subsequent increase in frequency and dissipation per attached cell may be due to the time-dependent establishment of a more intimate contact between the cell and the surface, reaching a maximum contact area. Although ionic strength does not seem to influence ongoing interactions of attached cells, its important role in determining the initial contact of bacteria with a surface may influence the subsequent colonization of surfaces and biofilm formation.
In summary, by simultaneously measuring the parameters of mass increase (Δf) and energy dissipation (ΔD) we were able to characterize the different binding mechanisms of fimbriated and nonfimbriated E. coli cells. Although the interpretation of frequency and dissipation shifts is not yet fully understood, changes in these parameters reflect clearly the dynamic character of interactions between bacterial cells and surfaces. Our study gives a better understanding of the influence of dominant cell surface structures on the adhesion behavior of bacterial strains and introduces the extended QCM technique as a useful tool to monitor bacterial adhesion.
ACKNOWLEDGMENTS
We thank Per Klemm, Danish Technical University, Lyngby, Denmark, for providing the strains; Omer Nur, Chalmers University of Technology, Göteborg, Sweden, for contributing silicon oxide surfaces; and Anna-Kerstin Thell for excellent technical assistance. Staffan Wall, Chalmers University of Technology, Göteborg, Sweden, is greatly acknowledged for his help with the zeta potential analysis, and we thank Fredrik Höök for valuable discussions.
This work was financially supported by the Foundation for Strategic Research through the Marine Science and Technology (MASTEC) program.
REFERENCES
- 1.Absolom D R, Lamberti F V, Policova Z, Zingg W, van Oss C J, Neumann A W. Surface thermodynamics of bacterial adhesion. Appl Environ Microbiol. 1983;46:90–97. doi: 10.1128/aem.46.1.90-97.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison D G, Evans D J, Brown M R W, Gilbert P. Possible involvement of the division cycle in dispersal of Escherichia coli from biofilms. J Bacteriol. 1990;172:1667–1669. doi: 10.1128/jb.172.3.1667-1669.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angell P, Arrage A A, Mittelman M W, White D C. On line, non-destructive biomass determination of bacterial biofilms by fluorometry. J Microbiol Methods. 1993;18:317–327. [Google Scholar]
- 4.Bao L L, Deng L, Nie L H, Yao S Z, Wei W Z. Determination of microorganisms with a quartz crystal microbalance sensor. Anal Chim Acta. 1996;319:97–101. [Google Scholar]
- 5.Bellon-Fontaine M-N, Mozes N, van der Mei H C, Sjollema J, Cerf O, Rouxhet P G, Busscher H J. A comparison of thermodynamic approaches to predict the adhesion of dairy microorganisms to solid substrata. Cell Biophys. 1990;17:93–106. doi: 10.1007/BF02989805. [DOI] [PubMed] [Google Scholar]
- 6.Brinton C C, Buzzelli A, Lauffer M A. Electrophoresis and phage susceptibility studies on a filament-producing variant of the E. coli B bacterium. Biochim Biophys Acta. 1954;15:533–542. doi: 10.1016/0006-3002(54)90011-6. [DOI] [PubMed] [Google Scholar]
- 7.Cady W G. Piezoelectricity. New York, N.Y: Dover Publications; 1964. pp. 1–9. [Google Scholar]
- 8.Camper A K, Jones W L, Hayes J T. Effect of growth conditions and substratum composition on the persistence of coliforms in mixed-population biofilms. Appl Environ Microbiol. 1996;62:4014–4018. doi: 10.1128/aem.62.11.4014-4018.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter R M, Mekalanos J J, Jacobs M B, Lubrano G J, Guilbault G G. Quartz crystal microbalance detection of Vibrio cholerae O139 serotype. J Immunol Methods. 1995;187:121–125. doi: 10.1016/0022-1759(95)00176-b. [DOI] [PubMed] [Google Scholar]
- 10.Cassidy M B, Lee H, Trevors J T. Environmental applications of immobilized microbial cells: a review. J Ind Microbiol. 1996;16:79–101. [Google Scholar]
- 11.Chen K, Le D, Zhang H, Nie L H, Yao S Z. Model of quartz crystal microbe growth sensor and its application to estimation of microbial populations in mineral waters. Anal Chim Acta. 1996;329:83–89. [Google Scholar]
- 12.Costerton J W, Lewandowski Z. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 13.Deng L, Bao L L, Yang Z Y, Nie L H, Yao S Z. In situ continuous detection of bacteria on the surface of solid medium with a bulk acoustic wave-impedance sensor. J Microbiol Methods. 1996;26:197–203. [Google Scholar]
- 14.Derjaguin B V, Landau L. Theory of the stability of strongly charged hydrophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Acta Physicochim URSS. 1941;14:633–662. [Google Scholar]
- 15.Ebersole R C, Foss R P, Ward M D. Piezoelectric cell growth sensor. Bio/Technology. 1991;9:450–454. doi: 10.1038/nbt0591-450. [DOI] [PubMed] [Google Scholar]
- 16.Elwing H, Welin S, Askendal A, Lundström I. Adsorption of fibrinogen as a measure of the distribution of methyl groups on silicon wafers. J Colloid Interface Sci. 1988;123:306–308. [Google Scholar]
- 17.Espinosa-Urgel M, Kolter R. Escherichia coli genes expressed preferentially in an aquatic environment. Mol Microbiol. 1998;28:325–332. doi: 10.1046/j.1365-2958.1998.00796.x. [DOI] [PubMed] [Google Scholar]
- 18.Gannon J, Tan Y, Baveye P, Alexander M. Effect of sodium chloride on transport of bacteria in a saturated aquifer material. Appl Environ Microbiol. 1991;57:2497–2501. doi: 10.1128/aem.57.9.2497-2501.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon A S, Millero F J. Electrolyte effects on attachment of an estuarine bacterium. Appl Environ Microbiol. 1984;47:495–499. doi: 10.1128/aem.47.3.495-499.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon J G, Kanazawa K K. Frequency of a quartz microbalance in contact with liquid. Anal Chem. 1985;57:1770–1771. [Google Scholar]
- 21.Habash M B, van der Mei H C, Reid G, Busscher H J. Adhesion of Pseudomonas aeruginosa to silicone rubber in a parallel plate flow chamber in the absence and presence of nutrient broth. Microbiology. 1997;143:2569–2574. doi: 10.1099/00221287-143-8-2569. [DOI] [PubMed] [Google Scholar]
- 22.Hermansson, M. The DLVO theory in microbial adhesion. Colloids Surf. B Biointerfaces, in press.
- 23.Hiemenz P C. Electrophoresis and other electrokinetic phenomena. In: Lagowski J J, editor. Principles of colloid and surface chemistry. New York, N.Y: Dekker; 1977. pp. 452–487. [Google Scholar]
- 24.Höök F, Rodahl M, Kasemo B. Energy dissipation kinetics for protein and antibody-antigen adsorption under shear oscillation on a quartz crystal microbalance. Langmuir. 1998;14:729–734. [Google Scholar]
- 25.Jönsson U, Ivarsson B, Lundström I, Berghem L. Adsorption behaviour of fibronectin on well-characterized silicon surfaces. J Colloid Interface Sci. 1982;90:148–163. [Google Scholar]
- 26.Jucker B A, Harms H, Zehnder A J B. Adhesion of the positively charged bacterium Stenotrophomonas (Xanthomonas) maltophilia 70401 to glass and teflon. J Bacteriol. 1996;178:5472–5479. doi: 10.1128/jb.178.18.5472-5479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 28.Nimeri G, Fredriksson C, Elwing H, Liu L, Rodahl M, Kasemo B. Neutrophil interaction with protein-coated surfaces studied by an extended quartz crystal microbalance technique. Colloids Surf B Biointerfaces. 1998;11:255–264. [Google Scholar]
- 29.Nivens D E, Chambers J Q, Anderson T R, Tunlid A, Smit J, White D C. Monitoring microbial adhesion and biofilm formation by attenuated total reflection/Fourier transform infrared spectroscopy. J Microbiol Methods. 1993;17:199–213. [Google Scholar]
- 30.Ochoa J L. Hydrophobic (interaction) chromatography. Biochimie. 1978;60:1–15. doi: 10.1016/s0300-9084(78)80193-x. [DOI] [PubMed] [Google Scholar]
- 31.Olofsson A C, Zita A, Hermansson M. Floc stability and adhesion of GFP marked bacteria to flocs in activated sludge. Microbiology. 1998;144:519–528. doi: 10.1099/00221287-144-2-519. [DOI] [PubMed] [Google Scholar]
- 32.Otto, K., H. Elwing, and M. Hermansson. The role of type-1 fimbriae in adhesion of Escherichia coli to hydrophilic and hydrophobic surfaces. Colloids Surf. B Biointerfaces, in press.
- 33.Piette J-P G, Idziak E S. A model study of factors involved in adhesion of Pseudomonas fluorescens to meat. Appl Environ Microbiol. 1992;58:2783–2791. doi: 10.1128/aem.58.9.2783-2791.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pratt-Terpstra I H, Weerkamp A H, Busscher H J. On a relation between interfacial free energy-dependent and non-interfacial free energy-dependent adherence of oral streptococci to solid surfaces. Curr Microbiol. 1988;16:311–313. [Google Scholar]
- 35.Prime K L, Whitesides G M. Self-assembled organic monolayers: model systems for studying adsorption of proteins at surfaces. Science. 1991;252:1164–1167. doi: 10.1126/science.252.5009.1164. [DOI] [PubMed] [Google Scholar]
- 36.Redepenning J, Schlesinger T K, Mechalke E J, Puleo D A, Bizios R. Osteoblast attachment monitored with a quartz crystal microbalance. Anal Chem. 1993;65:3378–3381. doi: 10.1021/ac00071a008. [DOI] [PubMed] [Google Scholar]
- 37.Rijnaarts H H M, Norde W, Bouwer E J, Lyklema J, Zehnder A J B. Reversibility and mechanism of bacterial adhesion. Colloids Surf B Biointerfaces. 1995;4:5–22. [Google Scholar]
- 38.Rijnaarts H H M, Norde W, Lyklema J, Zehnder A J B. The isoelectric point of bacteria as an indicator for the presence of cell surface polymers that inhibit adhesion. Colloids Surf B Biointerfaces. 1995;4:191–197. [Google Scholar]
- 39.Rodahl M, Höök F, Fredriksson C, Keller C A, Krozer A, Brzezinski P, Voinova M, Kasemo B. Simultaneous frequency and dissipation factor QCM measurements of biomolecular adsorption and cell adhesion. Faraday Discuss. 1997;107:229–246. doi: 10.1039/a703137h. [DOI] [PubMed] [Google Scholar]
- 40.Rodahl M, Höök F, Kasemo B. QCM operations in liquids: an explanation of measured variations in frequency and Q factor with liquid conductivity. Anal Chem. 1996;68:2219–2227. doi: 10.1021/ac951203m. [DOI] [PubMed] [Google Scholar]
- 41.Rodahl M, Höök F, Krozer A, Brzezinski P, Kasemo B. Quartz crystal microbalance setup for frequency and Q-factor measurements in gaseous and liquid environments. Rev Sci Instrum. 1995;66:3924–3930. [Google Scholar]
- 42.Sauerbrey G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z Phys. 1959;155:206–222. [Google Scholar]
- 43.Schembri M A, Pallesen L, Connell H, Hasty D L, Klemm P. Linker insertion analysis of the FimH adhesin of type 1 fimbriae in an Escherichia coli fimH-null background. FEMS Microbiol Lett. 1996;137:257–263. doi: 10.1111/j.1574-6968.1996.tb08115.x. [DOI] [PubMed] [Google Scholar]
- 44.Sjollema J, van der Mei H C, Uyen H M W, Busscher H J. The influence of collector and bacterial cell surface properties on the deposition of oral streptococci in a parallel plate flow cell. J Adhesion Sci Technol. 1990;4:765–777. [Google Scholar]
- 45.Stentebjerg-Olesen B, Pallesen L, Bogø Jensen L, Christiansen G, Klemm P. Authentic display of a cholera toxin epitope by chimeric type 1 fimbriae: effects of insert position and host background. Microbiology. 1997;143:2027–2038. doi: 10.1099/00221287-143-6-2027. [DOI] [PubMed] [Google Scholar]
- 46.Van der Mei H. Physico-chemical surface properties of oral streptococci. Ph.D. thesis. Groningen, Groningen: Rijksuniversiteit; 1989. , The Netherlands. [Google Scholar]
- 47.Van Oss C J. Energetics of cell-cell and cell-biopolymer interactions. Cell Biophys. 1989;14:1–16. doi: 10.1007/BF02797387. [DOI] [PubMed] [Google Scholar]
- 48.Verwey E J W, Overbeek J T G. Theory of the stability of lyophobic colloids. Amsterdam, The Netherlands: Elsevier; 1948. [Google Scholar]
- 49.Vigeant M A S, Ford R M. Interactions between motile Escherichia coli and glass in media with various ionic strengths, as observed with a three-dimensional-tracking microscope. Appl Environ Microbiol. 1997;63:3474–3479. doi: 10.1128/aem.63.9.3474-3479.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wiencek K M, Fletcher M. Bacterial adhesion to hydroxyl- and methyl-terminated alkanethiol self-assembled monolayers. J Bacteriol. 1995;177:1959–1966. doi: 10.1128/jb.177.8.1959-1966.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zita A, Hermansson M. Effects of bacterial cell surface structures and hydrophobicity on attachment to activated sludge flocs. Appl Environ Microbiol. 1997;63:1168–1170. doi: 10.1128/aem.63.3.1168-1170.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]