Abstract
This single-arm pilot study (NCT03329937) evaluated neoadjuvant niraparib antitumor activity and safety in patients with localized HER2-negative, BRCA-mutated breast cancer. Twenty-one patients received niraparib 200 mg once daily in 28-day cycles. After 2 cycles, tumor response (≥30% reduction from baseline) by MRI was 90.5% and 40.0% (6 of 15) of patients who received only niraparib (2–6 cycles) had pathological complete response; no new safety signals were identified. High niraparib intratumoral concentration was observed.
Subject terms: Breast cancer, Targeted therapies, Cancer, Cancer therapy
Isakoff and colleagues demonstrate the safety and feasibility, as well as the clinical efficacy, of neoadjuvant niraparib in a phase II study in patients with HER2-negative and BRCA-altered breast cancer.
Main
Neoadjuvant therapy for locally advanced breast cancer (BC) aims to downstage tumors and enable breast-conserving surgery1. Pathological complete response (pCR) is associated with lower recurrence rates than residual invasive cancer at surgery after neoadjuvant therapy1. Poly(ADP-ribose) polymerase (PARP) inhibitors provide new, effective treatment options for BRCA1/2-mutated advanced/metastatic breast cancer2 by targeting homologous recombination deficiency (HRd)3. Talazoparib and olaparib are approved for HER2-negative, germline BRCA-mutated (gBRCA-mut) metastatic BC4,5.
Niraparib is a PARP-1/2 inhibitor approved for recurrent or advanced ovarian cancers6. Preliminary pharmacokinetic data showed higher niraparib concentrations in tumors than in plasma, including in BRCA-mut, triple-negative breast cancer (TNBC) and BRCA-wild-type ovarian xenograft models7–9, which may facilitate primary tumor penetration in the neoadjuvant setting.
This pilot study (NCT03329937) explored the antitumor activity of neoadjuvant niraparib for localized HER2-negative, BRCA-mut BC and assessed niraparib concentration in tumor versus plasma. Duration of niraparib treatment beyond cycle 2 was determined by clinician decision and based on observed patient responses.
As of 30 June 2020, efficacy-evaluable (two or more cycles) and safety (one or more niraparib dose) populations included 21 of 24 enrolled patients with tumor BRCA mutations. One patient discontinued due to protocol noncompliance after completing two niraparib cycles. No patients received fewer than two cycles of niraparib, 19.0% received two cycles and 81.0% received more than two cycles. Six patients (28.6%) received post-niraparib neoadjuvant chemotherapy (NACT); all patients underwent surgery: 14 patients had BRCA1mut, 6 had BRCA2mut and 1 had BRCA1/2mut; 15 patients (71.4%) had TNBC and 6 patients (28.6%) had hormone-receptor positive (HR+) BC (Supplementary Table 1).
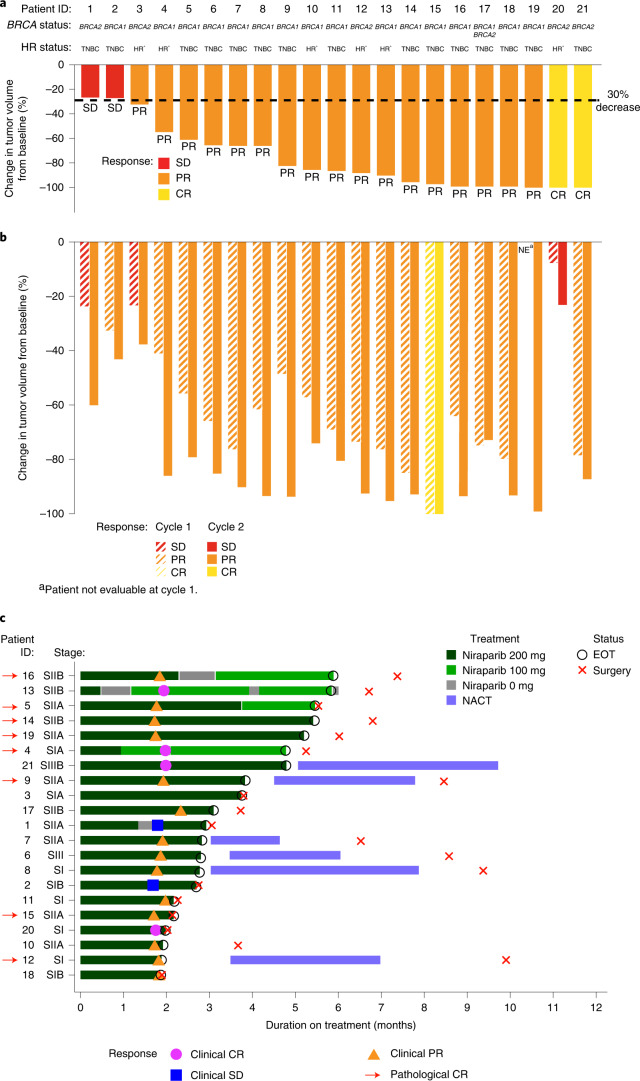
Tumor response by magnetic resonance imaging (MRI) after 2 cycles (primary endpoint) was 90.5% (95% confidence interval (CI): 69.6, 98.8%), with 2 CRs and 17 partial responses (PRs) (Fig. 1a) (86.7% in TNBC, 100% in HR+). By ultrasound, 81.0% (95% CI: 58.1, 94.6%) of tumors responded (1 CR, 16 PRs) after 1 cycle of niraparib and 95.2% (95% CI: 76.2, 99.9%) (1 CR, 19 PRs) responded after 2 cycles (Fig. 1b). Median (range) decrease in tumor volume after 2 cycles was 86.4% (26–100%) by MRI and 87.2% (23–100%) by ultrasound; best response by ultrasound (≥2 cycles) was a 92.5% (23–100%) decrease.
Fig. 1. Clinical response and change in tumor volume by MRI and ultrasound, and clinical and pathological response patient journeys by MRI.
a, Response by MRI at the end of cycle 2 of niraparib. b, Response by ultrasound after cycles 1 and 2. c, Presence of pCR, defined as ypT0/Tis ypN0, made at the time of surgery (n = 21 patients). EOT, end of treatment; NE, not evaluable; SI, stage I; SII, stage II; SIII; stage III.
Overall, eight patients (38.1%; 95% CI: 18.1, 61.6%) had pCR after neoadjuvant niraparib (niraparib duration, 1.9–5.9 months) (Fig. 1c). Of 15 patients, 6 (40.0%; 95% CI: 16.3, 67.7%; 5 TNBC, 1 HR+) who received only niraparib for 2–6 cycles had pCR; 2 of 6 patients (33.3%; 95% CI: 4.3, 77.7%; 1 TNBC, 1 HR+) who received NACT after niraparib had pCR. Six patients with pCR had BRCA1mut; 2 had BRCA2mut. Of 15 patients 6 (40.0%; 95% CI: 16.3, 67.7%) with TNBC and 2/6 (33.3%; 95% CI: 4.3, 77.7%) with HR+ BC had pCR. A summary of patient response, tumor characteristics and niraparib exposure can be found in Supplementary Table 2.
Median (range) duration of niraparib exposure was 2.9 (1.8–5.9) months. Overall, 19 of 21 patients (90.5%) experienced any-grade, niraparib-related, treatment-emergent adverse events (TEAEs; Supplementary Table 3). Grade ≥3, niraparib-related TEAEs included anemia (n = 3), neutropenia (n = 2), decreased neutrophil count (n = 2), hypertension (n = 1) and thrombocytopenia (n = 1). Two patients (9.5%) had a niraparib-related serious adverse event (AE: 1 thrombocytopenia, 1 fetal ventricular septal defect (grade 2) in the fetus of a patient with ~3 weeks’ niraparib exposure during pregnancy identified at the end-of-treatment visit). TEAEs led to niraparib dose reduction in 4 patients (19.0%; neutropenia, n = 1; thrombocytopenia, n = 1; neutrophil count decreased, n = 2). No patients discontinued treatment due to TEAEs and there were no deaths during the study.
In 10 patients with time-matched plasma/tumor samples collected after 2 cycles, mean (±s.d.) intratumoral niraparib concentrations were 35.2 ± 37.2-fold higher versus plasma (Wilcoxon’s matched-pairs signed ranks test, P = 0.002; Fig. 2a). A post-hoc analysis of the association of tumor:plasma niraparib concentration and tumor response was assessed by linear regression (Fig. 2b; R2 = 0.088; Spearman’s rank correlation ρ = −0.26, two-sided P = 0.36) including 95% confidence bands of best fit. Other parameters analyzed included total tumor niraparib concentration, which demonstrated a similar trend but was not statistically significant (Extended Data Fig. 1). However, due to the small sample size (n = 14), conclusive statements cannot be drawn from these data.
Fig. 2. Niraparib concentration in plasma and tumor and association between reduction in tumor volume and tumor:plasma niraparib concentration.
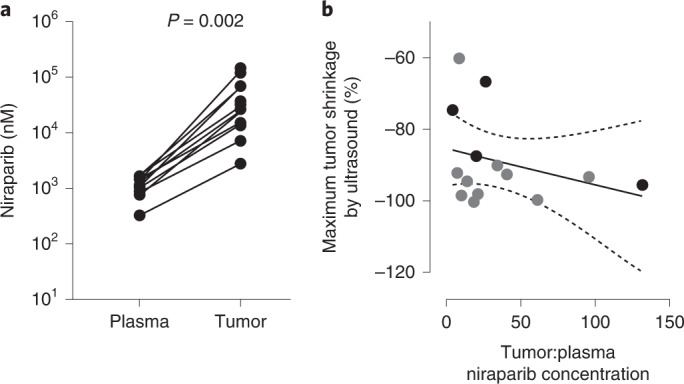
a, Niraparib concentration in patient plasma and tumor samples collected at the end of cycle 2 of niraparib, day 28 (n = 10 patients with time-matched samples; two-sided Wilcoxon’s matched-pairs, signed-rank test, P = 0.002). One patient in the analysis had a dose reduction to 100 mg before the end of cycle 2. b, Maximum tumor volume reduction based on ultrasound measurement after ≥2 months of niraparib treatment (maximal tumor reduction was −100%) and the fold difference in tumor versus matched plasma niraparib concentration (where available; for patients without available matched plasma samples, the plasma niraparib Cmax value from C2D1 was used instead to estimate the fold difference), using a linear regression model R2 = 0.088; Spearman’s rank correlation (ρ = −0.26, two-sided P = 0.36). The gray dot indicates patients with time-matched tumor and plasma samples (n = 10 patients) and the black dot patients without time-matched plasma samples (n = 4 patients), for whom fold difference in tumor versus plasma niraparib concentration was estimated based on the plasma Cmax. The dashed lines indicate 95% CIs.
Extended Data Fig. 1. Association between reduction in tumor volume and total tumor niraparib concentration.
Maximum tumor volume reduction based on ultrasound measurement after ≥2 months of niraparib treatment (maximal tumor reduction was −100%) and the fold difference in tumor versus total tumor niraparib concentration using a linear regression model R2 = 0.076; P = 0.34.•indicate patients with time-matched tumor and plasma samples (n = 14 patients) Dashed lines indicate 95% confidence intervals. Cmax, maximum concentration.
Neoadjuvant niraparib was highly active in patients with localized HER2-negative, BRCA-mut BC. There were no new safety signals and no discontinuations due to TEAEs.
After 2 cycles, >90% of patients experienced a clinical response; 38% had pCR after neoadjuvant niraparib, most of whom received only niraparib. Intratumoral niraparib concentrations were >30-fold higher than in plasma. Tumor penetration may be associated with reduced tumor volume, warranting further investigation. This is consistent with preclinical data showing superior tumor penetration by niraparib (3.3-fold higher exposure than plasma) versus other PARP inhibitors (for example, olaparib: 0.6- to 0.7-fold plasma concentration)7. In addition, niraparib concentrates in tumor and other tissues rather than circulating in the plasma; dose-normalized niraparib exposure was 10-, 51- and 100-fold higher versus olaparib in plasma, tumor and brain, respectively7. This, combined with the low clearance and high volume of distribution of niraparib, further supports a higher tendency of niraparib to concentrate in the peripheral body compartment and solid tumors, rather than in plasma7.
A phase II pilot study of neoadjuvant talazoparib also demonstrated clinical activity. All patients with gBRCA-mut BC received 6 months of neoadjuvant talazoparib; 53% (10/19) had pCR (primary endpoint) and 9 patients had dose reductions due to TEAEs5.
In our study, physicians could make treatment decisions based on observed responses at the end of cycle 2 by MRI or ultrasound, before receipt of additional therapy. Of 15 patients, 6 (40.0%) who received niraparib only (no NACT) had pCR; these patients received 2–6 cycles of niraparib. Given that five of the six patients achieving pCR in our study received four or more cycles of niraparib (no NACT), the rate of pCR achieved in this population is consistent with that of the neoadjuvant talazoparib study5. Furthermore, the INFORM trial reported that 18–26% of patients with stage I–III, BRCA-mut, HER2-negative BC had pCR with NACT (cisplatin or doxorubicin–cyclophosphamide)10. These promising results, determined from imaging and pCR rates, highlight the efficacy of neoadjuvant niraparib in BRCA-mut BC and support the use of pCR as a primary endpoint for future studies using niraparib. In addition, these results also suggest that chemotherapy use could potentially be de-escalated, reducing toxicity.
Sensitivity to PARP inhibitors has also been shown in somatic BRCA-mut ovarian cancer and in patients with mutations in other HRd-related genes11. Up to 69% of patients with TNBC have HRd and PALB2 mutations are also associated with HRd12. A phase II trial of olaparib showed antitumor activity in metastatic BC with somatic BRCA1/2 and germline PALB2 mutations13. In addition, a phase II study of talazoparib monotherapy demonstrated activity of PARP inhibitors in patients with advanced HER2-negative BC and a HR pathway gene mutation, beyond BRCA1/2. RECIST response was seen in 3 of 12 BC patients who had a RECIST response (objective response rate 25%; 2 gPALB2, 1 gCHEK2/gFANCA/sPTEN) and 3 additional patients (gPALB2, sATR, sPTEN) had stable disease (SD) for ≥6 months14. Further investigations may identify additional genetic subgroups that are likely to respond to PARP inhibitors. Limitations of our study included small sample size and heterogeneity in treatment after neoadjuvant niraparib and the number of cycles of niraparib, limiting conclusions about pCR. However, this targeted, chemotherapy-sparing approach showed favorable pCR rates and tolerability, supporting future investigations.
In this pilot study, single-agent neoadjuvant niraparib demonstrated promising antitumor activity and high levels of tumor penetration in HER2-negative, BRCA-mut, localized BC. No new safety signals were identified.
Methods
The study was conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines following approval by ethics committees and institutional review boards at each study site (Moffitt Cancer Center, Tampa, FL; Mayo Clinic Rochester, Rochester, MN; Sarah Cannon Research Institute/Tennessee Oncology, Nashville, TN; Icahn School of Medicine at Mount Sinai, New York, NY; Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD; Florida Cancer Specialists-South, Fort Myers, FL; Pacific Shores Medical Group, Long Beach, CA; Memorial Health Care System, Hollywood, FL; Baylor College of Medicine, Houston, TX; Providence Portland Medical Center, Portland, OR; and Massachusetts General Hospital, Boston, MA). All patients provided written informed consent.
The first subject was enrolled on 12 April 2018 and the last on 15 May 2019. All 24 patients were recruited from 7 of 11 active sites (site 1: 3 patients; site 2: 5 patients; site 3: 2 patients; site 4: 6 patients; site 5: 5 patients; site 6: 2 patients; and site 7: 1 patient). Eligible patients were female or male adults with: primary operable, histologically confirmed, HER2-negative, localized BC; deleterious/suspected deleterious BRCA1/2 mutations (germline, may include somatic); primary tumor size ≥1 cm; and Eastern Cooperative Oncology Group performance status 0–1. Patients were excluded for previous therapy for current malignancy, previous PARP inhibitor use or distant metastases.
Niraparib 200 mg orally once daily was given in 28-day cycles. This dose was chosen to reduce the likelihood of dose interruptions due to AEs, which predominantly occurred within cycles 1–3 in a previous study15. Patients with progressive disease (increase in tumor volume >20% per ultrasound) after cycle 1 discontinued; patients with CR, PR or SD continued into cycle 2. The primary endpoint was tumor response rate (change in tumor volume by breast MRI by investigator after two cycles). A clinical response was defined as ≥30% reduction in tumor volume from baseline without new lesions (≥PR). After cycle 2, patients proceeded directly to surgery, received NACT and then surgery, or received up to 6 cycles of niraparib before surgery with or without subsequent NACT, at the physician’s discretion.
Secondary endpoints were tumor response rate by breast ultrasound (≥30% reduction in tumor volume from baseline), change in tumor volume from baseline after cycle 2 by MRI and ultrasound, pCR at time of surgery (ypT0/Tis ypN0 by American Joint Committee on Cancer staging v.7.0) and safety/tolerability until 30 d after last niraparib dose. Niraparib intratumoral and plasma concentrations (via qualified liquid chromatography–tandem mass spectrometry at cycle 2) were exploratory endpoints.
Tumor volume was calculated as (length × width × height × π)/6 (ref. 16). If too small to measure, change from baseline was imputed as 99%. TEAEs were graded using Common Terminology Criteria for Adverse Events v.4.03. Differences between plasma and tumor niraparib concentrations were assessed using Wilcoxon’s matched-pair, signed-rank test (significance level P < 0.05). Maximum concentration (Cmax) was used to estimate niraparib tumor/plasma ratio when time-matched plasma samples were missing. Linear regression (GraphPad Prism v.8.0) assessed the correlation between response and niraparib tumor:plasma ratio. Spearman’s rank correlation was also performed.
Statistics and reproducibility
All statistical analyses were performed using SAS statistical software v.9.3 or later unless otherwise noted; data distribution was assumed to be normal, but this was not formally tested. No statistical methods were used to predetermine sample sizes, but our sample sizes are similar to those reported in previous publications17. Data collection and analysis were not performed blind to the conditions of the experiments. Clinical exclusion criteria were pre-specified and patients were not eligible for the study if any of these were met; no data points were excluded from the analyses.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Supplementary Tables 1–3.
Acknowledgements
The present study was funded by GlaxoSmithKline (GSK). GSK contributed to study design, implementation, data collection, interpretation and analysis. All the authors had full access to the data upon request and had final responsibility for the decision to submit for publication. Medical writing support was provided by E. Mercadante and C. Kelly, of Fishawack Indicia Ltd, UK, part of Fishawack Health, funded by GSK.
Extended data
Source data
Raw data for Fig. 1.
Raw data for Fig. 2.
Raw data for Extended Data Fig. 1.
Author contributions
L.M.S., H.H., J.R.G., L.W.E. and S.J.I. contributed to the conception or design of the study. L.M.S., H.H., M.C.L., E.H., H.I., C.A.S.-M., J.R., L.W.E. and S.J.I. contributed to the acquisition of data. All authors were involved in data analysis or interpretation. L.M.S. and S.J.I. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Peer review
Peer review information
Nature Cancer thanks Joshua Gruber and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Data availability
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies that evaluate medicines, on approval of proposals submitted to www.clinicalstudydatarequest.com and a data access agreement will be required. To access data for other types of GSK-sponsored research, for study documents without patient-level data, and for clinical studies not listed, please submit an inquiry via this website. Source data are provided with this paper.
Competing interests
L.M.S. reports personal fees from Novartis, Puma, Lumicell and Avrobio; travel support from Tesaro and Merck; research funding to institution from Tesaro and Merck. H.H. reports research funding to institution from Arvinas, AbbVie, Bristol-Myers Squibb, Daiichi Pharma, GSK, Karyopharm, Prescient, G1 Therapeutics, Marker Therapeutics, Novartis, Horizon Pharma, Quantum Leap Healthcare Collaborative, Pfizer, Zymeworks and Seattle Genetics; grants from the Department of Defense; and personal fees from Speaker’s Bureau for Lilly. M.C.L. reports research funding to institution from Eisai, Exact Sciences, Genentech, Genomic Health, GRAIL, Menarini Silicon Biosystems, Merck, Novartis, Seattle Genetics and Tesaro; funding to institution for participation in advisory boards for Celgene, Roche/Genentech, Genomic Health, GRAIL, Ionis, Merck, Pfizer, Seattle Genetics and Syndax. E.H. reports research funding to institution from OncoMed, Genentech/Roche, Zymeworks, Rgenix, ArQule, Clovis, Silverback Therapeutics, Millennium, Medivation, Acerta Pharma, Sermonix Pharmaceuticals, Aravive, Torque, Black Diamond, Karyopharm, Infinity Pharmaceuticals, Curis, Syndax, Novartis, Boehringer Ingelheim, Immunomedics, Fujifilm, Taiho, Deciphera, Fochon, Molecular Templates, Onconova Therapeutics, Dana Farber Cancer Hospital, Hutchinson Medipharma, MedImmune, Seagen, Puma Biotechnology, Compugen, TapImmune, Lilly, Pfizer, H3 Biomedicines, Takeda, Merus, Regeneron, Arvinas, StemcentRx, Verastem, eFFECTOR Therapeutics, CytomX, InventisBio, Lycera, Mersana, Radius Health, AbbVie, Nucana, Leap Therapeutics, Zenith Epigenetics, Harpoon, Orinove, AstraZeneca, Tesaro, Macrogenics, EMD Serono, Daiichi Sankyo, Syros, Sutro, GI Therapeutics, Merck, PharmaMar, Olema, Polyphor, Immunogen, Plexxikon and Amgen; fees for consulting/advisory role to institution from Genentech/Roche, Boehringer Ingelheim, Novartis, Dantari, Lilly, Merck, Puma Biotechnology, Silverback Therapeutics, CytomX, Pfizer, Mersana, Black Diamond, H3 Biomedicine, Daiichi Sankyo and AstraZeneca; travel/accommodation expenses from AstraZeneca, Lilly, Pfizer, Puma and Daiichi Sankyo. H.I. reports no conflicts of interest. C.A.S.-M. reports grants from Pfizer, AstraZeneca and Bristol-Myers Squibb; research funding to institution from Novartis; and advisory boards for Bristol-Myers Squibb, Seattle Genetics, Athenex and Genomic Health. J.R. reports grants to institution from Eli Lilly, Tesaro, Sarah Cannon, TG Therapeutics, Genentech, Celgene, Merck, Bristol-Myers Squibb, Boston Biomedical Inc., AstraZeneca, Novocure, Calithera Biosciences, Novartis, Guardant Health, Acerta Pharma, Rhizen Pharmaceuticals, Takeda Pharmaceuticals, Onconova Therapeutics, Sanofi and CTI Biopharma; grants from Daiichi Sankyo, Seattle Genetics, Taiho Oncology, Odonate, Boehringer Ingelheim, Macrogenics, Ipsen/Medpace, Beigene, Acerta, Verastem, Pharmacyclics, Medimmune, Jiangsu Hengrui Med, Arcus Biosciences, Calethera, Mirati, Pfizer, Immunogen, Karyopharm and GSK; speaker bureau fees and travel expenses from Eisai and Janssen; personal fees for advisory boards for Karyopharm, Polyphor and Bayer. P.P., M.S., S.H. and Y.T. are employees of and owners of shares/options in GSK. J.R.G. is an employee of and owner of shares/options in GSK, and reports stock ownership in Pfizer. L.W.E. reports no conflicts of interest. S.J.I. reports personal fees for consulting from AbbVie, Hengrui, Immunomedics, Mylan, Myriad, Puma, Seattle Genetics and Novartis.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Laura M. Spring, Hyo Han.
Change history
8/5/2022
A Correction to this paper has been published: 10.1038/s43018-022-00430-w
Extended data
are available for this paper at 10.1038/s43018-022-00400-2.
Supplementary information
The online version contains supplementary material available at 10.1038/s43018-022-00400-2.
References
- 1.Selli C, Sims AH. Neoadjuvant therapy for breast cancer as a model for translational research. Breast Cancer. 2019;13:1178223419829072. doi: 10.1177/1178223419829072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee A, Moon BI, Kim TH. BRCA1/BRCA2 pathogenic variant breast cancer: treatment and prevention strategies. Ann. Lab. Med. 2020;40:114–121. doi: 10.3343/alm.2020.40.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Litton JK, et al. A feasibility study of neoadjuvant talazoparib for operable breast cancer patients with a germline BRCA mutation demonstrates marked activity. NPJ Breast Cancer. 2017;3:49. doi: 10.1038/s41523-017-0052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LYNPARZA (package insert) (AstraZeneca Pharmaceuticals LP, 2020).
- 5.Litton JK, et al. Neoadjuvant talazoparib for patients with operable breast cancer with a germline BRCA pathogenic variant. J. Clin. Oncol. 2020;38:388–394. doi: 10.1200/JCO.19.01304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ZEJULA (package insert) (GlaxoSmithKline, 2020).
- 7.Sun K, et al. A comparative pharmacokinetic study of PARP inhibitors demonstrates favorable properties for niraparib efficacy in preclinical tumor models. Oncotarget. 2018;9:37080–37096. doi: 10.18632/oncotarget.26354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Andel L, et al. Determination of the absolute oral bioavailability of niraparib by simultaneous administration of a 14C-microtracer and therapeutic dose in cancer patients. Cancer Chemother. Pharmacol. 2018;81:39–46. doi: 10.1007/s00280-017-3455-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morosi L, et al. Quantitative determination of niraparib and olaparib tumor distribution by mass spectrometry imaging. Int. J. Biol. Sci. 2020;16:1363–1375. doi: 10.7150/ijbs.41395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tung N, et al. TBCRC 031: randomized phase II study of neoadjuvant cisplatin versus doxorubicin–cyclophosphamide in germline BRCA carriers with HER2-negative breast cancer (the INFORM trial) J. Clin. Oncol. 2020;38:1539–1548. doi: 10.1200/JCO.19.03292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faraoni I, Graziani G. Role of BRCA mutations in cancer treatment with poly(ADP-ribose) polymerase (PARP) inhibitors. Cancers. 2018;10:487. doi: 10.3390/cancers10120487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chopra N, et al. Homologous recombination DNA repair deficiency and PARP inhibition activity in primary triple negative breast cancer. Nat. Commun. 2020;11:2662. doi: 10.1038/s41467-020-16142-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tung, N. M. et al. TBCRC 048: phase II study of olaparib for metastatic breast cancer and mutations in homologous recombination-related genes. J Clin. Oncol. 10.1200/JCO.20.02151 (2020). [DOI] [PubMed]
- 14.Gruber JJ, et al. Talazoparib beyond BRCA: a phase II trial of talazoparib monotherapy in BRCA1 and BRCA2 wild-type patients with advanced HER2-negative breast cancer or other solid tumors with a mutation in homologous recombination (HR) pathway genes. J. Clin. Oncol. 2019;37:15. doi: 10.1200/JCO.2019.37.15_suppl.3006. [DOI] [Google Scholar]
- 15.Mirza MR, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N. Engl. J. Med. 2016;375:2154–2164. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 16.Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother. Pharmacol. 1989;24:148–154. doi: 10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]
- 17.Stringer-Reasor EM, et al. An open-label, pilot study of veliparib and lapatinib in patients with metastatic, triple-negative breast cancer. Breast Cancer Res. 2021;23:30. doi: 10.1186/s13058-021-01408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables 1–3.
Data Availability Statement
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies that evaluate medicines, on approval of proposals submitted to www.clinicalstudydatarequest.com and a data access agreement will be required. To access data for other types of GSK-sponsored research, for study documents without patient-level data, and for clinical studies not listed, please submit an inquiry via this website. Source data are provided with this paper.