Abstract
Purpose
To review the outcomes of patients with insertional Achilles tendinopathy who underwent a minimally invasive surgery: fluoroscopic and endoscopic calcaneal exostosis resection and Achilles tendon debridement.
Methods
The medical records of consecutive patients who underwent this surgery from February 2017 to July 2019 were reviewed. The inclusion criterion was ≥2-year follow-up. The exclusion criterion was another surgery performed on the ipsilateral or contralateral foot. Haglund deformity resection was not combined with this surgery. The outcomes were assessed using the visual analog scale (VAS) score and the Japanese Society for Surgery of the Foot (JSSF) scores for all patients and the Victorian Institute of Sport Assessment self-administered Achilles (VISA-A) scores for patients participating in sports activities. The Wilcoxon signed-rank test and the thresholds of minimal clinically important difference (MCID) and patient acceptable symptom state (PASS) were used for statistical analyses.
Results
Forty-four patients with a mean age of 55.7 ± 11.0 years and mean body mass index of 26.0 ± 4.0 kg/m2 were included. The mean follow-up duration was 2.8 ± 0.7 years. Of all participants, 22 participated in sports activities. The overall median VAS and JSSF scores improved from 64.5 to 6.5 mm and from 67.0 to 100 points, respectively (P < .001). The percentages of patients who achieved the MCID for the VAS, JSSF, and VISA-A scores were 100%, 93.2%, and 100%, respectively, and the percentages of patients who achieved the PASS for the VAS, JSSF, and VISA-A scores were 77.3%, 86.4%, and 81.8%, respectively. The median VISA-A scores improved from 40.5 to 95.0 points (P < .001). The median time to return to sport was 4.5 months. Complications included five cases of reoperation and two cases of scar sensitivity.
Conclusion
For patients with insertional Achilles tendinopathy, fluoroscopic and endoscopic calcaneal exostosis resection and Achilles tendon debridement resulted in good outcomes, early return to sports activities, and few wound complications.
Level of Evidence
IV, therapeutic case series
Introduction
Insertional Achilles tendinopathy is defined as posterior heel pain associated with the degenerative change in the distal Achilles tendon and the generation of calcaneal exostosis at its insertion. Conservative treatment, including eccentric exercise, orthosis, and medication, for insertional Achilles tendinopathy may be applied for 3–6 months1; however, this approach may be less effective when there is severe degeneration.2
Surgical treatment is chosen after patients fail to respond to conservative treatment. For Achilles insertional tendinopathy, surgical treatment includes the medial or lateral paratendinous approach, transtendinous approach, calcaneal exostosis resection, Achilles tendon debridement, resection of Haglund deformity, reattachment of the distal Achilles tendon using periosteum sutures, bone anchors, or knotless footprint reconstruction, and flexor hallucis longus (FHL) tendon transfer augmentation, in cases with >50% resection of the Achilles attachment.1,3, 4, 5
Good outcomes have been reported4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16; Elias et al. reported on 40 patients treated with Achilles tendon reattachment using 2 bone anchors, FHL tendon transfer augmentation, Haglund resection with a mean improvement in the visual analog scale (VAS) score from 75 to 3 mm and a mean improvement in the American Orthopedic Foot & Ankle Society (AOFAS) score from 56 points preoperatively to 96 points postoperatively.6 Hunt et al. reported on 39 patients treated with FHL tendon transfer augmentation and Achilles tendon reattachment using 2 bone anchors with a mean improvement in the VAS score from 68 mm preoperatively to 15 mm postoperatively and a mean improvement in the AOFAS score from 57 points preoperatively to 91 points postoperatively.7 With regard to patients engaging in sports activities, Maffulli et al. reported on 30 patients treated with Achilles tendon debridement and Haglund resection through a transverse incision with a mean improvement in the Victorian Institute of Sport Assessment self-administered Achilles (VISA-A) score from 62 points preoperatively to 88 points postoperatively,8 and Miao et al. reported on 34 patients treated with Achilles tendon reattachment using 2 bone anchors and Haglund resection with a mean VISA-A score from 48 points preoperatively to 88 points postoperatively.9
Despite the good outcomes, many wound complications have been reported, including scar sensitivity in 40%10 and 9%,11 scar numbness in 19%,11 scar hypersensitivity in 14%,12 delayed wound healing in 13%,5 wound infection in 10%13 and 20% of patients.8 In addition, delayed return to normal daily activities6 or full sports activities13, 14, 15, 16 has been reported. Carmont et al. reported that the average time to return to previous activities was 9 months for 40 patients treated with Achilles tendon reattachment using transosseous sutures or anchors and Haglund resection,13 and Miyamoto et al. indicated that the average time to return to full sports activities was 13.5 months for 49 patients treated with augmentation using bone–patellar tendon graft and Haglund resection.14 These findings may have been a result of the invasiveness of the open techniques. However, reports of minimally invasive surgery for pathologies associated with Achilles tendon insertion have been limited to those of endoscopic surgery in which the Haglund deformity is resected, and reports of endoscopic surgery involving the resection of calcaneal exostosis are scarce.
The purpose of this study was to review the outcomes of patients with insertional Achilles tendinopathy who underwent a minimally invasive surgery: fluoroscopic and endoscopic calcaneal exostosis resection and Achilles tendon debridement. We hypothesized that minimally invasive surgery would result in good outcomes, early return to sport activities, and few wound complications. This study was approved by the Institutional Review Board of Yashio Central General Hospital (approval number: YIHCE2021-02).
Methods
Participants and Inclusion/Exclusion Criteria
After obtaining approval from the institutional review board of our hospital, we retrospectively reviewed the medical records of consecutive patients with insertional Achilles tendinopathy who underwent surgical treatment from February 2017 to July 2019 after failing to respond to conservative treatment, such as eccentric exercise, orthosis, or medication, for more than 6 months.17 Informed consent for the use of medical record data was acquired from all patients before surgery.17 The inclusion criterion was ≥2-year follow-up. Because one affected or operated foot might affect the postoperative outcome of the other foot, the exclusion criteria were other surgeries on the ipsilateral foot or this surgery for bilateral feet. Fluoroscopic and endoscopic calcaneal exostosis resection and Achilles tendon debridement described below was performed for all patients with insertional Achilles tendinopathy, and no other procedure was conducted. Haglund deformity resection was not combined with this surgery.
Surgical Approach
All surgeries were performed by the author. Prior to surgery, the surgeon confirmed the shape of the exostosis and intratendon ossification using three-dimensional computed tomography (3D-CT; Fig 1A), and the extent of the Achilles tendon attachment, volume of the Achilles tendon at the level of intratendon ossification, and extent of the degenerated Achilles tendon by magnetic resonance imaging (MRI; Fig 2A). Regardless of their preoperative condition, all patients underwent the endoscopic surgery described below. Reoperations were performed using the same technique as the primary surgery.
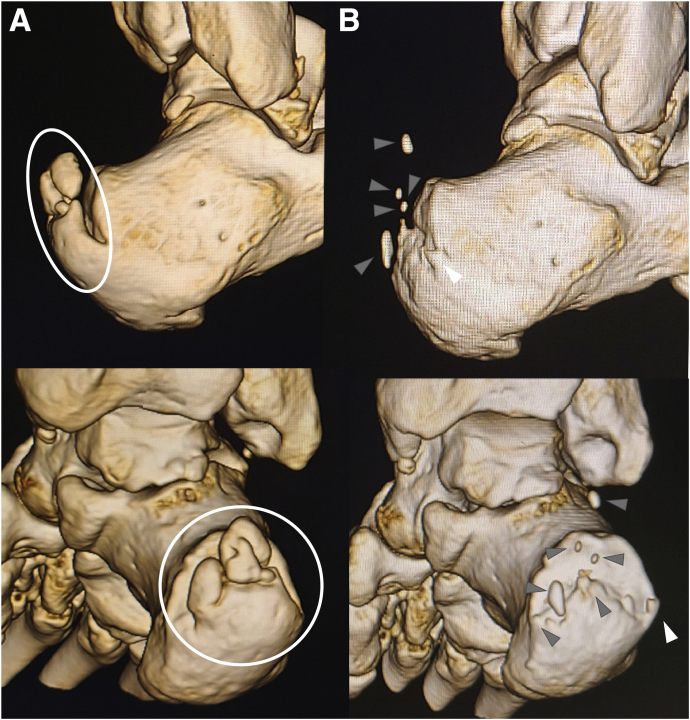
Fig 1.
Preoperative and postoperative 3-dimensional computed tomography (3D-CT) of the right calcaneus of a patient with insertional Achilles tendinopathy who underwent fluoroscopic and endoscopic calcaneal exostosis resection and Achilles tendon debridement. (A) Preoperative 3D-CT. Before surgery, the surgeon should confirm the shape of the exostosis (especially medial and lateral edge) and intratendon ossifications (circles). (B) Postoperative 3D-CT. The medial and lateral edge of the calcaneal exostosis and the portion of intratendon ossification were left unresected (gray arrowheads). The patient had moderate postoperative pain at the lateral edge of the unresected exostosis (white arrowheads), underwent the same endoscopic surgery again, and achieved pain relief.
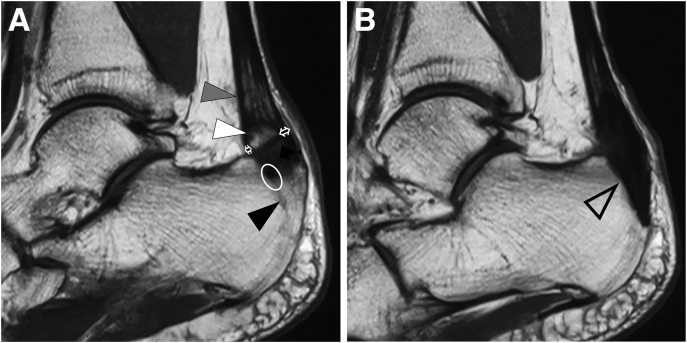
Fig 2.
Preoperative and postoperative T1-weighted magnetic resonance imaging (MRI) of the lateral view of the left calcaneus of a patient with insertional Achilles tendinopathy who underwent fluoroscopic and endoscopic calcaneal exostosis resection and Achilles tendon debridement. (A) Preoperative MRI. Calcaneal bone exostosis (black arrowhead), intratendon ossification (white arrowhead), and degenerative Achilles tendon (gray arrowhead) were visible. Before surgery, the surgeon should confirm the area of the Achilles tendon attachment (circle) and take care not to damage the portion of the attachment other than the exostosis during fluoroscopic resection of the exostosis. In addition, the surgeon should confirm the volume of the tendon at the level of the intratendon ossification (white arrows) and the range of the degenerated Achilles tendon (gray arrowhead) to be debrided. (B) MRI at 1 year after surgery. The calcaneal exostosis and intratendon ossification were resected, and the defect that remained after resection was filled with soft tissue showing the same signal as the Achilles tendon (arrowhead).
The patient was placed in the prone position. The leg was externally rotated so as to obtain a lateral view of the operated foot using fluoroscopy. An arthroscopic monitor, a fluoroscopic monitor, and an image intensifier were placed along the unoperated side, and the surgeon stood at the end of the operating table. A thigh tourniquet was not used.
Planned portals were marked using a surgical pen at 1 cm proximal and 1 cm distal to the calcaneal spur on the lateral side of the heel under fluoroscopic guidance. In addition, the prominence of the spur was palpated, and the region was also marked using a surgical pen (Fig 3, A and B).
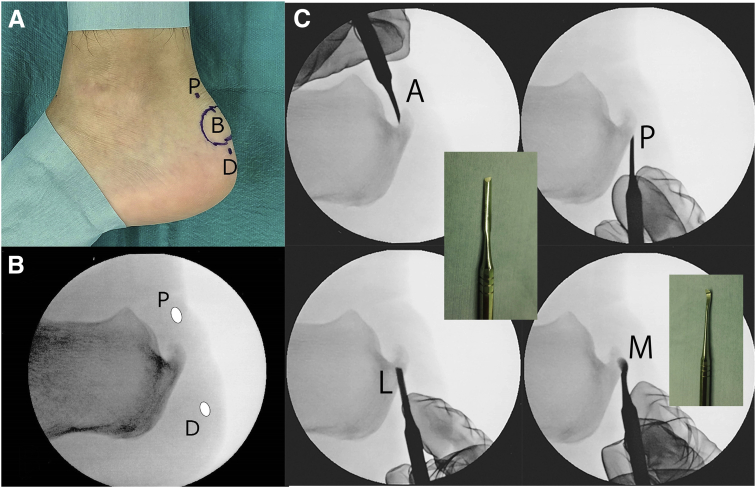
Fig 3.
Position of the portals and the exostosis, and blunt dissection for fluoroscopic and endoscopic calcaneal exostosis resection and Achilles tendon debridement for insertional Achilles tendinopathy. (A) The lateral side of the left foot. The patient is placed in the prone position with the medial foot down on the table. Planned portals and the palpable bony prominence are marked as follows: B, bony prominence; D, distal portal; and P, proximal portal. (B) Preoperative fluoroscopic view of the left calcaneus. The planned portals were marked 1 cm proximal and 1 cm distal to the calcaneal exostosis as follows: D, distal portal and P, proximal portal. (C) Blunt dissection around the calcaneal exostosis under fluoroscopic guidance. Blunt dissection on the anterior (A), posterior (P), and lateral aspects (L) of the exostosis was performed using the flat end of a raspatorium (Mineshima Co.), and blunt dissection on the medial aspect (M) of the exostosis was performed using its curved end.
Skin incisions at the planned portals were made, and blunt dissection was performed around the exostosis using a raspatorium (Art-Line, Mineshima Co., Niigata, Japan) to make space for the next step: the introduction of an abrasion bur (Fig 3C). A 3.0-mm hooded abrasion bur (Formula, Stryker, Kalamazoo, MI) was introduced through the distal portal; then, the exostosis was abraded (Fig 4). Care was taken not to abrade the insertion of the Achilles tendon; it is critical for surgeons to preoperatively confirm the insertion using MRI (Fig 2A). In cases where the abrasion bur did not reach the medial side of the exostosis, a third portal was made near the unreached exostosis, and further abrasion was subsequently performed. With internal and external calcaneus rotation, the calcaneal exostosis was completely resected under fluoroscopic guidance.
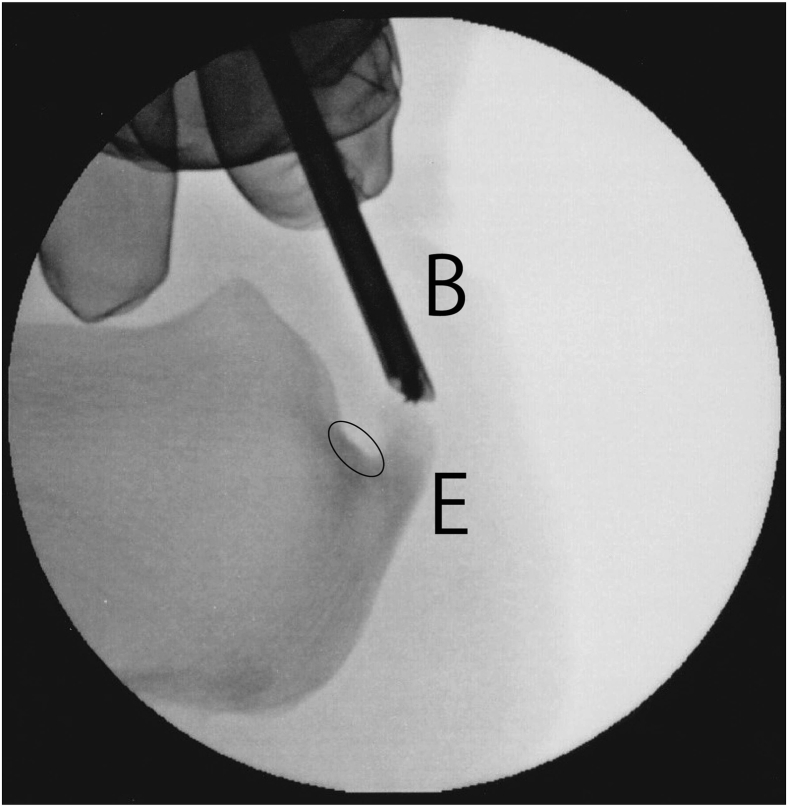
Fig 4.
Calcaneal exostosis resection of the left calcaneus using a 3.0-mm hooded abrasion bur (Stryker) under fluoroscopic guidance for fluoroscopic and endoscopic calcaneal exostosis resection and Achilles tendon debridement for insertional Achilles tendinopathy. Care was taken not to abrade the insertion of the Achilles tendon (circle). In cases for which reaching the medial side of the exostosis was difficult using the bur, an additional portal was made near the unreached exostosis.
The void space that remained following the exostosis resection was used as a working space for endoscopy. The bur was extracted; then, a 2.3-mm 30° arthroscope (Stryker) was introduced through the distal portal. An infusion pump (FloSteady, Stryker) was set to the autocalibration mode.17 A 3.5-mm cutter (Formula, Stryker) was introduced through the proximal portal to clear the debris (Fig 5A). After the debris was cleared, the degenerated Achilles tendon and the calcified paratenon, which was not visible by fluoroscopy, were identified and resected using the cutter; they appeared to be a hybrid of tendon and bone (Fig 5, B and C). In cases where intratendon ossification was fluoroscopically found, but not endoscopically identified, the surgeon touched the ossification with a bone curette under fluoroscopic guidance, extracted the ossification from the tendon, and removed it endoscopically. The surgeon palpated the marked area and confirmed that the bony prominence was perfectly resected. Before wound closure, a postoperative radiograph was obtained to confirm if the resection was accurately performed (Fig 6). The skin incision was sutured using a 4-0 nylon. After surgery, a below-knee posterior splint was applied with the ankle in the neutral position.
Fig 5.
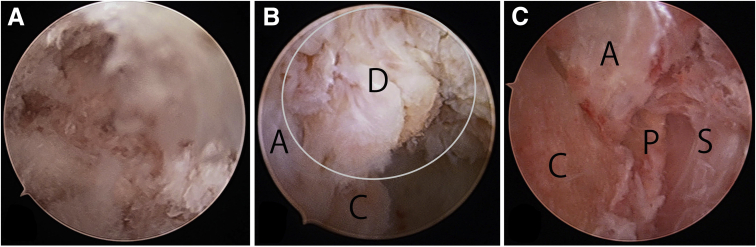
Endoscopic views of the left calcaneus from the distal portal using a 2.3-mm. 30° arthroscope (Stryker) for fluoroscopic and endoscopic calcaneal exostosis resection and Achilles tendon debridement for insertional Achilles tendinopathy. (A) View immediately after introducing the endoscope. Debris of the abraded spur interfered with the view. (B) View after cleaning the debris using a 3.5-mm cutter (Stryker) introduced through the proximal portal. The space left after the fluoroscopic resection of the calcaneal bone exostosis was a working space for endoscopy. The exostosis was resected, and the cancellous bone of the calcaneus was exposed (C). The intact anterior portion of the Achilles tendon was visible (A). The distal portion of the Achilles tendon that had attached to the exostosis was released by resecting the exostosis (D, ellipse). This portion was neither a normal tendon nor a normal bone, but was rather a hybrid of bone and tendon, which was debrided endoscopically. A, Anterior portion of the Achilles tendon; C, calcaneus; D, distal portion of the Achilles tendon that had attached the exostosis. (C) After endoscopic Achilles tendon debridement. A, Achilles tendon; C, calcaneus; P, paratenon calcified; and S, skin.
Fig 6.
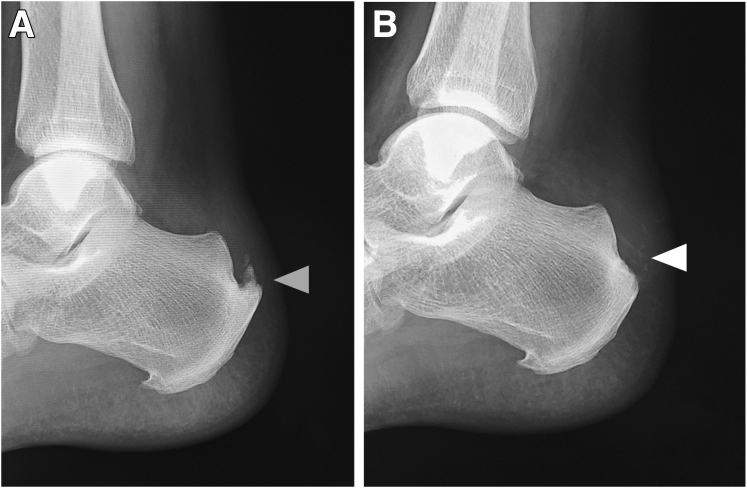
Preoperative and postoperative radiographs of the left calcaneus of the patient with insertional Achilles tendinopathy who underwent fluoroscopic and endoscopic calcaneal exostosis resection and Achilles tendon debridement. (A) Preoperative radiograph. A large exostosis was visible (gray arrowhead). (B) Postoperative radiograph. The exostosis was resected (white arrowhead).
Postoperative Management
Full weight-bearing with the splint and active range of motion exercises without the splint were initiated 1 day postoperatively, whereas passive range of motion exercises were avoided until 3 weeks after surgery. The physical therapists at our hospital provided instructions regarding postoperative rehabilitation to the patients. The splint was removed at 3 weeks after surgery. For cases with intratendon calcification and degenerative Achilles tendon on preoperative MRI, an MRI was taken 1 year postoperatively to confirm the condition of the Achilles tendon. For patients with intratendon ossification detected on MRI, the splint was removed at 4 weeks after surgery. For patients who typically participated in sports activities, jogging was initiated 2 months postoperatively, and unrestricted sports activities were initiated 3 months postoperatively as tolerated.
Data Collection
Baseline patient characteristics included sex, age, height, weight, and body mass index (BMI).17 The study outcomes were assessed using the VAS score to rate pain and Japanese Society for Surgery of the Foot (JSSF) scale scores18,19 for all patients and the VISA-A questionnaire scores20 for patients who participated in sports activities.
Preoperative data for analyses included the data recorded within 1 month before surgery, and postoperative data for analyses included the data recorded at final follow-up. The VAS score represented the worst pain felt during recent activities of daily living and sports.17
Statistical Analyses
The Wilcoxon signed-rank test was used to investigate whether the VAS, JSSF, and VISA-A scores improved postoperatively.17 All tests were two-tailed, and differences were considered to be significant at a P value of < .05.17 In addition, the thresholds of minimal clinically important improvement difference (MCID) and patient acceptable symptom state (PASS) for the scales were assessed. The MCID for a scale was defined as the one-half standard deviation of the scale improvements for all patients,21, 22, 23, 24 and the percentage of patients who achieved the MCID was calculated. The PASS for a scale was defined as follows: 1) the patients answered the following anchor question at the latest follow-up: “Taking into account all the activities you have in your daily life, your level of pain, and also your functional impairment, do you consider that your current state is satisfactory?”21 by choosing “very satisfied”, “satisfied”, “neutral”, “dissatisfied”, or “very dissatisfied”. The patients who chose “very satisfied” and “satisfied” were regarded as those with a postoperative acceptable symptom state. 2) The threshold of a scale was calculated using a receiver operating characteristic curve analysis with maximized sensitivity and specificity, and the threshold was defined as the PASS for the scale.21,25, 26, 27 Finally, the percentage of patients who achieved the PASS was calculated.
All statistical analyses were performed using EZR28 version 1.54 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a modified version of R version 4.0.3 (The R Foundation for Statistical Computing, Vienna, Austria) commander designed to add statistical functions frequently used in biostatistics.15
Results
Demographics
Endoscopic surgery was performed in 52 consecutive patients (total: 56 feet) between February 2017 and July 2019. Among these patients, four (total: 8 feet) underwent this surgery on both feet, two (total: 2 feet) underwent another surgery on the ipsilateral foot, and two (total: two feet) were lost to follow-up. Therefore, data were analyzed for the remaining 44 patients (total: 44 feet).
The baseline characteristics for the 44 patients were as follows: mean age: 55.7 ± 11.0 (35–77) years; mean BMI: 26.0 ± 4.0 (16.6–35.1) kg/m2; and mean duration of follow-up: 2.8 ± 0.7 (2.0–4.5) years. Sex distribution was 31 male and 13 female patients. Participation in sports activities was equally split among the 44 patients (although it differed by sex): 22 patients (19 male and 3 female) participated, and 22 patients (12 male and 10 female) did not participate (Table 1).
Table 1.
Patient Demographics
| Patients | 44 (31 Male, 13 Female) |
|---|---|
| Age (years) | 55.7 ± 11.0 (35–77) |
| BMI (kg/m2) | 26.0 ± 4.0 (16.6–35.1) |
| Duration of follow-up (years) | 2.8 ± 0.7 (2.0–4.5) |
| Patients participating in sports activities | 22 (19 male, 3 female) |
BMI, body mass index.
Overall Outcomes
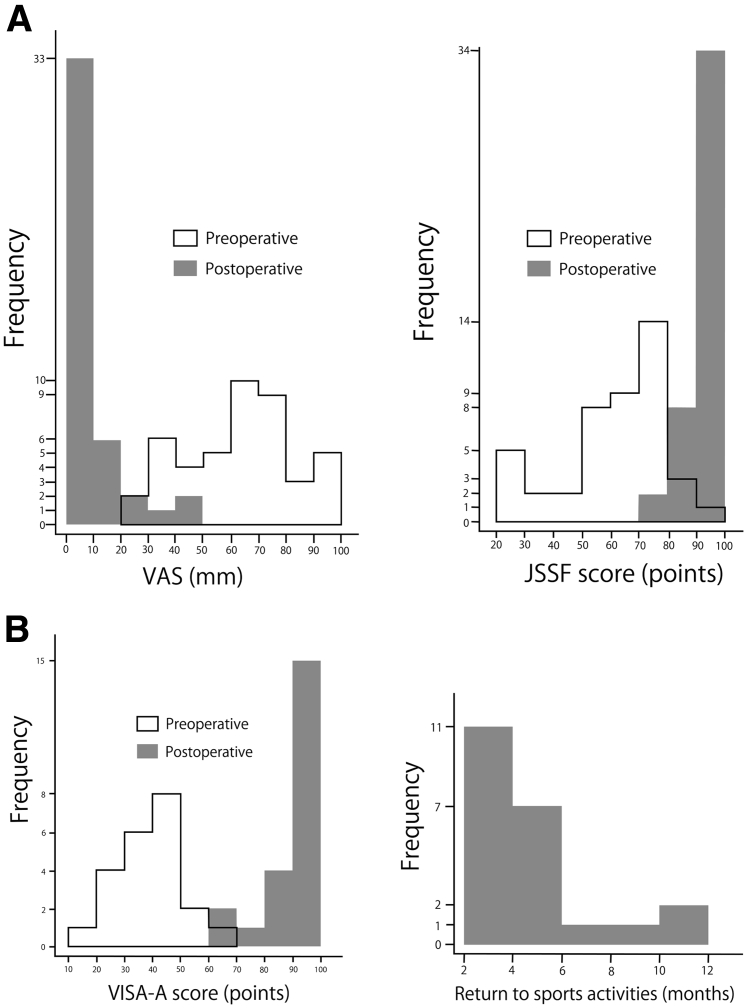
Outcomes are presented in Table 2. Overall, the VAS and JSSF scores improved and were highly statistically significant (P < .001) (Table 2, Fig 7A).
Table 2.
Outcomes for Patients with Insertional Achilles Tendinopathy Who Underwent Minimally Invasive Surgery with Fluoroscopic and Endoscopic Calcaneal Exostosis Resection and Achilles Tendon Debridement
| Assessment | Class (n) | Subclass (n) | Preoperative (points) | Postoperative (points) | P Value | MCID (points) | Patients Achieving the MCID (%) (n/n) |
PASS (points) (AUC) |
Patients Achieving the PASS (%) (n/n) |
|---|---|---|---|---|---|---|---|---|---|
| VAS (0–100 points) | Overall (44) | 64.5 (25–100) | 6.5 (0–46) | P < .001 | 10.6 | 100 (44/44) | 14 (0.94) | 77.3 (34/44) | |
| Sports + (22) | 64.5 (25–100) | 6.5 (0–36) | P < .001 | 11.7 | 100 (22/22) | 14 (0.91) | 77.3 (17/22) | ||
| Sports -(22) | 68.5 (31–99) | 5.0 (0–46) | P < .001 | 9.6 | 100 (22/22) | 43 (1.00) | 95.4 (21/22) | ||
| JSSF (0–100 points) | Overall | 67.0 (22–92) | 100 (78–100) | P < .001 | 8.9 | 93.2 (41/44) | 85 (0.98) | 86.4 (38/44) | |
| Sports + (22) | 67.0 (22–92) | 100 (78–100) | P < .001 | 9.6 | 86.4 (19/22) | 85 (0.98) | 86.4 (19/22) | ||
| Sports -(22) | 66.0 (27–82) | 100 (75–100) | P < .001 | 8.2 | 100 (22/22) | 83 (1.00) | 95.4 (21/22) | ||
| VISA-A (0–100 points) | Sports + (22) | 40.5 (12–65) | 95.0 (64–100) | P < .001 | 6.8 | 100 (22/22) | 86 (0.95) | 81.8 (18/22) | |
| Time to RTSA (months) | 4.5 (2–12) |
NOTE: The clinical assessment values were described with median and range because these values were not normally distributed. The improvement between the preoperative and postoperative values was tested using the Wilcoxon signed-rank test.
AUC, area under the curve; JSSF, Japanese Society for Surgery of the Foot; Sports +/–, patients who participated/did not participate in sports activities; MCID, minimal clinically important improvement difference; PASS, patient acceptable symptom state; RTSA, return to sports activities; VAS, visual analog scale for rating pain (0–100 points); VISA-A, Victorian Institute of Sport Assessment self-administered Achilles.
Fig 7.
Preoperative and postoperative results of 44 patients with insertional Achilles tendinopathy who underwent fluoroscopic and endoscopic calcaneal exostosis resection and Achille tendon debridement. (A) Histograms showing the results of the visual analog scale (VAS) and Japanese Society for Surgery of the Foot (JSSF) scores. (B) Histograms showing the results of the Victorian Institute of Sport Assessment-Achilles (VISA-A) score and time to return to sports activities.
Outcomes Regarding Sports
Among the 44 patients, 22 participated in recreational sports activities. All patients returned to their sports activities after surgery. The median VISA-A scores improved from 40.5 (12–65) points preoperatively to 95.0 (64–100) points postoperatively (P < .001). The median time to return to sports activities was 4.5 (2–12) months (Table 2, Fig 7B).
MRI Findings
Postoperative MRI demonstrated that the defect that remained after resecting the exostosis, and the debridement of the Achilles tendon was filled with soft tissue showing the same signal as that of the Achilles tendon (Fig 2B).17
Complications
Overall, five patients (11%) had moderate pain 1 year after primary surgery and opted for reoperation. Incomplete removal of the exostosis and intratendon ossification was regarded as the cause of the persistent pain. The abovementioned five cases were early cases and had severe exostosis and intratendon ossification. For these five patients, 3D-CT was obtained to confirm the leftovers of the exostosis and intratendon ossification (Fig 1B). The patients experienced pain at the site of residual exostosis. After reoperations, all patients achieved pain relief; the outcomes after reoperation were used for analyses. The other complication was scar sensitivity, which occurred in two patients at the proximal portal. No other complications occurred.
Discussion
In this study, a minimally invasive surgery resulted in good outcomes, early return to sports activities, and few wound complications. The overall median VAS score improved from 64.5 mm preoperatively to 6.5 mm postoperatively, and the overall median JSSF score improved from 67.0 points preoperatively to 100 points postoperatively. The percentages of patients who achieved the MCID for the VAS and JSSF scores were 100% and 93.2%, respectively, and the percentages of patients who achieved the PASS for the VAS and JSSF scores were 77.3% and 86.4%, respectively. For patients participating in sports activities, the median VISA-A score improved from 40.5 points preoperatively to 95.0 points postoperatively; the percentages of patients who achieved the MCID and PASS for VISA-A score were 100% and 81.6%, respectively; and the median time to return to sports activities was 4.5 months. Complications included five (11%) cases of reoperation and two (4.5%) cases of scar sensitivity. In the Introduction, we have discussed previous studies on open surgery for insertional Achilles tendinopathy with similar outcome indicators (e.g., VAS and VISA-A scores) to our study. 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 Overall outcomes were as good as those reports. 6, 7, 8, 9 Endoscopic surgery provided an earlier return to sports activities than that noted in previous reports.13,14 This may be because our technique did not entirely release the attachment of the Achilles tendon and did not sacrifice the FHL tendon. Furthermore, the rate of wound complications was lower than that in the literature; 5,8,10, 11, 12, 13 the low rate of wound complications for patients treated using our approach was a result of the small incision used for endoscopic surgery.
Our technique enabled the use of endoscopic surgery for the exostosis of the Achilles tendon attachment. In this technique, the void space that remained after exostosis resection was used as a working space for endoscopy. This allowed the degenerated Achilles tendon to be debrided endoscopically. Current reports discuss endoscopic Haglund deformity resection as a surgical technique for pathologies associated with Achilles tendon insertion. 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47 This method involves an endoscopic dissection of the soft tissue surrounding the Haglund deformity and resection of the bone. 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47 However, it should be noted that this technique was entirely different from our technique. Endoscopic Haglund deformity resection has been reported only for Haglund’s disease; no report has discussed the use of endoscopic Haglund deformity resection alone for insertional Achilles tendinopathy. Some surgeons believe that the Haglund deformity is pathologically associated with insertional Achilles tendinopathy, and, thus, performed the resection of the Haglund deformity alongside open calcaneal exostosis resection and Achilles tendon repair for insertional Achilles tendinopathy. 4, 5, 6,8,10,13, 14, 15, 16 The patients in our study did not have retrocalcaneal bursitis preoperatively, and therefore, we did not perform Haglund deformity resection. Nevertheless, because the patient outcomes were good, we believe that the Haglund deformity did not require resection in our cases. However, in cases where patients have retrocalcaneal bursitis, our technique can still be used for Haglund deformity resection.
Postoperative MRI suggested that the Achilles insertion was repaired by filling the defect that remained after exostosis and intratendon ossification resection with tendon-like tissue (Fig 2B). We believe that this natural repair occurred because the defect and surrounding soft tissue were well preserved owing to the use of minimally invasive endoscopic surgery. In the literature, FHL tendon transfer augmentation in cases with >50% resection of the Achilles attachment has been reported.3, 4, 5 We believe that the FHL tendon transfer augmentation is necessary in open surgery because the exostosis-resected space will not be preserved, and natural repair of the attachment will, thus, not be expected.
In this case series of 44 patients, reoperation occurred in 5 cases (11%). Their pain was alleviated by performing a reoperation to remove leftovers of the exostosis and intratendon ossification using the same procedure as that used in the first operation. Therefore, we believe that the patients’ residual pain after the primary surgery might have resulted from the leftovers. The factors influencing the leftovers may include 1) immature technique, 2) severe ossifications, and 3) difficulty in visualizing the ossification because of the low radiation dose in fluoroscopy. The main factor was that 1) all five cases were early cases. However, even if the surgeon uses a mature technique, leftovers can occur owing to factors 2 and 3. Some surgeons may prefer to completely remove the exostosis and intratendon ossification and perform FHL tendon transfer augmentation by employing one open surgery, but we believe that two minimally invasive surgeries are preferable over one invasive surgery. This is because 1) there is a possibility that treatment will be completed using one minimally invasive surgery, and 2) a conventional open surgery may sacrifice the FHL tendon and increase the probability of wound complications. In addition, reoperation was easy because most exostosis and intratendon ossifications were already resected, and what remained could be identified using 3D-CT. Therefore, especially in cases of severe ossification, the surgeon should consider reoperation as a good option and ensure that patients are well informed of the possibility of reoperation before they undergo primary surgery.
Limitations
Our study had several limitations. First, because of the small sample size, we were unable to perform subgroup analyses, such as comparison between male and female patients, between the patients with high and low BMI, and between the patients with and without complications. Second, the recurrence rate of ossification was unknown because follow-up radiography was not performed. Third, we were unable to identify the soft tissue that was generated in the void space of the resected spur and debrided Achilles tendon by biopsy. Fourth, the JSSF score was the score validated for ankle–hindfoot diseases and not for Achilles tendon diseases. Finally, the study did not include a comparative group because of the explorative nature of case series.
Conclusions
For patients with insertional Achilles tendinopathy, fluoroscopic and endoscopic calcaneal exostosis resection and Achilles tendon debridement resulted in good outcomes, early return to sports activities, and few wound complications.
Acknowledgments
The author would like to thank Enago (www.enago.jp) for the English language review.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: M.B. receives consulting fees from Arthrex and LifeNet Health, outside the submitted work. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
References
- 1.Barg A., Ludwig T. Surgical strategies for the treatment of insertional Achilles tendinopathy. Foot Ankle Clin N Am. 2019;24:533–559. doi: 10.1016/j.fcl.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Nicholson C.W., Berlet G.C., Lee T.H. Prediction of the success of nonoperative treatment of insertional Achilles tendinosis base on MRI. Foot Ankle Int. 2007;28:472–477. doi: 10.3113/FAI.2007.0472. [DOI] [PubMed] [Google Scholar]
- 3.Kolodziej P., Glisson R.R., Nunley J.A. Risk of avulsion of the Achilles tendon after partial excision for treatment of insertional tendonitis and Haglund's deformity: A biomechanical study. Foot Ankle Int. 1999;20:433–437. doi: 10.1177/107110079902000707. [DOI] [PubMed] [Google Scholar]
- 4.Calder J.D.F., Saxby T.S. Surgical treatment of insertional Achilles tendinosis. Foot Ankle Int. 2003;24:119–121. doi: 10.1177/107110070302400203. [DOI] [PubMed] [Google Scholar]
- 5.Schon L.C., Shores J.L., Faro F.D., Vora A.M., Camire L.M., Guyton G.P. Flexor hallucis longus tendon transfer in treatment of Achilles tendinosis. J Bone Joint Surg Am. 2013;95:54–60. doi: 10.2106/JBJS.K.00970. [DOI] [PubMed] [Google Scholar]
- 6.Elias I., Raikin S.M., Besser M.P., Nazarian L.N. Outcomes of chronic insertional Achilles tendinosis using FHL autograft through single incision. Foot Ankle Int. 2009;30:197–204. doi: 10.3113/FAI.2009.0197. [DOI] [PubMed] [Google Scholar]
- 7.Hunt K.J., Cohen B.E., Davis W.H., Anderson R.B., Jones C.P. Surgical treatment of insertional Achilles tendinopathy with or without flexor hallucis longus tendon transfer: A prospective, randomized study. Foot Ankle Int. 2015;36:998–1005. doi: 10.1177/1071100715586182. [DOI] [PubMed] [Google Scholar]
- 8.Maffulli N., Del Buono A., Testa V., Capasso G., Oliva F., Denaro V. Safety and outcome of surgical debridement of insertional Achilles tendinopathy using a transverse (Cincinnati) incision. J Bone Joint Surg Br. 2011;93:1503–1507. doi: 10.1302/0301-620X.93B11.27379. [DOI] [PubMed] [Google Scholar]
- 9.Miao X.D., Jiang H., Wu Y.P., Tao H.M., Yang D.S., Hu H. Treatment of calcified insertional Achilles tendinopathy by the posterior midline approach. J Foot Ankle Surg. 2016;55:529–534. doi: 10.1053/j.jfas.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Yodlowski M.L., Scheller A.D., Jr., Minos L. Surgical treatment of Achilles tendinitis by decompression of the retrocalcaneal bursa and the superior calcaneal tuberosity. Am J Sports Med. 2002;30:318–321. doi: 10.1177/03635465020300030301. [DOI] [PubMed] [Google Scholar]
- 11.McGarvey W.C., Palumbo R.C., Baxter D.E., Leibman B.D. Insertional Achilles tendinosis: Surgical treatment through a central tendon splitting approach. Foot Ankle Int. 2002;23:19–25. doi: 10.1177/107110070202300104. [DOI] [PubMed] [Google Scholar]
- 12.Maffulli N., Testa V., Capasso G., Sullo A. Calcific insertional Achilles tendinopathy: Re-attachment with bone anchors. Am J Sports Med. 2004;32:174–182. doi: 10.1177/0363546503258923. [DOI] [PubMed] [Google Scholar]
- 13.Carmont M.R., Maffulli N. Management of insertional Achilles tendinopathy through a Cincinnati incision. BMC Musculoskelet Disord. 2007;15:82. doi: 10.1186/1471-2474-8-82. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyamoto W., Takao M., Matsushita T. Reconstructive surgery using autologous bone-patellar tendon graft for insertional Achilles tendinopathy. Knee Surg Sports Traumatol Arthrosc. 2012;20:1863–1867. doi: 10.1007/s00167-011-1792-6. [DOI] [PubMed] [Google Scholar]
- 15.Nunley J.A., Ruskin G., Horst F. Long-term clinical outcomes following the central incision technique for insertional Achilles tendinopathy. Foot Ankle Int. 2011;32:850–855. doi: 10.3113/FAI.2011.0850. [DOI] [PubMed] [Google Scholar]
- 16.Watson A.D., Anderson R.B., Davis W.H. Comparison of results of retrocalcaneal decompression for retrocalcaneal bursitis and insertional Achilles tendinosis with calcific spur. Foot Ankle Int. 2000;21:638–642. doi: 10.1177/107110070002100802. [DOI] [PubMed] [Google Scholar]
- 17.Nakajima K. Arthroscopic sesamoidectomy for hallux sesamoid disorders. J Foot Ankle Surg. 2022;61:175–180. doi: 10.1053/j.jfas.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Niki H., Aoki H., Inokuchi S., et al. Development and reliability of a standard rating system for outcome measurement of foot and ankle disorders. II: Interclinician and intraclinician reliability and validity of the newly established standard rating scales and Japanese Orthopaedic Association rating scale. J Orthop Sci. 2005;10:466–474. doi: 10.1007/s00776-005-0937-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niki H., Aoki H., Inokuchi S., et al. Development and reliability of a standard rating system for outcome measurement of foot and ankle disorders. I: Development of standard rating system. J Orthop Sci. 2005;10:457–465. doi: 10.1007/s00776-005-0936-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson J.M., Cook J.L., Purdam C., et al. The VISA-A questionnaire: A valid and reliable index of the clinical severity of Achilles tendinopathy. Br J Sports Med. 2001;35:335–341. doi: 10.1136/bjsm.35.5.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kvien T.K., Heiberg T., Hagen K.B. Minimal clinically important improvement/difference (MCII/MCID) and patient acceptable symptom state (PASS): What do these concepts mean? Ann Rheum Dis. 2007;66:40–41. doi: 10.1136/ard.2007.079798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Revicki D., Hays R.D., Cella D., Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61:102–109. doi: 10.1016/j.jclinepi.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Katz N.P., Paillard F.C., Ekman E. Determining the clinical importance of treatment benefits for inventions for orthopedic conditions. J Orthop Surg Res. 2015;10:1–11. doi: 10.1186/s13018-014-0144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mouelhi Y., Jouve E., Catelli C., Gentile S. How is the minimal clinically important difference established in health-related quality of life instruments? Review of anchors and methods. Health Qual Life Outcomes. 2020;18:136. doi: 10.1186/s12955-020-01344-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galea V.P., Ingelsrud L.H., Florissi I., et al. A patient-acceptable symptom state for the Oxford hip score and forgotten joint score at 3 months, 1 year, and 2 years following total hip arthroplasty: a registry-based study of 597 cases. Acta Orthop. 2020;91:372–377. doi: 10.1080/17453674.2020.1750877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berliner J.L., Brodke D.J., Chan V., SooHoo N.F., Bozic K.J. Can preoperative patient-reported outcome measures be used to predict meaningful improvement in function after TKA? Clin Orthop Relat Res. 2017;475:149–157. doi: 10.1007/s11999-016-4770-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katz P., Kannowski C.L., Sun L., Michaud K. Estimation of minimally important differences and patient acceptable symptom state scores for the patient-reported outcomes measurement information system pain interference short form in rheumatoid arthritis. ACR Open Rheumatol. 2020;2:320–329. doi: 10.1002/acr2.11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jerosch J., Nasef N.M. Endoscopic calcaneoplasty—Rationale, surgical technique, and early results: A preliminary report. Knee Surg Sports Traumatol Arthrosc. 2003;11:190–195. doi: 10.1007/s00167-003-0365-8. [DOI] [PubMed] [Google Scholar]
- 30.Leitze Z., Sella E.J., Aversa J.M. Endoscopic decompression of the retrocalcaneal space. J Bone Joint Surg Am. 2003;85:1488–1496. doi: 10.2106/00004623-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Morag G., Maman E., Arbel R. Endoscopic treatment of hindfoot pathology. Arthroscopy. 2003;19:E13. doi: 10.1053/jars.2003.50063. [DOI] [PubMed] [Google Scholar]
- 32.Ortmann F.W., McBryde A.M. Endoscopic bony and soft-tissue decompression of the retrocalcaneal space for the treatment of Haglund deformity and retrocalcaneal bursitis. Foot Ankle Int. 2007;28:149–153. doi: 10.3113/FAI.2007.0149. [DOI] [PubMed] [Google Scholar]
- 33.Jerosch J., Schunck J., Sokkar S.H. Endoscopic calcaneoplasty (ECP) as a surgical treatment of Haglund’s syndrome. Knee Surg Sports Traumatol Arthrosc. 2007;15:927–934. doi: 10.1007/s00167-006-0279-3. [DOI] [PubMed] [Google Scholar]
- 34.Michels F., Guillo S., King A., Jambou S., de Lavigne C. Endoscopic calcaneoplasty combined with Achilles tendon repair. Knee Surg Sports Traumatol Arthrosc. 2008;16:1043–1046. doi: 10.1007/s00167-008-0602-2. [DOI] [PubMed] [Google Scholar]
- 35.Labib S.A., Pendleton A.M. Endoscopic calcaneoplasty: An improved technique. J Surg Orthop Adv. 2012;21:176–180. doi: 10.3113/jsoa.2012.0176. [DOI] [PubMed] [Google Scholar]
- 36.Phisitkul P. Endoscopic surgery of the Achilles tendon. Curr Rev Musculoskelet Med. 2012;5:156–163. doi: 10.1007/s12178-012-9115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Z., Hua Y., Li Y., Chen S. Endoscopic treatment of Haglund’s syndrome with a three portal technique. Int Orthop. 2012;36:1623–1627. doi: 10.1007/s00264-012-1518-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaynak G., Öğüt T., Yontar N.S., Botanlıoğlu H., Can A., Ünlü M.C. Endoscopic calcaneoplasty: 5-year results. Acta Orthop Traumatol Turc. 2013;47:261–265. doi: 10.3944/aott.2013.3003. [DOI] [PubMed] [Google Scholar]
- 39.Retrouvey H., Silvanathan J., Bleakney R.R., Anastakis D.J. A case of posterior tibial nerve injury after arthroscopic calcaneoplasty. J Foot Ankle Surg. 2018;57:587–592. doi: 10.1053/j.jfas.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Vega J., Baduell A., Malagelada F., Allmendinger J., Dalmau-Pastor M. Endoscopic Achilles tendon augmentation with suture anchors after calcaneal exostectomy in Haglund syndrome. Foot Ankle Int. 2018;39:551–559. doi: 10.1177/1071100717750888. [DOI] [PubMed] [Google Scholar]
- 41.Xu J.H., Ding S.L., Chen B., Wu S.C. Modified Bunnell suture expands the surgical indication of the treatment of Haglund’s syndrome heel pain with endoscope. Exp Ther Med. 2018;15:4817–4821. doi: 10.3892/etm.2018.6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michalski M.P., Gonzalez T.A., Metzger M.F., Nelson T.J., Eberlein S., Pfeffer G.B. Biomechanical comparison of Achilles tendon pullout strength following midline tendon-splitting and endoscopic approaches for calcaneoplasty. Foot Ankle Int. 2019;40:1219–1225. doi: 10.1177/1071100719856939. [DOI] [PubMed] [Google Scholar]
- 43.Yang Y.P., Li B.H., Wei Y.X., et al. Functional follow-up after endoscopic calcaneoplasty for Haglund’s deformity using the biodex isokinetic muscle testing system: A case series. Exp Ther Med. 2020;20:2805–2811. doi: 10.3892/etm.2020.9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lughi M. Haglund’s syndrome: Endoscopic or open treatment? Acta Biomed. 2020;30(91):167–171. doi: 10.23750/abm.v91i4-S.9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller C.P., McWilliam J.R., Michalski M.P., Acevedo J. Endoscopic Haglund’s resection and percutaneous double-row insertional Achilles repair. Foot Ankle Spec. 2021;14:534–543. doi: 10.1177/19386400211002707. [DOI] [PubMed] [Google Scholar]
- 46.Alessio-Mazzola M., Russo A., Capello A.G., et al. Endoscopic calcaneoplasty for the treatment of Haglund’s deformity provides better clinical functional outcomes, lower complication rate, and shorter recovery time compared to open procedures: A systematic review. Knee Surg Sports Traumatol Arthrosc. 2021;29:2462–2484. doi: 10.1007/s00167-020-06362-1. [DOI] [PubMed] [Google Scholar]
- 47.Pi Y., Hu Y., Guo Q., et al. Open versus endoscopic osteotomy of posterosuperior calcaneal tuberosity for Haglund syndrome: A retrospective cohort study. Orthop J Sports Med. 2021;19(9) doi: 10.1177/23259671211001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.