Abstract
Loss of cyclin-dependent kinase 5 (Cdk5) in the mitochondria-associated endoplasmic reticulum (ER) membranes (MAMs) increases ER–mitochondria tethering and ER Ca2+ transfer to the mitochondria, subsequently increasing mitochondrial Ca2+ concentration ([Ca2+]mt). This suggests a role for Cdk5 in regulating intracellular Ca2+ dynamics, but how Cdk5 is involved in this process remains to be explored. Using ex vivo primary mouse embryonic fibroblasts (MEFs) isolated from Cdk5−/− mouse embryos, we show here that loss of Cdk5 causes an increase in cytosolic Ca2+concentration ([Ca2+]cyt), which is not due to reduced internal Ca2+ store capacity or increased Ca2+ influx from the extracellular milieu. Instead, by stimulation with ATP that mediates release of Ca2+ from internal stores, we determined that the rise in [Ca2+]cyt in Cdk5−/− MEFs is due to increased inositol 1,4,5-trisphosphate receptor (IP3R)-mediated Ca2+ release from internal stores. Cdk5 interacts with the IP3R1 Ca2+ channel and phosphorylates it at Ser421. Such phosphorylation controls IP3R1-mediated Ca2+ release as loss of Cdk5, and thus, loss of IP3R1 Ser421 phosphorylation triggers an increase in IP3R1-mediated Ca2+ release in Cdk5−/− MEFs, resulting in elevated [Ca2+]cyt. Elevated [Ca2+]cyt in these cells further induces the production of reactive oxygen species (ROS), which upregulates the levels of Nrf2 and its targets, Prx1 and Prx2. Cdk5−/− MEFs, which have elevated [Ca2+]cyt, proliferate at a faster rate compared to wt, and Cdk5−/− embryos have increased body weight and size compared to their wt littermates. Taken together, we show that altered IP3R1-mediated Ca2+ dynamics due to Cdk5 loss correspond to accelerated cell proliferation that correlates with increased body weight and size in Cdk5−/− embryos.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-022-04515-8.
Keywords: Proliferation, Cdk5, Ca2+ signaling, IP3R
Introduction
Cdk5 belongs to the Cdk family of small serine/threonine kinases, which, together with their respective cyclin activators, regulate the eukaryotic cell cycle [1]. It was identified based on its structural similarity to Cdk1 (Cdc2) and Cdk2 [2–4], but most Cdk5 studies are directed at non-cell cycle events. Nonetheless, there is increasing evidence implicating a role for Cdk5 in cell cycle progression and proliferation [5–8]. For example, in human HeLa cervical epithelial cells, Cdk5 and its activator, p35, have been mapped to centrosomes and suggested to regulate centrosome-mediated cell cycle events [9]. In addition, Cdk5 was found to suppress the neuronal cell cycle [6, 10, 11], particularly at G1/S [5, 8, 10], and in non-neuronal cells, Cdk5 was found to be required for intra-S and G2/M cell-cycle checkpoints [7]. Cdk5 regulates cell-cycle progression by downregulating p21CIP1 [1, 12, 13] and p27KIP1 [1, 13], and cell proliferation through modulation of AKT [13, 14], STAT3 [15, 16] or ERK5 [17]. However, gaps remain in our understanding of how Cdk5 regulates the cell cycle and cell proliferation.
The manner in which the various intracellular Ca2+ channels, pumps and exchangers are distributed allows extracellular stimuli to induce [Ca2+]cyt oscillations in a highly defined spatial and temporal patterns, inducing specific cellular responses such as cell proliferation [18, 19]. Increases in [Ca2+]cyt are triggered through a number of mechanisms, including entry from the extracellular milieu, reduced internal Ca2+ store capacity, and Ca2+ release from internal stores, primarily the endoplasmic reticulum (ER). The role of Cdk5 in regulating intracellular Ca2+ dynamics has been primarily examined in neurons where it is abundantly expressed [3, 20]. For example, neuronal Cdk5 has been implicated in regulating external Ca2+ entry from the extracellular milieu. Cdk5 phosphorylates the P/Q-type voltage-dependent Ca2+ channel (VDCC), supressing external Ca2+ entry [21]. Cdk5 also phosphorylates the N-type VDCC [22] and the transient receptor potential cation channel subfamily V member 1 (TrpV1) [23–26], stimulating Ca2+ influx from the extracellular milieu. In nociceptive neurons, Cdk5-mediated phosphorylation of the purinergic P2X receptor 2 (P2X2)’s full-size isoform (P2X2aR) at Thr372 stimulates external Ca2+ entry [27], whereas P2X3R phosphorylation downregulates external Ca2+ influx [28]. Thus, Cdk5 regulation of Ca2+ influx from the extracellular milieu is dependent upon its target, indicating the need to understand the cellular context in various experimental model systems [29]. In mesencephalic neurons and NGF-differentiated sympathetic-like neuronal cells, ceramide induces stimulation of Cdk5 activity, which causes tau hyperphosphorylation, leading to the formation of paired helical filaments (PHFs) and subsequently neuronal cell death. Cdk5-mediated tau phosphorylation also causes an increase in Ca2+ transfer from the ER to mitochondria through enhancement of ER-mitochondria contacts [30]. In separate studies, using NGF-differentiated sympathetic-like neuronal cells, Choi and Chung investigated Cdk5 regulation of intracellular Ca2+ dynamics using the Cdk5 inhibitors, roscovitine (ros, 50 µM) and olomoucine (olo, 100 µM) [31]. However, the concentrations used to inhibit Cdk5 in this study lack specificity as other kinases such as Cdk1 (ros, IC50 = 0.65 µM; olo, IC50 = 7 µM), Cdk2 (ros, IC50 = 0.7 µM; olo, IC50 = 7 µM) and ERK1 (ros, IC50 = 34 µM; olo, IC50 = 25 µM) could have also been inhibited. In non-neuronal cells, the specific role of Cdk5 in regulating intracellular Ca2+ dynamics remains unknown.
The IP3R, an ER transmembrane protein that forms a Ca2+ channel in its transmembrane domain and an IP3-binding site on its cytosolic face [32], forms the major route for Ca2+ release from the ER. When extracellular soluble agonists bind a G protein-coupled receptor, phospholipase C (PLC) is activated, producing IP3 from the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2). IP3 binding to IP3R induces opening of this channel and release of Ca2+ from the ER. Ca2+ released from the ER is then mobilized to the mitochondria [33] through the voltage-dependent anion channels (VDACs) in the outer mitochondrial membrane (OMM) and the mitochondrial calcium uniporter (MCU) channels in the inner mitochondrial membrane (IMM). In previous studies [34], we demonstrated that loss of Cdk5 in MEFs increases ER–mitochondria tethering and ER Ca2+ transfer to the mitochondria, subsequently increasing [Ca2+]mt. This points to a role for Cdk5 in regulating intracellular Ca2+ dynamics. Indeed, Cdk5 localizes in the MAM ER-mitochondria interface [34], and thus is well placed to influence ER Ca2+ release through IP3R, which is regulated by IP3R phosphorylation [18]. Cdk5 has a preferred phosphorylation consensus sequence of (S/T)PX(K/H/R) [3], and among the IP3R isoforms, IP3R1 has two potential Cdk5 phosphorylation sites: S421PLK and T799PVK [35]. IP3R2 is insensitive to ATP and does not contain possible Cdk5 phosphorylation sites; IP3R3 has Thr799 but ATP-induced, IP3R3-mediated Ca2+ release is much less significant than that mediated by IP3R1 [36]. It is possible that Cdk5 interacts with and phosphorylates IP3R1, regulating its channel opening. In fact, the Cdk5-related kinase, Cdk1 phosphorylates IP3R1 at Thr799 causing IP3R1 opening [35, 37]. Conversely, ERK1/2 phosphorylation of IP3R1 at Ser436 decreases IP3 binding and thus IP3-induced Ca2+ release [38–40].
Interplay between [Ca2+]cyt and ROS signaling has been reported [41, 42]. Cellular ROS are metabolic byproducts and act as secondary messengers in signaling pathways at sub-toxic levels. Oxidative stress, however, activates the nuclear factor erythroid 2-related factor 2 (Nrf2) transcription factor by inhibiting its negative regulator, Keap1. Nuclear translocation of activated Nrf2 results in the production of the antioxidant enzymes, peroxiredoxins (Prx1 and Prx2), catalase, glutathione peroxidase (GPX), and heme oxygenase-1 (HO-1), to maintain optimal cellular redox balance [43].
In this study, we utilized the Cdk5−/− mouse model and corresponding ex vivo MEFs to explore the mechanisms by which Cdk5 regulates intracellular Ca2+ dynamics and Ca2+-mediated cell proliferation. We demonstrate that Cdk5 targets IP3R1 to control ER Ca2+ release and [Ca2+]cyt. These Cdk5-mediated Ca2+ dynamics are reflected in the disrupted Ca2+-mediated proliferation of Cdk5−/− MEFs and development of Cdk5−/− embryos.
Materials and methods
Materials
Dulbecco’s modified eagle’s medium (DMEM), heat-inactivated fetal bovine serum (FBS), EDTA-Trypsin, antibiotic–antimycotic, H2-DCFDA, MitoSOX red, MitoTracker green, Fluo-4 AM, Mag-Fluo-4 AM, ionomycin, GlutaMAX and an antibody against p27KIP1 (719,600) were from ThermoFisher Scientific (Waltham, MA). Mito-tempo and antibodies against Cdk5 (C-8), tubulin (D-10), IP3R1 (E-8), p21CIP1 (L-17), Prx1 (N-19) and actin (I-19) were from Santa Cruz Biotech (Manassas, VA, USA). The polyclonal antibodies against the two IP3R1 phosphopeptides, MLKIGTpS421VKEDKE and HVDRDPQEQVpT799PVK, were generated by GL Biochem. Ltd (Shanghai, China). The phosphoThr202/Tyr204-ERK1/2 antibody was from Cell Signaling (Danvers, MA, USA). The Ki67 (ab92353), Prx2 (ab109367), GAPDH (6C5) and Nrf2 (ab31163) antibodies and BAPTA-AM (ab120503) were from Abcam (Cambridge, MA, USA). The protease inhibitor cocktail, ATP and XeC were from Sigma (ON, Canada). Thapsigargin (TG) was a gift from Dr. Andrew Braun at the University of Calgary. The IP3R1 siRNAs were synthesized at the University of Calgary Core DNA Services. The peroxidase and serum-free protein block kits were from Dako (Glostrup, Denmark). The avidin and biotin block kit and DAB were from Zymed (CA, USA). The secondary antibodies were from Jackson ImmunoResearch Labs (Pennsylvania, USA). The Vectastain® ABC Reagent was from Vector Laboratories (CA, USA). ECL reagent was from GE Healthcare (Chicago, USA). Jet prime transfection reagent was from Polyplus transfection (NY, USA).
Animals
The Cdk5−/− embryos that we used in our studies were generated by intercrossing Cdk5+/− mice (Jackson Laboratory, Bar Harbor, ME, USA) maintained at the University of Calgary Animal Facility. Wt littermates were used as controls. All animal studies conformed to regulatory standards and were approved by the University of Calgary Health Sciences Animal Care Committee.
Isolation and culture of primary MEFs
Primary MEFs were isolated from E13.5 Cdk5+/+ and Cdk5−/−embryos as described previously [34]. Briefly, embryos were washed with 1 × PBS, decapitated and eviscerated, and then washed again with PBS. Embryos were minced using sterile forceps and placed in 3–5 ml of 0.05% trypsin–EDTA, pipetted up and down to get cells into suspension and incubated at 37 °C for 5 min. Cell suspensions were transferred to tubes containing MEF medium (DMEM-high glucose supplemented with 10% FBS, 100 U/ml penicillin and 100 U/ml streptomycin, and 2 mM GlutaMAX) and then centrifuged at 1000 rpm for 5 min. Cell pellets were resuspended in fresh media and plated in 10 cm cell culture dishes. Primary MEFs were maintained in DMEM supplemented with 10% FBS, 50 U/ml penicillin and 50 mg/ml streptomycin under hypoxic condition (5% O2 and 5% CO2) at 37 °C. All experiments were performed using passage 2 to 7 (P2-P7) MEFs.
Ca2+ measurement
(i) To measure resting [Ca2+]cyt, trypsinized 0.5 × 106 MEFs were loaded with 5 µM Fluo-4 AM in DMEM for 1 h at room temperature. Cells were then washed three times with Ca2+-free EGTA-containing KRH buffer (25 mM HEPES, pH 7.4, containing 125 mM NaCl, 5 mM KCl, 1.2 mM MgCl2 and 6 mM glucose) and analyzed using a Shimadzu RF 5301PC spectrofluorometer. To minimize background fluorescence, 40 µM EDTA was added before reading the F values. Fmax value was obtained after treatment with 0.02% saponin and addition of 2 µM CaCl2 three times (Supplementary Fig. 1A). Fmin value was taken upon addition of 4 mM EDTA. [Ca2+]cyt was calculated using the formula: free [Ca2+]cyt = Kd [F–Fmin]/[Fmax–F] [44], whereas Kd (for Fluo-4) = 345 nM. (ii) To measure [Ca2+]cyt transients by single-cell Ca2+ imaging, MEFs were seeded in 3.5 cm glass bottom petri dishes and stained with 5 µM Fluo-4 AM in HBSS (with 1.26 mM Ca2+) for 30 min at room temperature (RT). Cells were then washed three times with KRH buffer and analyzed by single-cell Ca2+ imaging using a Zeiss LSM 510 Meta confocal laser scanning microscope (20 × objective). Fluorescence signals were measured in 10–20 cells. Peak amplitudes were quantified as ratios of fluorescence (F/F0) after addition of ATP, XeC or TG. F0 represents basal fluorescence or fluorescence before stimulation. (iii) To measure ER Ca2+, MEFs were seeded in 3.5 cm glass bottom petri dishes and stained with 5 µM Mag-Fluo-4 AM in DMEM for 30 min at RT. Cells were then permeabilized with 0.1 mg/ml saponin for 45 s, washed with ICM buffer (10 mM HEPES, pH 7.4, containing 19 mM NaCl, 125 mM KCl, 1.5 mM Na2ATP, 0.735 mM MgCl2, 1 mM EGTA, 0.5 mM CaCl2) three times and analyzed using a Zeiss LSM 510 Meta confocal microscope (20 × objective). Fluorescence signals were measured in ten cells and quantified as ratios of fluorescence (F/F0) after addition of 500 nM IP3. F0 represents basal fluorescence or fluorescence before stimulation.
siRNA transfection
MEFs (2.5 × 105) seeded in 6 cm dishes were transfected with 100 nM IP3R1 siRNA #1 (AACATTGTGCAGAAAACAGCC) or #2 (AACAAAGAGATCCGTAGTAAG) for 48 h using Jet prime transfection reagent following the manufacturer’s protocol.
Transfection of S421A and S421D IP3R1
pcDNA 3.0 carrying rat IP3R1 was obtained from Dr. I. Bezprozvanny at the University of Texas Southwestern Medical Center at Dallas. S421A IP3R1 and S421D IP3R1 were custom-generated by Genscript (USA). The IP3R also carries silent mutations: 2118 G > A, 2121 C > A, 2122 C > A, 2124 T > G, 2125 A > T and 2126 G > C that do not alter the IP3R1 amino acid sequence but confer resistance to IP3R1 siRNA #2. MEFs (5 × 105) seeded in 6 cm dishes were co-transfected with IP3R1 siRNA (100 nM) and pcDNA 3.0 carrying S421A IP3R1 or S421D IP3R1 (2 µg) as per the Lonza nucleofector protocol (Basel Switzerland).
ROS measurement
(i) For live-cell imaging, MEFs seeded in 4-chamber cover glass (Lab-Tek) were stained with 5 µM DCFDA or 200 nM MitoTracker green + 5 µM MitoSOX red to measure cytoplasmic hydrogen peroxide or mitochondrial superoxide levels, respectively. Images were taken using an Olympus 1X71 fluorescence microscope at 160 × magnification. (ii) By flow cytometry, MEFs (2.5 × 105) seeded in 3.5 cm dishes were treated with 3 µM XeC, 10 µM ionomycin or 50 µM BAPTA-AM for 30 min. Cells were then washed with KRH buffer and harvested using trypsin. Cytoplasmic hydrogen peroxide and mitochondrial superoxide levels were measured by staining with 5 µM H2-DCFDA and 5 µM MitoSOX red, respectively, in KRH buffer for 30 min at 37 °C. Cells were then washed and resuspended in KRH buffer and analyzed by flow cytometry using a fluorescein isothiocyanate filter (530 nm) for DCFDA, a phycoerythrin filter (575 nm) for MitoSOX red.
Proliferation analysis
1 × 103 MEFs were seeded in 96 well plates (n = 3). Cdk5−/− MEFs were treated (or untreated) with 3 µM XeC. After 1, 3 or 6 days in culture, cells were harvested using trypsin and stained with trypan blue, and viable cells were counted using a hemocytometer under an Olympus CK40 microscope.
Immunoprecipitation and immunoblotting
Immunoprecipitation (IP) of clarified MEF lysates in lysis buffer (25 mM HEPES, pH 7.4, containing 250 mM NaCl, 1 mM PMSF, 1 mM EDTA, 1% Triton X-100, 1 µg/ml leupeptin, 1 µg/ml aprotinin, 1 µg/ml antipain and 15 µg/ml benzamidine) was performed using IP3R1 (E-8) antibody. IP samples or cell lysates were resolved by SDS–polyacrylamide gel electrophoresis (PAGE) and proteins were transferred onto nitrocellulose membranes, which were blocked in 5% skimmed milk and then incubated with the indicated primary antibody at 4 °C overnight. After washing with TBST buffer, containing 50 mM Tris–HCl, pH 7.6, 0.8% NaCl and 0.1% Tween-20, membranes were incubated with horseradish peroxidase-conjugated secondary antibody for 1 h. Immunoreactive bands were detected using ECL reagent (Amersham).
Immunohistochemistry
E13.5 mouse embryos fixed in 4% paraformaldehyde (PFA) were sectioned to 10 µM thickness using a Leica RM2235 microtome. Tissue sections mounted on glass slides were incubated initially with peroxidase and then with avidin and biotin using a block kit followed by a serum-free protein block kit to eliminate non-specific binding of the primary antibody. The slides were then incubated with a Ki67 antibody at 1:100 dilution for 2 h, washed, and incubated with a secondary antibody for 40 min. Ki67-positive cells were detected by incubating with Vectastain® ABC reagent for 30 min followed by DAB for 5 min. Tissue staining was visualized and photographed using an Olympus I ×71 light microscope with an attached Photometrics Coolsnap FX camera from Roper Scientific (Arizona, USA).
Statistical analysis
Cdk5-regulated (i) [Ca2+]cyt effect on ROS level, (ii) proliferation, and (iii) IP3-induced Ca2+ release were analyzed by one-way ANOVA. All other data were analyzed by Student’s t test. Significance was set at p < 0.05.
Results
Elevated ATP-evoked rise in [Ca2+]cyt in Cdk5−/− MEFs results from increased Ca2+ release from internal stores
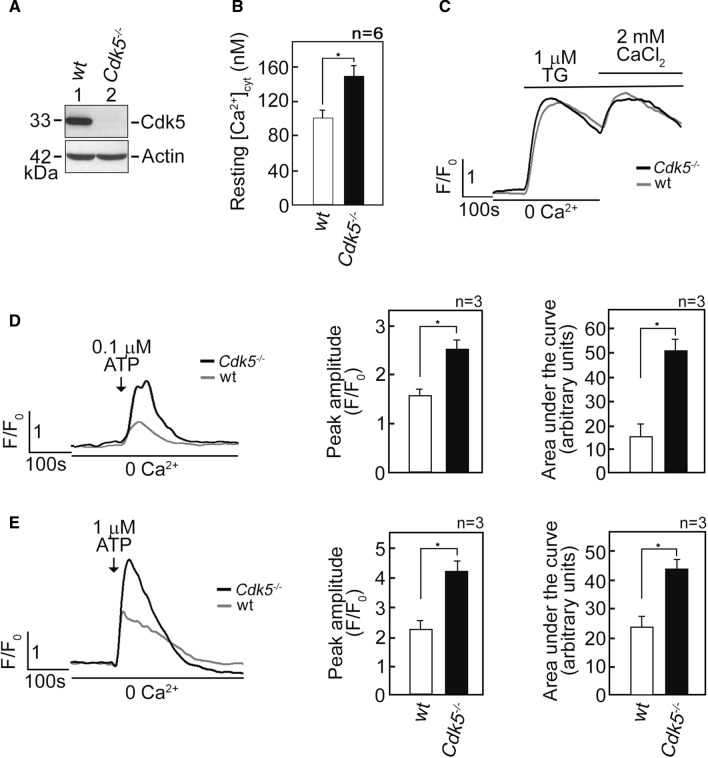
Our previous findings that Cdk5 regulates mitochondrial Ca2+ concentration ([Ca2+]mt) by controlling ER Ca2+ transfer to the mitochondria [34] led us to investigate whether Cdk5 also regulates free cytosolic Ca2+ concentration ([Ca2+]cyt). To do so, we initially performed spectrofluorometric analysis to measure resting [Ca2+]cyt in Fluo-4 AM-loaded primary MEFs [34] isolated from wt and Cdk5−/− mice embryos (Fig. 1A). As shown in Fig. 1B, the resting [Ca2+]cyt in Cdk5−/− MEFs was higher (p < 0.01) compared to that in wt MEFs (150 vs 100 nM), suggesting that loss of Cdk5 elicits a rise in free [Ca2+]cyt. We then examined three possible mechanisms that may have caused the rise in [Ca2+]cyt in Cdk5−/− MEFs: (i) reduced internal Ca2+ store capacity, (ii) increased Ca2+ influx from the extracellular milieu, and (iii) increased Ca2+ release from internal Ca2+ stores. We began by treating wt and Cdk5−/− MEFs with thapsigargin (TG), a potent non-competitive inhibitor of the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) pump [45], that empties internal Ca2+ stores, allowing measurement of internal Ca2+ store capacity. This was followed by subjecting cells to external 2 mM CaCl2 to measure capacitative Ca2+ entry from the extracellular milieu. As shown in Fig. 1C, there was no difference in internal Ca2+ store capacity and capacitative external Ca2+ entry in wt and Cdk5−/− MEFs. Since ATP, which binds cell surface purinergic receptors [46], induces Ca2+ release from internal stores [47] in Ca2+-free buffer, we examined whether increased [Ca2+]cyt in Cdk5−/− MEFs is due to internal store Ca2+ release by loading cells with Fluo-4 AM and treating them with ATP in Ca2+-free EDTA-containing buffer. By single-cell Ca2+ imaging analyses using a confocal laser scanning microscope, we found that 0.1 µM (Fig. 1D) and 1 µM (Fig. 1E) ATP caused greater [Ca2+]cyt transients in Cdk5−/− MEFs compared to wt, with greater rise in [Ca2+]cyt transient at 1 µM ATP. These findings suggest that elevated ATP-evoked rise in [Ca2+]cyt in Cdk5−/− MEFs results from increased Ca2+ release from internal stores.
Fig. 1.
Cdk5−/− MEFs exhibit increased [Ca2+]cyt and increased ATP-induced Ca2+ release from internal stores. A Lysates of MEFs isolated from wt and Cdk5−/− mouse embryos were analyzed by SDS-PAGE and immunoblotting for Cdk5. Actin blot was used as loading control. B Cdk5 loss caused an increase in free [Ca2+]cyt. Wt and Cdk5−/− MEFs loaded with the cell-permeable intracellular Ca2+ indicator, Fluo-4 AM, were analyzed for [Ca2+]cyt using spectrofluorometric Ca2+ imaging. Free [Ca2+]cyt levels were measured as described in Materials and methods. Data represent means ± SEM from six independent experiments (n = 6). * indicates p < 0.01. C Wt and Cdk5−/− MEFs loaded with Fluo-4 AM were analyzed for [Ca2+]cyt transients following the addition of TG (1 µM) by single-cell Ca2+ imaging as described in Materials and methods. TG-induced Ca2+ release from internal stores corresponds to internal Ca2+ store capacity. After initial analysis in [Ca2+]-free buffer, capacitative Ca2+ entry from the extracellular milieu was determined upon addition of 2 mM CaCl2 in the presence of TG. Data represent means of Ca2+ signal traces from 15 cells. D and E Loss of Cdk5 caused an increase in ATP-induced Ca2+ release from internal stores. [Ca2+]cyt in wt and Cdk5−/− MEFs was measured following stimulation with 0.1 µM (D) or 1 µM (E) ATP in [Ca2+]-free buffer by single-cell Ca2+ imaging. Graphs in (D) and (E), right panels, represent peak amplitude values and integrated Ca2+ signals, which is the area under the curve (AUC area that begins immediately after addition of 0.1 mM ATP and ends when the Ca2+ trace goes back to the baseline level). Data represent means of Ca2+ signal traces from 20 cells. All values are means ± SEM from three independent experiments (n = 3). *p < 0.05
Elevated ATP-evoked rise in [Ca2+]cyt in Cdk5−/− MEFs is due to increased Ca2+ release via IP3R channels
We next tested whether the ATP-induced rise in [Ca2+]cyt in Cdk5−/− MEFs occurs through IP3R. As shown in Fig. 2A, treatment of Fluo-4 AM-loaded and ATP-stimulated MEFs with xestospongin C (XeC) [48], a potent IP3R inhibitor, caused complete inhibition of the ATP-evoked [Ca2+]cyt increase in both wt and Cdk5−/− MEFs, indicating that such [Ca2+]cyt increase is mediated by IP3R, which forms Ca2+ channels in the internal Ca2+ stores [47]. To examine whether loss of Cdk5 alters the IP3-mediated Ca2+ release from the ER, cells loaded with the ER Ca2+ probe, Mag-Fluo-4 AM [49], were treated with IP3 after permeabilization with saponin to facilitate IP3 access to IP3R. By single-cell Ca2+ imaging, we found that IP3 induced a greater decline in Mag-Fluo-4 signal in Cdk5−/− MEFs compared to wt (Fig. 2B). Together, these results indicate that elevated ATP-evoked rise in [Ca2+]cyt in Cdk5−/− MEFs is due, at least in part, to increased ER Ca2+ release through IP3R channels.
Fig. 2.
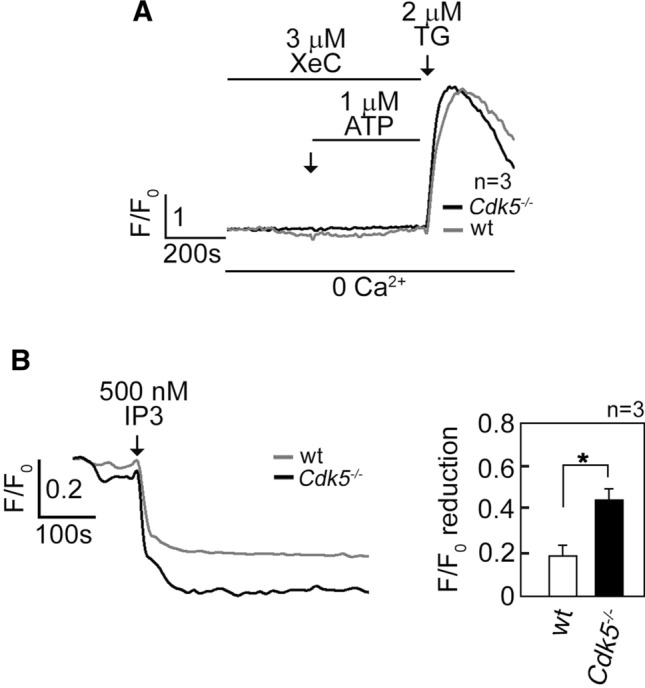
Increased [Ca2+]cyt in Cdk5−/− MEFs is due to increased IP3R-mediated Ca2+ release. A MEFs loaded with Fluo-4 AM and treated with 3 µM XeC followed by 1 µM ATP then 2 µM TG in Ca2+-free EGTA-containing KRH buffer were analyzed for [Ca2+]cyt transients by single-cell Ca2+ imaging analyses. The similar increase in [Ca2+]cyt in wt and Cdk5−/− MEFs upon treatment with TG, which was added after 15 min of treatment with XeC, indicates comparable viability of these cells during analysis. Data represent means of Ca2+ signal traces from 15 cells. B Measurement of Ca2+ release from internal stores upon IP3 treatment is described in Materials and methods. Ca2+ release was measured every 4 s by single-cell Ca2+ imaging. Data represent means of Ca2+ signal traces from ten cells, and are results from one of three independent experiments showing similar patterns. Values are means ± SEM from the three separate experiments (n = 3). *p < 0.05
Cdk5 interaction with and phosphorylation of IP3R1 at S421 downregulate IP3R1-mediated ER Ca2+ release
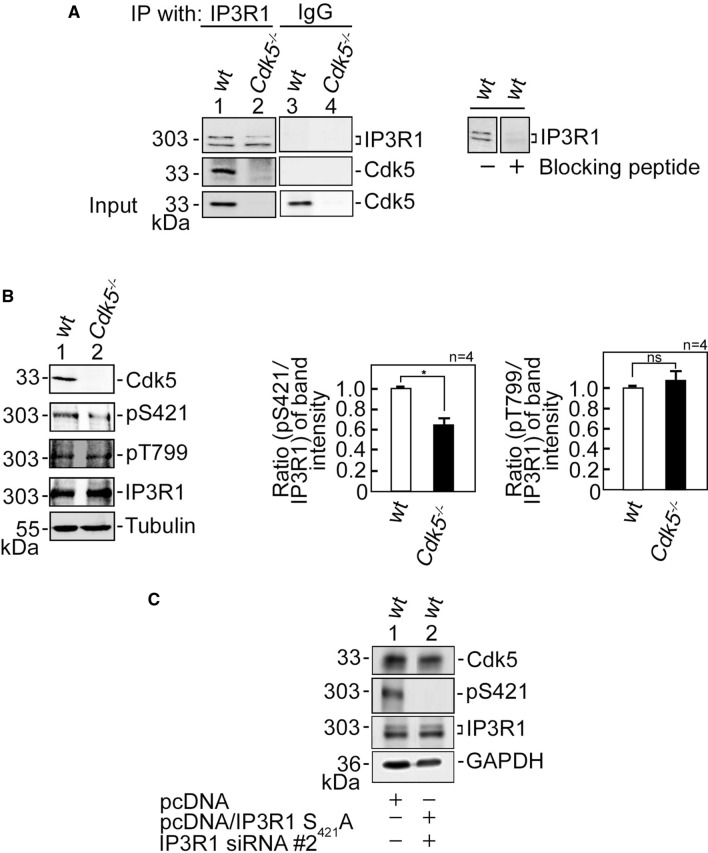
We then sought to further investigate how ATP-evoked IP3R-mediated ER Ca2+ release increases in Cdk5−/− MEFs. IP3R-mediated Ca2+ release is regulated by IP3R phosphorylation [18], and Cdk5, a Ser/Thr kinase with a (S/T)PX(K/H/R) preferred consensus phosphorylation site [3], localizes in MAMs [34] (Supplementary Fig. 3B) where it could interact with and phosphorylate IP3R. To test the possibility that Cdk5 associates with its likely IP3R isoform target, IP3R1, lysates of wt and Cdk5−/− MEFs were subjected to immunoprecipitation (IP) using an IP3R antibody, and the IPs were immunoblotted for Cdk5. Co-IP of Cdk5 with IP3R1 (Fig. 3A) indicates interaction between the two proteins. However, co-transfection of Cdk5, p35 and IP3R1 in HEK293T cells followed by immunoprecipitation of Cdk5 or p35 showed the presence of the Cdk5/p35-IP3R1 complex. However, co-transfection of Cdk5 and IP3R1, but not p35, also showed Cdk5 interaction with IP3R1, indicating that such interaction does not require p35 (Supplementary Fig. 3C). We then examined whether Cdk5 phosphorylates its potential targets in IP3R1, S421PLK and T799PVK, by analyzing lysates of wt and Cdk5−/− MEFs. Immunoblotting showed that while there was no difference in IP3R1 Thr799 phosphorylation in wt and Cdk5−/− MEFs, IP3R1 Ser421 phosphorylation was reduced (p < 0.05) in Cdk5−/− MEFs compared to wt (Fig. 3B), indicating that Cdk5 specifically targets Ser421 in IP3R1. The inability of IP3R1 Ser421 antibody to detect non-phosphorylatable IP3R1 S421A (Fig. 3C) confirms the specificity of the antibody.
Fig. 3.
Cdk5 associates with and phosphorylates IP3R1 at Ser421. A Cdk5 associates with IP3R1. Lysates of wt and Cdk5−/− MEFs were subjected to immunoprecipitation (IP) using IP3R1 antibody. The IPs were resolved by 4–20% gradient SDS-PAGE and then immunoblotted for IP3R1 and Cdk5 (left panel). To assess the specificity of the IP3R1 antibody, lysates of wt MEFs were blotted (right panel) with antibody blocked (lane 2) or not blocked (lane 1) with the peptide antigen that was used to raise the antibody. Lanes 3 and 4 represent IP control using normal IgG. B Cdk5 specifically phosphorylates IP3R1 at Ser421. Lysates of wt and Cdk5−/− MEFs were subjected to SDS-PAGE and then immunoblotted for IP3R1 phosphoSer421 and phosphoThr799, IP3R1, Cdk5 and tubulin. Tubulin blot was used as loading control. Representative blots are from one of four independent experiments (n = 4) showing similar results. Ratios of levels of IP3R1 phosphoSer421 (middle panel) and phosphoThr799 (right panel) vs total IP3R were calculated following densitometric analysis of blots using NIH Image J 1.61. Standard deviations were calculated based on the ratios obtained from the four independent sets of experiments. Values from wt MEFs were normalized to 1.0. *p < 0.05. ns: not significant. C Shows the specificity of the IP3R1 phosphoSer421 antibody. Lysates of wt MEFs depleted of endogenous IP3R1, but expressing exogenous IP3R1 S421A (res) were subjected to immunoblotting for IP3R1 phosphoSer421, IP3R1, and Cdk5. GAPDH blot was used as loading control
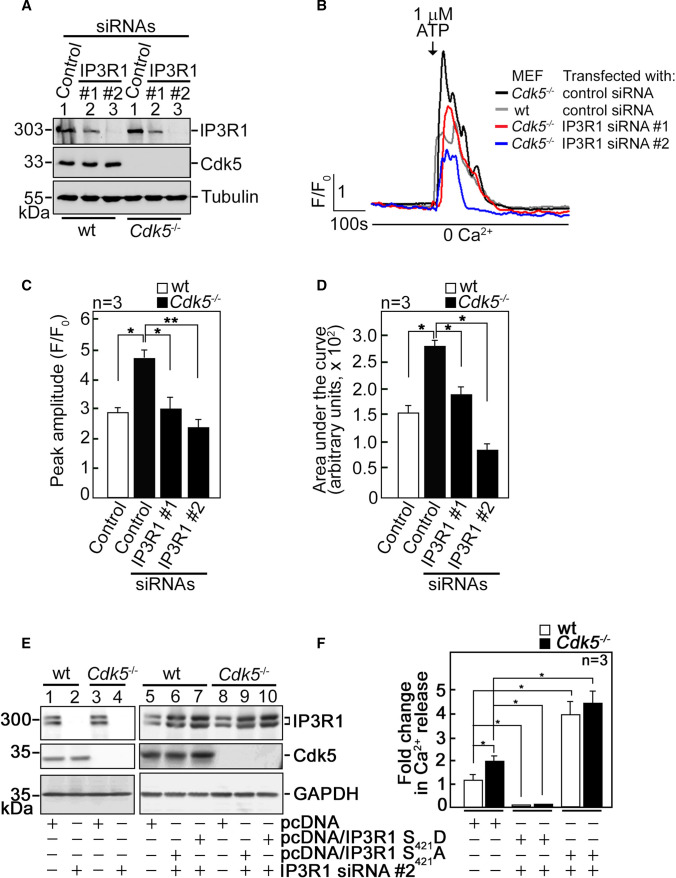
To further examine whether increased Ca2+ release in Cdk5−/− MEFs is regulated by IP3R1, we utilized wt and Cdk5−/− MEFs depleted of IP3R1 by siRNA #1 or #2 (Fig. 4A). We noted that siRNA #2 is more efficient at depleting IP3R1 compared to siRNA #1. IP3R1-depleted cells loaded with Fluo-4 AM then treated with 1 µM ATP in Ca2+-free buffer were analyzed by single-cell Ca2+ imaging. Consistent with our findings above, Cdk5−/− MEFs showed increased ATP-evoked [Ca2+]cyt transients compared to wt (Fig. 4B–D). Depletion of IP3R1 reversed the increase in [Ca2+]cyt transients in Cdk5−/− MEFs to levels close to those in wt MEFs. These [Ca2+]cyt transients were quantified by measuring their peak amplitudes (Fig. 4C) and calculating the areas under the curve (AUC), which correspond to the integrated Ca2+ signals (Fig. 4D). These data imply that increased ATP-evoked ER Ca2+ release in Cdk5−/− MEFs, as indicated by elevated ATP-evoked [Ca2+]cyt transients in these cells, is mediated by IP3R1.
Fig. 4.
IP3R1 loss inhibits the ATP-induced increase in [Ca2+]cyt in Cdk5−/− MEFs. A Lysates of cells transfected with IP3R1 siRNA #1 or #2 for 48 h were resolved by SDS-PAGE and immunoblotting for IP3R1 and Cdk5. Tubulin blot was used to assess protein loading. Representative blots are from one of three independent experiments showing similar result are shown. B wt and Cdk5−/− MEFs transfected with IP3R1 siRNA #1 or #2, loaded with Fluo-4 AM, and treated with 1 µM ATP were analyzed for [Ca2+]cyt transients by single-cell Ca2+ imaging analyses in Ca2+-free buffer. Data are means of Ca2+ signal traces from 20 cells and are from one of three independent experiments showing similar results. [Ca2+]cyt transients were further analyzed by measuring their peak amplitudes (C) and calculating the areas under the curve which begin immediately after addition of 1 mM ATP and ends when the Ca2+ trace goes back to the baseline level. D Values are means ± SEM from three independent experiments (n = 3). * and **Denote p < 0.05 and p < 0.01, respectively. E Wt and Cdk5−/− MEFs were co-transfected with the indicated vector and IP3R1 siRNA #2. Cell lysates (40 µg) were resolved by SDS-PAGE and immunoblotted for IP3R1 and Cdk5. GAPDH blot was used as loading control. F Wt and Cdk5−/− MEFs co-transfected with the indicated vector and siRNA #2 were loaded with Mag-Fluo-4 AM. ER Ca2+ release following IP3 treatment was measured by spectrofluorometry. Values, which represent the fold change in peak amplitudes, are means ± SEM from three independent experiments (n = 3). *p < 0.05
Next, we tested whether Cdk5-mediated phosphorylation of IP3R1 at S421 inhibits ER Ca2+ release. To do so, pcDNA carrying rat IP3R1, which shares 99.6% amino acid sequence identity with mouse IP3R1 (Supplementary Fig. 2), was subjected to site-directed mutagenesis to generate phosphomimetic IP3R1 S421D and non-phosphorylatable S421A, and for additional nucleotide substitutions to confer resistance to IP3R1 siRNA #2, but not alter the IP3R1 amino acid sequence (Supplementary Fig. 3A). Wt and Cdk5−/− MEFs were then co-transfected with IP3R1 siRNA #2 (to deplete endogenous IP3R1) and pcDNA3.0 carrying IP3R1(res) S421D or S421A (Fig. 4E), loaded with Mag-Fluo-4 AM, and analyzed for IP3-induced ER Ca2+ release. As shown in Fig. 4F, Cdk5−/− MEFs transfected with empty pcDNA3.0 displayed greater IP3-induced Ca2+ release than wt MEFs, which is consistent with our data in Fig. 2B. Expression of exogenous IP3R1 S421D caused complete inhibition of IP3-induced ER Ca2+ release in both wt and Cdk5−/− MEFs depleted of endogenous IP3R1, while expression of exogenous IP3R1 S421A caused further increase in ER Ca2+ release compared to control vector-transfected cells. These findings and our earlier data, showing that Cdk5 loss, which reduces inhibitory phosphorylation of IP3R1 S421 (Fig. 3B), causes elevated IP3-induced Ca2+ release suggest that Cdk5 serves to downregulate ER Ca2+ release through inhibitory phosphorylation of IP3R1 at S421.
Increased [Ca2+]cyt due to Cdk5 loss induces ROS production, which upregulates Nrf2 level
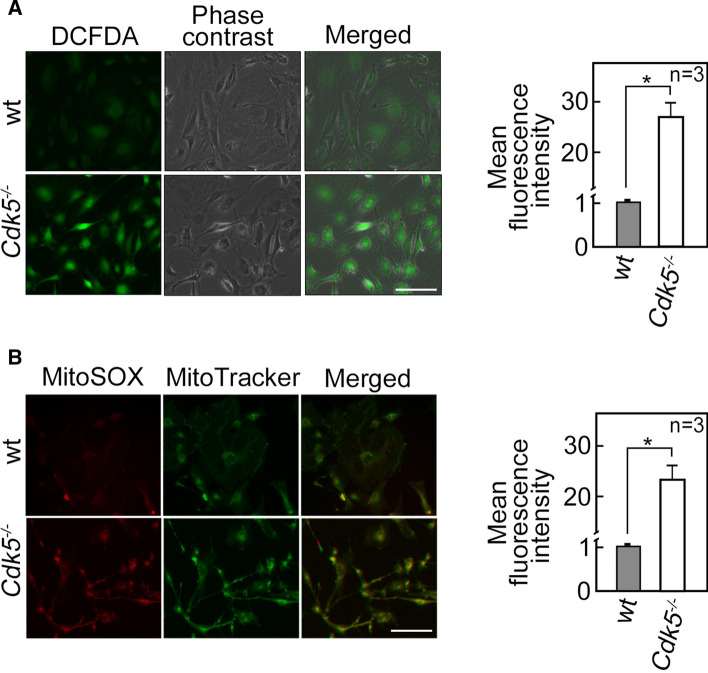
Since dysregulated [Ca2+]cyt homeostasis affects intracellular ROS level [50, 51], and loss of Cdk5 alters [Ca2+]cyt, we next sought to assess intracellular ROS levels in wt and Cdk5−/− MEFs. Cells stained with a fluorescent cytosolic ROS probe, 2',7'-dichlorodihydrofluorescein diacetate (DCFDA), were analyzed for intracellular hydrogen peroxide level by live-cell imaging using an Olympus 1X71 fluorescent microscope. As shown in Fig. 5A (left panel), Cdk5−/− MEFs displayed increased DCFDA staining compared to wt. Consistent with the microscopic data, flow cytometry analysis showed an increase (p < 0.05) in intracellular hydrogen peroxide level in Cdk5−/− MEFs compared to wt MEFs (Fig. 5A, right panel). Since mitochondria are a major source of ROS, we also examined ROS levels in this organelle by MitoSOX staining. Figure 5B (left panel) shows that mitochondrial superoxide anion levels were likewise higher in Cdk5−/− MEFs compared with wt. Similarly, flow cytometry analysis showed an increase in mitochondrial superoxide anions in Cdk5−/− MEFs compared to wt (Fig. 5B, right panel). These observations indicate that loss of Cdk5 induces ROS production.
Fig. 5.
Cdk5−/− MEFs exhibit increased ROS production. Wt and Cdk5−/− MEFs stained with 5 µM DCFDA (A) or 5 µM MitoSOX red and 200 nM MitoTracker green (B) for 30 min were analyzed by live-cell imaging using an Olympus I × 71 fluorescence microscope at 160X magnification (left panels) and by flow cytometry (right panels). The left panels’ scale bars are equivalent to 100 µm. The right panels show the percentage increase in mean fluorescence intensity. Values from wt MEFs were normalized to 1.0. Values are means ± SEM from three independent experiments (n = 3). *p < 0.05
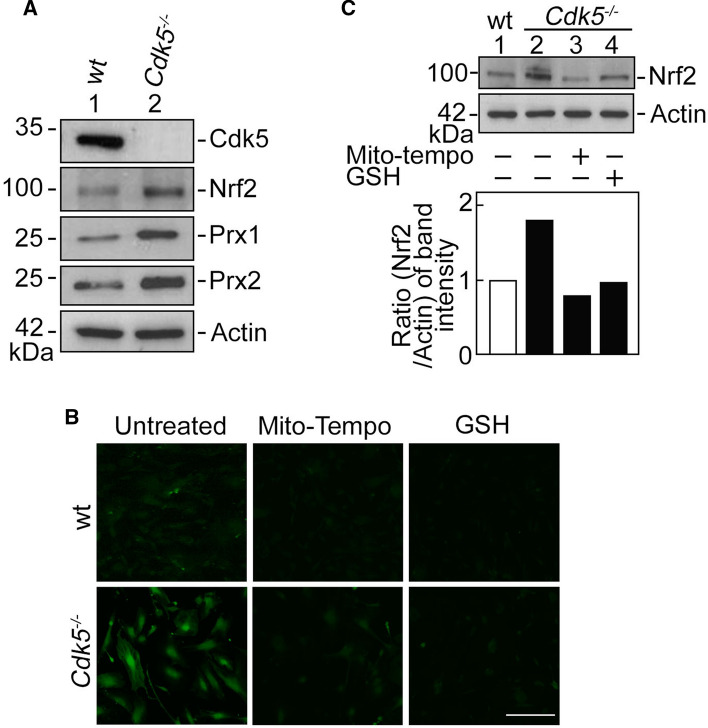
ROS tightly regulates the activity of nuclear factor erythroid 2-related factor 2 (Nrf2) [52], which responds to oxidative stress by binding to the antioxidant response element (ARE) in the promoter of genes coding for antioxidant enzymes such as peroxiredoxin 1 and 2 (Prx1 and Prx2). Thus, we further examined the levels of Nrf2 in wt and Cdk5−/− MEFs. Interestingly, Cdk5 loss, which induces ROS production, upregulates Nrf2 level as well as levels of the Nrf2 antioxidant protein targets, Prx1 and Prx2 (Fig. 6A). To establish a link between increased ROS level and upregulated Nrf2 level in Cdk5−/− MEFs, cells treated with a ROS scavenger, mito-tempo or reduced glutathione (GSH) were (i) stained with DCFDA and analyzed for cytoplasmic ROS level by live-cell imaging, and (ii) subjected to SDS-PAGE and immunoblotting for Nrf2. As shown in Fig. 6B, and C, mito-tempo and GSH prevented the increase in ROS (Fig. 6B) and Nrf2 (Fig. 6C) levels in Cdk5−/− MEFs. ROS and Nrf2 levels in these cells were reduced by the ROS scavengers to a level equivalent to that in untreated wt MEFs, indicating that increased ROS level in Cdk5−/− MEFs upregulates Nrf2 level in these cells.
Fig. 6.
Cdk5−/− MEFs exhibit increased Nrf2 level, and scavenging ROS with mito-tempo or GSH prevents the increase in ROS and Nrf2 level in these cells. A Cdk5−/− MEFs show upregulated expression of Nrf2 and its downstream targets, Prx1 and Prx2. Lysates of wt and Cdk5−/− MEFs were analyzed by SDS-PAGE and immunoblotting for Cdk5, Nrf2, Prx1 and Prx2. Actin blot was used to assess protein loading. B Wt and Cdk5−/− MEFs treated with an ROS scavenger, mito-tempo (10 µM) or GSH (10 µM), and then stained with 5 µM DCFDA for 30 min were examined for cytoplasmic ROS level by live-cell imaging using an Olympus I×71 fluorescence microscope at 160 × magnification. Scale bar = 100 µm. C MEFs treated with mito-tempo or GSH were also analyzed by SDS-PAGE and immunoblotting for Nrf2. The graph (lower panel) shows the ratios of levels of Nrf2 vs actin calculated following densitometric analysis of blots using NIH Image J 1.61
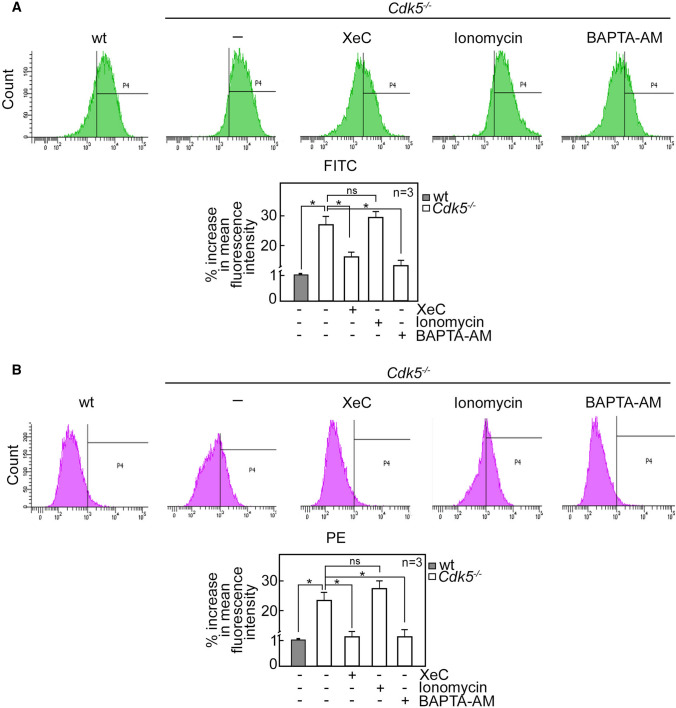
Our next step was to examine the effect of Cdk5-regulated [Ca2+]cyt on ROS level. To do so, wt and Cdk5−/− MEFs treated with XeC, ionomycin or BAPTA-AM and then stained with DCFDA (Fig. 7A) or mitoSOX red (Fig. 7B) were subjected to flow cytometry. As shown in Fig. 7A and B, inhibition of IP3R-mediated Ca2+ release with XeC and chelating Ca2+ with BAPTA-AM in Cdk5−/− MEFs reduced ROS production to a level close to that in wt. As expected, treatment of Cdk5−/− MEFs with the membrane permeable Ca2+ ionophore, ionomycin, increased ROS level. These findings indicate that increased [Ca2+]cyt in Cdk5−/− MEFs upregulates ROS production. We then examined the effect of scavenging ROS with GSH or mito-tempo on [Ca2+]cyt in Cdk5−/− MEFs. To do so, wt and Cdk5−/− MEFs treated with GSH or mito-tempo were stained with Fluo-4 AM to measure [Ca2+]cyt in these cells. As shown in Supplementary Fig. 4, GSH and mito-tempo had no effect on [Ca2+]cyt in Cdk5−/− MEFs under a condition where BAPTA-AM reduced [Ca2+]cyt to a level similar to that in wt. These results indicate that while [Ca2+]cyt regulates ROS production in Cdk5−/− MEFs, ROS level does not influence [Ca2+]cyt in these cells.
Fig. 7.
[Ca2+]cyt regulates ROS production in Cdk5−/− MEFs. Wt and Cdk5−/− MEFs treated with 3 µM XeC, 10 µM ionomycin or 50 µM BAPTA-AM then stained with DCFDA (A) or mitoSOX red (B) were subjected to flow cytometry to measure cytoplasmic and mitochondrial ROS levels, respectively. Graphs (lower panels) show the % increase in mean fluorescence intensity. Values from wt MEFs were normalized to 1.0. Values are means ± SEM from three independent experiments (n = 3). *p < 0.05. ns: not significant, FITC: Fluorescein isothiocyanate, PE: Phycoerythrin
Altered Ca2+ dynamics in Cdk5−/− MEFs correspond to accelerated cell proliferation that correlates with increased body weight and size in Cdk5−/− embryos
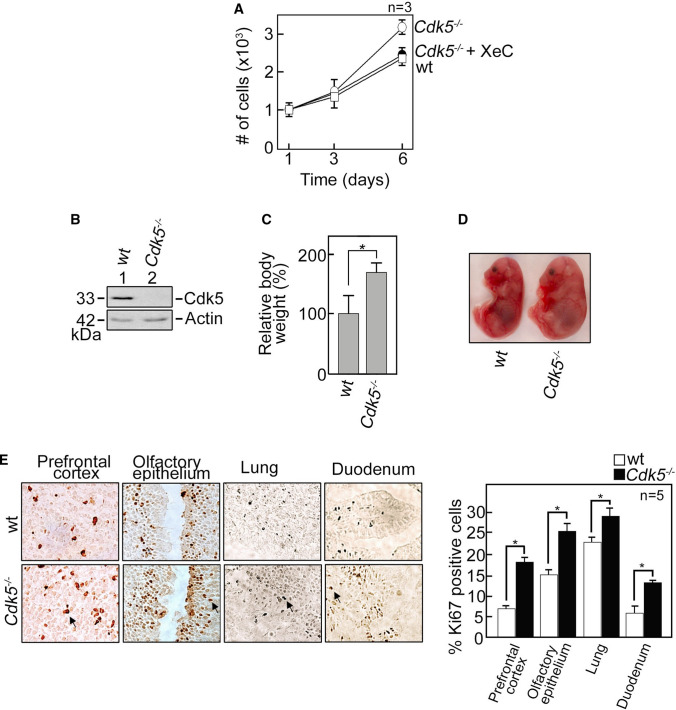
IP3R-mediated Ca2+ transients regulate G1/S transition during cell-cycle progression [53] and cell proliferation [18, 19], and Cdk5, which regulates intracellular Ca2+ dynamics, has been implicated in cell proliferation [7, 8]. This prompted us to test whether altered Ca2+ dynamics in ex vivo Cdk5−/− MEFs is associated with proliferation defect. As shown in Fig. 8A, Cdk5−/− MEFs proliferate at a faster rate compared to wt, indicating that Cdk5 loss, which triggers a rise in [Ca2+]cyt, accelerates proliferation in MEFs. To establish a link between altered Ca2+ dynamics and increased proliferation in Cdk5−/− MEFs, proliferation of cells treated with XeC was examined. As shown in Fig. 8A, treatment with XeC reversed the increase in Cdk5−/− MEF proliferation to a level equivalent to that in wt, indicating that accelerated proliferation in Cdk5−/− MEFs is linked to altered IP3R-mediated Ca2+ dynamics. We then tested whether the proliferation error in Cdk5−/− MEFs is recapitulated in mice. Since Cdk5−/− mice exhibit perinatal mortality (i.e., 64% die in utero and newborns are either dead or weak and die within 12 h after birth) [54], we isolated embryonic day 16.5 (E16.5) Cdk5+/+ and Cdk5−/− embryos (Fig. 8B) from pregnant Cdk5+/– mice, and body weights and sizes were compared. As shown in Fig. 8C, the Cdk5−/− embryos weighed more (p < 0.05) than their wt littermates. Figure 8D shows representative wt and Cdk5−/− littermate embryos with the Cdk5−/− embryo clearly bigger than the wt. By immunohistochemistry, we found increased staining for Ki67, a proliferation marker, in the prefrontal cortex, olfactory epithelium, lung, and duodenum of Cdk5−/− embryos compared to wt (Fig. 8E), which likely accounts for their increased body weight and size. In addition, Cdk5−/− MEFs have increased phospho-ERK1/2 but reduced p27KIP1 and p21CIP1 compared to wt (Supplementary Fig. 5). Taken together, our results indicate that altered Ca2+ dynamics due to Cdk5 loss correspond to accelerated cell proliferation that correlates with increased body weight and size in Cdk5−/− embryos.
Fig. 8.
Cdk5−/− MEFs show increased proliferation and Cdk5−/− mouse embryos are heavier and bigger than wt. A Cdk5−/− MEFs proliferate at a faster rate compared to wt, but treatment with XeC reverses this phenotype in Cdk5−/− MEFs. Proliferation was measured as described in Materials and methods. B Homogenates (20 μg) of tails from E16.5 wt and Cdk5−/− embryos were analyzed by SDS-PAGE and immunoblotting for Cdk5. Actin blot was used as loading control. C Body weights of E16.5 wt (n = 4) and Cdk5−/− (n = 5) embryos from four litters were measured. *p < 0.05. D Representative images of E16.5 wt and Cdk5−/− littermates. E Immunohistochemistry of prefrontal cortex, olfactory epithelium, lung and duodenum from wt and Cdk5−/− littermates. Embryos were sectioned to 10 μm thickness and stained for Ki67. Arrows are directed at Ki67-positive cells. The graph showing the percentage of Ki67-positive cells was calculated from five non-overlapping fields (n = 5). *p < 0.05
Discussion
Our previous finding that Cdk5 localizes in MAMs, and regulates [Ca2+]mt by controlling ER Ca2+ transfer to the mitochondria [34], points to a role for Cdk5 in regulating intracellular Ca2+ dynamics. However, the involvement of Cdk5 in this process remains to be investigated. In this study, using Cdk5−/− MEFs, we demonstrated that the IP3R1 Ca2+ channel is a downstream target of Cdk5, which interacts with and phosphorylates IP3R1 at Ser421, a target that lies in the IP3-binding site. As illustrated in our proposed model (Fig. 9), Cdk5 phosphorylation of IP3R1 Ser421 controls IP3R1-mediated internal Ca2+ release as loss of Cdk5 in MEFs, and thus, loss of IP3R1 Ser421 phosphorylation triggers an increase in IP3R1-mediated Ca2+ release from internal stores, resulting in elevated [Ca2+]cyt. This rise in [Ca2+]cyt causes accelerated proliferation in Cdk5−/− MEFs, which correlates with increased body weight and size in Cdk5−/− mouse embryos.
Fig. 9.
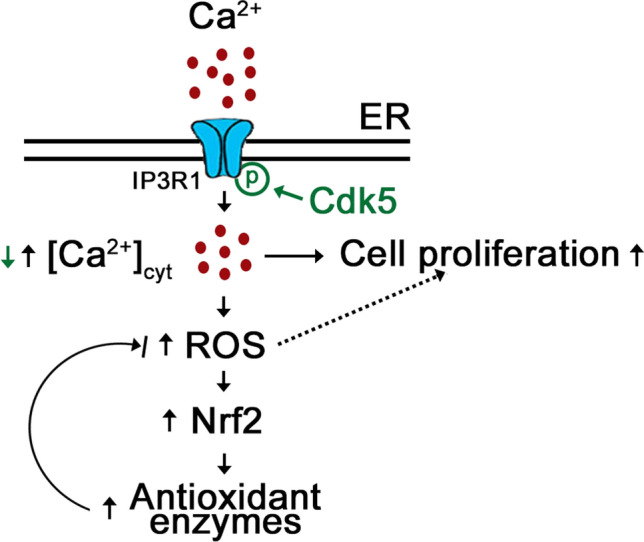
Proposed model illustrating how Cdk5 phosphorylation of IP3R1 (Ser421) controls IP3R1-mediated internal Ca2+ release and [Ca2+]cyt (green text and arrow) and how loss of Cdk5 in Cdk5−/− MEFs affects [Ca2+]cyt and Ca2+-mediated processes (black text and arrows). Loss of Cdk5 reduces the phosphorylation of IP3R1 Ser421, causing increased IP3R1-mediated Ca2+ release. Subsequent rise in [Ca2+]cyt increases ROS production, causing increased Nrf2 expression and activity, and increased expression of the NRF2 antioxidant targets such as Prx1 and Prx2. Adequate [Ca2+]cyt permits progression of Ca2+-mediated proliferation, but excess levels cause increased cell proliferation
While the rise in free [Ca2+]cyt in Cdk5−/− MEFs could be due to reduced internal Ca2+ store capacity or increased Ca2+ influx from the extracellular milieu, we did not observe either of these in Cdk5−/− MEFs. To test the possibility that loss of Cdk5 perturbs Ca2+ release from the ER to cause increased [Ca2+]cyt, we took advantage of the fact that ATP mediates Ca2+ release from internal stores [47], and that an ER Ca2+ probe, Mag-Fluo-4 AM, may be utilized to measure ER Ca2+ release. Using this approach, we found greater ATP-induced ER Ca2+ release in Cdk5−/− MEFs compared to wt. IP3R inhibition with XeC [55] completely blocks this ATP-evoked [Ca2+]cyt increase in both wt and Cdk5−/− MEFs, indicating that such [Ca2+]cyt increase is mediated by the IP3R Ca2+ channels in the ER. Our finding that IP3 induces a greater decline in Mag-Fluo-4 signal in Cdk5−/− MEFs compared to wt further supports our view that Cdk5 serves to control the IP3R-mediated ER Ca2+ release that leads to increased [Ca2+]cyt in Cdk5−/− MEFs.
Since IP3R-mediated Ca2+ release is regulated by IP3R phosphorylation [18], and Cdk5, which localizes in MAMs [34], has two potential phosphorylation target sites in IP3R1, S421PLK and T799PVK, that exist in the IP3-binding site, we examined whether Cdk5 interacts with and phosphorylates IP3R1. Indeed, we found that Cdk5 interacts with and specifically phosphorylates IP3R1 at Ser421. This was demonstrated by reduced IP3R1 Ser421 phosphorylation in Cdk5−/− MEFs, which increases in ER Ca2+ release. The specificity of IP3R1 immunoreactivity was verified by the loss of detectable IP3R1 in wt MEFs when the IP3R1 antibody was blocked with the peptide antigen that was used to generate the antibody. The detection of two IP3R1 immunoreactive bands may reflect immunoreactivity with (i) both unphosphorylated and phosphorylated forms, (ii) different isoforms, or (iii) intact and degraded forms of the protein. Partial reduction (p < 0.05) of IP3R1 S421 phosphorylation in Cdk5−/− MEFs compared to wt suggests the presence of at least one other IP3R1 S421 kinase. In fact, Cdk1 has been shown to phosphorylate IP3R1 S421 [37]. Although IP3R1 S421A substitution was shown to increase IP3 binding to IP3R1 [35], its effect on ER Ca2+ release has not been investigated. Our data show that in endogenous IP3R1-depleted cells, exogenous IP3R1 S421D (res) expression inhibits ER Ca2+ release, while IP3R1 S421A expression enhances ER Ca2+ release substantiate the importance of inhibitory phosphorylation of IP3R1 S421, which prevents Ca2+ release from internal stores. Apparently, Cdk5 plays a significant role in this process. Our findings support the idea that MAM-associated Cdk5 negatively regulates the opening of the IP3R1 Ca2+ channel through phosphorylation of IP3R1 Ser421, which, as indicated above, lies in the IP3-binding site. It is interesting that ERK1/2 phosphorylation of Ser436, which also lies in the IP3-binding site, inhibits the opening of the IP3R1 channel as well [39, 40]. Since a rise in [Ca2+]cyt can activate Ca2+-induced Ca2+ release (CICR) [56] from the internal stores, elevated ER Ca2+ release in Cdk5−/− MEFs may propagate Ca2+ signals to neighboring organelles, causing a further rise in [Ca2+]cyt.
In addition to triggering a rise in free [Ca2+]cyt, loss of Cdk5 in MEFs further induces ROS production. The ability of XeC and BAPTA-AM to reverse the increase in ROS level in Cdk5−/− MEFs indicates that ROS production occurs downstream of the IP3R1-mediated increase in [Ca2+]cyt in these cells. Interestingly, we found that increased ROS production in Cdk5−/− MEFs is associated with increased proliferation. Since increased ROS also induces apoptosis, it is possible that ROS-associated upregulation of Nrf2 and its antioxidant protein targets, Prx1 and Prx2, in Cdk5−/− MEFs acts in a feedback control loop, ensuring that the ROS level in these cells does not exceed the threshold level that triggers apoptosis. This notion indicates the adaptability of MEFs under increased oxidative stress condition.
Cdk5 and IP3R-mediated Ca2+ oscillations have been shown to regulate cell-cycle progression [6, 7, 10, 11, 53] and proliferation [12–19]. Thus, it is not surprising that Cdk5−/− MEFs, which have elevated [Ca2+]cyt through IP3R1, proliferate at a faster rate compared to wt. The ability of XeC to reverse the increase in Cdk5−/− MEF proliferation to a level equivalent to that in wt supports our view that accelerated proliferation in Cdk5−/− MEFs is linked to IP3R-mediated increase in [Ca2+]cyt. This is consistent with previous reports that Cdk5 plays an inhibitory role in the neuronal cell cycle [6, 10, 11]. In addition, reduced level of p27KIP1 in Cdk5−/− MEFs is consistent with the fact that IP3R-mediated Ca2+ oscillations stimulate proliferation through downregulation of p27KIP1 [1, 53]. Proliferation error in Cdk5−/− MEFs correlates with increased weight and size in E16.5 Cdk5−/− embryos, and increased number of Ki67-positive cells in various embryonic tissues, including prefrontal cortex, olfactory epithelium, lung and duodenum. Although we observed the same trend in body weight and size in earlier E13.5 Cdk5−/− embryos, we note that later E18.5 Cdk5−/− embryos were lighter and smaller than their wt counterparts. This may be due to the development of other abnormalities in Cdk5−/− embryos as they exhibit perinatal mortality. It is known that ~ 64% of Cdk5−/− embryos die in utero and newborns are either dead or weak and die within 12 h after birth [54]. Nonetheless, increased body weight and size in E16.5 Cdk5−/− embryos are consistent with reduced levels of the cell-cycle inhibitors, p21CIP1 and p27KIP1, and increased body weight in p27KIP1 knockout mice [57].
In summary, we provide evidence that Cdk5 controls intracellular Ca2+ dynamics through phosphorylation of IP3R1 at Ser421, and Ca2+-mediated cell proliferation as indicated by increased Cdk5−/− MEF proliferation that correlates with increased body weight and size in Cdk5−/− embryos.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Drs. I. Bezprozvanny at the University of Texas Southwestern Medical Center at Dallas for providing pcDNA 3.0-rat IP3R1 and Andrew Braun for providing thapsigargin and advice on our initial Ca2+ analysis.
Author contributions
SN performed the experiments for Figs. 1–5, 7, 8A, 9 and Supplementary Figs. 1, 2, 3 and 4, and wrote a draft of the manuscript. VL performed the experiments for Fig. 8B–E and Supplementary Fig. 5. JL performed the experiments for Fig. 6. KYL and JLR contributed to the analysis and interpretation of data and/or experimental design, critically revised the manuscript for important intellectual content, and wrote the final version of the manuscript.
Funding
This work was supported by a grant from NSERC (RGPIN/06270-2019) to KYL. SN was supported by a graduate studentship from the Alberta Cancer Foundation.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All studies involving mice, which were maintained at the University of Calgary Animal Facility, conformed to regulatory standards and were approved by the University of Calgary Health Sciences Animal Care Committee.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Morgan DO. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- 2.Lew J, Wang JH. Neuronal cdc2-like kinase. Trends Biochem Sci. 1995;20:33–37. doi: 10.1016/S0968-0004(00)88948-3. [DOI] [PubMed] [Google Scholar]
- 3.Lee KY, Qi Z, Yu YP, Wang JH. Neuronal Cdc2-like kinases: neuron-specific forms of Cdk5. Int J Biochem Cell Biol. 1997;29:951–958. doi: 10.1016/S1357-2725(97)00048-4. [DOI] [PubMed] [Google Scholar]
- 4.Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Herrup K. Cdk5 and the non-catalytic arrest of the neuronal cell cycle. Cell Cycle. 2008;7:3487–3490. doi: 10.4161/cc.7.22.7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J, Li H, Herrup K. Cdk5 nuclear localization is p27-dependent in nerve cells: implications for cell cycle suppression and caspase-3 activation. J Biol Chem. 2010;285:14052–14061. doi: 10.1074/jbc.M109.068262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner NC, et al. A synthetic lethal siRNA screen identifying genes mediating sensitivity to a PARP inhibitor. EMBO J. 2008;27:1368–1377. doi: 10.1038/emboj.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, et al. Cdk5 suppresses the neuronal cell cycle by disrupting the E2F1-DP1 complex. J Neurosci. 2010;30:5219–5228. doi: 10.1523/JNEUROSCI.5628-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosales JL, Rattner JB, Lee KY. The primary microcephaly 3 (MCPH3) interacting protein, p35 and its catalytic subunit, Cdk5, are centrosomal proteins. Cell Cycle. 2014;9:618–620. doi: 10.4161/cc.9.3.10597. [DOI] [PubMed] [Google Scholar]
- 10.Cicero S, Herrup K. Cyclin-dependent kinase 5 is essential for neuronal cell cycle arrest and differentiation. J neurosci. 2005;25:9658–9668. doi: 10.1523/JNEUROSCI.1773-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, et al. Nuclear localization of Cdk5 is a key determinant in the postmitotic state of neurons. Proc Natl Acad Sci USA. 2008;105:8772–8777. doi: 10.1073/pnas.0711355105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang PH, et al. Cdk5 directly targets nuclear p21CIP1 and promotes cancer cell growth. Cancer Res. 2016;76:6888–6900. doi: 10.1158/0008-5472.CAN-15-3253. [DOI] [PubMed] [Google Scholar]
- 13.Zhang S, et al. CDK5 regulates paclitaxel sensitivity in ovarian cancer cells by modulating AKT activation, p21Cip1- and p27Kip1-mediated G1 cell cycle arrest and apoptosis. PLoS One. 2015;10:e0131833. doi: 10.1371/journal.pone.0131833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindqvist J, et al. Cyclin-dependent kinase 5 acts as a critical determinant of AKT-dependent proliferation and regulates differential gene expression by the androgen receptor in prostate cancer cells. Mol Biol Cell. 2015;26:1971–1984. doi: 10.1091/mbc.E14-12-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu FN, et al. Cyclin-dependent kinase 5 modulates STAT3 and androgen receptor activation through phosphorylation of Ser(7)(2)(7) on STAT3 in prostate cancer cells. Am J Physiol Endocrinol Metab. 2013;305:E975–986. doi: 10.1152/ajpendo.00615.2012. [DOI] [PubMed] [Google Scholar]
- 16.Lin H, Chen MC, Chiu CY, Song YM, Lin SY. Cdk5 regulates STAT3 activation and cell proliferation in medullary thyroid carcinoma cells. J Biol Chem. 2007;282:2776–2784. doi: 10.1074/jbc.M607234200. [DOI] [PubMed] [Google Scholar]
- 17.Zhuang K, et al. CDK5 functions as a tumor promoter in human colorectal cancer via modulating the ERK5-AP-1 axis. Cell Death Dis. 2016;7:e2415. doi: 10.1038/cddis.2016.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanderheyden V, et al. Regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release by reversible phosphorylation and dephosphorylation. Biochem Biophys Acta. 2009;1793:959–970. doi: 10.1016/j.bbamcr.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinto MC, et al. Calcium signaling and cell proliferation. Cellular signaling. 2015;27:2139–2149. doi: 10.1016/j.cellsig.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Rosales JL, Lee KY. Extraneuronal roles of cyclin-dependent kinase 5. BioEssays. 2006;28:1023–1034. doi: 10.1002/bies.20473. [DOI] [PubMed] [Google Scholar]
- 21.Tomizawa K, et al. Cdk5/p35 regulates neurotransmitter release through phosphorylation and downregulation of P/Q-type voltage-dependent calcium channel activity. J Neurosci. 2002;22:2590–2597. doi: 10.1523/JNEUROSCI.22-07-02590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su SC, et al. Regulation of N-type voltage-gated calcium channels and presynaptic function by cyclin-dependent kinase 5. Neuron. 2012;75:675–687. doi: 10.1016/j.neuron.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jendryke T, et al. TRPV1 function is modulated by Cdk5-mediated phosphorylation: insights into the molecular mechanism of nociception. Sci Rep. 2016;6:22007. doi: 10.1038/srep22007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Du J, Yang Y, Wang Y. Phosphorylation of TRPV1 by cyclin-dependent kinase 5 promotes TRPV1 surface localization, leading to inflammatory thermal hyperalgesia. Exp Neurol. 2015;273:253–262. doi: 10.1016/j.expneurol.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Pareek TK, et al. Cyclin-dependent kinase 5 modulates nociceptive signaling through direct phosphorylation of transient receptor potential vanilloid 1. Proc Natl Acad Sci USA. 2007;104:660–665. doi: 10.1073/pnas.0609916104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rozas P, et al. Targeted overexpression of tumor necrosis factor-alpha increases cyclin-dependent kinase 5 activity and TRPV1-dependent Ca2+ influx in trigeminal neurons. Pain. 2016;157:1346–1362. doi: 10.1097/j.pain.0000000000000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coddou C, et al. Cyclin-dependent kinase 5 modulates the P2X2a receptor channel gating through phosphorylation of C-terminal threonine 372. Pain. 2017;158:2155–2168. doi: 10.1097/j.pain.0000000000001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nair A, Simonetti M, Fabbretti E, Nistri A. The Cdk5 kinase downregulates ATP-gated ionotropic P2X3 receptor function via serine phosphorylation. Cell Mol Neurobiol. 2010;30:505–509. doi: 10.1007/s10571-009-9483-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furusawa K, Asada A, Saito T, Hisanaga S. The effect of Cyclin-dependent kinase 5 on voltage-dependent calcium channels in PC12 cells varies according to channel type and cell differentiation state. J Neurochem. 2014;130:498–506. doi: 10.1111/jnc.12746. [DOI] [PubMed] [Google Scholar]
- 30.Darios F, Muriel MP, Khondiker ME, Brice A, Ruberg M. Neurotoxic calcium transfer from endoplasmic reticulum to mitochondria is regulated by cyclin-dependent kinase 5-dependent phosphorylation of tau. J Neurosci. 2005;25:4159–4168. doi: 10.1523/JNEUROSCI.0060-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi HS, Chung SH. Roscovitine increases intracellular calcium release and capacitative calcium entry in PC12 cells. Neurosci Lett. 2010;469:141–144. doi: 10.1016/j.neulet.2009.11.061. [DOI] [PubMed] [Google Scholar]
- 32.Seo MD, Enomoto M, Ishiyama N, Stathopulos PB, Ikura M. Structural insights into endoplasmic reticulum stored calcium regulation by inositol 1,4,5-trisphosphate and ryanodine receptors. Biochem Biophys Acta. 2015;1853:1980–1991. doi: 10.1016/j.bbamcr.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 33.Patergnani S, et al. Calcium signaling around Mitochondria Associated Membranes (MAMs) Cell Commun Signal. 2011;9:19. doi: 10.1186/1478-811X-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.NavaneethaKrishnan S, Rosales JL, Lee KY. mPTP opening caused by Cdk5 loss is due to increased mitochondrial Ca(2+) uptake. Oncogene. 2020;39:2797–2806. doi: 10.1038/s41388-020-1188-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malathi K, et al. Cdc2/cyclin B1 interacts with and modulates inositol 1,4,5-trisphosphate receptor (type 1) functions. J Immunol. 2005;175:6205–6210. doi: 10.4049/jimmunol.175.9.6205. [DOI] [PubMed] [Google Scholar]
- 36.Miyakawa T, et al. Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. EMBO J. 1999;18:1303–1308. doi: 10.1093/emboj/18.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malathi K, et al. Inositol 1,4,5-trisphosphate receptor (type 1) phosphorylation and modulation by Cdc2. J Cell Biochem. 2003;90:1186–1196. doi: 10.1002/jcb.10720. [DOI] [PubMed] [Google Scholar]
- 38.Bosanac I, Michikawa T, Mikoshiba K, Ikura M. Structural insights into the regulatory mechanism of IP3 receptor. Biochem Biophys Acta. 2004;1742:89–102. doi: 10.1016/j.bbamcr.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 39.Bai GR, Yang LH, Huang XY, Sun FZ. Inositol 1,4,5-trisphosphate receptor type 1 phosphorylation and regulation by extracellular signal-regulated kinase. Biochem Biophys Res Commun. 2006;348:1319–1327. doi: 10.1016/j.bbrc.2006.07.208. [DOI] [PubMed] [Google Scholar]
- 40.Yang LH, Bai GR, Huang XY, Sun FZ. ERK binds, phosphorylates InsP3 type 1 receptor and regulates intracellular calcium dynamics in DT40 cells. Biochem Biophys Res Commun. 2006;349:1339–1344. doi: 10.1016/j.bbrc.2006.08.185. [DOI] [PubMed] [Google Scholar]
- 41.Hempel N, Trebak M. Crosstalk between calcium and reactive oxygen species signaling in cancer. Cell Calcium. 2017;63:70–96. doi: 10.1016/j.ceca.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gorlach A, Bertram K, Hudecova S, Krizanova O. Calcium and ROS: a mutual interplay. Redox Biol. 2015;6:260–271. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.NavaneethaKrishnan S, Rosales JL, Lee KY. ROS-mediated cancer cell killing through dietary phytochemicals. Oxid Med Cell Longev. 2019;2019:9051542. doi: 10.1155/2019/9051542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaufmann R, Mussbach F, Henklein P, Settmacher U. Proteinase-activated receptor 2-mediated calcium signaling in hepatocellular carcinoma cells. J Cancer Res Clin Oncol. 2011;137:965–973. doi: 10.1007/s00432-010-0961-1. [DOI] [PubMed] [Google Scholar]
- 45.Lytton J, Westlin M, Hanley MR. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem. 1991;266:17067–17071. doi: 10.1016/S0021-9258(19)47340-7. [DOI] [PubMed] [Google Scholar]
- 46.Kaczmarek-Hajek K, Lorinczi E, Hausmann R, Nicke A. Molecular and functional properties of P2X receptors–recent progress and persisting challenges. Purinergic Signal. 2012;8:375–417. doi: 10.1007/s11302-012-9314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith JB, Smith L, Higgins BL. Temperature and nucleotide dependence of calcium release by myo-inositol 1,4,5-trisphosphate in cultured vascular smooth muscle cells. J Biol Chem. 1985;260:14413–14416. doi: 10.1016/S0021-9258(17)38581-2. [DOI] [PubMed] [Google Scholar]
- 48.Solanes P, et al. Space exploration by dendritic cells requires maintenance of myosin II activity by IP3 receptor 1. EMBO J. 2015;34:798–810. doi: 10.15252/embj.201489056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takahashi A, Camacho P, Lechleiter JD, Herman B. Measurement of intracellular calcium. Physiol Rev. 1999;79:1089–1125. doi: 10.1152/physrev.1999.79.4.1089. [DOI] [PubMed] [Google Scholar]
- 50.Yan Y, Wei CL, Zhang WR, Cheng HP, Liu J. Cross-talk between calcium and reactive oxygen species signaling. Acta Pharmacol Sin. 2006;27:821–826. doi: 10.1111/j.1745-7254.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 51.Adam-Vizi V, Starkov AA. Calcium and mitochondrial reactive oxygen species generation: how to read the facts. J Alzheimer's Dis. 2010;20(Suppl 2):S413–426. doi: 10.3233/JAD-2010-100465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vasconcelos AR, Dos Santos NB, Scavone C, Munhoz CD. Nrf2/ARE pathway modulation by dietary energy regulation in neurological disorders. Front Pharmacol. 2019;10:33. doi: 10.3389/fphar.2019.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Resende RR, Adhikari A, da Costa JL, Lorençon E, Ladeira MS, Guatimosim S, Kihara AH, Ladeira LO. Influence of spontaneous calcium events on cell-cycle progression in embryonal carcinoma and adult stem cells. Biochimica et Biophysica Acta (BBA) 2010;1803:246–260. doi: 10.1016/j.bbamcr.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 54.Ohshima T, et al. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci USA. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gafni J, et al. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron. 1997;19:723–733. doi: 10.1016/S0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- 56.Albrecht MA, et al. Multiple modes of calcium-induced calcium release in sympathetic neurons I: attenuation of endoplasmic reticulum Ca2+ accumulation at low [Ca2+](i) during weak depolarization. J Gen Physiol. 2001;118:83–100. doi: 10.1085/jgp.118.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qiu J, et al. p27Kip1 constrains proliferation of neural progenitor cells in adult brain under homeostatic and ischemic conditions. Stem Cells. 2009;27:920–927. doi: 10.1002/stem.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Not applicable.