Abstract
Background
Understanding the trend of global antifungal agent consumption could assist with identification of global healthcare policy inadequacies and promote accessibility and availability of antifungal agents.
Methods
Using pharmaceutical sales data from the IQVIA-multinational integrated data analysis system database, we assessed use of systemic antifungal agents in humans in 27 middle- and 38 high-income countries from 2008 through 2018.
Results
Consumption of systemic antifungal agents increased from 0.50 (in 2008) to 0.92 defined daily dose (DDD)/1000 inhabitants/day (in 2018), with a compound annual growth rate of 6.2%. High-income countries remain major consumers of antifungal agents with large variance in quantities consumed, with a gradual decline in consumption in recent years. Consumption in middle-income countries increased. Itraconazole (0.32 DDD/1000 inhabitants/day), terbinafine (0.30 DDD/1000 inhabitants/day), and fluconazole (0.23 DDD/1000 inhabitants/day) were the most commonly used antifungal agents in middle- and high-income countries in 2018. Following incorporation into the World Health Organization Essential Medicines List, itraconazole consumption in middle-income countries surged. Consumption of ketoconazole slowly declined, with 5.04% annual decrease, probably due to labelling changes in 2013 to reflect hepatotoxicity concerns. The use of polyenes (0.004 DDD/1000 inhabitants/day) and echinocandins (0.003 DDD/1000 inhabitants/day) were lowest among all the antifungal drug classes.
Conclusion
Global consumption of triazoles and terbinafine has gradually increased in middle- and high-income countries. Life-saving antifungal agents, including echinocandins and polyenes, are available only parenterally and may be underutilized, mainly in middle-income countries. Future research on country-specific epidemiology is warranted to guide health policy coordination to ensure equitable access to appropriate use of antifungal agents.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40265-022-01751-x.
Key Points
| Using global pharmaceutical sales data, the study compared consumption trends of antifungal agents in middle- and high-income countries. |
| Consumption trends of antifungal agents in recent years reflect changes in antifungal resistance epidemiology, prescribing practices, and global health policies. |
Introduction
Fungal infections are one of the most underrated global health concerns, despite an annual death toll of 1.7 million, which is three-fold the deaths from malaria and comparable to that of tuberculosis [1]. Judicious use of antifungal agents could potentially reduce nearly 80% of deaths from fungal infections [2]. Immunocompromised individuals such as those with human immunodeficiency virus (HIV) disease, cancer, solid organ or hematopoietic stem cell transplantation, long-term corticosteroid use, and primary immunodeficiency are at a higher risk of invasive fungal infections. Hence, ensuring equitable access to life-saving antifungal agents across countries of different income levels is essential. Global healthcare policies in this area are currently limited.
The World Health Organization (WHO) currently does not have any global fungal infection surveillance program. Notably, the Global Action Fund for Fungal Infections (GAFFI), a non-profit organization, is actively investigating the burden of fungal infections in more than 80% of the world’s population, availability of antifungal agents, and preventive measures against the development of resistant fungal infections [2]. In 2015, GAFFI launched an aspiring global program to improve the diagnosis of fungal infections and access to antifungal agents for 95% of patients with serious fungal infections by 2025 [2]. Recently, GAFFI assessed availability of antifungal drug formulations in 159 countries with populations > 1 million, and reported wide variability in the access, availability, and costs of life-saving antifungal agents [3]. However, only selected antifungal agents used for life-threatening fungal infections (namely, fluconazole, itraconazole, amphotericin B [AmB], and flucytosine) were assessed [3]. Global trends regarding the use of more recent and more costly antifungal agents, particularly extended-spectrum azoles (voriconazole, posaconazole, and isavuconazole), newer antifungal agents such as echinocandins, and antifungal agents used for superficial fungal infections such as terbinafine, are unclear.
Currently, AmB, clotrimazole, fluconazole, flucytosine, griseofulvin, itraconazole, nystatin, and voriconazole are listed in the WHO Essential Medicines List (EML) [4]. Since cost and availability of antifungal agents vary greatly, there is a need to understand the global consumption of antifungal agents to safeguard public health and design global health policies, especially in middle-income countries where access may be limited. In this paper, we aimed to assess the global consumption trends of antifungal agents in 65 countries over an 11-year period, comparing middle- versus high-income countries, which might shed light on the design and implementation of global health policies relating to the use of antifungal agents.
Methods
Data Source
We assessed the global consumption trend of systemic antifungal agents using the IQVIA-multinational integrated data analysis system (MIDAS). Data on topical formulations were not available and not included in the analysis. Description of the amphotericin B formulation was not available and systemic amphotericin B includes all formulations. The database includes annual drug sales data of oral and parenteral antifungal formulations from wholesalers in 65 countries (Supplementary Table 1, see electronic supplementary material [ESM]), of which 27 were middle-income and 38 were high-income countries. This includes the generic name, brand name, pharmaceutical formulation, and sales volume of antifungal agents in retail and hospital pharmacy sectors. Individual patient-level data such as demographics, diagnosis, indication, and concomitant medications, and data from low-income countries are not available. Previous studies have used IQVIA-MIDAS successfully to assess the global trend of medicines, such as antibiotics [5, 6] and psychotropic agents [7]. Ethics approval was not required.
Data Analysis
Sales data of antifungal agents between January 2008 and December 2018 were obtained from the IQVIA-MIDAS database. Countries were categorized as middle- or high-income country based on the World Bank 2018 income classification [8]. Population estimates were obtained from the United Nations world population prospects (2019 report) [9]. Consumption of antifungal agents was measured using defined daily dose (DDD) per 1000 inhabitants per day (as defined by the WHO) [10] to allow comparison of antifungal agent consumption across time and between countries. Similar to Van Boeckel et al. [11], consumption trends were characterized by calculating the compound annual growth rate, defined as . The global consumption trend of antifungal agents was assessed by country, drug, and pharmacologic drug class. Consumption trend was assessed using Mann-Kendall test (Supplementary Table 2, see ESM). Statistical analyses were performed using R 4.0.1 (RStudio, Boston, MA, USA), and independent crosscheck of analysis was conducted by two co-authors (S.P and V.K.C.Y) for quality assurance.
Results
Overall Global Consumption of Antifungal Agents in 65 Countries (2008–2018)
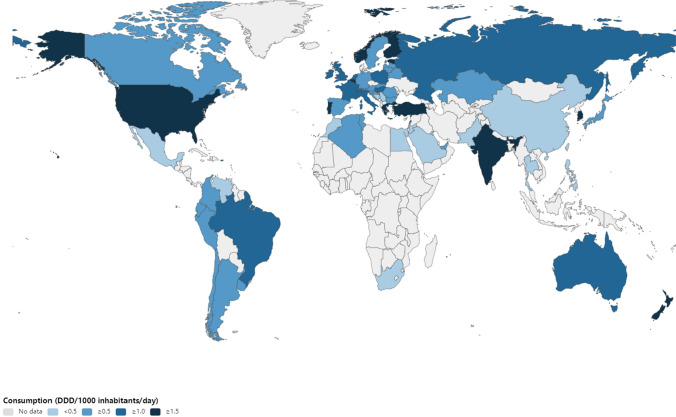
The global consumption of antifungal agents increased from 0.50 in 2008 to 0.92 DDD/1000 inhabitants/day in 2018, with CAGR of 6.2% (Table 1). The consumption of antifungal agents in 2018 ranged from 0.03 (Philippines) to 3.37 (Belgium) DDD/1000 inhabitants/day (Figs. 1 and 2). There was a slow downward trend in the consumption of agents in the high-income countries, however the consumption of antifungal agents was increasing in middle-income countries.
Table 1.
Global consumption of antifungal agents from 2008 to 2018 in 65 countries
| Drug name | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | CAGRa 2008–2018 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Echinocandins | ||||||||||||
| Anidulafungin | 0.000189 | 0.000227 | 0.000228 | 0.000233 | 0.000249 | 0.000311 | 0.000378 | 0.000468 | 0.000548 | 0.000595 | 0.000612 | 12.45 |
| Caspofungin | 0.000957 | 0.000912 | 0.000896 | 0.000874 | 0.000908 | 0.000945 | 0.000981 | 0.000991 | 0.001014 | 0.001033 | 0.001230 | 2.54 |
| Micafungin | 0.000936 | 0.001167 | 0.001364 | 0.001520 | 0.001614 | 0.001620 | 0.001636 | 0.001677 | 0.001743 | 0.001741 | 0.001718 | 6.26 |
| Imidazoles | ||||||||||||
| Ketoconazole | 0.061129 | 0.060694 | 0.062981 | 0.061724 | 0.063225 | 0.060383 | 0.047105 | 0.044064 | 0.040954 | 0.039156 | 0.036422 | − 5.04 |
| Miconazole | 0.000078 | 0.000065 | 0.0000595 | 0.000053 | 0.000051 | 0.000043 | 0.000040 | 0.0000373 | 0.000028 | 0.000030 | 0.000029 | − 9.34 |
| Polyeneb | ||||||||||||
| Amphotericin B | 0.003285 | 0.003399 | 0.003613 | 0.003663 | 0.003741 | 0.003804 | 0.003696 | 0.003682 | 0.003659 | 0.003754 | 0.00394 | 1.82 |
| Triazoles | ||||||||||||
| Fluconazole | 0.144044 | 0.170108 | 0.181778 | 0.197279 | 0.182574 | 0.186588 | 0.194213 | 0.203014 | 0.208883 | 0.220232 | 0.229617 | 4.77 |
| Isavuconazole | 0.000498 | 0.002039 | 0.003811 | 0.005622 | 124.27 | |||||||
| Itraconazole | 0.066135 | 0.065450 | 0.066679 | 0.070892 | 0.076629 | 0.077068 | 0.081803 | 0.096189 | 0.141494 | 0.237012 | 0.324027 | 17.22 |
| Posaconazole | 0.003072 | 0.003226 | 0.003449 | 0.004237 | 0.004768 | 0.004990 | 0.005433 | 0.004434 | 0.003986 | 0.004010 | 0.004503 | 3.90 |
| Voriconazole | 0.003507 | 0.003700 | 0.004022 | 0.004631 | 0.005160 | 0.005461 | 0.005522 | 0.005862 | 0.006260 | 0.006711 | 0.007383 | 7.73 |
| Others | ||||||||||||
| Terbinafine | 0.217883 | 0.221046 | 0.226815 | 0.232156 | 0.233199 | 0.237763 | 0.254827 | 0.271679 | 0.289722 | 0.298927 | 0.302938 | 3.35 |
| Flucytosine | 0.000053 | 0.000082 | 0.000188 | 0.000208 | 0.000151 | 0.000130 | 0.000133 | 0.000132 | 0.000113 | 0.000110 | 0.000119 | 8.37 |
Consumption shown as defined daily dose per 1000 inhabitants per day
CAGR compound annual growth rate
aData on isavuconazole was not available from 2008 to 2014 since it was approved later
bAmphotericin B formulations include both conventional and lipophilic formulations of amphotericin B
Fig. 1.
Global consumption of antifungal agents in 2018. DDD defined daily dose
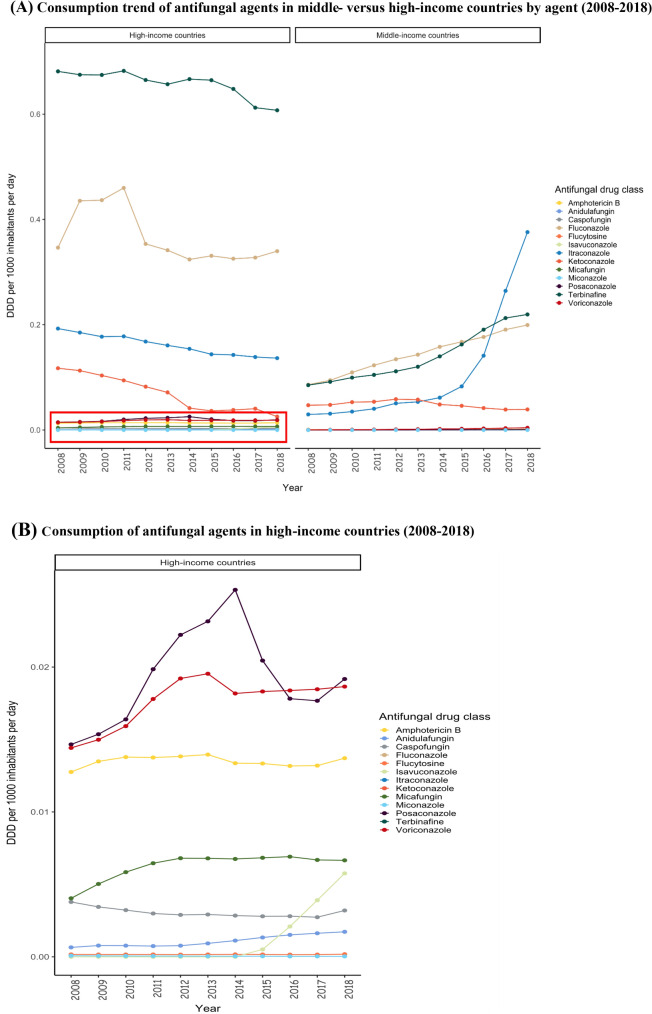
Fig. 2.
A Consumption trend of antifungal agents in middle- versus high-income countries by agent (2008–2018). B Consumption of antifungal agents in high-income countries (2008–2018). DDD defined daily dose
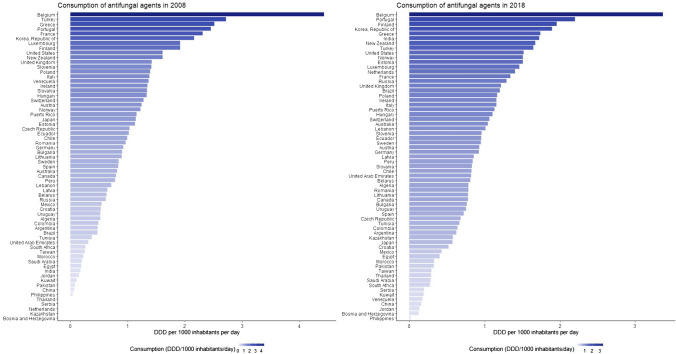
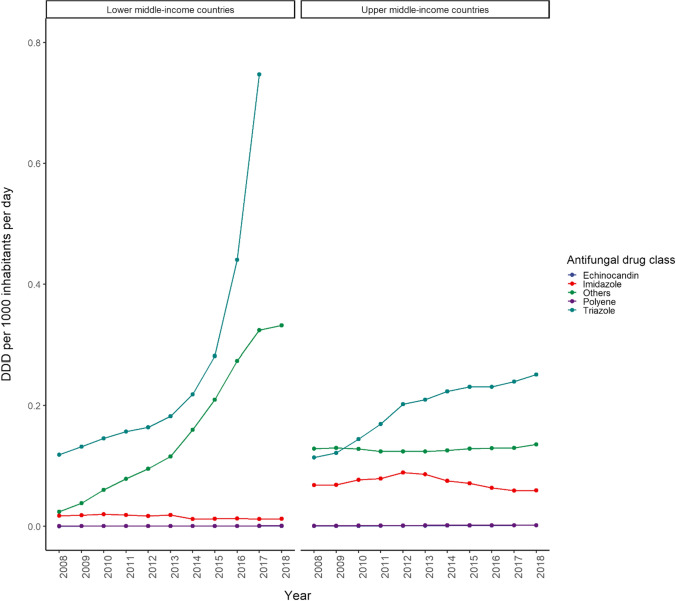
Despite the slow downward trend in the consumption of antifungal agents, high-income countries were major drivers of global antifungal consumption (Fig. 2). The consumption of antifungal agents was highest for high-income countries (1.18 DDD/1000 inhabitants/day) with a slow declining consumption trend in recent years; Belgium, Portugal, Finland, South Korea, and France were the top five countries in 2018 (Fig. 3). The consumption of antifungal agents in middle-income countries (0.84 DDD/1000 inhabitants/day) was lower in comparison, with Philippines, Bosnia and Herzegovina, Jordan, China, and Venezuela having the lowest antifungal agent consumption in 2018. Belgium was noted to be the top consumer of antifungal agents during the study period. We observed an increasing trend in the consumption of antifungal agents in middle-income countries, which seems to be driven mainly by lower middle-income countries (Fig. 4).
Fig. 3.
Global consumption of antifungal agents in 2008 and 2018 in 65 countries. DDD defined daily dose
Fig. 4.
Consumption of antifungal agents by antifungal drug class in lower middle- and upper middle-income countries (2008–2018). Others includes flucytosine and terbinafine. DDD defined daily dose
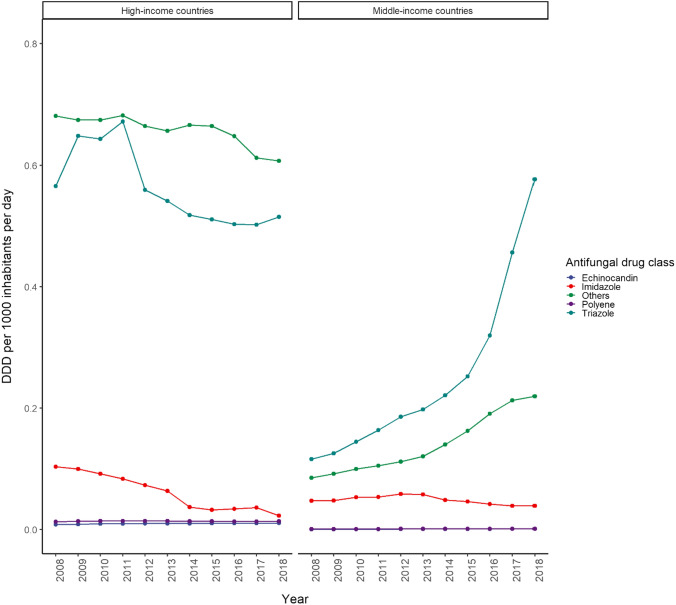
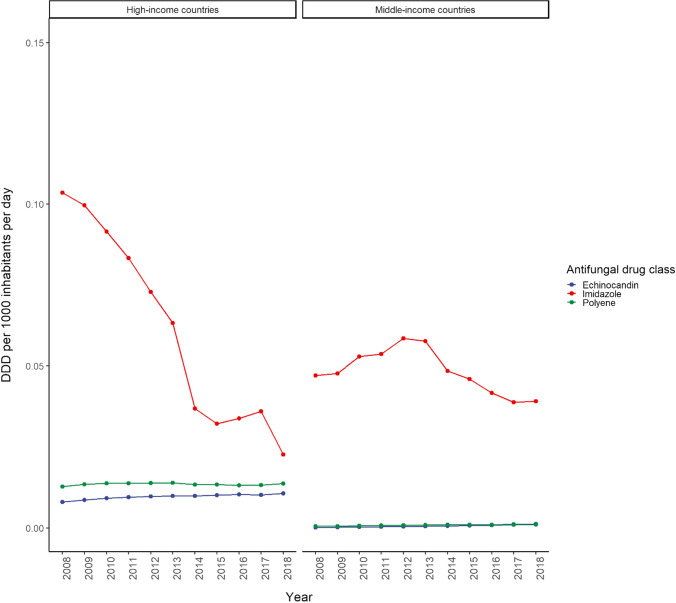
In 2018, the consumption of triazole was similar in middle-income (0.58 DDD/1000 inhabitants/day) and high-income countries (0.52 DDD/1000 inhabitants/day), with a sharp increase in triazole since 2014 in middle-income countries, while a slow decline was observed in high-income countries (Fig. 5). The consumption of oral triazoles increased in middle-income countries, however the consumption of parenteral triazoles was similar in middle- and high-income countries (Supplementary Figs 1 and 2, see ESM). In general, terbinafine was the most commonly used antifungal agent followed by fluconazole and itraconazole in high-income countries; whereas the consumption of itraconazole exceeded terbinafine and fluconazole in middle-income countries in 2016 (Fig. 6). Since approval in 2015, we observed a 10-fold increase in the consumption of isavuconazole in high-income countries, yet consumption was nil in middle-income countries (Table 1). While the consumption of polyenes and echinocandins remained steady during the study period in all countries, the consumption of imidazoles consistently declined throughout the same period in high-income countries, with a similar observed trend in middle-income countries since 2013 (Fig. 7). Although polyenes and echinocandins had the lowest consumption, in high-income countries (0.01 DDD/1000 inhabitants/day) the consumption of these agents was 10 times higher than in middle-income countries (0.001 DDD/1000 inhabitants/day).
Fig. 5.
Consumption trend of antifungal agents in middle- versus high-income countries by antifungal drug class (2008–2018). Others includes flucytosine and terbinafine. DDD defined daily dose
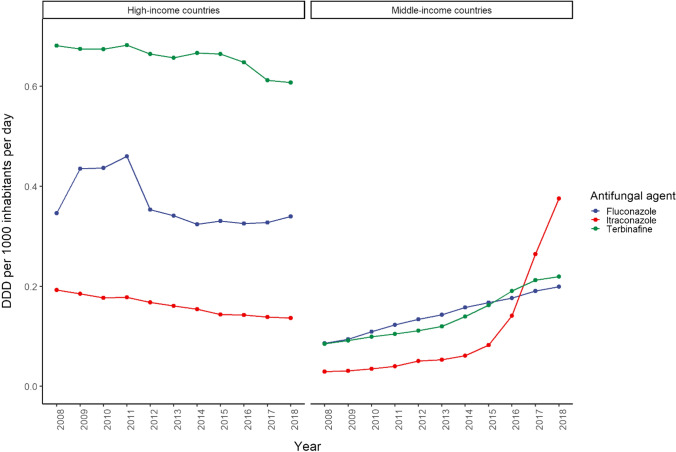
Fig. 6.
Consumption trend of fluconazole, itraconazole, and terbinafine in middle- versus high-income countries (2008–2018). Fluconazole, itraconazole, and terbinafine are the most commonly used antifungal agents. DDD defined daily dose
Fig. 7.
Consumption trend of echinocandins, imidazole, and polyenes in middle- versus high-income countries (2008–2018). The consumption of echinocandins, imidazole, and polyene were the lowest among antifungal drug classes. DDD defined daily dose
Discussion
The global consumption of antifungal agents could be influenced by several factors, including access and availability of antifungal agents, acquisition cost of antifungal agents, the incidence and type of fungal infections, adherence to clinical practice guidelines, provision of organ transplantation services, local antifungal prescribing practices, potential toxicity, and antifungal drug resistance.
The ongoing antifungal agent surveillance program in 28 European countries from 2009 to 2018 reported no change in antifungal agent consumption, with a population-weighted mean consumption of 20.1 DDD/1000 persons/day [12]. Conversely, our study findings revealed that global consumption of antifungal agents increased by 6.2% each year over the last decade. Over the study period, the consumption of antifungal agents increased in volume, which is closely linked with an increase in the global burden of fungal infections [13]. In middle-income countries, the lack of healthcare resources, high burden of immunocompromised individuals, and lack of appropriate infection preventive measures may have resulted in higher infection rates [14]. Other contributing factors to an increased consumption of antifungal agents might be the growing population of immunocompromised individuals, recent reduction in the price of azoles, and the use of broad-spectrum antifungal agents as a prophylactic and pre-emptive strategy for fungal infections.
Itraconazole, terbinafine, and fluconazole were the most commonly used antifungal agents, which is congruent to results reported from 28 European countries [12]. This is anticipated given the high prevalence of superficial infections, wide availability, and low retail cost of these agents.
In high-income countries, terbinafine was the most commonly used antifungal agent, and the second most common in middle-income countries. This is in line with the high prevalence of superficial fungal infections and reflects the important role of terbinafine in their treatment. Superficial nail and skin infections are the most common type of fungal infections [13] with a prevalence higher than other skin diseases affecting more than one-fourth of the world’s population, and is currently listed as one of 15 prevalent global medical problems [15, 16]. Approximately one billion cases of superficial fungal infections and 134 million cases of mucosal infections (oral candidiasis, oesophageal candidiasis, and vulvovaginal candidiasis) were documented in 2017 [15]. Although terbinafine has a minor role in the treatment of invasive fungal infections, it is the drug of choice for dermatophyte infections and cutaneous fungal infections, resulting in high consumption compared with other antifungal agents.
While the consumption of fluconazole is higher than itraconazole in high-income countries, the consumption of itraconazole surpassed fluconazole in middle-income countries from 2016. This consumption pattern may reflect changes in antifungal resistance, prescribing practices, and global health policies in recent years. Fluconazole is licensed worldwide [3], used mainly for the treatment of fungal infections such as cryptococcal meningitis and candidiasis, and was one of the most widely used antifungal agents. Fluconazole and itraconazole are the agents of choice for the treatment of vulvovaginal candidiasis, which affects nearly 75% of women at least once in their lifetime, contributing to a substantial proportion of their sales [17]. However, due to increasing resistance, fluconazole is no longer the mainstay treatment for invasive candidemia, and is recommended only as an alternative to echinocandins for non-neutropenic clinically stable patients with no prior azole exposure [18, 19]. Fluconazole has no activity against the Aspergillus species and is inferior to itraconazole and other extended-spectrum azoles for the treatment of blastomycosis, histoplasmosis, and paracoccidioidomycosis. Global consumption of itraconazole was increasing before its introduction into the EML, mainly in middle-income countries. Itraconazole is an antifungal agent of choice against aspergillosis, histoplasmosis, blastomycosis, sporotrichosis, and eosinophilic folliculitis in patients with AIDS, and was added to the EML in 2017 for selected indications [4]. Yet, it is still not licensed in 72 countries and is unavailable to 78 million people (1.07% of the world population) [3].
Other azole antifungal agents such as voriconazole, isavuconazole, and posaconazole with extended spectrum may not be licensed or available worldwide [3]. Hence, their uptake is much lower than other antifungal agents. While these azoles (e.g. voriconazole) are used for aspergillosis as first-line therapy, our results revealed potential underutilization of these medications in middle-income countries. This could be due to the unaffordability of these antifungal agents, which are generally more expensive. However, the patents for some of these antifungal agents have expired in recent years (e.g. voriconazole) and are likely to result in the availability of more economical generic products. Ensuring affordability and access to these medications in low- and middle-income countries is important to support optimal treatment of fungal infections.
Due to increasing resistance to azoles ranging from 3% to 18% and a shift in candidemia towards non-albicans species [20], the echinocandins (Approved: caspofungin, 2001; micafungin, 2005; anidulafungin, 2006) have become the preferred initial therapy in febrile neutropenic patients and as empiric antifungal therapy in suspected and confirmed invasive candidiasis, with the exception of the central nervous system, eye, and urinary tract infections, since 2016 [18, 19]. However, the consumption of echinocandins was lowest among all antifungal agents. This is possibly given that, except when compared with novel AmB formulations, they are considerably more expensive than other antifungals, including the novel triazoles; added to that, they are not listed in the EML. Although the patent for caspofungin expired in March 2017, it may only be accessible to patients who can afford it. Hence, availability and affordability could potentially limit the use of these life-saving antifungal agents, especially in middle-income countries, where the cost of medical care is usually paid out of pocket. As such, ensuring equitable access to these newer agents in low- and middle-income countries is essential. However, increased exposure has been linked to the development of resistance towards echinocandins [21, 22], and overuse of antifungal agents has also been reported. Therefore, caution should be exercised to avoid overuse and subsequent antifungal resistance while promoting equitable access [23].
Despite the successful WHO-EML inclusion of AmB and flucytosine for the treatment of cryptococcal meningitis, histoplasmosis, and aspergillosis, there were no observable changes in the consumption of these two antifungal agents from 2008 to 2018. This may be attributed to the high costs of the liposomal AmB formulation, relatively low prevalence of severe fungal infections requiring AmB treatment, as well as associated serious adverse effects, including nephrotoxicity [19, 24]. Conventional AmB, which has higher toxicity compared with liposomal AmB, is primarily used in developing countries [24]. Efforts are being carried out to improve access to safer liposomal AmB in low- and middle-income countries in recent years [25]. While the consumption of AmB formulations in the current study was slightly higher than flucytosine in high-income countries, the flucytosine plus AmB combination remains an effective treatment, particularly against infections such as cryptococcal meningitis, where it is the therapy of choice. This is important as nearly half of patients with AIDS are from low- and middle-income countries, and despite the expansion of antiretroviral treatment, die within 10 days of contracting cryptococcal meningitis [26]. Hence, ensuring affordability of and access to these life-saving antifungal agents in cases of severe fungal infections is essential. AmB is unavailable in nearly one in every four countries, and up to 40% of the world’s population have no access to flucytosine, with unavailability in nearly 80% of countries [3]. Additionally, polyenes and echinocandins are parenteral formulations, which may also limit consumption despite the patent expiry.
In the current study, we observed an upward trend in the consumption of all antifungal agents from 2008 to 2018, except imidazoles (ketoconazole and miconazole) which show declining consumption since 2013. This is expected following the announcement in 2013 by the US Food and Drug Administration (FDA) that oral ketoconazole should no longer be used as first-line treatment of fungal infections due to risk of hepatotoxicity, adrenal insufficiency, and potential for harmful drug–drug interactions [27]. Along with labelling changes by the FDA, the use of ketoconazole was suspended throughout the European Union in 2013 [28]. Oral formulations of ketoconazole are now indicated only for endemic mycoses intolerant to other antifungal agents. The FDA warning was further reinforced in 2016, following a death due to liver injury [29], and the FDA no longer recommends using ketoconazole for the treatment of nail and skin fungal infections.
This study provides an important overview of antifungal agents used in 27 middle- and 38 high-income countries over a decade. However, there are limitations with our approach. Due to the nature of pharmaceutical sales data, relevant patient-level data is unavailable. This, together with differences between countries in access to resources, infection control practices, the burden of immunocompromised individuals, and susceptibility towards antifungal agents, will result in wide variations of antifungal prescribing practices that may undermine our interpretations of observed consumption patterns. Large variability in consumption of systemic antifungal agents in community settings was noted within EU/EEA countries, ranging from 0.38 DDD/1000 inhabitants/day to 3.0 DDD/1000 inhabitants/day (Belgium), varying by a factor of 8 [12]. Due to lack of patient-level data, we are unable to link antifungal consumption with clinical data. Further studies investigating antifungal stewardship and the appropriateness of antifungal prescribing globally are warranted. No stratification by indication, whether prophylactic, empiric, pre-emptive or targeted, or based on settings such as adult versus pediatric, surgical versus intensive care, and transplantation versus cancer care, was considered in this study. Since details about the dosage form of antifungal agents are not available, adverse drug events and the cost of antifungal agent formulations, which will influence consumption trends, especially the type of amphotericin B formulation, were not assessed.
Currently, there are limited resources from the WHO for antifungal agent surveillance at an individual country level, and there are no actively funded programs investigating the burden of fungal diseases or the use of antifungal agents. Except for fluconazole, most antifungal agents are not available worldwide. Although efforts have been made by GAFFI to include itraconazole and other antifungal agents into the EML by the WHO, these agents are not listed in the national EMLs in nearly one-third of countries with low resources [30]. Efforts are needed to implement the inclusion of antifungal agents into the national EMLs of countries.
Conclusion
The global consumption of antifungal agents is on the rise, especially in middle-income countries. However, there was wide variability in consumption of different antifungal agents in countries with different income levels from 2008 to 2018. The consumption patterns of triazoles and ketoconazole reflect changes in antifungal resistance epidemiology, prescribing practices, and global health policies in recent years. More importantly, certain life-saving antifungal agents indicated in severe fungal infections such as echinocandins and polyenes may be underutilized, especially in middle-income countries. Conversely, it does not appear that their use is rapidly expanding in high-income countries. High-income countries have variances in antifungal consumption that may be further investigated. Effort and coordination in health policies at global and national levels are needed to ensure equitable access to these medications, and an antifungal stewardship program is needed to optimize the use of antifungal agents.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgement
We thank L.Y. Lam for proofreading this manuscript.
Declarations
Funding
This study was supported through internal seed funding from the Li Ka Shing Faculty of Medicine, The University of Hong Kong. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
EWC has received research grants from the Research Grants Council (RGC, Hong Kong), Narcotics Division of the Security Bureau of the Government of the Hong Kong SAR, Research Fund Secretariat of the Food and Health Bureau, National Natural Science Fund of China, Wellcome Trust, Bayer, Bristol-Myers Squibb, Pfizer, Janssen, Amgen, and Takeda, outside the submitted work. ICKW has received research grants from Research Grants Council (RGC, Hong Kong), Innovative Medicines Initiative (IMI), Shire, Janssen-Cilag, Eli-Lily, Pfizer, Bayer, and grants from European Union FP7 program, outside the submitted work. DCMK has sat on advisory boards for Becton Dickinson Pty Ltd and Merck Sharp & Dohme (MSD), and received financial support from MSD and F2G, outside the submitted work. MS has received grants from Gilead Sciences, Merck, F2G, and Pfizer unrelated to the submitted work. BJC has received funding from Sanofi Pasteur and Roche, outside the submitted work. The other author(s) declare no conflict of interest.
Author contributions
EWC, SP, and VKCY conceptualized the study. EWC provided resources, acquired the data and supervised this study. SP and VKCY conducted analysis and formulated the original draft. All authors reviewed the manuscript critically and commented on manuscript drafts.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Ethics approval
Not applicable.
Informed consent
Not applicable.
Data availability
Not applicable.
Code availability
Not applicable.
Footnotes
Swathi Pathadka and Vincent K. C. Yan contributed equally to the manuscript.
Contributor Information
Swathi Pathadka, Email: swathi.pathadka@gmail.com.
Esther W. Chan, Email: ewchan@hku.hk
References
- 1.Stop neglecting fungi Nat Microbiol. 2017;2:17120. doi: 10.1038/nmicrobiol.2017.120. [DOI] [PubMed] [Google Scholar]
- 2.Global Action Fund for Fungal Infections. 95–95 by 2025. Improving outcomes for patients with fungal infections across the world: a roadmap for the next decade. 2020. https://www.gaffi.org/. Accessed 19 Feb 2020.
- 3.Kneale M, Bartholomew JS, Davies E, Denning DW. Global access to antifungal therapy and its variable cost. J Antimicrob Chemother. 2016;71(12):3599–3606. doi: 10.1093/jac/dkw325. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. WHO model list of essential medicine, 21st list. 2019. https://www.who.int/medicines/publications/essentialmedicines/en/. Accessed 17 Aug 2020.
- 5.Hsia Y, Sharland M, Jackson C, Wong ICK, Magrini N, Bielicki JA. Consumption of oral antibiotic formulations for young children according to the WHO Access, Watch, Reserve (AWaRe) antibiotic groups: an analysis of sales data from 70 middle-income and high-income countries. Lancet Infect Dis. 2019;19(1):67–75. doi: 10.1016/S1473-3099(18)30547-4. [DOI] [PubMed] [Google Scholar]
- 6.Jackson C, Hsia Y, Bielicki JA, et al. Estimating global trends in total and childhood antibiotic consumption, 2011–2015. BMJ Glob Health. 2019;4:1. doi: 10.1136/bmjgh-2018-001241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong ICK, Murray ML, Camilleri-Novak D, Stephens P. Increased prescribing trends of paediatric psychotropic medications. Arch Dis Child. 2004;89(12):1131. doi: 10.1136/adc.2004.050468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Bank. World Bank Country and Lending Groups. 2020. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed 7 Aug 2020.
- 9.United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2019. 2020. https://population.un.org/wpp/. Accessed 14 Jul 2020.
- 10.WHO Collaborating Centre for Drug Statistics Methodology. Use of ATC/DDD: DDD indicators. 2020. https://www.whocc.no/use_of_atc_ddd/#indica. Accessed 9 Aug 2020.
- 11.Van Boeckel TP, Gandra S, Ashok A, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis. 2014;14(8):742–750. doi: 10.1016/S1473-3099(14)70780-7. [DOI] [PubMed] [Google Scholar]
- 12.European Centre for Disease Prevention and Control. Antimicrobial consumption—Annual Epidemiological Report for 2018. 2020. https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-consumption-europe-2018. Accessed 6 Mar 2020.
- 13.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med. 2012;4(165):165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 14.Kaur H, Chakrabarti A. Strategies to reduce mortality in adult and neonatal candidemia in developing countries. J Fungi (Basel) 2017;3:3. doi: 10.3390/jof3030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bongomin F, Gago S, Oladele RO, Denning DW. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi (Basel) 2017;3:4. doi: 10.3390/jof3040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392(10159):1789–858. [DOI] [PMC free article] [PubMed]
- 17.Denning DW, Kneale M, Sobel JD, Rautemaa-Richardson R. Global burden of recurrent vulvovaginal candidiasis: a systematic review. Lancet Infect Dis. 2018;18(11):e339–e347. doi: 10.1016/S1473-3099(18)30103-8. [DOI] [PubMed] [Google Scholar]
- 18.Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis. Nat Rev Dis Primers. 2018;4:18026. doi: 10.1038/nrdp.2018.26. [DOI] [PubMed] [Google Scholar]
- 19.Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62(4):e1–50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamoth F, Lockhart SR, Berkow EL, Calandra T. Changes in the epidemiological landscape of invasive candidiasis. J Antimicrob Chemother. 2018;73(1):i4–i13. doi: 10.1093/jac/dkx444. [DOI] [PubMed] [Google Scholar]
- 21.Perlin DS. Echinocandin resistance in candida. Clin Infect Dis. 2015;61(Suppl 6):S612–S617. doi: 10.1093/cid/civ791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farmakiotis D, Kontoyiannis DP. Epidemiology of antifungal resistance in human pathogenic yeasts: current viewpoint and practical recommendations for management. Int J Antimicrob Agents. 2017;50(3):318–324. doi: 10.1016/j.ijantimicag.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 23.Munoz P, Bouza E, Group Cs. The current treatment landscape: the need for antifungal stewardship programmes. J Antimicrob Chemother 2016;71(suppl 2):ii5–ii12. [DOI] [PubMed]
- 24.Cavassin FB, Baú-Carneiro JL, Vilas-Boas RR, Queiroz-Telles F. Sixty years of amphotericin B: an overview of the main antifungal agent used to treat invasive fungal infections. Infect Dis Therapy. 2021;10(1):115–147. doi: 10.1007/s40121-020-00382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Global Action Fund for Fungal Infections. Gilead reduces price of AmBisome (liposomal amphotericin B) for cryptococcal meningitis in HIV/AIDS. 2022. https://gaffi.org/gilead-reduces-price-of-ambisome-liposomal-amphotericin-b-for-cryptococcal-meningitis-in-hiv-aids/. Accessed 1 Jul 2022.
- 26.Loyse A, Thangaraj H, Easterbrook P, et al. Cryptococcal meningitis: improving access to essential antifungal medicines in resource-poor countries. Lancet Infect Dis. 2013;13(7):629–637. doi: 10.1016/S1473-3099(13)70078-1. [DOI] [PubMed] [Google Scholar]
- 27.U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA limits usage of Nizoral (ketoconazole) oral tablets due to potentially fatal liver injury and risk of drug interactions and adrenal gland problems. 2020. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-limits-usage-nizoral-ketoconazole-oral-tablets-due-potentially. Accessed 12 Mar 2020.
- 28.European Medicines Agency. Ketoconazole-containing medicines. 2020. https://www.ema.europa.eu/en/medicines/human/referrals/ketoconazole-containing-medicines. Accessed 20 Apr 2020.
- 29.U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA warns that prescribing of Nizoral (ketoconazole) oral tablets for unapproved uses including skin and nail infections continues; linked to patient death. 2020. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-warns-prescribing-nizoral-ketoconazole-oral-tablets-unapproved. Accessed 27 Mar 2020.
- 30.Kirby J, Ojha RP, Johnson KM, Bittner EC, Caniza MA. Challenges in managing infections among pediatric cancer patients: Suboptimal national essential medicines lists for low and middle income countries. Pediatr Blood Cancer. 2015;62(2):204–207. doi: 10.1002/pbc.25273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.