Abstract
Background
The standard treatment of rectal carcinoma is surgical resection according to the total mesorectal excision principle, either by open, laparoscopic, robot-assisted or transanal technique. No clear consensus exists regarding the length of the learning curve for the minimal invasive techniques. This systematic review aims to provide an overview of the current literature regarding the learning curve of minimal invasive TME.
Methods
A systematic literature search was performed. PubMed, Embase and Cochrane Library were searched for studies with the primary or secondary aim to assess the learning curve of either laparoscopic, robot-assisted or transanal TME for rectal cancer. The primary outcome was length of the learning curve per minimal invasive technique. Descriptive statistics were used to present results and the MINORS tool was used to assess risk of bias.
Results
45 studies, with 7562 patients, were included in this systematic review. Length of the learning curve based on intraoperative complications, postoperative complications, pathological outcomes, or a composite endpoint using a risk-adjusted CUSUM analysis was 50 procedures for the laparoscopic technique, 32–75 procedures for the robot-assisted technique and 36–54 procedures for the transanal technique. Due to the low quality of studies and a high level of heterogeneity a meta-analysis could not be performed. Heterogeneity was caused by patient-related factors, surgeon-related factors and differences in statistical methods.
Conclusion
Current high-quality literature regarding length of the learning curve of minimal invasive TME techniques is scarce. Available literature suggests equal lengths of the learning curves of laparoscopic, robot-assisted and transanal TME. Well-designed studies, using adequate statistical methods are required to properly assess the learning curve, while taking into account patient-related and surgeon-related factors.
Keywords: Total mesorectal excision, Laparoscopy, Robot-assisted, Transanal, Learning curve
The cornerstone of therapeutic management of rectal cancer is surgical resection by total mesorectal excision (TME). This can be performed using several surgical approaches: open, laparoscopic (L-TME), robot-assisted (R-TME) and transanal TME (TaTME) [1–4]. Whereas the first TME was performed using open surgery, minimal invasive approaches are increasingly used since the introduction of laparoscopic rectal resections in the mid 90’s. The R-TME and TaTME technique were introduced in the beginning of the 00’s and 10’s respectively, in order to overcome technical limitations of the L-TME procedure.
With the introduction and implementation of a new surgical approach, surgeons need to climb a learning curve. This is the amount of procedures required to achieve an adequate surgical performance, regarding safety, efficacy and efficiency [5]. The ideal minimal invasive procedure has a short learning curve, and is therefore easy to master. In addition, the period in which the surgeon ‘climbs’ the learning curve should not result in additional morbidity, worsened oncological outcomes or mortality for the patient [6, 7].
It is suggested that L-TME and TaTME have a relatively long learning curve of around 50–90 procedures per surgeon [8–12], while R-TME is suggested to have a shorter learning curve [13–15]. Despite the number of papers reporting on learning curves of these approaches, the quality of evidence is limited. Patient populations are heterogeneous by including both benign and malignant diseases. Experience with previous techniques is mostly not taken into account, and some studies do not make a clear distinction between colonic and rectal resections. Additionally, multiple designs and statistical methods are used to assess the learning curve. Finally, although systematic reviews are available, some are outdated, or not restricted to rectal cancer surgery, while others do not evaluate the learning curve of all three minimal invasive techniques [16–20].
The aim of this systematic review is two-fold: First, we aim to create an overview of the current available literature regarding the learning curve of L-TME, R-TME and TaTME for patients with rectal carcinoma. Second, we aim to explore the impact of the learning curve on clinical outcomes in L-TME, R-TME and TaTME.
Materials and methods
This systematic review was conducted and reported according to the PRISMA 2020 statement [21]. Approval of the Institutional Review Board (IRB) was deemed unnecessary, due to the nature of the study. Inclusion and exclusion criteria, as well as search strategies, the used critical appraisal tool, and outcomes of interest were prespecified. We did not register a review protocol in advance.
Eligibility criteria
In order to create an overview of studies regarding the learning curve of L-TME, R-TME, and TaTME, studies were deemed eligible if: (1) the studies included patients with primary rectal cancer, or patients with colorectal cancer in which rectal cancer patients could be distinguished, (2) the patients underwent a TME, (3) the primary or secondary aim of the paper was to obtain the learning curve of either L-TME, R-TME or TaTME. Studies were excluded if they: (1) were written in other languages than English, German, French or Dutch, or if the studies (2) did not resemble an original article.
Literature search and study selection
Two researchers independently conducted a systematic search (TAB and DJS) in PubMed, Embase and Cochrane Library on August 10, 2021. The following search terms were used: (rectum cancer OR colorectal cancer OR rectal OR colorectal) AND (learning curve OR learning), without limiting the search (for example to year of publication). After undoubling, title and abstract of all studies were screened for inclusion, and full text reading of the remaining studies was performed by two researchers independently. Finally, the reference lists of included studies were screened for possible eligible studies. Systematic reviews emerging in the literature search were excluded, but reference lists were screened for possible eligible studies. Disagreement between the two independent researchers was resolved through discussion until consensus was reached.
Data collection
The primary outcome was length of the learning curve for L-TME, R-TME, and TaTME. Secondary outcomes included intraoperative, postoperative and oncological outcomes of patients operated during the learning curve, compared with patients operated after completion of the learning curve. In addition, statistical methods used to obtain the learning curve, as well as the outcome variables used to obtain the learning curve were recorded. A prespecified form was used to capture data of studies. This form contained the following data: author, year, country, study design, surgical technique, number of participating centers and surgeons, number of patients included, exclusion criteria and aim of the study. Additionally, surgeon-based or institute-based learning curve analysis, prior experience with the surgical technique, length of the learning curve based on intraoperative complications, length of the learning curve based on postoperative complications, length of the learning curve based on positive pathological circumferential margin (CRM), length of the learning curve based on operative time, length of the learning curve based on other variables or a compound variable, and used statistical methods for learning curve analysis were registered. Finally, if a comparison was performed between patients operated during the learning curve and after the learning curve was achieved, the following outcomes were compared: intraoperative complications, postoperative complications, positive CRM rate and operative time. All data was extracted by two researchers independently and disagreement was resolved through discussion.
Outcomes
Length of the learning curve was specified as the number of procedures necessary to reach proficiency as identified by the specific study. Since studies used different clinical outcomes and statistical methods to assess proficiency of the surgical technique, length of the learning curve was reported per clinical outcome and statistical method used. Used clinical outcomes were: intraoperative complications; postoperative complications within 30 days; positive CRM rate, defined as a margin ≤ 1 mm; operative time, defined as time from incision to skin closure, or a composite of multiple clinical outcomes (i.e., conversion, local recurrence and postoperative complications). We registered length of the learning curve for each specific statistical method, and for CUSUM or RA-CUSUM analyses we differentiated between length of the learning curve based on deflection of the graph and stabilization of the graph, as the point at which the learning curve was achieved. Furthermore, a final conclusion per technique regarding length of the learning curve was defined as the reported lengths of the learning curve per technique as estimated only by RA-CUSUM analyses.
Risk of bias
The MINORS tool [22] was used to assess the quality of the studies. Both researchers (TAB and DJS) recorded the data independently. Disagreement was resolved through discussion until consensus was reached.
Results
Study selection
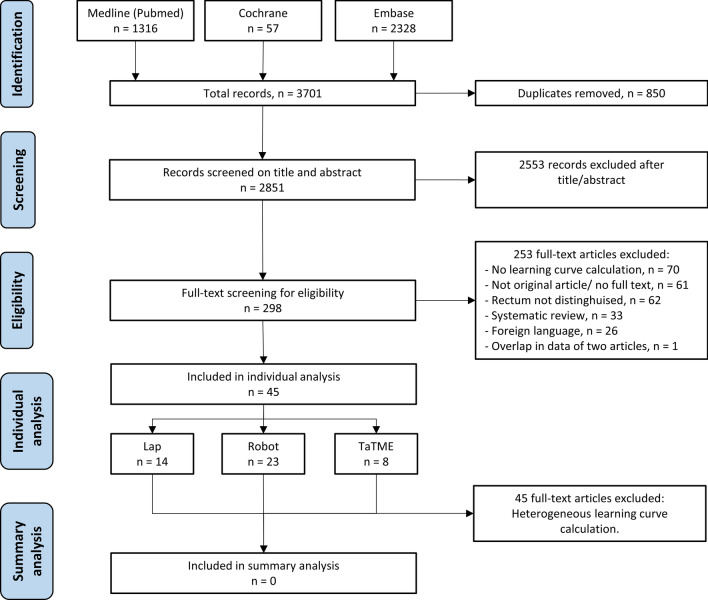
PubMed, Embase and Cochrane Library were searched on August 10, 2021 and yielded 3701 records. After undoubling 2851 records remained. Screening title and abstract for eligibility resulted in 298 records. After full text screening, an additional 253 records were excluded. This resulted in 45 records that were included in this systematic review. Studies were too heterogeneous, both clinically and methodologically, to perform a meta-analysis (Fig. 1).
Fig. 1.
Flow diagram of study selection. Lap Studies involving laparoscopic total mesorectal excision, Robot Studies involving robot-assisted total mesorectal excision, TaTME Studies involving transanal total mesorectal excision
Study characteristics
The characteristics of included studies are presented in Table 1. Studies were published between 2009 and 2021, with a total of six prospective studies [11, 23–27], 34 retrospective studies [9, 10, 12, 14, 15, 28–56], and five studies in which the design was not clearly described [8, 13, 57–59]. Thirteen studies reported on the learning curve of L-TME [10–12, 26, 27, 39–44, 58, 59], twenty on the learning curve of R-TME [13–15, 23–25, 28–35, 52–57], eight on the learning curve of TaTME [8, 9, 36–38, 48–50], and four reported on the comparison of the learning curve of two approaches [45–47, 51].
Table 1.
Study characteristics of included studies
| Author, year | Country | Study design | Technique | Centers | Surgeons | Patients | Exclusion criteria | Learning curve study aim |
|---|---|---|---|---|---|---|---|---|
| Kim (2014) [14] | South Korea | Retrospective | R-TME | 1 | 1 | 167 | None | Primary aim |
| Akmal (2012) [23] | South Korea | Prospective | R-TME | 1 | 1 | 80 | None | Primary aim |
| Foo (2015) [24] | Hong Kong | Prospective | R-TME | 1 | 1 | 39 | Abdominoperineal resection, Hartmann resection | Primary aim |
| Sng (2013) [52] | South Korea | Retrospective | R-TME | 1 | 1 | 197 |
Low rectal tumor, > 5 cm size Male, T4b, anterior invasion |
Primary aim |
| Jiménez-Rodriguez (2013) [13] | Spain | Not mentioned | R-TME | 1 | 3 | 43 | None | Primary aim |
| Yamaguchi (2015) [53] | Japan | Retrospective | R-TME | 1 | 1 | 80 | None | Primary aim |
| Kim (2014) [54] | South Korea | Retrospective | R-TME | 1 | 2 | 200 | None | Primary aim |
| Odermatt (2017) [55] | United Kingdom | Retrospective | R-TME | 1 | 2 | 90 | None | Primary aim |
| Kawai (2018) [56] | Japan | Retrospective | R-TME | 1 | 1 | 131 | None | Primary aim |
| Park (2014) [15] | South Korea | Retrospective | R-TME | 1 | 1 | 130 |
Synchronous procedure Lateral lymph node dissection |
Primary aim |
| Byrn (2014) [28] | United States | Retrospective | R-TME | 1 | 1 | 51 |
History of laparotomy for abdominopelvic surgery Large risk of conversion, extreme age or comorbidities |
Primary aim |
| Morelli (2016) [29] | Italy | Retrospective | R-TME | 1 | 1 | 50 | None | Secondary aim |
| Kim (2012) [25] | South Korea | Prospective | R-TME | 1 | 1 | 62 |
Acute surgery, acute obstruction History of abdominal surgery, severe cardiopulmonary disease |
Primary aim |
| Kuo (2014) [30] | Taiwan | Retrospective | R-TME | 1 | 1 | 36 | None | Secondary aim |
| D’Annibale (2013) [31] | Italy | Retrospective | R-TME | 1 | 1 | 50 | None | Secondary aim |
| Lee (2020) [35] | South Korea | Retrospective | R-TME | 1 | 1 | 506 | No adenocarcinoma, palliative intent | Primary aim |
| Olthof (2020) [32] | The Netherlands | Retrospective | R-TME | 1 | 2 | 100 | None | Primary aim |
| Aghayeva (2020) [33] | Turkey | Retrospective | R-TME | 1 | unclear | 96 |
Abdominoperineal resection Missing value for operative time |
Primary aim |
| Gachabayov (2020) [57] | USA, South Korea, Spain, Taiwan, Italy, Russia | Not mentioned | R-TME | 5 | 5 | 235 | None | Primary aim |
| Noh (2020) [34] | South Korea | Retrospective | R-TME | 1 | 5 | 662 |
Abdominoperineal resection, other synchronous surgical procedures Palliative intent, R2 resection for macroscopic residual disease |
Primary aim |
| Koedam (2018) [8] | The Netherlands | Not mentioned | TaTME | 1 | 3 | 138 | None | Primary aim |
| Lee (2018) [9] | United States | Retrospective | TaTME | 1 | 4 | 87 |
High rectum carcinoma Benign lesions or lesions fit for local excision |
Primary aim |
| Mege (2018) [36] | France | Retrospective | TaTME | 1 | 1 | 34 | Tumor in mid or high rectum, Abdominoperineal resection | Primary aim |
| Rubinkiewicz (2020) [37] | Poland | Retrospective | TaTME | 1 | 1 | 66 | None | Primary aim |
| Persiani,2020[38] | Italy | Retrospective | TaTME | 1 | 1 | 121 |
TaTME for IBD or locoregional recurrence after previous rectal surgery High rectal cancer |
Primary aim |
| Caycedo-Marulanda (2020) [48] | Canada | Retrospective | TaTME | 1 | 1 | 100 | High rectal cancer | Primary aim |
| Zeng (2021) [50] | China | Retrospective | TaTME | 1 | 1 | 171 | T4b, stage IV tumors, emergency surgery | Primary aim |
| Oostendorp, 2021 [49] | The Netherlands | Retrospective | TaTME | 6 | Unclear | 624 | None | Primary aim |
| Balik (2010) [39] | Turkey | Retrospective | L-TME | 1 | 3 | 284 | Emergency surgery, inoperability | Primary aim |
| Tsai (2015) [40] | Taiwan | Retrospective | L-TME | 1 | 1 | 39 |
Abdominoperineal resection, Hartmann resection Conversion and single port laparoscopy |
Primary aim |
| Bege (2010) [11] | France | Prospective | L-TME | 1 | 1 | 127 |
T4 or fixed tumor, synchronous liver resection Abdominoperineal resection Medical contraindication or refusal for laparoscopy |
Primary aim |
| Lujan (2014) [41] | Spain | Retrospective | L-TME | 1 | 2 | 120 | BMI > 35, carcinoma in lower 1/3 of the rectum | Primary aim |
| Kayano (2011) [58] | Japan | Not mentioned | L-TME | 1 | 1 | 250 | Combined resections (cholecystectomy, hepatectomy, hysterectomy) | Primary aim |
| Agha, 2008[42] | Germany | Retrospective | L-TME | 1 | 6 | 300 |
Acute resection, transanal local resections Local recurrent disease |
Secondary aim |
| Ito (2009) [59] | Japan | Not mentioned | L-TME | 1 | Multiple | 200 | T3-T4 tumor, T2 carcinoma in middle or lower rectum | Secondary aim |
| Son (2010) [12] | South Korea | Retrospective | L-TME | 1 | 1 | 431 | Inoperable disease | Primary aim |
| Fukunaga (2008) [26] | Japan | Prospective | L-TME | 1 | 1 | 97 |
Emergency resection, abdominoperineal resection, obstruction Morbid obesity, prior major lower abdominal surgery Tumor occupying most of the pelvis, carcinoma below peritoneal deflection Lateral lymph node dissection, |
Secondary aim |
| Kim (2014) [10] | South Korea | Retrospective | L-TME | 1 | 1 | 512 | Palliative resection, Abdominoperineal resection, Hartmann resection | Primary aim |
| Park (2009) [27] | South Korea | Prospective | L-TME | 1 | 1 | Unknown | None | Secondary aim |
| Kuo (2013) [43] | Taiwan | Retrospective | L-TME | 1 | 2 | 28 | Low anterior resection without need for intersphincteric resection | Secondary aim |
| Wu (2017) [44] | China | Retrospective | L-TME | 1 | 3 | 281 |
ASA 4, BMI > 35, Neoadjuvant therapy, pregnancy History of major abdominal surgery, malignancy within 5 years Metastatic or in situ disease, palliative resection, emergency resection |
Primary aim |
| Melich (2015) [45] | South Korea | Retrospective | R-TME vs L-TME | 1 | 1 | 92 vs 106 | Combined procedure | Primary aim |
| Morelli (2018) [46] | Italy | Retrospective | R-TME Si vs R-TME Xi | 1 | 1 | 40 vs 40 | None | Secondary aim |
| Park (2014) [47] | South Korea | Retrospective | R-TME vs L-TME | 1 | 1 | 89 vs 89 |
Synchronous operation Lateral lymph node dissection |
Primary aim |
| Wang (2021) [51] | China | Retrospective | R-TME vs L-TME | 1 | 1 | 40 vs 65 | Combined resections, palliative resections, ASA IV, previous abdominal pelvic surgery | Primary aim |
TME Total mesorectal excision, L-TME Laparoscopic TME, R-TME Robot-assisted TME, TaTME Transanal TME, ASA American Society of Anesthesiology classification, BMI Body mass index
In total 7562 patients were included in this systematic review. The average number of included patients was 150 for R-TME studies, 168 for TaTME studies and 205 for L-TME studies. Most studies’ primary aim was to define the learning curve, though for nine studies it was a secondary aim [26, 27, 29–31, 42, 43, 46, 59]. Thirteen studies reported on institutional learning curves [8, 9, 11, 13, 31–33, 39, 41–43, 49, 59], while the others reported on surgeons’ individual learning curves. Previous experience with colorectal surgery was mentioned in twenty-one studies [8–10, 13, 15, 24, 25, 28, 29, 31, 35, 37, 39–42, 45–47, 51–56]. The majority of studies defined exclusion criteria, while seventeen did not exclude patients during the learning curve [8, 13, 14, 23, 27, 29–32, 37, 46, 48, 49, 53–57].
Risk of bias
None of the studies scored high on all criteria of the MINORS tool. Nineteen out of 41 non-comparative studies adequately reported more than half of the required criteria [9–12, 15, 24, 25, 27, 33, 35, 37, 38, 48–50, 53, 55]. Study quality was highest among the TaTME studies, and varied most among the R-TME and L-TME studies. All comparative studies adequately reported more than half of the MINORS criteria [45–47]. One study prospectively calculated the study size [9] and seventeen used adequate statistical analyses [8–10, 12–14, 24, 35, 37, 38, 44, 45, 47, 50, 52, 55]. Regarding the use of adequate definitions of clinical outcome variables, nineteen studies adequately reported unbiased assessment of endpoints [8, 10, 15, 24, 25, 27, 33, 35, 38, 39, 47–57, 60] (Table 2).
Table 2.
Risk of bias assessment according to MINORS tool
| Author/year | Clearly stated aim | Inclusion of consecutive patients | Prospective collection of data | Endpoints appropriate to the aim | Unbiased assessment of endpoints | FU appropriate for study aim | Loss to follow up < 5% | Prospective calculation of the study size | Adequate control group | Contemporary groups | Baseline equivalence of groups | Adequate statistical analyses |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kim (2014) [14] | 2 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | NA | NA | NA | 2 |
| Akmal (2012) [23] | 2 | 2 | 2 | 1 | 0 | NA | 0 | 0 | NA | NA | NA | 1 |
| Foo (2015) [24] | 2 | 1 | 2 | 1 | 2 | NA | 0 | 0 | NA | NA | NA | 2 |
| Sng (2013) [52] | 2 | 1 | 1 | 1 | 2 | NA | 0 | 0 | NA | NA | NA | 2 |
| Jiménez-Rodriguez (2013) [13] | 2 | 2 | 0 | 2 | 1 | 0 | 0 | 0 | NA | NA | NA | 2 |
| Yamaguchi (2015) [53] | 2 | 2 | 1 | 1 | 2 | NA | 0 | 0 | NA | NA | NA | 2 |
| Kim (2014) [54] | 2 | 2 | 1 | 1 | 2 | NA | 0 | 0 | NA | NA | NA | 2 |
| Odermatt (2017) [55] | 2 | 2 | 1 | 2 | 2 | 2 | 0 | 0 | NA | NA | NA | 2 |
| Kawai (2018) [56] | 2 | 2 | 1 | 1 | 2 | NA | 0 | 0 | NA | NA | NA | 2 |
| Park (2014) [15] | 2 | 2 | 1 | 2 | 2 | 1 | 0 | 0 | NA | NA | NA | 2 |
| Byrn (2014) [28] | 1 | 1 | 1 | 1 | 0 | NA | 0 | 0 | NA | NA | NA | 1 |
| Morelli (2016) [29] | 2 | 2 | 1 | 1 | 1 | NA | 0 | 0 | NA | NA | NA | 2 |
| Kim (2012) [25] | 2 | 2 | 2 | 1 | 2 | NA | 0 | 0 | NA | NA | NA | 1 |
| Kuo (2014) [30] | 2 | 0 | 1 | 1 | 1 | NA | 0 | 0 | NA | NA | NA | 1 |
| D’Annibale (2013) [31] | 2 | 2 | 1 | 1 | 1 | NA | 0 | 0 | NA | NA | NA | 1 |
| Lee (2020) [35] | 2 | 2 | 1 | 2 | 2 | 2 | 0 | 0 | NA | NA | NA | 2 |
| Olthof (2020) [32] | 2 | 2 | 1 | 2 | 2 | NA | 0 | 0 | NA | NA | NA | 2 |
| Aghayeva (2020) [33] | 2 | 2 | 1 | 1 | 2 | NA | 0 | 0 | NA | NA | NA | 2 |
| Gachabayov (2020) [57] | 2 | 2 | 0 | 1 | 2 | NA | 0 | 0 | NA | NA | NA | 2 |
| Noh (2020) [34] | 2 | 2 | 1 | 2 | 2 | 2 | 0 | 0 | NA | NA | NA | 2 |
| Koedam (2018) [8] | 2 | 2 | 0 | 2 | 2 | 2 | 0 | 0 | NA | NA | NA | 2 |
| Lee (2018) [9] | 2 | 2 | 1 | 2 | 1 | 2 | 0 | 2 | NA | NA | NA | 2 |
| Caycedo-Marulanda (2020) [48] | 2 | 2 | 1 | 1 | 2 | 2 | 0 | 0 | NA | NA | NA | 2 |
| Mege (2018) [36] | 2 | 2 | 1 | 1 | 1 | NA | 0 | 0 | NA | NA | NA | 1 |
| Rubinkiewicz (2020) [37] | 2 | 2 | 1 | 2 | 1 | NA | 0 | 0 | NA | NA | NA | 2 |
| Persiani (2020) [38] | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | NA | NA | NA | 2 |
| Zeng (2021) [50] | 2 | 2 | 1 | 1 | 2 | NA | 0 | 0 | NA | NA | NA | 2 |
| Oostendorp (2021) [49] | 2 | 2 | 1 | 2 | 2 | 2 | 0 | 0 | NA | NA | NA | 1 |
| Balik (2010) [39] | 2 | 0 | 1 | 1 | 2 | 1 | 0 | 0 | NA | NA | NA | 1 |
| Tsai (2015) [40] | 2 | 1 | 1 | 1 | 1 | NA | 0 | 0 | NA | NA | NA | 1 |
| Bege (2010) [11] | 1 | 1 | 2 | 2 | 1 | 1 | 0 | 0 | NA | NA | NA | 2 |
| Lujan (2014) [41] | 2 | 1 | 1 | 1 | 1 | 2 | 0 | 0 | NA | NA | NA | 1 |
| Kayano (2011) [58] | 2 | 2 | 0 | 1 | 1 | 0 | 0 | 0 | NA | NA | NA | 1 |
| Agha (2008) [42] | 1 | 0 | 1 | 1 | 1 | NA | 0 | 0 | NA | NA | NA | 1 |
| Ito (2009) [59] | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | NA | NA | NA | 1 |
| Son (2010) [12] | 2 | 2 | 1 | 2 | 1 | 0 | 0 | 0 | NA | NA | NA | 2 |
| Fukunaga (2008) [26] | 1 | 1 | 2 | 1 | 1 | NA | 0 | 0 | NA | NA | NA | 1 |
| Kim (2014) [10] | 2 | 1 | 0 | 2 | 2 | 2 | 2 | 0 | NA | NA | NA | 2 |
| Park (2009) [27] | 1 | 0 | 2 | 2 | 2 | 2 | 0 | 0 | NA | NA | NA | 1 |
| Kuo (2013) [43] | 2 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | NA | NA | NA | 1 |
| Wu (2017) [44] | 2 | 1 | 1 | 1 | 1 | NA | 0 | 0 | NA | NA | NA | 2 |
| Melich (2015) [45] | 2 | 2 | 1 | 2 | 1 | 0 | 0 | 0 | 2 | 2 | 1 | 2 |
| Morelli (2018) [46] | 2 | 2 | 1 | 1 | 1 | NA | 0 | 0 | 2 | 1 | 1 | 1 |
| Park (2014) [47] | 2 | 1 | 1 | 1 | 2 | NA | 0 | 0 | 2 | 2 | 1 | 2 |
| Wang (2021) [51] | 2 | 1 | 1 | 1 | 2 | NA | 0 | 0 | 2 | 2 | 2 | 1 |
NA not applicable (Assessment score: 2 = adequately reported, 1 = inadequately reported, 0 = not reported)
Statistical methods of learning curve analyses
Most studies used a combination of different learning curve analyses. No clear learning curve analysis was used in three studies [23, 36, 54], eleven studies used split group analyses (SGA) or sequence analysis for one or more clinical outcome variables [12, 25–28, 39, 41–43, 49, 58, 59] and twelve studies used the moving average analysis (MAA) [10, 12, 14, 30, 40, 44, 45, 47, 58, 60]. Eighteen studies used the CUSUM analysis based on operative time [8, 9, 13, 15, 24, 29, 31–34, 37, 44, 46, 47, 50–53, 55–57]. Two studies used the CUSUM analysis based on intraoperative complications [12, 37], six studies used the CUSUM analysis based on postoperative complications [11, 12, 32, 37, 45, 48], one study based the CUSUM analysis on positive CRM rate [45] and seven studies used the CUSUM analysis based on a composite outcome [9, 11, 13–15, 34, 35].
One or more risk-adjusted CUSUM analyses (RA-CUSUM) were used in eight studies: three studies used postoperative morbidity [8, 9, 35, 38], two studies used positive CRM rate [10, 35], one study used local recurrence [10]. Another study used conversion [12] and three studies used a composite outcome [14, 15, 35]. Finally, some studies used the first deflection in the (RA-)CUSUM or MAA graph as the point at which proficiency was reached [8, 10, 12, 14, 15, 27, 29, 31, 33, 34, 44, 46, 47, 52, 53, 55–57], while others defined proficiency as the point at which stabilization was reached [9, 13, 24, 30, 35, 37, 38, 40, 58] (Tables 3 and 4).
Table 3.
Results of individual studies regarding statistical analysis and learning curve
| Author, year | Technique | Learning curve characteristics | Learning curve analysis | Conclusion according to article | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analysis | Previous experience with surgical technique | Variable (IOC) | Analysis | Variable (POC) | Analysis | CRM rate | Analysis | Operative time | Analysis | Other variable | Analysis | Length | |||
| Kim (2014) [14] | R-TME | Per surgeon | Not mentioned | – | – | – | – | – | – | Operative time Console time |
MAAD: 33 MAAS: 72 |
Combination: Conversion, IOC, POC, CRM + , OT > 2 SD | RA-CUSUMD: 32 | 32 | |
| Akmal (2012) [23] | R-TME | Per surgeon | Not mentioned | – | – | – | – | – | – | – | – | – | – | ||
| Foo (2015) [24] | R-TME | Per surgeon |
30 robotic RR assisted < 5 open/lap RR |
– | – | – | – | – | – | Operative time |
CUSUMD: 8 CUSUMS: 25 |
– | – | 25 | |
| Sng (2013) [52] | R-TME | Per surgeon |
> 2000 lap CRR > 1000 lap RR |
– | – | – | – | – | – | Console time |
CUSUMD: 35 CUSUMS: 128 |
– | – | 35 | |
| Jiménez-Rodriguez (2013) [13] | R-TME | Per institute |
Long experience in lap Robot training |
– | – | – | – | – | – | Operative time |
CUSUMD: 11 CUSUMS: 21 |
Combination: Conversion, IOC, POC, mortality |
CUSUMD: 11 CUSUMS: 23 |
23 | |
| Yamaguchi (2015) [53] | R-TME | Per surgeon | Expert in RR | – | – | – | – | – | – | Operative time |
CUSUMD: 25 CUSUMS: 50 |
– | – | 25 | |
| Kim (2014) [54] | R-TME | Per surgeon |
Surg A 200 open CRR, < 30 lap Surg B 800 open CRR, > 300 lap Robot training |
– | – | – | – | – | – | Operative time | – | – | – | – | |
| Odermatt (2017) [55] | R-TME | Per surgeon |
Surg A: 1500 CRR Surg B: 400 CRR Robot training |
– | – |
Morbidity (CD 3b-5) |
– | – | – | Operative time |
CUSUMD: 7 (Surg A) CUSUMD: 15 (Surg B) |
– | – | 15 | |
| Kawai (2018) [56] | R-TME | Per surgeon |
Substantial lap CRR Robot training |
– | – | – | Console time |
CUSUMD: 19 CUSUMS: 78 |
– | – | 19 | ||||
| Park (2014) [15] | R-TME | Per surgeon |
2 year CRC fellowship 6 lap, 10 open CRR |
– | – | – | – | – | – | Operative time |
CUSUMD: 44 CUSUMS: 78 |
Combination: Conversion, R1, < 12 LN, LR, POC | RA-CUSUMD: 75 | 75 | |
| Byrn (2014) [28] | R-TME | Per surgeon | 1 year staff level experience of lap pelvic dissection | – | – | – | – | – | – | Operative time | SGA: - | – | – | – | |
| Morelli (2016) [29] | R-TME | Per surgeon | > 500 lap procedures | – | – | – | – | – | – | Operative time | CUSUMD: 19 | – | – | 19 | |
| Kim (2012) [25] | R-TME | Per surgeon | > 20 year experience in open RR | – | – | – | – | – | – | Operative time | SGA: 20 | – | – | 20 | |
| Kuo (2014) [30] | R-TME | Per surgeon | Not mentioned | – | – | – | – | – | – | Operative time | MAA: 19 | – | – | 19 | |
| D’Annibale (2013) [31] | R-TME | Per institute | Not mentioned | – | – | – | – | – | – | Operative time |
Sequence: 25 CUSUMD: 22 |
– | – | – | |
| Lee (2020) [35] | R-TME | Per surgeon | 3000 lap TMEs | – | – | Morbidity (CD 3–5) | RA-CUSUMS: 191 | CRM + DRM + | RA-CUSUMS: 418 | – | – | Combination: Conversion, CD 3–5, R1, < 12 LN or < 8 LN (CRT) | RA-CUSUMD: 177 | 177 | |
| Olthof (2020) [32] | R-TME | Per Institute |
Intuitive training program Experienced colorectal center |
– | – |
CCI Major morbidity (CD 3–5) |
CUSUM: 40 CUSUM: 40 |
Operative time | CUSUM: 20 | Anastomotic leakage | CUSUM: 30–40 | 40 | |||
| Aghayeva (2020) [33] | R-TME | Per institute | Not mentioned | – | – | – | – | – | – | Operative time | CUSUMD: 52 | – | – | 52 | |
| Gachabayov (2020) [57] | R-TME | Per surgeon | Not mentioned | – | – | – | – | – | – | Operative time |
CUSUMD: 8–25 CUSUMS: 12–56 |
– | – | – | |
| Noh (2020) [34] | R-TME | Per surgeon |
Not mentioned Different previous lap experience |
– | – | – | – | – | – | Operative time | CUSUMD: 23–110 |
Local failure (CRM + , LR) Surgical failure (conversion, AL) |
CUSUMD: - CUSUMD: - |
23–110 | |
| Koedam (2018) [8] | TaTME | Per Institute |
> 75 lap CRR resect annual > 30 TAMIS annual |
– | – |
Morbidity (CD 3b-5) |
RA-CUSUMD: 40 | – | – | Operative time | CUSUMD: 80 | – | – | 40 | |
| Lee (2018) [9] | TaTME | Per institute |
Proficient in lap RR Proficient in TAMIS |
- | - | Morbidity |
RA-CUSUMD:29 RA-CUSUMS: 36 |
- | Operative time |
CUSUMD: 36 CUSUMS: 51 |
Combination: R1, incomplete TME quality, |
CUSUMD: 36 CUSUMS: 51 |
51 | ||
| Caycedo-Marulanda (2020) [48] | TaTME | Per institute | Not mentioned | – | – | – | – | – | – | – | – | Anastomotic leakage | CUSUM: 50 | 45–51 | |
| Mege (2018) [36] | TaTME | Per surgeon | Not mentioned | – | – | – | – | – | – | – | – | – | – | – | |
| Rubinkiewicz (2020) [37] | TaTME | Per surgeon | Training in reference centers | Yes | CUSUMS: 40 | Morbidity | CUSUMS:30 | – | – | Operative time | CUSUMS: 40 | TME quality | CUSUMS: - | 40 | |
| Persiani (2020) [38] | TaTME | Per Institute | Not mentioned | – | – | Morbidity |
RA- CUSUMD:24 RA- CUSUMS:69 Bernoulli CUSUM: 21 Reference CUSUM:108 |
Operative time |
RA- CUSUMD:54 RA- CUSUMS:87 Bernoulli CUSUM: 71 |
Reoperation rate |
RA- CUSUMD:54 RA- CUSUMS:54 Bernoulli CUSUM: 31 Reference CUSUM: 69 |
71 | |||
| Major Morbidity |
RA- CUSUMD:54 RA- CUSUMS:54 Bernoulli CUSUM: 55 Reference CUSUM: 54 |
Anastomotic leakage |
RA- CUSUMD:78 RA- CUSUMS:78 Bernoulli CUSUM: 42 Reference CUSUM: 42 |
||||||||||||
| Zeng (2021) [50] | TaTME | Per surgeon | Not mentioned | – | – | – | – | – | – | Operative time |
CUSUMD: 42 CUSUMS: 95 |
– | – | 42–95 | |
| Oostendorp (2021) [49] | TaTME | Per Institute | Not mentioned | – | – | – | – | – | – | – | – | Local recurrence | SGA: - | – | |
POC Postoperative complication, IOC Intraoperative complication, RR Rectal resection, CRR Colorectal resection, CR Colon resection, R-TME robot-assisted TME, TaTME transanal TME, TAMIS Transanal minimal invasive surgery, CUSUM Cumulative sum analysis, RA-CUSUM Risk-adjusted cumulative sum analysis, CUSUMD Deflection point in CUSUM, CUSUMS Stabilization point in CUSUM, MAA Moving average analysis, MAAS Moving average stabilization point, MAAD Moving average deflection point, SGA Split group analysis, CD Clavien–Dindo classification, CRM Circumferential resection margin, LN Lymph nodes, AL Anastomotic leakage, LR Local recurrence, AL Anastomotic leakage, SSI Surgical site infection, LR Local recurrence, CRT Neoadjuvant chemoradiotherapy, CCI Comprehensive complication index
Table 4.
Results of individual studies regarding statistical analysis and learning curve
| Author, year | Technique | Learning curve characteristics | Learning curve analysis | Conclusion according to article | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analysis | Previous experience with surgical technique | Variable (IOC) | Analysis | Variable (POC) | Analysis | CRM rate | Analysis | Operative time | Analysis | Other variable | Analysis | Length | ||
| Balik (2010) [39] | L-TME | Per institute | 305 open + lap CR | – | – | – | – | – | – | Operative time |
SGA: - Sequence: - |
– | – | – |
| Tsai (2015) [40] | L-TME | Per surgeon |
Fellowship completed Little experience in lap |
– | – | – | – | – | – | Operative time | MAA: 22 | – | – | 22 |
| Bege (2010) [11] | L-TME | Per Institute | Not mentioned | – | – | Morbidity | CUSUMD:45 | – | – | – | – | Combination (comb): POC, LR, Conversion, R1 | CUSUMD: 50 | 50 |
| Lujan (2014) [41] | L-TME | Per institute |
Ample experience in open CRR Skilled advanced lap |
– | – | – | – | – | – | Operative time |
Sequence: - SGA: - |
– | – | – |
| Kayano (2011) [58] | L-TME | Per surgeon | Not mentioned | – | Morbidity | SGA: 200 | – | – | Operative time | MAA: 50 | Conversion | SGA: 150 | – | |
| Agha (2008) [42] | L-TME | Per institute |
Experience with lap CR No experience with lap RR |
– | – | SSI | SGA: 20 | – | – | Operative time | SGA: 40 | – | – | – |
| Ito (2009) [59] | L-TME | Per institute | Not mentioned | – | – | Morbidity | SGA: - | – | – | Operative time | SGA: 40 | – | – | – |
| Son (2010) [12] | L-TME | Per surgeon | Not mentioned | Yes | CUSUMD: 243 | Morbidity | CUSUMD: 79 | – | – | Operative time | MAA:61 |
Conversion Transfusion volume |
RA-CUSUMDl: 61 (Conv) SGA:75 (Transfusion volume) |
79 |
| Fukunaga (2008) [26] | L-TME | Per surgeon | Not mentioned | – | – | – | – | – | Operative time | Sequence: - | – | – | – | |
| Kim (2014) [10] | L-TME | Per surgeon |
A: Fast experience B: Trained by A |
– | – | – | – | CRM + |
RA-CUSUMD:50 (A) RA-CUSUMD:70 (B) |
Operative time |
MAAA: 90 MAAB: 90 |
LR |
RA- CUSUMD:110 (A) RA- CUSUMD: 110 (B) |
110 |
| Park (2009) [27] | L-TME | Per surgeon | Not mentioned | – | – | – | – | – | – | Operative time | MAA: 30 |
LR Conversion |
SGA: 69 (LR) CUSUMD: 13 (Conversion) |
69 |
| Kuo (2013) [43] | L-TME | Per institute | Not mentioned | – | – | – | – | – | – | Operative time | SGA: 17 | – | – | 17 |
| Wu (2017) [44] | L-TME | Unclear | Not mentioned | – | – | – | – | – | – | Operative time |
CUSUMD: 36–42 MAA: 36–47 |
– | – | 40 |
| Melich (2015) [45] | R-TME vs L-TME | Per surgeon | 700 open CR, 50 open RR, 150 lap CR | – | – | AL, intra-abdominal abscess | CUSUM: - | CRM + | CUSUM: - | Operative time | MAA: - | – | – | – |
| Morelli (2018) [46] | R-TMESi vs R-TME Xi | Per surgeon |
> 100 RR > 100 lap surgery |
– | – | – | – | – | – | Operative time | CUSUMD: 19 | – | – | 19 |
| Park (2014) [47] | R-TME vs L-TME | Per surgeon | 2 year lap CRR fellowship | – | – | – | – | – | – | Operative time |
CUSUMD: 44 (robot) MAA: 21 (robot) MAA: 69 (lap) CUSUMD: 41 (lap) |
– | – |
44 (robot) 41 (lap) |
| Wang (2021) [51] | R-TME vs L-TME | Per surgeon |
> 300 open CRR > 150 lap CRR Robot training |
– | – | – | – | – | – | Operative time |
CUSUMD: 17 (rob) CUSUMD: 34 (lap) |
– | – |
17 (rob) 34 (lap) |
POC Postoperative complication, IOC Intraoperative complication, RR Rectal resection, CRR Colorectal resection, CR Colon resection, lap Laparoscopy, TAMIS Transanal minimal invasive surgery, CUSUM Cumulative sum analysis, RA-CUSUM Risk-adjusted cumulative sum analysis, CUSUMD Deflection point in CUSUM, CUSUMS Stabilization point in CUSUM, MAA Moving average analysis, MAAS Moving average stabilization point, MAAD Moving average deflection point, SGA Split group analysis, CD Clavien–Dindo classification, CRM Circumferential resection margin, LN Lymph nodes, AL Anastomotic leakage, LR Local recurrence, AL Anastomotic leakage, SSI Surgical site infection, LR Local recurrence, CRT Neoadjuvant chemoradiotherapy, R-TME robot assisted TME, L-TME laparoscopic TME
Length of the learning curve
Despite the fact that all studies assessed the learning curve as their primary or secondary outcome, only 31 studies defined the number of procedures necessary to complete the learning curve based on their results [8, 9, 11, 13–15, 24, 25, 29, 30, 32, 34, 35, 37, 38, 40, 48, 50–53, 55, 56]. CUSUM analyses for length of the learning curve based on operative time differed between 19 and 128 for R-TME, between 51 and 95 for TaTME and between 36 and 42 for L-TME. The only study using RA-CUSUM for length of the learning curve based on operative time showed 87 procedures to be the learning curve for TaTME [38].
Length of the learning curve based on specific clinical outcomes differed widely. Two studies used intraoperative complications as the variable for the calculation of the learning curve: a TaTME study and a L-TME study estimated the learning curve to be respectively 40 and 243 patients using the CUSUM method [12, 37]. Additionally, two studies used positive CRM as oncological variable for the analyses of the learning curve, both using RA-CUSUM analyses: Length of the learning curve was 418 in a R-TME study [35] and 50–70 in a L-TME study [10]. Most studies calculated the learning curve based on postoperative morbidity: using CUSUM analyses lengths differed between 45 and 79 for L-TME studies [11, 12], 40–191 for R-TME studies [32, 35], and 21–108 for TaTME studies [8, 9, 37, 38]. When only taking into account RA-CUSUM analyses, lengths were 191 for R-TME [35] and between 24 and 54 for TaTME [8, 9, 38]. No RA-CUSUM analysis was conducted for L-TME.
Lengths of the learning curve using (RA-)CUSUM analyses based on compound outcome of clinical variables, were 11, 32, 75 and 177 for four R-TME studies and 36 for a TaTME study. No RA-CUSUM analysis was conducted for L-TME. A CUSUM analysis based on compound outcomes showed a length of 50 procedures in a L-TME study [9, 11, 35, 61]. When only taking into account RA-CUSUM analyses based on a compound outcome, length of the learning curve was between 32 and 177 for R-TME, 36 for TaTME, while this was not performed for L-TME.
Finally, taking into account all RA-CUSUM analyses of clinical outcomes only, length of the learning curve was between 50 and 70 for L-TME, 32–418 for R-TME and 36–54 for TaTME.
Before-after learning curve comparison
After establishing a learning curve, 23 studies reported on the comparison of outcomes between patients that had been operated during the learning curve and patients that had been operated after completing the learning curve [8, 9, 11, 13–15, 24, 25, 29, 30, 32, 35, 37, 38, 43, 46, 47, 51–53, 56–58]. Bege et al., who used postoperative complications to assess the learning curve, showed a decline in postoperative morbidity after the learning curve for L-TME was reached [11]. Rubinkiewicz et al., who used postoperative morbidity, intraoperative morbidity, operative time and a composite outcome to assess the learning curve of TaTME, showed a significant decline in postoperative morbidity and intraoperative morbidity after the learning curve was reached [37]. Operative times were significantly reduced in thirteen studies after the learning curve was reached [8, 14, 15, 24, 33, 37, 38, 46, 47, 51–53, 56, 57] (Table 5). Eight of these studies used operative time to assess the learning curve. While in three R-TME studies and two TaTME studies the learning curve was based on clinical outcomes [8, 14, 15, 35, 38].
Table 5.
Comparison of outcomes during the learning curve and after the learning curve
| Author, year | Comparison | Technique | During learning curve | After learning curve | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intraop complications | Postop complications | CRM + | Operative time | Intraop complications | Postop compicationsl | CRM + | Operative time | |||
| Kim (2014) [14] | 32 vs 135 | R-TME | 3 (9.4%) | 5 (15.6%) | 3 (9.4%) | 252 (42) * | 7 (5.2%) | 23 (17.0%) | 5 (3.7%) | 203 (46) * |
| Foo (2015) [24] | 25 vs 14 | R-TME | – | 4 (16%) | 0 | 446 (102) * | – | 0 | 2 (14.3%) | 311 (165) * |
| Sng (2013) [52] | 35 vs 162 | R-TME | – | 6 (17.1%) | 0 | 265 (190–470) * | – | 68 (42.0%) | 2 (1.2%) | 270 (145–515) * |
| Jiménez-Rodriguez (2013) [13] | 23 vs 20 | R-TME | 3 (13.0%) | 5 (21.7%) | – | 189 (39) | 0 | 1 (5.0%) | – | 208 (44) |
| Yamaguchi (2015) [53] | 25 vs 55 | R-TME | – | 3 (12.0%) | – | 415 (156–683) * | – | 5 (9.1%) | – | 240 (135–529) * |
| Kawai (2018) [56] | 19 vs 111 | R-TME | – | 2 (11.8%) | – | 305 (111) * | – | 13 (11.7%) | – | 227 (112) * |
| Park (2014) [15] | 78 vs 52 | R-TME | – | 8 (10.3%) | 6 (7.7%) | 212 (110–338) * | – | 15 (28.8%) | 3 (5.8%) | 182 (109–376) * |
| Morelli, 2016[29] | 19 vs 31 | R-TME | – | 7 (35.0%) | – | – | – | 9 (29.0%) | – | – |
| Kim (2012) [25] | 20 vs 42 | R-TME | – | 3 (15.0%) | – | 454 (112) | – | 5 (11.9%) | – | 359 (62) |
| Kuo (2014) [30] | 19 vs 17 | R-TME | – | – | 1 (5.3%) | 520 (360–720) | – | – | 3 (17.6%) | 448 (315–585) |
| Lee (2020) [35] | 177 vs 329 | R-TME | – | 48 (27.1%) | 10 (5.4%) | 361 (313–432) | – | 77 (23.5% | 19 (5.9%) | 337 (292–398) |
| Aghayeva (2020) [33] | 52 vs 44 | R-TME | – | 15 (28.8%) | 2 (3.9%) | 380 (109)* | – | 7 (15.9%) | 1 (2.7%) | 323 (103)* |
| Gachabayov (2020) [57] | 83 vs 152 | R-TME | – | 20 (24.1%) | – | 244 (123)* | – | 51 (33.5%) | – | 192 (100) * |
| Koedam (2018) [8] | 40 vs 98 | TaTME | – | 23 (57.5%) | 1 (2.5%) | 199 (95–329) * | – | 53 (54.1%) | 1 (1.0%) | 153 (80–261) * |
| Lee (2018) [9] | 51 vs 36 | TaTME | 6 (12%) | 23 (45%) | 2 (4%) | 278 (84) | 2 (6%) | 15 (42%) | 0 | 270 (73) |
| Rubinkiewicz (2020) [37] | 40 vs 26 | TaTME | 5 (20%) * | 13 (33%) * | – | 270 (240–300) * | 1 (13%) * | 2 (8%) * | – | 210 (170–240) * |
| Persiani (2020) [38] |
69 vs 52 87 vs 34 |
TaTME | – | 31 (45%) | – |
– 294 (59)* |
– | 15 (25%) | – |
– 259 (46)* |
| Bege (2010) [11] | 50 vs 77 | L-TME | – | 26 (52%)* | 5 (10%) | 445 (117) | – | 27 (35.1%) * | 7 (9%) | 414 (97) |
| Kayano (2011) [58] | 50 vs 200 | L-TME | – | 14 (28%) | 0 | – | – | 44 (22%) | 0 | – |
| Kuo (2013) [43] | 17 vs 11 | L-TME | – | – | 3 (17.6%) | 402 (210–570) * | – | – | 1 (9.1%) | 331 (210–450) |
| Morelli (2018) [46](Si) | 19 vs 21 | R-TME | – | 7 (36.8%) | – | 335 (64) * | – | 7 (33.3%) | – | 289 (42) * |
| Morelli (2018) [46](Xi) | 19 vs 21 | R-TME | – | 4 (21.1%) | – | 305 (51) * | – | 6 (28.6%) | – | 264 (39) * |
| Park (2014) [47] | 44 vs 45 | R-TME | – | 5 (11.4%) | 4 (9.1%) | 230 (49) * | – | 4 (8.9%) | 2 (4.4%) | 188 (53) * |
| Park (2014) [47] | 41 vs 48 | L-TME | – | 8 (19.5%) | 2 (4.9%) | 242 (81) * | – | 15 (31.3%) | 4 (8.3%) | 169 (53) * |
| Wang (2021) [51] | 17 vs 23 | R-TME | – | 1 (5.9%) | 1 (5.9%) | 361 (41)* | – | 2 (8.7%) | 1 (4.3%) | 324 (43) * |
| Wang (2021) [51] | 34 vs 31 | L-TME | – | 2 (5.9%) | 0 (0.0%) | 338 (47) * | – | 2 (6.5%) | 0 (0.0%) | 302 (53) * |
CRM Circumferential resection margin 1 is composed outcome of CRM and DRM. * Significant difference between during and after learning curve. L-TME laparoscopic TME, R-TME robot-assisted TME, TaTME transanal TME
Discussion
This systematic review aimed to provide an overview of the current literature regarding the learning curve of L-TME, R-TME and TaTME, and reveals the paucity of high-quality studies. The few available studies using a high-quality RA-CUSUM analysis based on intraoperative complications, postoperative complications or oncological outcomes show similar lengths of the learning curve for L-TME, R-TME, and TaTME. Additionally, although length of the learning curve is suggested to be similar, L-TME and TaTME might bear the risk of additional morbidity while obtaining the learning curve.
Only one L-TME study, three R-TME studies and three TaTME studies used the RA-CUSUM analysis based on clinically relevant outcomes such as intraoperative morbidity, postoperative morbidity or oncological outcomes [8, 9, 12, 14, 15, 35, 38]. Length of the learning curve was 50–70 for L-TME, 32–418 for R-TME and 36–54 for TaTME. This might suggest that the learning curve for R-TME is considerably longer than the learning curve of L-TME and TaTME. However, the results are influenced by the study of Lee et al., who found a learning curve of 177–418 procedures for R-TME [35]. As the authors state in their discussion, the substantial length of the learning curve might be due to the high number of examined cases: with increasing number of consecutive cases, length of the learning curve increases as well [5, 35, 62]. Taking this into account, the learning curve shows similar lengths between techniques: 50–70 procedures for L-TME, 32–75 procedures for R-TME and 36–54 procedures for TaTME [9, 11, 13–15]. This is in line with other systematic reviews evaluating the learning curve of minimal invasive techniques. A systematic review estimated the learning curve to be between 30 and 50 procedures in TaTME [16], and another systematic review estimated the learning curve of R-TME to be 37 procedures [17]. Furthermore, two systematic reviews compared length of the learning curve between L-TME and R-TME. One included studies with colorectal patients, both having benign and malign disease and reported a length between 5 and 310 for L-TME and 15–30 for R-TME [19]. A more recent systematic review only included studies with surgeons without laparoscopic experience and showed equal length of the learning curve: 44–55 for L-TME, and 41–55 for R-TME [20].
Although the length of the learning curve might not differ between the three techniques, L-TME and TaTME might bear the risk of additional morbidity while obtaining the learning curve. A L-TME and a TaTME study show higher rates of intraoperative and postoperative complications before reaching the learning curve, while no R-TME study shows a difference between these two phases [11, 18, 37]. Additionally, a systematic review comparing outcomes before and after the learning curve of TaTME showed less intraoperative complications, less anastomotic leakages and better quality of the TME specimen after the learning curve was obtained [16]. The evidence is scarce, but this might be in line with recently published data showing additional morbidity and higher local recurrence rates during the learning curve of TaTME [49, 50, 63–65]. This has also been suggested in a study assessing the learning curve of L-TME [10]. Perhaps the learning curve of L-TME and TaTME bear the risk of worsened oncological outcomes as these techniques differ significantly from the preceding ‘standard’ technique, while R-TME shows a high degree of similarity with the preceding L-TME technique. Subsequently, since most surgeons starting with R-TME have preceding experience with the L-TME technique, this influences the learning curve. While, on the other hand, surgeons starting with L-TME or TaTME start with a completely new technique, which might cause the additional morbidity during the learning curve.
The statements regarding length of the learning curve and additional morbidity during the learning curve should be interpreted cautious. Since, only limited amount of high-quality evidence exists, with lack of comparative studies, and a large amount of heterogeneity among studies. This is mainly caused by differences in patient-related factors, surgeon-related factors and statistical methods. First, regarding patient-related factors, inclusion- and exclusion criteria differ among studies, resulting in selection bias between studies. Furthermore, case-mix changes over the course of the learning curve: mostly an overrepresentation of “easy” patients is seen while climbing the learning curve, and more “difficult” patients are operated at the middle of the learning curve [14, 15]. Although case-mix can be controlled for by using a risk-adjusted analysis using the RA-CUSUM, this is only performed in a small number of studies.
Secondly, heterogeneity due to surgeon-related factors among studies exists as well: while some studies report on learning curves for individual surgeons, others report on institutional learning curves. As institutional learning curves might indicate the experience of the whole surgical team, they fail to address differences between individual surgeons. In addition, it is known that the first surgeon mastering the technique within an institution has a longer learning curve than the ones following, due to the institutional experience [66]. Furthermore, as experience with the minimal invasive technique and TME in general influences the learning curve, it is important to describe this. And although most studies reported the experience of the surgeon with the minimal invasive technique, details were lacking. Young surgeons who are at the start of their career, might have a longer learning curve than senior surgeons mastering minimal invasive surgery since the latter might have experience in performing open or L-TME [67]. Additionally, as R-TME and TaTME have been introduced 10–15 years later than L-TME, most studies addressing the learning curve of R-TME and TaTME included surgeons who already had experience with L-TME. This might be an important confounder while assessing the learning curve of L-TME with R-TME or TaTME, but it is inherent to the clinical practice. Finally, since TaTME is generally not used for an abdominoperineal resection, while this is performed using L-TME or R-TME, differences regarding the indication of the technique complicate the comparability of these techniques.
Thirdly, regarding heterogeneity among studies caused by the used statistical analyses, differences could be due to the used outcome measure to establish the learning curve, the used statistical technique and the used cut-off point. Regarding the used outcome measure to establish the learning curve, operative time is often used for the learning curve. However the outcome is said to be a poor surrogate for clinical outcomes, and mere a reflection of efficiency [5, 68]. Instead, clinical outcomes that are of interest for patients should be used to assess the learning curve [5, 68]. For example, intraoperative complications, major postoperative complications [69], positive CRM rate and for the long term local recurrence rate [70]. Additionally, in order to provide comparable outcomes, clear definitions according to international standards should be used [71].
Regarding the used statistical technique, several methods for the analyses are used: split group analysis (SGA), moving average analysis (MAA), CUSUM and RA-CUSUM. For SGA, patients are arbitrarily divided into two or more groups, based on the chronological order. Since these learning curves are dependent on how groups were divided, it could be doubted whether SGA is suitable for analyzing learning curves [5, 28, 39, 41]. MAA learning curves are based on operative time alone. As operative time might not be an adequate indicator of proficiency, this technique might not be suitable either [72]. CUSUM and RA-CUSUM analyses are more complex methods used to continuously monitor outcomes. The CUSUM is a chronically ordered cumulative sum of the difference between the outcome of the procedure and the average of the studied cohort or a predefined cut-off point based on literature [14, 15]. The RA-CUSUM analysis is the more sophisticated method, correcting for case-mix that may influence the risk of an event [14, 15, 35, 73]. However, both methods have been developed to monitor processes known to be adequate, while signaling inadequacy. For surgeons carrying out a procedure they have not yet performed regularly the learning curve CUSUM (LC-CUSUM) might be more suitable. This analysis assumes inadequacy of the surgeon, while signaling adequacy [62]. This method could be used when the surgeon has no experience with the procedure, as is the case with young surgeons starting with L-TME or R-TME. Or it can be used for describing the learning curve of an experienced colorectal surgeon starting with TaTME, since this procedure is to a large extent different from the “top-down” approach used in open, L-TME and R-TME.
Finally, regarding the used cut-off points, all CUSUM methods can be performed using limits based on averages of the cohort or using literature-based limits. Using averages of the cohort complicates comparison with other studies. And, as mentioned earlier, using averages causes the length of the learning curve to increase with larger cohort size [5, 62]. Therefore, literature-based limits are preferred. Furthermore, the point at which ‘proficiency’ is reached influences the length of the learning curve as well. Studies used two different points to identify proficiency: the point at which the graph deflects or the point at which a stabilization occurs. Both methods are used, while different outcomes are produced [13, 24, 52]. Therefore, it has been proposed that a learning plateau (i.e., stabilization) should reach a predefined competency level, based on estimates available in literature [5]. As not all studies included in this systematic review provided the point of stabilization, while the point of deflection was provided in every study, this was used in our analysis for assessing the length of the learning curve.
Although this is the first systematic review to provide an overview of the literature regarding the learning curves of all three minimal invasive techniques of TME and the methods used to establish them, it cannot draw a definite conclusion regarding differences in length of the learning curves and differences in additional morbidity during the learning curve of L-TME, R-TME and TaTME. Clearly, more high-quality studies are necessary to shed light on the learning curve of minimally invasive techniques for rectal resections. We suggest that this should preferably be performed with comparative studies, while controlling for patient-related factors (i.e., risk-adjusted analysis), and surgeon-related factors such as experience with TME in general and experience with the specific minimal invasive technique. In addition, if former experience with the TME procedure is limited (i.e., beginning surgeon or adhering to a new technique like TaTME) the LC-CUSUM should be used. Furthermore, we propose that learning curves should be established for individual surgeons, based on the following clinically relevant outcome variables: intraoperative morbidity, (major) postoperative morbidity and positive CRM. Additionally, clear outcome definitions should be reported and learning curves should be estimated using literature-based limits. Finally, comparison of outcomes during and after the learning curve should be performed, to investigate whether the learning curve is associated with additional morbidity.
Author contributions
TAB, ECJC, PMV, MelM and RH contributed to the study conception and design, literature search and data analysis was performed by TAB and DJS. The first draft was written by TAB, and all authors critically revised the work.
Funding
No funding was received for conducting this study.
Data availability
Data is available on request, this includes template data collection forms, data extracted from included studies, data used for analyses and any other materials used in the review.
Code availability
Not applicable.
Declarations
Disclosure
Paulus Menno Verheijen and Esther Catharina Josephina Consten receive fees from Intuitive Surgical. Roel Hompes receives fees from Applied Medical. Thijs Adriaan Burghgraef, Daan Jeroen Sikkenk and Mostafa El Moumni have no relevant financial or non-financial interests to disclose.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Heald RJ, Ryall RDH. Recurence and survival after total mesorectal excision for rectal cancer. Lancet. 1986 doi: 10.1016/S0140-6736(86)91510-2. [DOI] [PubMed] [Google Scholar]
- 2.van der Pas MHGM, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WCJ, Bonjer HJ. Laparoscopic versus open surgery for rectal cancer (COLOR II): Short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14:210–218. doi: 10.1016/S1470-2045(13)70016-0. [DOI] [PubMed] [Google Scholar]
- 3.Jayne D, Pigazzi A, Marshall H, Croft J, Corrigan N, Copeland J, Quirke P, West N, Rautio T, Thomassen N, Tilney H, Gudgeon M, Pietro BP, Edlin R, Hulme C, Brown J. Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer. JAMA. 2017;318:1569. doi: 10.1001/jama.2017.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Detering R, Roodbeen SX, van Oostendorp SE, Dekker JWT, Sietses C, Bemelman WA, Tanis PJ, Hompes R, Tuynman JB, Aalbers AGJ, Beets-Tan RGH, den Boer FC, Breukink SO, Coene PPLO, Doornebosch PG, Gelderblom AJ, Karsten TM, Ledeboer M, Manusama ER, Marijnen CAM, Nagtegaal ID, Peeters KCMJ, Tollenaar RAEM, van de Velde CJH, Wagner A, Westerterp M, van Westreenen HL. Three-year nationwide experience with transanal total mesorectal excision for rectal cancer in the netherlands: a propensity score-matched comparison with conventional laparoscopic total mesorectal excision. J Am Coll Surg. 2019;228:235–244.e1. doi: 10.1016/j.jamcollsurg.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Khan N, Abboudi H, Khan MS, Dasgupta P, Ahmed K. Measuring the surgical “learning curve”: methods, variables and competency. BJU Int. 2014;113:504–508. doi: 10.1111/bju.12197. [DOI] [PubMed] [Google Scholar]
- 6.State of New York Department of Health Memorandum - series 92–20. https://www.health.ny.gov/professionals. Accessed 22 Jun 2021
- 7.Le Morvan P, Stock B. Medical learning curves and the Kantian ideal. J Med Ethics. 2005;31:513–518. doi: 10.1136/jme.2004.009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koedam TWA, Veltcamp Helbach M, van de Ven PM, Kruyt PM, van Heek NT, Bonjer HJ, Tuynman JB, Sietses C. Transanal total mesorectal excision for rectal cancer: evaluation of the learning curve. Tech Coloproctol. 2018;22:279–287. doi: 10.1007/s10151-018-1771-8. [DOI] [PubMed] [Google Scholar]
- 9.Lee L, Kelly J, Nassif GJ, DeBeche-Adams TC, Albert MR, Monson JRTT. Defining the learning curve for transanal total mesorectal excision for rectal adenocarcinoma. Surg Endosc Other Interv Tech. 2018 doi: 10.1007/s00464-018-6360-4. [DOI] [PubMed] [Google Scholar]
- 10.Kim CH, Kim HJ, Huh JW, Kim YJ, Kim HR. Learning curve of laparoscopic low anterior resection in terms of local recurrence. J Surg Oncol. 2014;110:989–996. doi: 10.1002/jso.23757. [DOI] [PubMed] [Google Scholar]
- 11.Bege T, Lelong B, Esterni B, Turrini O, Guiramand J, Francon D, Mokart D, Houvenaeghel G, Giovannini M, Delpero JR. The learning curve for the laparoscopic approach to conservative mesorectal excision for rectal cancer: Lessons drawn from a single institution’s experience. Ann Surg. 2010;251:249–253. doi: 10.1097/SLA.0b013e3181b7fdb0. [DOI] [PubMed] [Google Scholar]
- 12.Son G-M, Kim J-G, Lee J-C, Suh Y-J, Cho H-M, Lee Y-S, Lee I-K, Chun C-S. Multidimensional analysis of the learning curve for laparoscopic rectal cancer surgery. J Laparoendosc Adv Surg Tech. 2010;20:609–617. doi: 10.1089/lap.2010.0007. [DOI] [PubMed] [Google Scholar]
- 13.Jiménez-Rodríguez RM, Díaz-Pavón JM, de la Portilla de Juan F, Prendes-Sillero E, Dussort HC, Padillo J (2013) Learning curve for robotic-assisted laparoscopic rectal cancer surgery. Int J Colorectal Dis 28:815–821. 10.1007/s00384-012-1620-6 [DOI] [PubMed]
- 14.Kim HJ, Choi G-SS, Park JS, Park SY. Multidimensional analysis of the learning curve for robotic total mesorectal excision for rectal cancer: Lessons from a single surgeon’s experience. Dis Colon Rectum. 2014;57:1066–1074. doi: 10.1097/DCR.0000000000000174. [DOI] [PubMed] [Google Scholar]
- 15.Park EJ, Kim CW, Cho MS, Baik SH, Kim DW, Min BS, Lee KY, Kim NK. Multidimensional analyses of the learning curve of robotic low anterior resection for rectal cancer: 3-phase learning process comparison. Surg Endosc. 2014;28:2821–2831. doi: 10.1007/s00464-014-3569-8. [DOI] [PubMed] [Google Scholar]
- 16.Lau SYC, Choy KT, Yang TWW, Heriot A, Warrier SK, Guest GD, Kong JC (2021) Defining the learning curve of transanal total mesorectal excision: a systematic review and meta-analysis. ANZ J. Surg. [DOI] [PubMed]
- 17.Jiménez-rodríguez RM, Rubio-Dorado-Manzanares M, Díaz-pavón JM, Reyes-díaz ML, Vazquez-Monchul JM, Garcia-Cabrera AM, Padillo J, De la Portilla F, Portilla F De, R.M. J-R, M. R-D-M, J.M. D-P, M.L. R-D, J.M. V-M, A.M. G-C, J. P, F. D la P, Jiménez-rodríguez RM, Rubio-Dorado-Manzanares M, Díaz-pavón JM, Reyes-díaz ML, Vazquez-Monchul JM, Garcia-Cabrera AM, Padillo J, De la Portilla F, Jimenez-Rodriguez RM, Rubio-Dorado-Manzanares M, Diaz-Pavon JM, Reyes-Diaz ML, Vazquez-Monchul JM, Garcia-Cabrera AM, Padillo J, De la Portilla F (2016) Learning curve in robotic rectal cancer surgery: current state of affairs. Int J Colorectal Dis 31:1807–1815 . 10.1007/s00384-016-2660-0 [DOI] [PubMed]
- 18.Gachabayov M, You K, Kuo LJ, Kim SH, Cianchi F, Yamaguchi T, Staderini F, Jimenez-Rodriguez R, Bergamaschi R. Meta-analysis of the impact of the learning curve in robotic rectal cancer surgery on histopathologic outcomes. Surg Technol Int. 2019;34:139–155. [PubMed] [Google Scholar]
- 19.Barrie J, Jayne DG, Wright J, Murray CJC, Collinson FJ, Pavitt SH. Attaining surgical competency and its implications in surgical clinical trial design: a systematic review of the learning curve in laparoscopic and robot-assisted laparoscopic colorectal cancer surgery. Ann Surg Oncol. 2014;21:829–840. doi: 10.1245/s10434-013-3348-0. [DOI] [PubMed] [Google Scholar]
- 20.Flynn J, Larach JT, Kong JCH, Waters PS, Warrier SK, Heriot A. The learning curve in robotic colorectal surgery compared with laparoscopic colorectal surgery: a systematic review. Color Dis. 2021;23:2806–2820. doi: 10.1111/codi.15843. [DOI] [PubMed] [Google Scholar]
- 21.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372
- 22.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (Minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 23.Akmal Y, Baek J-HH, McKenzie S, Garcia-Aguilar J, Pigazzi A. Robot-assisted total mesorectal excision: is there a learning curve? Surg Endosc. 2012;26:2471–2476. doi: 10.1007/s00464-012-2216-5. [DOI] [PubMed] [Google Scholar]
- 24.Foo CC, Law WL. The learning curve of robotic-assisted low rectal resection of a novice rectal surgeon. World J Surg. 2015;40:456–462. doi: 10.1007/s00268-015-3251-x. [DOI] [PubMed] [Google Scholar]
- 25.Kim YW, Lee HM, Kim NK, Min BS, Lee KY. The learning curve for robot-assisted total mesorectal excision for rectal cancer. Surg Laparosc Endosc Percutaneous Tech. 2012;22:400–405. doi: 10.1097/SLE.0b013e3182622c2d. [DOI] [PubMed] [Google Scholar]
- 26.Fukunaga Y, Higashino M, Tanimura S, Takemura M, Osugi H. Laparoscopic colorectal surgery for neoplasm. A large series by a single surgeon. Surg Endosc Other Interv Tech. 2008;22:1452–1458. doi: 10.1007/s00464-007-9630-0. [DOI] [PubMed] [Google Scholar]
- 27.Park IJ, Choi G-S, Lim K-H, Kang B-M, Jun S-H. Multidimensional analysis of the learning curve for laparoscopic colorectal surgery: lessons from 1,000 cases of laparoscopic colorectal surgery. Surg Endosc Other Interv Tech. 2009;23:839–846. doi: 10.1007/s00464-008-0259-4. [DOI] [PubMed] [Google Scholar]
- 28.Byrn JC, Hrabe JE, Charlton ME. An initial experience with 85 consecutive robotic-assisted rectal dissections: improved operating times and lower costs with experience. Surg Endosc. 2014;28:3101–3107. doi: 10.1007/s00464-014-3591-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morelli L, Guadagni S, Lorenzoni V, Di Franco G, Cobuccio L, Palmeri M, Caprili G, D’Isidoro C, Moglia A, Ferrari V, Di Candio G, Mosca F, Turchetti G, D’Isidoro C, Moglia A, Ferrari V, Di Candio G, Mosca F, Turchetti G. Robot-assisted versus laparoscopic rectal resection for cancer in a single surgeon’s experience: a cost analysis covering the initial 50 robotic cases with the da Vinci Si. Int J Colorectal Dis. 2016;31:1639–1648. doi: 10.1007/s00384-016-2631-5. [DOI] [PubMed] [Google Scholar]
- 30.Kuo L-JJ, Lin Y-KK, Chang C-CC, Tai C-JJ, Chiou J-FF, Chang Y-JJ. Clinical outcomes of robot-assisted intersphincteric resection for low rectal cancer: Comparison with conventional laparoscopy and multifactorial analysis of the learning curve for robotic surgery. Int J Colorectal Dis. 2014;29:555–562. doi: 10.1007/s00384-014-1841-y. [DOI] [PubMed] [Google Scholar]
- 31.D’Annibale A, Pernazza G, Monsellato I, Pende V, Lucandri G, Mazzocchi P, Alfano G, D’Annibale A, Pernazza G, Monsellato I, Pende V, Lucandri G, Mazzocchi P, Alfano G. Total mesorectal excision: a comparison of oncological and functional outcomes between robotic and laparoscopic surgery for rectal cancer. Surg Endosc. 2013;27:1887–1895. doi: 10.1007/s00464-012-2731-4. [DOI] [PubMed] [Google Scholar]
- 32.Olthof PB, Giesen LJX, Vijfvinkel TS, Roos D, Dekker JWT. Transition from laparoscopic to robotic rectal resection: outcomes and learning curve of the initial 100 cases. Surg Endosc. 2020;1:3. doi: 10.1007/s00464-020-07731-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aghayeva A, Baca B. Robotic sphincter saving rectal cancer surgery: a learning curve analysis. Int J Med Robot Comput Assist Surg. 2020 doi: 10.1002/rcs.2112. [DOI] [PubMed] [Google Scholar]
- 34.Noh GT, Han M, Hur H, Baik SH, Lee KY, Kim NK, Min BS. Impact of laparoscopic surgical experience on the learning curve of robotic rectal cancer surgery. Surg Endosc. 2020;1:3. doi: 10.1007/s00464-020-08059-5. [DOI] [PubMed] [Google Scholar]
- 35.Lee JM, Yang SY, Han YD, Cho MS, Hur H, Min BS, Lee KY, Kim NK. Can better surgical outcomes be obtained in the learning process of robotic rectal cancer surgery? a propensity score-matched comparison between learning phases. Surg Endosc. 2020;1:3. doi: 10.1007/s00464-020-07445-3. [DOI] [PubMed] [Google Scholar]
- 36.Mege D, Hain E, Lakkis Z, Maggiori L, Prost à la Denise J, Panis Y, D. M, E. H, Z. L, L. M, J. P à la D, Y. P, Mege D, Hain E, Lakkis Z, Maggiori L, Prost à la Denise J, Panis Y (2018) Is trans-anal total mesorectal excision really safe and better than laparoscopic total mesorectal excision with a perineal approach first in patients with low rectal cancer? a learning curve with case-matched study in 68 patients. Color Dis 20:O143–O151. 10.1111/codi.14238 [DOI] [PubMed]
- 37.Rubinkiewicz M, Truszkiewicz K, Wysocki M, Witowski J, Torbicz G, Nowakowski MM, Budzynski A, Pędziwiatr M. Evaluation of the learning curve of transanal total mesorectal excision: single-centre experience. Wideochirurgia I Inne Tech Maloinwazyjne. 2020;15:36–42. doi: 10.5114/wiitm.2019.82733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Persiani R, Agnes A, Belia F, D’Ugo D, Biondi A. The learning curve of TaTME for mid-low rectal cancer: a comprehensive analysis from a five-year institutional experience. Surg Endosc. 2020;1:3. doi: 10.1007/s00464-020-08115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balik E, Asoglu O, Saglam S, Yamaner S, Akyuz A, Buyukuncu Y, Gulluoglu M, Bulut T, Bugra D. Effects of surgical laparoscopic experience on the short-term postoperative outcome of rectal cancer: results of a high volume single center institution. Surg Laparosc Endosc Percutan Tech. 2010;20:93–99. doi: 10.1097/SLE.0b013e3181d83e20. [DOI] [PubMed] [Google Scholar]
- 40.Tsai K-YY, Kiu K-TT, Te H-T, Wu C-HH, Chang T-CC. The learning curve for laparoscopic colectomy in colorectal cancer at a new regional hospital. Asian J Surg. 2015;39:34–40. doi: 10.1016/j.asjsur.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 41.Lujan J, Gonzalez A, Abrisqueta JJ, Hernandez Q, Valero G, Abellan I, Frutos MDMD, Parrilla P, Luján J, Gonzalez A, Abrisqueta JJ, Hernandez Q, Valero G, Abellán I, Frutos MDMD, Parrilla P (2014) The Learning Curve of Laparoscopic Treatment of Rectal Cancer Does Not Increase Morbidity. Cirugía Española (English Ed 92:485–490 . 10.1016/j.cireng.2013.03.008 [DOI] [PubMed]
- 42.Agha A, Furst A, Iesalnieks I, Fichtner-Feigl S, Ghali N, Krenz D, Anthuber M, Jauch KW, Piso P, Schlitt HJ. Conversion rate in 300 laparoscopic rectal resections and its influence on morbidity and oncological outcome. Int J Colorectal Dis. 2008;23:409–417. doi: 10.1007/s00384-007-0425-5. [DOI] [PubMed] [Google Scholar]
- 43.Kuo L-J, Hung C-S, Wang W, Tam K-W, Lee H-C, Liang H-H, Chang Y-J, Huang M-T, Wei P-L. Intersphincteric resection for very low rectal cancer: clinical outcomes of open versus laparoscopic approach and multidimensional analysis of the learning curve for laparoscopic surgery. J Surg Res. 2013;183:524–530. doi: 10.1016/j.jss.2013.01.049. [DOI] [PubMed] [Google Scholar]
- 44.Wu Y, Sun X, Qi J, Wei G, Cui F, Gao Q, Yu J, Wang K, Zheng J. Comparative study of short- and long-term outcomes of laparoscopic-assisted versus open rectal cancer resection during and after the learning curve period. Medicine (Baltimore) 2017 doi: 10.1097/MD.0000000000006909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Melich G, Hong YK, Kim J, Hur H, Baik SH, Kim NK, Sender Liberman A, Min BS. Simultaneous development of laparoscopy and robotics provides acceptable perioperative outcomes and shows robotics to have a faster learning curve and to be overall faster in rectal cancer surgery: analysis of novice MIS surgeon learning curves. Surg Endosc. 2015;29:558–568. doi: 10.1007/s00464-014-3698-0. [DOI] [PubMed] [Google Scholar]
- 46.Morelli L, Di Franco G, Lorenzoni V, Guadagni S, Palmeri M, Furbetta N, Gianardi D, Bianchini M, Caprili G, Mosca F, Turchetti G, Cuschieri A. Structured cost analysis of robotic TME resection for rectal cancer: a comparison between the da Vinci Si and Xi in a single surgeon’s experience. Surg Endosc Other Interv Tech. 2018 doi: 10.1007/s00464-018-6465-9. [DOI] [PubMed] [Google Scholar]
- 47.Park EJ, Kim CW, Cho MS, Kim DW, Min BS, Baik SH, Lee KY, Kim NK. Is the learning curve of robotic low anterior resection shorter than laparoscopic low anterior resection for rectal cancer?: a comparative analysis of clinicopathologic outcomes between robotic and laparoscopic surgeries. Med (United States) 2014 doi: 10.1097/MD.0000000000000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caycedo-Marulanda A, Verschoor CP. Experience beyond the learning curve of transanal total mesorectal excision (taTME) and its effect on the incidence of anastomotic leak. Tech Coloproctol. 2020;24:309–316. doi: 10.1007/s10151-020-02160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Oostendorp S, Belgers H, Hol J, Doornebosch P, Belt E, Oosterling S, Kusters M, Bonjer H, Sietses C, Tuynman J. Learning curve of TaTME for rectal cancer is associated with local recurrence; results from a multicentre external audit. Color Dis. 2021 doi: 10.1111/codi.15722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeng Z, Liu Z, Huang L, Liu H, Jie H, Luo S, Zhang X, Kang L. Transanal total mesorectal excision in mid-low rectal cancer: evaluation of the learning curve and comparison of short-term results with standard laparoscopic total mesorectal excision. Dis Colon Rectum. 2021;64:380–388. doi: 10.1097/DCR.0000000000001816. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Wang G, Li Z, Ling H, Yi B, Zhu S. Comparison of the operative outcomes and learning curves between laparoscopic and “Micro Hand S” robot-assisted total mesorectal excision for rectal cancer: a retrospective study. BMC Gastroenterol. 2021 doi: 10.1186/s12876-021-01834-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sng KK, Hara M, Shin J-WW, Yoo B-EE, Yang K-SS, Kim S-HH. The multiphasic learning curve for robot-assisted rectal surgery. Surg Endosc. 2013;27:3297–3307. doi: 10.1007/s00464-013-2909-4. [DOI] [PubMed] [Google Scholar]
- 53.Yamaguchi T, Kinugasa Y, Shiomi A, Sato S, Yamakawa Y, Kagawa H, Tomioka H, Mori K. Learning curve for robotic-assisted surgery for rectal cancer: use of the cumulative sum method. Surg Endosc. 2015;29:1679–1685. doi: 10.1007/s00464-014-3855-5. [DOI] [PubMed] [Google Scholar]
- 54.Kim I, Kang J, Park YA, Kim NK, Sohn S-K, Lee KY. Is prior laparoscopy experience required for adaptation to robotic rectal surgery?: feasibility of one-step transition from open to robotic surgery. Int J Colorectal Dis. 2014;29:693–699. doi: 10.1007/s00384-014-1858-2. [DOI] [PubMed] [Google Scholar]
- 55.Odermatt M, Ahmed J, Panteleimonitis S, Khan J, Parvaiz A. Prior experience in laparoscopic rectal surgery can minimise the learning curve for robotic rectal resections: a cumulative sum analysis. Surg Endosc. 2017;31:4067–4076. doi: 10.1007/s00464-017-5453-9. [DOI] [PubMed] [Google Scholar]
- 56.Kawai K, Hata K, Tanaka T, Nishikawa T, Otani K, Murono K, Sasaki K, Kaneko M, Emoto S, Nozawa H. Learning curve of robotic rectal surgery with lateral lymph node dissection: cumulative sum and multiple regression analyses. J Surg Educ. 2018;75:1598–1605. doi: 10.1016/j.jsurg.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 57.Gachabayov M, Kim SH, Jimenez-Rodriguez R, Kuo LJ, Cianchi F, Tulina I, Tsarkov P, Bergamaschi R. Impact of robotic learning curve on histopathology in rectal cancer: a pooled analysis. Surg Oncol. 2020;34:121–125. doi: 10.1016/j.suronc.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 58.Kayano H, Okuda J, Tanaka K, Kondo K, Tanigawa N. Evaluation of the learning curve in laparoscopic low anterior resection for rectal cancer. Surg Endosc. 2011;25:2972–2979. doi: 10.1007/s00464-011-1655-8. [DOI] [PubMed] [Google Scholar]
- 59.Ito M, Sugito M, Kobayashi A, Nishizawa Y, Tsunoda Y, Saito N. Influence of learning curve on short-term results after laparoscopic resection for rectal cancer. Coloproctology. 2009;23:403–408. doi: 10.1007/s00464-008-9912-1. [DOI] [PubMed] [Google Scholar]
- 60.Gkionis IG, Flamourakis ME, Tsagkataki ES, Kaloeidi EI, Spiridakis KG, Kostakis GE, Alegkakis AK, Christodoulakis MS. Multidimensional analysis of the learning curve for laparoscopic colorectal surgery in a regional hospital: the implementation of a standardized surgical procedure counterbalances the lack of experience. BMC Surg. 2020 doi: 10.1186/s12893-020-00975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jeong SY, Park JW, Nam BH, Kim S, Kang SB, Lim SB, Choi HS, Kim DW, Chang HJ, Kim DY, Jung KH, Kim TY, Kang GH, Chie EK, Kim SY, Sohn DK, Kim DH, Kim JS, Lee HS, Kim JH, Oh JH. Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): Survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol. 2014;15:767–774. doi: 10.1016/S1470-2045(14)70205-0. [DOI] [PubMed] [Google Scholar]
- 62.Biau DJ, Porcher R. A method for monitoring a process from an out of control to an in control state: application to the learning curve. Stat Med. 2010;29:1900–1909. doi: 10.1002/sim.3947. [DOI] [PubMed] [Google Scholar]
- 63.van Oostendorp SE, Belgers HJ, Bootsma BT, Hol JC, Belt EJTH, Bleeker W, Den Boer FC, Demirkiran A, Dunker MS, Fabry HFJ, Graaf EJR, Knol JJ, Oosterling SJ, Slooter GD, Sonneveld DJA, Talsma AK, Van Westreenen HL, Kusters M, Hompes R, Bonjer HJ, Sietses C, Tuynman JB. Locoregional recurrences after transanal total mesorectal excision of rectal cancer during implementation. Br J Surg. 2020 doi: 10.1002/bjs.11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wasmuth HH, Færden AE, Myklebust T, Pfeffer F, Norderval S, Riis R, Olsen OC, Lambrecht JR, Kørner H, Larsen SG, Forsmo HM, Bækkelund O, Lavik S, Knapp JC, Sjo O, Rashid G. Transanal total mesorectal excision for rectal cancer has been suspended in Norway. Br J Surg. 2020;107:121–130. doi: 10.1002/bjs.11459. [DOI] [PubMed] [Google Scholar]
- 65.Atallah S, Sylla P, Wexner SD. Norway versus The Netherlands: will taTME stand the test of time? Tech Coloproctol. 2019;23:803–806. doi: 10.1007/s10151-019-02097-5. [DOI] [PubMed] [Google Scholar]
- 66.Guend H, Widmar M, Patel S, Nash GM, Paty PB, Guillem JGJG, Temple LK, Garcia-Aguilar J, Weiser MR. Developing a robotic colorectal cancer surgery program: understanding institutional and individual learning curves. Surg Endosc. 2017;31:2820–2828. doi: 10.1007/s00464-016-5292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang X-M, Wang Z, Liang J-W, Zhou Z-X. Seniors have a better learning curve for laparoscopic colorectal cancer resection. Asian Pac J Cancer Prev. 2014;15:5395–5399. doi: 10.7314/apjcp.2014.15.13.5395. [DOI] [PubMed] [Google Scholar]
- 68.Soomro NA, Hashimoto DA, Porteous AJ, Ridley CJA, Marsh WJ, Ditto R, Roy S. Systematic review of learning curves in robot-assisted surgery. BJS open. 2020;4:27–44. doi: 10.1002/bjs5.50235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications. Ann Surg 240:205–213. 10.17116/hirurgia2018090162 [DOI] [PMC free article] [PubMed]
- 70.Couwenberg AM, de Beer FSA, Intven MPW, Burbach JPM, Smits AB, Consten ECJ, Schiphorst AHW, Wijffels NAT, de Roos MAJ, Hamaker ME, van Grevenstein WMU, Verkooijen HM. The impact of postoperative complications on health-related quality of life in older patients with rectal cancer; a prospective cohort study. J Geriatr Oncol. 2018;9:102–109. doi: 10.1016/j.jgo.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 71.Kassite I, Bejan-Angoulvant T, Lardy H, Binet A. A systematic review of the learning curve in robotic surgery: range and heterogeneity. Surg Endosc. 2019;33:353–365. doi: 10.1007/s00464-018-6473-9. [DOI] [PubMed] [Google Scholar]
- 72.Kirchhoff P, Clavien PA, Hahnloser D. Complications in colorectal surgery: risk factors and preventive strategies. Patient Saf Surg. 2010;4:1–13. doi: 10.1186/1754-9493-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Novick RJ, Fox SA, Stitt LW, Forbes TL, Steiner S. Direct comparison of risk-adjusted and non-risk-adjusted CUSUM analyses of coronary artery bypass surgery outcomes. J Thorac Cardiovasc Surg. 2006;132:386–391. doi: 10.1016/j.jtcvs.2006.02.053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available on request, this includes template data collection forms, data extracted from included studies, data used for analyses and any other materials used in the review.
Not applicable.