Abstract
Diploid Saccharomyces cerevisiae cells induce YVH1 expression and enter the developmental pathway, leading to sporulation when starved for nitrogen. We show that yvh1 disruption causes a defect in spore maturation; overexpression of MCK1 or IME1 suppresses this yvh1 phenotype. While mck1 mutations are epistatic to those in yvh1 relative to spore maturation, overexpression of MCK1 does not suppress the yvh1 slow-vegetative-growth phenotype. We conclude that (i) Yvh1p functions earlier than Mck1p and Ime1p in the signal transduction cascade that regulates sporulation and is triggered by nitrogen starvation and (ii) the role of Yvh1p in gametogenesis can be genetically distinguished from its role in vegetative growth.
Starvation for nitrogen, in the absence of a fermentable carbon source, causes diploid strains of Saccharomyces cerevisiae to sporulate. Sporulation is a complex developmental pathway involving many genes, whose products mediate progression through two meiotic divisions, spore morphogenesis and packaging (reviewed in reference 17). Initiation of meiosis requires that both nutritional and cell-type-specific signals be detected, integrated, and transmitted to modulate the expression of the sporulation master regulator, IME1, and/or the transcriptional activation activity of its product (12, 15, 17). The mechanism, which ensures that only diploid cells sporulate, is well understood. Diploid cells produce a1/α2, a transcriptional repressor that prevents transcription of the RME1 gene (19). Since Rme1p binds to a 21-bp sequence upstream of IME1 (designated RRE) and prevents its expression, a1/α2 repression of RME1 expression removes its negative regulation of IME1 expression (8). This view is supported by the observation that MATα/MATα diploids cannot sporulate unless Rme1p is absent or nonfunctional (23).
Sagee et al. have analyzed the IME1 promoter and identified four distinct upstream controlling sequences (UCS1 to -4) (24). UCS1, -3, and -4 exhibit the characteristics of negative elements, whereas UCS2 is a positive element required for IME1 expression. Both carbon signaling and nitrogen signaling are transmitted to IME1 but by different mechanisms. Glucose signaling is transmitted to IME1 via the Ras-cyclic AMP pathway in conjunction with Msn2p. Msn2p is a transcriptional activator binding to IRE, an element that is repeated in the IME1 promoter and shares homology to the stress response element (16, 25). Nitrogen signaling acts through the TATA-proximal UCS1, but the mechanism of this control is unknown (24).
Another protein known to regulate IME1 expression in response to nutritional signals is Mck1p, a dual-specificity protein kinase of the glycogen synthase kinase family (1, 21). Mck1p is a key regulator of entry into meiosis, as well as a participant in centromere function in vegetatively growing cells (11, 26). mck1 null mutations reduce but do not abolish IME1 expression and also possess an Ime1p-independent defect in spore morphogenesis (21). The fact that Mck1p is a dual-specificity protein kinase raises the possibility that phosphorylation has a regulatory function similar to those of other protein kinases. Further, Mck1p directly interacts with and negatively regulates pyruvate kinase, a key glycolytic enzyme, likely by phosphorylation of Pyk1p (3). Although Mck1p activity is required for a variety of regulatory functions, the mechanism by which it modulates IME1 expression in response to nutritional signals, or how it promotes spore morphogenesis independently of Ime1p, is unknown. The mechanisms, however, are likely to be posttranslational because MCK1 expression is not regulated by the cell’s nutritional state (21). Genetic experiments argue against Mck1p functioning in the same control pathways as either Rme1p, which transmits cell-type signals, or Ime4p, another protein known to affect IME1 expression and thought to transmit both nutrient and cell type signals (21, 28).
Here we demonstrate that in addition to the previously characterized phenotype of slow progression through meiosis, yvh1 disruption strains are defective in synthesizing dityrosine, a major constituent of ascospore walls. This observation indicates that dual-specificity Yvh1p phosphatase is required for spore maturation. Overexpression of MCK1 or IME1 was found to suppress the yvh1 mutant phenotype associated with sporulation. Neither of these genes, however, suppressed the slow-vegetative-growth phenotype of a yvh1 disruption, suggesting that the role of Yvh1p in vegetative growth is genetically distinct from its role in sporulation.
MATERIALS AND METHODS
Fluorescence assay.
The fluorescence assay, which monitors spore maturation, was performed as described by Wagner et al. (29), with the modification of using Glusulase (Sigma type H-2) and increasing the volume of ammonium hydroxide to 0.6 ml prior to photography. Strains GYC86, HPY120, and HPY123 were patched directly onto sterile nylon colony/plaque screen (NEN Life Sciences) membranes. The nylon membranes were transferred to a YEPD plate and allowed to grow at 30°C for 12 to 18 hours. Here and in all instances described below, the membranes were oriented with the cells side up. Thereafter, the nylon membrane was transferred to a plate containing sporulation medium and incubated at 25°C for 144 h or the time indicated. Lifts were then removed from the plate and transferred to a plastic petri dish containing cell wall lysis buffer (350 μl of 0.1 M sodium citrate [pH 5.8], 0.01 M EDTA, 15 μl of 2-mercaptoethanol, 70 μl of Glusulase [Sigma type H-2]). After being digested at 37°C for 3 to 4 h, the nylon membranes were quickly blotted onto a piece of Whatmann 3MM chromatography paper and transferred to a clean petri dish containing 0.6 ml of concentrated ammonium hydroxide. The top of the petri dish was replaced, and the nylon membrane incubated for 1 min before being placed on a piece of black paper. The nylon lift was illuminated with UV from above and photographed with a Kodak 47B Wratten filter and Polaroid type 57 film (type 55 film would not work).
To test the ability of transformants to rescue the spore maturation defect, transformants were patched onto nylon lifts, overlaid onto selective medium, grown for 12 to 18 h under selective conditions, and then transferred to YEPD plates and processed as described above. It is important to note that the relative levels of fluorescence differ from lift to lift. We attribute this variability to the directness of light used for excitation and the degree to which the cells were completely digested by Glusulase. Therefore, quantitatively comparing fluorescence observed on one membrane to that on another is strongly discouraged. We also have not attempted to quantitate the effectiveness of suppression for particular alleles and have scored all alleles only as plus or minus with respect to the same strain transformed with the parent control vector. For precisely this reason, control transformants containing the parent vector plasmid are included in every lift.
A slightly different method was used for screening of the genomic library. Diploid strain HPY120 (yvh1::HIS3) was transformed with a genomic bank contained on the vector Yep13, selecting for leucine prototrophy. A total of 7,500 independent Leu+ colonies were patched directly onto grids (1,500 independent transformants per large colony/plaque screen membrane) and placed on the surface of a large petri plate containing medium that selects for growth only of strains containing a genomic library plasmid; these plates were the master plates. When the cells had grown sufficiently, a large colony/plaque screen membrane was carefully laid upon the emerging colonies. It was then carefully peeled off the plate and transferred to a second plate containing selective medium (one lacking leucine) overnight before being transferred again to a YEPD plate. After 12 to 18 h incubation, the membrane was transferred to a sporulation plate for 144 h. The fluorescence assay was then used as described for the smaller lifts except that the volumes of cell wall lysis buffer and concentrated ammonium hydroxide were scaled up. Once putative suppressors were identified by fluorescence, corresponding live cells were taken from the master plate and retested to confirm the suppression phenotype. Putative suppressor plasmids, designated SYF (suppressor of YVH1-associated fluorescence [e.g., pSYF60]), were recovered from the transformants by using standard genetic methods.
Plasmid construction.
To construct plasmid pAB7, containing an epitope-tagged YVH1 gene, the 0.7-kb XbaI/XhoI fragment of plasmid pHP175 (22) was subcloned into plasmid Litmus28 (New England Biolabs) to generate plasmid pAB5 which was mutagenized with the MORPH mutagenesis system, using a mutagenic oligonucleotide to generate an in-frame NdeI site coincident with the initiation methionine (boldface), CAT ATG. The entire XbaI/XhoI fragment of the resulting plasmid (pAB5-NdeI) was sequenced to confirm that no additional mutations had been introduced. This plasmid was digested with NdeI, and a DNA fragment encoding the hemagglutinin (HA) epitope was introduced. The resulting plasmid was digested with NsiI and BglII, and the 400-bp fragment (containing the in-frame HA tag) was isolated and cloned into NsiI-BglII-digested plasmid vector pAB6 (2.2-kb XbaI fragment from plasmid pHP145 cloned into XbaI-digested plasmid pBluescript KS+) to generate plasmid pAB7. Therefore, plasmid pAB7 consists of an N-terminal HA-tagged allele of YVH1, transcribed from its own promoter. When the insert of plasmid pAB7 is cloned into plasmid pRS316 (27) to yield plasmid pRSHAYVH1, the latter plasmid complements the yvh1 disruption mutation (data not shown). Plasmid pHAYVH1 contains the 2.2-kb XbaI fragment of plasmid pAB7 cloned into vector plasmid Yep24 linearized with NheI.
A truncated version of YVH1 (containing amino acids 1 to 214) was generated by using oligonucleotides containing unique BamHI sites and plasmid pAB7 as the template. After the PCR product was completely sequenced to ensure that no additional mutations were present, the BamHI fragment was cloned into plasmid Yep24 digested with BamHI.
Since MCK1 was predicted to be contained on plasmid pSYF60, it was digested with StuI and the resulting 2.1-kb band was isolated and cloned into plasmid Litmus28 previously digested with EcoRV, generating plasmid pAB27. The insert contained on pAB27 was sequenced and found to contain an unexpected BamHI site so that a 2.1-kb fragment containing the MCK1 gene and its promoter could be excised and moved into other vectors, using restriction endonuclease BamHI. This 2.1-kb BamHI fragment from plasmid pAB27 was subcloned and ligated into vector plasmid Yep24 to generate plasmid pMCK1. This 2.1-kb BamHI fragment was also ligated into centromere-based vector plasmid pRS316 to yield plasmid p316AB27.
To generate the catalytic null allele of MCK1, plasmid pAB27 was digested with ApaI and SacII; the backbone was isolated and ligated to a 250-bp fragment generated by PCR. This introduced a change at codon 68 from AAA (Lys) to AGG (Arg). The resulting plasmid, pAB50, was sequenced through the SacII and ApaI sites to confirm the presence of only the desired mutation. To construct a plasmid that could be used to generate a mck1 deletion, plasmid pAB27 was digested with SacII and XmnI, resulting in the removal of 25% of the MCK1 coding sequence, including all of the conserved kinase domains VII, VIII, IX and portions of VI and X (21). A TRP1 allele carried on a DNA fragment with SacII and XmnI termini was generated by PCR. The resulting PCR product was cloned into plasmid pAB27 similarly digested to generate plasmid pAB34.
To construct plasmid pIME1, the 6.0-kb BamHI-XhoI fragment of plasmid pIME1BX (gift from M. Clancy) was isolated and ligated into vector plasmid Yep24 previously digested with BamHI and SalI.
Sporulation frequency.
The extent of sporulation was determined by using transformants that were processed identically to those used in the fluorescence assay. The transformants were grown on Ura− medium, transferred to YEPD medium for 12 to 18 h, and then transferred onto sporulation plates for 144 h. After 144 h on sporulation plates, the cells were scraped from the lift membrane and 1.0 ml of 70% ethanol was added to them. The cells were vortexed and stored at 4°C. The cells were then processed for propidium iodide staining as described by Nash et al. (20).
RESULTS AND DISCUSSION
Yvh1 protein phosphatase is specifically required for spore maturation.
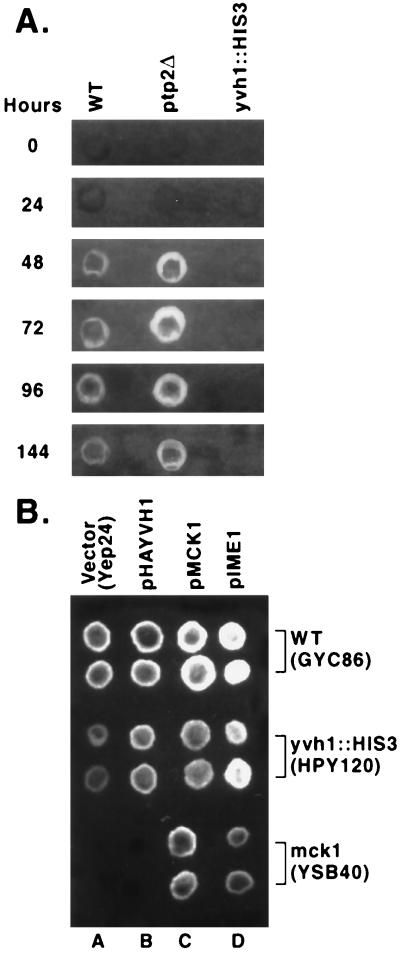
We have previously demonstrated that Yvh1p participates in controlling the onset and progression of S. cerevisiae through meiosis (22). yvh1 mutant strains pass through meiosis I and II and initiate spore development, but do so more slowly and less frequently than wild-type cells (22). These observations prompted us to seek a genetically tractable assay of the yvh1 phenotype. Therefore, we investigated events that occur late in spore maturation. Dityrosine production is such an event. Dityrosine is a major component of the spore wall but is completely absent in vegetative cells or in cells with certain defects in spore maturation (4, 6, 7, 9). The presence of dityrosine can be detected by the molecule’s inherent pH-dependent fluorescence. Sporulated yvh1 disruption mutants are unable to fluoresce, whereas ptp2 (encoding a related protein phosphatase implicated in the Hog1p osmosensing mitogen-activated protein kinase cascade) deletion strains possessed no such defect and fluoresced just like wild-type cells (Fig. 1A). As expected of a marker for spore maturation, this fluorescence is cell type restricted (Fig. 1A). The time at which fluorescence appears (Fig. 2A) also correlates well with the onset of spore development (22). This effect is not merely temporal, however, because yvh1 disruption strains lack the ability to fluoresce even if left in sporulation medium for up to 12 days (data not shown).
FIG. 1.
(A) Strain- and cell-type-restricted dityrosine production. Wild-type (WT) diploid (2n; GYC86), wild-type haploid (n; GYC121 and GYC122), yvh1 disruption diploid (2n; HPY120), yvh1 disruption haploid (n; GYC123 and GYC124), and diploid ptp2 deletion (2n; HPY123) strains were assayed after 144 h on sporulation medium as described in Materials and Methods. (B) Genetic screen for suppressors of a yvh1 disruption. The fluorescence assay was as described for the larger lifts in Materials and Methods. The presence of fluorescent colonies (arrows) in a background of nonfluorescing colonies was taken as indicative of suppression.
FIG. 2.
(A) Appearance of dityrosine fluorescence in diploid wild-type (WT; GYC86), ptp2 deletion (HPY123), and yvh1 disruption (HPY120) strains incubated for increasing amounts of time on sporulation plates. (B) Epistatic relationships of three genes which regulate sporulation in S. cerevisiae. Wild-type (GYC86), yvh1 disruption (HPY120), and mck1 deletion (YSB40) strains were transformed with parent vector plasmid Yep24 alone (column A) or carrying HA-tagged YVH1 (pHAYVH1; column B), MCK1 (pMCK1; column C), or IME1 (pIME1; column D). All three genes were expressed from their native promoters. Each assay appears in duplicate. There were reproducible strain-specific differences in the vector plasmid lane (column A); the mck1 deletion exhibited a more extreme phenotype than the yvh1 deletion mutant. The total lack of fluorescence with the mck1 deletion (column A) did not derive from a lack of cells on the membrane. Suppression capacity was always scored with respect to the test strain carrying the parent vector plasmid (Yep24 [column A]).
Multiple copies of MCK1 can suppress the fluorescence defect associated with yvh1 disruption.
Although a smaller percentage of yvh1 mutant cells sporulate compared with wild type and proceed through sporulation more slowly, these characteristics are too subtle to support extensive genetic screening. However, the lack of fluorescence associated with yvh1 disruption (Fig. 1A) permitted us to use this assay to identify high-copy-number suppressors of its phenotype. This approach yielded suppressors that we arbitrarily divided into strong, moderate, and weak based on the fluorescence intensity of the patched cells (Fig. 1B). One of the strongly suppressing plasmids (pSYF60) was characterized further.
Sequence data from plasmid pSYF60, which carried an approximately 7-kb insert, indicated that it contained the MCK1, YNL306W, and YNL305C genes and portions of YNL308C and YNL304W. The requirement of Mck1p (protein kinase) for maximal IME1 expression, as well as its Ime1p-independent role in spore maturation (21), made it a good candidate as the source of plasmid pSYF60’s ability to suppress the yvh1 fluorescence defect. To determine whether suppression was due exclusively to MCK1, the gene was subcloned into an episomal vector to yield plasmid pMCK1. When plasmid pMCK1 was used to transform yvh1 disruption mutant HPY120, the transformants fluoresced, indicating that MCK1 suppressed the yvh1 defect (Fig. 2B; compare columns A and C for strain HPY120). To ensure that Mck1p’s ability to suppress the yvh1 phenotype reflected a physiological role in dityrosine production, a homozygous diploid mck1 deletion strain (YSB40) was constructed. This deletion mutant, which failed to produce detectable MCK1 mRNA (data not shown), was unable to fluoresce (Fig. 2B; compare columns A and C for strain YSB40); i.e., it possessed a phenotype similar that observed with yvh1 disruption strains (Fig. 2B; compare column A for strain HPY120 and YSB40). Further, it appears as though the defect in spore maturation is more pronounced in the mck1 null strain than in the yvh1 disruption strain, an observation that occurs reproducibly (Fig. 2B; compare strain HPY120 [column A] with strain YSB40 [column A]).
Sporulation occurs more slowly and two- to threefold less frequently in a yvh1 disruption strain than in the wild type (22). To ascertain whether overexpression of MCK1 could suppress this characteristic, we monitored sporulation microscopically in a diploid yvh1 mutant (strain HPY120) transformed with one of four plasmids. Following sporulation of these transformants for 144 h, the percentage of sporulated cells was determined. About 7% of transformants carrying the vector plasmid (Yep24) sporulated (Fig. 3). In contrast, sporulation increased twofold when the mutant was transformed with plasmid Yep24 carrying either a wild-type allele of YVH1 (plasmid Yep24-YVH1) or MCK1 (plasmid Yep24-MCK1). Therefore, overexpression of YVH1 or MCK1 suppressed the yvh1 disruption to the same degree as observed when the mutation was complemented with a wild-type YVH1 allele.
FIG. 3.
Effect of episomal expression of fluorescence suppressors on sporulation. Strain HPY120 cells transformed with parent vector Yep24 or plasmid Yep24-IME1, Yep24-MCK1, or Yep24-YVH1 were processed as described in Materials and Methods. The extent of sporulation, determined as cells which contained three or four spores, was determined by visual inspection of at least 1,000 cells per determination.
Our genetic assays identified MCK1 as the gene carried on plasmid pSYF60 that was responsible for suppressing the yvh1 spore maturation defect. To ensure that Mck1p-dependent suppression of yvh1 required Mck1p protein kinase activity (14), we constructed a MCK1 allele (MCK1K68R), which has been shown to abolish ATP binding in related protein kinases (2). This allele and the cognate wild type were cloned into high-copy-number plasmid vector Yep24, and the resulting plasmids (pMCK1K68R and pMCK1, respectively) were used to transform yvh1 disruption (HPY120) and mck1 deletion (YSB40) strains. Dityrosine formation was tested in these six transformants by using the fluorescence assay (Fig. 4). Only the wild-type MCK1 allele (plasmid Yep24/MCK1) suppressed the fluorescence defects of yvh1 and mck1 mutant strains.
FIG. 4.
Mck1 protein kinase activity is required to suppress the fluorescence defect of a yvh1 disruption (HPY120) or mck1 YSB40 (YSB40) mutations. The strains were transformed the vector (plasmid Yep24), wild-type MCK1 (plasmid Yep24MCK1), or mck1 catalytic null allele (plasmid Yep24MCK1K68R), and the fluorescence assay was performed as described in Materials and Methods; each transformant was assayed in duplicate. The degree to which fluorescence is lacking in a mck1 mutant is similar to that seen in Fig. 2B; i.e., deletion of MCK1 has a stronger fluorescence defect than disruption of YVH1.
Mck1p and Ime1p lie downstream of Yvh1p in the spore development pathway.
Since both Mck1p and Yvh1p are required for fluorescence, we established the epistatic relationship of these two genes. As shown in Fig. 2B, and as suggested by the isolation of plasmid pSYF60, high-level expression of MCK1 suppresses the fluorescence defect of yvh1-disrupted strains, whereas the parent vector Yep24 does not (compare columns A and C for strain HPY120). The IME1 gene was not isolated in our screening. However, we were prompted, by virtue of its relationship to Mck1p (17), to test whether its overexpression could suppress the fluorescence defect of a yvh1 mutant. IME1 was able to suppress the fluorescence defect (Fig. 2B; compare columns A and D for strain HYP120) as well as the yvh1 sporulation phenotype (Fig. 3; compare bars for plasmids Yep24 and Yep24-IME1). Although overexpression of MCK1 could suppress the yvh1 disruption phenotype, overexpression of YVH1 could not suppress the fluorescence defect of a mck1 deletion mutant (Fig. 2B; compare columns A and B for strain YSB40). On the other hand, Ime1p, shown to act downstream of Mck1p in the promotion of meiosis, did suppress the mck1 deletion phenotype (Fig. 2B; compare columns A and D for strain YSB40). These experiments argue that Yvh1p is situated upstream of Mck1p and Ime1p in the spore maturation regulatory pathway. They also identify Yvh1 as the protein (of those currently known) most proximal to the spore maturation signal.
The roles of Yvh1p in sporulation and vegetative growth are genetically distinct.
In addition to its defect in sporulation, a yvh1 disruption mutant grows slowly relative to wild type (10). In view of this second defect, we determined whether suppressors of the fluorescence defect also suppressed slow vegetative growth. Episomal expression of MCK1, which suppressed the fluorescence defect, was incapable of suppressing the growth defect irrespective of whether it was expressed from a high-copy-number, episomal, or centromere-based vector plasmid (Fig. 5); i.e., growth was no greater in transformants carrying plasmid pMCK1 than in those carrying a yvh1 deletion mutant or the vector alone (Fig. 5). A truncated form of Yvh1p, containing the N-terminal 214 amino acids, was similarly unable to complement the yvh1 growth phenotype (Fig. 5). These results suggest that the role Yvh1p plays in promoting vegetative growth can be genetically distinguished from its role in spore morphogenesis.
FIG. 5.
The slow-growth phenotype of a yvh1 disruption mutant cannot be suppressed by overexpression of MCK1. (A) Strain HPY120 was transformed with plasmids Yep24, pHAYVH1, and pHAyvh1. All plasmids were Yep24 vector based. Transformants were streaked onto yeast nitrogen base-glucose-ammonia-Casamino Acids medium. Both plates were incubated for the same length of time. a.a., amino acids. (B) Strain HPY120 was transformed with plasmids pRS316 (centromere [CEN]-based vector plasmid) (27), pYVH1 (22), and p316AB27 (Materials and Methods). The latter two plasmids contain wild-type alleles of YVH1 and MCK1, respectively, cloned into plasmid pRS316. Transformation and plating conditions are as for panel A.
The experiments described above allowed us to establish the order of function: nitrogen starvation signal-Yvh1p-Mck1p-Ime1p-spore maturation. In other words, Yvh1p precedes Mck1p in the nitrogen starvation signal transduction cascade. It is important to emphasize, however, that our genetic assays cannot establish whether Yvh1p and Mck1p are contiguous steps in the cascade. As would be expected of a developmental pathway controlled by the phosphorylation state of a key regulator, a variant of MCK1 which has been shown by others to be catalytically inactive (14) is incapable of suppressing the spore development defects of yvh1 disruption or mck1 deletion.
From our data it can be suggested that Yvh1p functions in spore maturation by virtue of promoting IME1 and IME2 expression, a characteristic shared in common with Mck1p. Consistent with this interpretation is the observation that overexpression of IME1 suppresses the spore maturation defects (that we measured as dityrosine fluorescence) of both yvh1 and mck1 mutations. This is not quite congruent with the conclusion derived from the experiments of Neigeborn and Mitchell, who reported an IME1 expression-independent spore maturation defect (measured by spore morphology) for mck1 mutants (21). We can offer two possible ways of resolving the seeming incongruence and the manner in which IME1 was overexpressed. The first and most obvious difference was the strains used for the experiments. Another possibility is that the assays of events upon which the two conclusions were derived are quite different. In short, the incongruence of our results with those from Mitchell’s laboratory may well be more apparent than real.
Yvh1p is not the only regulatory protein to participate in the early and late phases of the sporulation pathway as well as in vegetative growth. Mck1p and Cak1p possess similar characteristics. Mck1p has a role in vegetative growth that is associated with centromere function; it also modulates the activity of a key glycolytic enzyme, pyruvate kinase (3). Mck1, also like Yvh1p, is required for maximal expression of IME1 and later in spore maturation. Cak1p (cyclin-dependent kinase-activating kinase 1) has been shown to participate in vegetative growth by activating Cdc28p, the cyclin-dependent protein kinase required for cell cycle progression, and has also been identified as a suppressor of the spore maturation defect caused by the smk1-2 mutation (29). There are important differences, however, in how YVH1, MCK1, and CAK1 gene expression is regulated. Mck1p production is not induced by nutritional signals. CAK1 is expressed in vegetative cells, but transfer of cells to sporulation medium causes a decrease in steady state levels of CAK1 mRNA followed by its reappearance in a way that is temporally similar to two known late sporulation genes, SMK1 and SPS1 (29). Yvh1p production, on the other hand, is rapidly induced following nitrogen starvation and thus would not have been a priori a likely candidate for a late sporulation specific gene such as SMK1, SPS1, DIT1, or DIT2 (5, 13). Nevertheless, disruption of YVH1 has a similar terminal phenotype as deletion of these late sporulation-specific genes.
A second important conclusion to derive from these experiments is that the Yvh1p function in the nitrogen starvation-associated signal transduction pathway that is suppressed by Mck1p overproduction appears to be distinct from its role in vegetative growth. These roles are genetically distinguished by the observation that overproduction of MCK1, which suppresses the yvh1 fluorescence defect but not the slow-vegetative-growth phenotype of yvh1 disruption mutants. The use of signal transduction pathway components for multiple physiological purposes is an increasingly common finding and makes good sense in terms of a cell’s economical use of its resources.
ACKNOWLEDGMENTS
We thank members of the UT Yeast Group who read the manuscript and offered suggestions for improvement. Oligonucleotides were prepared by the UT Molecular Resource Center.
This work was supported by Public Health Service grant GM-35642.
REFERENCES
- 1.Bianchi M W, Plyte S E, Kreis M, Woodgett J R. A Saccharomyces cerevisiae protein-serine kinase related to mammalian glycogen synthase kinase-3 and the Drosophila melanogaster gene shaggy product. Gene. 1993;134:51–56. doi: 10.1016/0378-1119(93)90173-z. [DOI] [PubMed] [Google Scholar]
- 2.Booher R, Beach D. Site-specific mutagenesis of cdc2+, a cell cycle control gene of the fission yeast Schizosaccharomyces pombe. Mol Cell Biol. 1986;6:3523–3530. doi: 10.1128/mcb.6.10.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brazill D T, Thorner J, Martin G S. Mck1, a member of the glycogen synthase kinase 3 family of protein kinases, is a negative regulator of pyruvate kinase in the yeast Saccharomyces cerevisiae. J Bacteriol. 1997;179:4415–4418. doi: 10.1128/jb.179.13.4415-4418.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briza P, Breitenbach M, Ellinger A, Segall J. Isolation of two developmentally regulated genes involved in spore wall maturation in Saccharomyces cerevisiae. Genes Dev. 1990;4:1775–1789. doi: 10.1101/gad.4.10.1775. [DOI] [PubMed] [Google Scholar]
- 5.Briza P, Eckerstorfer M, Breitenbach M. The sporulation-specific enzymes encoded by the DIT1 and DIT2 genes catalyze a two-step reaction leading to a soluble ll-dityrosine-containing precursor of the yeast spore wall. Proc Natl Acad Sci USA. 1994;91:4524–4528. doi: 10.1073/pnas.91.10.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briza P, Ellinger A, Winkler G, Breitenbach M. Chemical composition of the yeast ascospore wall. The second outer layer consists of chitosan. J Biol Chem. 1988;263:11569–11574. [PubMed] [Google Scholar]
- 7.Briza P, Winkler G, Kalchhauser H, Breitenbach M. Dityrosine is a prominent component of the yeast ascospore wall. A proof of its structure. J Biol Chem. 1986;261:4288–4294. [PubMed] [Google Scholar]
- 8.Covitz P A, Mitchell A P. Repression by the yeast meiotic inhibitor RME1. Genes Dev. 1993;7:1598–1608. doi: 10.1101/gad.7.8.1598. [DOI] [PubMed] [Google Scholar]
- 9.Esposito R E, Dresser M, Breitenbach M. Identifying sporulation genes, visualizing synaptonemal complexes, and large-scale spore and spore wall purification. Methods Enzymol. 1991;194:110–131. doi: 10.1016/0076-6879(91)94010-a. [DOI] [PubMed] [Google Scholar]
- 10.Guan K, Hakes D J, Wang Y, Park H D, Cooper T G, Dixon J E. A yeast protein phosphatase related to the vaccinia virus VH1 phosphatase is induced by nitrogen starvation. Proc Natl Acad Sci USA. 1992;89:12175–12179. doi: 10.1073/pnas.89.24.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang W, Lim M Y, Yoon H J, Thorner J, Martin G S, Carbon J. Overexpression of the yeast MCK1 protein kinase suppresses conditional mutations in centromere-binding protein genes CBF2 and CBF5. Mol Gen Genet. 1995;246:360–366. doi: 10.1007/BF00288609. [DOI] [PubMed] [Google Scholar]
- 12.Kassir Y, Granot D, Simchen G. IME1, a positive regulator gene of meiosis in S. cerevisiae. Cell. 1988;52:853–862. doi: 10.1016/0092-8674(88)90427-8. [DOI] [PubMed] [Google Scholar]
- 13.Krisak L, Strich R, Winters R S, Hall J P, Mallory M J, Kreitzer D, Tuan R S, Winter E. SMK1, a developmentally regulated MAP kinase, is required for spore wall assembly in Saccharomyces cerevisiae. Genes Dev. 1994;8:2151–2161. doi: 10.1101/gad.8.18.2151. [DOI] [PubMed] [Google Scholar]
- 14.Lim M Y, Dailey D, Martin G S, Thorner J. Yeast MCK1 protein kinase autophosphorylates at tyrosine and serine but phosphorylates exogenous substrates at serine and threonine. J Biol Chem. 1993;268:21155–21164. [PubMed] [Google Scholar]
- 15.Mandel S, Robzyk K, Kassir Y. IME1 gene encodes a transcription factor which is required to induce meiosis in Saccharomyces cerevisiae. Dev Genet. 1994;15:139–147. doi: 10.1002/dvg.1020150204. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Pastor M T, Marchler G, Schuller C, Marchler-Bauer A, Ruis H, Estruch F. The Saccharomyces cerevisiae zinc finger proteins Msn2p and Msn4p are required for transcriptional induction through the stress response element (STRE) EMBO J. 1996;15:2227–2235. [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell A P. Control of meiotic gene expression in Saccharomyces cerevisiae. Microbiol Rev. 1994;58:56–70. doi: 10.1128/mr.58.1.56-70.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell A P, Driscoll S E, Smith H E. Positive control of sporulation-specific genes by the IME1 and IME2 products in Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:2104–2110. doi: 10.1128/mcb.10.5.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell A P, Herskowitz I. Activation of meiosis and sporulation by repression of the RME1 product in yeast. Nature. 1986;319:738–742. doi: 10.1038/319738a0. [DOI] [PubMed] [Google Scholar]
- 20.Nash R, Tokiwa G, Anand S, Erickson K, Futcher B. The WHI1+ gene of Saccharomyces cerevisiae tethers cell division to cell size and is a cyclin homolog. EMBO J. 1988;7:4335–4346. doi: 10.1002/j.1460-2075.1988.tb03332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neigeborn L, Mitchell A P. The yeast MCK1 gene encodes a protein kinase homolog that activates early meiotic gene expression. Genes Dev. 1991;5:533–548. doi: 10.1101/gad.5.4.533. [DOI] [PubMed] [Google Scholar]
- 22.Park H D, Beeser A E, Clancy M J, Cooper T G. The S. cerevisiae nitrogen starvation-induced Yvh1p and Ptp2p phosphatases play a role in control of sporulation. Yeast. 1996;12:1135–1151. doi: 10.1002/(sici)1097-0061(19960915)12:11<1135::aid-yea11>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 23.Rine J, Sprague G F, Jr, Herskowitz I. rme1 mutation of Saccharomyces cerevisiae: map position and bypass of mating type locus control of sporulation. Mol Cell Biol. 1981;1:958–960. doi: 10.1128/mcb.1.10.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sagee S, Sherman A, Shenhar G, Robzyk K, Ben-Doy N, Simchen G, Kassir Y. Multiple and distinct activation and repression sequences mediate the regulated transcription of IME1, a transcriptional activator of meiosis-specific genes in Saccharomyces cerevisiae. Mol Cell Biol. 1998;18:1985–1995. doi: 10.1128/mcb.18.4.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitt A P, McEntee K. Msn2p, a zinc finger DNA-binding protein, is the transcriptional activator of the multistress response in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1996;93:5777–5782. doi: 10.1073/pnas.93.12.5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shero J H, Hieter P. A suppressor of a centromere DNA mutation encodes a putative protein kinase (MCK1) Genes Dev. 1991;5:549–560. doi: 10.1101/gad.5.4.549. [DOI] [PubMed] [Google Scholar]
- 27.Sikorski R S, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su S S, Mitchell A P. Molecular characterization of the yeast meiotic regulatory gene RIM1. Nucleic Acids Res. 1993;21:3789–3797. doi: 10.1093/nar/21.16.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagner M, Pierce M, Winter E. The CDK-activating kinase CAK1 can dosage suppress sporulation defects of smk1 MAP kinase mutants and is required for spore wall morphogenesis in Saccharomyces cerevisiae. EMBO J. 1997;16:1305–1317. doi: 10.1093/emboj/16.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]