Abstract
Interstitial lung diseases (ILD) encompasses a heterogeneous group of parenchymal lung diseases characterized by variable amounts of inflammation and fibrosis. The targeting of fibroblasts and myofibroblasts with antifibrotic treatments is a potential therapeutic target for these potentially fatal diseases. Treprostinil is unique among the prostacyclin mimetics in that it has distinct actions at additional prostaglandin receptors. Preclinical and clinical evidence suggests that treprostinil has antifibrotic effects through the activation of the prostaglandin E receptor 2 (EP2), the prostaglandin D receptor 1 (DP1), and peroxisome proliferator-activated receptors (PPAR). In vivo studies of EP2 and the DP1 have found that administration of treprostinil resulted in a reduction in cell proliferation, reduced collagen secretion and synthesis, and reduced lung inflammation and fibrosis. In vitro and in vivo studies of PPARβ and PPARγ demonstrated that treprostinil inhibited fibroblast proliferation in a dose-dependent manner. Clinical data from a post hoc analysis of the INCREASE trial found that inhaled treprostinil improved forced vital capacity in the overall population as well as in idiopathic interstitial pneumonia and idiopathic pulmonary fibrosis subgroups. These preclinical and clinical findings suggest a dual benefit of treprostinil through the amelioration of both lung fibrosis and pulmonary hypertension.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-022-02229-8.
Keywords: Antifibrotic, Idiopathic pulmonary fibrosis, Interstitial lung diseases, Pulmonary fibrosis
Key Summary Points
| Treprostinil is approved for the treatment of pulmonary arterial hypertension and pulmonary hypertension associated with interstitial lung disease. |
| The antifibrotic effects of treprostinil are mediated through the activation of the prostaglandin E receptor 2, the prostaglandin D receptor 1, and peroxisome proliferator-activated receptors. |
| Preclinical and clinical data provide evidence for the antifibrotic effects of treprostinil. |
| Treprostinil may have a role in mitigating the effects of fibrosis caused by vascular remodeling, cytokine overexpression, and alveolar wall thickening. |
Digital Features
This article is published with digital features, including an infographic, to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.20073086.
Introduction
The broad category of interstitial lung diseases (ILD) encompasses a heterogeneous group of parenchymal lung diseases that are characterized by varying amounts of inflammation and fibrosis of the bronchioles and interstitium of the lungs [1, 2]. Lung fibrosis may be the result of inflammation in certain ILDs, while in others it may result from fibroblastic proliferation. Fibrosis tends to be progressive and invariably results in the destruction of the normal extracellular lung matrix with architectural distortion [3–5]. The resulting fibrotic tissue hinders gas exchange and reduces lung compliance, leading to progressive shortness of breath, functional impairment, and ultimately respiratory failure and death [6–8].
Numerous therapies have been used off-label to treat ILDs, but there are only four therapies specifically approved for distinct ILD indications. Pirfenidone and nintedanib, which have antifibrotic properties, are both approved for the treatment of idiopathic pulmonary fibrosis (IPF), while nintedanib carries a broader label to include ILD due to scleroderma (SSc-ILD) and patients with a progressive fibrotic phenotype [9, 10]. More recently, the interleukin-6 (IL-6) receptor antagonist tocilizumab was approved for SSc-ILD [11]. There have been numerous clinical trials of pulmonary vasodilators for the treatment of ILD, which were based on the hypothesis that these drugs may ameliorate the vascular component of ILD [12]. Unfortunately, studies of endothelin receptor antagonists and phosphodiesterase 5 inhibitors have been largely negative, with a few even suggesting harm in patients with IPF.
One exception is sildenafil which has demonstrated a quality-of-life benefit and a trend toward improvement in mortality in the STEP-IPF trial [13] as well as lower risk of forced vital capacity (FVC) decline when combined with nintedanib in the INSTAGE trial [14]. These results suggested a potential role for pulmonary vasodilators for ILDs and set the stage for the landmark INCREASE trial, which was a randomized controlled study evaluating inhaled treprostinil in pulmonary hypertension associated with ILD (PH-ILD) [15]. This study met its primary endpoint, demonstrating a 31-m improvement in 6-min walk distance (6MWD), leading to US Food and Drug Administration approval of inhaled treprostinil as the first and only treatment for PH-ILD on April 1, 2021. Interestingly, a post hoc analysis of the INCREASE trial suggested that inhaled treprostinil was associated with an improvement in FVC, which has never been demonstrated in any well-controlled study of ILD [16]. There may be several reasons for this FVC improvement, but one potential mechanism is the antifibrotic property of treprostinil; treprostinil’s role in reducing fibrosis and thereby acting as a potential treatment option for pulmonary fibrosis has been illuminated through recent studies and will be discussed herein. A summary of the preclinical and clinical studies attesting to the antifibrotic effects of treprostinil is provided in Table 1. This review is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Table 1.
Preclinical and clinical evidence for the antifibrotic effects of treprostinil
| Study | Methods and purpose | Main findings |
|---|---|---|
| Preclinical | ||
| In vitro | ||
| Wilborn 1995 [42] | Comparison of lung fibroblasts isolated from patients with IPF and patients undergoing resectional surgery for lung cancer | Demonstrated diminished capacity to synthesize PGE2 and express COX-2 |
| Kolodsick 2003 [33] | Normal human fetal lung fibroblasts were examined to determine if PGE2 could modulate the transition of lung fibroblasts to myofibroblasts | PGE2 inhibits the transition of fibroblasts to myofibroblasts |
| Burgess 2004 [37] | Cultured human airway smooth muscle cells from asthmatic and nonasthmatic patients tested to see if PGE2 can inhibit proliferation and identify the receptors involved | EP2 receptor is responsible for the antiproliferative effects of PGE2 in airway smooth muscle cells |
| Moore 2005 [34] | Fibroblasts from bleomycin-treated C57BL/6 mice were analyzed for prostanoid receptor changes | Loss of PGE2 suppression is associated with reduced expression of EP2 receptors. This resulted in fibroblasts that were unresponsive to PGE2 |
| White 2005 [32] | Cultured normal human fetal lung fibroblasts and embryonic pten-null murine fibroblast cells were analyzed to analyze the effects of treatment with PGE2 | Treatment with PGE2 inhibited fibroblast migration via the EP2 receptor, leading to increased PTEN and diminished fibroblast migration |
| Ali 2006 [44] | Cultured lung tissue from IP- or PPARβ-deficient murine models was examined to assess the role of IP and PPARβ as therapeutic agents for PH | Antiproliferative effects of treprostinil are mediated by PPARβ, not IP |
| Ali 2006 [29] | Blood samples from healthy patients were tested to investigate the presence and function of PPARβ in human platelets | Activation of PPARβ was found to have anti-inflammatory effects and PPARγ was found to inhibit cell proliferation |
| Falcetti 2007 [45] | HEK-293 cells were transfected with human IP to investigate whether PGI2 analogues regulate PPARγ | Prostacyclin analogues activated PPARγ in an IP-dependent manner |
| van den Brule 2010 [43] | Cultured lung fibroblasts from bleomycin-treated female C57BL/6 mice to examine the effects of a DP agonist | Activation of DP1 receptors reduced lung inflammation and fibrosis |
| Ayabe 2013 [55] | Primary human fetal lung fibroblasts were stimulated with TGFβ and treated with PGD2, DP receptor agonists, DP receptor antagonists, or CRTH2 to assess the effects on collagen synthesis and secretion | PGD2 inhibits TGFβ induced collagen secretion via activation of the DP receptor and intracellular cAMP accumulation |
| Dagouassat 2013 [40] | Primary lung fibroblasts from patients with COPD, male C57BL/6 mice, and p53−/− mice to analyze the role of PGE2 in inducing senescence and inflammation | COPD lung fibroblasts had higher levels of EP2 and EP4 receptors than healthy smoking and non-smoking controls and displayed increased senescent markers. p53−/− murine models showed that PGE2 is responsible for this increased senescence and inflammation |
| Safholm 2015 [56] | Cultured healthy human lung tissue samples were treated PGE2 with or without an EP4 receptor antagonist to characterize the effects of PGE2 | EP2 receptors were shown to inhibit mast cell-mediated bronchoconstriction |
| Horikiri 2017 [41] | PGE-MUM levels were analyzed via radioimmunoassay in controls and patients with lung diseases. Human bronchial epithelial cells and lung fibroblast samples were treated with TGFβ to analyze its role in EP2 receptor expression | PGE-MUM levels were increased in patients with chronic lung fibrosis and were correlated with fibrosis scores |
| Lambers 2018 [36] | Human peripheral lung fibroblasts were stimulated with PDGF or TGFβ1, or both and incubated with treprostinil, forskolin, DDA, or vehicle to investigate their effects on PDGF-BB and TGFβ activated intracellular signaling | Treprostinil activated cAMP, preventing PDGF-BB-induced proliferation and TGFβ1 secretion |
| Patel 2018 [38] | Human PASMCs from patients with PAH were treated with agonists, antagonists, or EP2 receptor siRNAs to assess the effects on receptor expression, cell proliferation, and cAMP | EP2 receptors were elevated in PAH cells and treprostinil demonstrated EP2-dependent antiproliferative actions |
| Roberts 2018 [46] | Normal human lung fibroblasts were used to test the function of select Gs-coupled GPCR agonists and their ability to inhibit fibroblast proliferation and differentiation | Formoterol, PGE2, treprostinil, and forskolin all elicited maximal cAMP responses. BAY60-6583 and MRE-269 fully inhibited fibroblast proliferation and differentiation and were partial cAMP agonists. The magnitude of cAMP response was not predictive of antifibrotic efficacy |
| Blumer 2021 [47] | Fibroblasts from human lung tissue from patients with end-stage ILD were isolated and treated with TGFβ1 or TGFβ1 + treprostinil | Phosphorylation of ERK1/2 MAPK was significantly reduced with treprostinil. Treatment with treprostinil also increased the expression of DUSP1, which was decreased by TGFβ1. This resulted in a concentration-dependent reduction in TGFβ1-induced proliferation |
| In vivo | ||
| Corboz 2018 [49] | Rats with bleomycin-induced pulmonary fibrosis were intranasally administered INS1009 to evaluate potential antifibrotic effects and cultured human lung fibroblasts were treated with treprostinil to determine the effects on genes associated with collagen synthesis and secretion | INS1009 dose-dependently reduced lung hydroxyproline, demonstrating an antifibrotic effect of inhaled treprostinil by mechanisms likely involving suppression of collagen production from lung fibroblasts |
| Nikitopoulou 2018 [50] | Mice with bleomycin-induced injury were treated with orotracheally administered treprostinil or vehicle to determine if treprostinil has downstream effects on inflammation and pulmonary fibrosis | Treprostinil reduced bleomycin-induced lung dysfunction and attenuated lung injury compared with mice receiving placebo. Mice treated with inhaled treprostinil showed less inflammation, focal alveolar thickening, and reduced collagen deposition |
| Clinical | ||
| Nathan 2021 Lancet [16] | Evaluated the change in FVC in the overall population from the INCREASE study and subgroup analysis | Treatment with inhaled treprostinil resulted in FVC improvements in patients with PH-ILD. Patients with IIP and IPF also demonstrated FVC improvements |
| Nathan 2021 Chest [52] | Comparison of lung function changes in the INCREASE and TRIUMPH studies | Findings suggest that the pulmonary function test response to inhaled treprostinil differs mechanistically between PAH and PH-ILD, with significant improvements seen in % predicted FVC in patients with PH-ILD but not patients with PAH |
| Waxman 2021 [15] | Evaluated the efficacy and safety of inhaled treprostinil in patients with PH-ILD | Treatment with inhaled treprostinil significantly improved exercise capacity for patients with PH-ILD and was associated with a lower risk of clinical worsening |
| TETON (NCT04708782) [57] | Evaluate the safety and efficacy of inhaled treprostinil in patients with IPF | Ongoing |
ATP adenosine triphosphate, cAMP cyclic adenosine monophosphate, COPD chronic obstructive pulmonary disorder, COX-2 cyclooxygenase-2, CRTH2 chemoattractant receptor–homologous molecule expressed on Th2 cells, DDA dideoxyadenosine, DP prostaglandin D receptor, EP2 prostaglandin E receptor 2, FVC forced vital capacity, GPCR G protein-coupled receptors, IP prostacyclin receptor, IIP idiopathic interstitial pneumonia, IPF idiopathic pulmonary fibrosis, IIP idiopathic interstitial pneumonia, mPGES-1 microsomal prostaglandin E2 synthase 1, MAPK mitogen-activated protein kinase, mRNA messenger ribonucleic acid, PAH pulmonary arterial hypertension, PASMC pulmonary arterial smooth muscle cell, PGE2 prostaglandin E2, PGE-MUM prostaglandin E major urinary metabolite, PH pulmonary hypertension, PH-ILD interstitial lung disease with pulmonary hypertension, PPARβ peroxisome proliferator-activated receptor β, PTEN phosphatase and tensin homolog on chromosome ten, siRNA small interfering ribonucleic acids, SP substance P
Pathophysiology
Pathologic vascular findings are common in ILD and may consist of changes to arteries, arterioles, and venules, as well as the destruction of capillary beds [17]. Increased vascular resistance in pre-capillary vessels leads to increased pulmonary arterial pressure and pulmonary hypertension [18].
Cytokines and cytokine-like molecules, such as transforming growth factor beta 1 (TGFβ1), platelet-derived growth factor (PDGF), and endothelin-1 (a potent vasoconstrictor) [19], have been shown to regulate pulmonary fibrosis [20, 21]. Their overexpression may contribute to vascular remodeling and increased extracellular matrix deposition, leading to increased fibrosis and increased pulmonary arterial pressures [7, 20]. Impaired angiogenesis in fibrotic lung tissue may impact vascular remodeling further and contribute to the rise in pulmonary arterial pressures [22].
Given the complex interplay between the lung interstitium and pulmonary vasculature in ILD, the intersection between the two is an area of emerging interest.
Treprostinil Mechanism of Action
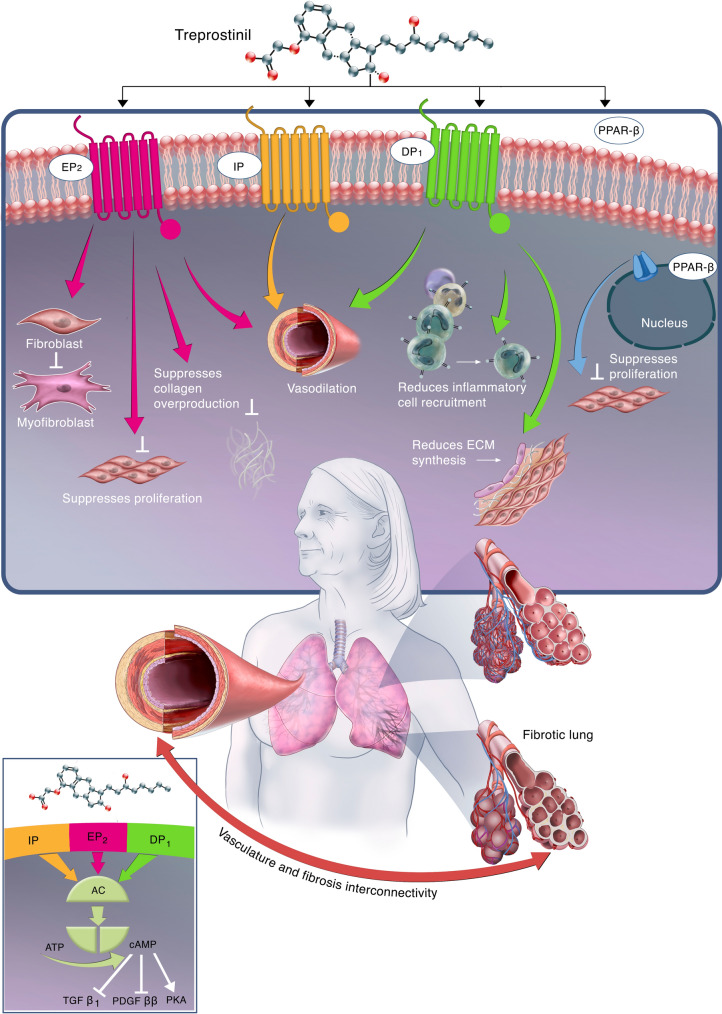
Treprostinil is currently approved for the treatment of pulmonary arterial hypertension (PAH; WHO Group 1) and PH-ILD (WHO Group 3) to improve exercise ability [23]. It is a full prostacyclin receptor (IP) agonist and also has a high affinity for prostaglandin E receptor 2 (EP2) and the prostaglandin D receptor 1 (DP1) [24]. Figure 1 depicts the mechanism of action of treprostinil and physiologic effects resulting from each receptor.
Fig. 1.
Schematic depicting the impact of treprostinil on the relationship between vascular remodeling, cytokine overexpression, and the development of fibrosis within the lungs. Treprostinil binds and activates the EP2, IP, and DP1 receptors and activates the PPARβ receptors to produce antifibrotic effects. Activation of the EP2, IP, and DP1 receptors leads to vasodilation. Activation of EP2 additionally inhibits fibroblast to myofibroblast differentiation, suppresses fibroblast proliferation, and suppresses collagen overproduction. Activation of DP1 additionally reduces inflammatory cell recruitment and reduces extracellular matrix synthesis. Activation of the nuclear receptor PPARβ leads to suppressed fibroblast proliferation. Collectively, treprostinil activates EP2, IP, DP1, and PPARβ and causes vasodilation, reduced vascular remodeling, reduced fibroblast activity, proliferation and collagen deposition, and reduced inflammation, thereby promoting antifibrotic activity. Mechanistically, when IP, EP2, and DP1 are activated, G protein-coupled signaling triggers adenylyl cyclase and converts ATP to cAMP, which drives the activation of TGFβ1, PDGFββ, and PKA, leading to therapeutic effects. Treprostinil activation of PPARβ drives therapeutic effects via an anti-inflammatory pathway, leading to activation of retinoid X receptor, suppression of B cell lymphoma 6, and suppression of protein kinase C-α (not shown). IP prostacyclin receptor, EP2 prostaglandin E type 2 receptor, DP1 prostaglandin D type 1 receptor, PPARβ peroxisome proliferator-activated receptor β, ECM extracellular matrix, AC adenylyl cyclase, ATP adenosine triphosphate, cAMP cyclic adenosine monophosphate, TGFβ1 transforming growth factor β1, PDGFββ platelet-derived growth factor ββ, PKA protein kinase A
Upon activation, IP couples with adenylate cyclase to convert adenosine triphosphate to cyclic adenosine monophosphate (cAMP), activating protein kinase A [25]. A secondary pathway involves activation of peroxisome proliferator-activated receptor β (PPARβ), activating anti-inflammatory mechanisms including activation of the retinoid X receptor to drive gene transcription, suppression of B cell lymphoma 6, and suppression of protein kinase C-α [25]. Both pathways lead to vasorelaxation, reduced thrombosis from platelet inhibition, reduced vascular remodeling and inflammation, and contribute to treprostinil’s antifibrotic effects [25].
When activated, IP, EP2, and DP1 induce a G protein-coupled cascade that results in an increase in protein kinase A which produces treprostinil’s therapeutic effects of vasodilation [26, 27], platelet inhibition [28–30], antiproliferation [31–35], anti-inflammation, and antifibrosis [23, 24, 36]. Activation of these receptors has been shown to inhibit fibroblast proliferation, collagen secretion, and fibroblast-to-myofibroblast differentiation. The following section will review the specific receptors, transcription factors, growth factors, and physiology possibly involved in the antifibrotic process of treprostinil.
Preclinical Data to Support the Antifibrotic Effects of Treprostinil
Role of the EP2 Receptor
The EP2 receptor, an important receptor for its role in fibrosis, is responsible for the antiproliferative effects of prostaglandin E2 (PGE2) in airway smooth muscle cells [37], In PAH, EP2 receptor expression is upregulated compared to lung tissue samples from healthy controls, while IP expression is decreased [38]. This observation may imply that EP2 is upregulated as a consequence of disease, or that pulmonary disease may negatively impact IP expression [38–41]. Therefore, it is important to consider the role of EP2 as a negative modulator of vascular tone, proliferation, and fibrosis.
Experiments have shown that treprostinil’s antifibrotic effects are independent from IP receptors, being more dependent on other receptors such as EP2 and DP1. When human pulmonary arterial smooth muscle cells (PASMCs) were incubated with an IP receptor antagonist (R01138452), the antiproliferative effects of a non-prostanoid IP receptor agonist (MRE-269) were abolished [38]. However, increasing concentrations of treprostinil continued to produce a marked reduction in cell proliferation despite the presence of the IP receptor antagonist [38]. Incubating PASMCs with an EP2 receptor antagonist resulted in the opposite effect, significantly reducing the antiproliferative effects of treprostinil, supporting the activation of EP2 by treprostinil [38].
Activation of EP2 receptors has a range of inhibitory effects on fibroblast function that could have beneficial effects in patients with pulmonary fibrosis [39]. Through different in vitro experiments, fibroblast treatment with PGE2 has displayed inhibition of fibroblast-to-myofibroblast transition and suppression of fibroblast proliferation and reduced collagen synthesis [33, 34]. Because patients with IPF have a diminished capacity to synthesize PGE2 [42], supplementation with PGE2 or a small molecule that activates EP2 receptors, such as treprostinil, could therefore have therapeutic potential for patients.
Role of the DP1 Receptor
In addition to EP2, treprostinil also has a high affinity for the lesser-known DP1, which also contributes to the antifibrotic action of treprostinil through mechanisms similar to EP2.
Further experiments showed that activation of DP1 receptors reduced lung inflammation and fibrosis. C57BL/6 mice were treated with 500 nmol/kg of BW245C, a DP1 agonist, or placebo for 2 days before receiving bleomycin treatment, followed by continued treatment with BW245C or placebo three times a week [43]. Lactate dehydrogenase, a measurement of cell damage, was significantly decreased with BW245C compared with placebo. Treatment with BW245C also reversed bleomycin-induced lymphocyte recruitment and significantly reduced collagen accumulation [43], Overall, these results show that activation of DP1 significantly decreases both inflammatory cell recruitment and pulmonary collagen accumulation, thus providing evidence for the involvement of the DP1 receptor in reducing fibrosis.
As treprostinil has a high affinity for DP1, it follows that treatment with treprostinil would result in decreasing inflammatory cell recruitment and collagen accumulation through the activation of DP1 receptors.
Role of PPARs
PPARs are a family of transcription factors that influence lipid and energy metabolism, epidermal wound repair, inflammation responses, and atherosclerotic plaque formation. There are three major isoforms, PPARα, PPARβ, and PPARγ. The role of PPARs in the antifibrotic effect of treprostinil has been described though is still being explored. A study by Ali et al. showed that treprostinil increased PPARβ which resulted in inhibition of fibroblast proliferation [44].
The effect of treprostinil on PPARγ is not as well defined. A 2006 study by Ali et al. found that PPARγ was not activated by treprostinil [29], but studies by Falcetti et al. found that prostacyclin analogues do activate PPARγ in an IP-dependent manner [45]. These findings were confirmed using the PPARγ antagonist GW9662, which significantly reduced the antiproliferative effects of treprostinil. The PPAR pathway is intriguing and presents an area warranting further exploration.
Cytokines and Growth Factors
When found in healthy tissue, TGFβ1 and PDGF have antiproliferative and apoptotic effects; however, in patients with PAH, TGFβ1 causes excess proliferation of PASMCs [24]. Additionally, patients with PAH have elevated levels of growth factors, including TGFβ1, PDGF, vascular endothelial growth factor, epithelial growth factor, and angiopoietin [24].
A study by Lambers et al. investigated the effects of treprostinil on PDGF and TGFβ1 intracellular signaling in fibroblasts cells from patients with IPF [36]. The study demonstrated that PDGF-BB and TGFβ1 induced α-smooth muscle actin, a marker of activated fibrogenic cells, and that this effect was dose-dependently reduced with treprostinil.
Furthermore, these inhibitory effects on PDGF and TGFβ1 appear to be mediated by the increased activation of cAMP; a downstream effect of relaxant prostanoid receptor activation [36]. Together, these results show that treprostinil can prevent PDGF and TGFβ1-mediated profibrotic proliferation and extracellular matrix synthesis in IPF fibroblasts.
Further research into the effects of prostacyclin agonists elucidated some of the mechanisms behind these downstream effects of cAMP. Roberts et al. found that PDGF-induced fibroblast proliferation was inhibited by treatment with the prostacyclin agonists treprostinil, MRE-269, and iloprost [46]. Treprostinil partially inhibited both processes while generating maximal cAMP; however, iloprost did not block PDGF-induced proliferation despite also causing maximal cAMP signaling.
Recent research has shown that the microRNAs negatively regulate the downstream fibrotic effects of TGFβ1, but clusters of microRNAs are dysregulated in patients with IPF and other fibrotic lung diseases, leading to increases in Erk1/2 mitogen-activated protein kinase [47]. When lung tissue samples from patients with end-stage ILD were treated with treprostinil, this dysregulation was overcome in a concentration-dependent manner through the upregulation of DUSP1, an Erk1/2 mitogen-activated protein kinase inhibitor, reducing TGFβ1-induced proliferation [47].
Bleomycin Lung Models
To further evaluate the antifibrotic effects of treprostinil, animal models were simulated in two significant studies using bleomycin-induced pulmonary fibrosis models [48]. In a study by Corboz et al., rats were treated with 10, 30, or 100 μg/kg of INS1009, an inhaled prodrug of treprostinil, inhaled phosphate buffer saline (PBS), or 100 mg/kg pirfenidone administered orally twice daily for 17 days, beginning 10 days post-bleomycin challenge [49].
All doses of INS1009 significantly reduced hydroxyproline, a marker of collagen deposition and synthesis, in the lungs in a dose-dependent manner. The lowest dose of treprostinil, 10 μg/kg, produced a 44% reduction in hydroxyproline levels, while the medium and high doses resulted in 68% and 88% reductions, respectively. Orally administered pirfenidone also reduced hydroxyproline, but to a lesser extent than the upper doses of treprostinil (60%) [49].
In the same study, bleomycin-induced pulmonary fibrosis was also analyzed by measuring changes in lung mass to assess fibrotic changes and collagen deposition. Bleomycin-challenged animals treated with inhaled PBS had a significant increase in right caudal lung mass; whereas, rats treated with an inhaled prodrug of treprostinil exhibited a dose-dependent reduction in right caudal lung mass. These results demonstrate an antifibrotic effect of inhaled treprostinil in a rat model of bleomycin-induced pulmonary fibrosis by mechanisms involving the suppression of collagen production from lung fibroblasts [49].
Additional animal model studies by Nikitopoulou et al. evaluated the effect of inhaled treprostinil on inflammation, pulmonary fibrosis, and vascular remodeling in a bleomycin-induced model of pulmonary fibrosis [50]. Inhaled treprostinil or placebo were administered to mice twice daily beginning 1 day prior to bleomycin or saline challenge. Impaired breathing and lung function were assessed by measuring tissue elasticity and static compliance on days 7, 14, and 21. Daily treatment with treprostinil reduced bleomycin-induced lung dysfunction compared with mice receiving placebo. A statistically significant change in elasticity was achieved at all three time points, and on day 21 for static compliance. Histological analyses of the mice showed that inhaled treprostinil attenuated lung injury. Tissue samples displayed less inflammation as well as a reduction of alveolar wall thickening and collagen deposition [50].
Overall, inhaled treprostinil (40 μg/kg twice daily) maintained lung function and prevented bleomycin-induced lung injury, fibrosis, and vascular remodeling. The authors concluded that these findings suggest treprostinil potentially has therapeutic efficacy in pulmonary fibrosis and pulmonary hypertension related to chronic lung diseases [50].
Clinical Data
In addition to the previously described in vitro data and animal experiments, the antifibrotic effects of treprostinil have been brought to light through clinical studies as well. A post hoc analysis of the INCREASE study assessed FVC for the overall study population and subgroups, including patients with idiopathic interstitial pneumonia (IIP) and IPF [16]. Inhaled treprostinil treatment resulted in an overall FVC improvement of 28.5 mL (P = 0.35) and 44.4 mL (P = 0.21) at weeks 8 and 16, respectively, compared to placebo. Greater differences in FVC were seen in subgroups of patients with IIP (108.2 mL, P = 0.0229) and IPF (168.5 mL, P = 0.0108) at week 16. These larger numeric differences in FVC in the subgroups of patients with IIP and IPF compared to placebo are likely due to the IIP and IPF populations having a greater propensity for progression [16].
A question that these results raise is whether this difference in the FVC was due to an improvement of vascular compliance or if it indicates a true antifibrotic effect. To shed further light on this issue, a comparison of the FVC changes in the INCREASE and TRIUMPH studies was performed. The TRIUMPH study evaluated inhaled treprostinil over 12 weeks in patients with PAH [51]. Pulmonary function tests (PFTs) were conducted at baseline and week 12 in TRIUMPH and percentage change in predicted FVC and forced expiratory volume in 1 s (FEV1) were evaluated using analysis of covariance (ANCOVA). In INCREASE, PFTs were completed at baseline and weeks 8 and 16. The mixed model repeated measurement was used to evaluate the change in percentage predicted FVC and FEV1 [52]. This post hoc analysis found that PFT response to inhaled treprostinil differs between PAH and PH-ILD as improvements in percentage predicted FVC were seen in patients with PH-ILD but not in those with PAH [52]. These differences support treprostinil’s antifibrotic mechanism of action, suggesting amelioration in the fibrotic lung disease rather than through its effects on the pulmonary vasculature, further promoting the potential therapeutic role of treprostinil for patients with PH-ILD.
Conclusion
Treprostinil’s pharmacological profile via its ability to activate IP, DP1, and EP2 receptors, coupled with the robust expression of the last two receptors in PAH, suggests that its use could provide additional benefits as an antifibrotic agent. Inhibition of both TGFβ1 and PDGF is unique to treprostinil as other approved therapies for ILD only affected either TGFβ1 or PDGF [53, 54].
Treprostinil also had a significant effect on PPARs. PPARβ and PPARγ both demonstrated antiproliferative effects on human lung fibroblasts with in vitro treprostinil treatment.
Preclinical and animal model research supports that treprostinil may exert its therapeutic effect via multiple pathways, including inhibiting fibroblast-to-myofibroblast differentiation, fibroblast proliferation [33, 44, 49, 55], fibroblast migration [32, 39], fibronectin deposition [36], and mast cell-mediator release [56].
The in vitro and in vivo evidence of treprostinil’s antiproliferative potential is intriguing evidence for its therapeutic potential as an inhaled treatment for lung fibrosis. Treprostinil is unique among the prostacyclin mimetics approved for treating PAH in that it has distinct actions at additional prostaglandin receptors that contribute to its therapeutic benefit.
There is yet to be an inhaled therapy with proven antifibrotic effects. Patients with IPF may benefit from an inhaled therapy owing to targeted deposition of drug at the disease site, rapid onset of action, and fewer systemic side effects than are seen with oral administration. In addition, with fibrosis causing lung architectural distortion and associated vascular ablation, it is uncertain where in the lung any systemically delivered agent is actually deposited and if deposition is to the target areas of activity to facilitate amelioration of the fibrosis.
In summary, this overview provides a sound biologic and physiologic basis for a clinical trial which is currently underway to assess the efficacy of inhaled treprostinil in patients with pulmonary fibrosis (NCT04708782) [57].
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Funding
This work, in addition to the Rapid Service and Open Access fees was funded by United Therapeutics Corporation.
Medical Writing Assistance
The authors thank Dorothy Keine, PhD, of 3Prime Medical Writing, LLC for providing medical writing support, compensated by United Therapeutics Corporation.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the work’s integrity as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the study conception, design, and manuscript preparation.
Disclosures
Martin Kolb has received grants from the Canadian Institute for Health Research, the Canadian Pulmonary Fibrosis Foundation, Roche, Boehringer Ingelheim, Pieris, Prometic, and personal fees from Boehringer Ingelheim, Roche, the European Respiratory Journal, Bellerophon Therapeutics, United Therapeutics, Nitto Denko, MitoImmune, Pieris, AbbVie, DevPro Biopharma, Horizon, Algernon, and CSL Behring. Stylianos E. Orfanos has received research grants, honoraria, and travel awards from United Therapeutics and Ferrer-Galenica. Christopher Lambers received an unrestricted research Grant from United Therapeutics. Kevin Flaherty has received grants from Boehringer Ingelheim and consulting fees from Roche/Genentech, Bellerophonm, Respivant, Shionogi, DevPro, AstraZeneca, Pure Health, Horizon, Fibrogen, Sun Pharmaceuticals, Pliant, United Therapeutics, Arrowhead, Lupin, Polarean, PureTech, Trevi, CSL Behring, Daewong, Dispersol, Immumet, NeRRe Therapeutics. Steven D. Nathan is a consultant and is on the speaker's bureau for United Therapeutics and Ferrer-Galenica. Lisa Lancaster is a consultant with United Therapeutics. Alison Masters has nothing to disclose. Adam Silverstein has nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data supporting the findings in this manuscript have been cited appropriately and may be found in the References section. Further questions may be directed to the corresponding author.
References
- 1.Travis WD, Costabel U, Hansell DM, et al. An Official American Thoracic Society/European Respiratory Society Statement: update of the International Multidisciplinary Classification of the Idiopathic Interstitial Pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seeger W, Adir Y, Barberà JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62(25):D109–D116. doi: 10.1016/j.jacc.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 3.Wollin L, Distler JHW, Redente EF, et al. Potential of nintedanib in treatment of progressive fibrosing interstitial lung diseases. Eur Respir J. 2019;54(3):1900161. doi: 10.1183/13993003.00161-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghumman M, Dhamecha D, Gonsalves A, et al. Emerging drug delivery strategies for idiopathic pulmonary fibrosis treatment. Eur J Pharm Biopharm. 2021;164:1–12. doi: 10.1016/j.ejpb.2021.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambers C, Kornauth C, Oberndorfer F, et al. Mechanism of anti-remodelling action of treprostinil in human pulmonary arterial smooth muscle cells. PLoS One. 2018;13(11):e0205195. doi: 10.1371/journal.pone.0205195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wells AU, Brown KK, Flaherty KR, Kolb M, Victor J. What's in a name? That which we call IPF, by any other name would act the same. Eur Respir J. 2018;51(5):1800692. doi: 10.1183/13993003.00692-2018. [DOI] [PubMed] [Google Scholar]
- 7.Luzina IG, Todd NW, Sundararajan S, Atamas SP. The cytokines of pulmonary fibrosis: much learned, much more to learn. Cytokine. 2015;74(1):88–100. doi: 10.1016/j.cyto.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Zafrani L, Lemiale V, Lapidus N, Lorillon G, Schlemmer B, Azoulay E. Acute respiratory failure in critically ill patients with interstitial lung disease. PLoS One. 2014;9(8):e104897. doi: 10.1371/journal.pone.0104897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ESBRIET (pirfenidone) [Prescribing Information]. South San Francisco: Genentech, Inc; 2019.
- 10.OFEV® (nintedanib capsules) [Prescribing Information]. Ridgefield: Boehringer Ingelheim Pharmaceuticals, Inc; 2020.
- 11.ACTEMRA (tocilizumab) [Prescribing Information]. South San Francisco; Genetech, Inc; 2021.
- 12.Behr J, Ryu JH. Pulmonary hypertension in interstitial lung disease. Eur Respir J. 2008;31(6):1357–1367. doi: 10.1183/09031936.00171307. [DOI] [PubMed] [Google Scholar]
- 13.IPFCR Network. Zisman DA, Schwarz M, et al. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med. 2010;363(7):620–628. doi: 10.1056/NEJMoa1002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolb M, Raghu G, Wells AU, et al. Nintedanib plus sildenafil in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2018;379(18):1722–1731. doi: 10.1056/NEJMoa1811737. [DOI] [PubMed] [Google Scholar]
- 15.Waxman A, Restrepo-Jaramillo R, Thenappan T, et al. Inhaled treprostinil in pulmonary hypertension due to interstitial lung disease. N Engl J Med. 2021;384(4):325–334. doi: 10.1056/NEJMoa2008470. [DOI] [PubMed] [Google Scholar]
- 16.Nathan SD, Waxman A, Rajagopal S, et al. Inhaled treprostinil and forced vital capacity in patients with interstitial lung disease and associated pulmonary hypertension: a post-hoc analysis of the INCREASE study. Lancet Respir Med. 2021;9:1266–1274. doi: 10.1016/S2213-2600(21)00165-X. [DOI] [PubMed] [Google Scholar]
- 17.Patel NM, Lederer DJ, Borczuk AC, Kawut SM. Pulmonary hypertension in idiopathic pulmonary fibrosis. Chest. 2007;132(3):998–1006. doi: 10.1378/chest.06-3087. [DOI] [PubMed] [Google Scholar]
- 18.Panagiotou M, Church AC, Johnson MK, Peacock AJ. Pulmonary vascular and cardiac impairment in interstitial lung disease. Eur Respir Rev. 2017;26(143):160053. doi: 10.1183/16000617.0053-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park SH, Saleh D, Giaid A, Michel RP. Increased endothelin-1 in bleomycin-induced pulmonary fibrosis and the effect of an endothelin receptor antagonist. Am J Respir Crit Care Med. 1997;156(2 Pt 1):600–608. doi: 10.1164/ajrccm.156.2.9607123. [DOI] [PubMed] [Google Scholar]
- 20.Bellaye P-S, Yanagihara T, Granton E, et al. Macitentan reduces progression of TGF-β1-induced pulmonary fibrosis and pulmonary hypertension. Eur Respir J. 2018;52(2):1701857. doi: 10.1183/13993003.01857-2017. [DOI] [PubMed] [Google Scholar]
- 21.Hassoun PM, Mouthon L, Barbera JA, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54(1 Suppl):S10–S19. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Farkas L, Gauldie J, Voelkel NF, Kolb M. Pulmonary hypertension and idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;45(1):1–15. doi: 10.1165/rcmb.2010-0365TR. [DOI] [PubMed] [Google Scholar]
- 23.TYVASO (treprostinil) [Prescribing Information]. Research Triangle Park: United Therapeutics Corp; 2017.
- 24.Clapp LH, Gurung R. The mechanistic basis of prostacyclin and its stable analogues in pulmonary arterial hypertension: role of membrane versus nuclear receptors. Prostaglandins Other Lipid Mediat. 2015;120:56–71. doi: 10.1016/j.prostaglandins.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell JA, Ahmetaj-Shala B, Kirkby NS, et al. Role of prostacyclin in pulmonary hypertension. Glob Cardiol Sci Pract. 2014;2014(4):382–393. doi: 10.5339/gcsp.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norel X, Walch L, Labat C, Gascard J-P, Dulmet E, Brink C. Prostanoid receptors involved in the relaxation of human bronchial preparations. Br J Pharmacol. 1999;126(4):867–872. doi: 10.1038/sj.bjp.0702392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benyahia C, Gomez I, Kanyinda L, et al. PGE2 receptor (EP4) agonists: potent dilators of human bronchi and future asthma therapy? Pulm Pharmacol Ther. 2012;25(1):115–118. doi: 10.1016/j.pupt.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Petrucci G, De Cristofaro R, Rutella S, et al. Prostaglandin E2 differentially modulates human platelet function through the prostanoid EP2 and EP3 receptors. J Pharmacol Exp Ther. 2011;336(2):391–402. doi: 10.1124/jpet.110.174821. [DOI] [PubMed] [Google Scholar]
- 29.Ali FY, Davidson SJ, Moraes LA, et al. Role of nuclear receptor signaling in platelets: antithrombotic effects of PPARβ. FASEB J. 2006;20(2):326–328. doi: 10.1096/fj.05-4395fje. [DOI] [PubMed] [Google Scholar]
- 30.Hubertus K, Mischnik M, Timmer J, et al. Reciprocal regulation of human platelet function by endogenous prostanoids and through multiple prostanoid receptors. Eur J Pharmacol. 2014;740:15–27. doi: 10.1016/j.ejphar.2014.06.030. [DOI] [PubMed] [Google Scholar]
- 31.Huang S, Wettlaufer SH, Hogaboam C, Aronoff DM, Peters-Golden M. Prostaglandin E(2) inhibits collagen expression and proliferation in patient-derived normal lung fibroblasts via E prostanoid 2 receptor and cAMP signaling. Am J Physiol Lung Cell Mol Physiol. 2007;292(2):L405–L413. doi: 10.1152/ajplung.00232.2006. [DOI] [PubMed] [Google Scholar]
- 32.White ES, Atrasz RG, Dickie EG, et al. Prostaglandin E2 inhibits fibroblast migration by E-prostanoid 2 receptor-mediated increase in PTEN activity. Am J Respir Cell Mol Biol. 2005;32(2):135–141. doi: 10.1165/rcmb.2004-0126OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolodsick JE, Peters-Golden M, Larios J, Toews GB, Thannickal VJ, Moore BB. Prostaglandin E2 inhibits fibroblast to myofibroblast transition via E. prostanoid receptor 2 signaling and cyclic adenosine monophosphate elevation. Am J Respir Cell Mol Biol. 2003;29(5):537–544. doi: 10.1165/rcmb.2002-0243OC. [DOI] [PubMed] [Google Scholar]
- 34.Moore BB, Ballinger MN, White ES, et al. Bleomycin-induced E prostanoid receptor changes alter fibroblast responses to prostaglandin E2. J Immunol. 2005;174(9):5644–5649. doi: 10.4049/jimmunol.174.9.5644. [DOI] [PubMed] [Google Scholar]
- 35.Mori A, Ito S, Morioka M, et al. Effects of specific prostanoid EP receptor agonists on cell proliferation and intracellular Ca2+ concentrations in human airway smooth muscle cells. Eur J Pharmacol. 2011;659(1):72–78. doi: 10.1016/j.ejphar.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Lambers C, Roth M, Jaksch P, et al. Treprostinil inhibits proliferation and extracellular matrix deposition by fibroblasts through cAMP activation. Sci Rep. 2018;8(1):1087. doi: 10.1038/s41598-018-19294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burgess JK, Ge Q, Boustany S, Black JL, Johnson PRA. Increased sensitivity of asthmatic airway smooth muscle cells to prostaglandin E2 might be mediated by increased numbers of E-prostanoid receptors. J Allergy Clin Immunol. 2004;113(5):876–881. doi: 10.1016/j.jaci.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 38.Patel J, Shen L, Hall S, et al. Prostanoid EP2 receptors are up-regulated in human pulmonary arterial hypertension: a key anti-proliferative target for treprostinil in smooth muscle cells. Int J Mol Sci. 2018;19(8):2372. doi: 10.3390/ijms19082372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clapp LH, Abu-Hanna JHJ, Patel JA. Diverse pharmacology of prostacyclin mimetics: implications for pulmonary hypertension. Molecular mechanism of congenital heart disease and pulmonary hypertension. Singapore: Springer; 2020. pp. 31–61. [Google Scholar]
- 40.Dagouassat M, Gagliolo J-M, Chrusciel S, et al. The cyclooxygenase-2-prostaglandin E2 pathway maintains senescence of chronic obstructive pulmonary disease fibroblasts. Am J Respir Crit Care Med. 2013;187(7):703–714. doi: 10.1164/rccm.201208-1361OC. [DOI] [PubMed] [Google Scholar]
- 41.Horikiri T, Hara H, Saito N, et al. Increased levels of prostaglandin E-major urinary metabolite (PGE-MUM) in chronic fibrosing interstitial pneumonia. Respir Med. 2017;122:43–50. doi: 10.1016/j.rmed.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 42.Wilborn J, Crofford LJ, Burdick MD, Kunkel SL, Strieter RM, Peters-Golden M. Cultured lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis have a diminished capacity to synthesize prostaglandin E2 and to express cyclooxygenase-2. J Clin Investig. 1995;95(4):1861–1868. doi: 10.1172/JCI117866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van den Brule S, Wallemme L, Uwambayinema F, Huaux F, Lison D. The D prostanoid receptor agonist BW245C [(4S)-(3-[(3R, S)-3-cyclohexyl-3-hydroxypropyl]-2,5-dioxo)-4-imidazolidineheptanoic acid] inhibits fibroblast proliferation and bleomycin-induced lung fibrosis in mice. J Pharmacol Exp Ther. 2010;335(2):472–479. doi: 10.1124/jpet.110.169250. [DOI] [PubMed] [Google Scholar]
- 44.Ali FY, Egan K, Fitzgerald GA, et al. Role of prostacyclin versus peroxisome proliferator-activated receptor β receptors in prostacyclin sensing by lung fibroblasts. Am J Respir Cell Mol Biol. 2006;34(2):242–246. doi: 10.1165/rcmb.2005-0289OC. [DOI] [PubMed] [Google Scholar]
- 45.Falcetti E, Flavell DM, Staels B, Tinker A, Haworth SG, Clapp LH. IP receptor-dependent activation of PPARgamma by stable prostacyclin analogues. Biochem Biophys Res Commun. 2007;360(4):821–827. doi: 10.1016/j.bbrc.2007.06.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roberts MJ, Broome RE, Kent TC, Charlton SJ, Rosethorne EM. The inhibition of human lung fibroblast proliferation and differentiation by Gs-coupled receptors is not predicted by the magnitude of cAMP response. Respir Res. 2018;19(1):1–13. doi: 10.1186/s12931-018-0759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blumer S, Fang L, Chen W-C, et al. IPF-fibroblast Erk1/2 activity is independent from microRNA cluster 17–92 but can be inhibited by treprostinil through DUSP1. Cells. 2021;10(11):2836. doi: 10.3390/cells10112836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williamson JD, Sadofsky LR, Hart SP. The pathogenesis of bleomycin-induced lung injury in animals and its applicability to human idiopathic pulmonary fibrosis. Exp Lung Res. 2015;41(2):57–73. doi: 10.3109/01902148.2014.979516. [DOI] [PubMed] [Google Scholar]
- 49.Corboz MR, Zhang J, Lasala D, et al. Therapeutic administration of inhaled INS1009, a treprostinil prodrug formulation, inhibits bleomycin-induced pulmonary fibrosis in rats. Pulm Pharmacol Ther. 2018;49:95–103. doi: 10.1016/j.pupt.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 50.Nikitopoulou I, Manitsopoulos N, Kotanidou A, et al. Orotracheal treprostinil administration attenuates bleomycin-induced lung injury, vascular remodeling, and fibrosis in mice. Pulm Circ. 2019;9(4):204589401988195. doi: 10.1177/2045894019881954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mclaughlin VV, Benza RL, Rubin LJ, et al. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2010;55(18):1915–1922. doi: 10.1016/j.jacc.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 52.Nathan S, Tapson V, Ramani G, et al. Comparison of effects of inhaled treprostinil on lung function in patients with pulmonary hypertension associated with interstitial lung disease and pulmonary arterial hype. Chest. 2021;160:A2244–A2246. doi: 10.1016/j.chest.2021.07.1976. [DOI] [Google Scholar]
- 53.Hostettler KE, Zhong J, Papakonstantinou E, et al. Anti-fibrotic effects of nintedanib in lung fibroblasts derived from patients with idiopathic pulmonary fibrosis. Respir Res. 2014;15(1):1–9. doi: 10.1186/s12931-014-0157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Conte E, Gili E, Fagone E, Fruciano M, Iemmolo M, Vancheri C. Effect of pirfenidone on proliferation, TGF-β-induced myofibroblast differentiation and fibrogenic activity of primary human lung fibroblasts. Eur J Pharm Sci. 2014;58:13–19. doi: 10.1016/j.ejps.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 55.Ayabe S, Kida T, Hori M, Ozaki H, Murata T. Prostaglandin D2 inhibits collagen secretion from lung fibroblasts by activating the DP receptor. J Pharmacol Sci. 2013;121(4):312–317. doi: 10.1254/jphs.12275FP. [DOI] [PubMed] [Google Scholar]
- 56.Säfholm J, Manson ML, Bood J, et al. Prostaglandin E2 inhibits mast cell-dependent bronchoconstriction in human small airways through the E prostanoid subtype 2 receptor. J Allergy Clin Immunol. 2015;136(5):1232–9.e1. doi: 10.1016/j.jaci.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 57.Study of efficacy and safety of inhaled treprostinil in subjects with idiopathic pulmonary fibrosis (TETON). ClinicalTrials.gov identifier: NCT04708782. Updated July 20, 2021. https://clinicaltrials.gov/ct2/show/NCT04708782. Accessed Aug 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings in this manuscript have been cited appropriately and may be found in the References section. Further questions may be directed to the corresponding author.