Abstract
The aim of the present research was to understand the impacts of foliar nitrogen and α-oxoglutarate on proline accumulation, photosynthesis, and ammonium assimilation of soybean seedlings subjected to drought stress. The data in the present study demonstrated that foliar α-oxoglutarate and nitrogen significantly enhanced leaf glutamine synthetase (GS) activity, glutamate dehydrogenase (GDH) activity, glutamate content, proline content, relative water content (RWC) and photosynthesis of soybean seedlings exposed to drought stress at each stage. Accordingly, the ammonium content was significantly reduced by foliar α-oxoglutarate and nitrogen. These results suggested that a combination of foliar nitrogen plus α-oxoglutarate had an advantage over either foliar nitrogen or foliar α-oxoglutarate in increasing the proline accumulation under drought stress and a combination of foliar nitrogen plus α-oxoglutarate could better mitigate the adverse impacts of drought stress.
Subject terms: Biochemistry, Physiology, Plant sciences
Introduction
In recent years, the influence of frequent drought hazards on crop production has been increasing in Northeast China. Soybean (Glycine max (L.) Merr.) is the second most important crop in Northeast China (following corn), and frequently subjected to drought stress (DS) during its growth and development, which results in a significant reduction in soybean seed yield. Soybean is a vital crop which needs a sufficient supply of water during its growth process to achieve high seed yield1. Plants have evolved many different strategies to cope with abiotic stress. Osmoregulation is a vital mechanism for plants to acclimate to adverse osmotic stresses1. Proline (Pro) is reported to act as a key osmolyte in increasing the tolerance of plants2. Pro accumulation has been revealed to take place after drought stress in plants3. Drought stress leads to a wide range of physiological changes, such as stomata to close and cells to lose water. These changes disturb regular nitrogen metabolism, carbon metabolism, and result in plant death as a consequence of the decrease in relative water content (RWC). The RWC is an important parameter of plant water status4–6. Drought stress causes the decrease in photosynthesis rate, and alters carbon metabolism in plant, leading to depleted energy and reduced yield7.
Nitrogen (N) metabolism is one of the most basic metabolic processes, which affects the growth state, yield and stress tolerance of plants. There has been report on the association between proline accumulation and the acquisition of stress tolerance8. It is a fact that proline is eventually synthesized from glutamate (Glu) that acts an important role in the amino acid metabolism of plant9,10. Glu biosynthesis is much associated with ammonium uptake10. Excessive ammonium leads to the toxic impacts on plants when subjected to adverse abiotic stresses11. The GDH pathway is considered as a complementary route when plants are exposed to abiotic stresses12,13. Reducing the accumulation of NH4+ in plant tissues is considered to be an important ability to resist drought stress. Glutamine synthetase (GS) and glutamate dehydrogenase (GDH) are key rate limiting enzymes in the process of NH4+ assimilation and plants use NH4+ to catalyze the biosynthesis of Glu14,15. Nitrogen (N) is required by plants in relatively large amounts, unlike the other plant nutrients. In addition to supplying a nutrient for plant growth, nitrogen application could increase the drought tolerance of plant to enhance yield when exposed to drought stress6,16. Adequate nitrogen supply can enhance the drought tolerance of plants16,17. Yang et al.18 reported that nitrogen application to wheat (Triticum aestivum L.) during grain filling enhanced the remobilization of stored carbohydrates from vegetative plant part to grain under moderate drought stress. Several studies have provided the evidence of the role of nitrogen in ameliorating the effects of drought stress by enhancing proline accumulation, glycine betaine, and soluble protein18–21. Foliar application of nitrogen much more raised the RWC and the nitrate reductase activity in maize under short-term drought stress22.
α-oxoglutarate, an important organic acid of the tricarboxylic acid (TCA) cycle23,24, participates in a wide array of reactions in distinct plant cell compartments25, also being a key metabolite at the crossroads of carbon/nitrogen (C/N) metabolism as it is needed for assimilating ammonia26,27. There are some reports on the impacts of exogenous α-oxoglutarate on nitrogen assimilation in crops, such as wheat28, tobacco29,30, and rice31 when exposed to abiotic stress. There is a report32 indicating that exogenous application of α-oxoglutarate sharply reduced the NH4+ concentrations and increased the ammonium assimilation in tomato roots and shoots as compared to control (CK). The previous study indicated that exogenous α-oxoglutarate significantly enhanced the cold resistance by increasing leaf ammonium assimilation, proline accumulation and photosynthesis of soybean seedling when subjected to cold stress33.
However, the effects of foliar nitrogen or α-oxoglutarate, particularly the combination of foliar nitrogen plus α-oxoglutarate and the underlying mechanism were not reported in crops exposed to drought stress. This study is the first report on the effects of foliar nitrogen plus α-oxoglutarate on soybean subjected to abiotic stress. We hypothesize that: (1) the combination of foliar nitrogen plus α-oxoglutarate could improve the drought tolerance of soybean; (2) exogenous α-oxoglutarate or nitrogen helps to enhance nitrogen metabolism under drought stress.
Materials and methods
Plant materials and growth conditions
A pot experiment was conducted in the rain shelter at the experimental station of Northeast Agricultural University, China. The experimental pot diameter was 16 cm, and the depth was 18 cm. Each pot was filled with 600 g of typical black soil. Two spring soybean varieties, Henong51 (drought resistant) and Henong43 (drought sensitive), were used in the present study and the experiment has adopted Randomized Block Design. Henong51 was released in 2006 with 100-seed weight of 19.0–20.0 g and the plant height was 80–85 cm. The content of its protein and oil was 40.15% and 21.31%, respectively. The effective accumulative temperature for maturity (≥ 10 °C) was about 2200 °C. Henong43 was released in 2002. The 100-seed weight was 20.0–22.0 g and the plant height was 90–100 cm. The content of its protein and oil was 42.05% and 20.52%, respectively. The growth habit of the two soybean varieties was semi-determinate with purple flowers.
Soil was collected at a depth of 20 cm from a corn field. The soil was analyzed in Sangjiang River Key Laboratory of Cultivation and Breeding of Main Crops. The properties of soil were as follows: total nitrogen 2.19 g/kg; available nitrogen 139.21 mg/kg; total phosphorus 1.72 g/kg; available phosphorus 37.25 mg/kg; total potassium 27.03 g/kg; available potassium 123.25 mg/kg; organic matter 30.13 g/kg; pH 6.98.
We state that our experimental research on soybeans comply with the relevant institutional, national, and international guidelines and legislation.
Experimental design and sampling
Twelve seeds of each soybean variety were seeded by hand in the pots. Soybean was not inoculated or dressed before planting. The main treatments included two soil water levels: (1) CK, that is, soil water content which was maintained at 19 ± 1% of water content (85 ± 5% of water-holding capacity); and (2) drought stress (DS) which was maintained at 15 ± 1% of water content (65 ± 5% of water-holding capacity). Subtreatments were (1) 0.5% Urea (N 46%), (2) 5 mmol/L α-oxoglutarate, and (3) 0.5% Urea + 5 mmol/L α-oxoglutarate. Foliar α-oxoglutarate and nitrogen were applied to soybean seedlings at V2 stage (completely unrolled leaf at the second node on the main stem). There were five treatments in the present experiment: DS1 (foliar N), DS2 (foliar α-oxoglutarate), DS3 (foliar N plus α-oxoglutarate), CK1 (foliar water under DS), CK2 (normal water content). Leaf samples for all treatments were collected after drought stress of 24 h (S1 stage), 48 h (S2 stage) and 72 h (S3 stage). By using a weighing method, the weight of each pot was recorded once every day. The amount of watering required was then calculated. When the soil RWC was lower than the lower limit of the water control treatment, water was precisely added to the pot.
We state that we have permission for foliar spraying and collecting leaves at V2 stage and there is no issues on ethics.
Determination of nitrogen metabolism
The activity of GS and NADH-GDH was measured with the method of Lu et al.34. One unit of NADH-GDH activity was the reduction amount of 1 µmol of coenzyme (NADH) per min under 30 °C and one unit of GS activity was defined as the amount of enzyme catalyzing the formation of 1 µmol γ-glutamylhydroxamate per min under 37 °C.
Determination of photosynthetic index
Photosynthetic rate was measured according to Gai et al.35. Photosynthetic rate was determined by using the CI-340 portable photosynthesis measuring system (CID, Inc., USA). Relative chlorophyll content (SPAD) in the middle leaflet of the trifoliate leaf at V2 stage was determined by SPAD-502 Chlorophyll Meter produced by Konica Minolta Inc., Japan.
Determination of proline
Free proline was determined with the method of Bates et al.36. Fresh leaf (0.5 g) was used to extract proline with 3% sulphosalicylic acid, ninhydrin reagent containing glacial acetic acid and incubated under 100 °C for 1 h. The reaction mixture was rapidly cooled by using ice water. The toluene was used to extract the colored reaction product, and the toluene phase absorbance was determined at 520 nm.
Determination of ammonium content
The ammonium content in soybean leaf was measured by using HPLC (Agilent 1100, USA) with the method of Lu et al.35.
Determination of relative water content
The leaf RWC was determined through recording the turgid weight of 1.0 g fresh leaf samples by making them stay in water for 4 h, followed by drying in the hot air oven until stationary weight was derived. The following equation was used to determine RWC37.
The FW is defined as sample fresh weight, TW is defined as the sample turgid weight, and DW is defined as the sample dry weight.
Statistical analyses
Data analysis was conducted using SPSS 22 (SPSS Inc., Chicago, IL, US). Test for significance was carried out by analysis of variance, and LSD test was used to determine the significant differences (P < 0.05) between different treatments.
Results and discussion
Effects of foliar α-oxoglutarate and nitrogen on soybean fresh weight (FW)
The growth of soybean seedlings was evaluated by measuring the fresh weight. Inhibition in soybean fresh weight was significantly mitigated by foliar addition of nitrogen and α-oxoglutarate under drought stress. The data in Table 1 indicated that foliar nitrogen and α-oxoglutarate significantly enhanced the fresh weight of soybean seedling under drought stress compared to CK1 at S1, S2, and S3 stages. The fresh weight of soybean seedling under DS1, DS2, and DS3 was significantly lower than that under CK2 at each stage. There was no significant difference in fresh weight among DS1, DS2, and DS3 treatments. With the stress duration prolonged, the fresh weight of soybean seedling gradually decreased under drought stress. The fresh weight of soybean seedling under CK2 gradually increased from S1 to S3 stage, while the fresh weight of soybean seedling under DS1, DS2, DS3, and CK1 gradually decreased from S1 to S3 stage. When drought stress was applied, the fresh weight of drought-treated plants was reduced for two soybean varieties. The fresh weight of Hefeng51 was significantly lower than that for Hefeng43 at each stage. The data in the present study indicated that there was a lower reduction in fresh weight for Hefeng51 than that for Hefeng43 from S1 to S3 stage when exposed to drought stress.
Table 1.
Effects of foliar α-oxoglutarate and nitrogen on fresh weight under drought stress (g plant−1).
| Treatment | S1 stage | S2 stage | S3 stage | |||
|---|---|---|---|---|---|---|
| Hefeng51 | Hefeng43 | Hefeng51 | Hefeng43 | Hefeng51 | Hefeng43 | |
| DS1 | 3.31b | 3.82b | 3.25b | 3.69b | 2.98b | 3.21b |
| DS2 | 3.36b | 3.86b | 3.31b | 3.70b | 3.01b | 3.32b |
| DS3 | 3.41b | 3.91b | 3.37b | 3.72b | 3.08b | 3.38b |
| CK1 | 3.02c | 3.59c | 2.78c | 3.28c | 2.48c | 2.71c |
| CK2 | 3.84a | 4.25a | 3.95a | 4.31a | 4.11a | 4.49a |
| Analysis of variance (ANOVA) | ||||||
| Treatment (T) | ** | ** | ** | *** | *** | *** |
| Cultivar (C) | *** | *** | ** | |||
| C × T | ** | *** | ** | |||
Means with same letter within columns are not significantly different (P < 0.05) using LSD test.
***, **Significance at 0.001 and 0.01, respectively.
Drought stress is one of the important environmental stresses and has adverse impacts on the crop productivity. Foliar application of fertilizers could enhance the use efficiency of a nutrient urgently needed for the growth and development of plant38–40. Urea is a widely utilized foliar N fertilizer, with characteristics of high leaf penetration rate and low cost41. Foliar application of nitrogen was sometimes carried when abiotic stress occurred22. It has been reported that improving plant nutrition by increasing the supply of nutrients, including nitrogen may ameliorate the negative effects of abiotic stress42,43. Supplemental foliar application of urea could effectively enhance the growth, yield and yield components of sunflower subjected to drought stress44. These results were in agreement with the work of Zhang et al.22, who reported that foliar application of nitrogen posed positive impacts on the growth of maize under short-term drought stress. The previous study demonstrated that foliar application of α-oxoglutarate significantly increased the fresh weight of soybean seedling exposed to cold stress33. Similarly, the present study indicated that foliar nitrogen, foliar α-oxoglutarate and a combination of foliar nitrogen plus α-oxoglutarate resulted in a marked rise in the fresh weight of soybean seedling exposed to drought stress as compared to CK1 at S1, S2, and S3 stages.
Effects of foliar α-oxoglutarate and nitrogen on relative water content
Leaf RWC is reported to be an alternative measure of evaluating plant water status. As shown in Table 2, foliar application of nitrogen and α-oxoglutarate significantly increased RWC of soybean leaf under drought stress compared to CK1 at S1, S2 and S3 stages. Leaf RWC under DS1, DS2, and DS3 was significantly greater than that under CK2 at each stage. There was no significant difference in RWC between DS1 and DS2 treatments and the RWC under DS3 was significantly higher than that under DS1 and DS2 at S1 and S2 stages. The leaf RWC under drought stress gradually decreased with the stress duration prolonged. The leaf RWC under DS1, DS2, DS3, and CK1 gradually decreased from S1 to S3 stage. The RWC of Hefeng51 was significantly greater than that for Hefeng43 at each stage. The data as shown in Table 2 showed that there was a lower reduction in RWC for Hefeng51 than that for Hefeng43 from S1 to S3 stage when subjected to drought stress. The RWC was significantly affected by treatment (T), cultivar (C) and their interaction (C × T) at each stage.
Table 2.
Effects of foliar α-oxoglutarate and nitrogen on leaf RWC under drought stress (%).
| Treatment | S1 stage | S2 stage | S3 stage | |||
|---|---|---|---|---|---|---|
| Hefeng51 | Hefeng43 | Hefeng51 | Hefeng43 | Hefeng51 | Hefeng43 | |
| DS1 | 58.24c | 53.66c | 55.25c | 50.09c | 52.25b | 46.25b |
| DS2 | 59.36c | 53.81c | 56.18c | 50.21c | 53.02b | 46.65b |
| DS3 | 63.85b | 59.95b | 61.27b | 54.16b | 53.56b | 47.34b |
| CK1 | 54.67d | 50.52d | 52.92d | 46.67d | 45.25c | 38.43c |
| CK2 | 68.21a | 63.21a | 67.98a | 64.12a | 68.46a | 63.56a |
| Analysis of variance (ANOVA) | ||||||
| Treatment (T) | * | ** | ** | *** | *** | *** |
| Cultivar (C) | ** | ** | *** | |||
| C × T | * | ** | *** | |||
Means with same letter within columns are not significantly different (P < 0.05) using LSD test.
***, **, *Significance at 0.001, 0.01, and 0.05, respectively.
It is important for plants to keep desirable RWC, which relieves the adverse impacts of drought stress5,45,46. This study is similar to the work of Taiz et al.5 who reported that the RWC was greater for plants under control conditions than those under drought stress. It has been proved that foliar application of nitrogen could cause a marked rise in RWC of both maize varieties exposed to drought stress22, which is similar to the results obtained in the present study. No information is available on the effects of a combination of foliar nitrogen plus α-oxoglutarate on the RWC of plant under abiotic stress. The data in this study showed that foliar nitrogen significantly enhanced the leaf RWC of soybean compared to CK1 at each stage, and the combination of foliar nitrogen plus α-oxoglutarate showed a much more positive effect than that under foliar nitrogen or α-oxoglutarate at S1 and S2 stages, while there is no significant difference in leaf RWC among DS1, DS2, and DS3 treatments at S3 stage.
Effects of foliar α-oxoglutarate and nitrogen on proline content
Proline accumulation is found to play adaptive roles in stress resistance47. The proline content was significantly influenced by treatment (T), cultivar (C) and their interaction (C × T) at each stage (Table 3). The proline content of Hefeng51 was significantly greater than that for Hefeng43 at each stage. The data in Table 3 showed that there was higher proline content for Hefeng51 than that for Hefeng43 at each stage when exposed to drought stress. That suggested that Hefeng51 could better adapt to drought stress than Hefeng43 in the present study. Similar to the work conducted by You et al.47 who found that the drought-tolerant sesame accumulates higher levels of proline, lysine, arginine, and allantoin under drought conditions.
Table 3.
Effects of foliar α-oxoglutarate and nitrogen on leaf proline content under drought stress (µg g−1 FW).
| Treatment | S1 stage | S2 stage | S3 stage | |||
|---|---|---|---|---|---|---|
| Hefeng51 | Hefeng43 | Hefeng51 | Hefeng43 | Hefeng51 | Hefeng43 | |
| DS1 | 60.51b | 56.09b | 75.99b | 71.12b | 86.93b | 79.23b |
| DS2 | 61.80b | 57.15b | 74.42b | 72.04b | 86.77b | 78.86b |
| DS3 | 70.30a | 64.67a | 85.95a | 83.94a | 92.85a | 87.22a |
| CK1 | 50.36c | 43.86c | 68.28c | 58.04c | 77.12c | 64.67c |
| CK2 | 35.91d | 31.97d | 35.23d | 32.06d | 36.58d | 31.67d |
| Treatment (T) | *** | *** | *** | *** | *** | *** |
| Cultivar (C) | *** | *** | *** | |||
| C × T | ** | *** | *** | |||
Means with same letter within columns are not significantly different (P < 0.05) using LSD test.
***, **Significance at 0.001, and 0.01, respectively.
Results obtained from the present study demonstrated that foliar application of nitrogen and α-oxoglutarate significantly enhanced leaf proline content of soybean under drought stress compared to CK1 at S1, S2, and S3 stages (Table 3). Leaf proline content of soybean under DS1, DS2, and DS3 was significantly higher than that under CK2 at each stage. There was no significant difference in leaf proline content between DS1 and DS2 treatments at each stage, while the leaf proline content under DS3 was significantly greater than that under DS1 and DS2 at S1 and S2 stages. The leaf proline content under drought stress gradually increased with the stress duration prolonged. This could be greatly vital for the crop to adapt to drought stress48. In addition, lettuce plants under salt stress were sprayed with urea, and the contents of glycine and proline increased further49. N nutrition can alleviate crop water stress by maintaining metabolic activities. Under deficit irrigation, nitrogen treated plants had higher growth and yield characteristics. Compared with the plants without nitrogen treatment, free proline was significantly improved due to nitrogen application, which effectively reduced the damage of wheat plants under drought stress50.
The role of proline in drought resistance has been investigated and positive associations between improved drought tolerance and proline accumulation have been found in the published report51. To date, there have been no reports on the effects of a combination of foliar nitrogen plus α-oxoglutarate on the regulation of proline accumulation in crops. However, in increasing the proline accumulation under drought stress, a combination of foliar nitrogen plus α-oxoglutarate had an advantage over either foliar nitrogen or foliar α-oxoglutarate in the present study (Table 3). It is important to take preventive actions to alleviate the adverse effects of drought stress. The data demonstrated that foliar application of nitrogen and α-oxoglutarate significantly increased leaf proline content at each stage. These findings supported several studies that have provided the experimental evidence of the role of nitrogen in ameliorating the adverse impacts of drought stress by enhancing proline accumulation and soluble protein18–21. Li et al.28 pointed out that foliar application of exogenous α-oxoglutarate could enhance the proline content in wheat leaf and mitigate the adverse impacts of drought stress on wheat growth and development. Their results revealed the importance of exogenous α-oxoglutarate for wheat exposed to drought stress.
Effects of foliar α-oxoglutarate and nitrogen on glutamate content
As shown in Table 4, the glutamate content was significantly affected by treatment (T), cultivar (C) and their interaction (C × T) at each stage. The glutamate content of Hefeng51 was significantly higher than that for Hefeng43 at each stage. The data obtained in the present study demonstrated that there was a lower reduction in glutamate content for Hefeng51 than that for Hefeng43 from S1 to S3 stage when exposed to drought stress. Data from the present study indicated that foliar application of nitrogen and α-oxoglutarate caused a significant rise in leaf glutamate content of soybean seedling under drought stress as compared with CK1 at each stage (Table 4). Leaf glutamate content of soybean seedling under DS1, DS2, and DS3 was significantly greater than that under CK2 at S1, S2, and S3 stages. There was no significant difference in leaf glutamate content between DS1 and DS2 treatments at each stage, while the leaf glutamate content under DS3 was significantly greater than that under DS1 and DS2 at S1 and S2 stages. The leaf glutamate content under DS1, DS2, DS3, and CK1 gradually decreased from S1 to S3 stage.
Table 4.
Effects of foliar α-oxoglutarate and nitrogen on leaf glutamate content under drought stress (µg g−1 FW).
| Treatment | S1 stage | S2 stage | S3 stage | |||
|---|---|---|---|---|---|---|
| Hefeng51 | Hefeng43 | Hefeng51 | Hefeng43 | Hefeng51 | Hefeng43 | |
| DS1 | 189.08b | 175.32b | 179.04b | 163.79b | 165.88a | 139.25a |
| DS2 | 191.53b | 176.01b | 181.65b | 164.38b | 166.15a | 140.58a |
| DS3 | 211.25a | 198.36a | 204.78a | 178.27a | 170.86a | 141.89a |
| CK1 | 175.85c | 154.25c | 164.45c | 140.24c | 150.03b | 112.21b |
| CK2 | 116.91d | 105.21d | 117.34d | 104.69d | 116.25c | 103.28c |
| Analysis of variance (ANOVA) | ||||||
| Treatment (T) | *** | *** | *** | ** | ** | * |
| Cultivar (C) | *** | ** | ** | |||
| C × T | *** | ** | ** | |||
Means with same letter within columns are not significantly different (P < 0.05) using LSD test.
***, **, *Significance at 0.001, 0.01, and 0.05, respectively.
An adequate supply of glutamate (Glu) is required for proline synthesis rate46. Similar to the present study, foliar application of α-oxoglutarate for plant had a positive impact on the rise of glutamate content33,52. This study also suggested foliar application of nitrogen plus α-oxoglutarate much more significantly enhanced leaf glutamate content under drought stress than that at DS1 or DS2 at S1 and S2 stages (Table 4).
Effects of foliar α-oxoglutarate and nitrogen on GS and GDH activity
The data in Tables 5 and 6 indicated there was no significant difference in leaf GS and GDH between DS1 and DS2 treatments at each stage, while the leaf activity of GS and GDH under DS3 was significantly greater than that under DS1 and DS2 at S1 and S2 stages. With the stress duration prolonged, the leaf activity of GS and GDH gradually decreased under drought stress. Drought stress resulted in a significant decrease in GS activity compared to CK2 at each stage, while drought stress led to a marked rise in GDH activity as compared with CK2 at S1, S2, and S3 stages. The data obtained in this study showed that foliar application of nitrogen and α-oxoglutarate significantly enhanced the leaf activity of GS and GDH of soybean seedling under drought stress as compared with CK1 at each stage (Tables 5, 6). The leaf activity of GS and GDH under DS1, DS2, and DS3 treatments with foliar nitrogen and α-oxoglutarate exhibited a lower reduction than that under CK1 from S1 to S3 stage.
Table 5.
Effects of foliar α-oxoglutarate and nitrogen on leaf GS activity under drought stress (µmol h−1 g−1 FW).
| Treatment | S1 stage | S2 stage | S3 stage | |||
|---|---|---|---|---|---|---|
| Hefeng51 | Hefeng43 | Hefeng51 | Hefeng43 | Hefeng51 | Hefeng43 | |
| DS1 | 180.98c | 151.25c | 171.25c | 140.33b | 152.32b | 120.03b |
| DS2 | 183.26c | 153.02c | 172.37c | 141.19b | 155.18b | 121.65b |
| DS3 | 191.95b | 171.43b | 182.49b | 155.83b | 158.87b | 123.29b |
| CK1 | 165.47d | 142.35d | 148.46d | 122.34c | 136.14c | 102.87c |
| CK2 | 218.13a | 182.67a | 219.81a | 184.11a | 220.54a | 185.05a |
| Analysis of variance (ANOVA) | ||||||
| Treatment (T) | *** | *** | *** | *** | *** | ** |
| Cultivar (C) | *** | *** | ** | |||
| C × T | *** | ** | ** | |||
Means with same letter within columns are not significantly different (P < 0.05) using LSD test.
***, **Significance at 0.001, and 0.01, respectively.
Table 6.
Effects of foliar α-oxoglutarate and nitrogen on GDH activity under drought stress (µmol h−1 g−1 FW).
| Treatment | S1 stage | S2 stage | S3 stage | |||
|---|---|---|---|---|---|---|
| Hefeng51 | Hefeng43 | Hefeng51 | Hefeng43 | Hefeng51 | Hefeng43 | |
| DS1 | 19.26b | 15.96b | 18.18b | 13.18b | 16.95a | 11.05a |
| DS2 | 19.05b | 16.01b | 18.21b | 13.21b | 16.91a | 11.11a |
| DS3 | 20.87a | 16.92a | 19.25a | 14.25a | 17.06a | 11.26a |
| CK1 | 16.23c | 13.65c | 15.02c | 11.92c | 13.15b | 9.15b |
| CK2 | 11.21d | 9.18d | 11.12d | 9.12d | 11.31c | 9.07b |
| Analysis of variance (ANOVA) | ||||||
| Treatment (T) | *** | *** | ** | *** | ** | * |
| Cultivar (C) | ** | *** | ** | |||
| C × T | ** | ** | * | |||
Means with same letter within columns are not significantly different (P < 0.05) using LSD test.
***, **, *Significance at 0.001, 0.01, and 0.05, respectively.
Little research has been done on the effects of nitrogen and α-oxoglutarate on leaf GS and GDH activity of soybean exposed to drought stress. An early published report demonstrated that the GS/GOGAT pathway was reported to play a key role in glutamate synthesis when proline is required53. There were some studies indicating that the increase in NADH-GDH activity and its vital role in providing glutamate for proline biosynthesis54,55. Luo et al.56 pointed out that foliar application of α-oxoglutarate could effectively enhance the leaf activity of GDH and GS in wheat when exposed to drought stress. The data in the present study clearly demonstrated that foliar α-oxoglutarate, foliar nitrogen and a combination of foliar nitrogen plus α-oxoglutarate imposed positive effects on the rise of GS and GDH activity for soybean seedling subjected to drought stress (Tables 5, 6). This finding was also similar to the work conducted by Zhang et al.22 that foliar application of nitrogen was beneficial to plant growth and the increase of nitrogen metabolism in both maize varieties when subjected to short-term drought stress. In the present study, a rise in the activity of GS and GDH caused by foliar nitrogen and α-oxoglutarate could explain the reason why ammonium content in soybean leaf was accordingly reduced when soybean seedlings were exposed to drought stress (Tables 5, 6, 7). The effect of foliar nitrogen and α-oxoglutarate on nitrogen assimilation was also reflected by a markedly lower level of ammonium in the present study (Table 7).
Table 7.
Effects of foliar α-oxoglutarate and nitrogen on ammonium content under drought stress (µmol h−1 g−1 FW).
| Treatment | S1 stage | S2 stage | S3 stage | |||
|---|---|---|---|---|---|---|
| Hefeng51 | Hefeng43 | Hefeng51 | Hefeng43 | Hefeng51 | Hefeng43 | |
| DS1 | 1.27c | 1.85c | 1.37b | 2.11b | 1.58b | 2.45b |
| DS2 | 1.22c | 1.81c | 1.35b | 2.08b | 1.60b | 2.51b |
| DS3 | 1.01b | 1.54b | 1.19c | 1.81b | 1.69b | 2.60b |
| CK1 | 1.52a | 2.38a | 1.78a | 2.87a | 2.04a | 3.27a |
| CK2 | 0.65d | 0.68d | 0.63d | 0.71c | 0.64c | 0.67c |
| Analysis of variance (ANOVA) | ||||||
| Treatment (T) | * | ** | ** | *** | *** | *** |
| Cultivar (C) | *** | *** | *** | |||
| C × T | ** | ** | *** | |||
Means with same letter within columns are not significantly different (P < 0.05) using LSD test.
***, **, *Significance at 0.001, 0.01, and 0.05, respectively.
Effects of foliar α-oxoglutarate and nitrogen on ammonium content
The data in Table 7 demonstrated that leaf ammonium content was significantly reduced by foliar application of α-oxoglutarate and nitrogen at each stage. There was a lower increment in leaf ammonium content after exposure to foliar α-oxoglutarate and nitrogen at DS1, DS2 and DS3 than that at CK1 from S1 to S3 stage. The proline ammonium content was significantly influenced by treatment (T), cultivar (C) and their interaction (C × T) at each stage (Table 7). The ammonium content in Hefeng51 was significantly lower compared to Hefeng43 when exposed to drought stress at each stage. The data in Table 7 showed that there was a lower increment in ammonium content for Hefeng51 than that for Hefeng43 from S1 to S3 stage when exposed to drought stress.
Excessive ammonium could pose toxic effects on crops11. The GDH pathway is considered as a complementary route when plants are exposed to abiotic stresses12,33. The data in the present study indicated that foliar nitrogen and α-oxoglutarate enhanced GS activity, GDH activity, and resulted in the reduction in ammonium content in soybean leaf. Similarly, an early report showed that foliar application of α-oxoglutarate could promote the ammonium assimilation in roots and shoots of tomato, suggesting that the availability of carbon skeletons is a key limiting factor for ammonium uptake in tomato plants32. Yuan et al. also observed the increase in GS activity and the ammonium uptake when exogenous α-oxoglutarate was applied to rice exposed to abiotic stress31. The data in the present study indicated that foliar α-oxoglutarate significantly increased proline content and reduced ammonium content of soybean seedlings exposed to drought stress. Moreover, a combination of foliar α-oxoglutarate plus nitrogen mitigated the adverse effects of drought stress on soybean seedling better than foliar α-oxoglutarate or nitrogen at S1 and S2 stages. In the end, an increase in proline content and RWC could explain the reason why foliar nitrogen and α-oxoglutarate could alleviate the adverse impacts of drought stress on soybean seedlings.
Effects of foliar α-oxoglutarate and nitrogen on chlorophyll content (Ch) and photosynthetic rate (Pn)
Since photosynthesis is closely related to plant growth, photosynthesis-related parameters such as photosynthetic rate and chlorophyll content were determined in the present study. The data obtained in the present study indicated that the chlorophyll content and photosynthetic rate were significantly affected by foliar α-oxoglutarate and nitrogen (Tables 8, 9). Results from this study showed that there was no significant difference in leaf chlorophyll content and photosynthetic rate between DS1 and DS2 treatments at each stage, while the leaf chlorophyll content and photosynthetic rate at DS3 were significantly greater than that at DS1 and DS2 at S1 and S2 stages. The leaf chlorophyll content and photosynthetic rate under drought stress gradually decreased with the stress duration prolonged. The data obtained in this study showed that foliar application of nitrogen and α-oxoglutarate significantly enhanced the leaf chlorophyll content and photosynthetic rate of soybean seedling under drought stress compared to CK1 at each stage. The leaf chlorophyll content and photosynthetic rate under DS1, DS2, and DS3 treatments with foliar nitrogen and α-oxoglutarate resulted in a lower reduction than that under CK1 from S1 to S3 stage. A combination of foliar nitrogen plus α-oxoglutarate was better in improving photosynthesis under drought stress than either foliar nitrogen or foliar α-oxoglutarate.
Table 8.
Effects of foliar α-oxoglutarate and nitrogen on leaf relative chlorophyll content under drought stress (SPAD).
| Treatment | S1 stage | S2 stage | S3 stage | |||
|---|---|---|---|---|---|---|
| Hefeng51 | Hefeng43 | Hefeng51 | Hefeng43 | Hefeng51 | Hefeng43 | |
| DS1 | 46.25c | 43.06c | 44.25c | 40.85c | 42.57b | 37.35b |
| DS2 | 46.49c | 43.19c | 44.49c | 40.97c | 42.69b | 37.62b |
| DS3 | 48.36b | 45.08b | 46.46b | 41.28b | 43.26b | 37.78b |
| CK1 | 43.05d | 37.17d | 41.55d | 36.37d | 38.21c | 33.01c |
| CK2 | 52.26a | 48.57a | 52.69a | 49.07a | 53.19a | 49.43a |
| Analysis of variance (ANOVA) | ||||||
| Treatment (T) | * | ** | * | ** | ** | *** |
| Cultivar (C) | ** | ** | *** | |||
| C × T | * | ** | ** | |||
Means with same letter within columns are not significantly different (P < 0.05) using LSD test.
***, **, *Significance at 0.001, 0.01, and 0.05, respectively.
Table 9.
Effects of foliar α-oxoglutarate and nitrogen on photosynthetic rate under drought stress (µmol CO2 m−2 s−1).
| Treatment | S1 stage | S2 stage | S3 stage | |||
|---|---|---|---|---|---|---|
| Hefeng51 | Hefeng43 | Hefeng51 | Hefeng43 | Hefeng51 | Hefeng43 | |
| DS1 | 23.79b | 20.89c | 22.09c | 18.54c | 20.67c | 16.38c |
| DS2 | 24.08b | 21.06c | 22.18c | 18.86c | 20.71c | 16.25c |
| DS3 | 25.75b | 23.51b | 24.36b | 20.31b | 22.94b | 18.06b |
| CK1 | 21.85c | 19.87d | 19.84d | 16.25d | 17.80d | 14.01d |
| CK2 | 28.28a | 22.12a | 28.31a | 22.38a | 26.48a | 22.38a |
| Analysis of variance (ANOVA) | ||||||
| Treatment (T) | * | * | * | ** | ** | *** |
| Cultivar (C) | * | ** | ** | |||
| C × T | * | * | ** | |||
Means with same letter within columns are not significantly different (P < 0.05) using LSD test.
***, **, *Significance at 0.001, 0.01, and 0.05, respectively.
The photosynthetic rate and chlorophyll content (SPAD) are two key parameters of photosynthesis. It has been observed in previous reports demonstrating that foliar application of α-oxoglutarate posed positive influence on leaf photosynthesis57–60. The data in this study was in agreement with the study by Ge59 who pointed out that foliar application of α-oxoglutarate enhanced the leaf photosynthetic rate and chlorophyll content of winter-wheat when subjected to drought stress. In the present study, foliar application of α-oxoglutarate and nitrogen enhanced the nitrogen metabolism, assimilated more ammonium and thus resulted in the increase of proline content at S1, S2, and S3 stages. NADPH is required when proline biosynthesis takes place and NADP+ which is needed for photosynthesis is derived from NADPH decomposition61. Based on the results above, it was found that exogenous α-oxoglutarate and nitrogen might enhance photosynthetic rate in leaf through increasing the proline content of soybean seedling under drought stress.
Analysis of correlation between physio-chemical traits under drought stress
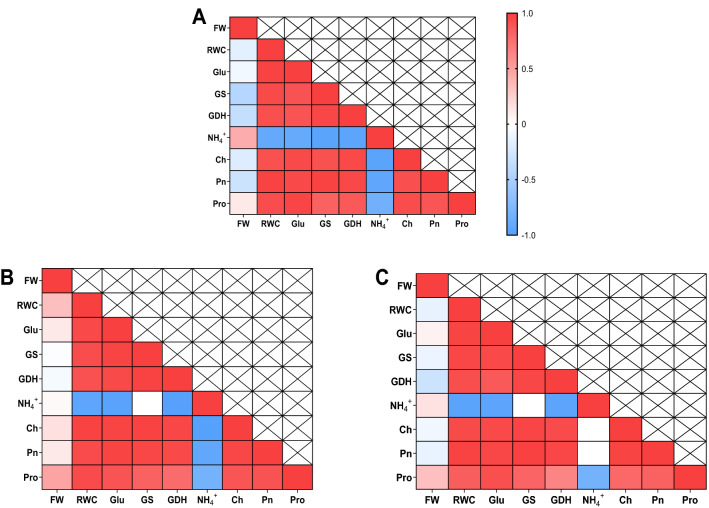
Analysis of correlation between all parameters under drought stress was performed to understand the relationship among all variables in soybean seedling exposed to drought stress with the application of foliar α-oxoglutarate and nitrogen. As shown in Fig. 1, all parameters exhibited statistically positive correlations with proline except for ammonium (NH4+) at each stage. The finding in this study was consistent with the study by Ren et al.52 who confirmed the role of proline in drought resistance and found the positive correlations between proline accumulation and improved drought tolerance. There has been a report that glutamate biosynthesis is greatly linked with ammonium assimilation10. The data obtained in this study suggested that there was positively significant relationship between glutamate and proline, which were negatively correlated with ammonium (NH4+) at each stage. Positive correlation between glutamate and proline was observed at S1, S2, and S3 stages with a correlation coefficient of 0.971**, 0.919**, and 0.893**, respectively, which supported glutamate is required when the rate of proline biosynthesis is enhanced47. Under drought stress, GDH activity showed positively significant relationship with glutamate but negatively significant relationship with ammonium (NH4+), indicating that GDH pathway could be considered as a complementary role in assimilating ammonium under abiotic stress13–15. NADPH is required when proline biosynthesis take place and NADP+ which is needed for photosynthesis, is derived from NADPH decomposition. Similarly, this study indicated that proline showed statistically positive relationship with Pn and Ch at each stage. This revealed that an increase in proline content was beneficial to photosynthesis under drought stress.
Figure 1.
The correlation between physio-chemical traits of soybean leaf at S1 (A), S2 (B), and S3 (C) stages under drought stress.
Conclusions
The present study has added new information that foliar α-oxoglutarate and nitrogen improved the drought tolerance in two soybean cultivars. Foliar α-oxoglutarate and nitrogen significantly increased leaf GS activity, GDH activity, glutamate content, proline content and photosynthesis of soybean seedlings exposed to drought stress at each stage. Accordingly, the ammonium content was significantly reduced by foliar α-oxoglutarate and nitrogen. These results suggested that in increasing the proline accumulation under drought stress, a combination of foliar nitrogen plus α-oxoglutarate had an advantage over either foliar nitrogen or foliar α-oxoglutarate, and a combination of foliar nitrogen plus α-oxoglutarate could mitigate the adverse effects of drought stress.
Acknowledgements
We are grateful to Tenglong Xie who made it possible to carry out the experiment.
Author contributions
Z.J.G. and L.L conceptualized and designed the research; Z.J.G. and L. L. carried out the experiment; Z.J.G., J.T.Z., L.J.C., and J.Q.L. analyzed all the data; Z.J.G. and J.Q.L wrote the manuscript. All authors have read and agreed to the published version of this manuscript.
Funding
This work was supported by the Heilongjiang Postdoctoral Science Foundation (LBH-Q21041).
Data availability
All data are contained in this article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Buezo J, Sanz-Saez Á, Moran JF, Soba D, Aranjuelo I, Esteban R. Drought tolerance response of high-yielding soybean varieties to mild drought: physiological and photochemical adjustments. Physiol. Plant. 2019;166(1):88–104. doi: 10.1111/ppl.12864. [DOI] [PubMed] [Google Scholar]
- 2.Verbruggen N, Hermans C. Proline accumulation in plants: a review. Amino Acids. 2008;35:753–759. doi: 10.1007/s00726-008-0061-6. [DOI] [PubMed] [Google Scholar]
- 3.Xin Z, Browse J. Eskimo1 mutants of Arabidopsis are constitutively freezing-tolerant. Proc. Natl. Acad. Sci. U. S. A. 1998;95:7799–7804. doi: 10.1073/pnas.95.13.7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyer JS. Plant productivity and environment. Science. 1982;218:443–448. doi: 10.1126/science.218.4571.443. [DOI] [PubMed] [Google Scholar]
- 5.Taiz L, Zeiger E. Plant Physiology. 3. Sinauer Associates; 2002. [Google Scholar]
- 6.Li SX. Dryland Agriculture in China. Science Press; 2007. [Google Scholar]
- 7.Cuellar-Ortiz SM, De La Paz Arrieta-Montiel M, Acosta-Gallegos J, Covarrubias AA. Relationship between carbohydrate partitioning and drought resistance in common bean. Plant Cell Environ. 2008;31:1399–1409. doi: 10.1111/j.1365-3040.2008.01853.x. [DOI] [PubMed] [Google Scholar]
- 8.Nanjo T, Fujita M, Seki M, Kato T, Tabata S, Shinozaki KT. Toxicity of free proline revealed in an Arabidopsis T-DNA-tagged mutant deficient in proline dehydrogenase. Plant Cell Physiol. 2003;44:541–548. doi: 10.1093/pcp/pcg066. [DOI] [PubMed] [Google Scholar]
- 9.Kishor PBK, Sangam S, Amrutha RN, Sreenivasulu N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005;88:424–438. [Google Scholar]
- 10.Forde BG, Lea PJ. Glutamate in plants: metabolism, regulation, and signalling. J. Exp. Bot. 2007;58:2339–2358. doi: 10.1093/jxb/erm121. [DOI] [PubMed] [Google Scholar]
- 11.Miflin BJ, Lea PJ. Ammonia Assimilation. Academic Press; 1980. [Google Scholar]
- 12.Cammaerts D, Jacobs MA. study of the role of glutamate dehydrogenase in the nitrogen metabolism of Arabidopsis thaliana. Planta. 1985;163:517–526. doi: 10.1007/BF00392709. [DOI] [PubMed] [Google Scholar]
- 13.Stitt M. Steps towards an integrated view of nitrogen metabolism. J. Exp. Bot. 2002;53:959–970. doi: 10.1093/jexbot/53.370.959. [DOI] [PubMed] [Google Scholar]
- 14.Ferrario-Mery S, Hodges M, Hirel B, Foyer CH. Photorespiration-dependent increases in phosphoenolpyruvate carboxylase, isocitrate dehydrogenase and glutamate dehydrogenase in transformed tobacco plants deficient in ferredoxin-dependent glutamine-α-ketoglutarate aminotransferase. Planta. 2002;214:877–886. doi: 10.1007/s00425-001-0692-2. [DOI] [PubMed] [Google Scholar]
- 15.Di Martino C, Delfine S, Alvino A, Loreto F. Photorespiration rate in spinach leaves under moderate NaCl stress. Photosynthetica. 1999;36:233. doi: 10.1023/A:1007099627285. [DOI] [Google Scholar]
- 16.Chipman RB, David RC, Patterson RP. Allocation of N and dry matter for two soybean genotypes in response to water stress during reproduction growth. J Plant Nutr. 2001;24:873–884. doi: 10.1081/PLN-100103779. [DOI] [Google Scholar]
- 17.Yang J, Zhang J, Huang Z, Zhu Q, Wang L. Remobilization of carbon reserves is improved by controlled soil-drying during grain filling of wheat. Crop Sci. 2000;40:1645–1655. doi: 10.2135/cropsci2000.4061645x. [DOI] [Google Scholar]
- 18.Zhang SG, Liu GL. Plant nutrition and drought resistance of crops. Chin. Bull. Bot. 2001;18:64–69. [Google Scholar]
- 19.Saneoka H, Moghaieb RE, Premachandra GS, Fujita K. Nutrition and water stress effects on cell membrane stability and leaf water relation in Agrostis palustris Hud. Environ. Exp. Bot. 2004;52:131–138. doi: 10.1016/j.envexpbot.2004.01.011. [DOI] [Google Scholar]
- 20.Zhang LX, Li SX, Zhang H, Liang ZS. Nitrogen rates and water stress effects on production, lipid peroxidation, and antioxidative enzyme activities in two maize (Zea mays L.) genotypes. J. Agron. Crop Sci. 2007;193:387–397. doi: 10.1111/j.1439-037X.2007.00276.x. [DOI] [Google Scholar]
- 21.Monrea AI, Jimenez E, Remesal TE, Morillo-Velarde R. Proline content of sugar beet storage roots: response to water deficit and nitrogen fertilization at field conditions. Environ. Exp. Bot. 2007;60:257–267. doi: 10.1016/j.envexpbot.2006.11.002. [DOI] [Google Scholar]
- 22.Zhang LX, Li SX, Liang ZS, Li SQ. Effect of foliar nitrogen application on nitrogen metabolism, water status, and plant growth in two maize cultivars under short-term moderate stress. J. Plant Nutr. 2009;32:1861–1881. doi: 10.1080/01904160903242367. [DOI] [Google Scholar]
- 23.Scheible WR, Krapp A, Stitt M. Reciprocal diurnal changes of phosphoenolpyruvate carboxylase expression and cytosolic pyruvate kinase, citrate synthase and NADP-isocitrate dehydrogenase expression regulate organic acid metabolism during nitrate assimilation in tobacco leaves. Plant Cell Environ. 2000;23:1155–1167. doi: 10.1046/j.1365-3040.2000.00634.x. [DOI] [Google Scholar]
- 24.Lancien M, Gadal P, Hodges M. Enzyme redundancy and the importance of 2-oxoglutarate in higher plant ammonium assimilation. Plant Physiol. 2000;123:817–824. doi: 10.1104/pp.123.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foyer CH, Noctor G. Photosynthetic Nitrogen Assimilation and Associated Carbon and Respiratory Metabolism. Springer; 2006. [Google Scholar]
- 26.Hodges M. Enzyme redundancy and the importance of 2-oxoglutarate in plant ammonium assimilation. J. Exp. Bot. 2002;53:905–916. doi: 10.1093/jexbot/53.370.905. [DOI] [PubMed] [Google Scholar]
- 27.Kawai Y, Ono E, Mizutani M. Evolution and diversity of the 2-oxoglutarate-dependent dioxygenase superfamily in plants. Plant J. 2014;78:328–343. doi: 10.1111/tpj.12479. [DOI] [PubMed] [Google Scholar]
- 28.Li H. Effects of Nitrogen Application on Nitrogen Metabolism in Winter Wheat Under Drought Stress Condition and the Regulation Metabolism of α-Ketoglutarate. Henan Agricultural University; 2016. [Google Scholar]
- 29.Morcuende R, Krapp A, Hurry V, Stitt M. Sucrose-feeding leads to increased rates of nitrate assimilation, increased rates of α-oxoglutarate synthesis, and increased synthesis of a wide spectrum of amino acids in tobacco leaves. Planta. 1998;206:394–409. doi: 10.1007/s004250050415. [DOI] [Google Scholar]
- 30.Ferrario-Méry S, Masclaux C, Suzuki A, Valadier M, Hirel B, Foyer CH. Glutamine and α-ketoglutarate are metabolite signals involved in nitrate reductase gene transcription in untransformed and transformed tobacco plants deficient in ferredoxin-glutamine-α-ketoglutarate aminotransferase. Planta. 2001;213:265–271. doi: 10.1007/s004250000504. [DOI] [PubMed] [Google Scholar]
- 31.Yuan Y, Du JQ, Wang ZQ, Zhang CF, Zhou ZP, Lin QH. Regulation of carbon and nitrogen metabolisms in rice roots by 2-oxoglutarate at the level of hexokinase. Physiol. Plant. 2007;129:296–306. doi: 10.1111/j.1399-3054.2006.00806.x. [DOI] [Google Scholar]
- 32.Magalhaes J, Huber D, Tsai C. Evidence of increased 15N-ammonium assimilation in tomato plants with exogenous α-ketoglutarate. Plant Sci. 1992;85:135–141. doi: 10.1016/0168-9452(92)90108-X. [DOI] [Google Scholar]
- 33.Gai ZJ, Liu L, Zhang JT, Liu JQ, Cai LJ. Effects of exogenous α-oxoglutarate on proline accumulation, ammonium assimilation and photosynthesis of soybean seedling (Glycine max(L.) Merr.) exposed to cold stress. Sci. Rep. 2020;10:17017. doi: 10.1038/s41598-020-74094-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu BB, Yuan YZ, Zhang CF, Ou JQ, Zhou W, Lin QH. Modulation of key enzymes involved in ammonium assimilation and carbon metabolism by low temperature in rice (Oryza sativa L.) roots. Plant Sci. 2005;169:295–302. doi: 10.1016/j.plantsci.2004.09.031. [DOI] [Google Scholar]
- 35.Gai ZJ, Zhang JT, Li CF. Effects of starter nitrogen fertilizer on soybean root activity, leaf photosynthesis and grain yield. PLoS ONE. 2017;12(4):e0174841. doi: 10.1371/journal.pone.0174841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bates LS, Waldren RP, Teare I. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- 37.Gao JF. Experiment Technique of Plant Physiology. Xi’an World Books Press; 2000. [Google Scholar]
- 38.Habib M. Effect of supplementary nutrition with Fe, Zn chelates and urea on wheat quality and quantity. Afr. J. Biotechnol. 2012;11:2661–2665. [Google Scholar]
- 39.Oosterhuis DM, Weir BL. Foliar fertilization of cotton. In: Stewart JM, Oosterhuis DM, Heitholt JJ, Mauney JR, editors. Physiology of Cotton. National Cotton Council of America and Springer; 2009. pp. 272–288. [Google Scholar]
- 40.Oosterhuis DM. Effects of PGR-IV on the growth and yield of cotton: a review. In: Constable GA, Forrester NW, editors. Challenging the Future: Proceedings of the World Cotton Research Conference. CSIRO; 1995. pp. 29–39. [Google Scholar]
- 41.Witte C, Tiller SA, Taylor MA, Davies H. Leaf urea metabolism in potato. Urease activity and patterns of recovery and distribution of 15N after foliar urea application in wild-type and urease-antisense transgenics. Plant Physiol. 2002;129:1129–1136. doi: 10.1104/pp.010506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ding XD, Tian CY, Zhang SR, Song J, Zhang FS, Mi GH, Feng G. Effects of NO3−-N on the growth and salinity tolerance of Tamarix laxa Willd. Plant Soil. 2010;331:57–67. doi: 10.1007/s11104-009-0231-7. [DOI] [Google Scholar]
- 43.Ebert G, Eberle J, Ali-Dinar H, Ludders P. Ameliorating effects of Ca(NO3)2 on growth, mineral uptake and photosynthesis of NaCl-stressed guava seedlings (Psidium guajava L.) Sci. Hortic. 2002;93:125–135. doi: 10.1016/S0304-4238(01)00325-9. [DOI] [Google Scholar]
- 44.Haseeb M, Maqbool N. Influence of foliar applied nitrogen on reproductive growth of sunflower (Helianthus annuus L.) under water stress. Agric. Sci. 2015;6:1413–1420. [Google Scholar]
- 45.Hare PD, Cress WA, Van Staden J. Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ. 1998;21:535–553. doi: 10.1046/j.1365-3040.1998.00309.x. [DOI] [Google Scholar]
- 46.Lutts S, Majerus V, Kinet JM. NaCl effects on proline metabolism in rice (Oryza sativa) seedlings. Physiol. Plant. 1999;105:450–458. doi: 10.1034/j.1399-3054.1999.105309.x. [DOI] [Google Scholar]
- 47.You J, Zhang Y, Liu A, Li D, Wang X, Dossa K, Zhou R, Yu J, Zhang Y, Wang L, Zhang X. Transcriptomic and metabolomic profiling of drought tolerant and susceptible sesame genotypes in response to drought stress. BMC Plant Biol. 2019;19:267. doi: 10.1186/s12870-019-1880-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan M, Hassan MJ, Peng Y, Feng G, Huang L, Liu L, Liu W, Han L, Li Z. Polyamines metabolism interacts with γ-aminobutyric acid, proline and nitrogen metabolisms to affect drought tolerance of creeping bentgrass. Int. J. Mol. Sci. 2022;23:2779. doi: 10.3390/ijms23052779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Younis ME, Hasaneen MNA, Kazamel AMS. Plant growth, metabolism and adaptation in relation to stress conditions: further studies supporting nullification of harmful effects of salinity in lettuce plants by urea treatment. Protoplasma. 2009;235:37–47. doi: 10.1007/s00709-008-0025-4. [DOI] [PubMed] [Google Scholar]
- 50.Agami RA, Alamri SA, Abd El-Mageed TA, Abousekken MS, Hashem M. Role of exogenous nitrogen supply in alleviating the deficit irrigation stress in wheat plants. Agric. Water Manag. 2018;210:261–270. doi: 10.1016/j.agwat.2018.08.034. [DOI] [Google Scholar]
- 51.Ren YB, Miao M, Cao JS, Fan TT, Yue JY, Xiao FM, Liu YS. DFR1-mediated inhibition of proline degradation pathway regulates drought and freezing tolerance in Arabidopsis. Cell Rep. 2018;23:3960–3974. doi: 10.1016/j.celrep.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 52.Forde BG, Lea PJ. Glutamate in plants: metabolism, regulation, and signaling. J. Exp. Bot. 2007;58:2339–2358. doi: 10.1093/jxb/erm121. [DOI] [PubMed] [Google Scholar]
- 53.Zhang H, Wang Z, Cui G, Lin T. Difference in seedlings ammonium assimilation of wheat cultivars with different drought resistance under osmotic stress. J. Appl. Ecol. 2009;20:2406–2410. [PubMed] [Google Scholar]
- 54.Maurin C, Gal YL. Glutamine synthetase in the marine coccolithophorid Emiliania huxleyi (Prymnesiophyceae): regulation of activity in relation to light and nitrogen availability. Plant Sci. 1997;122:1–69. doi: 10.1016/S0168-9452(96)04539-6. [DOI] [Google Scholar]
- 55.Mattioni C, Gabbrielli R, Vangronsveld J, Clijsters H. Nickel and cadmium toxicity and enzymatic activity in nitolerant and non-tolerant populations of Silene italica Pers. J. Plant Physiol. 1997;150:173–177. doi: 10.1016/S0176-1617(97)80198-8. [DOI] [Google Scholar]
- 56.Luo Z, Kong XQ, Dai JL, Dong HZ. Soil plus foliar nitrogen application increases cotton growth and salinity tolerance. J. Plant Nutr. 2015;38(3):443–455. doi: 10.1080/01904167.2014.912324. [DOI] [Google Scholar]
- 57.Jabeen R, Ahmad R. Alleviation of the adverse effects of salt stress by foliar application of sodium antagonistic essential minerals on cotton (Gossypium hirsutum L.) Pak. J. Bot. 2009;41:2199–2208. [Google Scholar]
- 58.Brugière N, Dubois F, Limami A, Lelandais M, Roux Y, Sangwan R, Hirel B. Glutamine synthetase in the phloem plays a major role in controlling proline production. Plant Cell. 1999;11:1995–2011. doi: 10.1105/tpc.11.10.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ge L. The Effect of α-Ketoglutarate on Winter Wheat Under Drought Stress. Henan Agricultural University; 2011. [Google Scholar]
- 60.Sun QLW, Jia L, Wang ZQ, Lin TB. Effects of exogenous α-oxoglutarate on yield traits of wheat under low water potential and low nitrogen stress. J. Anhui Agric. Sci. 2014;42:671–676. [Google Scholar]
- 61.Fehér-Juhász E, Majer P, Sass L, Lantos C, Csiszár J, Turóczy Z, Mihály R, Mai A, Horváth GV, Vass I, Dudits D. Phenotyping shows improved physiological traits and seed yield of transgenic wheat plants expressing the alfalfa aldose reductase under permanent drought stress. Acta Physiol. Plant. 2014;36:663–673. doi: 10.1007/s11738-013-1445-0. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are contained in this article.