Abstract
Background
We examined community- and hospital-acquired bloodstream infections (BSIs) in coronavirus disease 2019 (COVID-19) and non–COVID-19 patients across 2 epidemic waves.
Methods
We analyzed blood cultures of patients presenting to a London hospital group between January 2020 and February 2021. We reported BSI incidence, changes in sampling, case mix, healthcare capacity, and COVID-19 variants.
Results
We identified 1047 BSIs from 34 044 blood cultures, including 653 (62.4%) community-acquired and 394 (37.6%) hospital-acquired. Important pattern changes were seen. Community-acquired Escherichia coli BSIs remained below prepandemic level during COVID-19 waves, but peaked following lockdown easing in May 2020, deviating from the historical trend of peaking in August. The hospital-acquired BSI rate was 100.4 per 100 000 patient-days across the pandemic, increasing to 132.3 during the first wave and 190.9 during the second, with significant increase in elective inpatients. Patients with a hospital-acquired BSI, including those without COVID-19, experienced 20.2 excess days of hospital stay and 26.7% higher mortality, higher than reported in prepandemic literature. In intensive care, the BSI rate was 421.0 per 100 000 intensive care unit patient-days during the second wave, compared to 101.3 pre–COVID-19. The BSI incidence in those infected with the severe acute respiratory syndrome coronavirus 2 Alpha variant was similar to that seen with earlier variants.
Conclusions
The pandemic have impacted the patterns of community- and hospital-acquired BSIs, in COVID-19 and non–COVID-19 patients. Factors driving the patterns are complex. Infection surveillance needs to consider key aspects of pandemic response and changes in healthcare practice.
Keywords: healthcare-associated infection, bacteremia, antimicrobial resistance, SARS-CoV-2
The COVID-19 pandemic has had impact on patterns of community- and hospital-acquired bloodstream infections in COVID-19 and non–COVID-19 patients. High incidence of hospital-acquired bacteremia was seen during the COVID-19 waves, particularly in SARS-CoV-2–negative elective patients.
Existing assessment of the impact of the coronavirus disease 2019 (COVID-19) pandemic on healthcare-associated infections (HCAIs) has been limited to bacterial and fungal coinfections and secondary infections in hospitalized COVID-19 patients [1, 2] and based upon data emerged from the first pandemic wave. These initial analyses did not consider the broader context of potentially shifting patterns of infections in non–COVID-19 patients, nor the changes in dominant COVID-19 variants, health capacity, and practice across the pandemic waves.
In the United Kingdom (UK), delivery of care was disrupted significantly during the 2 COVID-19 waves. Within acute care, factors such as change in patient mix, including those who are critically ill, and prolonged duration of intensive care unit (ICU) admission may have collectively influenced the incidence of HCAIs [3]. Expanded use of antimicrobials and invasive procedures such as mechanical ventilation, alongside disruption of routine infection prevention and control (IPC) activities, may have intensified the emergence and transmission of resistant pathogens [4–6]. COVID-19 patients have symptoms and biomarkers that can mimic bacterial and fungal infection. This, plus use of immunomodulating drugs, has made it challenging to diagnose bacterial and fungal infection and monitor response to treatment. Analysis of how the pandemic has affected the epidemiology of other HCAIs in both patients with and without COVID-19 is urgently needed, and such analysis must consider important variables including changes in hospital and intensive care admission rates, capacity, culture sampling, and screening practices. In addition, the impact of the Alpha (B.1.1.7) variant, which dominated the UK’s second COVID-19 surge, and other variants on bacterial and fungal infections also requires characterization [5]. Investigating HCAIs in different patient groups will help elucidate the impact of the pandemic and strengthen targeted IPC interventions to improve care for patients.
In this study, we aimed to assess community- and hospital-acquired bloodstream infections (BSIs) identified in patients with and without COVID-19 presenting to a hospital group in London across 2 COVID-19 waves, while considering the changes in healthcare access and practice and the shifted dominant COVID-19 variants.
METHODS
Data
We analyzed de-identified blood cultures, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) test results, hospital episode, and prescribing records of patients admitted to a large teaching hospital group (Imperial College Healthcare National Health Service [NHS] Trust) between 1 January 2020 and 28 February 2021.
Descriptive and Statistical Analysis
We reported BSI incidence rates and susceptibility profiles for different patient groups over time, alongside hospital and ICU occupancy. We compared average length of stay (LOS) and all-cause in-hospital mortality for patients with and without hospital-acquired BSI, and performed Mann-Whitney and Pearson χ2 tests to determine whether the differences in LOS and mortality were significant. We considered a P value < .05 statistically significant.
Definitions
Hospital admission was defined as the continued stay within 1 hospital as an inpatient. The methods of admission were elective, or nonelective (including emergency and maternity admission), as per NHS National Codes [7].
Patient characteristics included gender (female, male, other), age group (children aged <18 years, adults aged 18–64 years, elderly aged ≥65 years), and ethnicity (non-white, white, unknown).
For bacterial and fungal bloodstream infection, a BSI was confirmed by bacterial or fungal isolates identified in blood cultures. If the organism is a common skin commensal, we followed the US Centers for Disease Control and Prevention (CDC) criteria [8] to define a true BSI by identifying at least 2 cultures of the same species isolated within 24 hours. Single cultures of skin commensals within 24 hours were considered contaminants. An “episode” relates to the 14-day period following the initial specimen (or subsequent specimens >14 days apart from the previous sample). Positive blood cultures taken within 14 days of the first sample were considered of the same episode, unless a negative blood culture has been obtained in the interim. If >1 pathogen was isolated from a blood culture, each was recorded individually at species level. A community-acquired infection episode was defined if the first positive blood culture was taken within 0–48 hours of admission; a hospital-acquired infection episode was defined if the first positive blood culture was taken after 48 hours of admission [9] and prior to discharge. Sensitivity testing was performed by disk diffusion and minimum inhibitory concentration strips, and results were de-duplicated by keeping the worst-case scenario for each antimicrobial agent. A patient with central line (central venous cannula/catheter, hemodialysis cannula, extended dwell peripheral catheter, and peripherally inserted central catheter) in place between 2 and 7 days before a BSI onset was considered to have a central line–associated bloodstream infection (CLABSI), as per CDC criteria [8].
COVID-19 status was confirmed by at least 1 positive SARS-CoV-2 nasopharyngeal and oral swab polymerase chain reaction test. A patient was considered “admitted with COVID-19 infection” if at least 1 positive SARS-CoV-2 test was identified during the first 7 days of admission. S-gene target failure (SGTF) was used as a proxy of infection caused by the Alpha (B.1.1.7) variant. A patient was grouped as without SGTF if all SARS-CoV-2 swabs were tested without SGTF. Bacterial or fungal coinfection was defined by coexistence of confirmed infection from blood or respiratory samples and at least 1 positive SARS-CoV-2 test sampled from the same day up to 21 days before the blood/respiratory sampling date; that is, we consider bacterial or fungal infections confirmed before a positive SARS-CoV-2 result, or >21 days after a positive SARS-CoV-2 result, not relevant to the COVID-19 infection.
Ethical Considerations
This study was approved by the Imperial Academic Health Science Centre COVID Research Committee, the COVID-19 North West London Data Prioritisation Group, and the Discover Research Advisory Group.
RESULTS
Blood Culture and Patient Characteristics
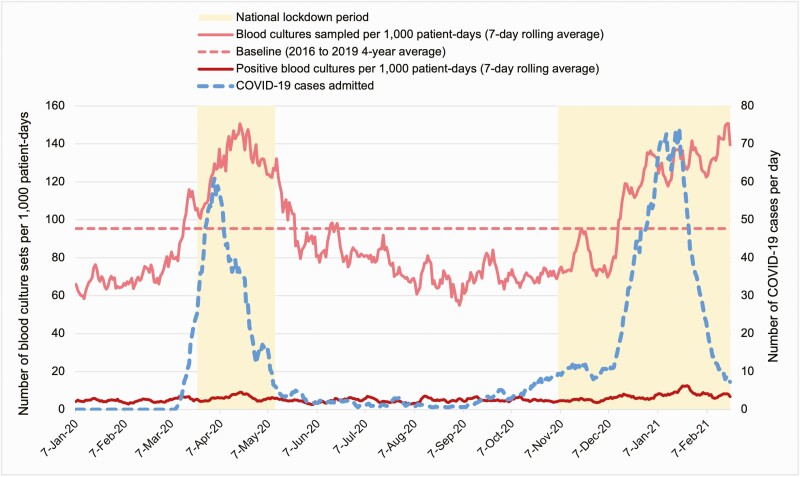
From 1 January 2020 to 28 February 2021, 34044 blood cultures were identified. Blood cultures were sampled from 19.9% (n=15077) patients admitted to hospital (compared to 16.8% prepandemic), and 59.9% (n=2311) patients were admitted to ICU. A total of 9743 (64.6%) admitted patients had blood cultures taken during the first 48 hours of admission. During the study, the average blood culture sampling rate was 86.8 sets per 1000 patient-days, which increased to 150.7 during the 2 COVID-19 surges (Figure 1).
Figure 1.
Blood culture sets per 1000 patient-days, January 2020–February 2021. Abbreviation: COVID-19, coronavirus disease 2019.
Organism growth was detected in 6.8% (2317/34044) of blood cultures, slightly below the pre–COVID-19 figure of 7.3%. Positive blood cultures were from a cohort of 1667 patients, with a mean age of 58.1 years (standard deviation [SD],24.1 years), 56.9% male (949/1667), 57.2% SARS-CoV-2 negative (954/1667), and 31.0% admitted to ICU (517/1667) (Table 1).
Table 1.
Characteristics of Patients Who Had Growth Detected in Blood Cultures
| Characteristic | Total (N=1667) | SARS-CoV-2 Positive (n=395) | SARS-CoV-2 Negative (n=1272) |
|---|---|---|---|
| Gender identity | |||
| Female | 718 (43.1) | 154 (39.0) | 564 (44.3) |
| Male | 949 (56.9) | 241 (61.0) | 708 (55.7) |
| Other | 0 | 0 | 0 |
| Age group, y | |||
| Children (<18) | 139 (8.3) | 6 (1.5) | 133 (10.5) |
| Adult (18–64) | 760 (45.6) | 211 (53.4) | 549 (43.2) |
| Elderly (≥65) | 768 (46.1) | 178 (45.1) | 590 (46.4) |
| Ethnicity | |||
| Non-white | 677 (40.6) | 204 (30.1) | 473 (69.9) |
| White | 656 (39.4) | 110 (16.8) | 546 (83.2) |
| Unknown | 334 (20.0) | 81 (20.5) | 253 (19.9) |
| ICU admission | |||
| Admitted to ICU | 517 (31.0) | 192 (48.6) | 325 (25.6) |
| Not admitted to ICU | 1150 (69.0) | 203 (51.4) | 947 (74.4) |
| Infection status | |||
| Developed hospital-acquired BSI | 314 (18.8) | 89 (28.3) | 225 (72.7) |
| Developed community-acquired BSI | 557 (33.4) | 80 (14.4) | 477 (85.6) |
| Had contaminants in blood culture | 796 (47.8) | 226 (28.4) | 570 (71.6) |
| COVID-19 status | |||
| Had SARS-CoV-2 test, positive | 395 (23.7) | NA | NA |
| Had SARS-CoV-2 test, negative | 954 (57.2) | NA | NA |
| Had no SARS-CoV-2 test | 318 (19.1) | NA | NA |
| In-hospital mortality | |||
| Deceased | 449 (26.9) | 134 (33.9) | 315 (24.8) |
| Alive | 1218 (73.1) | 261 (66.1) | 957 (75.2) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BSI, bloodstream infection; COVID-19, coronavirus disease 2019; ICU, intensive care unit; NA, not applicable; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Causative Organisms Identified in Blood Cultures
The most common organisms in blood culture were staphylococci (differentiated as Staphylococcus aureus and coagulase-negative staphylococci [CoNS]), Enterobacterales (including Citrobacter, Enterobacter, Escherichia coli, Hafnia, Klebsiella, Morganella, Proteus, and Serratia species), enterococci, streptococci, Pseudomonas sp, Corynebacterium sp, and Candida sp, which were isolated from 2129 cultures. CoNS was detected from 47.8% positive blood cultures, compared to 24.8% pre–COVID-19, followed by enterococci (increased by 3.6%) and streptococci (increased by 2.2%). Escherichia coli was detected in 15.5% of the positive blood cultures, decreased by 0.9% compared to pre–COVID-19. Of positive blood cultures, 41.3% were contaminants, compared to 31.5% prepandemic. The 1250 noncontaminant cultures were grouped into 1047 BSI episodes, including 394 (37.6%) hospital-acquired BSIs and 653 (62.4%) community-acquired BSIs (Table 2).
Table 2.
Summary of Positive Blood Cultures (January 2020–February 2021)
| Pathogen | Positive Blood Culture Isolates (n=2129), No. (%) Positive Blood Cultures | Positive Blood Culture Isolates (Pre–COVID-19), % Positive Blood Cultures | Contaminants (n=879), No. (%) Positive Blood Cultures With the Pathogen | Hospital-Acquired BSI (n=394), No. (%) Hospital-Acquired BSI | Community-Acquired BSI (n=653), No. (%) Community-Acquired BSI |
|---|---|---|---|---|---|
| CoNS | 1017 (47.8) | 24.8 | 797 (90.7) | 48 (12.2) | 25 (3.8) |
| Escherichia coli | 331 (15.5) | 16.4 | NA | 55 (14.0) | 246 (37.7) |
| Staphylococcus aureus | 212 (10.0) | 9.6 | NA | 34 (8.6) | 74 (11.3) |
| Enterococcus spp | 183 (8.6) | 5.0 | NA | 90 (22.8) | 48 (7.4) |
| Streptococcus spp | 147 (6.9) | 4.7 | 56 (96.4) | 11 (2.8) | 68 (10.3) |
| Klebsiella spp | 129 (6.1) | 5.5 | NA | 49 (12.4) | 60 (9.2) |
| Pseudomonas spp | 119 (5.6) | 3.9 | NA | 42 (10.7) | 49 (7.5) |
| Corynebacterium spp | 41 (1.9) | 0.8 | 41 (4.7) | 0 | 0 |
| Candida spp | 40 (1.9) | 1.0 | NA | 30 (7.6) | 6 (0.9) |
| Enterobacter spp | 40 (1.9) | 1.3 | NA | 14 (3.6) | 18 (2.8) |
| Proteus spp | 38 (1.8) | 1.5 | NA | 4 (1.0) | 30 (4.6) |
| Citrobacter spp | 21 (1.0) | 0.3 | NA | 8 (2.0) | 13 (2.0) |
| Serratia spp | 21 (1.0) | 0.6 | NA | 6 (1.5) | 11 (1.7) |
| Morganella spp | 7 (0.3) | 0.2 | NA | 2 (0.5) | 5 (0.8) |
| Hafnia spp | 2 (0.1) | 0.0 | NA | 1 (0.3) | 0 |
Abbreviations: BSI, bloodstream infection; CoNS, coagulase-negative staphylococci; COVID-19, coronavirus disease 2019; NA, not applicable.
Community-Acquired Bloodstream Infections
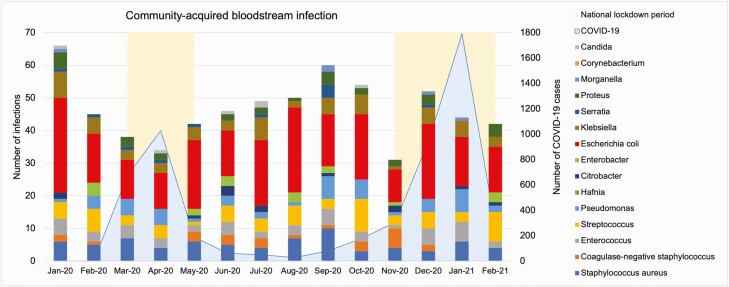
A total of 653 (62.4%) community-acquired BSIs presented during the study period (Figure 2). Monthly counts are shown in Figure 2, with 3 national lockdowns imposed in March–May 2020, November–December 2020, and January–February 2021 [10].
Figure 2.
Monthly counts of community-acquired bloodstream infections. Abbreviation: COVID-19, coronavirus disease 2019.
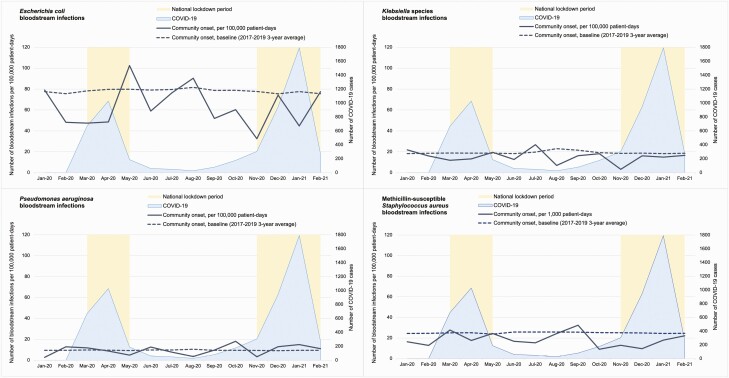
Gram-negative bacteria (E. coli, Klebsiella spp, and Pseudomonas aeruginosa) and methicillin-susceptible S. aureus (MSSA) were the most common causative pathogens, similar to pre–COVID-19. The overall rates of community-acquired BSI caused by gram-negative bacteria and MSSA were lower than the pre–COVID-19 level (Figure 3). During the study period, there were 87.7 gram-negative BSIs and 18.5 MSSA BSIs per 100000 patient-days, compared to pre–COVID-19 rates of 107.0 and 24.6. Between COVID-19 surges, during easing of the national lockdowns beginning on 10 May 2020, the gram-negative BSI rate rose to 126.8 per 100000 patient-days, in contrast to the pre–COVID-19 annual trend of peaking in the quarter of July to September [11].
Figure 3.
Community-onset bloodstream infections caused by Escherichia coli, Klebsiella spp, Pseudomonas aeruginosa, and methicillin-susceptible Staphylococcus aureus (January 2020–February 2021). Abbreviation: COVID-19, coronavirus disease 2019.
Hospital-Acquired Bloodstream Infections in Hospital Patients With and Without COVID-19
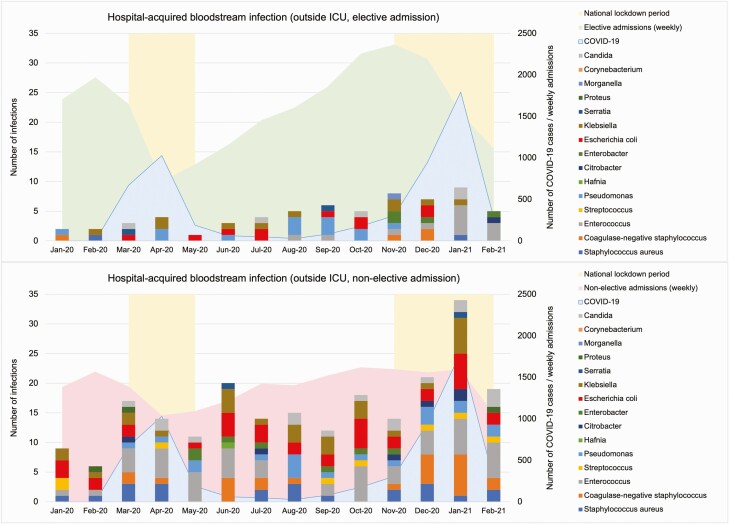
Between January 2020 and February 2021, 75798 patients were admitted to the hospital group. A total of 3510 (4.6%) patients were admitted with COVID-19. Three hundred fourteen (0.4%) patients had at least 1 hospital-acquired BSI. There were 394 hospital-acquired BSIs, of which 288 occurred outside the ICU (Figure 4), and 106 in the ICU. Daily case counts of BSIs in patients admitted with and without COVID-19 are shown in Supplementary Materials 1.
Figure 4.
Hospital-acquired bloodstream infections in patients (with or without coronavirus disease 2019 [COVID-19]) outside the intensive care unit (ICU), January 2020–February 2021.
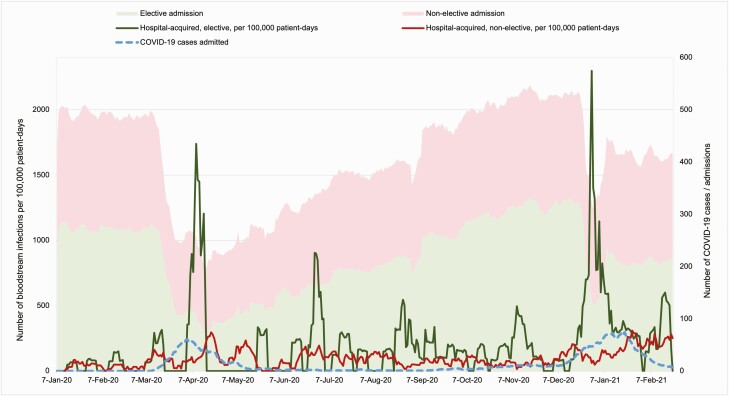
During the first COVID-19 surge in April 2020, both elective and nonelective admissions reached their lowest levels (Figure 5). Hospital admissions decreased by 53.6% from 15178 admissions in January to 7040 in April, with a 65.0% reduction in elective admissions. The overall hospital-acquired BSI rate was 100.4 episodes per 100000 patient-days during the study period across all levels of care, compared to 97.3 pre–COVID-19. The hospital-acquired BSI rate increased during both COVID-19 surges despite reduced number of hospital admissions. The BSI rate was 79.4 per 100000 patient-days during the first COVID-19 wave, and 132.8 during the second. Patients with COVID-19 had 170.2 BSIs per 100000 patient-days, whereas patients without COVID-19 had 90.1 BSIs per 100000 patient-days (P<.05). More significant increases were observed among elective admissions during the 2 COVID-19 surges, between 1 and 14 April 2020, and between 1 and 14 January 2021 (Figure 5), of which 98.8% of the patients in the elective cohort admitted were without COVID-19.
Figure 5.
Hospital admissions, coronavirus disease 2019 (COVID-19) cases, and hospital-acquired bloodstream infection incidence rates across all levels of care, January 2020–February 2021.
After adjusted for time to event, the average LOS was 26.1 days (SD,26.0 days) after BSI onset (27.4 days for COVID-19 patients, 25.6 days for non–COVID-19 patients). The crude excess LOS in patients with hospital-acquired BSI was 20.2 days (Mann-Whitney test, P<.05). A total of 4153 of 75798 (5.5%) admitted patients died during hospital stay. The all-cause in-hospital mortality rate was 32.1% (101/314) in patients with hospital-acquired BSI, and 5.4% (4052/75483) in patients without hospital-acquired BSI (Pearson χ2 test, P<.05). Of those 314 patients who had hospital-acquired BSI, 162 (51.6%) patients developed BSI in ICU, and 89 (28.3%) were diagnosed with COVID-19.
Hospital-acquired BSI caused by methicillin-resistant S. aureus (MRSA) had the largest increase among all causative pathogens in both COVID-19 and non–COVID-19 patients, compared to pre–COVID-19 figures. The MRSA BSI rate rose from 0.8 per 100000 patient-days pre–COVID-19 to 4.9 during the first COVID-19 wave and 6.0 during the second wave.
Hospital-Acquired Bloodstream Infections in Intensive Care
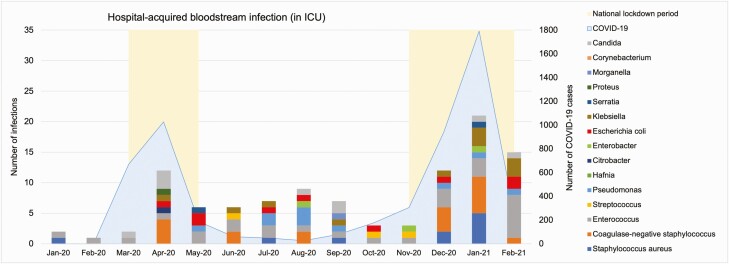
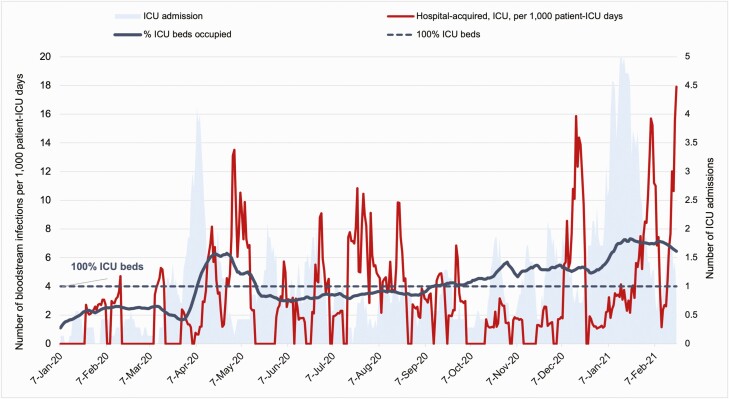
A total of 3856 patients were admitted to ICU during the study period, of which 26.8% (1035) had documented central venous access. Forty-three CLABSIs were identified. The overall CLABSI rate was 3.2 per 1000 line-days, and peaked at 8.4 during the second COVID-19 wave, compared to 2.5 pre–COVID-19. One hundred six hospital-acquired BSIs occurred in intensive care. The overall hospital-acquired BSI rate in ICU was 311.8 per 100000 patient-ICU days. Individuals with COVID-19 had 403.2 BSIs per 100000 patient-ICU days, while the patients without COVID-19 had 268.3 (P=.051). Outside ICU, the hospital-acquired BSI rate was 88.5 per 100000 patient-days, 92.7 in COVID-19 patients, and 66.7 in non–COVID-19 patients (P<.05). BSI rate in ICU remained stable during the first COVID-19 wave (304.3 per 100000 patient-ICU days), but increased to 421.0 during the second wave (Figure 6). A time lag of approximately a week between ICU admission and hospital-acquired BSI onset occurred throughout the study period (Figure 7).
Figure 6.
Hospital-acquired bloodstream infections in patients (with or without coronavirus disease 2019 [COVID-19]) in the intensive care unit (ICU), January 2020–February 2021.
Figure 7.
Admissions, bed occupancy, and hospital-acquired bloodstream infection incidence rates in the intensive care unit (ICU), January 2020–February 2021.
In intensive care, the average ICU bed occupancy was 95.1% compared to 83.1% pre–COVID-19. Bed occupancy increased to 157.6% in the first surge, with 47.3% occupied by COVID-19 patients, and 182.8% in the second surge, with 64.0% occupied by COVID-19 patients. The number of ICU beds were expanded by 70.5%, from 88 before 2020 to 150 in December 2020. However, the monthly staff hours of registered ICU nurses only expanded by 27.5%, from 41197.9 hours in July to 52522.6 hours in December 2020, including the redeployed non-ICU staff. Reporting of nurse and midwife staffing levels was discontinued between March and May [12].
Bacterial and Fungal Infection in COVID-19 Patients Infected by the Alpha (B.1.1.7) Variant
A total of 1171 SARS-CoV-2–positive nasopharyngeal and oral swabs from 850 patients were tested for SGTF using Thermo Fisher assays. Three hundred ninety-eight (46.8%) patients were infected with SGTF isolates, suggesting the Alpha variant. Thirty-eight (4.5%) SARS-CoV-2–positive patients had infections caused by other pathogens confirmed within 21 days following a positive SARS-CoV-2 test. Seventeen (4.3%) patients with SGTF had cultures yielding, compared to 15 (3.3%) patients without SGTF. The difference in the proportion of patients with bacterial and fungal infections in respiratory tract and bloodstream following a positive SARS-CoV-2 test was not significant in SGTF and non-SGTF groups (P=.452).
DISCUSSION
The COVID-19 pandemic has exerted high pressure on health systems [13]. The pandemic and the national response measures may have influenced the epidemiology of other infections, reflected by altered presentation of bacteremia to healthcare. Between January 2020 and February 2021, the average blood culture sampling rate and percentage of blood cultures with growth were lower than prepandemic. However, the blood sampling rate increased during COVID-19 surges, as the serving laboratory reduced the blood culture incubation period from 5 days to 3 days to increase sample processing capacity. Blood contaminants and noncontaminant isolates (as percentage of all blood cultures) both increased compared to pre–COVID-19.
Community-acquired infections identified in acute care have decreased, potentially associated with physical distancing and emphasized IPC measures [14]. However, an alternative explanation for the reported fall in community-acquired BSIs was reduced elective procedures [15]. There was a concern that patients with symptoms of infection were not seeking medical attention, leading to missed episodes of bacterial infection such as urinary tract–associated sepsis [16]. The Office for National Statistics reported one-third excess mortality in private homes during the first pandemic wave [17], of which 25.6% were due to non–COVID-19 causes including untreated sepsis [16, 18]. In our analysis, although the average community-acquired BSI rate across the pandemic was below the pre–COVID-19 level, we saw a rise in gram-negative bacteremia occurring between COVID-19 surges, suggesting potentially suppressed or delayed presentation during the pandemic waves.
Elective admissions were reduced by 65.0% in the hospital group during the first wave, and by 27.0% during the second. Despite the reduced number of admissions, hospital-acquired BSI rate increased in both surges, with a more significant rise occurring in the non–COVID-19 elective admissions. The patients with hospital-acquired BSI had crude excess LOS of 20.2 days, and 26.7% higher all-cause in-hospital mortality, both higher than what has been reported in previous literature, with LOS of 16.9 days and mortality ranging between 1.4% and 24% [19]. One explanation could be that the case mix was skewed during the COVID-19 surges as elective admission has been restricted to those who were critically ill and more likely to acquire infections and to have longer hospital stay. In ICU, a high hospital-acquired BSI rate was observed in both COVID-19 and non–COVID-19 patients. CLABSI rate increased during the second COVID-19 wave.
No significant difference in the prevalence of bacterial and fungal infections was detected in bloodstream or respiratory tract after a positive SARS-CoV-2 test of Alpha and other variants, suggesting that the Alpha variant, which dominated the UK’s second COVID-19 surge, was not directly contributing to the increase in-hospital-acquired BSI.
Factors driving reported variation in bacteremia incidence are highly complex. Earlier concerns have been raised over disrupted routine screening, laboratories having less capacity to process samples, and infections directly attributable to SARS-CoV-2. In this hospital group, blood culture sampling practice was maintained during the pandemic, although the capacity to collect data on vascular line-days was compromised. The variation in epidemiology during the pandemic waves is associated with changes in patient case mix and adjustments in healthcare access and practice. During the pandemic, the hospital group expanded the ICU capacity, however, without additional trained staff and facilities built to the Health Technical Memorandum standards. During the first wave, acute trusts in England increased their patient-to-nurse ratio from 1:1 to 6:1, and consultant-to-patient ratio from no more than 15:1 to 30:1, as the NHS sought to expand capacity rapidly [20]. As winter approached and a second wave arrived in November 2020, NHS hospitals temporarily widened the patient-to-nurse ratio again from 1:1 to 2:1 [21]. Widening patient-to-staff ratios, proning patients, and excess or suboptimal personal protective equipment use might have had a negative impact on IPC practices and subsequently on HCAIs, including the rise in hospital-acquired bacteremia reported in this analysis. Excess skin organisms yielded from clinical samples with overrepresentations in critical care, such as CoNS and Corynebacterium species (in particular C. striatum), compared to previous were observed within the institutes. Increased rates of contaminants in blood cultures suggested a breakdown of IPC practices and pointed to a direction for future intervention [15].
During the pandemic, elective admission was suspended and microbiology laboratories had less capacity to process non–SARS-CoV-2 samples. Analysis of microbiology data needs to be contextualized to ensure robust interpretation of the observed infection incidence. However, existing HCAI surveillance systems rarely included metrics to monitor pressure on health systems. Standardized metrics, which could improve the external validity of studies in infection epidemiology, enable comparison across locations and settings, and widen the evidence base of IPC intervention design, are yet to be developed and tested [22].
Our study has some limitations. First, datasets at the same level of granularity prior to 2020 were not available. Second, monitoring of device-associated BSI was not available between January to March 2020 due to operational challenges in capturing accurate data on indwelling device days. Third, the time lag between reporting from hospital laboratories to the central data registry might have caused missed identification of blood cultures toward the end of the study period.
To conclude, our study provides a comprehensive analysis of the impact of the pandemic on BSIs arising from the community and from acute care in both COVID-19 and non–COVID-19 patients, and across 2 COVID-19 waves in the UK. Existing infection surveillance needs to consider key aspects of the pandemic response and changes in healthcare access and practice to ensure learning and introduction of appropriate interventions to minimize unintended consequences to care.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author Contributions. N. Z., T. M. R., and A. H. developed the concept and methodology for this research. N. Z. undertook data extraction and analysis. T. M. R., M. G., and F. D. supported with additional data. N. Z. drafted the initial manuscript. T. M. R., S. M., J. R. P., M. G., F. D., J. O., P. A., Y. S., and A. H. contributed significantly to data interpretation, revision of the manuscript, and finalization for submission. A. H. is the guarantor of the study.
Acknowledgments. The authors thank Damien Ming and Isa Ahmad for the support in data, and Paul Randell for the assistance in interpreting severe acute respiratory syndrome coronavirus 2 virology test results. The authors thank Dimitri Papadimitriou from the Imperial Colleague Healthcare National Health Service (NHS) Trust for the assistance in conducting analysis within the iCARE environment.
Disclaimer. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research (NIHR), the Department of Health and Social Care, or Public Health England (PHE).
Financial support. This work was supported by the World Health Organization (WHO); the NIHR Health Protection Research Unit in Healthcare Associated Infections and Antimicrobial Resistance at Imperial College London in partnership with PHE, in collaboration with Imperial Healthcare Partners, University of Cambridge, and University of Warwick; the Department of Health and Social Care, which funded the Centre for Antimicrobial Optimisation at Imperial College London; and the NIHR Imperial Biomedical Research Centre, which developed and funded the iCARE high-performance analytics environment to provide data used in this research. A. H. is an NIHR Senior Investigator. P. A. is supported by the NIHR Applied Research Collaboration Northwest London and Telstra Health UK. F. D. receives funding from the Medical Research Council Clinical Academic Research Partnership Scheme.
Potential conflicts of interest. A. H. reports participation on WHO Health Emergencies Program Ad-Hoc Advisory Panel of Infection Prevention and Control Experts for Preparedness, Readiness and Response to COVID-19 (current); Scientific Advisory Group for Emergencies Coronavirus Response working group on nosocomial transmission (current); serving as board member for Wellcome-Surveillance and Epidemiology of Drug-resistant Infections Consortium (2017–2020); serving as committee member of Scientific Steering Committee on Antimicrobial Resistance organized by Academy of Medical Sciences, supported by the Yusuf and Farida Hamied Foundation (2018–2019); serving as expert advisory board group member (2017–2018) for De-linking Reimbursement of Antimicrobials from Volumes Sold: Implications for National Institute for Health and Care Excellence (NICE) Appraisal (contributor to report entitled “Framework for value assessment of new antimicrobials—implications of alternative funding arrangements for NICE Appraisal,” published in September 2018); and serving as executive committee member and president of the International Society for Infectious Diseases (current). R. H. reports honoraria editing for the Journal of Antimicrobial Chemotherapy. T. M. R. reports personal consultancy fees (2020) from Sandoz and personal consultancy fees (2021) and personal educational honoraria from bioMérieux. M. G. reports payment or honoraria from Pfizer and Sanofi. J. O. reports consulting payments from Gama Healthcare Ltd and Pfizer Ltd; payment or honoraria for giving talks from Antimicrobial Stewardship Progamme, Diversey and Clean Hospitals; and academic fees from Imperial College London and Nursing Times. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Nina J Zhu, National Institute for Health Research Health Protection Research Unit in Healthcare Associated Infections and Antimicrobial Resistance, Imperial College London, London, United Kingdom.
Timothy M Rawson, National Institute for Health Research Health Protection Research Unit in Healthcare Associated Infections and Antimicrobial Resistance, Imperial College London, London, United Kingdom; Imperial College Healthcare National Health Service Trust, Imperial College London, London, United Kingdom; Centre for Antimicrobial Optimisation, Imperial College London, London, United Kingdom.
Siddharth Mookerjee, Imperial College Healthcare National Health Service Trust, Imperial College London, London, United Kingdom.
James R Price, National Institute for Health Research Health Protection Research Unit in Healthcare Associated Infections and Antimicrobial Resistance, Imperial College London, London, United Kingdom; Imperial College Healthcare National Health Service Trust, Imperial College London, London, United Kingdom.
Frances Davies, Imperial College Healthcare National Health Service Trust, Imperial College London, London, United Kingdom.
Jonathan Otter, National Institute for Health Research Health Protection Research Unit in Healthcare Associated Infections and Antimicrobial Resistance, Imperial College London, London, United Kingdom; Imperial College Healthcare National Health Service Trust, Imperial College London, London, United Kingdom.
Paul Aylin, National Institute for Health Research Health Protection Research Unit in Healthcare Associated Infections and Antimicrobial Resistance, Imperial College London, London, United Kingdom; Department of Primary Care and Public Health, School of Public Health, Imperial College London, London, United Kingdomand.
Russell Hope, Division of Healthcare Associated Infection and Antimicrobial Resistance, Public Health England, London, United Kingdom.
Mark Gilchrist, National Institute for Health Research Health Protection Research Unit in Healthcare Associated Infections and Antimicrobial Resistance, Imperial College London, London, United Kingdom; Imperial College Healthcare National Health Service Trust, Imperial College London, London, United Kingdom; Centre for Antimicrobial Optimisation, Imperial College London, London, United Kingdom.
Yeeshika Shersing, Imperial College Healthcare National Health Service Trust, Imperial College London, London, United Kingdom.
Alison Holmes, National Institute for Health Research Health Protection Research Unit in Healthcare Associated Infections and Antimicrobial Resistance, Imperial College London, London, United Kingdom; Imperial College Healthcare National Health Service Trust, Imperial College London, London, United Kingdom; Centre for Antimicrobial Optimisation, Imperial College London, London, United Kingdom.
References
- 1. Gerver SM, Guy R, Wilson K, et al. . National surveillance of bacterial and fungal co- and secondary infection in COVID-19 patients in England—lessons from the first wave [manuscript published online ahead of print 8 June 2021]. Clin Microbiol Infect 2021. doi: 10.1016/j.cmi.2021.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Russell CD, Fairfield CJ, Drake TM, et al. . Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. Lancet Microbe 2021; 2:e354–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baker MA, Sands KE, Huang SS, et al. . The impact of COVID-19 on healthcare-associated infections [manuscript published online ahead of print 9 August 2021]. Clin Infect Dis 2021. doi: 10.1093/cid/ciab688. [DOI] [Google Scholar]
- 4. Hsu J. How Covid-19 is accelerating the threat of antimicrobial resistance. BMJ 2020; 369:e1983. [DOI] [PubMed] [Google Scholar]
- 5. Rawson TM, Wilson RC, Holmes A.. Understanding the role of bacterial and fungal infection in COVID-19. Clin Microbiol Infect 2021; 27:9–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patel A, Emerick M, Cabunoc MK, et al. . Rapid spread and control of multidrug-resistant gram-negative bacteria in COVID-19 patient care units. Emerg Infect Dis 2021; 27:1234–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. National Health Service. Admission method. NHS data model and dictionary. 2020. Available at: https://datadictionary.nhs.uk/attributes/admission_method.html?hl=admission%2Cmethod. Accessed 20 February 2020.
- 8. Centers for Disease Control and Prevention. Bloodstream infection event (central line-associated bloodstream infection and non-central line associated bloodstream infection). 2021. Available at: https://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf. Accessed 20 April 2021.
- 9. National Health Service England. Surveillance, management and reporting of infections. 2015. Available at: http://www.doncasterccg.nhs.uk/wp-content/uploads/2015/09/Surveillance-management-and-reporting-of-infections-June-2014.pdf. Accessed 20 March 2021.
- 10. UK Government. Prime Minister’s statement on coronavirus (COVID-19): 23 March 2020. 2020. Available at: https://www.gov.uk/government/speeches/pm-address-to-the-nation-on-coronavirus-23-march-2020. Accessed 20 September 2020.
- 11. Public Health England. Annual epidemiological commentary: gram-negative bacteraemia, MRSA bacteraemia, MSSA bacteraemia and C. difficile infections, up to and including financial year April 2019 to March 2020. 2020. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/940716/Annual_epidemiology_commentary_April_2019_March_2020.pdf. Accessed 20 May 2021.
- 12. Imperial College Healthcare NHS Trust. Nurse and midwife staffing levels. 2020. Available at: https://www.imperial.nhs.uk/about-us/who-we-are/publications/safe-nurse-and-midwife-staffing. Accessed 25 Feburary 2021.
- 13. Willan J, King AJ, Jeffery K, Bienz N.. Challenges for NHS hospitals during Covid-19 epidemic. BMJ 2020; 368:m1117. [DOI] [PubMed] [Google Scholar]
- 14. Rawson TM, Moore LSP, Castro-Sanchez E, et al. . COVID-19 and the potential long-term impact on antimicrobial resistance. J Antimicrob Chemother 2020; 75:1681–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Denny S, Rawson TM, Hart P, et al. . Bacteraemia variation during the COVID-19 pandemic; a multi-centre UK secondary care ecological analysis. BMC Infect Dis 2021; 21:556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. British Society for Antimicrobial Chemotherapy. Plummeting number of UTI diagnoses since the advent of COVID brings huge uncertainty to the management of serious infections in vulnerable patients. 2020. Available at: https://bsac.org.uk/plummeting-number-of-uti-diagnoses-since-the-advent-of-covid-brings-huge-uncertainty-to-the-management-of-serious-infections-in-vulnerable-patients/. Accessed 20 February 2021.
- 17. Office for National Statistics. Analysis of death registrations not involving coronavirus (COVID-19), England and Wales: 28 December 2019 to 10 July 2020. 2020. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/analysisofdeathregistrationsnotinvolvingcoronaviruscovid19englandandwales28december2019to1may2020/28december2019to10july2020. Accessed 20 February 2021.
- 18. Kontopantelis E, Mamas MA, Webb RT, et al. . Excess deaths from COVID-19 and other causes by region, neighbourhood deprivation level and place of death during the first 30 weeks of the pandemic in England and Wales: a retrospective registry study. Lancet Reg Health Eur 2021; 7:100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karagiannidou S, Triantafyllou C, Zaoutis TE, Papaevangelou V, Maniadakis N, Kourlaba G.. Length of stay, cost, and mortality of healthcare-acquired bloodstream infections in children and neonates: a systematic review and meta-analysis. Infect Control Hosp Epidemiol 2020; 41:342–54. [DOI] [PubMed] [Google Scholar]
- 20. Hill B. Changes to nurse-to-patient ratios in intensive care during the pandemic. Br J Nurs 2020; 29:1238–40. [DOI] [PubMed] [Google Scholar]
- 21. Dunhill L. Intensive care staffing ratios dramatically diluted. HSJ. 2020. Available at: https://tinyurl.com/y34pf53y. Accessed 20 January 2021. [Google Scholar]
- 22. Tacconelli E, Cataldo MA, Paul M, et al. . STROBE-AMS: recommendations to optimise reporting of epidemiological studies on antimicrobial resistance and informing improvement in antimicrobial stewardship. BMJ Open 2016; 6:e010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.