Abstract
Background
The development of effective vaccines against coronavirus disease 2019 is a global priority. CoronaVac is an inactivated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine with promising safety and immunogenicity profiles. This article reports safety and immunogenicity results obtained for healthy Chilean adults aged ≥18 years in a phase 3 clinical trial.
Methods
Volunteers randomly received 2 doses of CoronaVac or placebo, separated by 2 weeks. A total of 434 volunteers were enrolled, 397 aged 18–59 years and 37 aged ≥60 years. Solicited and unsolicited adverse reactions were registered from all volunteers. Blood samples were obtained from a subset of volunteers and analyzed for humoral and cellular measures of immunogenicity.
Results
The primary adverse reaction in the 434 volunteers was pain at the injection site, with a higher incidence in the vaccine than in the placebo arm. Adverse reactions observed were mostly mild and local. No severe adverse events were reported. The humoral evaluation was performed on 81 volunteers. Seroconversion rates for specific anti-S1-receptor binding domain (RBD) immunoglobulin G (IgG) were 82.22% and 84.44% in the 18–59 year age group and 62.69% and 70.37% in the ≥60 year age group, 2 and 4 weeks after the second dose, respectively. A significant increase in circulating neutralizing antibodies was detected 2 and 4 weeks after the second dose. The cellular evaluation was performed on 47 volunteers. We detected a significant induction of T-cell responses characterized by the secretion of interferon-γ (IFN-γ) upon stimulation with Mega Pools of peptides from SARS-CoV-2.
Conclusions
Immunization with CoronaVac in a 0–14 schedule in Chilean adults aged ≥18 years is safe, induces anti-S1-RBD IgG with neutralizing capacity, activates T cells, and promotes the secretion of IFN-γ upon stimulation with SARS-CoV-2 antigens.
Keywords: CoronaVac, phase 3 clinical trial, SARS-CoV-2, COVID-19, vaccines
Vaccination of Chilean adults ≥18 years old with CoronaVac (Sinovac Life Sciences) in a 0–14 immunization schedule is safe and immunogenic, inducing anti-S1-RBD antibodies with neutralizing capacity and the activation of T cells secreting IFN-γ upon recognition of SARS-CoV-2 peptides.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the emerging pathogen responsible for coronavirus disease 2019 (COVID-19) [1–3]. This virus was first described in December 2019 in Wuhan, China, and it is the source of an ongoing pandemic, which by September 2021 has resulted in almost 221 million infection cases and more than 4.5 million deaths worldwide [4]. International efforts are focused on generating vaccines to counteract COVID-19. Epidemiological studies show that individuals aged ≥60 years and those with chronic conditions are more susceptible to severe disease, frequently resulting in death [5, 6]. More than 294 vaccines are under development, with 37 undergoing phase 3 or 4 clinical trials and 10 approved for emergency use [7]. Although many different vaccine platforms are being used and explored, most of them rely on a single viral component, the full-length Spike (S) protein or the receptor binding domain (RBD) of the S protein [7, 8]. Whole virus inactivated platforms are a mature technology widely used against different viruses, and they can be easily stored and shipped at 4ºC for several years, which is a significant advantage for developing countries [9, 10]. Whole inactivated vaccines carry a wider diversity of antigens that are more prone to be conserved than the S protein in circulating variants, as is the case for the nucleocapsid (N) protein that has shown to promote protective T-cell immunity against related SARS-CoV viruses. Thus, including the N, envelope (E), and matrix (M) proteins of SARS-CoV-2 as additional antigenic targets could boost protection for whole inactivated vaccines [11].
CoronaVac is a whole inactivated SARS-CoV-2 vaccine developed by Sinovac Life Sciences Co., Ltd. (Beijing, China) [12]. Phase 1/2 clinical trials carried out in China evaluated 2 vaccination schedules with 2 doses separated by 14 days (0–14) or 28 days (0–28) [13, 14]. Both trials showed that this vaccine induces neutralizing antibodies 14 days after the second dose, suggesting that this vaccine is safe and likely induces a protective immune response against SARS-CoV-2 [13, 14]. Currently, 4 phase 3 clinical trials are evaluating the efficacy of CoronaVac and are being carried out in Brazil, Turkey, Indonesia, and Chile. Here, we report an interim analysis of safety and immunogenicity parameters upon immunization of a group of healthy Chilean adults with CoronaVac or placebo aged 18–59 years and ≥60 years in a 0–14 day vaccination schedule. The safety was evaluated in the total 434 volunteers recruited, and a subgroup was included in immunogenicity analysis. Given that this vaccine carries multiple SARS-CoV-2 antigens, the characterization of the humoral and cellular immune response was extended to components of the viral proteome beyond the S protein. Taken together, this is the first report characterizing the cellular and humoral immune responses elicited by CoronaVac in a population other than the Chinese against several viral antigens. Our results indicate that CoronaVac is safe and immunogenic in healthy Chilean adults.
MATERIALS AND METHODS
Study Design, Randomization, and Volunteers
This clinical trial (clinicaltrials.gov NCT04651790) was conducted in Chile at 8 different sites. The study protocol was performed according to the current Tripartite Guidelines for Good Clinical Practices, the Declaration of Helsinki [15], and local regulations. The trial protocol was reviewed and approved by the Institutional Scientific Ethical Committee of Health Sciences, Pontificia Universidad Católica de Chile (#200708006). Trial execution was approved by the Chilean Public Health Institute (#24204/20). Written informed consent was obtained from each volunteer before enrollment. The study included healthy Chilean adults aged ≥18 years. Volunteers were inoculated with either 2 doses of CoronaVac or placebo separated by 2 weeks.
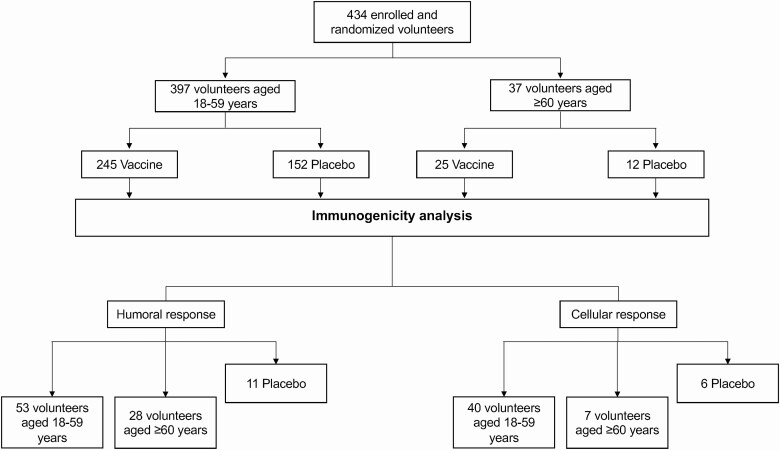
A complete list of inclusion/exclusion criteria is provided in the annexed study protocol. Volunteers were randomly assigned to immunization with CoronaVac or injection with placebo in a 1:1 ratio. A subgroup of volunteers was assigned to the immunogenicity arm and randomly received CoronaVac or placebo (3:1 ratio). Randomization was done using a sealed enveloped system integrated into the electronic case report forms in the OpenClinica platform. To collect adverse events (AEs), volunteers were instructed and trained to log in information on the platform until 28 days after the second dose at the same hour each day. Local and systemic symptoms were requested for 7 days after each dose or until they ceased. Other AEs, drugs used, severe adverse events (SAEs), events of special interest, and symptoms of SARS-CoV-2 were also requested until the end of the study. Daily reminders were sent via email and SMS until 28 days after the second dose and then weekly until the end of the study. Table 1 summarizes the characteristics of the volunteers, and Figure 1 shows the study profile.
Table 1.
Characteristics of the Volunteers at Baseline
| Characteristic | 18–59 y (n = 397) |
≥ 60 y (n = 37) |
Total (n = 434) |
P Value |
|---|---|---|---|---|
| Age, mean ± standard deviation | 38.2 ± 9.7 | 64.0 ± 4.3 | 40.4 ± 11.8 | |
| Inoculation | .482 | |||
| Vaccine, n (%) | 245 (61.7) | 25 (67.6) | 270 (62.2) | |
| Placebo, n (%) | 152 (38.3) | 12 (32.4) | 164 (37.8) | |
| Sex | .039 | |||
| Female, n (%) | 251 (63.2) | 17 (45.9) | 268 (61.8) | |
| Male, n (%) | 146 (36.8) | 20 (54.1) | 166 (38.2) | |
| Ethnicity | .152 | |||
| White, n (%) | 370 (93.2) | 37 (100.0) | 407 (93.8) | |
| Other, n (%) | 27 (6.8) | 0 (0.0) | 27 (6.2) |
P values are for comparison between total numbers in each characteristic.
Figure 1.
Study profile. Recruitment of volunteers for the phase 3 clinical trial as of February 10, 2021.
Procedures
CoronaVac consists of 3 µg of β-propiolactone inactivated SARS-CoV-2 (strain CZ02) with aluminum hydroxide as an adjuvant in 0.5 mL [12]. A study nurse administered unblinded ready-to-use syringes with CoronaVac or placebo (visually indistinguishable among them) intramuscularly in the deltoid area. To avoid any influence on the volunteers, the interaction with the nurse was restricted only to immunization. Then, safety evaluations were performed by the blinded clinical team. Blood samples were obtained at different time points for the immunogenicity arm and used to isolate sera and peripheral blood mononuclear cells (PBMCs). Further details can be found in the supplementary information.
To assess the presence of anti-SARS-CoV-2 antibodies, blood samples obtained before the first and second dose and 2 and 4 weeks after the second dose were analyzed. The quantitative measurement of human immunoglobulin G (IgG) antibodies against the RBD of the S1 protein (S1-RBD) and the N protein of SARS-CoV-2 was performed using the RayBio COVID-19 (SARS-CoV-2) Human Antibody Detection Kit (catalog #IEQ-CoVS1RBD-IgG and #IEQ-CovN-IgG). Arbitrary units obtained for these analyses were converted into World Health Organization (WHO) international units through a standard curve (National Institute for Biological Standards and Control code 20/268). The neutralizing capacities of circulating antibodies were evaluated by 3 different techniques: surrogate virus neutralization test (sVNT) (Genscript catalog #L00847-A), conventional virus neutralization test (cVNT), and pseudotyped virus neutralization test (pVNT) [16]. Further details on the methodology associated with these techniques can be found in the supplementary information.
To assess the cellular immune response, enzyme-linked immunospot (ELISPOT) and flow cytometry assays were performed using isolated PBMCs. ELISPOT assays were performed to evaluate changes in the numbers of interferon-γ (IFN-γ) secreting cells. Flow cytometry assays were performed to characterize T cells and the expression of activation-induced markers (AIMs) on these cells. The stimulus included in these assays considered the use of Mega Pools (MPs) of peptides derived from SARS-CoV-2 proteins [17]. Corresponding controls were held. Further details on the ELISPOT assays, antibodies used for flow cytometry, and the respective protocols can be found in the supplementary information.
Outcomes
The primary aim was to evaluate the frequency of solicited and unsolicited AEs occurring 7 days after each dose by age group (aged 18–59 and ≥60 years). Grading for solicited and unsolicited AEs can be found in detail in Tables S1–S4. Secondary immunogenicity endpoints considered assessing the presence of anti-SARS-CoV-2 antibodies and the cellular immune response elicited by the vaccine in a subgroup of volunteers. A complete list of outcomes can be found in the study protocol.
Statistical Analysis
Information regarding the determination of sample size, AE analysis test, and immunogenicity analysis test can be found in the supplementary information.
RESULTS
Safety Assessment
Volunteers on this study were recruited between November 27, 2020, and February 10, 2021 (Figure 1). On February 24, 2021, the last volunteer included in this analysis was inoculated with the second dose. As of February 24, 2021, only 80 volunteers from the placebo arm had received their second dose. Circulating SARS-CoV-2 strains detected during this time mainly were wild-type strains (original L strain) and the B.1.1.7 strain. Remarkably, the P1 or Gamma variant was detected for the first time in Chile by the end of January 2021 [18]. A total of 434 volunteers were enrolled in this study; 390 volunteers received 2 doses of CoronaVac and 44 received a placebo. The vaccination schedule for both groups was 0–14. A list of local and systemic solicited AEs reported is shown in Table 2. The most reported solicited local AEs was pain at the injection site (mostly grade 1), with an incidence of 55.6% in the vaccine arm compared with 40.0% in the placebo arm. Headaches (grade 1 or 2) were the most common solicited systemic AEs with a frequency of 48.5% in the vaccine arm and 48.8% in the placebo arm. No SAEs or events of special interest were reported. Significant differences were observed between age groups regarding the frequency of local and systemic AEs (Table S4). A total of 55 unsolicited AEs were reported. During the study period, 3 COVID-19 cases occurred in the vaccinated group (breakthrough cases). One of them had a clinical progression score of 1 (asymptomatic), and the other 2 had a score of 2 (symptomatic) [19].
Table 2.
Solicited Local and Systemic Adverse Events after Inoculation in Volunteers, Classified by Arm and Age Group
| First Dose | Second Dose | Both Doses | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (n = 434) | (n = 319) | (n = 319) | |||||||
| Adverse Reaction | Placebo (n = 164) | Vaccine (n = 270) | P Value | Placebo (n = 80)a |
Vaccine (n = 239) | P Value | Placebo (n = 80) | Vaccine (n = 239) | P Value |
| Local reactions | |||||||||
| Pain, n (%)b | 39 (23.8) | 117 (43.3) | <.001 | 16 (20.0) | 73 (30.5) | .069 | 32 (40.0) | 133 (55.6) | .015 |
| <60 y | 37 (24.3) | 113 (46.1) | <.001 | 15 (20.8) | 68 (31.8) | .077 | 30 (41.7) | 125 (58.4) | .014 |
| ≥60 y | 2 (16.7) | 4 (16.0) | .999 | 1 (12.5) | 5 (20.0) | .999 | 2 (25.0) | 8 (32.0) | .999 |
| Induration (%) | 1 (0.6) | 8 (3.0) | .163 | 0 (0.0) | 15 (6.3) | .015 | 0 (0.0) | 21 (8.8) | .006 |
| <60 y | 1 (0.7) | 7 (2.9) | .161 | 0 (0.0) | 13 (6.1) | .043 | 0 (0.0) | 18 (8.4) | .009 |
| ≥60 y | 0 (0.0) | 1 (4.0) | .999 | 0 (0.0) | 2 (8.0) | .999 | 0 (0.0) | 3 (12.0) | .56 |
| Pruritus (%) | 4 (2.4) | 15 (5.6) | .124 | 2 (2.5) | 6 (2.5) | .099 | 3 (3.8) | 17 (7.1) | .283 |
| <60 y | 4 (2.6) | 15 (6.1) | .113 | 2 (2.8) | 6 (2.8) | .099 | 3 (4.2) | 17 (7.9) | .277 |
| ≥60 y | 0 (0.0) | 0 (0.0) | … | 0 (0.0) | 0 (0.0) | … | 0 (0.0) | 0 (0.0) | … |
| Erythema (%) | 3 (1.8) | 10 (3.7) | .386 | 2 (2.5) | 3 (1.3) | .602 | 2 (2.5) | 10 (4.2) | .737 |
| <60 y | 3 (2.0) | 10 (4.1) | .385 | 1 (1.4) | 3 (1.4) | .999 | 1 (1.4) | 10 (4.7) | .301 |
| ≥60 y | 0 (0.0) | 0 (0.0) | … | 1 (12.5) | 0 (0.0) | .242 | 1 (12.5) | 0 (0,0.0) | .242 |
| Swelling (%) | 3 (1.8) | 5 (1.9) | .999 | 1 (1.3) | 5 (2.1) | .999 | 1 (1.3) | 9 (3.8) | .461 |
| <60 y | 3 (2.0) | 5 (2.0) | .999 | 1 (1.4) | 4 (1.9) | .999 | 1 (1.4) | 8 (3.7) | .458 |
| ≥60 y | 0 (0.0) | 0 (0.0) | … | 0 (0.0) | 1 (4.0) | .999 | 0 (0.0) | 1 (4.0) | .999 |
| Systemic reactions | |||||||||
| Headache (%) | 50 (30.5) | 107 (39.6) | .055 | 15 (18.8) | 46 (19.2) | .922 | 39 (48.8) | 116 (48.5) | .974 |
| <60 y | 49 (32.2) | 102 (41.6) | .061 | 12 (16.7) | 42 (19.6) | .579 | 36 (50.0) | 109 (50.9) | .891 |
| ≥60 y | 1 (8.3) | 5 (20.0) | .641 | 3 (37.5) | 4 (16.0) | .32 | 3 (37.5) | 7 (28.0) | .673 |
| Fatigue (%) | 32 (19.5) | 58 (21.5) | .624 | 10 (12.5) | 25 (10.5) | .613 | 22 (27.5) | 64 (26.8) | .9 |
| <60 y | 31 (20.4) | 55 (22.4) | .629 | 8 (11.1) | 23 (10.7) | .932 | 20 (27.8) | 60 (28.0) | .966 |
| ≥60 y | 1 (8.3) | 3 (12.0) | .999 | 2 (25.0) | 2 (8.0) | .241 | 2 (25.0) | 4 (16.0) | .616 |
| Myalgia (%) | 23 (14.0) | 48 (17.8) | .305 | 9 (11.3) | 19 (7.9) | .367 | 19 (23.8) | 54 (22.6) | .831 |
| <60 y | 22 (14.5) | 46 (18.8) | .269 | 8 (11.1) | 16 (7.5) | .336 | 18 (25.0) | 50 (23.4) | .778 |
| ≥60 y | 1 (8.3) | 2 (8.0) | .999 | 1 (12.5) | 3 (12.0) | .999 | 1 (12.5) | 4 (16.0) | .999 |
| Diarrhea (%) | 18 (11.0) | 36 (13.3) | .471 | 5 (6.3) | 18 (7.5) | .701 | 15 (18.8) | 44 (18.4) | .946 |
| <60 y | 17 (11.2) | 36 (14.7) | .318 | 4 (5.6) | 16 (7.5) | .58 | 14 (19.4) | 42 (19.6) | .973 |
| ≥60 y | 1 (8.3) | 0 (0.0) | .324 | 1 (12.5) | 2 (8.0) | .999 | 1 (12.5) | 2 (8.0) | .999 |
| Nausea (%) | 18 (11.0) | 25 (9.3) | .562 | 3 (3.8) | 9 (3.8) | .999 | 11 (13.8) | 27 (11.3) | .558 |
| <60 y | 18 (11.8) | 22 (9.0) | .357 | 2 (2.8) | 9 (4.2) | .736 | 10 (13.9) | 24 (11.2) | .544 |
| ≥60 y | 0 (0.0) | 3 (12.0) | .537 | 1 (12.5) | 0 (0.0) | .242 | 1 (12.5) | 3 (12.0) | .999 |
| Arthralgia (%) | 10 (6.1) | 14 (5.2) | .687 | 2 (2.5) | 7 (2.9) | .999 | 7 (8.8) | 18 (7.5) | .726 |
| <60 y | 10 (6.6) | 13 (5.3) | .596 | 2 (2.8) | 6 (2.8) | .999 | 7 (9.7) | 16 (7.5) | .544 |
| ≥60 y | 0 (0.0) | 1 (4.0) | .999 | 0 (0.0) | 1 (4.0) | .999 | 0 (0.0) | 2 (8.0) | .999 |
| Anorexia (%) | 10 (6.1) | 18 (6.7) | .815 | 3 (3.8) | 3 (1.3) | .169 | 6 (7.5) | 16 (6.7) | .806 |
| <60 y | 10 (6.6) | 16 (6.5) | .985 | 2 (2.8) | 3 (1.4) | .603 | 5 (6.9) | 14 (6.5) | .999 |
| ≥60 y | 0 (0.0) | 2 (8.0) | .999 | 1 (12.5) | 0 (0.0) | .242 | 1 (12.5) | 2 (8.0) | .999 |
| Pruritus (%) | 2 (1.2) | 10 (3.7) | .225 | 0 (0.0) | 4 (1.7) | .575 | 1 (1.3) | 14 (5.9) | .127 |
| <60 y | 2 (1.3) | 9 (3.7) | .217 | 0 (0.0) | 4 (1.9) | .575 | 1 (1.4) | 13 (6.1) | .202 |
| ≥60 y | 0 (0.0) | 1 (4.0) | .999 | 0 (0.0) | 0 (0.0) | … | 0 (0.0) | 1 (4.0) | .999 |
| Exanthema (%) | 1 (0.6) | 7 (2.6) | .268 | 0 (0.0) | 1 (0.4) | .999 | 1 (1.3) | 8 (3.3) | .459 |
| <60 y | 1 (0.7) | 7 (2.9) | .161 | 0 (0.0) | 1 (0.5) | .999 | 1 (1.4) | 8 (3.7) | .458 |
| ≥60 y | 0 (0.0) | 0 (0.0) | … | 0 (0.0) | 0 (0.0) | … | 0 (0.0) | 0 (0.0) | … |
| Allergy (%) | 1 (0.6) | 6 (2.2) | .262 | 0 (0.0) | 3 (1.3) | .575 | 0 (0.0) | 8 (3.3) | .209 |
| <60 y | 0 (0.0) | 5 (2.0) | .161 | 0 (0.0) | 2 (0.9) | .999 | 0 (0.0) | 4 (1.9) | .575 |
| ≥60 y | 1 (8.3) | 1 (4.0) | .999 | 0 (0.0) | 1 (4.0) | .999 | 0 (0.0) | 1 (4.0) | .999 |
| Vomiting (%) | 3 (1.8) | 1 (0.4) | .154 | 0 (0.0) | 4 (1.7) | .575 | 0 (0.0) | 4 (1.7) | .575 |
| <60 y | 3 (2.0) | 1 (0.4) | .159 | 0 (0.0) | 4 (1.9) | .575 | 0 (0.0) | 4 (1.9) | .575 |
| ≥60 y | 0 (0.0) | 0 (0.0) | … | 0 (0.0) | 0 (0.0) | … | 0 (0.0) | 0 (0.0) | … |
| Fever (>37.8ºC) (%) | 1 (0.6) | 1 (0.4) | .999 | 0 (0.0) | 0 (0.0) | … | 1 (1.3) | 1 (0.4) | .439 |
| <60 y | 1 (0.7) | 1 (0.4) | .999 | 0 (0.0) | 0 (0.0) | … | 1 (1.4) | 1 (0.5) | .441 |
| ≥60 y | 0 (0.0) | 0 (0.0) | … | 0 (0.0) | 0 (0.0) | … | 0 (0.0) | 0 (0.0) | … |
Data in the table were reported within 7 days after any of the 2 doses. Sample sizes: placebo < 60 first dose: n = 152; placebo < 60 second dose: n = 72; vaccine < 60 first dose: n = 245; vaccine < 60 second dose: n = 214; placebo ≥ 60 first dose: n = 12; placebo ≥ 60 second dose: n = 8; vaccine ≥ 60 first dose: n = 25; vaccine ≥ 60 years second dose: n = 25.
aAs of February 24, 2021, only 80 volunteers from the placebo arm had received their second dose.
bPercentages were calculated from the total number of volunteers in each group.
Immunization With CoronaVac Induces the Secretion of anti-S1-RBD IgG, anti-N IgG, and Circulating Neutralizing Antibodies in Chilean Adults
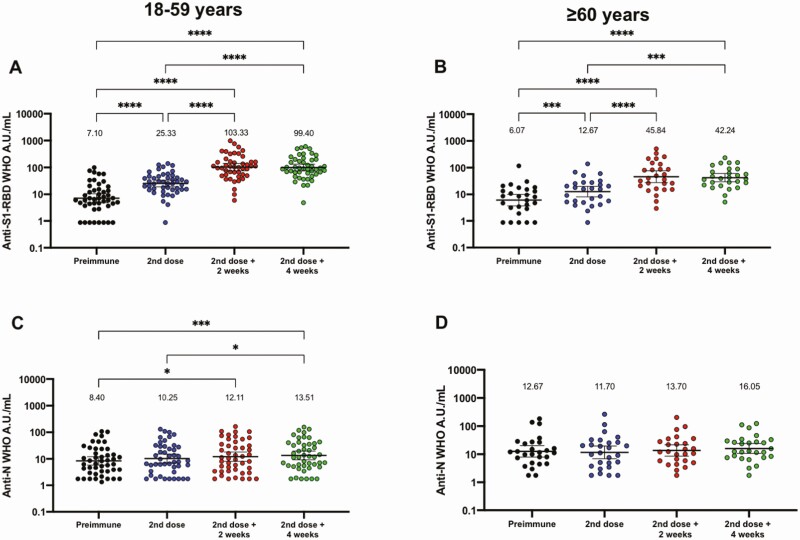
Evaluation of IgG-specific against S1-RBD and the N protein of SARS-CoV-2 was performed independently through enzyme-linked immunosorbent assays (Figure 2). This humoral evaluation was performed on serum samples from 81 volunteers, 53 of whom were aged 18–59 years, and 28 of whom were aged ≥60 years. The data are shown in international WHO arbitrary units. Increased levels of anti-S1-RBD circulating antibodies were detected at all times evaluated after the first dose for both age groups (Figure 2A and 2B). These changes were also detected in fold change analyses normalized to preimmune samples (Figure S1A and S1B). These results suggest that immunization with CoronaVac induces a significant production of S1-RBD specific IgG after vaccination with a 0–14 schedule. A modest increase in IgG specific against the N protein was detected (Figure 2C and 2D), with fold change analyses showing similar results to those for the international WHO arbitrary units (Figure S1C and S1D). We confirmed that doses of CoronaVac contain significant amounts of the N protein (Figure S2). Seroconversion rates for S1-RBD and N protein specific IgG can be found in Table 3. Results obtained for seropositive volunteers at enrollment (not included in this analysis) and breakthrough cases are shown in Table S5.
Figure 2.
Immunization with CoronaVac induces specific IgG against SARS-CoV-2 antigens in participants aged 18–59 years and ≥ 60 years after 2 immunizations in a 0–14 schedule. Titers of IgG antibodies after 2 doses of CoronaVac were evaluated for immunized participants (excluding seropositive participants at recruitment and placebo participants) before the first and second dose, and 2 (second dose + 2 weeks) and 4 weeks (second dose + 4 weeks) after the second dose for adults aged (A, C) 18–59 years and (B, D) ≥60 years. Specific IgG against the S1-RBD (upper panel) and the N protein (lower panel) of SARS-CoV-2 were measured. Data are expressed as the log10 of international WHO arbitrary units versus time after each dose. Error bars indicate the 95% CI of the geometric mean units (GMUs). The spots represent the individual values of antibody units for each volunteer, with the numbers above each time showing the GMU estimates. The graph illustrates the results obtained for 45 participants in the 18–59 years group and 27 participants in the ≥60 years group. One-way ANOVAs with repeated measures and post hoc Tukey tests were performed to evaluate statistical differences among the groups; *P < .05, **P < .005, ***P < .0005, ****P < .0001. Abbreviations: ANOVA, analysis of variance; CI, confidence interval; IgG, immunoglobulin G; N, nucleocapsid; RBD, receptor binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WHO, World Health Organization.
Table 3.
Seroconversion Rates and Geometric Median Units (GMU) of Circulating Antibodies Against SARS-CoV-2 Proteins
| Antibodies Detected | Group | Indicators | Second Dose | Second Dose + 2 wk | Second Dose + 4 wk |
|---|---|---|---|---|---|
| Anti-S1-RBD IgG (WHO A.U./mL) | Total vaccine | Seroconversion n/N | 23/72 | 54/72 | 57/72 |
| (%) | (31.94) | (75.00) | (79.17) | ||
| GMU | 19.60 | 76.50 | 72.43 | ||
| (95% CI) | (15.24–25.22) | (57.67–101.5) | (56.96–92.11) | ||
| 18–59 years | Seroconversion n/N | 18/45 | 37/45 | 38/45 | |
| (%) | (40.00) | (82.22) | (84.44) | ||
| GMU | 25.33 | 103.33 | 99.40 | ||
| (95% CI) | (19.07–33.64) | (75.31–141.8) | (74.53–132.6) | ||
| ≥ 60 years | Seroconversion n/N | 5/27 | 17/27 | 19/27 | |
| (%) | (18.52) | (62.96) | (70.37) | ||
| GMU | 12.67 | 45.84 | 42.24 | ||
| (95% CI) | (08.03–19.99) | (27.51–76.36) | (29.44–60.61) | ||
| Placebo | Seroconversion n/N | 0/12 | 0/9 | 0/0 | |
| (%) | (0) | (0) | N/D | ||
| GMU | 10.43 | 6.19 | N/D | ||
| (95% CI) | (04.33–25.10) | (01.85–20.76) | N/D | ||
| Anti-N IgG (WHO A.U./mL) | Total vaccine | Seroconversion n/N | 2/72 | 5/72 | 7/72 |
| (%) | (2.78) | (6.94) | (9.72) | ||
| GMU | 10.77 | 12.66 | 14.4 | ||
| (95% CI) | (07.95–14.57) | (09.36–17.12) | (10.89–19.04) | ||
| 18–59 years | Seroconversion n/N | 2/45 | 5/45 | 6/45 | |
| (%) | (4.44) | (11.11) | (13.33) | ||
| GMU | 10.25 | 12.11 | 13.51 | ||
| (95% CI) | (06.97–15.08) | (08.07–18.16) | (09.21–19.81) | ||
| ≥ 60 years | Seroconversion n/N | 0/27 | 0/27 | 1/27 | |
| (%) | (0) | (0) | (3.70) | ||
| GMU | 11.70 | 13.70 | 16.05 | ||
| (95% CI) | (06.96–19.67) | (08.60–21.82) | (10.65–24.18) | ||
| Placebo | Seroconversion n/N | 1/12 | 0/10 | 0/0 | |
| (%) | (8.3) | (0) | (-) | ||
| GMU | 11.06 | 9.61 | N/D | ||
| (95% CI) | (04.03–30.35) | (02.90–31.90) | (-) |
Timepoints refer to the number of days after the first dose of vaccine or placebo in the schedule.
Abbreviations: A.U., arbitrary unit; CI, confidence interval; GMU, geometric median unit; IgG, immunoglobulin G; N, nucleoprotein; N/D, not determined; RBD, receptor binding domain; S, Spike protein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WHO, World Health Organization.
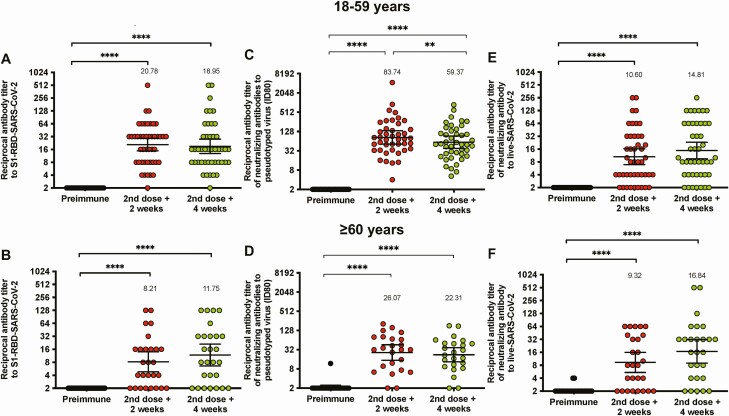
To evaluate the neutralizing capacities of circulating antibodies, sVNTs (Figure 3A and 3B), pVNTs (Figure 3C and 3D), and cVNTs for the D614G variant (Figure 3E and 3F) were performed. This additional humoral evaluation was performed on serum samples from the same 81 volunteers, 53 of whom were aged 18–59 years, and 28 of whom were aged ≥60 years. Both sVNTs and cVNTs showed a significant increase in the neutralizing (or surrogate neutralizing) capacities of circulating antibodies against SARS-CoV-2 2 and 4 weeks after the second dose. This could also be detected in fold change analyses (Figures S3 and S4). The geometric mean titers and seropositivity rates for the sVNT, pVNT, and cVNT can be found in Table 4. These results suggest that immunization with CoronaVac in a 0–14 schedule promotes anti-S1-RBD IgG with neutralizing capacities in both age groups.
Figure 3.
Immunization with CoronaVac induces neutralizing antibodies against SARS-CoV-2 in participants aged 18–59 years and ≥60 years after 2 immunizations in a 0–14 schedule. (A-B) Neutralizing antibody titers were evaluated with a surrogate virus neutralization assay, which quantifies the interaction between the S1-RBD and hACE2 precoated on enzyme-linked immunosorbent assay plates. Results were obtained from (A) 45 participants aged 18–59 years and (B) 27 ≥ 60 years before the first and second dose, and 2 (second dose + 2 weeks) and 4 weeks (second dose + 4 weeks) after the second dose. (C-D) Titers of neutralizing antibodies were evaluated with a pseudotyped viral system. Data are represented as the reciprocal dilution of sera that prevented infection by 80% (ID80) after the first dose. Numbers above the bars show the geometric mean titer (GMT), and the error bars indicate the 95% CI. Results were obtained from 45 participants (C) aged 18–59 years and (D) 24 ≥ 60 years before the first and second dose, and 2 (second dose + 2 weeks) and 4 weeks (second dose + 4 weeks) after the second dose. (E-F) Titers of neutralizing antibodies evaluated with a conventional neutralization assay using an ancestral D614G variant strain of SARS-CoV-2. Data are represented as the reciprocal dilution of sera that prevented infection after the first dose. Numbers above the bars show the GMT, and the error bars indicate the 95% CI. Results were obtained from 45 participants aged (E) 18–59 year and (F) 27 ≥ 60 years before the first and second dose, and 2 (second dose + 2 weeks) and 4 weeks (second dose + 4 weeks) after the second dose. Data are represented as the reciprocal antibody titer versus time after each dose. Numbers above the bars show the GMT, and the error bars indicate the 95% CI. Data were analyzed by a Wilcoxon test to evaluate statistical differences among the groups; *P < .05, **P < .005, ***P < .0005, ****P < .0001. Abbreviations: CI, confidence interval; RBD, receptor binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 4.
Seropositivity Rates and GMTs of Circulating Neutralizing Antibodies Against SARS-CoV-2 Proteins
| Antibodies Detected | Group | Indicators | Second Dose + 2 wk | Second Dose + 4 wk |
|---|---|---|---|---|
| Surrogate virus neutralization | Total vaccine | Seropositivity n/N | 63/72 | 59/72 |
| (%) | (87.5) | (81.94) | ||
| GMT | 14.23 | 15.54 | ||
| (95% CI) | (10.54–19.21) | (11.23–21.51) | ||
| 18–59 y | Seropositivity n/N | 44/45 | 39/45 | |
| (%) | (97.78) | (86.67) | ||
| GMT | 20.78 | 18.95 | ||
| (95% CI) | (14.81–29.18) | (12.87–27.92) | ||
| ≥ 60 y | Seropositivity n/N | 19/27 | 20/27 | |
| (%) | (70.37) | (74.07) | ||
| GMT | 8.21 | 11.75 | ||
| (95% CI) | (04.83–13.94) | (06.55–21.12) | ||
| Placebo | Seropositivity n/N | 0/11 | N/D | |
| (%) | (0) | (-) | ||
| GMT | 0 | N/D | ||
| (95% CI) | (0) | (-) | ||
| Pseudotyped virus neutralization | Total vaccine | Seropositivity n/N | 66/69 | 66/69 |
| (%) | (95.65) | (95.65) | ||
| GMT | 52.22 | 41.33 | ||
| (95% CI) | (35.12–77.65) | (29.10–56.69) | ||
| 18–59 y | Seropositivity n/N | 44/45 | 44/45 | |
| (%) | (97.78) | (97.78) | ||
| GMT | 83.74 | 59.37 | ||
| (95% CI) | (51.78–135.4) | (38.08–92.58) | ||
| ≥ 60 y | Seropositivity n/N | 22/24 | 22/24 | |
| (%) | (91.67) | (91.67) | ||
| GMT | 26.07 | 22.31 | ||
| (95% CI) | (14.91–45.59) | (13.39–37.18) | ||
| Placebo | Seropositivity n/N | 0/10 | N/D | |
| (%) | (0) | (-) | ||
| GMT | 0 | N/D | ||
| (95%CI) | (0) | (-) | ||
| Conventional virus neutralization |
Total vaccine | Seropositivity n/N | 55/72 | 60/72 |
| (%) | (76.39) | (83.33) | ||
| GMT | 10.10 | 15.54 | ||
| (95% CI) | (7.28–14.01) | (22.18) | ||
| 18–59 y | Seropositivity n/N | 36/45 | 38/45 | |
| (%) | (80.0) | (84.44) | ||
| GMT | 10.60 | 14.81 | ||
| (95% CI) | (6.92–16.26) | (9.49–23.09) | ||
| ≥ 60 y | Seropositivity n/N | 19/27 | 22/27 | |
| (%) | (70.37) | (81.48) | ||
| GMT | 9.32 | 16.84 | ||
| (95% CI) | (5.43–15.99) | (8.95–31.67) | ||
| Placebo | Seropositivity n/N | 6/11 | N/D | |
| (%) | (54.54) | (-) | ||
| GMT | 5.48 | N/D | ||
| (95% CI) | (1.84–16.29) | (-) |
Timepoints refer to the number of days after the first dose of vaccine or placebo in the schedule.
Abbreviations: CI, confidence interval; GMT, geometric mean titer; N, nucleoprotein; N/D, not determined; S, Spike protein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Immunization With CoronaVac Induces IFN-γ-Producing T cells Specific for SARS-CoV-2 Antigens in Chilean Adults
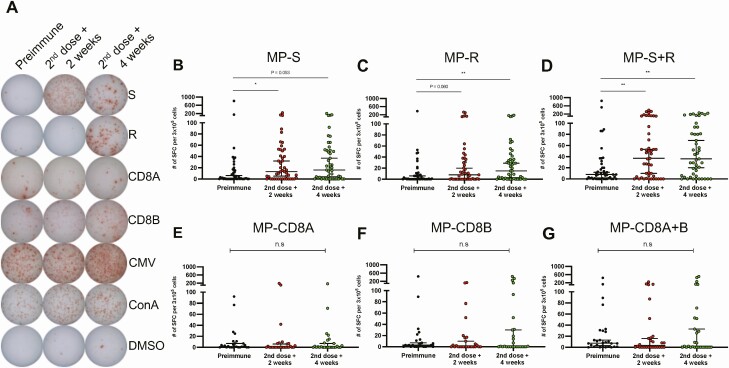
To evaluate the cellular immune response elicited upon vaccination with CoronaVac, the specific T-cell responses induced upon stimulation of PBMCs with MPs of 15-mer peptides derived from the S protein of SARS-CoV-2 (MP-S) and the remaining proteins of this virus (MP-R) were evaluated by ELISPOT in a total of 47 volunteers. Representative images of spot forming cells (SFCs) are shown (Figure 4A). We observed an increase in the number of SFCs for IFN-γ 2 and 4 weeks after the second dose (Figure 4D). Individual data from these MP also resulted in partial increases in SFC numbers (Figure 4B and 4C). Similar trends were observed with fold change analyses (Figure S5). The specific T-cell responses against MPs of 9- to 11-mer peptides from the whole proteome of SARS-CoV-2 (MP-CD8A and MP-CD8B) were also evaluated in 27 volunteers. Stimulation with these MPs resulted in a modest nonstatistically significant increase in SFCs for IFN-γ (Figure 4E and 4G). There was a subtle fold increase of SFCs for IFN-γ in volunteers stimulated with these 9- to 11-mer MPs (Figure S5). No changes were detected for the placebo group (Figure S6). These results suggest that immunization with CoronaVac induces a T-cell response polarized toward a Th1 immune profile, as the secretion of interleukin-4 by T cells was mainly undetected (Figure S7). As a positive control, PBMCs from volunteers were stimulated with an MP of peptides derived from cytomegalovirus (Figure S8).
Figure 4.
Evaluation of cellular immune response through ELISPOT upon stimulation with Mega Pools of peptides derived from SARS-CoV-2 proteins in volunteers immunized with CoronaVac. Numbers of IFN-γ-secreting cells, determined through ELISPOT as spot forming cells (SFCs) were determined. (A) Representative pictures for each stimulus are shown. PBMCs were stimulated with (B) MP-S, (C) MP-R, (D) MP-S + R, (E) MP-CD8A, (F) MP-CD8B, and (G) MP-CD8A + B for 48 h for samples obtained before the first dose, and 2 (second dose + 2 weeks) and 4 weeks (second dose + 4 weeks) after the second dose. A total of 47 volunteers were evaluated for MP-S and MP-R and 27 volunteers for MP-CD8A and MP-CD8B. Data shown represent median ± 95% CI. Statistical differences were evaluated by a Friedman test for repeated measures, followed by a post hoc Dunn test corrected for multiple comparisons against day preimmune samples; n.s. = no statistical differences, *P < .05, **P < .005. Abbreviations: CI, confidence interval; ELISPOT, enzyme-linked immunospot; IFN, interferon; MP, Mega Pools; PBMC, peripheral blood mononuclear cell; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
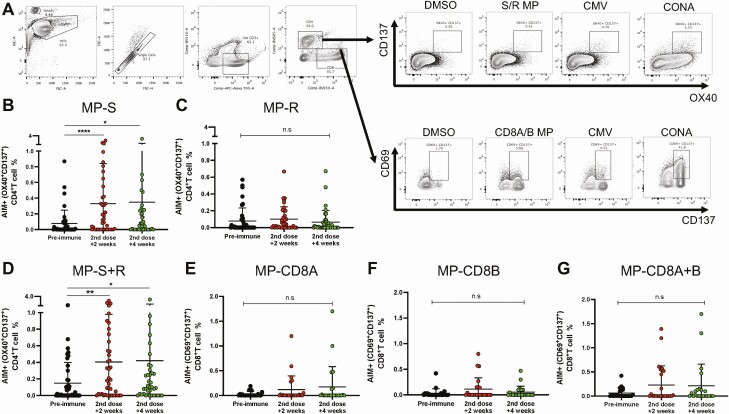
The expression of AIMs upon stimulation of PBMCs with these MPs was evaluated by flow cytometry. Because MP-S and MP-R were initially determined in silico to stimulate CD4+ T cells optimally, the expression of AIMs was assessed on these cells for 43 volunteers. The gating strategy is shown in Figure 5A, and stimulation with MP-S and consolidated data from both MP-S + R resulted in increased expression of AIMs (Figure 5B and 5D). No changes were detected when stimulating with MP-R alone (Figure 5C). Because MP-CD8A and MP-CD8B were determined in silico to stimulate CD8+ T cells, the expression of AIMs was evaluated on these cells for 21 volunteers. Modest increases in the expression of AIMs were detected for both MP-CD8A and MP-CD8B (Figure 5E and 5F). No changes were detected for the placebo group (Figure S9). Stimulation with cytomegalovirus and Concanavalin A confirmed the capacity of these cells to express AIMs (Figure S10). Although more volunteers must be evaluated, ELISPOT and flow cytometry results suggest that stimulation with these MPs induces a cellular immune response in volunteers immunized with CoronaVac.
Figure 5.
Changes in activation-induced markers (AIMs) expression in T cells through flow cytometry upon stimulation with Mega Pools of peptides derived from SARS-CoV-2 in volunteers immunized with CoronaVac. (A) The gating strategy used to evaluate changes in the expression of AIMs upon stimulation of PBMCs is shown. PBMCs were stimulated with (B) MP-S, (C) MP-R, (D) MP-S + R, (E) MP-CD8A, (F) MP-CD8B, and (G) MP-CD8A + B for 24 h for samples obtained before the first dose, and 2 (second dose + 2 weeks) and 4 weeks (second dose + 4 weeks) after the second dose. Changes in the expression of AIMs for CD4+ T cells (OX40+ CD137+) were measured upon stimulation with (B) MP-S, (C) MP-R, and (D) MP-S + R. Changes in the expression of AIMs for CD8+ T cells (CD69+ CD137+) were measured upon stimulation with (E) MP-CD8A, (F) MP-CD8B, and (G) MP-CD8A + B. A total of 43 volunteers were evaluated for MP-S and MP-R and 21 volunteers for MP-CD8A and MP-CD8B. Data shown represent mean ± standard deviation. Statistical differences were evaluated by a Friedman test for repeated measures, followed by a post hoc Dunn test corrected for multiple comparisons against preimmune samples. n.s. = no statistical differences, *P < .05, **P < .005, ****P < .0001. Abbreviations: MP, Mega Pools; PBMC, peripheral blood mononuclear cell; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
DISCUSSION
This study is a preliminary analysis of a phase 3 clinical trial performed in Chile with CoronaVac, an inactivated SARS-CoV-2 vaccine. We found that 2 doses of CoronaVac, in a 0–14 schedule, were safe and capable of inducing a humoral and cellular immune response in both age groups evaluated (18–59 and ≥60 years), which is in line with the phase 3 trial conducted in Turkey using the same vaccination schedule [21]. However, other studies using CoronaVac support the idea that a vaccination schedule with each dose separated by 4 weeks (0–28) induces better immune responses and shows a better efficacy profile [13]. A phase 2 trial conducted in China with CoronaVac compared both vaccination schedules and reported better immunogenicity in subjects vaccinated with a 0–28 schedule [13]. A recent study evaluating immune responses 6 months after the second dose in volunteers from both vaccination schedules reported higher seropositivity in individuals from the 0–28 schedule [22]. These results are consistent with published data from subjects vaccinated with messenger RNA (mRNA) vaccines, in which higher efficacy has been reported with longer intervals between doses [23, 24]. Therefore, a different immunization schedule considering a booster 4 weeks after the first dose instead of 2 weeks is being tested.
This study has relevant limitations that must be addressed, such as the reduced samples size evaluated for the immunogenicity profile. Also, although the high immunogenicity described here is encouraging, efficacy and death prevention data will be needed to guide the use of this vaccine in clinical and public health settings [13, 14, 20]. It is also important to note that further analyses are required to evaluate the relevance of this vaccine on emerging circulating variants.
Adverse reactions observed were primarily mild and local, which coincides with previous reports with this vaccine. No SAEs were reported for either the vaccine or placebo arm. We detected differences between the age groups in local and systemic AEs, being more frequent in the 18–59 age group than in the ≥60 age group.
Seroconversion rates for S1-RBD-specific IgG and seropositivity of neutralizing antibodies in this study are consistent with the data reported in the phase 2 trial conducted in China for the same immunization schedule, dose, and age [13]. The geometric median unit values obtained for anti-S1-RBD and anti-N antibodies in this study are somewhat lower than those described for the BNT162b2 (490.17 and 34.40 after the second dose, respectively) and the mRNA-1273 (659.91 and 37.03 after the second dose, respectively) vaccines when using the same international WHO units [25]. Possible differences in these values may be linked to a higher production of antibodies against a single antigen by mRNA vaccines compared with inactivated vaccines, which aim to induce a polyclonal response against several viral proteins [26]. The low production of anti-N antibodies compared with IgG induced against the S1-RBD is not related to the absence of the N protein in CoronaVac. Previous reports indicate that humans naturally infected with SARS-CoV-2 develop antibody responses mainly against the S and N proteins, in somewhat similar levels [12]. However, immunization studies of mice, rats, and nonhuman primates with CoronaVac showed that antibodies induced mainly were directed against the S protein and the S1-RBD, with a reduced number of antibodies against the N protein [12]. This is in line with our findings, suggesting that the enhanced secretion of antibodies against the S protein by CoronaVac, rather than against the N protein, may be playing a role in the protective response.
This is the first time a characterization of the cellular response against proteins other than the S protein of SARS-CoV-2 has been reported in humans immunized with CoronaVac. Unlike previous studies [13], we detected a robust T-cell response upon stimulation of PBMCs with MPs of peptides from S (MP-S). We also evaluated the response elicited upon stimulation with 2 MPs of peptides designed to stimulate a CD8+ T-cell response. Although more volunteers are required to raise more robust conclusions, the results suggest that the CD8+ immune response detected in vaccinated volunteers is not as robust as the CD4+ response. Because increased numbers of IFN-γ secreting cells and reduced amounts of interleukin-4 secreting cells align with a well-balanced Th1 immune response that could lead to virus clearance, immunization with CoronaVac shows promising capacities of inducing an antiviral response in the host. This IFN-γ response has also been sought and observed in other vaccines against SARS-CoV-2, such as the BNT162b1 designed by BioNTech [27] and the recombinant adenovirus type-5 vectored COVID-19 vaccine designed by CanSino [28].
In summary, immunization with CoronaVac is safe and induces robust humoral and cellular responses, characterized by increased antibody titers against the S1-RBD with neutralizing capacities and the production of T cells specific for several SARS-CoV-2 antigens and were characterized by the secretion of Th1 cytokines.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author Contributions. Conceptualization: S. M. B., K. A., P. A. G., G. Z., W. M., J. V. G.-A., and A. M. K. Visualization: S. M. B., N. M. S. G., J. A. S., L. F. D., B. M. S., and G. A.P. Methodology: S. M. B., K. A., P. A. G., N. M. S. G., J. A. S., L. F. D., B. M. S., G. A.P., L. A. G., Y. V., M. R., F. M.-G., D. R.-P., C. I., M. U., A. D., C. A. P., R. V. B., G. C.-M., C. C., D. M.-T., F. S., O. P. V., R. F., J. F., J. M., E. R., A. G.-A., A. O.-A., F. V.-E., R. S.-R., D. W., A. S., J. V. G.-A., and A. M. K. Investigation: N. M. S. G., J. A. S., L. F. D., B. M. S., G. A.P., L. A. G., Y. V., M. R., F. M.-G., D. R.-P., C. I., M. U., A. D., C. A. P., R. V. B., G. C.-M., C. C., D. M.-T., F. S., O. P. V., P. D., P. E., D. F., M. G., P. G., P. M.-V., C. M. P., M. P., A. R., R. F., J. F., J. M., E. R., A. G.-A., A. O.-A., F. V.-E., R. S.-R., and D. W. Funding acquisition: A. M. K. Project administration: S. M. B., K. A., P. A. G., G. Z., W. M., J. V. G.-A., and A. M. K. Supervision: S. M. B., K. A., P. A. G., C. I., M. U., J. V. G.-A., and A. M. K. Writing—original draft: S. M. B., P. A. G., N. M. S. G., J. A. S., L. F. D., B. M. S., G. A.P., and A. M. K. Writing—review and editing: S. M. B., K. A., P. A. G., N. M. S. G., J. A. S., L. F. D., B. M. S., G. A.P., L. A. G., Y. V., M. R., F. M.-G., D. R.-P., C. I., M. U., A. D., C. A. P., R. V. B., G. C.-M., C. C., D. M.-T., F. S., O. P. V., P. D., P. E., D. F., M. G., P. G., P. M.-V., C. M. P., M. P., A. R., R. F., J. F., J. M., E. R., A. G.-A., A. O.-A., F. V.-E., R. S.-R., D. W., A. S., J. V. G.-A., and A. M. K.
Acknowledgments. The authors thank the Ministry of Health, Government of Chile; Ministry of Science, Technology, Knowledge, and Innovation, Government of Chile; and The Ministry of Foreign Affairs, Government of Chile and the Chilean Public Health Institute (ISP). They also thank PATH for their support and sharing the First WHO International Standard for anti-SARS-CoV-2 immunoglobulin. We are grateful to Rami Scharf, Jessica White, Jorge Flores, and Miren Iturriza-Gomara from PATH for their support on experimental design and discussion; Alex Cabrera and Sergio Bustos from the Flow Cytometry Facility at Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile for support with flow cytometry; and Sebastián Silva from the same Faculty for support with statistical analyses. The authors also thank the Vice Presidency of Research (VRI), the Direction of Technology Transfer and Development (DTD), and the Legal Affairs Department (DAJ) of the Pontificia Universidad Católica de Chile and are also grateful to the Administrative Directions of the School of Biological Sciences and the School of Medicine of the Pontificia Universidad Católica de Chile for their administrative support. Special thanks to the members of the independent data safety monitoring committee (members in the supplementary Appendix) for their oversight, and the subjects enrolled in the study for their participation and commitment to this trial. Members of the CoronaVac03CL Study Team are listed in the supplemental Appendix. All data are available in the main text or the supplementary information and upon request to the corresponding authors.
Financial support. The Ministry of Health, Government of Chile, supported the funding of the CoronaVac03CL Study; The Confederation of Production and Commerce (CPC), Chile, supported the funding of the CoronaVac03CL Study; The Millennium Institute on Immunology and Immunotherapy, ANID—Millennium Science Initiative Program ICN09_016 (former P09/016-F) supports S. M. B., K.A., P. A. G., and A. M. K.; NIH contracts and 75N9301900065 awarded to A. S. and D. W.; The Innovation Fund for Competitiveness FIC-R 2017 (BIP Code: 30488811–0) supports S. M. B., P. A. G., and A. M. K.; FONDECYT grants Nº 1190156 awarded to R. S. R. and Nº 1180798 awarded to F. V. E.; SINOVAC Life Science Co contributed to this study with the investigational vaccine and placebo, and experimental reagents.
Potential conflicts of interest. Z. G. and M. W. are SINOVAC employees and contributed to the conceptualization of the study (clinical protocol and electronic case report form design) and did not participate in the analysis or interpretation of the data presented in the manuscript. P. M. V. reports funding and study materials from the trial sponsor, during the conduct of the study; reports support from Agencia Nacional de Investigación y Desarrollo (Fondecyt regular); reports being a member of scientific advisory committee for COVID Vaccines, Ministry of Science, technology, knowledge, and innovation, outside of the submitted work. A. G. A. reports receiving ANID/Conicyt Chile grant no. 21181508 (doctoral fellowship awarded by the Agencia Nacional de Investigación y Desarrollo [ANID] Chile), outside the submitted work. F. V.-E. reports receiving Fondecyt 1180798 – 1211547 (National Research and Development Agency), during the conduct of the study; served as Chilean Microbiology Society President 2021–2022. RS-R reports receiving research grant (Nº 1190156) from the FONDECYT Program (funds from this research grant were employed in neutralization assays involving the pseudotyped virus) (ANID Chile), during the conduct of the study. D. W. reports receiving NIH contract no. 75N9301900065 (D. W., A. S.).; reports that LJI has filed for patent protection for various aspects of T-cell epitope and vaccine design work. A. S. reports receiving NIH contract no. 75N9301900065 (D. W., A. S.); A. S. is a paid consultant for Gritstone, Flow Pharma, Arcturus, Immunoscape, CellCarta, OxfordImmunotech and Avalia; LJI has filed for patent protection for various aspects of T-cell epitope and vaccine design work. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Susan M Bueno, Millennium Institute on Immunology and Immunotherapy, Santiago, Chile; Departamento de Genética Molecular y Microbiología, Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile.
Katia Abarca, Millennium Institute on Immunology and Immunotherapy, Santiago, Chile; Departamento de Enfermedades Infecciosas e Inmunología Pediátrica, División de Pediatría, Escuela de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile.
Pablo A González, Millennium Institute on Immunology and Immunotherapy, Santiago, Chile; Departamento de Genética Molecular y Microbiología, Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile.
Nicolás M S Gálvez, Millennium Institute on Immunology and Immunotherapy, Santiago, Chile; Departamento de Genética Molecular y Microbiología, Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile.
Jorge A Soto, Millennium Institute on Immunology and Immunotherapy, Santiago, Chile; Departamento de Genética Molecular y Microbiología, Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile.
Luisa F Duarte, Millennium Institute on Immunology and Immunotherapy, Santiago, Chile; Departamento de Genética Molecular y Microbiología, Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile.
Bárbara M Schultz, Millennium Institute on Immunology and Immunotherapy, Santiago, Chile; Departamento de Genética Molecular y Microbiología, Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile.
Gaspar A Pacheco, Millennium Institute on Immunology and Immunotherapy, Santiago, Chile; Departamento de Genética Molecular y Microbiología, Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile.
Liliana A González, Millennium Institute on Immunology and Immunotherapy, Santiago, Chile; Departamento de Genética Molecular y Microbiología, Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile.
Yaneisi Vázquez, Millennium Institute on Immunology and Immunotherapy, Santiago, Chile; Departamento de Genética Molecular y Microbiología, Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile.
Mariana Ríos, Millennium Institute on Immunology and Immunotherapy, Santiago, Chile; Departamento de Genética Molecular y Microbiología, Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile.
Felipe Melo-González, Millennium Institute on Immunology and Immunotherapy, Santiago, Chile; Departamento de Genética Molecular y Microbiología, Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile.
Daniela Rivera-Pérez, Millennium Institute on Immunology and Immunotherapy, Santiago, Chile; Departamento de Genética Molecular y Microbiología, Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile.
Carolina Iturriaga, Departamento de Enfermedades Infecciosas e Inmunología Pediátrica, División de Pediatría, Escuela de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile.
Marcela Urzúa, Departamento de Enfermedades Infecciosas e Inmunología Pediátrica, División de Pediatría, Escuela de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile.
Angélica Domínguez, Departamento de Salud Pública, Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile.
Catalina A Andrade, Millennium Institute on Immunology and Immunotherapy, Santiago, Chile; Departamento de Genética Molecular y Microbiología, Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile.
Roslye V Berríos-Rojas, Millennium Institute on Immunology and Immunotherapy, Santiago, Chile; Departamento de Genética Molecular y Microbiología, Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile.
Gisela Canedo-Marroquín, Millennium Institute on Immunology and Immunotherapy, Santiago, Chile; Departamento de Genética Molecular y Microbiología, Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile.
Camila Covián, Millennium Institute on Immunology and Immunotherapy, Santiago, Chile; Departamento de Genética Molecular y Microbiología, Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile.
Daniela Moreno-Tapia, Millennium Institute on Immunology and Immunotherapy, Santiago, Chile; Departamento de Genética Molecular y Microbiología, Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile.
Farides Saavedra, Millennium Institute on Immunology and Immunotherapy, Santiago, Chile; Departamento de Genética Molecular y Microbiología, Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile.
Omar P Vallejos, Millennium Institute on Immunology and Immunotherapy, Santiago, Chile; Departamento de Genética Molecular y Microbiología, Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile.
Paulina Donato, Complejo Asistencial Dr. Sótero del Rio, Santiago, Chile.
Pilar Espinoza, Hospital Clínico Félix Bulnes, Santiago, Chile; Facultad de Medicina y Ciencia y Facultad de Ciencias para el Cuidado de la Salud. Universidad San Sebastián, Santiago, Chile.
Daniela Fuentes, Hospital Carlos Van Buren, V Región, Chile; Departamento de Pediatría, Universidad de Valparaíso, Valparaiso, Chile.
Marcela González, Hospital Dr. Gustavo Fricke, V Región, Chile; Departamento de Pediatría, Universidad de Valparaíso, Valparaiso, Chile.
Paula Guzmán, Clínica Los Andes, Universidad de Los Andes, Santiago, Chile.
Paula Muñoz Venturelli, Centro de Estudios Clínicos, Instituto de Ciencias e Innovación en Medicina, Facultad de Medicina Clínica Alemana Universidad del Desarrollo, Santiago, Chile; The George Institute for Global Health, Faculty of Medicine, University of New South Wales, Sydney, Australia.
Carlos M Pérez, Hospital Clínico Félix Bulnes, Santiago, Chile; Facultad de Medicina y Ciencia y Facultad de Ciencias para el Cuidado de la Salud. Universidad San Sebastián, Santiago, Chile.
Marcela Potin, Clínica San Carlos de Apoquindo, Red de Salud UC Christus, Santiago, Chile.
Álvaro Rojas, Departamento de Enfermedades Infecciosas del Adulto, División de Medicina, Escuela de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile.
Rodrigo A Fasce, Departamento de Laboratorio Biomédico, Instituto de Salud Pública de, Chile, Santiago, Chile.
Jorge Fernández, Departamento de Laboratorio Biomédico, Instituto de Salud Pública de, Chile, Santiago, Chile.
Judith Mora, Departamento de Laboratorio Biomédico, Instituto de Salud Pública de, Chile, Santiago, Chile.
Eugenio Ramírez, Departamento de Laboratorio Biomédico, Instituto de Salud Pública de, Chile, Santiago, Chile.
Aracelly Gaete-Argel, Laboratory of Molecular and Cellular Virology, Virology Program, Institute of Biomedical Sciences, Faculty of Medicine, Universidad de Chile, Santiago, Chile.
Aarón Oyarzún-Arrau, Laboratory of Molecular and Cellular Virology, Virology Program, Institute of Biomedical Sciences, Faculty of Medicine, Universidad de Chile, Santiago, Chile.
Fernando Valiente-Echeverría, Laboratory of Molecular and Cellular Virology, Virology Program, Institute of Biomedical Sciences, Faculty of Medicine, Universidad de Chile, Santiago, Chile.
Ricardo Soto-Rifo, Laboratory of Molecular and Cellular Virology, Virology Program, Institute of Biomedical Sciences, Faculty of Medicine, Universidad de Chile, Santiago, Chile.
Daniela Weiskopf, Center for Infectious Disease and Vaccine Research, La Jolla Institute for Immunology, La Jolla, California, USA.
Alessandro Sette, Center for Infectious Disease and Vaccine Research, La Jolla Institute for Immunology, La Jolla, California, USA; Department of Medicine, Division of Infectious Diseases and Global Public Health, University of California, San Diego (UCSD), La Jolla, CA, USA.
Gang Zeng, Sinovac Biotech, Beijing, China.
Weining Meng, Sinovac Biotech, Beijing, China.
José V González-Aramundiz, Departamento de Farmacia, Facultad de Química y de Farmacia. Pontificia Universidad Católica de Chile, Santiago, Chile.
Alexis M Kalergis, Millennium Institute on Immunology and Immunotherapy, Santiago, Chile; Departamento de Genética Molecular y Microbiología, Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile; Departamento de Farmacia, Facultad de Química y de Farmacia. Pontificia Universidad Católica de Chile, Santiago, Chile; Departamento de Endocrinología, Facultad de Medicina, Escuela de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile.
References
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382:727–733. Available at: http://www.ncbi.nlm.nih.gov/pubmed/31978945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim D, Lee J-Y, Yang J-S, Kim JW, Kim VN, Chang H. The architecture of SARS-CoV-2 transcriptome. Cell 2020; 181:914–21.e10. Available at: http://www.ncbi.nlm.nih.gov/pubmed/32330414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579:270–3. Available at: http://www.ncbi.nlm.nih.gov/pubmed/32015507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020; 20:533–4. Available at: http://www.ncbi.nlm.nih.gov/pubmed/32087114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Perez-Saez J, Lauer SA, Kaiser L, et al. Serology-informed estimates of SARS-CoV-2 infection fatality risk in Geneva, Switzerland. Lancet Infect Dis 2020; Available at: http://www.ncbi.nlm.nih.gov/pubmed/32679085. [DOI] [PMC free article] [PubMed]
- 6. O’Driscoll M, Ribeiro Dos Santos G, Wang L, et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature 2021; 590:140–5. Available at: http://www.ncbi.nlm.nih.gov/pubmed/33137809. [DOI] [PubMed] [Google Scholar]
- 7. World Health Organization. Draft landscape of COVID-19 candidate vaccines. 2020. Available at: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines. Accessed 6 October 2021.
- 8. Krammer F. SARS-CoV-2 vaccines in development. Nature 2020; 586:516–27. Available at: http://www.nature.com/articles/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 9. Stern PL. Key steps in vaccine development. Ann Allergy Asthma Immunol 2020; 125:17–27. Available at: http://www.ncbi.nlm.nih.gov/pubmed/32044451. [DOI] [PubMed] [Google Scholar]
- 10. Al Kaabi N, Zhang Y, Xia S, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA 2021; 326:35–45. Available at: http://www.ncbi.nlm.nih.gov/pubmed/34037666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li Y-D, Chi W-Y, Su J-H, Ferrall L, Hung C-F, Wu T-C. Coronavirus vaccine development: from SARS and MERS to COVID-19. J Biomed Sci 2020; 27:104. Available at: http://www.ncbi.nlm.nih.gov/pubmed/33341119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gao Q, Bao L, Mao H, et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020; 369:77–81. Available at: http://www.ncbi.nlm.nih.gov/pubmed/32376603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis 2021; 21:181–92. Available at: http://www.ncbi.nlm.nih.gov/pubmed/33217362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu Z, Hu Y, Xu M, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis 2021; Available at: http://www.ncbi.nlm.nih.gov/pubmed/33548194. [DOI] [PMC free article] [PubMed]
- 15. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310:2191–4. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24141714. [DOI] [PubMed] [Google Scholar]
- 16. Beltrán-Pavez C, Riquelme-Barrios S, Oyarzún-Arrau A, et al. Insights into neutralizing antibody responses in individuals exposed to SARS-CoV-2 in Chile. Sci Adv 2021; 7:eabe6855. Available at: http://www.ncbi.nlm.nih.gov/pubmed/33579701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 2020; 181:1489–501.e15. Available at: http://www.ncbi.nlm.nih.gov/pubmed/32473127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Departamento de Epidemiología G de C. Reporte de la circulación de variantes de SARS-CoV-2 en Chile. Santiago: 2021. Available at: https://www.minsal.cl/wp-content/uploads/2021/02/Reporte-circulacion-variantes-26-02-21.pdf. Accessed 14 August 2021.
- 19. WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis 2020; 20:e192–7. Available at: http://www.ncbi.nlm.nih.gov/pubmed/32539990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. He Q, Mao Q, Zhang J, et al. COVID-19 vaccines: current understanding on immunogenicity, safety, and further considerations. Front Immunol 2021; 12:669339. Available at: http://www.ncbi.nlm.nih.gov/pubmed/33912196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tanriover MD, Doğanay HL, Akova M, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 2021; 398:213–22. Available at: http://www.ncbi.nlm.nih.gov/pubmed/34246358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pan H, Wu Q, Zeng G, et al. Immunogenicity and safety of a third dose, and immune persistence of CoronaVac vaccine in healthy adults aged 18-59 years: interim results from a double-blind, randomized, placebo-controlled phase 2 clinical trial. medRxiv 2021; 2021.07.23.21261026. Available at: http://medrxiv.org/content/early/2021/07/25/2021.07.23.21261026.abstract.
- 23. Voysey M, Costa Clemens SA, Madhi SA, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet 2021; 397:881–91. Available at: http://www.ncbi.nlm.nih.gov/pubmed/33617777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parry H, Bruton R, Stephens C, et al. Extended interval BNT162b2 vaccination enhances peak antibody generation in older people. medRxiv 2021;2021.05.15.21257017. Available at: http://medrxiv.org/content/early/2021/05/17/2021.05.15.21257017.abstract. [DOI] [PMC free article] [PubMed]
- 25. Kung Y-A, Huang C-G, Huang S-Y, et al. Antibody titers measured by commercial assays are correlated with neutralizing antibody titers calibrated by international standards. medRxiv 2021;2021.07.16.21260618. Available at: http://medrxiv.org/content/early/2021/07/22/2021.07.16.21260618.abstract.
- 26. Kyriakidis NC, López-Cortés A, González EV, Grimaldos AB, Prado EO. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. NPJ Vaccines 2021; 6:28. Available at: http://www.ncbi.nlm.nih.gov/pubmed/33619260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020; 586:594–9. Available at: http://www.nature.com/articles/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 28. Zhu F-C, Li Y-H, Guan X-H, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020; 395:1845–54. Available at: http://www.ncbi.nlm.nih.gov/pubmed/32450106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.