Abstract
Background
We previously reported lower bone mineral density (BMD) among premenopausal women with HIV (WWH) compared to women without HIV (HIV−). Rate of bone loss may be even greater for WWH during the menopausal transition.
Methods
Pre-, peri- and postmenopausal women in the Women\'s Interagency HIV Study (WIHS) underwent whole body DXA and central quantitative computed tomography to measure areal BMD (aBMD) and volumetric BMD (vBMD), respectively. Multivariable regression models with covariates associated with low aBMD (T score < −1.0) in univariate analyses (P≤.05) and known risk factors for low BMD assessed contributions of HIV and menopausal stage to the prediction of aBMD.
Results
Compared to HIV− women, in unadjusted analyses, WWH had 5–9% lower aBMD at the lumbar spine (P=.001), femoral neck (P=.04), total hip (P=.003) and the ultradistal radius (P=.004), and higher osteoporosis prevalence (T score<−2.5) at the ultradistal radius only (13.5% vs 0%, P=.0003). WWH also had lower vBMD at the spine and hip. In fully adjusted models, HIV independently predicted reduced aBMD at the lumbar spine, total hip, femoral neck, and ultradistal radius; menopausal stage remained a significant predictor of lumbar spine and ultradistal radius aBMD.
Conclusions
HIV infection and menopausal stage were independent predictors of lower BMD, and had an additive effect on lumbar spine and total hip BMD. Additional research is needed to better understand underlying mechanisms by which HIV impacts BMD as women age and transition through menopause, and develop strategies to mitigate osteoporosis and fracture risk in this growing population.
Keywords: bone mineral density, osteoporosis, menopause, HIV, aging, women
Among pre-, peri- and postmenopausal women, low BMD was more common in women with HIV than without HIV. HIV infection and menopausal stage were independent predictors of lower BMD and had additive effects on lumbar spine and total hip BMD.
With successful antiretroviral therapy (ART), women with human immunodeficiency virus (HIV; WWH) are increasingly transitioning through menopause and surviving into older age. Menopause is marked by estrogen depletion and accelerated bone loss, ultimately leading to musculoskeletal senescence, including clinical features of frailty, falls, and fractures [1, 2]. Menopause is associated with increased production of bone-resorbing cytokines such as receptor activator of nuclear factor-κB ligand (RANKL) and tumor necrosis factor α (TNF-α) [3]. Moreover, T cells are now thought to play a fundamental role in the mechanisms by which estrogen deficiency causes early postmenopausal bone loss [4, 5]. Since HIV infection is associated with persistent T-cell activation as well as increased production of RANKL and TNF-α [6, 7], the adverse skeletal effects of estrogen deficiency may be exacerbated in WWH, particularly as they transition through menopause.
Our previous analyses of premenopausal women in the Women’s Interagency HIV Study (WIHS) found lower bone mineral density (BMD) among WWH than women without HIV; however, serum proinflammatory cytokines, bone resorption markers, and rate of bone loss did not differ significantly by HIV status [8]. In contrast, a separate study found that postmenopausal WWH had higher rates of bone loss as well as higher serum levels of bone resorption markers and TNF-α than postmenopausal women without HIV; higher serum TNF-α level was also associated with low BMD [9, 10].
Estrogen downregulates T-cell activation and mitigates the effects of T-cell–derived, proinflammatory cytokines on osteoclast-mediated bone resorption [1]. We therefore hypothesized that estrogen attenuates the adverse skeletal effects of HIV infection in premenopausal women [11] and that the combined effects of declining estrogen levels and persistent inflammation associated with HIV infection accelerates bone remodeling and bone loss during the menopausal transition to a greater extent in WWH.
METHODS
Participants
Participants were enrolled in a Musculoskeletal Study (MSK) during 2012–2016 nested in the WIHS, an ongoing, prospective cohort study of cis-gender women with HIV and demographically similar women without HIV who were initially enrolled in 1994–1995 at 6 sites nationally (Bronx/Manhattan, New York; Brooklyn, New York; Chicago, Illinoic; Washington, DC; San Francisco, California; and Los Angeles, California), with additional enrollment in 2001–2002 and 2011–2012 [12]. Women’s Interagency HIV Study semiannual visits included an interviewer-administered structured questionnaire, physical examination, and collection of specimens for laboratory testing.
The MSK enrolled 252 pre-, peri-, and postmenopausal WIHS participants aged 40–60 years with HIV (65%, n=164) and current CD4 >100 cells/μL on ART for more than 1 year and those without HIV (35%, n=88) from 3 WIHS sites (San Francisco, Bronx, and Chicago), with similar age, ethnicity, and risk behaviors by HIV serostatus [13]. The MSK exclusion criteria included weight greater than 264 pounds, height more than 6’1” (per dual-energy X-ray absorptiometry [DXA] limitations), currently pregnant or lactating, postpartum within 6 months, estimated glomerular filtration rate less than 60mL/minute, and ever use of bisphosphonates, hormone replacement therapy or hormonal birth control within 3 months, and growth hormone or glucocorticoid use within 12 months. A total of 244 participants with complete DXA data were included in analysis. The institutional review boards of all participating institutions approved WIHS and MSK study protocols, and all participants provided written informed consent.
Menopausal Stages/Status
Menopausal stages were defined using SWAN (Study of Women’s Health Across the Nation) study criteria [14]: early perimenopause (at least 1 menstrual period in the last 3 months with some change in the regularity over last 12 months), late perimenopause (no bleeding in 3–11 of the last 12 months), or early postmenopausal (no bleeding for >1 but <5 years). Because the rate of bone loss is similar in premenopause and early perimenopause, but lower in late perimenopause and early postmenopause [14], we dichotomized menopausal comparison groups as premenopause and early perimenopause versus late perimenopause and early postmenopause.
Dual-Energy X-ray Absorptiometery
Participants underwent whole-body DXA to measure areal BMD (aBMD) at 5 sites (lumbar spine, total hip, femoral neck, 1/3 distal radius, and ultradistal radius) and appendicular lean mass and total lean mass. DXA scans were performed using Lunar Prodigy densitometers (GE Medical Systems, Madison WI) at all study sites and read centrally at the Image Analysis Lab (New York, NY). Short-term in vivo precision met standards specified by the International Society for Clinical Densitometry. T scores, which compare subjects with the peak bone mass of young individuals of the same sex and race, were derived for the hip from the third National Health and Nutrition Examination Survey (NHANES-III) and the manufacturer’s normative database. Osteoporosis and osteopenia were defined by World Health Organization criteria: T scores less than −2.5 represent osteoporosis; T scores between −1.0 and −2.49 represent osteopenia [15]. Height and weight were measured by a Harpenden stadiometer and Detecto balance beam scale, respectively.
Quantitative Computed Tomography of the Hip and Spine
DXA measures of aBMD may be artifactually increased by adipose tissue absorption of x-rays, especially in obese individuals [16]. Central quantitative computed tomography (cQCT) was utilized to measure volumetric BMD in MSK participants between January 2012 and April 2016. Participants underwent helical (pitch=1) cQCT scanning of the proximal femur and spine (L1–L2) at 80 kVp, 2.5-mm slice thickness (GE CTi and GE Light Speed VCT 64; General Electric Medical Systems, Milwaukee WI) with a calibration phantom (3-Bar; Image Analysis, Columbia, KY) used to calibrate cQCT images to an equivalent concentration of calcium hydroxyapatite. QCT PRO Bone Investigational Toolkit software (v2; Mindways, Austin, TX) was used to compute total (integral) volumetric density of the proximal femur and total volumetric density of lumbar spine vertebrae (L1–L2) [17]. Additional structural data from cQCT analyses are presented separately.
Laboratory Methods
Fasting morning serum stored at −80°C was batch-analyzed the Irving Institute Biomarkers Core at Columbia University Irving Medical Center. We measured 25(OH)D2 and 25(OH)D3 by liquid chromatography–tandem mass spectrometry, intact parathyroid hormone by radioimmunoassay (RIA; Scantibodies, Santee, CA), N-terminal propeptide of procollagen type 1 (P1NP; Immunodiagnostic Systems [IDS], Scottsdale, AZ) by RIA, C-telopeptide (CTX; IDS) by enzyme-linked immunosorbent assay (ELISA), TNF-α (R&D Systems, Minneapolis, MN) by ELISA, high-sensitivity interleukin-6 (IL-6; R&D Systems) by ELISA, follicle-stimulating hormone (FSH; Siemens Healthcare, Los Angeles, CA) by immunoassay, estradiol (Siemens Healthcare) by immunoassay, osteoprotegerin (OPG; Biomedica, Vienna, Austria) by ELISA, and RANKL (Immundiagnostik, Bensheim, Germany) by ELISA. Except for 25(OH)D, biomarkers were measured in duplicate and values averaged for analysis.
Statistical Analysis
The primary outcome comparisons were DXA-derived aBMD at each of 5 sites (lumbar spine, total hip, femoral neck, 1/3 distal radius, and ultradistal radius) by HIV serostatus without adjustment for testing multiple outcomes. Continuous variables were examined for normality; RANKL was log-transformed due to right skewness. Between-group differences in categorical measures were assessed with Fisher’s exact test. Spearman’s and Pearson’s correlation coefficients were calculated for continuous measures. Data are presented as means + standard errors unless otherwise noted. P values less than .05 were considered statistically significant.
Univariate associations of demographic, anthropometric, reproductive, medical, and lifestyle variables with aBMD at each site were examined to identify variables associated with low aBMD/osteopenia (T score<−1.0). Statistically significant (P≤.05) variables were entered into a multiple regression model along with HIV and menopausal status, to assess the contribution of HIV and menopausal status on aBMD after accounting for other known risk factors for low BMD, and to assess for interaction (SAS Proc REG; SAS Institute, Cary, NC). Final multivariate models adjusted for body mass index (BMI), race/ethnicity, hepatitis C virus (HCV), diabetes mellitus, heavy alcohol use, cigarette smoking, and WIHS site. Bone turnover markers and cytokine levels were evaluated as modifiers of the association of HIV status with aBMD at each site. We next constructed multiple regression models with CT-measured vBMD at total hip and lumbar spine (L1 and L2) as outcomes, using the same statistical approach, as a means to confirm findings of aBMD.
RESULTS
Characteristics of the Study Population
Among WWH, the mean age was 50 years; 65% were African American, 19% Hispanic, and 16% White; 45% were obese (BMI≥30kg/m2); and 36% were premenopausal/early perimenopausal and 64% were late peri-/postmenopausal (Table 1). Women with HIV had a mean CD4+ cell count of 576 + 270 cells/mm3, and 58% had HIV RNA levels below the limit of detection; mean ART duration was 9 years. Most WWH were on protease-inhibitor (49%) or non-nucleoside reverse transcriptase inhibitor–containing regimens (46%); 51% were on tenofovir (TDF)-containing regimens. Women without HIV were similar to those with HIV in terms of age, race, BMI, menopausal status, opioid use, and prevalence of diabetes, HCV, renal insufficiency, prior fracture, and glucocorticoid use. Women with HIV were less likely to be current smokers (49% vs 65%; P=.01) and more likely to take calcium supplementation (12% vs 1%; P=.004) than women without HIV.
Table 1.
Women’s Interagency HIV Study Musculoskeletal Study Participant Characteristics
| Clinical Characteristics | HIV− (n=86) | HIV+ (n=158) | P |
|---|---|---|---|
| Age, years | 49±6 | 50±5 | .26 |
| Race/ethnicity | .55 | ||
| White | 11 (13%) | 25 (16%) | |
| Black | 62 (72%) | 103 (65%) | |
| Hispanic/other | 13 (15%) | 30 (19%) | |
| WIHS site | .92 | ||
| Bronx | 32 (37%) | 60 (38%) | |
| San Francisco | 37 (43%) | 64 (41%) | |
| Chicago | 17 (20%) | 34 (21%) | |
| BMI | .31 | ||
| 18.0–24.9kg/m2 | 16% | 23% | |
| 25.0–29.9kg/m2 | 30% | 33% | |
| >30kg/m2 | 54% | 45% | |
| Menopausal status | .91 | ||
| Premenopause | 20 (23%) | 34 (22%) | |
| Early perimenopause | 13 (15%) | 22 (14%) | |
| Late perimenopause | 12 (14%) | 19 (12%) | |
| Postmenopause | 41 (48%) | 83(52%) | |
| Smoking status | .01 | ||
| Never | 13 (15%) | 20 (13%) | |
| Past | 17 (20%) | 60 (38%) | |
| Current | 56 (65%) | 78 (49%) | |
| Alcohol >12 drinks/wk | 12 (14%) | 13 (8%) | .16 |
| Injection drug use at index visit | 1 (1%) | 3 (2%) | .67 |
| Opiate use at index visit | 2 (2%) | 2 (2%) | .53 |
| Cocaine use at index visit | 8 (9%) | 3 (2%) | .008 |
| Diabetes | 24 (28%) | 45 (28%) | .92 |
| Hepatitis C infection (RNA+) | 19 (22%) | 41 (26%) | .50 |
| eGFR<60 cc/minutes | 2 (2%) | 8 (5%) | .30 |
| Prior fracture before index visit | 16 (19%) | 30 (19%) | .94 |
| Glucocorticoid use at index visit | 15 (17%) | 27 (17%) | .94 |
| Calcium supplement at index visit | 1 (1%) | 18 (12%) | .004 |
| Vitamin D supplement at index visit | 11 (13%) | 36 (23%) | .06 |
| HIV parameters | |||
| Current CD4 at index, cells/mm3 | NA | 576±270 | NA |
| Undetectable HIV RNA at index (<50 copies/mL) | NA | 103 (65%) | NA |
| Antiretroviral use | |||
| Cumulative years of ART | NA | 9.0±5.7 | NA |
| Cumulative years of TDF | NA | 3.5±3.6 | NA |
| TDF use at index visit | NA | 81 (51%) | NA |
| NRTI use at index visit | NA | 151 (96%) | NA |
| PI use at index visit | NA | 77 (49%) | NA |
| NNRTI use at index visit | NA | 73 (46%) | NA |
| INSTI use at index visit | NA | 18 (12%) | NA |
| Biochemical markers | |||
| Estradiol, pg/mL | 79.8±59.5 | 74.8±65.4 | .29 |
| 25-Hydroxyvitamin D, ng/mL | 20.8±11.1 | 25.4±13.3 | .009 |
| CTX, median (IQR), ng/mL | 0.25 (0.15, 0.39) | 0.31 (0.22, 0.45) | .01 |
| P1NP, median (IQR), ng/mL | 45.5 (34.3, 59.7) | 53.3 (39.6, 75.5) | .005 |
| TNF-α, median (IQR), pg/mL | 1.17 (0.87, 1.53) | 1.27 (0.93, 1.71) | .07 |
| IL-6, median (IQR), pg/mL | 3.47 (2.01, 5.37) | 2.70 (1.68, 4.85) | .18 |
| RANKL, median (IQR), pg/mL | 462 (370, 30038) | 662 (370, 30000) | .85 |
| OPG, median (IQR), pmol/mL | 6.36 (4.73, 8.48) | 6.52 (4.77, 8.87) | .57 |
| Body composition by DXA | |||
| Total body fat, kg | 31.2±11.3 | 31.1±11.4 | .89 |
| Trunk fat, kg | 17.3±6.6 | 16.9±6.5 | .45 |
| Total body lean mass, kg | 42.9±5.6 | 43.0±6.2 | .90 |
| Lower extremity lean mass, kg | 14.5±2.1 | 14.3±2.4 | .65 |
Data are presented as mean ± SD or n (%) unless otherwise noted. Abbreviations: AMH, anti-Müllerian hormone; ART, antiretroviral therapy; BMI, body mass index; CTX, C-terminal telopeptide of type 1 collagen; DXA, dual-energy X-ray absorptiometry; eGFR, estimated glomerular filtration rate; HIV, human immunodeficiency virus; IL-6, interleukin-6; INSTI, integrase strand transfer inhibitor; IQR, interquartile range; NA, not applicable; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; OPG, osteoprotegerin; PI, protease inhibitor; P1NP, procollagen type 1N propeptide; RANKL, receptor activator of nuclear factor–κΒ ligand; TDF, tenofovir; TNF-α, tumor necrosis factor α; WIHS, Women’s Interagency HIV Study.
Calciotropic Hormones, Bone Turnover Markers, and Cytokine Levels
Serum 25(OH)D levels were higher in WWH than in women without HIV (Table 1); however, prevalence of severe vitamin D deficiency (<10ng/mL) did not differ (16% WWH vs 11% HIV−; P=.28). Serum bone turnover marker levels (CTX and P1NP) were higher in WWH than in women without HIV (Table 1). There was a trend towards higher serum TNF-α levels in WWH than in women without HIV, but IL-6, RANKL, and OPG levels did not differ by HIV status (Table 1). We found no statistically significant associations with levels of TNF-α, RANKL, or OPG with BMD in regression analyses, or between RANKL-to-OPG ratio and BMD at any site (data not shown).
Areal Bone Mineral Density
Compared with women without HIV, in unadjusted analyses, WWH had 5–9% lower aBMD at the lumbar spine (P=.001), femoral neck (P=.04), total hip (P=.003), and the ultradistal radius (P=.004) (Table 2), but 1/3 distal radius aBMD did not differ by HIV serostatus. T scores were lower in WWH than in women without HIV at all sites by 0.4 to 0.8 standard deviations (SDs), with the largest between-group difference observed at the lumbar spine. All P values were less than .01 including for 1/3 distal radius. Prevalence of osteoporosis (T score<−2.5) was low and was significantly higher in WWH than in women without HIV only at the ultradistal radius (13.5% vs 0%; P=.0003). Prevalence of low BMD/osteopenia (T score<−1.0) was significantly higher in WWH than in women without HIV at every site (Table 2).
Table 2.
Bone Mineral Density Among Women With and Without HIV
| DXA Parameters | HIV− (n=86) | HIV+ (n=158) | P |
|---|---|---|---|
| Areal BMD, mean±SD, g/cm2 | |||
| Lumbar spine | 1.30±0.21 | 1.20±0.19 | .001 |
| Femoral neck | 1.15±0.18 | 1.09±0.17 | .04 |
| Total hip | 1.09±0.17 | 1.01±0.15 | .003 |
| 1/3 Distal radius | 0.84±0.11 | 0.81±0.14 | .20 |
| Ultradistal radius | 0.46±0.07 | 0.42±0.09 | .004 |
| Areal BMD T scores (mean±SD) | |||
| Lumbar spine | 0.94±1.76 | 0.16±1.58 | .002 |
| Femoral neck | 0.08±1.32 | −0.40±0.99 | .009 |
| Total hip | 0.62±1.32 | 0.05±1.20 | .004 |
| 1/3 Distal radius | 0.20±0.92 | −0.21±1.24 | .006 |
| Ultradistal radius | 0.46±1.46 | −0.46±1.85 | .0004 |
| Osteoporosis (T score<−2.5), n (%) | |||
| Lumbar spine | 2 (2.4%) | 7 (4.6%) | .50 |
| Femoral neck | 0% | 2 (1.3%) | .55 |
| Total hip | 0% | 2 (1.3%) | .54 |
| 1/3 Distal radius | 1 (1.4%) | 7 (4.7%) | .27 |
| Ultradistal radius | 0% | 20 (13.5%) | .0003 |
| Low BMD (T score<−1.0),a n (%) | |||
| Lumbar spine | 7 (8.5%) | 35 (22.9%) | .007 |
| Femoral neck | 12 (14.8%) | 45 (29.4%) | .02 |
| Total hip | 7 (8.6%) | 31 (20.4%) | .03 |
| 1/3 Distal radius | 7 (9.5%) | 33 (22.3%) | .025 |
| Ultradistal radius | 11 (14.9%) | 58 (39.2%) | .0002 |
| CT parameters: volumetric BMD, mean±SD, g/cm3 | |||
| Lumbar spine (first vertebral body) | 173.2±46.1 | 157.2±41.8 | .02 |
| Lumbar spine (second vertebral body) | 168.7±43.6 | 155.3±41.9 | .03 |
| Total hip | 355.9±58.9 | 331.1±61.9 | .04 |
Abbreviations: BMD, bone mineral density; CT, computed tomography; DXA, dual-energy X-ray absorptiometry; HIV, human immunodeficiency virus.
Reflects both osteopenia and osteoporosis.
Volumetric Bone Mineral Density
In unadjusted analyses of CT-derived volumetric BMD (vBMD), WWH had significantly lower vBMD (mean±SD) compared with women without HIV at the L1 lumbar spine (173.2±46.1 vs 157.2±41.8; P=.019), L2 lumbar spine (168.7±43.6 vs 155.3±41.9; P=.30), and total hip (355.9±58.9 vs 331.1±61.9; P=.035) (Table 2).
Determinants of Areal Bone Mineral Density Among Women With and Without HIV
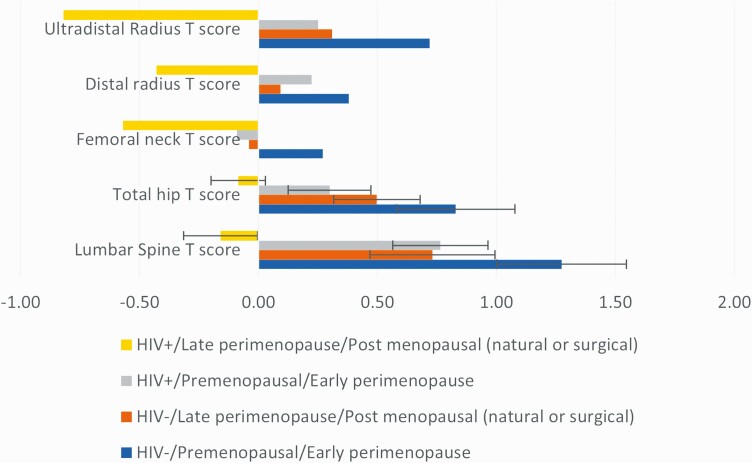
We examined the impact of HIV and menopausal status on aBMD, and they appeared to be additive at the lumbar spine and total hip, with the lowest T scores in the HIV+ late perimenopause/early postmenopause group and the highest T scores in the HIV− premenopause/early perimenopause group (Figure 1). Next, we used regression analysis to assess whether HIV status and menopausal status remained independent predictors of aBMD after adjustment for known determinants of aBMD. After adjustment for race, BMI, WIHS site, smoking, alcohol use, diabetes, and HCV antibody status, HIV remained an independent predictor of reduced aBMD at the lumbar spine, total hip, femoral neck, and ultradistal radius; menopausal status also remained an independent predictor of lumbar spine aBMD and ultradistal radius aBMD (Table 3). To further clarify the role of HIV and menopause, we examined multivariate models with combined categories for HIV and menopausal status. Our findings suggested a cumulative effect at the lumbar spine and total hip, with significant differences between groups by HIV and menopausal status (Figure 1). There was no statistically significant interaction between menopausal status and HIV at any aBMD site, although the study was not adequately powered to detect an interaction between menopause and HIV.
Figure 1.
Association of HIV and menopausal status with low BMD. Abbreviations: BMD, bone mineral density; HIV, human immunodeficiency virus.
Table 3.
Factors Associated With Areal Bone Mineral Density Among Women With and Without HIV Measured by Dual-Energy X-ray Absorptiometry
| Lumbar Spine Coefficient (95% CI) | Total Hip Coefficient (95% CI) | Femoral Neck Coefficient (95% CI) | 1/3 Distal Radius Coefficient (95% CI) | Ultradistal Radius Coefficient (95% CI) | |
|---|---|---|---|---|---|
| HIV seropositive | −.090a (−.142, −.037) | −.071a (−.111, −.031) | −.058b (−.104, −.012) | −.016 (−.052, .020) | −.031b (−.053, −.009) |
| Menopausal statusc | −.087b (−.140, −.034) | −.037 (−.078, .003) | −.020 (−.066, .027) | −.029 (−.065, .007) | −.029d (−.052, −.007) |
| BMI (per 5kg/m2) | .020 (−.002, .041) | .040e (.024, .056) | .029b (.010, .048) | .025b (.010, .040) | .024e (.014, .033) |
| Race (Ref: Black) | |||||
| White | −.059 (−.131, .013) | −.042 (−.096, .011) | −.054 (−.117, .008) | −.042 (−.091, .006) | −.024 (−.054, .006) |
| Other | −.091b (−.159, −.023) | −.027 (−.080, .026) | −.023 (−.083, .038) | −.020 (−.065, .026) | .006 (−.022, .034) |
| Hepatitis C virus | −.015b (−.072, .043) | −.007 (−.051, .038) | .016 (−.035, .068) | −.018 (−.057, .022) | −.014 (−.038, .011) |
All models are additionally adjusted for WIHS site, heavy alcohol use, cigarette smoking, and diabetes mellitus. Abbreviations: BMI, body mass index; CI, confidence interval; HIV, human immunodeficiency virus; Ref, reference; WIHS, Women’s Interagency HIV Study.
Menopausal status defined dichotomously (premenopause and early perimenopause vs late perimenopause and postmenopause).
P<.001.
P<.01.
P<.05.
Determinants of Volumetric Bone Mineral Density Among Women With and Without HIV
In multivariable models of vBMD, after adjustment for race, BMI, WIHS site, smoking, alcohol use, diabetes, and HCV status, HIV serostatus remained an independent predictor of reduced vBMD at L1, L2, and total hip (all P<.05) (Table 4). Menopause status was significantly associated with lower lumbar spine vBMD at both L1 and L2 but was not independently statistically associated with total hip vBMD. Other factors independently associated with reduced vBMD in multivariable models included race (at the total hip) and HCV (at L1 lumbar spine); BMI was not independently associated with vBMD.
Table 4.
Factors Associated with Volumetric Bone Mineral Density Measured by Computed Tomography Among Women With and Without HIV
| L1 Lumbar Spine Coefficient (95% CI) | L2 Lumbar Spine Coefficient (95% CI) | Total Hip Coefficient (95% CI) | |
|---|---|---|---|
| HIV seropositive | −16.92 (−30.99, −2.857)a | −13.91 (−27.71, −.122)a | −30.95 (−49.52, −12.38)b |
| Menopausal statusc | −27.46 (−42.41, −12.51)d | −26.89 (−41.55, −12.24)d | −18.49 (−38.38, 1.399) |
| BMI (per 5kg/m2) | 1.747 (−3.774, 7.269) | 2.804 (−2.610, 8.218) | 4.482 (−2.935, 11.899) |
| Race (Ref: Black) | |||
| White | −16.49 (−34.78, 1.793) | −16.01(−33.94, 1.920) | −24.38 (−48.42, −.331)a |
| Other | −3.711 (−20.20, 12.778) | −4.875 (−21.04, 11.293) | 6.251 (−15.71, 28.212) |
| Hepatitis C virus | −15.44 (−30.46, −.415)a | −12.99 (−27.72, 1.740) | −9.504 (−29.30, 10.288) |
All models are additionally adjusted for WIHS site, heavy alcohol use, cigarette smoking, and diabetes mellitus. Abbreviations: BMI, body mass index; CI, confidence interval; HIV, human immunodeficiency virus; Ref, reference; WIHS, Women’s Interagency HIV Study.
P<.05.
P<.01.
Menopausal status defined dichotomously (premenopause and early perimenopause vs late perimenopause and postmenopause).
P<.001.
P<.0001.
Determinants of Areal Bone Mineral Density Among Women With HIV Only
In univariate analyses, among the subset of WWH there were no significant associations between aBMD at any of 5 sites (lumbar spine, total hip, femoral neck, 1/3 distal radius, and ultradistal radius) with current or nadir CD4 count, current HIV plasma RNA level, prior AIDS-defining illness, or ART class (either current or cumulative use). In univariate models, only cumulative TDF use was significantly associated with reduced aBMD (P=.03 at the 1/3 distal radius and ultradistal radius) and approached significance at the lumbar spine (P=.059). In multivariable models adjusting for race/ethnicity, menopausal status, BMI, smoking status, heavy alcohol use, HCV seropositivity, and diabetes, cumulative TDF use was no longer statistically associated with aBMD at any of the 5 sites (data not shown).
DISCUSSION
Women with HIV had lower BMD at the spine, hip, and radius when compared with women without HIV, with the greatest between-group difference observed at the spine. HIV seropositivity and postmenopausal status had independent negative effects on BMD, particularly at the spine and the total hip where these associations remained statistically significant after adjustment for traditional risk factors for osteoporosis including race, BMI, cigarette smoking, alcohol use, and HCV infection. While bone turnover markers were higher in WWH than in those without HIV, they were not associated with BMD, and similarly, pro-resorptive inflammatory cytokines were not associated with BMD. Although not statistically significant, deficits in BMD by menopausal status appear to be greater between women with HIV than in those without HIV, such that the difference in BMD between late peri-/postmenopausal women and pre-/early perimenopasual women is nonsignificantly wider for WWH, particularly at the distal radius and spine; a larger sample size would be needed to determine whether these differences truly exist. In our analyses, these differences cannot be attributed to traditional osteoporosis risk factors or increased inflammatory cytokines.
The between-serostatus-group differences in BMD observed in this study are generally consistent with, although perhaps slightly higher than, those observed in other studies of older persons living with HIV (PLWH). The 5–9% unadjusted difference in BMD by HIV serostatus in our analyses is slightly greater than the 2–5% difference reported in studies of middle-age women with and without HIV (mean ages of 41 and 44 years, respectively) by Arnsten [18] and Dolan [19], but is similar to the 5.9% lower total hip BMD in a New York City cohort of postmenopausal WWH [10]. However, among WWH with a median age of 50 years, we found a 23% prevalence of low BMD at the lumbar spine, which is substantially less than reports among older PLWH (67%; median age of 61) [20] or postmenopausal WWH (78%; median age of 57) [10]. These wide variations in low BMD prevalence may be due to important differences in the composition of participants within each study with respect to characteristics that affect BMD, such as age, sex, menopausal status, and race.
HIV and menopausal status appeared to be additive in their association with BMD in unadjusted models. In multivariate analysis, HIV and menopausal status remained independent predictors of BMD at the lumbar spine, total hip, and ultradistal radius. The association of HIV was similar for both lumbar spine and total hip BMD, while the association of menopausal status was much greater at the lumbar spine than total hip. Because lumbar spine is predominantly composed of the more metabolically active trabecular bone, whereas total hip BMD reflects both cortical and trabecular bone, BMD changes at the lumbar spine are usually greater with the menopausal transition than at sites that contain cortical bone [21, 22].
Lower body weight is a powerful determinant of BMD. In their meta-analysis, Bolland et al [23] argue that published differences in BMD between persons with and without HIV are largely related to lower body weight of PLWH and are clinically insignificant (2.2–2.8%) after adjustment for group differences in weight. In our study, BMD remained lower in WWH than in those without HIV after adjusting for race/ethnicity and BMI, although it is noteworthy that most participants had an overweight or obese BMI, and few had a low body weight.
Surprisingly, serum levels of inflammatory cytokines known to induce bone resorption (TNF-α, IL-6, RANKL, and RANKL:OPG) were not associated with low BMD in this study. The OPG/RANK/RANKL system is the dominant, final mediator of osteoclastogenesis [24], with the rate of bone resorption determined by the relative amounts of RANKL and OPG produced by osteoblasts [25]. Systemic hormones such as estrogen and locally secreted bone-active cytokines including TNF-α and IL-6 that increase bone resorption do so, in part, by increasing RANKL expression by bone marrow stromal cells and osteoblasts. In a previous study of postmenopausal women, TNF-α levels were higher among WWH and correlated with low BMD [10]. In our study, serum TNF-α and IL-6 levels were not higher in WWH than in women without HIV, likely reflecting effective ART use with immune reconstitution among WWH. Additionally, serum cytokine levels may not reflect cellular activation or proinflammatory cytokine level in the bone microenvironment.
The strengths of this study include its focus on menopausal WWH, a growing population at high risk of bone loss and fractures, with a well-matched uninfected comparison group. Limitations include the relatively modest sample size, which, although large enough to permit group comparisons between women with and without HIV, is too small to conduct detailed explorations of all determinants of BMD.
In conclusion, low BMD was more common in WWH than in women without HIV. Despite adjusting for BMI and traditional osteoporosis risk factors, HIV infection and menopausal status were each independently associated with lower BMD, and had additive effects on lumbar spine and total hip BMD. However, it is worth noting that, while low BMD was more common among WWH compared with women without HIV, the prevalence of osteoporosis, the most severe form of reduced BMD, did not differ by HIV status. Additional research is needed to better understand mechanisms by which HIV may impact BMD with age and menopausal transition in women, and to develop strategies to mitigate osteoporosis and fracture risk in this growing population.
Notes
Disclaimer. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Financial support. The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). The MSK study was additionally supported by NIAID (grant number AI059884; to M. T. Y.).
Data in this manuscript were collected by 5 sites of the Women’s Interagency HIV Study (WIHS). WIHS (Principal Investigators): Bronx WIHS (Kathryn Anastos and Anjali Sharma) (U01-AI-035004); Brooklyn WIHS (Howard Minkoff and Deborah Gustafson) (U01-AI-031834); Chicago WIHS (Mardge Cohen and Audrey French) (U01-AI-034993); Metropolitan Washington WIHS (Seble Kassaye) (U01-AI-034994); Connie Wofsy Women’s HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien) (U01-AI-034989); WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub) (U01-AI-042590).
Potential conflicts of interest. P. C. T. reports grants or contracts paid to their institution from Merck and Gilead, outside of the submitted work. A. S. has received funding from Gilead Sciences, Inc. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Anjali Sharma, Department of Medicine, Albert Einstein College of Medicine, Bronx, New York, USA.
Donald R Hoover, Department of Statistics, Institute for Health, Health Care Policy and Aging Research, Rutgers University, Piscataway, New Jersey, USA.
Qiuhu Shi, School of Health Sciences and Practice, New York Medical College, Valhalla, New York, USA.
Phyllis C Tien, Department of Medicine, San Francisco VA Medical Center, San Francisco, California, USA; University of California San Francisco, San Francisco, California, USA.
Kathleen M Weber, Cook County Health/Hektoen Institute of Medicine, Chicago, Illinois, USAand.
Jayesh G Shah, Department of Medicine, College of Physicians and Surgeons, Columbia University Medical Center, New York, New York, USA.
Michael T Yin, Department of Medicine, College of Physicians and Surgeons, Columbia University Medical Center, New York, New York, USA.
References
- 1. Rapuri PB, Gallagher JC, Haynatzki G.. Endogenous levels of serum estradiol and sex hormone binding globulin determine bone mineral density, bone remodeling, the rate of bone loss, and response to treatment with estrogen in elderly women. J Clin Endocrinol Metab 2004; 89:4954–62. [DOI] [PubMed] [Google Scholar]
- 2. Cummings SR, Browner WS, Bauer D, et al. ; Study of Osteoporotic Fractures Research Group. . Endogenous hormones and the risk of hip and vertebral fractures among older women. N Engl J Med 1998; 339:733–8. [DOI] [PubMed] [Google Scholar]
- 3. D’Amelio P, Grimaldi A, Di Bella S, et al. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: a key mechanism in osteoporosis. Bone 2008; 43:92–100. [DOI] [PubMed] [Google Scholar]
- 4. Pacifici R. T cells: critical bone regulators in health and disease. Bone 2010; 47:461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weitzmann MN, Pacifici R.. T cells: unexpected players in the bone loss induced by estrogen deficiency and in basal bone homeostasis. Ann N Y Acad Sci 2007; 1116:360–75. [DOI] [PubMed] [Google Scholar]
- 6. Gazzola L, Bellistri GM, Tincati C, et al. Association between peripheral T-lymphocyte activation and impaired bone mineral density in HIV-infected patients. J Transl Med 2013; 11:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Titanji K, Vunnava A, Foster A, et al. T-cell receptor activator of nuclear factor-κB ligand/osteoprotegerin imbalance is associated with HIV-induced bone loss in patients with higher CD4+ T-cell counts. AIDS 2018; 32:885–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yin MT, Lu D, Cremers S, et al. Short-term bone loss in HIV-infected premenopausal women. J Acquir Immune Defic Syndr 2010; 53:202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yin MT, McMahon DJ, Ferris DC, et al. Bone loss in HIV+ postmenopausal women. Presented at: American Society for Bone and Mineral Research 31st Annual Meeting; Denver, Colorado; September 2009. [Google Scholar]
- 10. Yin MT, McMahon DJ, Ferris DC, et al. Low bone mass and high bone turnover in postmenopausal human immunodeficiency virus-infected women. J Clin Endocrinol Metab 2010; 95:620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pacifici R. Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. J Bone Miner Res 1996; 11:1043–51. [DOI] [PubMed] [Google Scholar]
- 12. Adimora AA, Ramirez C, Benning L, et al. Cohort profile: the Women’s Interagency HIV Study (WIHS). Int J Epidemiol 2018; 47:393–394i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ross RD, Sharma A, Shi Q, et al. Circulating sclerostin is associated with bone mineral density independent of HIV-serostatus. Bone Rep 2020; 12:100279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Finkelstein JS, Brockwell SE, Mehta V, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab 2008; 93:861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. Lancet 2002; 359:1929–36. [DOI] [PubMed] [Google Scholar]
- 16. Javed F, Yu W, Thornton J, Colt E.. Effect of fat on measurement of bone mineral density. Int J Body Compos Res 2009; 7:37–40. [PMC free article] [PubMed] [Google Scholar]
- 17. Poole KE, Mayhew PM, Rose CM, et al. Changing structure of the femoral neck across the adult female lifespan. J Bone Miner Res 2010; 25:482–91. [DOI] [PubMed] [Google Scholar]
- 18. Arnsten JH, Freeman R, Howard AA, Floris-Moore M, Santoro N, Schoenbaum EE.. HIV infection and bone mineral density in middle-aged women. Clin Infect Dis 2006; 42:1014–20. [DOI] [PubMed] [Google Scholar]
- 19. Dolan SE, Huang JS, Killilea KM, Sullivan MP, Aliabadi N, Grinspoon S.. Reduced bone density in HIV-infected women. AIDS 2004; 18:475–83. [DOI] [PubMed] [Google Scholar]
- 20. Jones S, Restrepo D, Kasowitz A, et al. Risk factors for decreased bone density and effects of HIV on bone in the elderly. Osteoporos Int 2008; 19:913–8. [DOI] [PubMed] [Google Scholar]
- 21. Greendale GA, Sowers M, Han W, et al. Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: results from the Study of Women’s Health Across the Nation (SWAN). J Bone Miner Res 2012; 27:111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Riggs BL, Melton LJ 3rd, Robb RA, et al. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res 2004; 19:1945–54. [DOI] [PubMed] [Google Scholar]
- 23. Bolland MJ, Grey AB, Gamble GD, Reid IR.. CLINICAL review: low body weight mediates the relationship between HIV infection and low bone mineral density: a meta-analysis. J Clin Endocrinol Metab 2007; 92:4522–8. [DOI] [PubMed] [Google Scholar]
- 24. Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology 2001; 142:5050–5. [DOI] [PubMed] [Google Scholar]
- 25. Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998; 93:165–76. [DOI] [PubMed] [Google Scholar]