Abstract
Background
Takeda’s live attenuated tetravalent dengue vaccine candidate (TAK-003) is under evaluation in a long-term clinical trial across 8 dengue-endemic countries. Previously, we have reported its efficacy and safety in both seronegative and seropositive participants and that its performance varies by serotype, with some decline in efficacy from first to second year postvaccination. This exploratory analysis provides an update with cumulative and third-year data.
Methods
Healthy 4–16 year olds (n = 20099) were randomized 2:1 to receive TAK-003 or placebo (0, 3 month schedule). The protocol included baseline serostatus testing of all participants and detection of all symptomatic dengue throughout the trial with a serotype specific reverse transcriptase-polymerase chain reaction.
Results
Cumulative efficacy after 3 years was 62.0% (95% confidence interval, 56.6–66.7) against virologically confirmed dengue (VCD) and 83.6% (76.8–88.4) against hospitalized VCD. Efficacy was 54.3% (41.9–64.1) against VCD and 77.1% (58.6–87.3) against hospitalized VCD in baseline seronegatives, and 65.0% (58.9–70.1) against VCD and 86.0% (78.4–91.0) against hospitalized VCD in baseline seropositives. Efficacy against VCD during the third year declined to 44.7% (32.5–54.7), whereas efficacy against hospitalized VCD was sustained at 70.8% (49.6–83.0). Rates of serious adverse events were 2.9% in TAK-003 group and 3.5% in placebo group during the ongoing long-term follow-up (ie, second half of the 3 years following vaccination), but none were related. No important safety risks were identified.
Conclusions
TAK-003 was efficacious against symptomatic dengue over 3 years. Efficacy declined over time but remained robust against hospitalized dengue. A booster dose evaluation is planned.
Keywords: children, dengue, efficacy, safety, vaccine
This ongoing phase 3 study demonstrated that TAK-003 was efficacious against symptomatic dengue in children and adolescents in a varied epidemiological setting across 8 dengue-endemic countries over 3 years postvaccination, and supports the utility of TAK-003 in dengue control.
Vaccination is one of the major public health developments of the past century and has greatly reduced the burden of many infectious diseases [1]. Dengue, a mosquito-borne viral infection, leads to millions of symptomatic cases annually and has major impacts on public health and economies [2]. Dengue vaccine development has been extremely challenging with the complexities of formulating a vaccine against 4 serotypes (DENV-1, DENV-2, DENV-3, and DENV-4) and evaluating it in large field studies over multiple years to demonstrate durable protection and assess any increased risk of dengue or severe dengue in vaccinated vs unvaccinated individuals following a wild-type infection (ie, disease enhancement). Unlike many vaccine-preventable diseases, there is no immune correlate of protection (CoP) for dengue [3]. The past decade saw progress in the field of dengue vaccine development with the licensure of Dengvaxia (Sanofi Pasteur). However, that vaccine was associated with increased risk of severe or hospitalized disease in dengue-naïve recipients, necessitating prevaccination screening to vaccinate only those with evidence of a past infection [4]. There remains an unmet need for a dengue vaccine that can be administered without prevaccination screening and is beneficial regardless of dengue serostatus.
Takeda’s tetravalent dengue vaccine (TAK-003), a live attenuated vaccine based on a DENV-2 backbone [5], is the most advanced among the candidate dengue vaccines under development. It has been assessed in a number of clinical trials since 2010 [6-15] and is currently under evaluation in a multiyear multicountry efficacy study in 4–16 year olds (NCT02747927). The study met the primary endpoint assessed a year after completion of 2 doses given at months 0 and 3, with an overall vaccine efficacy (VE) against virologically confirmed dengue (VCD) of 80.2% (95% confidence interval [95% CI], 73.3–85.3), and the secondary efficacy endpoints assessed over 18 months with VE of 76.1% (68.5–81.9) in baseline seropositives, 66.2% (49.1–77.5) in baseline seronegatives, and 90.4% (82.6–94.7) against hospitalized VCD [16, 17]. Vaccine performance was variable against individual serotypes and exploratory analysis suggested a lack of efficacy against DENV-3 in baseline seronegatives, warranting continued monitoring over a longer term [16]. After 2 years of follow-up, some decline in efficacy was observed, with the largest decline in the youngest age group (4–5 years of age) [18].
In this report, we present the exploratory long-term VE, safety, and immunogenicity 3 years after completing vaccination.
METHODS
Study Design and Participants
This phase 3, double-blind, placebo-controlled, randomized clinical study of TAK-003 vs placebo in healthy 4–16 year olds is being performed across 8 countries. Full details of the study design and participant inclusion and exclusion criteria have been published previously [16-18]. The study is being conducted in accordance with the Declaration of Helsinki and the principles of Good Clinical Practice. Written informed assent/consent was obtained from participants and/or their parents or legal guardians before enrollment in the study. The protocol, its amendments, and informed consent and assent forms were approved by local ethics committees or institutional review boards, as appropriate, before study commencement.
Study Procedures
Eligible participants were randomized 2:1 to receive 2 doses of TAK-003 or placebo at month 0 and month 3, with randomization stratified by region and age group (see [17] for full details). This ongoing study has approximately 4–4.5 years of follow-up after the second dose (1 year in part 1, 6 months in part 2, and 2.5–3 years in part 3) for individual participants, and another 25 months of follow-up is planned for those participating in the booster evaluation phase. All participants were sampled at baseline to assess baseline serostatus, and at month 4 to aid in the evaluation of a correlate of protection, respectively using a microneutralization test (MNT). There are weekly contacts to enable robust identification of symptomatic dengue (both hospitalized and nonhospitalized) throughout the trial. Cases are confirmed by serotype-specific reverse transcriptase-polymerase chain reaction. In view of the diverse etiology of febrile illnesses and practical considerations, hospitalization and case management are as per local practices. Further details of the febrile surveillance methodology are provided in the supplementary materials.
In a randomly selected subset of 4000 participants, MNT blood samples were assessed at months 1, 3, 9, and 15, and then annually for immunogenicity assessments. Serious adverse events (SAEs) are being collected throughout the trial.
Outcomes
This analysis reports the cumulative VE up to approximately 39 months after the first dose (3 years after the second dose), together with VE in the third year after the second dose (ie, year 3), safety from month 19 to month 36 after the second dose, and persistence of immunogenicity. In a related analysis, MNT titers at months 4 and 9 in baseline seronegative participants were compared with those in seronegative adults from a separate study conducted in the United States (NCT03423173) (see supplementary materials).
Statistical Analysis
VE was defined as 1 − (λV/λC), where λV and λC denote the hazard rates for the TAK-003 and placebo groups, respectively, and was expressed as a percentage. Hazard ratios (λV/λC) and corresponding 95% CIs were estimated using a Cox proportional hazard model with treatment group as a factor, adjusted for age, and stratified by region. Further details of the analysis sets and exploratory analysis are provided in the supplementary materials.
Additional details of study design, outcomes, statistical methodology, study procedures, and safety reporting are available in previously published reports [16-18].
RESULTS
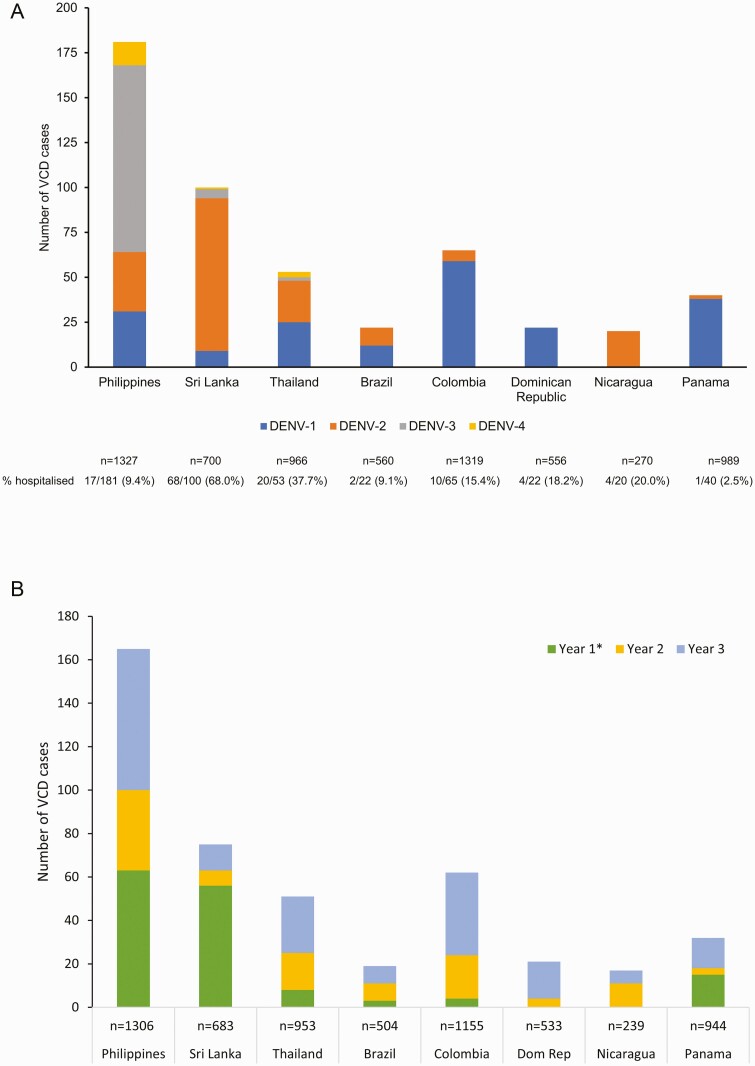
A total of 20071 of 20099 randomized participants received the first dose of TAK-003 or placebo between September 2016 and March 2017, and 94.6% completed 3 years of follow-up after the second dose (supplementary Figure 1). Cumulatively, 23693 febrile illnesses were reported and 895 cases of VCD were identified. In the placebo group, all 4 serotypes were identified in Asia, whereas only DENV-1 or DENV-2 were identified in Latin America (Figure 1A). At the study level, DENV-1 was the most common (39%) and DENV-4 the least common (3.4%) serotype. In the placebo group, hospitalization rates were 16.3% (32/196) for DENV-1, 41.9% (75/179) for DENV-2, 14.4% (16/111) for DENV-3, and 17.6% (3/17) for DENV-4. Hospitalization rates also varied among the trial countries (placebo group data: Latin America, from 2.5% [1/40] in Panama to 20% [4/20] in Nicaragua; Asia, from 9.4% [17/181] in the Philippines to 68% in Sri Lanka [68/100]). In a year-by-year comparison, the highest number of VCD cases in the placebo group were reported during year 3 (Figure 1B).
Figure 1.
(A) Number of virologically confirmed dengue (VCD) cases by serotype and total number of hospitalized VCD cases in the placebo group occurring from the first dose to 3 years after the second dose (approximately month 39 after first dose; safety set data) and (B) the number of VCD cases in the placebo group in each year of the study after completing vaccination by country (per protocol set data).
∗Thirty days after second dose to end of first year after second dose. (Dom Rep: Dominican Republic).
Cumulative VE from First Dose to 3 Years After the Second Dose, by Serostatus, Age, and Region (Safety Set Data)
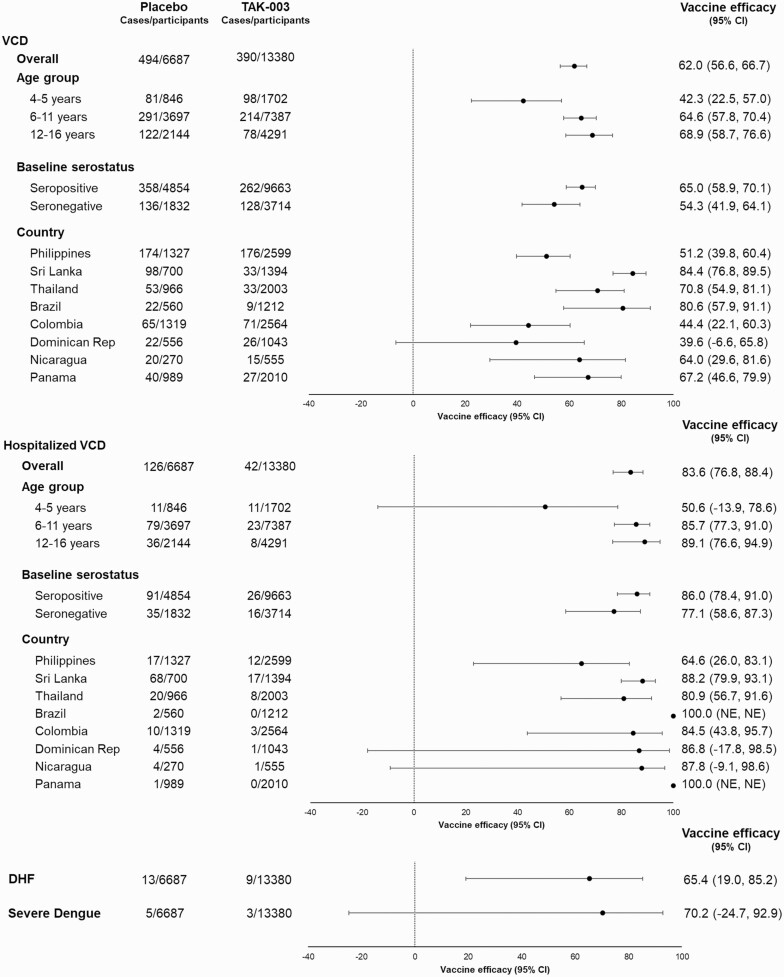
Cumulative VE of TAK-003 was 62.0% (95% CI, 56.6–66.7) against VCD, and 83.6% (76.8–88.4) against hospitalized VCD (Table 1). In baseline seropositives, TAK-003 had a VE against VCD of 52.3–83.4 across the 4 serotypes. For the first time, a positive lower bound of the 95% CI (16.0–81.6) was observed, with a VE of 60.7% against DENV-4 in baseline seropositives. In baseline seronegatives, TAK-003 was efficacious against DENV-1 (VE 43.5%; 21.5–59.3) and DENV-2 (91.9%; 83.6–96.0), but no efficacy was observed against DENV-3 (−23.4%; −125.3 to 32.4). Only 10 DENV-4 cases were reported in baseline seronegatives, most of which occurred in the latter part of the study, precluding a robust interpretation.
Table 1.
Vaccine efficacy (95% CI) of TAK-003 in preventing VCD and hospitalized VCD from the first dose to 3 years after the second dose (approximately month 39 after first dose; safety set data) by baseline serostatus
| Placebo (n = 6687) | TAK-003 (n = 13380) | Efficacy % (95% CI) | |
|---|---|---|---|
| VCD | |||
| Overall | 494/6687 (2.4) | 390/13380 (0.9) | 62.0 (56.6–66.7) |
| Seropositive | 358/4854 (2.4) | 262/9663 (0.9) | 65.0 (58.9–70.1) |
| DENV-1 | 130/4854 (0.9) | 114/9663 (0.4) | 56.2 (43.7–66.0) |
| DENV-2 | 124/4854 (0.8) | 42/9663 (0.1) | 83.4 (76.4–88.3) |
| DENV-3 | 95/4854 (0.6) | 94/9663 (0.3) | 52.3 (36.6–64.2) |
| DENV-4 | 15/4854 (<0.1) | 12/9663 (<0.1) | 60.7 (16.0–81.6) |
| Seronegative | 136/1832 (2.4) | 128/3714 (1.1) | 54.3 (41.9–64.1) |
| DENV-1 | 66/1832 (1.2) | 77/3714 (0.7) | 43.5 (21.5–59.3) |
| DENV-2 | 55/1832 (1.0) | 9/3714 (<0.1) | 91.9 (83.6–96.0) |
| DENV-3a | 15/1832 (0.3) | 36/3714 (0.3) | −23.4 (−125.3 to 32.4) |
| DENV-4 | 2/1832 (<0.1) | 8/3714 (<0.1) | −105.5 (−867.5 to 56.4) |
| Hospitalized VCD | |||
| Overall | 126/6687 (0.6) | 42/13380 (<0.1) | 83.6 (76.8–88.4) |
| Seropositive | 91/4854 (0.6) | 26/9663 (<0.1) | 86.0 (78.4–91.0) |
| DENV-1 | 21/4854 (0.1) | 13/9663 (<0.1) | 69.2 (38.5–84.6) |
| DENV-2 | 53/4854 (0.3) | 5/9663 (<0.1) | 95.3 (88.4–98.1) |
| DENV-3 | 14/4854 (<0.1) | 8/9663 (<0.1) | 72.1 (33.6–88.3) |
| DENV-4 | 3/4854 (<0.1) | 0/9663 (0) | 100.0 (NE–NE) |
| Seronegative | 35/1832 (0.6) | 16/3714 (0.1) | 77.1 (58.6–87.3) |
| DENV-1 | 11/1832 (0.2) | 5/3714 (<0.1) | 77.2 (34.3–92.1) |
| DENV-2 | 22/1832 (0.4) | 0/3714 (0) | 100.0 (NE–NE) |
| DENV-3b | 2/1832 (<0.1) | 11/3714 (<0.1) | −183.4 (−1178.3 to 37.2) |
| DENV-4 | 0/1832 (0) | 0/3714 (0) | NE (NE–NE) |
Data under the placebo and TAK-003 groups are presented as number of VCD or hospitalized VCD/number of evaluable participants (number of VCD cases per 100 person-years at risk).
Participants were classified as seronegative when testing seronegative for all dengue serotypes at baseline. Participants were classified as seropositive when demonstrating a reciprocal neutralizing antibody titer ≥ 10 against at least 1 dengue serotype at baseline. Cases of severe VCD were determined according to Dengue Case Adjudication Committee (DCAC) criteria. Cases of DHF were determined according to WHO 1997 criteria. Only the first instance of VCD was included in efficacy evaluation. For serotype-specific efficacy calculations, only the first instance of VCD from the individual serotype in question was included, regardless of previous instances of VCD from other serotypes.
Abbreviations: CI, confidence interval; DHF, dengue hemorrhagic fever; NE, non estimable; VCD, virologically confirmed dengue; WHO, World Health Organization.
Forty-three were reported at the sites in the Philippines (29 in the TAK-003 group and 14 in the placebo group). Six cases were reported at a single site in Sri Lanka (all in the TAK-003 group). The remaining 2 cases were reported at the sites in Thailand (1 each in the TAK-003 and placebo groups).
Two of the 11 hospitalized VCD in the TAK-003 group were DCAC-defined severe dengue (0.05% of 3714 participants). Four of the 11 hospitalized VCD (0.11% of 3714 participants) in the TAK-003 group and 1 of the 2 hospitalized VCD (0.05% of the 1832 participants) in the placebo group were classified as DHF as per WHO 1997 criteria. Of note, 1 of these 4 DHF cases in the TAK-003 group was also classified as DCAC-defined severe dengue. There were no DCAC-defined severe dengue or DHF cases caused by other serotypes in baseline seronegative participants.
VE against VCD and hospitalized VCD varied by country (Figure 2) in line with the predominant circulating serotypes and the duration between the cases and vaccination. In all countries, VE against hospitalized VCD was higher than for VCD. Overall VE against dengue hemorrhagic fever (DHF) and Dengue Case Severity Adjudication Committee (DCAC) defined severe dengue (see supplementary materials for details of definition) was 65.4% (19.0–85.2) and 70.2% (−24.7 to 92.9), respectively (Figure 2). Further details of cases are provided in footnotes to Table 1.
Figure 2.
Forest plots of vaccine efficacy of TAK-003 vs placebo in preventing virologically confirmed dengue (VCD), hospitalized VCD, severe dengue, and DHF from the first dose to 3 years after the second dose (approximately month 39 after first dose; safety set data). Only the first instance of VCD was included in efficacy evaluation. Participants were classified as seronegative when testing seronegative for all dengue serotypes at baseline. Participants were classified as seropositive when demonstrating a reciprocal neutralizing antibody titer ≥ 10 against at least 1 dengue serotype at baseline. DHF included cases of virologically confirmed dengue meeting World Health Organization 1997 criteria for dengue hemorrhagic fever in a programmed algorithm to analyze data. DHF cases: Philippines: DENV-3 (n = 5), DENV-4 (n = 1); Sri Lanka: DENV-1 (n = 1), DENV-2 (n = 4), DENV-3 (n = 4); Thailand: DENV-1 (n = 1), DENV-2 (n = 1), DENV-3 (n = 1); Colombia: DENV-1 (n = 3); Nicaragua: DENV-2 (n = 1). Except for 1 case of DHF, all required hospitalization. Cases of severe dengue were determined by the Dengue Case Adjudication Committee (DCAC). Severe dengue cases: Philippines: DENV-3 (n = 6); Nicaragua: DENV-2 (n = 1); Colombia: DENV-1 (n = 1). All cases required hospitalization. One case in the placebo group and 2 cases in the TAK-003 group met criteria for both DCAC-defined severe dengue and DHF. Abbreviations: DHF, dengue hemorrhagic fever; NE, not estimable; VCD, virologically confirmed dengue.
Cumulative VE appeared lower in the youngest age group (Figure 2). However, no consistent age effects were apparent when analyzed by individual age (supplementary Tables 1 and 2).
VE During Year 3, by Serostatus and Age Group (per Protocol set Data)
VE against VCD continued to decline in year 3, as observed in the previous year (ie, year 2) of the study [18] (Table 2, Figure 3, supplementary Table 3), but the extent varied by serotype. Efficacy against hospitalized VCD remained high against all serotypes in baseline seropositives, and against DENV-1 and DENV-2 in baseline seronegatives. The data from year 3 did not suggest an age effect, with conflicting patterns when comparing the 3 age groups by baseline serostatus (Table 2).
Table 2.
Vaccine efficacy (95% CI) of TAK-003 in preventing VCD and hospitalized VCD during year 3 after the second dose (per protocol set data)
| Placebo (N = 6317) | TAK-003 (N = 12704) | Efficacy % (95% CI) | ||
|---|---|---|---|---|
| VCD | ||||
| Overall | 179/6201 (3.1) | 208/12435 (1.7) | 44.7 (32.5–54.7) | |
| SP | 128/4502 (3.1) | 139/8968 (1.6) | 48.3 (34.2–59.3) | |
| SN | 51/1698 (3.2) | 69/3465 (2.1) | 35.5 (7.3–55.1) | |
| SP | DENV-1 | 69/4502 (1.7) | 77/8968 (0.9) | 45.4 (24.5–60.6) |
| DENV-2 | 34/4502 (0.8) | 20/8968 (0.2) | 72.1 (51.6–84.0) | |
| DENV-3 | 20/4502 (0.5) | 37/8968 (0.4) | 15.2 (−46.1 to 50.8) | |
| DENV-4 | 6/4502 (0.1) | 5/8968 (<0.1) | 61.9 (−24.9 to 88.4) | |
| SN | DENV-1 | 28/1698 (1.8) | 49/3465 (1.5) | 17.2 (−31.8 to 47.9) |
| DENV-2 | 16/1698 (1.0) | 5/3465 (0.1) | 84.9 (58.7–94.5) | |
| DENV-3 | 6/1698 (0.4) | 11/3465 (0.3) | 9.5 (−144.7 to 66.5) | |
| DENV-4 | 1/1698 (<0.1) | 4/3465 (0.1) | −99.0 (−1680.3 to 77.8) | |
| SP | 4–5 y | 16/457 (3.9) | 34/941 (3.8) | 2.5 (−76.6 to 46.2) |
| 6–11 y | 75/2401 (3.4) | 75/4743 (1.6) | 51.9 (33.8–65.1) | |
| 12–16 y | 37/1644 (2.4) | 30/3284 (0.9) | 61.1 (37.1–76.0) | |
| SN | 4–5 y | 16/331 (5.2) | 17/652(2.8) | 47.4 (−4.3 to 73.4) |
| 6–11 y | 27/1051 (2.8) | 40/2165 (1.9) | 30.0 (−14.0 to 57.1) | |
| 12–16 y | 8/316 (2.7) | 12/648 (1.9) | 29.0 (−73.6 to 71.0) | |
| Hospitalized VCD | ||||
| Overall | 34/6201 (0.6) | 21/12435 (0.2) | 70.8 (49.6–83.0) | |
| SP | 26/4502 (0.6) | 12/8968 (0.1) | 78.4 (57.1–89.1) | |
| SN | 8/1698 (0.5) | 9/3465 (0.3) | 45.0 (−42.6 to 78.8) | |
| SP | DENV-1 | 10/4502 (0.2) | 6/8968 (<0.1) | 71.6 (21.7–89.7) |
| DENV-2 | 9/4502 (0.2) | 2/8968 (<0.1) | 89.4 (51.1–97.7) | |
| DENV-3 | 6/4502 (0.1) | 4/8968 (<0.1) | 69.6 (−7.9 to 91.4) | |
| DENV-4 | 1/4502 (<0.1) | 0/8968(0) | 100.0 (NE–NE) | |
| SN | DENV-1 | 5/1698 (0.3) | 2/3465 (<0.1) | 80.6 (−0.1 to 96.2) |
| DENV-2 | 2/1698 (0.1) | 0/3465 (0) | 100.0 (NE–NE) | |
| DENV-3 | 1/1698 (<0.1) | 7/3465 (0.2) | −246.6 (−2716.1 to 57.3) | |
| DENV-4 | 0/1698 (0) | 0/3465 (0) | NE | |
| SP | 4–5 y | 1/457 (0.2) | 4/941 (0.4) | −80.7 (−1517.3 to 79.8) |
| 6–11 y | 18/2401 (0.8) | 5/4743 (0.1) | 87.0 (65.0–95.2) | |
| 12–16 y | 7/1644 (0.5) | 3/3284 (<0.1) | 79.9 (22.1–94.8) | |
| SN | 4–5 y | 2/331 (0.6) | 1/652 (0.2) | 73.0 (−197.4 to 97.6) |
| 6–11 y | 5/1051 (0.5) | 7/2165 (0.3) | 28.7 (−124.9 to 77.4) | |
| 12–16 y | 1/316 (0.3) | 1/648 (0.2) | 52.3 (−669.7 to 97.0) | |
Data under the placebo and TAK-003 groups are presented as number of VCD or hospitalized VCD/number of evaluable participants (number of VCD cases per 100 person years at risk). Only the first instance of VCD was included in efficacy evaluation. For serotype-specific efficacy calculations, only the first instance of VCD from the individual serotype in question was included, regardless of previous instances of VCD from other serotypes. Participants were classified as seronegative when testing seronegative for all dengue serotypes at baseline. Participants were classified as seropositive when demonstrating a reciprocal neutralizing antibody titer ≥ 10 against at least one dengue serotype at baseline.
Abbreviations: CI, confidence interval; NE, not estimable; SN, seronegative at baseline; SP, seropositive at baseline; VCD, virologically confirmed dengue.
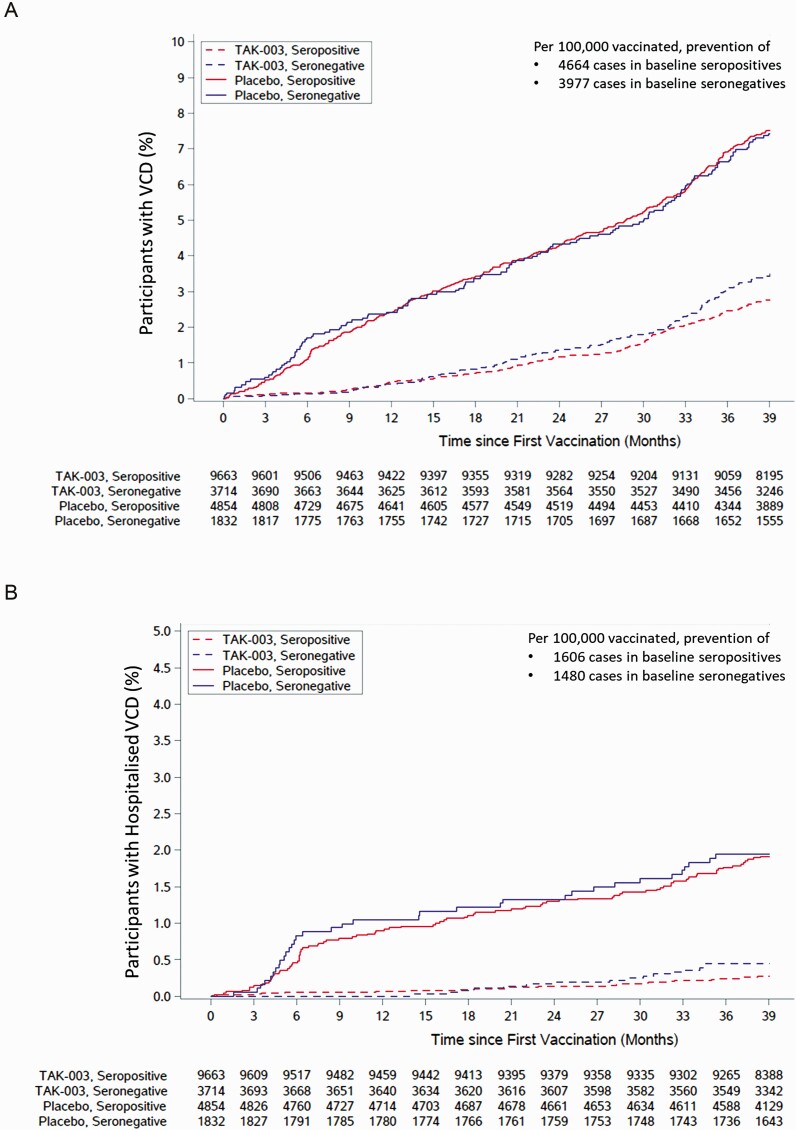
Figure 3.
Cumulative incidence of (A) virologically confirmed dengue (VCD) cases and (B) hospitalized VCD cases, from the first dose to 3 years after the second dose (approximately month 39 after first dose; safety set data). Number of cases prevented per 100000 participants vaccinated is calculated as 100000/number needed to treat (NNT). NNT is calculated as the reciprocal of risk difference. Risk difference is calculated as the number of events divided by the number of participants evaluated in the placebo group, subtracted by the number of events divided by the number of participants evaluated in the TAK-003 group.
VE Against DENV-3 in Baseline Seronegatives
The previously reported lack of efficacy against DENV-3 in baseline seronegatives continued through to year 3 [16-18] (Table 2). In the cumulative period, there were 36 VCD cases in the TAK-003 group and 15 in the placebo group, in line with the 2:1 participant randomization. However, proportionally more TAK-003 recipients were hospitalized (11 TAK-003 recipients vs 2 placebo recipients). In year 3, as in previous years, most of the DENV-3 cases (11/17) occurred in the Philippines, whereas the other 6 occurred at a single Sri Lankan site and all were hospitalized in line with the high hospitalization rate for dengue cases observed locally (Figure 1). See the Discussion section.
Clinical Characteristics of Breakthrough VCD Cases and Dengue Viremia
In the cumulative data, the percentage of VCD with signs of bleeding, plasma leakage, or thrombocytopenia (platelet count ≤ 100 × 109/L) was lower in the TAK-003 group vs the placebo group (supplementary Table 4). This observation was mainly driven by data from baseline seropositives and DENV-1 and DENV-2 cases in baseline seronegatives (supplementary Tables 5 and 6). Numerical imbalance in DENV-3 cases with plasma leakage (7 in TAK-003 vs 1 in placebo groups) and thrombocytopenia (8 in TAK-003 vs 1 in placebo groups) was noted in baseline seronegatives. The cases in Sri Lanka accounted for 4/7 with plasma leakage and 5/8 with thrombocytopenia in the TAK-003 group. Clinical practice differences and analyses including and excluding Sri Lanka data are presented in supplementary Tables 7 and 8, respectively. See the Discussion section.
Levels of viremia were broadly similar or lower in TAK-003 vs placebo groups, (supplementary Table 9). However, the small case numbers in some of the comparisons and variability in the interval between fever onset and sampling for reverse transcriptase-polymerase chain reaction limit robust interpretation.
Safety
Seven deaths were reported (5 in TAK-003 recipients; 2 in placebo recipients) and SAEs were reported by 2.9% of TAK-003 recipients and 3.5% of placebo recipients in the first half of part 3 (Table 3, supplementary Table 10). None of the deaths or SAEs were considered related to the study vaccine. No important safety risks have been identified during the study.
Table 3.
Numbers (%) of participants experiencing serious adverse events during first half of part 3 (approximately months 22–39 after the first dose/months 19–36 after the second dose; safety set data)
| Placebo (n = 6687) | TAK-003 (n = 13380) | |
|---|---|---|
| Any | 234 (3.5%) | 386 (2.9%) |
| Mild | 21 (0.3%) | 48 (0.4%) |
| Moderate | 182 (2.7%) | 291 (2.2%) |
| Severe | 31 (0.5%) | 47 (0.4%) |
| Related to investigational producta | 0 (0) | 0 (0) |
| Related to study procedures | 0 (0) | 0 (0) |
| Leading to withdrawal of investigational product or study discontinuation | 2 (<0.1%) | 5 (<0.1%) |
| Deaths | 2 (<0.1%) | 5 (<0.1%) |
| Related to investigational productb | 0 (0.0%) | 0 (0.0%) |
As assessed by the sponsor or by the investigator (blinded).
Causes of death were adenocarcinoma of colon, road traffic accident, wound by sharp element, traumatic lung injury secondary to drowning, completed suicide, multiple organ dysfunction secondary to suicide attempt, and traumatic brain injury.
Immunogenicity
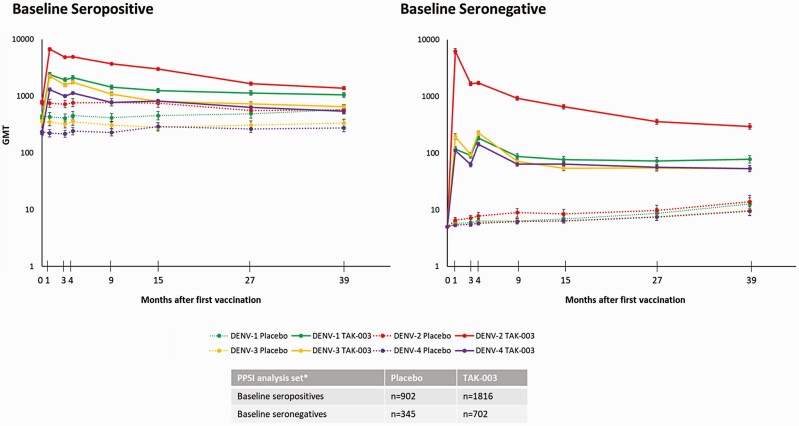
Geometric mean titers (GMTs) in baseline seronegative participants remained broadly similar to those at month 27, as reported previously [18] (Figure 4). At month 39, tetravalent seropositivity was 80.5% in baseline seronegatives and 98.4% in baseline seropositives.
Figure 4.
Serotype-specific geometric mean titers (GMTs; 95% confidence interval) by serostatus at baseline (per protocol set for immunogenicity data). Number of participants evaluated at each timepoint may vary. MNT results were expressed as the reciprocal of the highest dilution of test serum that shows a 50% reduction in plaque counts compared with that of virus controls (MNT50).
Abbreviations: GMT, geometric mean titer; PPSI, per-protocol subset for immunogenicity.
Preliminary analysis did not show a clear threshold of neutralizing antibody titers at month 4 that distinguish participants who experienced VCD (ie, cases) from the controls, although titers were lower in cases (supplementary Figure 2), largely driven by data from baseline seropositive participants. In further exploratory analysis assessing the risk of VCD based on low, medium, and high terciles of titer levels pooled across the 4 serotypes, a trend suggestive of lower risk of VCD with higher antibody titers was observed (supplementary Table 11). Immunobridging analysis with seronegative adults from a separate phase 3 study indicated broadly similar GMTs 1 and 6 months after the second dose for all serotypes (supplementary Table 12).
DISCUSSION
Three to 5 years of postvaccination follow-up is recommended for candidate dengue vaccines to allow evaluation over multiple dengue seasons [19]. In this context, this report marks an important milestone in the clinical development of TAK-003. Two years after vaccination, 3 major questions remained (ie, persistence of efficacy, potential age effect, and performance against DENV-3 and DENV-4 in baseline seronegatives) [18]. We investigated these closely in the year 3 data.
Efficacy declined to a variable extent by serotype but with the encouraging observation that it remained robust against hospitalized dengue. Notably, although waning of efficacy to a variable extent was observed at VCD level against DENV-1 and -2 in baseline seronegatives in a year-by-year comparison, there was not any major change in efficacy estimates against hospitalized VCD nor was there any DHF or DCAC-defined severe case in the TAK-003 group caused by DENV-1 and -2. This indicates that waning of efficacy may not be associated with disease enhancement. Notably, despite varying background rates of hospitalization in trial countries, a consistent pattern of high efficacy was observed against hospitalized VCD.
A booster dose may have the potential to reverse some of the observed waning efficacy. Data from a phase 2 study that evaluated different dosing schedules (single dose, 0- and 3-month, and 0- and 12-month) showed considerable boosting of titers in baseline seronegatives when the second dose was administered at 12 months, whereas the second dose at 3 months largely improved vaccine responders [10]. Although GMTs were similar between schedules in the longer term [14], a potential booster effect on antibody specificity or affinity maturation and its related efficacy is plausible. In the current study, a booster is planned to be administered approximately 4 years after the second dose, which was the earliest operationally feasible time. Subsequently, there will be follow-up over 25 months. Additionally, booster evaluation in nonendemic settings (NCT03999996) is ongoing in participants from 2 earlier conducted studies in the United States (NCT03423173) and Mexico City (NCT03341637).
Previous analysis of data in the 4–5 year age group had shown VE of 46.6% (−12.7 to 74.5) in baseline seropositives and −23.7 (−219.1 to 52.0) in baseline seronegatives during year 2, whereas during year 3 it was 2.5% (−76.6 to 46.2) and 47.4% (−4.3 to 73.4), respectively. These fluctuating estimates emphasize the need for caution in interpreting sub-group analyses. No clear age effect was evident in either the year 3 or cumulative data.
We had hoped that the geographical distribution of DENV-3 cases would be more varied during year 3; however, the majority continued to be reported in the Philippines. Six cases of DENV-3 were identified in baseline seronegative vaccinees in Sri Lanka, where the local practice to hospitalize most dengue cases based on local laboratory (NS1) confirmation of dengue complicated data interpretation and skewed a number of exploratory endpoints. Hospitalizations and case management were as per local guidelines and resource availability, resulting in heterogeneity in hospitalization rates as observed in the placebo arm (eg, 68.0% in Sri Lanka vs 9.4% in the Philippines [cumulative across serotypes]). During year 3, the case distribution by treatment arm contrasted sharply between the Philippines and Sri Lanka (Philippines: 5 [1 hospitalized] in TAK-003 vs 6 [1 hospitalized] in placebo; Sri Lanka: 6 [all hospitalized] in TAK-003 vs 0 in placebo). Cumulatively through year 3, cases remained largely proportionate to the 2:1 randomization ratio (36 in TAK-003 vs 15 in placebo); however, the distribution of hospitalized cases was affected by those identified in Sri Lanka (11 [5 outside Sri Lanka] in TAK-003 vs 2 [both outside Sri Lanka] in placebo). None of these Sri Lankan cases was DCAC-defined severe dengue.
Additionally, dengue hospitalization in Sri Lanka involved periodic ultrasonography for early detection of plasma leakage and close monitoring of platelets counts (supplementary Table 7). This likely led to to detection of plasma leakage or thrombocytopenia in most of the previously mentioned 6 DENV-3 cases. Impact of local clinical practice is also reflected at the trial level with a higher number of DHF detection in Sri Lanka (9/22 DHF cases, 4 in TAK-003, and 5 in placebo). Interestingly, all of the cases that were DCAC-defined severe or met DHF criteria in baseline seronegatives (see Table 1 footnotes) were caused by DENV-3. This is consistent with a meta-analysis that reported DENV-3 from Southeast Asia displaying the greatest proportion of severe cases in primary infection or being highly associated with DHF [20]. Although an imbalance in DHF or severe dengue caused by DENV-3 was observed, the overall number of cases were very small (5 in TAK-003 [3 outside Sri Lanka] vs 1 in placebo [outside Sri Lanka] groups). Because there were 36 VCD cases in the TAK-003 group vs 15 in the placebo group because of the 2:1 randomization, there is a higher likelihood of these infrequent events (DHF or DCAC-defined severe cases) occurring in the TAK-003 group merely by chance assuming no vaccine effect. Overall, the totality of the DENV-3 data in baseline seronegatives most likely represent a lack of efficacy rather than an increased safety risk but warrant continued monitoring for any firm conclusion.
Evaluation of vaccine performance against DENV-4 in baseline seronegatives was limited by the small number of cases, even though the trial is ongoing in 8 countries. Additionally, most of these cases occurred in the latter part when there was indication of waning efficacy against other serotypes, which also precluded robust interpretation.
In line with our previous reports [16-18], there were no important safety risks identified in the first half of the long-term follow-up phase.
A vaccine with a profile of rapid onset of protection [16], efficacy against all 4 serotypes in dengue preexposed, against DENV-1 and -2 in dengue-naïve, and durable protection against hospitalized dengue is a step forward in addressing the current unmet needs in dengue control. Importantly, the net benefit in baseline seronegatives, though driven by protection against DENV-1 and DENV-2, together with no disease enhancement, could eliminate the need for a prescreening test and thereby facilitate community vaccination.
One of the key challenges to dengue vaccine development is the lack of a CoP, which complicates direct extrapolation of efficacy to populations not studied in the efficacy study. In endemic countries, the adult population generally has a lower burden than children or adolescents. Additionally, most adults are preexposed to dengue, which limits efficacy evaluation in a reasonably sized study in dengue-naive adults. Preliminary assessment did not suggest a clear CoP based on the MNT data, although the data indicated an association of increased titers with a lower risk of dengue. Across a number of studies of TAK-003, broadly similar levels of neutralizing antibody titers were elicited in the adult and pediatric populations. Additionally, qualitatively comparable levels of a number of exploratory immunological parameters have been observed in both populations (data on file). These data overall support a reasonable assumption about efficacy in adults.
Incorporation of potential dengue vaccines with other preventive interventions has been identified by the World Health Organization as key in the reduction of dengue-related morbidity and mortality [21]. In addition to reliable data on vaccine characteristics, information on effective vaccination strategies, their likely impact on disease burden, and cost-effectiveness will be required. Hence, in addition to the decade already spent in clinical development, considerable work remains. Ongoing efforts include further immune response profile characterization of TAK-003; CoP estimation; investigating the mechanisms underlying the lack of efficacy to DENV-3 in baseline seronegatives; determinants of efficacy persistence; booster evaluation; and health economics analysis to guide vaccination strategies.
Overall, this ongoing phase 3 study demonstrates that TAK-003 was efficacious against symptomatic dengue in children and adolescents in a varied epidemiological setting across 8 dengue-endemic countries. Efficacy, which was variable by serotype, declined over time but remained durable against hospitalized dengue. These data support the utility of TAK-003 in dengue control.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author Contributions. Investigators were L. R., X. S.-L., H. R., E. L.-M., C. B.-T., L. B., C. S., P. K., L. M. V., D. Y., V. W., F. E., R. D., L. F., P. W., E. D. M., A. D. F., D. G., K. L., and R. V. d. C. Study design was carried out by S. B., M. R., D. W., and A. B. The trial was managed by S. B. and V. T. Data analysis and interpretation was carried out by S. B., M. R., O. Z., V. T., M. L., E. H., I. L., D. W., and A. B. Publication management was by S. B. All authors had full access to the presented data, provided critical input during manuscript preparation, and approved the manuscript for submission.
Acknowledgments. The authors thank: the trial participants and their parents/guardians who agreed to take part in the trial, the data monitoring committee members, the Dengue Case Severity Adjudication Committee members, the Takeda expanded study team, the study team at PPD, and all the trial staff in each of the countries. The authors would also like to acknowledge the contribution of the late Dan Stinchcomb, PhD, and Inviragen Inc. (Fort Collins, Colorado, USA) in the initial developmental work of TAK-003 (Inviragen Inc. was subsequently acquired by Takeda). The authors are grateful to Jenny Engelmoer, PhD (Sula Communications, The Netherlands), for editorial assistance in the preparation of this manuscript.
Financial support. This work was supported by Takeda Vaccines; Takeda Pharmaceuticals International AG.
Potential conflicts of interest. S. B., V. T., M. R., O. Z., M. L., E. H., I. L., D. W., and A.B. were permanent employees of Takeda group of companies at the time of writing this manuscript. D. W. has patents WO2017/179017 and WO2020/051334 (with I. L.) pending. X. S-L., P. K., and L. B. have served as advisory board members for Takeda. X. S.-L. reports a research grant by Takeda given to Cevaxin, where they have been the principal investigator of current study outside of the submitted work. E. L. M. reports grants or contracts from Janssen and GSK Senofi Pasteur outside of the conduct of the study and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Sanofi Pasteur, MSD. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Luis Rivera, Hospital Maternidad Nuestra Senora de Altagracia, Santo Domingo, Dominican Republic.
Shibadas Biswal, Takeda Vaccines, Inc., Boston, Massachusetts, USA.
Xavier Sáez-Llorens, Hospital del Niño Dr. José Renán Esquivel, Sistema Nacional de Investigación at SENACYT, Centro de Vacunación Internacional (Cevaxin), Panama City, Panama.
Humberto Reynales, Centro de Atención e Investigación Médica, CAIMED, Bogotá, Colombia.
Eduardo López-Medina, Centro de Estudios en Infectología Pediátrica, Universidad del Valle and Centro Medico Imbanaco, Cali, Colombia.
Charissa Borja-Tabora, Research Institute For Tropical Medicine, Muntinlupa, Philippines.
Lulu Bravo, University of the Philippines Manila, Ermita, Philippines.
Chukiat Sirivichayakul, Department of Tropical Pediatrics, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand.
Pope Kosalaraksa, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand.
Luis Martinez Vargas, CAIMED, Dominicana, Santo Domingo, Dominican Republic.
Delia Yu, De La Salle Medical and Health Sciences Institute, Dasmariñas, Philippines.
Veerachai Watanaveeradej, Phramongkutklao Hospital, Bangkok, Thailand.
Felix Espinoza, National Autonomous University of Nicaragua, León, Nicaragua.
Reynaldo Dietze, Núcleo de Doenças Infecciosas, Centro de Ciencias da Saude-UFES, Vitória, Brazil.
LakKumar Fernando, Centre for Clinical Management of Dengue & Dengue Haemorrhagic Fever, Negombo General Hospital, Negombo, Sri Lanka.
Pujitha Wickramasinghe, University of Colombo, Colombo, Sri Lanka.
Edson Duarte MoreiraJr, Associação Obras Sociais Irmã Dulce Hospital Santo Antônio and Oswaldo Cruz Foundation, Bahia, Brazil.
Asvini D Fernando, Faculty of Medicine, University of Kelaniya, Colombo, Sri Lanka.
Dulanie Gunasekera, Faculty of Medical Sciences, University of Sri Jayawardenenpura, Colombo, Sri Lanka.
Kleber Luz, Instituto de Medicina Tropical da Universidade Federal do Rio Grande do Norte, Natal, Brazil.
Rivaldo Venâncioda Cunha, Universidade Federal de Mato Grosso do Sul, Campo Grande, Brazil.
Martina Rauscher, Takeda Pharmaceuticals International AG, Zurich, Switzerland.
Olaf Zent, Takeda Pharmaceuticals International AG, Zurich, Switzerland.
Mengya Liu, Takeda Vaccines, Inc., Boston, Massachusetts, USA.
Elaine Hoffman, Takeda Vaccines, Inc., Boston, Massachusetts, USA.
Inge LeFevre, Takeda Pharmaceuticals International AG, Zurich, Switzerland.
Vianney Tricou, Takeda Pharmaceuticals International AG, Zurich, Switzerland.
Derek Wallace, Takeda Vaccines, Inc., Boston, Massachusetts, USA.
MariaTheresa Alera, Philippines-Armed Forces Research Institute of Medical Sciences Virology Research Unit, Cebu City, Philippines.
Astrid Borkowski, Takeda Pharmaceuticals International AG, Zurich, Switzerland.
References
- 1. Andre FE, Booy R, Bock HL, et al. . Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull World Health Organ 2008; 86:140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bhatt S, Gething PW, Brady OJ, et al. . The global distribution and burden of dengue. Nature 2013; 496:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol 2010; 17:1055–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sridhar S, Luedtke A, Langevin E, et al. . Effect of dengue serostatus on dengue vaccine safety and efficacy. N Engl J Med 2018; 379:327–40. [DOI] [PubMed] [Google Scholar]
- 5. Huang CY, Kinney RM, Livengood JA, et al. . Genetic and phenotypic characterization of manufacturing seeds for a tetravalent dengue vaccine (DENVax). PLoS Negl Trop Dis 2013; 7:e2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. George SL, Wong MA, Dube TJ, et al. . Safety and immunogenicity of a live attenuated tetravalent dengue vaccine candidate in flavivirus-naive adults: a randomized, double-blinded phase 1 clinical trial. J Infect Dis 2015; 212:1032–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jackson LA, Rupp R, Papadimitriou A, Wallace D, Raanan M, Moss KJ.. A phase 1 study of safety and immunogenicity following intradermal administration of a tetravalent dengue vaccine candidate. Vaccine 2018; 36:3976–83. [DOI] [PubMed] [Google Scholar]
- 8. Osorio JE, Velez ID, Thomson C, et al. . Safety and immunogenicity of a recombinant live attenuated tetravalent dengue vaccine (DENVax) in flavivirus-naive healthy adults in Colombia: a randomised, placebo-controlled, phase 1 study. Lancet Infect Dis 2014; 14:830–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rupp R, Luckasen GJ, Kirstein JL, et al. . Safety and immunogenicity of different doses and schedules of a live attenuated tetravalent dengue vaccine (TDV) in healthy adults: a Phase 1b randomized study. Vaccine 2015; 33:6351–9. [DOI] [PubMed] [Google Scholar]
- 10. Sáez-Llorens X, Tricou V, Yu D, et al. . Immunogenicity and safety of one versus two doses of tetravalent dengue vaccine in healthy children aged 2-17 years in Asia and Latin America: 18-month interim data from a phase 2, randomised, placebo-controlled study. Lancet Infect Dis 2018; 18:162–70. [DOI] [PubMed] [Google Scholar]
- 11. Sáez-Llorens X, Tricou V, Yu D, et al. . Safety and immunogenicity of one versus two doses of Takeda’s tetravalent dengue vaccine in children in Asia and Latin America: interim results from a phase 2, randomised, placebo-controlled study. Lancet Infect Dis 2017; 17:615–25. [DOI] [PubMed] [Google Scholar]
- 12. Sirivichayakul C, Barranco-Santana EA, Esquilin-Rivera I, et al. . Safety and immunogenicity of a tetravalent dengue vaccine candidate in healthy children and adults in dengue-endemic regions: a randomized, placebo-controlled phase 2 study. J Infect Dis 2016; 213:1562–72. [DOI] [PubMed] [Google Scholar]
- 13. Tricou V, Low JG, Oh HM, et al. . Safety and immunogenicity of a single dose of a tetravalent dengue vaccine with two different serotype-2 potencies in adults in Singapore: a phase 2, double-blind, randomised, controlled trial. Vaccine 2020; 38:1513–9. [DOI] [PubMed] [Google Scholar]
- 14. Tricou V, Sáez-Llorens X, Yu D, et al. . Safety and immunogenicity of a tetravalent dengue vaccine in children aged 2-17 years: a randomised, placebo-controlled, phase 2 trial. Lancet 2020; 395:1434–43. [DOI] [PubMed] [Google Scholar]
- 15. Turner M, Papadimitriou A, Winkle P, et al. . Immunogenicity and safety of lyophilized and liquid dengue tetravalent vaccine candidate formulations in healthy adults: a randomized, phase 2 clinical trial. Hum Vaccin Immunother 2020; 16:2456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Biswal S, Borja-Tabora C, Martinez Vargas L, et al. ; TIDES study group. . Efficacy of a tetravalent dengue vaccine in healthy children aged 4-16 years: a randomised, placebo-controlled, phase 3 trial. Lancet 2020; 395:1423–33. [DOI] [PubMed] [Google Scholar]
- 17. Biswal S, Reynales H, Saez-Llorens X, et al. ; TIDES Study Group. . Efficacy of a tetravalent dengue vaccine in healthy children and adolescents. N Engl J Med 2019; 381:2009–19. [DOI] [PubMed] [Google Scholar]
- 18. López-Medina E, Biswal S, Saez-Llorens X, et al. . Efficacy of a dengue vaccine candidate (TAK-003) in healthy children and adolescents two years after vaccination. J Infect Dis 2022; 225:1521–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vannice KS, Wilder-Smith A, Barrett ADT, et al. . Clinical development and regulatory points for consideration for second-generation live attenuated dengue vaccines. Vaccine 2018; 36:3411–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Soo KM, Khalid B, Ching SM, Chee HY.. Meta-analysis of dengue severity during infection by different dengue virus serotypes in primary and secondary infections. PLoS One 2016; 11:e0154760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Health Organization. Global Strategy for Dengue Prevention and Control 2012-2020. Geneva, Switzerland: WHO Press, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.