Abstract
Background
Although severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infectious virus isolation in outpatients with coronavirus disease 2019 (COVID-19) has been associated with viral RNA levels and symptom duration, little is known about the host, disease, and viral determinants of infectious virus detection.
Methods
COVID-19 adult outpatients were enrolled within 7 days of symptom onset. Clinical symptoms were recorded via patient diary. Nasopharyngeal swabs were collected to quantitate SARS-CoV-2 RNA by reverse transcriptase polymerase chain reaction and for infectious virus isolation in Vero E6-cells. SARS-CoV-2 antibodies were measured in serum using a validated ELISA assay.
Results
Among 204 participants with mild-to-moderate symptomatic COVID-19, the median nasopharyngeal viral RNA was 6.5 (interquartile range [IQR] 4.7–7.6 log10 copies/mL), and 26% had detectable SARS-CoV-2 antibodies (immunoglobulin (Ig)A, IgM, IgG, and/or total Ig) at baseline. Infectious virus was recovered in 7% of participants with SARS-CoV-2 antibodies compared to 58% of participants without antibodies (prevalence ratio [PR] = 0.12, 95% confidence interval [CI]: .04, .36; P = .00016). Infectious virus isolation was also associated with higher levels of viral RNA (mean RNA difference +2.6 log10, 95% CI: 2.2, 3.0; P < .0001) and fewer days since symptom onset (PR = 0.79, 95% CI: .71, .88 per day; P < .0001).
Conclusions
The presence of SARS-CoV-2 antibodies is strongly associated with clearance of infectious virus. Seropositivity and viral RNA levels are likely more reliable markers of infectious virus clearance than subjective measure of COVID-19 symptom duration. Virus-targeted treatment and prevention strategies should be administered as early as possible and ideally before seroconversion.
Clinical Trials Registration
Keywords: COVID-19, infectious virus, outpatient, SARS-CoV-2, serostatus
Among COVID-19 outpatients within 7 days of symptom onset, the presence of SARS-CoV-2-specific antibodies was strongly associated with clearance of infectious virus. Seropositivity appears to be more reliable marker of infectious virus clearance than patient-reported COVID-19 symptoms.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the etiological agent of coronavirus disease 2019 (COVID-19), has caused more than 240 million infections and 4.9 million deaths worldwide [1, 2]. The US Centers for Disease Control and Prevention (CDC) recommends physical isolation of 10 days for individuals with mild-to-moderate COVID-19 and 20 days for individuals with severe disease [3]. However, infectious SARS-CoV-2 virus isolation has been reported beyond 20 days from patients with a compromised immune system [4–9]. Although prior studies have demonstrated an association between infectious virus isolation in the upper airway and high levels of nasal viral RNA measured by quantitative reverse transcription polymerase chain reaction (qRT-PCR) [10–14], limited data are available regarding the host and disease factors associated with the presence of infectious virus in persons with mild-to-moderate COVID-19.
An improved understanding of viral and host factors associated with shedding of infectious virus is essential to prevent transmission of SARS-CoV-2 and reliably evaluate the antiviral efficacy of novel therapies. Here we provide a comprehensive analysis of demographic, immunologic, virologic, and clinical disease factors associated with infectious virus isolation and levels of viral RNA in nasopharyngeal swab samples in the largest study of symptomatic outpatient adults with COVID-19.
METHODS
Study Design
This cross-sectional analysis was conducted at study entry among all participants who enrolled in a Phase IIa clinical trial of the oral antiviral agent molnupiravir [15, 16]. Adult outpatients with mild-to-moderate COVID-19 were enrolled at ten sites in the United States. The study protocol was approved by Western IRB (WIRB). All study participants provided written informed consent. Participants were eligible to enroll if they were ≥18 years of age, had SARS-CoV-2 infection with symptom onset within the past 7 days and were experiencing symptoms of COVID-19. Active SARS-CoV-2 infection was confirmed by molecular testing from a sample collected within 96 hours prior to enrollment. A complete list of eligibility criteria is available at clinicaltrials.gov (NCT04405570).
Measurement of SARS-CoV-2 RNA, Infectious Virus, and Antibodies
Nasopharyngeal (NP) swabs collected from each participant at enrollment were placed into virus transport medium (VTM; supplied by COPAN), stored at 5–8˚C and shipped to central laboratories within 5 days of collection, and stored at –80˚C until tested. Two NP swabs were collected (1 from each nostril from all but 31 participants where a single NP swab was collected) and VTM aliquoted for testing. SARS-CoV-2 RNA was measured using a qRT-PCR assay on NP swab VTM that targets the N1 region [17]; this assay was performed by Covance (Labcorp) with samples run in triplicate and the average cycle threshold value was interpolated from a standard curve (lower limit of quantification: 1018 copies/mL).
To measure the presence of infectious virus in each NP swab, samples were cultured in Vero E6 cells similar to prior studies [10]. This work was performed in a Biosafety Level 3 facility with operating and safety procedures approved by the UNC Department of Environmental Health/CDC. Briefly, VTM samples were thawed, diluted 1:1 in infection medium and added in duplicate to Vero cell cultures plated 24 hours prior. Quadruplicate negative (infection medium only) high (500 particle forming units [PFU]) and low (50 PFU) positive controls were included. On 2 and 5 days postinoculation (dpi), culture positivity was determined by measuring viral RNA in heat-inactivated culture medium by qRT-PCR using the Abbott m2000sp/rt quantitative SARS-CoV-2 RNA assay (limit of quantification: 100 copies/mL) [18]. The absolute limit of culture detection was 50 PFU (MOI 0.0008) using two criteria for positivity: (i) SARS-CoV-2 RNA copies >1000/mL in supernatant at 2 dpi, or (ii) a relative change of >5 in RNA copy number from 2 to 5 dpi (ie, [copies_5 dpi/copies_2 dpi] > 5) (Supplementary Figure 1). Additional laboratory methods are provided online in Supplementary Methods.
SARS-CoV-2-specific antibodies for the Spike protein in serum were analyzed using an antigen-capture enzyme-linked immunosorbent assay [19]. Based on reference panel performance, the following optical density thresholds for seropositivity were applied: total immunoglobulin (Ig) > 0.376, IgG > 0.376, IgA > 0.30, IgM > 0.31. SARS-CoV-2 antibody positive status was determined by a positive result on at least one of the following: total Ig, IgG, IgM, or IgA.
Covariates
Participant demographics and disease characteristics were collected at baseline (study entry or screening), including medical history, hematology, and chemistry laboratory tests. Obesity was defined as body mass index (BMI) ≥ 30 kg/m2. Days since symptom onset was measured by participant report; and COVID-19 symptom severity was assessed using a 4-point scale (absent, mild, moderate, or severe) in the participant-reported diary. Sixteen COVID-related symptoms were collected, as well as overall severity. D-dimer and C-reactive protein (CRP) were measured at local laboratories. D-dimer was dichotomized as <0.5 (within or near normal range) versus ≥0.5 mg/L and CRP was dichotomized as <10 versus ≥10 mg/L.
Data Analyses
Associations between infectious virus isolation and each characteristic were estimated with a prevalence ratio (PR) and corresponding 95% CI from a modified Poisson model for bivariate and multivariable analyses [20]. The following host characteristics were evaluated for associations with infectious virus isolation: presence of antibodies, symptom duration, age, sex, ethnicity (Hispanic versus non-Hispanic), BMI and obesity, frequent medical conditions known to be associated with worse COVID-19 illness (diabetes, hypertension, and asthma), overall symptom severity, and 5 COVID-19 respiratory-related symptoms (cough, shortness of breath, sore throat, nasal obstruction, nasal discharge). Race was not evaluated because few participants self-identified as Black or Asian. Enrollment of Latinx participants varied considerably by site, and post hoc sensitivity analyses accounting for site were conducted using a Mantel-Haenszel pooled PR. The same statistical approach was used to estimate PRs for associations of each characteristic with seropositive antibody status.
Furthermore, mean difference in SARS-CoV-2 RNA levels (log10 copies/mL) was estimated for each host characteristic listed above using a general linear model with heteroscedasticity-consistent standard errors. A receiver operator curve (ROC) analysis was applied to identify a SARS-CoV-2 RNA cutpoint for infectious virus detection that minimized the Euclidean distance between the ROC curve and the (0.1) point in the ROC plane, to maximize sensitivity and specificity. Wilcoxon rank-sum tests were used to compare hematology measures by infectious virus status, and by antibody status. A χ2 test was used to compare inflammatory markers (D-dimer, CRP) by infectious virus status, and by antibody status.
Missing data were excluded and typically were due to specimen cold-chain shipping abnormalities. 95% confidence intervals (CIs) and P-values are presented with no adjustment for multiplicity. Analyses were conducted in Windows SAS version 9.4 (Cary, North Carolina, USA) and Windows R version 4.0.2 or 4.0.4.
RESULTS
Patient Population
Between 19 June 2020 and 22 January 2021, 240 outpatients were screened and 204 participants were enrolled. Enrollment visits occurred a median of 5 (IQR 4–5) days after symptom onset. Median age was 40 (IQR 27–52) years, and 51% were women; 47% were White non-Hispanic, 42% Hispanic/Latinx, 5% Black non-Hispanic, and 3% Asian non-Hispanic (Table 1). Symptom severity was rated overall as mild (48%), moderate (45%), or severe (7%). The most frequent symptoms, regardless of severity, were fatigue (80%), cough (77%), stuffy nose (74%), headache (71%), muscle aches (64%), loss of smell or taste (54% each), and sore throat (50%) (Table 2).
Table 1.
Baseline Characteristics of Outpatients With Mild-to-Moderate COVID-19 (n = 204)
| Characteristic | Overall |
|---|---|
| Sex | |
| Female | 105 (51%) |
| Male | 99 (49%) |
| Age, y | |
| Median (Q1, Q3) | 40 (27, 52) |
| Min, Max | 18, 82 |
| Race-ethnicitya | |
| Hispanic or Latinx, regardless of race | 86/203 (42%) |
| 2 or more races, non-Hispanic | 3/203 (1%) |
| Asian, non-Hispanic | 6/203 (3%) |
| Black, non-Hispanic | 11/203 (5%) |
| White, non-Hispanic | 96/203 (47%) |
| Other, non-Hispanic | 1/203 (<1%) |
| Days since COVID-19 symptom onset | |
| Median (Q1, Q3) | 5 (4, 5) |
| Mean (SD) | 4.5 (1.4) |
| Min, Max | 0, 7 |
| WHO ordinal scaleb | |
| Ambulatory, limitation of activities | 151/194 (78%) |
| Ambulatory, no limitation of activities | 43/194 (22%) |
| Body mass index,a kg/m2 | |
| Median (Q1, Q3) | 27 (24, 31) |
| Min, Max | 18, 55 |
| Obesity: BMI ≥30 | 57/203 (28%) |
| Frequent medical diagnoses | |
| Hypertension | 28/204 (14%) |
| Diabetes | 20/204 (10%) |
| Asthma | 19/204 (9%) |
| Smoking/vaping statusa,c | |
| Current | 30/203 (15%) |
| Former | 34/203 (17%) |
| Never | 139/203 (68%) |
| Viral RNA from NP swab,b log10 copies/mL | |
| Median (Q1, Q3) | 6.51 (4.68, 7.61) |
| Mean (SD) | 6.19 (1.84) |
| Min, Max | BLQ-9.93 |
| Below limit of quantification (<1018 copies/mL) | 15/195 (8%) |
| Infectious virus from NP swabb | |
| Positive, PCR(+) | 78/175 (45%) |
| Negative, PCR(−) | 97/175 (55%) |
| SARS CoV-2 antibodiesb | |
| Seropositive on any: total Ig, IgM, IgG, or IgA | 46/177 (26%) |
| IgM(+) | 40/177 (23%) |
| IgG(+) | 36/177 (20%) |
| IgA(+) | 14/177 (8%) |
| D-dimer,b mg/L FEU | |
| Median (Q1, Q3) | 0.28 (0.20, 0.44) |
| Min, Max | 0.15, 4.43 |
| <0.5 mg/L FEU (normal) | 146/183 (80%) |
| C-reactive protein,b mg/L | |
| Median (Q1, Q3) | 3.0 (1.0, 8.0) |
| Min, Max | 0.3, 262.0 |
| <10 mg/L (normal) | 147/190 (77%) |
| White blood cell count, × 109/L | |
| Median (Q1, Q3) | 4.4 (3.7, 5.6) |
| Min, Max | 1.4, 18.3 |
| Absolute lymphocyte count,b × 109/L | |
| Median (Q1, Q3) | 1.4 (1.2, 1.9) |
| Min, Max | 0.5, 3.6 |
| Absolute neutrophil count,b × 109/L | |
| Median (Q1, Q3) | 2.4 (1.8, 3.3) |
| Min, Max | 0.4, 16.8 |
| Absolute monocyte count,b × 109/L | |
| Median (Q1, Q3) | 0.40 (0.30, 0.50) |
| Min, Max | 0.04, 1.10 |
| Hemoglobin, g/dL | |
| Median (Q1, Q3) | 14.3 (13.4, 15.3) |
| Min, Max | 8.1, 17.7 |
Abbreviations: BMI, body mass index; BLQ, below the limit of quantification; Ig, immunoglobulin; Max, maximum; Min, minimum; NP, nasopharyngeal; PCR, polymerase chain reaction; Q1, 25th percentile; Q3, 75th percentile; SARS, severe acute respiratory syndrome; SD, standard deviation; US, United States; WHO, World Health Organization.
n = 1 missing.
Missing data: WHO ordinal scale (n = 10), viral RNA (n = 9), infectious virus (n = 29), antibodies (n = 27), D-dimer (n = 21), C-reactive protein (n = 14), absolute lymphocyte count (n = 34), absolute neutrophil count (n = 34), absolute monocyte count (n = 35).
Smoking/vaping status is a composite of cigarette, marijuana, vaping, and cigar use.
Table 2.
Symptom Severity Among Outpatients With Mild-to-Moderate COVID-19 a
| Severity,b n (row %) | ||||
|---|---|---|---|---|
| Symptom type | Any Severity | Mild | Moderate | Severe |
| Respiratory symptoms (n = 202) | ||||
| Cough | 155 (77%) | 95 (47%) | 48 (24%) | 12 (6%) |
| Nasal obstruction (stuffy nose) | 149 (74%) | 84 (42%) | 53 (26%) | 12 (6%) |
| Sore throat | 101 (50%) | 69 (34%) | 28 (14%) | 4 (2%) |
| Nasal discharge (runny nose) | 91 (45%) | 64 (32%) | 22 (11%) | 5 (2%) |
| Shortness of breath | 83 (41%) | 56 (28%) | 22 (11%) | 5 (2%) |
| Other symptoms (n = 202) | ||||
| Fatigue | 162 (80%) | 73 (36%) | 59 (29%) | 30 (15%) |
| Headache | 144 (71%) | 70 (35%) | 52 (26%) | 22 (11%) |
| Muscle aches | 130 (64%) | 62 (31%) | 54 (27%) | 14 (7%) |
| Loss of smell | 109 (54%) | 23 (11%) | 34 (17%) | 52 (26%) |
| Loss of taste | 109 (54%) | 28 (14%) | 35 (17%) | 46 (23%) |
| Chills | 83 (41%) | 54 (27%) | 20 (10%) | 9 (4%) |
| Feeling feverish | 82 (41%) | 54 (27%) | 22 (11%) | 6 (3%) |
| Diarrhea | 64 (32%) | 43 (21%) | 18 (9%) | 3 (1%) |
| Nausea | 57 (28%) | 34 (17%) | 16 (8%) | 7 (3%) |
| Fainting | 15 (7%) | 13 (6%) | 1 (<1%) | 1 (<1%) |
| Vomiting | 14 (7%) | 6 (3%) | 6 (3%) | 2 (1%) |
| Overall symptom severity (n = 197) | 95 (48%) | 89 (45%) | 13 (7%) | |
The “Any Severity” column is a sum of mild, moderate, or severe (ie, regardless of severity level).
Abbreviation: COVID-19, coronavirus disease 2019.
n = 2 participants were missing a symptom diary, and n = 5 additional participants did not report overall symptom severity.
Participants ranked each reported symptom as absent, mild, moderate, or severe using a symptom diary.
Baseline laboratory testing was notable for a median lymphocyte count of 1.4 × 109/L (IQR 1.2–1.9). Most participants had baseline D-dimer and CRP levels within or near the normal range with 80% below 0.5 mg/L and 77% below 10 mg/L, respectively (Table 1). Baseline median SARS-CoV-2 viral RNA from NP swabs was 6.5 (IQR 4.7–7.6) log10 copies/mL overall (Table 1) and was highest within 3 days after symptom onset (Supplementary Figure 2).
SARS-CoV-2 Antibodies, Virus Isolation, and Viral RNA
Of 177 patients with evaluable measurements, 26% were antibody positive at baseline for at least one SARS-CoV-2 spike protein-specific immunoglobulin isotype, with 20% IgG+, 23% IgM+, and 8% IgA+ (Table 1). Three participants were IgG(+) with IgM(−) and IgA(−), and 1 was IgA+ only (Supplementary Table 1). In bivariate analyses, having SARS-CoV-2 specific antibodies was associated with longer time since symptom onset (prevalence ratio [PR] = 1.27 per day, 95% CI: 1.06, 1.52, P = .0091) and higher white blood cell counts, including total white blood cells and absolute neutrophil count (Supplementary Figure 3). Participants with SARS-CoV-2 antibodies were also more likely to have elevated D-dimer and CRP levels as compared to seronegative participants (D-dimer ≥ 0.5 mg/L: 39% versus 13%, P = .00037; CRP ≥ 10 mg/L: 44% versus 15%, P = .00011; Supplementary Table 2).
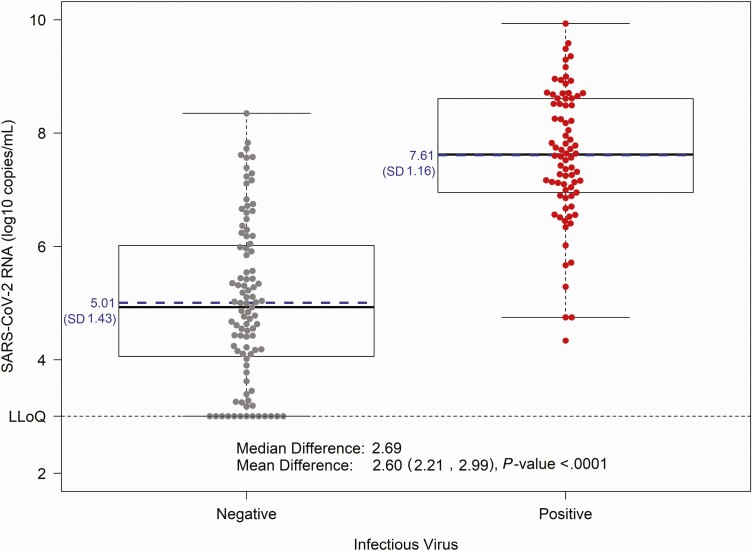
Infectious virus was isolated from 45% (78/175) of participants at baseline. Mean viral RNA levels were higher among participants with infectious virus isolation compared to those who were culture negative (mean: 7.6 versus 5.0 log10 copies/mL; mean difference [MD] = 2.6, 95% CI: 2.2, 3.0, P < .0001; Figure 1). An ROC curve analysis revealed that a viral RNA threshold of ≥6.4 log10 copies/mL was predictive of infectious virus isolation (Supplementary Figure 4), with an estimated positive predictive value of 80% and negative predictive value of 91% (Supplementary Table 3). Patients presenting later after symptom onset had lower prevalence of infectious virus isolation (PR = 0.79 per day, 95% CI: .71, .88) and lower viral RNA levels (MD = –0.5 log10 per day, 95% CI: –.6, –.3); however, antibody status played a critical role.
Figure 1.
SARS-CoV-2 viral RNA levels in nasopharyngeal swab by infectious virus status. Nasopharyngeal viral RNA levels measured via qRT-PCR are displayed by infectious virus status, with culture negative in gray (n = 95) and culture positive (n = 78) in red; each dot represents a participant. Solid lines on the boxplots display the median and 25th–75th percentile (mean = blue dashed line) and the whiskers extend to the extrema (no more than 1.5 times the IQR from the box). Abbreviations: IQR, interquartile range; LLoQ, lower limit of quantification; qRT-PCR, quantitative reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.
Infectious virus was isolated from 7% (3/43) of seropositive compared to 58% (73/125) of seronegative participants, consistent with an 88% lower prevalence of infectious virus for those with SARS-CoV-2 antibodies (PR = 0.12, 95% CI: .04, .36, P = .00016). The 3 seropositive individuals with infectious virus isolated were 3, 4, and 6 days from symptom onset (all 3 had SARS-CoV-2 specific IgM; 2 had IgG; and 0 had IgA). Likewise, antibody status and NP viral RNA levels were strongly associated (mean: 4.4 log10 copies/ml among seropositives versus 6.8 log10 copies/ml among seronegatives; MD = –2.4, 95% CI: –2.8, –1.9, P < .0001). All participants with viral RNA measurements in the top quartile (>7.6 log10) were seronegative (100%; 43/43) and 91% (39/43) had infectious virus isolated. However, participants in the bottom viral RNA quartile (<4.7 log10) were predominantly infectious virus negative (98%; 40/41), and 68% (28/41) were seropositive (Figure 2).
Figure 2.
SARS-CoV-2 viral RNA, time since symptom onset, and infectious virus by SARS-CoV-2 specific antibody serostatus. A, Seropositive participants. B, Seronegative participants. Seropositive is defined as having SARS-CoV-2 specific total Ig, IgG, IgM, or IgA antibodies. Nasopharyngeal viral RNA levels (log10 copies/mL) are shown on the y-axis and days since symptom onset on the x-axis, with infectious virus culture positive participants in red circles, and culture negative participants in gray squares. Overall viral RNA median (Q1, Q3) and LLoQ are indicated by dashed horizontal lines. Abbreviations: Ig, immunoglobulin; LLoQ, lower limit of quantification; Q1, 25th percentile; Q3, 75th percentile; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
In an exploratory model (including viral RNA, antibody status, and symptom duration), infectious virus isolation was more prevalent for those with higher viral RNA (PR = 1.52 per 1.0 log10 copies/mL, 95% CI: 1.37, 1.70, P < .0001) and less prevalent for those with antibodies (PR = 0.35 for seropositive versus seronegative, 95% CI: .11, 1.07, P = .066) and was no longer associated with days since symptom onset (PR = 1.01 per day, 95% CI: 0.91, 1.11, P = .90). When viral RNA was excluded from this model the other associations became stronger: infectious virus isolation was less prevalent for those with antibodies (PR = 0.13 for seropositive versus seronegative, 95% CI: .04, .40, P = .0003) and more prevalent for outpatients with fewer days since symptom onset (PR = 0.86 per day, 95% CI: .77, .95, P = .004).
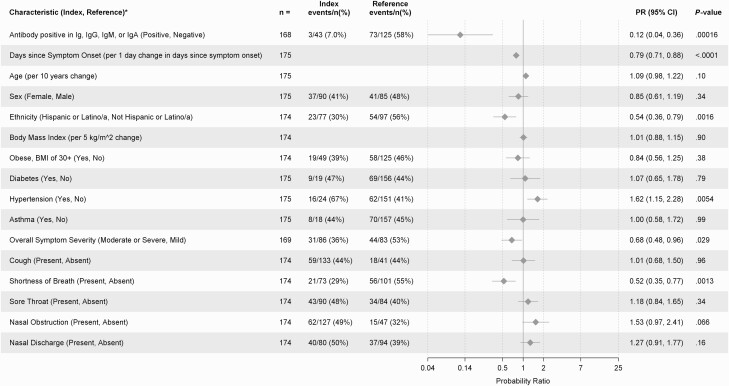
To evaluate the association between infectious virus isolation and host characteristics both bivariate and multivariable analyses were performed. In bivariate analyses, infectious virus isolation was more prevalent among participants with hypertension (PR = 1.62, 95% CI: 1.15, 2.28, P = .0054) and participants with nasal obstruction (PR = 1.53, 95% CI: .97, 2.41, P = .066). However, infectious virus isolation was less prevalent among participants who were further from symptom onset (PR = 0.79 per day, 95% CI: .71, .88, P < .0001), those with moderate-to-severe versus mild symptoms (PR = 0.68, 95% CI: .48, .96, P = .029), those with shortness of breath (PR = 0.52, 95% CI: .35, .77, P = .0013), and those who self-identified as Latinx compared to non-Latinx (PR = 0.54, 95% CI: .36, .79, P = .0016). In a sensitivity analysis adjusting for site, the association between Latinx ethnicity and lower prevalence of virus isolation remained (Mantel-Haenszel PR = 0.52, 95% CI: .28, .96, P = .032). Infectious virus isolation was not clearly associated with age (PR = 1.09 per 10 years, 95% CI: .98, 1.22, P = .10) or biological sex (PR = 0.85 women vs. men, 95% CI: .61, 1.19, P = .34) (Figure 3). In a multivariable model including host characteristics, seronegativity, fewer days since symptom onset, non-Latinx ethnicity, and hypertension remained associated with higher prevalence of infectious virus isolation (Supplementary Table 4).
Figure 3.
Host factor associations with infectious SARS-CoV-2 virus isolation. Bivariate analyses are shown. PRs for the probability of infectious virus isolation were estimated for each dichotomous characteristic and a prevalence ratio per unit change was estimated for each continuous characteristic with corresponding 95% CIs. Each continuous characteristic was fit as linear in the log-prevalence of infectious virus isolation. Abbreviations: CI, confidence interval; Ig, immunoglobulin; PR, prevalence ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Associations between infectious virus isolation and inflammatory and hematology markers were also evaluated. Elevated inflammatory markers were numerically less frequent among participants with infectious virus culture positive compared to culture negative (Supplementary Table 2). Participants with positive infectious virus culture had lower levels of white blood cells including total white blood cell count, absolute lymphocyte count, and absolute neutrophil count (Supplementary Figure 5).
Host factor associations with viral RNA level, a readily-available objective measure of SARS-CoV-2, are shown in Supplementary Figure 6. Higher viral RNA was associated with fewer days since symptom onset (mean difference (MD) −0.5 log10 per day, 95% CI: −.6, −.3, P < .0001). There was a trend toward higher viral RNA levels in those with mild symptom severity (MD: −0.5 log10 for moderate-to-severe versus mild symptoms, 95% CI: −1.0, .07, P = .086), with median viral RNA levels of 6.8 (IQR 4.8–8.1), 6.4 (4.6–7.3), and 5.7 (4.6–7.1) log10 copies/mL for participants with mild, moderate, and severe overall symptom severity, respectively. Viral RNA levels were higher on average for those with hypertension (MD: 1.0 log10, 95% CI: .3, 1.7, P = .0036) or nasal discharge (MD: 0.7 log10, 95% CI: .2, 1.2, P = .0052). Lower viral RNA levels were observed for those who self-identified as Latinx; however, in a sensitivity model adjusting for site, the association between Latinx ethnicity and viral RNA was no longer present (MD: -0.01 log10, 95% CI: −.7, .7, P = .98). Viral RNA was not associated with age (MD: 0.1 log10 per 10 years of age, 95% CI: −.06, .3, P = .20) or body mass index (MD: 0.02 log10 per 5 kg/m2, 95% CI: −.2, .3, P = .83). There was some evidence of lower levels of viral RNA in women compared to men (MD: −0.5 log10, 95% CI: −1.0, .02, P = .057).
DISCUSSION
SARS-CoV-2 infectious virus isolation within 7 days of symptom onset was prevalent and strongly associated with both viral RNA levels and the absence of SARS-CoV-2-specific antibodies. This suggests that humoral immunity plays a key role in limiting infectious virus shedding, and is further supported by studies identifying prolonged viral replication in individuals with immune deficiencies, nearly all of whom had specific deficiencies in B cell function [4–8, 21]. Importantly, the novel association, in this study, between seropositivity and clearance of infectious virus supports recent reports of increased efficacy of monoclonal antibodies in seronegative patients with COVID-19 [22], and indicates that seronegativity could serve to identify those who would benefit the most from antiviral therapy.
At entry, most outpatients in this study had fatigue, cough, nasal obstruction, and/or headache; about half reported sore throat or loss of taste/smell; and 41% reported feeling feverish, having chills, or experiencing shortness of breath. This study revealed associations between infectious virus isolation and milder symptoms, lower white blood cell populations, and not having shortness of breath, which is consistent with an early stage in the clinical course of infection. Presence of SARS-CoV-2 antibodies was associated with higher white blood cell and neutrophil counts, consistent with these findings. Prior studies report no virus isolation beyond 10 days from symptom onset in outpatients with mild-moderate COVID-19 [11, 14, 23, 24]. However, in this study, when accounting for NP RNA level and serologic response, duration of symptoms was no longer associated with infectious virus isolation, although only individuals within 7 days of symptom onset were enrolled.
Viral RNA was highest soon after symptom onset with a peak in the median levels at ≤ 3 days after symptom onset. SARS-CoV-2 RNA in NP swabs of 6.4 log10 copies/mL or higher was predictive of infectious virus detection, with a positive predictive value of 80% and negative predictive value of 91%, supporting prior studies suggesting a threshold of 6 log10 copies/mL [10, 11, 25]. However, infectious virus was isolated in three individuals with viral RNA below 5.0 log10 copies/mL (minimum: 4.3 log10 copies/mL), all 3 of whom were antibody negative men at 6 days from symptom onset, potentially suggesting a delayed immune response.
We did not identify associations between several established risk factors for severe COVID-19 –including age, obesity, diabetes, or asthma—and infectious virus isolation or higher levels of viral RNA. However, individuals with hypertension tended to have both higher levels of viral RNA and higher prevalence of infectious virus isolation, the mechanism of which remains unclear. Importantly, most outpatients with mild-to-moderate symptoms have a relatively low probability of developing severe COVID-19 disease [26–28].
This study describes novel associations between virologic and immunologic factors and isolation of infectious virus, however there are important limitations to consider. This was a cross-sectional sample of predominantly low risk, symptomatic outpatient adults, restricting our target population to people 18 years of age or older with mild-to-moderate COVID-19. The inclusion of only symptomatic participants precludes an assessment of factors associated with infectious virus among asymptomatic individuals. Additionally, symptoms were self-reported which can be subjective. This study enrolled a large number of participants who identified as Latinx, yet there was an under-representation of Blacks and Asian-Americans compared with the US burden of COVID-19 [29]. No multiplicity adjustments were included in the analyses presented; thus, confidence intervals and P-values should be interpreted with care.
Furthermore, seropositivity is not synonymous with virus neutralization. Infectious virus was isolated from 3 seropositive participants, 2 of whom also had RNA levels above 6.0 log10, but neutralization titers were not measured, thereby limiting this analysis to a qualitative assessment of seropositivity rather than antibody function. We also acknowledge that a negative culture does not entirely exclude the presence of infectious virus. The sensitivity of the virus isolation assay is 50 pfu/mL, and thus infectious virus below this limit may not be detected. Additionally, variability in swabbing technique can result in false negative cultures.
Nonetheless, the strong associations we observed between antibody status and viral RNA shedding with isolation of infectious virus provide important insight into viral and host factors associated with infectious SARS-CoV-2 virus. Measuring a patient’s serostatus and NP RNA level can help clinicians assess the likelihood of infectious virus detection, which can help guide isolation practices, therapy, and clinical follow-up. However, given the cross-sectional nature of this analysis we must be careful to not confer causality without further mechanistic research. Longitudinal research is needed to evaluate whether the early detection of SARS-CoV-2 antibodies can predict subsequent infectious virus clearance and whether early viral clearance decreases the likelihood of long-term COVID-19 symptoms [30]. As SARS-CoV-2 continues to spread globally, improved understanding of host, disease, and viral factors associated with infectious virus isolation is important to inform nonpharmacologic, vaccine, and therapeutic strategies to interrupt transmission and progression of disease.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Dr Sarah Reifeis for data carpentry support, Ms Ann Marie Weideman for statistical graphics advice, and Covance by Labcorp for data management support and quantification of viral RNA. They sincerely thank all the participants and sites for their dedication and contributions to the study: Valley Clinical Trials (Northridge, CA), University of North Carolina (Chapel Hill, North Carolina, USA), Fred Hutchinson (Seattle, Washington, USA), Care United Research (Forney, Texas, USA), Benchmark Research (Colton, California, USA), FOMAT Medical Research (Oxnard, California, USA), Indago Research and Health Center (Hiahleah, Florida, USA), Wake Forest Baptist Health (Winston-Salem, North Carolina, USA), Duke University (Durham, North Carolina, USA), and NOLA Research Works (New Orleans, Louisiana, USA). Molnupiravir was invented at Drug Innovations at Emory (DRIVE) LLC, a not-for-profit biotechnology company wholly owned by Emory University and is being developed by Merck & Co., Inc. in a collaboration with Ridgeback Biotherapeutics.
Author contributions. K. R. M., J. J. E., T. P. S., and W. A. F. led the conceptualization of the analysis and the writing of original manuscript draft. E. A. G., L. P., E. J. D. A., A. J. B., J. A. D., J. J. W., R. S. B., R. W. C., and T. P. S. conducted SARS-CoV-2 laboratory assessments. K. R. M., T. J. K., J. K., and M. G. H. conducted the statistical analyses. Study data were verified by K. R. M., T. J. K., W. P., L. J. S., L. F., and W. A. F.; K. R. M., J. J. E., W. P., L. J. S., R. S. B., M. G. H., L. F., D. A. W., R. S. B., T. P. S., and W. A. F. contributed to the study design. W. P., E. R. D., C. G. M., C. R. W., L. J. S., P. L. A., A. J. L., and W. A. F. oversaw data collection or protocol implementation. All authors reviewed the draft manuscript and approved the final version.
Data sharing statement. Upon approval from the corresponding author and Ridgeback Biotherapeutics, in collaboration with Merck & Co., de-identified participant level data may be shared given the investigator who requests the data has approval from an Institutional Review Board, Independent Ethics Committee, or Research Ethics Board, as applicable, and executes a data use/sharing agreement.
Financial support. This work was supported by Merck & Co., Inc. in collaboration with Ridgeback Biotherapeutics LP. Support was also provided in part by the National Institutes of Health (NIH) (grant numbers P30 AI050410, T32 ES007018 to T. J. K., AI-027757 to R. W. C., AI-106701 to R. W. C.), and a generous gift from the Chan Zuckerberg Initiative to R. S. B.
Potential conflicts of interest. Grants or contracts were awarded to the participating sites from Ridgeback Biotherapeutics LP. K. R. M., C. G. M., and R. S. B. have collaborations, unrelated to this study, with Gilead Sciences. J. J. E. receives consulting honoraria outside the current study from Merck, VIR/GSK, and is a DSMB member for Adagio. W. P. and L. J. S. are employed by Ridgeback Biotherapeutics LP. C.G.M. has collaborations, unrelated to this study, with Roche, Moderna, Johnson & Johnson, Covis Pharma, and Chimerix. D. A. W. has collaborations (advisory boards, consultancy, research funding), unrelated to this work, with Gilead Sciences. R. S. B. has collaborations, unrelated to this study, with Moderna, Pfizer, Takeda, Eli Lily, and VaxArt. C. R. W. has, unrelated to this study, financial disclosures for DSMB work with Biogen, Janssen, Merck, and Atea, consultancy paid by Enzychem Lifesciences, and honoraria paid by Abbott Laboratories. T. P. S. has contracts, unrelated to this work, from ViiV Healthcare and GSK. W. A. F. participates on adjudication committees for Janssen, Syneos, and provides consultancy to Roche and Merck. P. L. A. reports direct salary support for the period of the study from Dr. Billy Fischer II/UNC ID. R. S. B. reports grants or contracts outside of the submitted work with Rideback Biosciences to recover live viruses from COVID-19 patients treated with EIDD2801 or from controls and the NIH provided funds to research remdesivir in collaboration with Gilead. M. S. C. reports leadership or fiduciary role for HPTN, CoVPN, Fogarty, and McGill. R. W. C. reports payments made to University of Washington outside of the submitted work (UM1-AI-106701, UM1-AI-068618, P30-AI-027757). E. R. D. reports research contract with payments made to the institution from Merck & Co. W. A. F. reports financial interests from Regeneron (research funding related to COVID-19 but not related to work presented here and Lilly (research funding related to COVID-19 but not related to work presented here) and funding for research provided to UNC outside of the conduct of the study from Ridgeback Biopharmaceuticals. M. G. H. reports payment to the institution (UNC) outside of the conduct of the study from NIH and received support for attending meetings and/or travel from NIH. J. K. reports direct payment as salary from UNC CFAR during the conduct of the study. T. J. K. reports that their work on this paper was supported in part by a grant from the National Institute of Environmental Health Sciences (T32ES007018) and by the UNC-CH Center for AIDS Research. L. P. reports being co-investigator and receiving support for the present funding from UNC Gillings Innovation Laboratory Award, NCI-U54 CA260543, and NC Collaboratory fund and co-investigator for NIAID U01AI151788 outside of the conduct of the study. K. R. M. reports payment made to the institution (UNC) from the NIH outside of the conduct of the study and received support for attending meetings and/or travel from the NIH. C. G. M. reports COVID-19 clinical trials: NIH/NIAID, Duke Clinical Research Institute, Moderna, Genetech, Gilead Sciences, Chimerix outside of the conduct of the study and consulting fees for HIV: Theratechnologies, Inc., Viiv. W. P. reports that their spouse is an inventor and patent holder and employee of Emory University. T. P. S. reports payments made to them for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Albert Einstein School of Medicine, The Rockefeller University, Southern Society of Clinical Investigation, and American College of Veterinary Pathology. D. A. W. reports research grant funding to university from Eli Lily Inc. and participation on advisory boards for Gilead, Merck, Janssen, and ViiV. C. R. W. reports work on CoVID therapeutics (monoclonal antibodies) from Adagio Therapeutics. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Katie R Mollan, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, North Carolina, United States; School of Medicine, University of North Carolina at Chapel Hill, North Carolina, United States; Center for AIDS Research, University of North Carolina at Chapel Hill, North Carolina, United States.
Joseph J Eron, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, North Carolina, United States; School of Medicine, University of North Carolina at Chapel Hill, North Carolina, United States; Center for AIDS Research, University of North Carolina at Chapel Hill, North Carolina, United States.
Taylor J Krajewski, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, North Carolina, United States; Center for AIDS Research, University of North Carolina at Chapel Hill, North Carolina, United States.
Wendy Painter, Ridgeback Biotherapeutics LP, Miami, Florida, USA.
Elizabeth R Duke, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA.
Caryn G Morse, Wake Forest School of Medicine, Winston-Salem, North Carolina, USA.
Erin A Goecker, Department of Laboratory Medicine and Pathology, University of Washington, Seattle, Washington, USA.
Lakshmanane Premkumar, School of Medicine, University of North Carolina at Chapel Hill, North Carolina, United States.
Cameron R Wolfe, Duke University Medical Center, Durham, North Carolina, USA.
Laura J Szewczyk, Ridgeback Biotherapeutics LP, Miami, Florida, USA.
Paul L Alabanza, Center for AIDS Research, University of North Carolina at Chapel Hill, North Carolina, United States.
Amy James Loftis, School of Medicine, University of North Carolina at Chapel Hill, North Carolina, United States.
Emily J Degli-Angeli, Department of Laboratory Medicine and Pathology, University of Washington, Seattle, Washington, USA.
Ariane J Brown, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, North Carolina, United States.
Joan A Dragavon, Department of Laboratory Medicine and Pathology, University of Washington, Seattle, Washington, USA.
John J Won, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, North Carolina, United States.
Jessica Keys, Center for AIDS Research, University of North Carolina at Chapel Hill, North Carolina, United States.
Michael G Hudgens, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, North Carolina, United States; Center for AIDS Research, University of North Carolina at Chapel Hill, North Carolina, United States.
Lei Fang, Pharstat Inc., Raleigh, North Carolina, USA.
David A Wohl, School of Medicine, University of North Carolina at Chapel Hill, North Carolina, United States.
Myron S Cohen, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, North Carolina, United States; School of Medicine, University of North Carolina at Chapel Hill, North Carolina, United States; Center for AIDS Research, University of North Carolina at Chapel Hill, North Carolina, United States.
Ralph S Baric, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, North Carolina, United States.
Robert W Coombs, Department of Laboratory Medicine and Pathology, University of Washington, Seattle, Washington, USA.
Timothy P Sheahan, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, North Carolina, United States.
William A Fischer, II, School of Medicine, University of North Carolina at Chapel Hill, North Carolina, United States; Division of Pulmonary and Critical Care Medicine, University of North Carolina at Chapel Hill, North Carolina, USA.
References
- 1. Hu B, Guo H, Zhou P, Shi ZL.. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 2020. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. WHO coronavirus (COVID-19) dashboard. Available at: https://covid19.who.int/. Accessed 27 October 2021.
- 3. CDC. Interim guidance on duration of isolation and precautions for adults with COVID-19. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html. Accessed 12 April 2021.
- 4. Aydillo T, Gonzalez-Reiche AS, Aslam S, et al. . Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med 2020; 383:2586–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Avanzato VA, Matson MJ, Seifert SN, et al. . Case study: prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell. 2020; 183:1901–1912.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baang JH, Smith C, Mirabelli C, et al. . Prolonged severe acute respiratory syndrome coronavirus 2 replication in an immunocompromised patient. J Infect Dis 2021; 223:23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Choi B, Choudhary MC, Regan J, et al. . Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med 2020; 383:2291–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tarhini H, Recoing A, Bridier-Nahmias A, et al. . Long term SARS-CoV-2 infectiousness among three immunocompromised patients: from prolonged viral shedding to SARS-CoV-2 superinfection. J Infect Dis 2021. doi: 10.1093/infdis/jiab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walsh KA, Spillane S, Comber L, et al. . The duration of infectiousness of individuals infected with SARS-CoV-2. J Infect 2020; 81:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wölfel R, Corman VM, Guggemos W, et al. . Virological assessment of hospitalized patients with COVID-2019. Nature 2020; 581:465–469. [DOI] [PubMed] [Google Scholar]
- 11. van Kampen JJA, van de Vijver DAMC, Fraaij PLA, et al. . Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19). Nat Commun 2021; 12:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu Y, Yan LM, Wan L, et al. . Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis 2020; 20:656–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen J, Qi T, Liu L, et al. . Clinical progression of patients with COVID-19 in Shanghai, China. J Infect 2020; 80:e1–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bullard J, Dust K, Funk D, et al. . Predicting infectious severe acute respiratory syndrome coronavirus 2 from diagnostic samples. Clin Infect Dis 2020; 71:2663–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sheahan TP, Sims AC, Zhou S, et al. . An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med 2020; 12. doi: 10.1126/scitranslmed.abb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Painter WP, Holman W, Bush JA, et al. . Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2. Antimicrob Agents Chemother 2021. doi: 10.1128/AAC.02428-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Center for Disease Control and Prevention. CDC diagnostic test for COVID-19. 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/lab/virus-requests.html. Accessed 12 April 2021.
- 18. Berg MG, Zhen W, Lucic D, et al. . Development of the RealTime SARS-CoV-2 quantitative laboratory developed test and correlation with viral culture as a measure of infectivity. J Clin Virol 2021; 143:104945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Premkumar L, Segovia-Chumbez B, Jadi R, et al. . The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci Immunol 2020; 5:eabc8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159:702–6. [DOI] [PubMed] [Google Scholar]
- 21. Buckland MS, Galloway JB, Fhogartaigh CN, et al. . Treatment of COVID-19 with remdesivir in the absence of humoral immunity: a case report. Nat Commun 2020; 11:6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Horby PW, Mafham M, Peto L, et al. . Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv. 2021. doi: 10.1101/2021.06.15.21258542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Folgueira MD, Luczkowiak J, Lasala F, Pérez-Rivilla A, Delgado R.. Prolonged SARS-CoV-2 cell culture replication in respiratory samples from patients with severe COVID-19. Clin Microbiol Infect 2021. doi: 10.1016/j.cmi.2021.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Singanayagam A, Patel M, Charlett A, et al. . Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill 2020; 25. doi: 10.2807/1560-7917.ES.2020.25.32.2001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang CG, Lee KM, Hsiao MJ, et al. . Culture-based virus isolation to evaluate potential infectivity of clinical specimens tested for COVID-19. J Clin Microbiol 2020; 58:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gottlieb RL, Nirula A, Chen P, et al. . Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. J Am Med Assoc 2021; 325:632–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen P, Nirula A, Heller B, et al. . SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med 2021; 384:229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weinreich DM, Sivapalasingam S, Norton T, et al. . REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med 2021; 384:238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rossen LM, Branum AM, Ahmad FB, Sutton P, Anderson RN.. Excess deaths associated with COVID-19, by age and race and ethnicity—United States, January 26–October 3, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1522–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nalbandian A, Sehgal K, Gupta A, et al. . Post-acute COVID-19 syndrome. Nat Med 2021. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.