Abstract
Background
Due to concerns about the effects of the coronavirus disease 2019 (COVID-19 pandemic on health services, we examined its effects on human immunodeficiency virus (HIV) services in sub-Saharan Africa.
Methods
Quarterly data (Q1, 10/2019–12/2019; Q2, 1/2020–3/2020; Q3, 4/2020–6/2020; Q4, 7/2020–9/2020) from 1059 health facilities in 11 countries were analyzed and categorized by stringency of pandemic measures. We conducted a difference-in-differences assessment of HIV service changes from Q1–Q2 to Q3–Q4 by higher vs lower stringency.
Results
There was a 3.3% decrease in the number HIV tested from Q2 to Q3 (572 845 to 553 780), with the number testing HIV-positive declining by 4.9% from Q2 to Q3. From Q3 to Q4, the number tested increased by 10.6% (612 646), with an increase of 8.8% (23 457) in the number testing HIV-positive with similar yield (3.8%). New antiretroviral therapy (ART) initiations declined by 9.8% from Q2 to Q3 but increased in Q4 by 9.8%. Across all quarters, the number on ART increased (Q1, 419 028 to Q4, 476 010). The number receiving viral load (VL) testing in the prior 12 months increased (Q1, 255 290 to Q4, 312 869). No decrease was noted in VL suppression (Q1, 87.5% to Q4, 90.1%). HIV testing (P < .0001) and new ART initiations (P = .001) were inversely associated with stringency.
Conclusions
After initial declines, rebound was brisk, with increases noted in the number HIV tested, newly initiated or currently on ART, VL testing, and VL suppression throughout the period, demonstrating HIV program resilience in the face of the COVID-19 crisis.
Keywords: Africa, COVID-19, delivery of healthcare, HIV, SARS-CoV-2
While alarming predictions were made regarding potential impacts of the coronavirus disease 2019 (COVID-19) pandemic on human immunodeficiency virus services, few empiric data exist. Our findings demonstrate remarkable resilience. Lessons learned and how programs adapted can inform future responses.
Disruptions in health services, including human immunodeficiency virus (HIV) services, have been reported by various countries in the context of the coronavirus disease 2019 (COVID-19) pandemic associated with travel restrictions, avoidance of health facilities for fear of exposure, limitations in supply chains for various commodities, and changes in national policies and redirection of resources [1–4]. Modeling has also predicted that disruptions in access to HIV testing and antiretroviral therapy (ART) in sub-Saharan Africa, where 70% of the world’s people living with HIV (PLWH) reside, could lead to substantial increases in HIV-related deaths [5]. At the same time, reported cases, hospitalizations, and deaths due to COVID-19 have been lower across most African countries compared with other regions of the world with the exception of several surges in cases reported in some African countries [6]. However, these numbers likely represent an underenumeration due to limited testing and underreporting. Nonetheless, disruptions to HIV-related health services have been reported in Africa [1, 2, 4, 7, 8]. In an effort to avert anticipated disruptions in services, measures were put in place to enable persons to access HIV-related services through innovations such as adoption of differentiated service delivery models including multimonth dispensing of ART to limit the need for health facility visits along with other patient-, community-, and health system–level innovations [9–14].
To evaluate the effect of the COVID-19 pandemic on HIV services, we examined data on the HIV testing and treatment cascade, that is, HIV testing, initiation and use of ART, viral load (VL) testing, and VL suppression at 1059 health facilities supported by ICAP in 11 countries in sub-Saharan Africa.
METHODS
The data analyzed represented 4 quarters encompassing 2 quarters pre-COVID-19 and 2 quarters since COVID-19 was declared a pandemic, offering the opportunity to measure the effects of the pandemic on these critical HIV interventions in the initial pandemic period. We used routine aggregate HIV-related data from ICAP-supported health facilities that were reported for adults and children from 1 October 2019 to 30 September 2020. President’s Emergency Plan for AIDS Relief (PEPFAR)–supported programs report aggregate data for a set of predefined indicators into PEPFAR’s Data for Accountability, Transparency and Impact (DATIM) system. DATIM utilizes numerous data quality checks, and the data also undergo extensive human review. Data were downloaded from DATIM into ICAP’s DHIS2-based data management system in February 2021 and then exported into Excel and SAS for analysis. The data were examined by quarter (Q), with Q1 (October 2019–December 2019) and Q2 (January 2020–March 2020) data reflecting HIV service delivery provision before the implementation of any COVID-19–related restrictions. Q3 (April 2020–June 2020) data reflected HIV service delivery during the time period of restrictions, and Q4 (July 2020–September 2020) data reflected HIV service delivery as some countries began relaxing restrictions [15]. The data were from health facilities supported by ICAP at Columbia University in 11 African countries: Angola, Burundi, Cameroon, Cote d’Ivoire, Democratic Republic of the Congo (DRC), Eswatini, Ethiopia, Kenya, Mozambique, South Sudan, and Zambia. More information on ICAP’s support in each country can be found at https://icap.columbia.edu/where-we-work/. At these health facilities, ICAP provided technical support to strengthen various aspects of HIV service delivery including HIV testing and treatment as well as data monitoring and evaluation activities. In this analysis, supported health facilities were restricted to those that reported HIV testing data in all 4 quarters to ensure observed trends were not impacted by changes in programmatic support.
We examined quarterly trends along the HIV testing and treatment cascade. For HIV testing, the number of individuals who received an HIV test, the number newly diagnosed with HIV, and HIV testing yield (percent who tested positive) were examined. For HIV treatment, the number of PLWH newly initiating ART and those currently on ART, the number who had a VL test done in the past 12 months, and the percentage of those with VL suppression (defined as <1000 copies/mL) were evaluated. Descriptive analyses were used to examine trends during the 1-year period overall and stratified by country, age (<15 years vs ≥15 years), and sex for those aged ≥15 years. HIV testing was also stratified by point of service.
To evaluate the effect of COVID-19 mitigation measures, we examined the effects overall as well as based on median mitigation stringency score. We used the median score on a country’s Oxford COVID-19 Government Response Tracker Stringency index for the period between 1 April 2020 and 30 September 2020 [15]. This index is a daily composite measure (range, 0–100) based on 9 pandemic responses: school closure, workplace closure, cancellation of public events, restrictions on public gatherings, closure of public transport, public information campaigns, stay-at-home orders, restrictions on internal movement, and international travel bans [15]. Six countries (Angola, DRC, Eswatini, Ethiopia, Kenya, South Sudan) had median scores ≥75% (range across countries, 78%–84%) and were considered to have more stringent measures, while 5 countries (Burundi, Cameroon, Cote d’Ivoire, Mozambique, Zambia) had median scores <75% (range across countries, 14%–63%) and were considered to have less stringent measures. Sensitivity analyses explored different thresholds of exposure categorization. We chose this dichotomization because it enabled the largest gap between successive median scores (63% vs 78%) and created 2 distinct groups of countries.
A difference-in-differences design [16] was used to assess whether changes in HIV testing and treatment services differed from Q1–Q2 (premitigation measures) to Q3–Q4 (mitigation measures in place) by stringency category. Linear regression with generalized estimating equations to account for within–health facility correlation was used to assess whether the cross-product term describing the difference in the slope of the line for the relationship between reporting period and mean facility outcome measure was significant at a 5% level of significance. Sensitivity analyses were performed by comparing Q1–Q2 to Q4 to assess whether the Q3 period was only a temporary reduction.
The data analyzed in this study were collected as part of services supported by the PEPFAR through the US Centers for Disease Control and Prevention and US Agency for International Development. The funders were provided with the opportunity to review the manuscript as per funding agreements.
RESULTS
Supported Health Facilities
A total of 1059 supported health facilities with HIV testing data reported for all 4 quarters under analysis were included from the following countries: Angola (n = 17), Burundi (n = 88), Cameroon (n = 73), Cote d’Ivoire (n = 145), DRC (n = 199), Eswatini (n = 42), Ethiopia (n = 31), Kenya (n = 1), Mozambique (n = 59), South Sudan (n = 20), and Zambia (n = 384).
HIV Testing Services
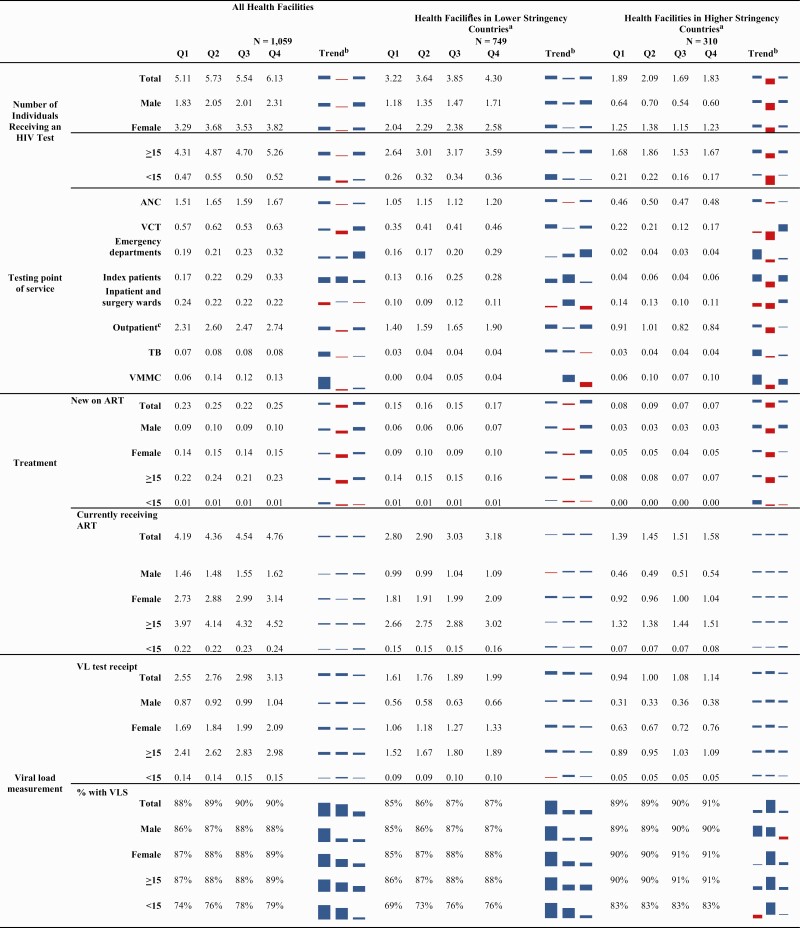
In Q1, 511 233 individuals received HIV testing services and 4.0% (20 525) tested HIV-positive at the included health facilities (Table 1). Overall, the health facilities reported a 12.1% increase in the total number of individuals receiving an HIV test from Q1 to Q2, a slight decrease of 3.3% in Q3, and a rebound in Q4 with a 10.6% increase to 612 646. When stratified by age and sex (for those aged ≥15 years), similar patterns were seen. Patterns did differ by the testing point of service, with the largest declines in Q3 for voluntary counseling and testing (13.6% decline) and testing occurring as part of voluntary medical male circumcision services (15.7% decline). In most countries, the same pattern was observed among the included health facilities, though the magnitudes of the changes differed. Exceptions to the pattern included Burundi, Cameroon, and Cote d’Ivoire (Supplementary Table 1). The number of individuals who had an HIV-positive test result followed the same overall testing pattern (Q1, 20 525; Q2, 22 662; Q3, 21 533; Q4, 23 478) with test yield remaining stable each quarter (Q1, 4.0%; Q2, 4.0%; Q3, 3.9%; and Q4, 3.8%). HIV testing yield also remained stable when stratified by age and sex, except for males aged ≥15 years where yield declined from 5.2% in Q1 to 4.7% in Q4.
Table 1.
Human Immunodeficiency Virus Testing and Antiretroviral Therapy Services at 1059 Health Facilities in 11 African Countries, October 2019–September 2020: Overall and Stratified by Coronavirus Disease 2019 Response Stringency Index
|
Table is presented in hundreds of thousands. Numbers may not sum to the total due to missing information. Q1, October 2019–December 2019; Q2, January 2020–March 2020; Q3, April 2020–June 2020; Q4, July 2020–September 2020.
Abbreviations: ANC, antenatal care; ART, antiretroviral therapy; TB, tuberculosis; VCT, voluntary counseling and testing; VL, viral load; VLS, viral load suppression; VMMC, voluntary medical male circumcision.
Lower stringency: Burundi, Cameroon, Cote d’Ivoire, Mozambique, Zambia. Higher stringency: Angola, Democratic Republic of the Congo, Eswatini, Ethiopia, Kenya, South Sudan.
Trend displays percentage change in indicator from the previous quarter. Blue indicates an increase; red indicates a decrease.
Outpatient includes testing that took place at clinics for patients aged <5 years, sexually transmitted infection, and malnutrition and other provider-initiated testing.
When stratified by mitigation stringency, supported health facilities in countries with higher stringency showed substantial decreases in HIV testing from Q2 (208 718 total HIV tests) to Q3 (169 260 total HIV tests; 18.9% decrease) and modest increases from Q3 to Q4 (183 129 total HIV tests; 8.2% increase), while supported health facilities in countries with lower stringency showed increases in each successive quarter (Q1, 321 803; Q2, 364 127; Q3, 384 520; Q4, 429 517). These patterns were consistent across age and sex (Table 1).
HIV Treatment Services
The overall pattern for new ART initiations reflected those for HIV testing and new diagnoses (Table 1). In Q1, 23 169 individuals newly initiated ART. This increased by 7.5% to 24 918 in Q2. The number then declined by 9.8% to 22 469 in Q3, then increased again by 9.8% in Q4 to 24 665. When stratified by age and sex, similar trends were seen. In the majority of the supported health facilities in each country, there was also a similar pattern; exceptions were noted in Burundi, Cameroon, DRC, and Eswatini (Supplementary Table 2). When stratified by mitigation stringency, countries with lower stringency indices had less decline from Q2 to Q3 (lower, 5.6% decrease in ART initiations; higher, 17.9% decrease) and a larger increase from Q3 to Q4 (lower, 11.8% increase; higher, 5.2% increase); these patterns were consistent across age and sex (Table 1).
The overall number of PLWH on ART, which reflects both new initiations and individuals continuing ART, increased in each quarter by ≥4.0%, from 419 028 in Q1 to 476 010 in Q4 (Table 1). When stratified by sex, age, and stringency of restrictions, similar patterns were seen. When stratified by country, the same pattern was noted except for Burundi and Kenya where there were very small declines from Q1 to Q2 (–1.0%) in Burundi and from Q2 to Q3 (–0.5%) in Kenya (Supplementary Table 3). Quantity of antiretrovirals (ARVs) dispensed for patients, that is, dispensing of a 3- or 6-month supply of medicines, increased over the analysis period, with the largest increase from Q2 to Q3. The proportion of those on ART who received ≥3 months of ARVs was 51% in Q1 and 59% in Q2, increasing to 78% in Q3 and 80% in Q4.
Viral Load Testing and Suppression
In Q1, 255 290 individuals on ART received a VL test in the past 12 months with 87.5% with VL suppression (Table 1). The number who received VL testing in the past 12 months increased each quarter, up to 312 869 tests in Q4. In terms of VL suppression, there was a slight increase over the year with 90.1% having VL suppression in Q4. Similar patterns were seen when stratified by sex, age, country, and stringency level (Table 1 and Supplementary Table 4).
Association of COVID-19 Mitigation Measures and HIV Services
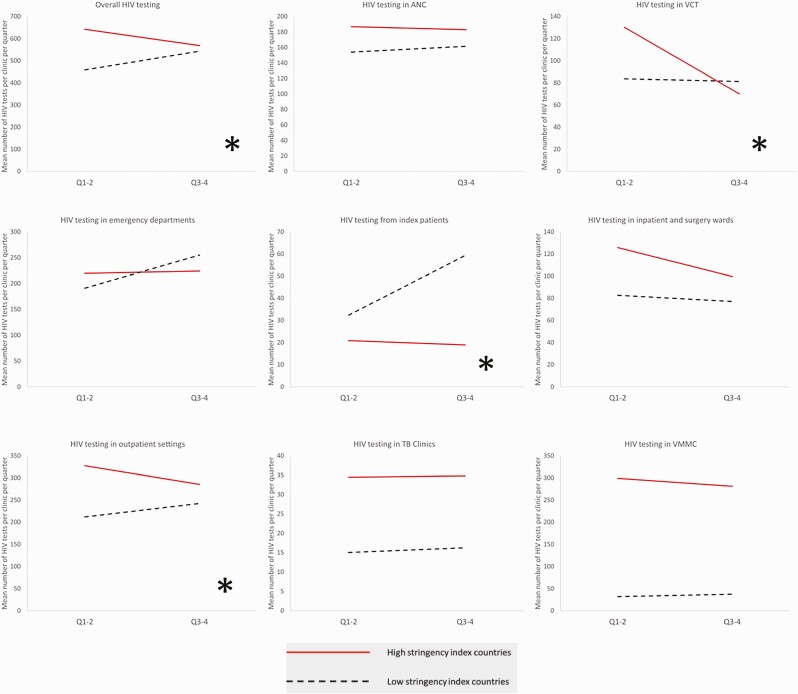
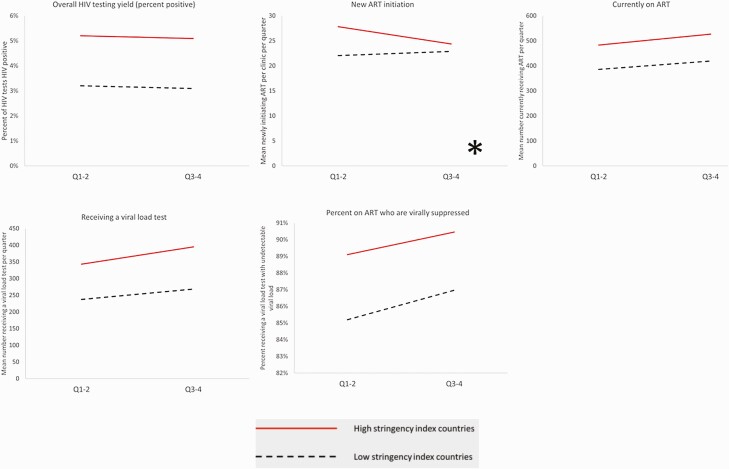
The results of the difference-in-differences analysis for HIV testing services overall and by point of service are shown in Figure 1 and for HIV yield and ART in Figure 2. Table 2 provides the results of the regression coefficients. Overall, supported health facilities in countries categorized as having lower stringency scores averaged 458 (standard deviation [SD] = 640) HIV tests per clinic per quarter in Q1–Q2 and 543 (SD = 834) HIV tests per clinic per quarter in Q3–Q4, while supported health facilities in countries with higher stringency scores showed a decline in average number of HIV tests per facility per quarter from 642 (SD = 1033) to 568 (SD = 820) from Q1–Q2 to Q3–Q4 (P < .001). Significant differences in Q1–Q2 vs Q3–Q4 in the number of HIV tests conducted were observed within specific points of testing, including voluntary counseling and testing, index patient testing, and outpatient testing (all P < .05), with marginal differences observed in testing at antenatal clinics (P value = .05 for interaction). No differences were observed in HIV testing volume between Q1–Q2 and Q3–Q4 at tuberculosis clinics, emergency departments, inpatient and surgery wards, and voluntary medical male circumcision services (all P value > .05).
Figure 1.
Difference-in-differences analysis: human immunodeficiency virus testing by point of service at 1059 health facilities in 11 African countries, October 2019–September 2020. ∗P <.05 for difference-in-difference. Abbreviations: ANC, antenatal care; HIV, human immunodeficiency virus; TB, tuberculosis; VCT, voluntary counseling and testing; VMMC, voluntary medical male circumcision.
Figure 2.
Difference-in-differences analysis: human immunodeficiency virus testing yield and ART at 1059 health facilities in 11 African countries, October 2019–September 2020. ∗P < .05 for difference-in-difference. Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus.
Table 2.
Results of Difference-in-Differences Analysis at 1059 Health Facilities in 11 African Countries, October 2019–September 2020
| Mean Value | Higher vs Lower Stringency Index | Q1–Q2 vs Q3–Q4a | Interaction | |||||
|---|---|---|---|---|---|---|---|---|
| Intercept | b | P Value | b | P Value | b | P Value | ||
| Human immunodeficiency virus testing | Overall | 457.9 | 184.3 | 0.003 | 85.5 | <0.0001 | –159. | <0.0001 |
| ANC | 154.0 | 32.8 | 0.12 | 7.5 | 0.001 | –11.2 | 0.05 | |
| VCT | 83.6 | 46.5 | 0.03 | –2.2 | 0.50 | –57.7 | <0.0001 | |
| Emergency department | 190.9 | 28.8 | 0.69 | 64.0 | 0.002 | –59.5 | 0.07 | |
| Index patients | 32.3 | –11.4 | 0.01 | 27.0 | <0.0001 | –28.9 | <0.0001 | |
| Inpatient | 82.7 | 43.1 | 0.16 | –5.6 | 0.64 | –20.5 | 0.25 | |
| Outpatient | 211.6 | 115.8 | 0.001 | 30.6 | 0.002 | –72.3 | 0.0001 | |
| TB | 15.0 | 19.4 | 0.0001 | 1.2 | 0.24 | –0.9 | 0.60 | |
| VMMC | 32.1 | 267.1 | <0.0001 | 5.2 | 0.28 | –23.2 | 0.13 | |
| ART services | New on ART | 22.0 | 5.8 | 0.06 | 0.8 | 0.25 | –4.3 | 0.001 |
| Currently receiving ART | 385.2 | 97.9 | 0.22 | 33.7 | <0.0001 | 9.4 | 0.07 | |
| VL test receipt | 237.3 | 105.8 | 0.15 | 31.0 | <0.0001 | 21.0 | 0.06 | |
| % with VLS | 85 | 3.9 | <0.0001 | 1.8 | 0.00001 | –0.40 | 0.41 | |
Abbreviations: ANC, antenatal care; ART, antiretroviral therapy; TB, tuberculosis; VCT, voluntary counseling and testing; VL, viral load; VLS, viral load suppression; VMMC, voluntary medical male circumcision.
Q1–Q2, October 2019–March 2020; Q3–Q4, April 2020–September 2020.
Overall, trends in HIV testing yield did not differ between stringency category (P value for interaction, .41; Figure 2 and Table 2), although the total number who received an HIV test and tested HIV-positive decreased more at supported health facilities in countries with a higher stringency index score, owing to the lower numbers receiving an HIV test. New ART initiations declined from Q1–Q2 (average = 28, SD = 43) to Q3–Q4 (average = 25, SD = 32) in high stringency countries but remained stable at supported health facilities in countries with low stringency scores (Q1–Q2, 22 [SD = 54]; Q3–Q4, 23 [SD = 51]); this difference was also statistically significant (P value = .001; Figure 2 and Table 2). There were no differences by stringency score category in the number currently on ART (P value = .07), the number who received viral load testing (P value = .06), or the percent virally suppressed (P value = .41; Table 2). In sensitivity analyses removing April 2020–June 2020 from the comparison, and therefore comparing October–March with July–September, differences in the trend between higher and lower stringency countries were similar (data not shown).
DISCUSSION
The COVID-19 pandemic has resulted in extensive disruptions globally, with many fearing a deleterious impact on HIV testing, prevention, and treatment programs [5, 12]. The findings from our study, which included more than 1000 ICAP-supported health facilities located in 11 sub-Saharan African countries, are reassuring. The data over 1 year spanning the advent of the COVID-19 pandemic showed a transient effect on HIV services, followed by rapid recovery in important measures related to the HIV testing and treatment cascade.
On average, ICAP-supported health facilities in countries with more stringent pandemic mitigation measures showed decreased HIV testing services in Q3–Q4 vs Q1–Q2, while supported health facilities with less stringent measures showed increased HIV testing services in Q3–Q4 vs Q1–Q2. In countries with more stringent restrictions, the most notable reductions in HIV testing services were observed in voluntary counseling and testing sites, index patients, and outpatient testing sites. Reasons for these reductions may include lockdown-related limitations in accessing testing sites, self-imposed reductions in voluntary travel to hospitals, and restrictions in community outreach activities that impacted index testing availability. Inpatient HIV testing and testing for individuals who require access to supported health facilities, such as patients with tuberculosis or pregnant women attending antenatal care clinics, were less affected, with less differential between high and low stringency countries. We also found that new ART initiations were more affected in countries with more stringent restrictions, likely due to declines in HIV testing and difficulty in accessing health facilities where ART is traditionally initiated.
It is heartening to note that over the entire year that spanned the advent of the COVID-19 pandemic, there was an increase in the overall number of persons on ART from 419 028 in Q1 to 476 010 in Q4. Importantly, VL suppression, a critical measure of ART effectiveness, remained high throughout the year. Overall, even at health facilities in countries with higher mitigation stringency scores, changes noted were consistent with a rebound in HIV service delivery.
Several countries have reported declines in HIV testing, including among pregnant women, with most not having fully rebounded by September 2020 [17], which is consistent with our findings when restricted to health facilities in countries with more stringent mitigation measures. A study of 65 primary care clinics in South Africa also found that HIV testing and ART initiations were the most affected services [18]. Another study that focused on services for men who have sex with men in 3 counties in Kenya also found impacts on HIV testing and ART initiations [19]. Decreases in HIV testing could have lasting impacts on efforts to achieve HIV elimination.
Expansion of differentiated service delivery models has been cited as an approach to reducing the impacts of the COVID-19 pandemic on HIV programs [9–14]. ICAP has supported the implementation of differentiated service delivery in several countries in sub-Saharan Africa over the past 5 years and supported the expansion and adaptation of such models during the COVID-19 pandemic [11]. The rebound in HIV services we observed may, in part, be due to the expansion of differentiated service delivery models and other innovations including multimonth dispensing of medications, ART distribution in the community, and virtual adherence consultations.
Our study has several limitations. First, it included select health facilities and may not be representative of all health facilities in the 11 countries. Second, it includes only 1 year of data and, thus, may not capture potential longer-term effects of the COVID-19 pandemic. Future studies should examine these effects, particularly those on VL suppression as the VL data in our analysis covers testing that was conducted in the past 12 months that, especially in Q1–Q2, will include testing done prior to October 2019. Third, the difference-in-differences assumptions of no time-varying confounding between groups and no within-group confounding across times are not strictly verifiable, particularly with the few time points used in this analysis. Future studies should also evaluate the differentiated service delivery models and other innovations put in place in response to the pandemic.
Our study has several strengths. It includes more than 1000 health facilities located in 11 countries from across sub-Saharan Africa, representing diverse settings. While the data represent programmatic data, ICAP performs extensive data quality assurance activities. The difference-in-differences approach allowed each health facility to serve as its own time-invariant control, while comparing health facilities in higher vs lower stringent measure countries allowed for some measure of time-varying trends unrelated to the pandemic response.
In summary, our study findings indicate that HIV programs in several sub-Saharan African countries demonstrated evidence of resilience in the face of the COVID-19 crisis. Health facilities in countries with more stringent COVID-19 restrictions had more substantial decreases in HIV testing and new ART initiations when compared with those in countries with less stringent restrictions. However, even in the latter settings, recovery was brisk. Looking ahead, efforts should focus on building resilient health systems that can withstand the shocks from various crises.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors acknowledge the partnership and efforts of the ministries of health in Angola, Burundi, Cameroon, Côte d’Ivoire, Democratic Republic of the Congo (DRC), Eswatini, Ethiopia, Kenya, Mozambique, South Sudan, and Zambia; the health facility staff; the headquarter and in-country teams for the US Agency for International Development (USAID) and the US Centers for Disease Control and Prevention (CDC); partner organizations and recipients of care; and ICAP in-country and headquarter staff.
Disclaimer. The findings and conclusions presented here are those of the authors and do not necessarily represent the official position of the funding agencies.
Financial support. This work was supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the US CDC (U2GGH002216, Angola, Cameroon, Zambia; U2GGH002015, Cote d’Ivoire; U2GGH001351, DRC; U2GGH001433, Eswatini; NU2GGH002156, Ethiopia; U2GGH001952, Kenya; NU2GGH001944, Mozambique; and U2GGH001335, South Sudan) and USAID (19-SBA-149, Burundi). T. G. H. reports grants to ICAP at Columbia University that supported the technical assistance provided by ICAP to the Ministry of Health human immunodeficiency virus (HIV) programs in the countries included in this article from the US CDC and USAID. P. K. reports CDC Zambia using PEPFAR funding on the Care and Treatment award supporting 2 provinces in Zambia (award to Columbia University) and support from the Ministry of Health in Zambia (health facility level, district, provincial health offices). B. N. reports USAID award to Jhpiego for which Columbia University is a subrecipient. R. S. reports the following: Strengthening Local Capacity To Deliver Sustainable Quality Assured Universal Coverage of Clinical HIV/TB Services in Manzini Region, and Provide Central Level Technical Assistance to the NTCP Services in the Kingdom of Swaziland under PEPFAR–Swaziland (ICAP received funding from CDC/ITF to strengthen health facilities in the Manzini Region to respond to the coronavirus disease 2019 [COVID-19] crisis); Strengthening HIV/TB Laboratory Quality Management Systems and Services in the Kingdom of Swaziland under PEPFAR–Swaziland (ICAP received funding from PEPFAR through CDC/ITF to support, improve, and integrate COVID-19 sample referral into the National Sample Transportation System in the Kingdom of Eswatini); and Resolve to Save Lives (RTSL) project (ICAP received funding to build capacity of frontline health providers to manage COVID-19 through training on Infection Prevention and Control). M. L. M. F. reports support from Instituto Nacional de Luta contra a SIDA Angola (INLS; serves as director of INLS that is responsible for HIV program data). M. A. B. A. reports the following support: Supporting Sustainable Implementation of HIV and TB Services for Epidemic Control in the Republic of Mozambique (under PEPFAR Co-Ag, NU2GGH001944). M. R. L. reports support from PEPFAR through the CDC- and USAID-funded Reaching Impact, Saturation, and Epidemic Control program. F. B. reports that technical assistance that was provided by ICAP and was supported by an award from the US CDC (paid to Columbia University). M. V. reports receiving Supporting Sustainable Implementation of HIV and TB Services for Epidemic Control in the Republic of Mozambique under PEPFAR (Co-Ag, NU2GGH001944; US CDC; paid to institution). G. L. E. and C. A. L. report that funding from US CDC (award to Columbia University) supported the technical assistance provided by ICAP in the included countries. H. B. reports that this work was funded by PEPFAR through the US CDC and USAID under the terms of U2GGH002015 (Cote d’Ivoire; funds provided to ICAP).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Contributor Information
Tiffany G Harris, ICAP and Department of Epidemiology, Columbia University, New York, New York, USA.
Edward Jaszi, ICAP Columbia University, New York, New York, USA.
Matthew R Lamb, ICAP and Department of Epidemiology, Columbia University, New York, New York, USA.
Carlos A Laudari, ICAP at Columbia University, Luanda, Angola.
Maria Lúcia Mendes Furtado, National AIDS Control Program, Luanda, Angola.
Bonaparte Nijirazana, ICAP at Columbia University, Bujumbura, Burundi.
Ndayizeye Aimé, Ministry of Public Health and the Fight Against AIDS, Bujumbura, Burundi.
Gabriel Loni Ekali, ICAP at Columbia University, Yaoundé, Cameroon.
Lifanda Ebiama Lifanda, National AIDS Control Committee, Yaoundé, Cameroon.
Hermann Brou, ICAP at Columbia University, Abidjan, Côte d’Ivoire.
Eboi Ehui, Ministry of Health and Public Hygiene, Abidjan, Côte d’Ivoire.
Faustin Malele Bazola, ICAP at Columbia University, Kinshasa, Democratic Republic of the Congo.
Aimé Mboyo, National AIDS Control Program, Kinshasa, Democratic Republic of the Congo.
Ruben Sahabo, ICAP at Columbia University, Mbabane, Eswatini.
Nkhosikhona Advocate Dlamini, Ministry of Health, Mbabane, Eswatini.
Zenebe Melaku, ICAP at Columbia University, Addis Ababa, Ethiopia.
Mirtie Getachew Meselu, Ministry of Health, Addis Ababa, Ethiopia.
Mark Hawken, ICAP at Columbia University, Nairobi, Kenya.
Catherine Ngugi, National AIDS and STIs Control Programme, Nairobi, Kenya.
Mirriah Vitale, ICAP at Columbia University, Maputo, Mozambique.
Munira Abubakar Bin Abudou, Ministry of Health, Nampula Province, Nampula, Mozambique.
Florence Bayoa, ICAP at Columbia University, Juba, South Sudan.
Victoria Achut, Ministry of Health, Juba, South Sudan.
Prisca Kasonde, ICAP at Columbia University, Lusaka, Zambiaand.
Paul Munsanje, Western Provincial Health Office, Mongu, Zambia.
Wafaa M El-Sadr, ICAP and Department of Epidemiology, Columbia University, New York, New York, USA.
References
- 1. World Health Organization. Pulse survey on continuity of essential health services during the COVID-19 pandemic: interim report, 27 August 2020. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-EHS_continuity-survey-2020.1. Accessed 22 April 2021.
- 2. World Health Organization. Second round of the national pulse survey on continuity of essential health services during the COVID-19 pandemic: January-March 2021. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-EHS-continuity-survey-2021.1. Accessed 28 April 2021.
- 3. Hrynick TA, Ripoll Lorenzo S, Carter SE.. COVID-19 response: mitigating negative impacts on other areas of health. BMJ Global Health 2021; 6:e004110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. The Global Fund. COVID-19 Situation Report #40. Available at: https://www.theglobalfund.org/en/covid-19/news/2021-03-12-situation-report/. Accessed 29 March 2021.
- 5. Jewell BL, Mudimu E, Stover J, et al. . Potential effects of disruption to HIV programmes in sub-Saharan Africa caused by COVID-19: results from multiple mathematical models. Lancet HIV 2020; 7:e629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization. WHO coronavirus (COVID-19) dashboard. Available at: https://covid19.who.int/. Accessed 23 May 2021.
- 7. Khan MS, Rego S, Rajal JB, et al. . Mitigating the impact of COVID-19 on tuberculosis and HIV services: a cross-sectional survey of 669 health professionals in 64 low and middle-income countries. PLoS One 2021; 16:e0244936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization. WHO: access to HIV medicines severely impacted by COVID-19 as AIDS response stalls. Available at: https://www.who.int/news/item/06-07-2020-who-access-to-hiv-medicines-severely-impacted-by-covid-19-as-aids-response-stalls. Accessed 29 March 2021.
- 9. Wilkinson L, Grimsrud A.. The time is now: expedited HIV differentiated service delivery during the COVID-19 pandemic. J Int AIDS Soc 2020; 23:e25503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Golin R, Godfrey C, Firth J, et al. . PEPFAR’s response to the convergence of the HIV and COVID-19 pandemics in Sub-Saharan Africa. J Int AIDS Soc 2020; 23:e25587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Preko P, Shongwe S, Abebe A, et al. . Rapid adaptation of HIV differentiated service delivery program design in response to COVID-19: results from 14 countries in sub-Saharan Africa (LBPEE44). AIDS 2020. Available from: https://cquin.icap.columbia.edu/wp-content/uploads/2020/06/CQUIN_DSD-design-in-response-to-COVID19_AIDS-2020-2.pdf Accessed 29 March 2021.
- 12. Nachega JB, Kapata N, Sam-Agudu NA, et al. . Minimizing the impact of the triple burden of COVID-19, tuberculosis and HIV on health services in sub-Saharan Africa. Int J Infect Dis 2021; 113:S16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hong SY, Ashipala LSN, Bikinesi L, et al. . Rapid adaptation of HIV treatment programs in response to COVID-19—Namibia, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1549–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grimsrud A, Wilkinson L.. Acceleration of differentiated service delivery for HIV treatment in sub-Saharan Africa during COVID-19. J Int AIDS Soc 2021; 24:e25704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hale T, Angrist N, Goldszmidt R, et al. . A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker). Nature Human Behaviour 2021; 5:529–38. [DOI] [PubMed] [Google Scholar]
- 16. Wing C, Simon K, Bello-Gomez RA.. Designing difference in difference studies: best practices for public health policy research. Annu Rev Public Health 2018; 39:453–69. [DOI] [PubMed] [Google Scholar]
- 17. UNAIDS. Prevailing against pandemics by putting people at the centre—World AIDS Day report 2020. Available at: https://www.unaids.org/en/resources/documents/2020/prevailing-against-pandemics. Accessed 30 March 2021.
- 18. Dorward J, Khubone T, Gate K, et al. . The impact of the COVID-19 lockdown on HIV care in 65 South African primary care clinics: an interrupted time series analysis. Lancet HIV 2021; 8:e158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Odinga MM, Kuria S, Muindi O, et al. . HIV testing amid COVID-19: community efforts to reach men who have sex with men in three Kenyan counties. Gates Open Res 2020; 4:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.