Abstract
Background
Based on interim analyses and modeling data, lower doses of bamlanivimab and etesevimab together (700/1400 mg) were investigated to determine optimal dose and expand availability of treatment.
Methods
This Phase 3 portion of the BLAZE-1 trial characterized the effect of bamlanivimab with etesevimab on overall patient clinical status and virologic outcomes in ambulatory patients ≥12 years old, with mild-to-moderate coronavirus disease 2019 (COVID-19), and ≥1 risk factor for progressing to severe COVID-19 and/or hospitalization. Bamlanivimab and etesevimab together (700/1400 mg) or placebo were infused intravenously within 3 days of patients’ first positive COVID-19 test.
Results
In total, 769 patients were infused (median age [range]; 56.0 years [12, 93], 30.3% of patients ≥65 years of age and median duration of symptoms; 4 days). By day 29, 4/511 patients (0.8%) in the antibody treatment group had a COVID-19-related hospitalization or any-cause death, as compared with 15/258 patients (5.8%) in the placebo group (Δ[95% confidence interval {CI}] = −5.0 [−8.0, −2.1], P < .001). No deaths occurred in the bamlanivimab and etesevimab group compared with 4 deaths (all COVID-19-related) in the placebo group. Patients receiving antibody treatment had a greater mean reduction in viral load from baseline to Day 7 (Δ[95% CI] = −0.99 [−1.33, −.66], P < .0001) compared with those receiving placebo. Persistently high viral load at Day 7 correlated with COVID-19-related hospitalization or any-cause death by Day 29 in all BLAZE-1 cohorts investigated.
Conclusions
These data support the use of bamlanivimab and etesevimab (700/1400 mg) for ambulatory patients at high risk for severe COVID-19. Evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants will require continued monitoring to determine the applicability of this treatment.
Clinical Trials Registration
Keywords: bamlanivimab, etesevimab, COVID-19, persistently high viral load
Bamlanivimab and etesevimab together (700 mg plus 1400 mg) reduced coronavirus disease 2019 (COVID-19)-related hospitalizations and viral load in patients with mild-to-moderate COVID-19, with persistently high viral load correlating with COVID-19-related hospitalizations or any-cause death.
The coronavirus disease 2019 (COVID-19) pandemic remains a hazard to public health globally. Although vaccination is the main strategy to protect against COVID-19, there remains a need for anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) therapies, both for individuals pending vaccination and for breakthrough infections in certain vulnerable vaccinated patients, particularly those at a high risk of developing severe disease [1]. COVID-19 has an unpredictable disease course, however; to date, several factors conferring a high risk of developing severe COVID-19 have been identified [2, 3]. Older age, respiratory disease, diabetes, obesity, hypertension, and an immunosuppressive condition have all been associated with severe illness from COVID-19 [3, 4]. In addition to demographic risk factors and baseline comorbidities, high SARS-CoV-2 viral load has also been associated with severe clinical outcomes [5, 6]. High viral load has been shown to be associated with mortality in hospitalized patients [6] and severe COVID-19 cases have been found to have higher viral load than milder cases [5, 7].
As of June 2021, the Food and Drug Administration (FDA) has granted emergency use authorization (EUA) to a number of therapies [8–10] including 3 neutralizing monoclonal antibody (mAb) therapies [11–13]. Neutralizing mAbs can provide passive immunization [14, 15] and may also function as a prophylactic therapy in patients at high risk of developing severe COVID-19 [1]. Bamlanivimab and etesevimab, 2 potent anti-spike neutralizing mAbs, received an EUA from the FDA for the treatment of mild-to-moderate COVID-19 in ambulatory patients (≥12 years of age) at high risk of developing severe COVID-19 in February 2021 [11, 16, 17].
To date, 2800 mg of bamlanivimab together with 2800 mg of etesevimab has been shown to significantly reduce COVID-19-related hospitalizations and deaths, reduce viral load at Day 7, and accelerate symptom resolution compared with placebo [18]. Based on pharmacokinetic-pharmacodynamic modeling and interim BLAZE-1 analyses, lower doses of bamlanivimab and etesevimab together (700/1400 mg) were investigated to determine optimal dose and expand treatment availability.
Here, we present results from the latest portion of the BLAZE-1 trial evaluating the impact of a lower dose of bamlanivimab and etesevimab (700/1400 mg), which was granted the EUA based on clinical outcomes and viral clearance in patients with mild-to-moderate COVID-19. We also present data on persistently high viral load (PHVL), first identified in the phase 2 portion of BLAZE-1 [7], validating it as a feature predictive of progression to severe COVID-19.
METHODS
Study Design
BLAZE-1 is an ongoing, phase 2/3, randomized, double-blind, placebo-controlled, single-dose study in patients with recently diagnosed mild-to-moderate COVID-19 in the outpatient setting. In this portion of BLAZE-1, patients with ≥1 risk factor for progression to severe COVID-19 received a single intravenous (IV) infusion of 700 mg bamlanivimab and 1400 mg etesevimab together or placebo. Patients were considered lost to follow-up if they repeatedly failed to participate in scheduled visits and were unable to be contacted by the study site. This study was conducted in accordance with the Declaration of Helsinki and Council for International Organizations of Medical Sciences International Ethical Guidelines, and applicable International Council for Harmonization Good Clinical Practice Guidelines, laws, and regulations. All participants or their legally authorized representative provided written, informed consent or child/adolescent assent prior to study initiation.
Participants
Patients in this study were ambulatory adolescents (12–17 years of age, inclusive) and adults (≥18 years of age) with ≥1 risk factor [19] for progression to severe COVID-19 illness that presented within 3 days of their first positive SARS-CoV-2 test (either reverse transcription polymerase chain reaction [RT-PCR] or direct antigen) with ≥1 mild or moderate COVID-19 symptoms. The full list of adolescent and adult risk factors, COVID-19 symptoms, inclusion criteria, and exclusion criteria are listed in the Supplementary Materials.
Randomization and Intervention
Participants were centrally randomized to either bamlanivimab and etesevimab together (700/1400 mg) or placebo (0.9% sodium chloride solution), using an interactive web response system. Randomization was stratified by patients’ symptom duration (≤8 days vs >8 days), and age at the time of screening (<18 years of age vs ≥18 years of age). All eligible patients were randomized in a 1:2 (placebo:bamlanivimab and etesevimab) allocation ratio between 9 December 2020 and 7 January 2021. Each patient was infused within 3 days of their first positive SARS-CoV-2 test and was monitored for ≥1 hour post infusion. The sample size provided over 90% power to detect a 60% reduction in hospitalization or death events relative to pooled placebo arms. Further details on the sample size and details on the dose rationale are in the Supplementary Materials.
Primary and Secondary Endpoints
The primary endpoint was the proportion of patients that experienced a COVID-19-related hospitalization (≥24 hours of acute care) or any-cause death by Day 29. The following four pre-specified key secondary outcomes were also evaluated: change in SARS-CoV-2 viral load from baseline to Day 7 (±2 days), the percentage of patients with PHVL (defined below) at Day 7 (±2 days), the percentage of patients that experienced a COVID-19-related hospitalization, emergency room (ER) visit, or any-cause death by Day 29, and the time to sustained symptom resolution. Additional secondary outcomes included change in SARS-CoV-2 viral load from baseline to Days 3 and 5, time to viral clearance, time to symptom improvement and resolution, and safety. Symptom severity was assessed using a questionnaire and sustained symptom resolution was defined as 2 consecutive assessments with, a score of 0 or 1 for fatigue or cough, and a score of 0 for other symptoms [20] (further details in the Supplementary materials).
Virology
Viral load and viral clearance were measured by nasopharyngeal swab followed by quantitative reverse transcription polymerase chain reaction (qRT-PCR), with an upper cycle threshold (Ct) limit of 45. Further details of qRT-PCR and viral load quantification are in the Supplementary Materials. SARS-CoV-2 clearance was defined as 2 consecutive negative qRT-PCR tests for the SARS-CoV-2 virus. To determine PHVL, a viral load threshold that correlated with hospitalization was defined by a cut-point analysis of the full phase 2 cohort. Based on this analysis, a PCR Ct value of <27.5 (or log10 viral load ≥5.27) [20] was prespecified as a secondary endpoint for the phase 3 portion.
Statistical Analysis
Variables were analyzed on the original scale on which they were measured except for SARS-CoV-2 viral load, the derivation for which is included in the Supplementary Materials. Primary and secondary endpoints were tested in a sequential manner at a 1-sided .025 significance level. Type 1 errors were controlled across primary and secondary endpoints using a hierarchical multiple comparison procedure. Treatment effect tests using frequentist approaches were conducted using 2-sided tests at an alpha level of .05, unless stated otherwise. The primary outcome was analyzed by logistic regression, which included treatment group and the duration of symptom onset to randomization (≤8 days vs >8 days) as factors. Treatment comparisons of continuous efficacy with multiple postbaseline measurements (including viral load) were made using mixed model repeat measure (MMRM) analysis including the following as fixed factors: (a) treatment group, (b) stratification factor of duration since symptom onset to randomization (≤8 days vs >8 days), (c) baseline value in the model, (d) visit, and (e) the interactions of treatment-by-visit. The Kaplan-Meier product limit method was used for time-to-event analyses. The proportion of patients with SARS-CoV-2 nasopharyngeal viral load >log 5.27 (PHVL) on Day 7 was analyzed using a logistic regression with a Firth penalized likelihood [21]. For PHVL analyses, if Day 7 (±2 days) SARS-CoV-2 viral load was missing (including due to hospitalization), data were imputed from the last available observation, including baseline. If only baseline viral load was present, this value was utilized. All statistical analyses were performed using SAS software (version 9.4 or higher), FACTS (version 6.0 or higher), and/or R (version 3.6 or higher).
RESULTS
Participant Demographics
At the time of database lock (26 February 2021), when the full cohort reached Day 29, 769 patients had been randomized to receive 700 mg bamlanivimab and 1400 mg etesevimab, or placebo (Figure 1). Overall, patient demographics and baseline disease characteristics were similar between those receiving placebo or bamlanivimab and etesevimab together (Table 1). Across the total cohort, the median (range) age of patients was 56.0 (12, 93) years with 30.3% of patients ≥65 years of age. The median body mass index was 33.2 kg/m2, and 53.1% of patients were female. The majority of patients were white (86.8%) with 27.2% identifying as Hispanic or LatinX, and 8.3% identifying as Black or African American. At the time of randomization, 75.7% of patients had mild COVID-19 symptoms, and patients were randomized within a median of 4 days of symptom onset. On the day of infusion, a mean qRT-PCR cycle threshold of 24.3 was observed (Table 1).
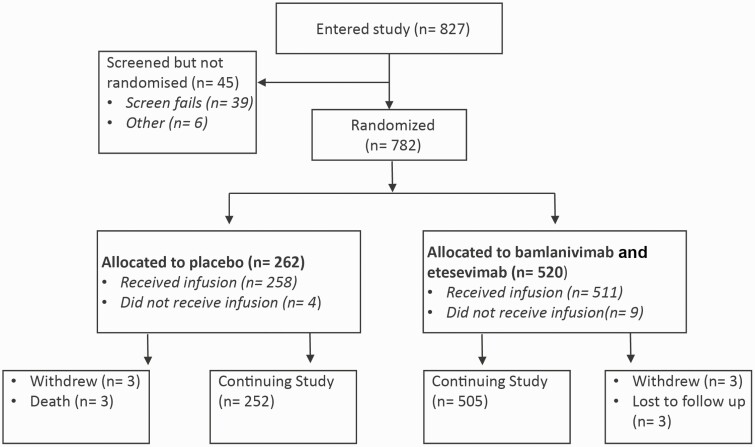
Figure 1.
Patient enrolment and treatment assignment.
Table 1.
Patient Demographics and Baseline Clinical Characteristics
| Placebo | Bamlanivimab and Etesevimab | Total | |
|---|---|---|---|
| (N = 258) | (N = 511) | (N = 769) | |
| Age | |||
| Median (min, max), y | 55.0 (13, 89) | 57 (12, 93) | 56.0 (12, 93) |
| Group, n (%) | |||
| ≥12 and <18 | 6 (2.3) | 10 (2.0) | 16 (2.1) |
| ≥18 and <35 | 32 (12.4) | 67 (13.1) | 99 (12.9) |
| ≥35 and <45 | 43 (16.7) | 63 (12.3) | 106 (13.8) |
| ≥45 and <55 | 44 (17.1) | 76 (14.9) | 120 (15.6) |
| ≥55 and <65 | 58 (22.5) | 137 (26.8) | 195 (25.4) |
| ≥65 | 75 (29.1) | 158 (30.9) | 233 (30.3) |
| Male sex, n (%) | 114 (44.2) | 247 (48.3) | 361 (46.9) |
| Race, na | 255 | 508 | 763 |
| n (%) | |||
| American Indian or Alaska Native | 1 (0.4) | 3 (0.6) | 4 (0.5) |
| Asian | 11 (4.3) | 18 (3.5) | 29 (3.8) |
| Black or African American | 22 (8.6) | 41 (8.1) | 63 (8.3) |
| Native Hawaiian or other Pacific Islander | 2 (0.8) | 1 (0.2) | 3 (0.4) |
| White | 219 (85.9) | 443 (87.2) | 662 (86.8) |
| Of multiple race decent | 0 | 2 (0.4) | 2 (0.3) |
| Missing | 3 | 3 | 6 |
| Ethnicity, na | 257 | 510 | 767 |
| Hispanic or LatinX, n (%) | 71 (27.6) | 138 (27.1) | 209 (27.2) |
| Body mass index, na | 258 | 510 | 768 |
| Median, kg/m2 | 34.4 | 32.5 | 33.2 |
| SpO2 category, n (%) | |||
| <96 | 56 (21.7) | 88 (17.2) | 144 (18.7) |
| ≥96 | 202 (78.3) | 423 (82.8) | 625 (81.3) |
| Duration of symptoms | |||
| Median no. days from symptom onset to randomization (min, max) | 3.0 (1, 15) | 4 (0, 19) | 4 (0, 19) |
| Days since COVID-19 symptom onset to randomization, n (%) | |||
| ≤8 | 245 (95.0) | 490 (95.9) | 735 (95.6) |
| >8 | 13 (5.0) | 21 (4.1) | 34 (4.4) |
| Baseline COVID-19 severity, n (%) | |||
| Mild | 202 (78.3) | 380 (74.4) | 582 (75.7) |
| Moderate | 56 (21.7) | 131 (25.6) | 187 (24.3) |
| High-risk status for severe COVID-19 illness, n (%) | |||
| High | 247 (95.7) | 485 (94.9) | 732 (95.2) |
| Low | 11 (4.3) | 26 (5.1) | 37 (4.8) |
| Number of high-risk criteria met (adults only), na | 252 | 501 | 753 |
| n (%) | |||
| 0 | 9 (3.6) | 25 (5.0) | 34 (4.5) |
| 1 | 145 (57.5) | 258 (51.5) | 403 (53.5) |
| 2 | 53 (21.0) | 122 (24.4) | 175 (23.2) |
| 3 | 34 (13.5) | 63 (12.6) | 97 (12.9) |
| 4 | 9 (3.6) | 28 (5.6) | 37 (4.9) |
| 5 | 2 (0.8) | 3 (0.6) | 5 (0.7) |
| 6 | 0 | 2 (0.4) | 2 (0.3) |
| Number of high-risk criteria met (adolescents only), na | 6 | 10 | 16 |
| n (%) | |||
| 0 | 2 (33.3) | 1 (10.0) | 3 (18.8) |
| 1 | 4 (66.7) | 9 (90.0) | 13 (81.3) |
| Medical history and preexisting conditions, n (%) | |||
| Chronic kidney disease | 3 (1.2) | 6 (1.2) | 9 (1.2) |
| Diabetes | 59 (22.9) | 139 (27.2) | 198 (25.7) |
| Immunosuppressive disease | 0 | 8 (1.6) | 8 (1.0) |
| Immunosuppressive treatment | 16 (6.2) | 28 (5.5) | 44 (5.7) |
| In adults aged ≥ 55, na | 252 | 501 | 753 |
| Cardiovascular disease | 18 (7.1) | 38 (7.6) | 56 (7.4) |
| Hypertension | 85 (33.7) | 191 (38.1) | 276 (36.7) |
| Chronic obstructive pulmonary disease | 15 (6.0) | 41 (8.2) | 56 (7.4) |
| Viral load, na | 237 | 468 | 705 |
| Mean Ct value (SD) | 24.7 (8.1) | 24.2 (7.3) | 24.3 (7.6) |
Abbreviations: COVID-19, coronavirus disease 2019; Ct, cycle threshold of the reverse-transcriptase polymerase-chain-reaction assay; N, number of subjects in the analysis population; n, number of patients in a specified category; SD, standard deviation; SpO2, saturation of peripheral oxygen.
Number of patients with nonmissing data used as the denominator.
Primary Outcome
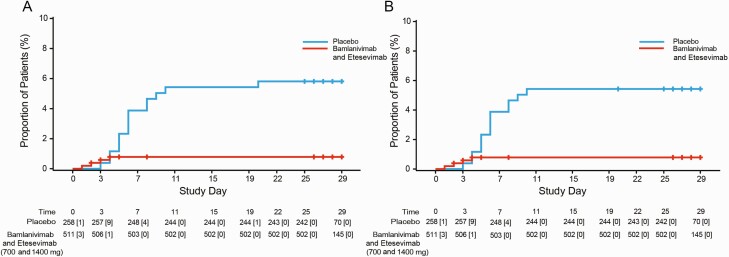
There was an 87% reduction in COVID-19-related hospitalization or all-cause death by Day 29 in patients that received bamlanivimab and etesevimab together as compared with those that received placebo (Figure 2A). A total of 4 hospitalizations or all-cause deaths by Day 29 were recorded in the bamlanivimab and etesevimab treatment group (0.8% [4/511]), compared with 15 events in the placebo group (5.8% [15/258]) (Δ [95% confidence interval {CI}] = −5.0 [−8.0, −2.1], P < .001; Figure 2A). In bamlanivimab and etesevimab treated patients, there was an 86% reduction in COVID-19-related hospitalizations (Figure 2B) and no deaths by Day 29. All deaths recorded were among those receiving placebo (4/258) and were considered COVID-19-related. Subgroup analyses of age and body mass index (BMI) are reported in the Supplementary Materials.
Figure 2.
Kaplan-Meier analysis of time to COVID-19-related hospitalization or any-cause death (A) and time to COVID-19-related hospitalization (B) among high-risk patients that received bamlanivimab and etesevimab (700 mg and 1400 mg) or placebo. Number of patients at risk are presented below each graph with the number of events occurring after each timepoint, up to and including the next timepoint, in brackets. Time to event analyses were calculated using the Kaplan-Meier product limit method. Patients were infused on Study Day 1. Abbreviation: COVID-19, coronavirus disease 2019.
Secondary Outcomes
The key secondary endpoints were met for the 700/1400 mg dose of bamlanivimab and etesevimab when compared to the concurrently enrolled placebo arm. For clinical outcomes, 1.2% (6/511) of patients that received the antibody treatment experienced COVID-19-related hospitalization, emergency room (ER) visit or death from any cause by Day 29, whereas 5.8% (15/258) of patients in the placebo group experienced these outcomes by Day 29 (Δ [95% CI] = −4.6% [−7.6, −1.6], P < .001). Similarly, the median time to sustained symptom resolution on two consecutive assessments was significantly decreased (P < .01) for patients receiving bamlanivimab and etesevimab together (days [95% CI] = 8.0 [7.0, 9.0]) compared with those receiving placebo (days [95% CI] = 10.0 [8.0, 11.0]) (Supplementary Figure 1). The prespecified key secondary analysis of sustained symptom resolution between 700/1400 mg (days [95% CI] = 8.0 [7.0, 9.0]) and all placebo patients (days [95% CI] = 9.0 [9.0, 10.0]) was not statistically significant (hazard ratio [HR] 1.1, P = .13). Additional clinical secondary outcomes including symptom improvement and resolution are in the Supplementary material (Supplementary Figures 2–5).
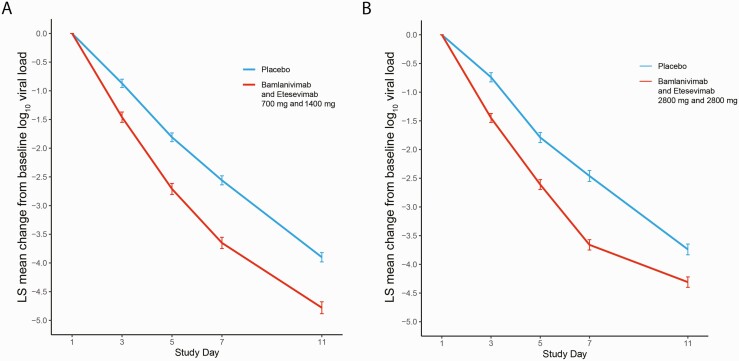
Patients receiving bamlanivimab and etesevimab together had a significantly greater mean reduction in log viral load from baseline to Day 7 as compared with placebo (Δ [95% CI] = -0.99 [-1.33, -0.66], P < .0001; Figure 3A). Evaluation of the viral load revealed a similar reduction in viral load at Day 7 for patients receiving 700/1400mg of bamlanivimab together with etesevimab compared to those receiving higher doses (2800/2800 mg) (Figure 3) [18]. A total of 14.9% of patients (76/510) receiving 700/1400mg of bamlanivimab and etesevimab together had PHVL on Day 7, compared with 41.1% (106/258) of patients receiving placebo (Δ [95% CI] = −26.2 [−32.9, −19.4], P < .0001). Additional viral secondary outcomes including viral clearance are included in the Supplementary Materials (Supplementary Figure 6).
Figure 3.
Least squares (LS) mean change in viral load from baseline. LS mean change in viral load from baseline following treatment with (A) 700 mg and 1400 mg of bamlanivimab and etesevimab together as compared with placebo and (B) 2800 mg and 2800 mg of bamlanivimab and etesevimab together, as compared with placebo (data from Dougan et al [18]). Change in viral load from baseline for patients treated with a combination of bamlanivimab and etesevimab is significantly lower than placebo at all time points investigated for both panels A and B. Data analyzed as a mixed model repeat measure (MMRM) model. Error bars represent standard error of the mean.
Origin of PHVL as Dichotomous Cut-Point for Secondary Analysis
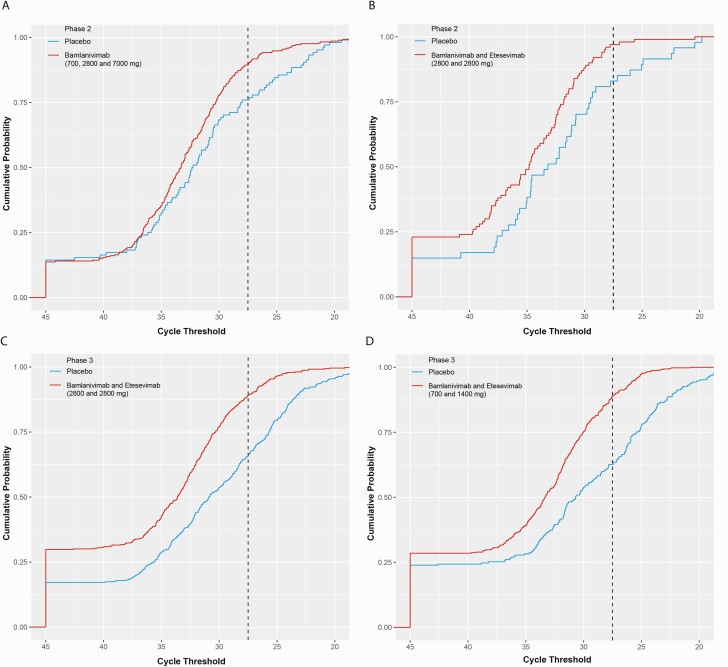
Post hoc analysis of pooled phase 2 data from all patients revealed an association between slower viral clearance and hospitalization of patients with COVID-19, with hospitalized patients having higher viral load than non-hospitalized patients at Day 7 [7, 20]. These data were used to discern a dichotomous PHVL cutoff threshold of <27.5 Ct (log10 viral load ≥5.27) that would stratify patients based on COVID-19-related hospitalization; this was then prespecified as a key secondary endpoint prior to the phase 3 portions of BLAZE-1. Figure 4 shows the cumulative probability that patients in phase 2 (A and B) and phase 3 (C and D) would have the indicated Ct value or higher on Day 7.
Figure 4.
SARS-COV-2 viral load in phase 2 and phase 3 BLAZE-1 cohorts on Day 7. A–D, The cumulative probability that patients would have the PCR cycle threshold at or above the specified cycle threshold value on Day 7. Phase 2 patients receiving bamlanivimab (700, 2800, and 7000 mg pooled) or placebo (data from Chen et al [7]) (A), and bamlanivimab and etesevimab (2800/2800 mg) or placebo (B). Phase 3 patients receiving bamlanivimab and etesevimab (2800/2800 mg) or placebo (C), and bamlanivimab and etesevimab (700/1400 mg) or placebo (D). Cycle threshold value of 27.5 (corresponding to PHVL) is indicated by the vertical dotted line. PHVL threshold was calculated using a cut-point analysis and PHVL and viral load were analyzed using a logistic regression. Abbreviations: PCR, polymerase chain reaction; PHVL, persistently high viral load; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
There was a significant association between PHVL at Day 7 and hospitalization or any-cause death by Day 29 in both phase 2 and phase 3 patients (P < .0001). In the phase 2 portion, 69% of patients (9/13) that experienced COVID-19-related hospitalization or all-cause death by Day 29 had PHVL at Day 7. This was verified in the phase 3 cohorts, where 70% of patients (32/46 events; pooled 2800/2800 mg + placebo) and 68% of patients (13/19 events; pooled 700/1400 mg + placebo) that experienced COVID-19-related hospitalization or all-cause death by Day 29 had PHVL at Day 7. In contrast, in the nonhospitalized group, 13% (74/564) of patients in the Phase 2 cohort had PHVL at Day 7, whereas 24% (234/985 events) and 22.6% (169/748 events) of patients in the phase 3 cohorts (pooled 2800/2800 mg + placebo and pooled 700/1400 mg + placebo respectively) had PHVL at Day 7. Of patients with PHVL at Day 7, 11% (9/83), 12% (32/266), and 7% (13/182) in the phase 2 and phase 3 (2800/2800 mg and 700/1400 mg) cohorts, respectively, experienced COVID-19 related hospitalization or all-cause death by Day 29. At least 84% of patients in each cohort had Day 7 viral load observed.
Safety
No significant differences were observed between the number of serious adverse events (SAEs) or treatment-emergent adverse events (TEAEs) between patients receiving bamlanivimab and etesevimab together (700/1400 mg) compared with those receiving placebo. SAEs occurred in 1.2% (6/511) of the patients receiving bamlanivimab and etesevimab together and in 0.8% (2/258) of patients receiving placebo (for clarity, admission for COVID-19 disease progression was treated, per protocol, as an endpoint rather than SAE). TEAEs were reported in 9% (46/511) of patients in the antibody treatment group and 9.7% (25/258) of patients in the placebo group (Table 2). No TEAE occurred in more than 1.2% in either group (Supplementary Table 1). Across both groups, the most common TEAEs (affecting at least 0.5% of patients) were increased liver function test, increased C-reactive protein, and dizziness (Supplementary Table 1).
Table 2.
Adverse Events
| Placebo (N = 258) | Bamlanivimab and Etesevimab (N = 511) | Total (N = 769) | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| SAE | 2 (0.8) | 6 (1.2) | 8 (1.0) |
| TEAE (total) | 25 (9.7) | 46 (9.0) | 71 (9.2) |
| Mild | 15 (5.8) | 26 (5.1) | 41 (5.3) |
| Moderate | 8 (3.1) | 16 (3.1) | 24 (3.1) |
| Severe | 1 (0.4) | 4 (0.8) | 5 (0.7) |
| Missing | 1 (0.4) | 0 | 1 (0.1) |
| Death due to AE | 0 | 0 | 0 |
Abbreviations: AE, adverse event; SAE, serious adverse event; TEAE, treatment-emergent adverse event.
DISCUSSION
Here we present data from the latest portion of the ongoing BLAZE-1 trial, evaluating the impact of bamlanivimab and etesevimab together (700/1400mg, the EUA dose) on viral clearance and clinical outcomes in high-risk patients with mild-to-moderate COVID-19 illness. Previous portions of the BLAZE-1 trial investigated higher doses of bamlanivimab and etesevimab together [18, 20] and the FDA authorized dose (700/1400 mg) was selected based on the synthesis of nonclinical, clinical, and virologic data and was supported by pharmacokinetic/pharmacodynamic modeling data [11]. Results presented here confirm and extend the previous BLAZE-1 findings and suggest that the authorized dose has a safety and efficacy profile comparable to that observed with the higher doses (2800/2800 mg) [18].
The EUA dose of bamlanivimab and etesevimab together (700/1400 mg) met both the primary and key secondary outcomes reducing COVID-19-related hospitalizations and any-cause death, time to symptom improvement, time to symptom resolution and accelerated viral clearance as compared with placebo. Indeed, both the clinical and virologic outcomes were similar for the EUA dose and the previously reported higher dose of treatment [18]. In terms of safety, data presented here confirm that the safety profile of the EUA dose was consistent with earlier analyses. The proportion of patients experiencing SAEs in this portion of the trial (0.8% placebo and 1.2% bamlanivimab and etesevimab) was comparable to those observed with the higher dose [18, 20].
The phase 3 portion of BLAZE-1 investigated meaningful clinical outcomes, specifically COVID-19–related hospitalizations or any-cause death, as the primary outcome [18]. This was a natural extension of the primary endpoint from the earlier phase 2 portion due to the clinical imperative to determine whether viral load translates into clinically meaningful outcomes for patients. Similarly, the primary endpoint in this portion of the trial did not include ER visits as not all ER visits lead to hospitalizations and the circumstances supporting a decision to hospitalize a patient vary among institutions and regions.
PHVL, which emerged as a meaningful virological endpoint and a potential surrogate endpoint for clinical outcomes during the interim analysis of the phase 2 portion of BLAZE-1 [7], was incorporated as a prespecified secondary endpoint for the phase 3 portions. Pooling data across phase 2 treatment and placebo groups revealed that viral load at Day 7 is associated with increased risk of COVID-19-related hospitalization. Across the phase 2 and phase 3 cohorts at least 68% of patients who experienced COVID-19 related hospitalization or any-cause death had PHVL (a Ct value <27.5) on Day 7. Initial data suggested that PHVL at Day 7 had a stronger association with clinical outcomes than PHVL at earlier time points. Data presented here from Phase 3 cohorts confirms that PHVL at Day 7 is associated with COVID-19-related hospitalization or any-cause death. Indeed, other studies have found that nasopharyngeal viral load is associated with increased inflammatory cytokines [22], risk of intubation [23] and increased risk of death [22]. Additionally, a treatment effect on PHVL rates at Day 7 has been strongly associated with a treatment effect on clinical outcomes across 2 phase 3 cohorts. As a result, PHVL may be a useful alternative endpoint in scenarios where collecting data on clinical outcomes is impractical.
It is important to acknowledge that different PCR protocols from the method used here may result in different Ct values for the same input of viral RNA. The cut-off Ct value of <27.5 on Day 7 for PHVL established for our assay may be slightly different for different PCR assays using other thermocycling equipment, viral gene targets, and PCR protocols. An alternative to using Ct values may be to use the 70th percentile of Day 7 viral load of patients receiving placebo, as this percentile may be more transferable across assays. Indeed, a multi-center investigation across several commonly used SARS-CoV-2 diagnostic PCR platforms and the assay used in this study is currently underway and may provide insight onto the interassay translation of our PHVL cutoff.
One limitation of this study is that there was a 1:2 randomization between placebo and antibody treatment. Thus, fewer patients received placebo concurrently with those that received bamlanivimab and etesevimab together. This was warranted given the severity of the pandemic and the results showing the clinical benefit of bamlanivimab and etesevimab during the previous 1:1 randomization of placebo and the higher dose of 2800 mg bamlanivimab and 2800 mg etesevimab together [18].
Overall, data from this portion of BLAZE-1 confirm the efficacy of 700/1400 mg of bamlanivimab and etesevimab on both clinical and viral outcomes in high-risk patients with mild-to-moderate COVID-19 and support the use of this dose in the ongoing fight against COVID-19. The utility of bamlanivimab and etesevimab together, as well as other antibodies and antibody combinations, can clearly be impacted by the development of resistant viral variants [24, 25]. Due to the prevalence of specific resistant variants, the distribution of bamlanivimab and etesevimab was temporarily restricted in the United States between June and September 2021 [26, 27]. Regardless, as the pandemic evolves globally and the burden on health care settings continues, reducing deaths, hospitalizations and clinical severity of disease will be crucial.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The names of the investigators and support staff that assisted in this study are listed in the Supplementary Materials. The authors thank Holly Green, PhD (Eli Lilly and company) for medical writing and editorial support. Eli Lilly and company designed the study, provided assistance with data acquisition, and carried out statistical analyses.
Disclaimer. Bamlanivimab emerged from the collaboration between Eli Lilly and Company and AbCellera Biologics to create antibody therapies for the prevention and treatment of COVID-19. Eli Lilly and Company developed the antibody after it was discovered by AbCellera Biologics and scientists at the National Institute of Allergy and Infectious Diseases Vaccine Research Center. Etesevimab emerged from the collaboration between Eli Lilly and Company and Junshi Biosciences.
Data sharing statement. Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Financial support. This work was supported by Eli Lilly and Company. The BLAZE-1 trial was sponsored and funded by Eli Lilly and Company.
Potential conflicts of interest. M. D. reports grants from Eli Lilly and Company and Novartis, consulting fees from Tillotts, Partner Therapeutics, SQZ Biotech, AzurRx, Moderna, ORIC Pharmaceuticals, honoria from Medscape and is an advisory board member and stockholder of Neoleukin Therapeutics. B. M. reports grants from Eli Lilly and Company to conduct clinical trial. R. L. G. reports consulting fees from Gilead Sciences and Johnson & Johnson and is an advisory board member for Eli Lilly and Company (COVID-19 Advisory Board), GSK Pharmaceuticals COVID-19 Advisory Board), Johnson & Johnson (Consulting fees to Baylor Scott & White Research Institute for service as National Coordinating PI for a COVID-19 Randomized Clinical Trial), and Roivant Sciences (Consulting fees to Baylor Scott & White Research Institute for COVID-19 Randomized Trial Steering Committee) and his research institution received a gift-in-kind of medication from Gilead Sciences for NCT03383419. P. C. reports grants from Eli Lilly and Company, National Institutes of Health (NIH), Gilead and Pliant Therapeutics, consulting fees from Eli Lilly and Company and Gilead, honoraria from Rockepointe, Frontier Collaborative, CME Outfitter, and Physician Education Resource. C. H. reports that Hebert Medical Consulting received clinical research grants and ancillary supplies from Eli Lilly and Company. R. P. reports honoraria from Genentech (Genentech Speakers Bureau 2018–2019). J. B. reports PI fees from Vitalink Pharmaceutical Research, contracted services fees from Eli Lilly and Company and speaker fees from GSK. J. M. reports clinical research grants to the research group from Eli Lilly and Company (Payments made to research group for enrolling and treating and following up with patients per study protocol). C. C. reports grants to Eastside Research Associates from Eli Lilly and company. G. H. reports institutional grants from Eli Lilly and Company, Gilead, ViiV, and Janssen, consulting fees from Trio Health, CME fees from Clinical Care Options, Practice Point Communications, Rockpointe, CME Outfitters and advisory board member for ViiV, Gilead, and Janssen. J. C. reports payment for ancillary supplies from Eli Lilly and Company. I. S. reports grants to the Franciscan Health-Indianapolis from Eli Lilly and Company. P. Kumar reports grants from Eli Lilly and Company, GSK, Merck, and Gilead, and is an advisory board member for Johnson & Johnson, ViiV Healthcare, Gilead, Theratechnologies, and Merck, and is a shareholder of Merck, Pfizer, Johnson & Johnson, GSK, and Gilead. A. B. reports clinical grants and ancillary supplies from Eli Lilly and Company. A. A., J. V. N., K. C., J. K., G. O. J., A. E. S., T. H., P. E., R. H., J. S., D. P., M. D., M. W., P. Klekotka, L. S., D. S., and A. N. are all employees and shareholders of Eli Lilly and Company. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Michael Dougan, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Masoud Azizad, Valley Clinical Trials Northridge, Northridge, California, USA.
Bharat Mocherla, Las Vegas Medical Research Center, Las Vegas, Nevada, USA.
Robert L Gottlieb, Baylor University Medical Center, Dallas, Texas, USA; Baylor Scott and White Research Institute, Dallas, Texas, USA.
Peter Chen, Department of Medicine, Women’s Guild Lung Institute, Cedars-Sinai Medical Center, Los Angeles, California, USA.
Corey Hebert, NOLA Research Works, New Orleans, Louisiana, USA.
Russell Perry, Gadolin Research, Beaumont, Texas, USA.
Joseph Boscia, Vitalink Research, Union, South Carolina, USA.
Barry Heller, Long Beach Clinical Trials, Long Beach, California, USA.
Jason Morris, Care Access, Lake Charles, California, USA.
Chad Crystal, Eastside Research Associates, Redmond, Washington, USA.
Awawu Igbinadolor, Monroe Biomedical Research, Monroe, North Carolina, USA.
Gregory Huhn, Cook County Health, Chicago, Illinois, USA.
Jose Cardona, Indago Research and Health Center, Hialeah, Florida, USA.
Imad Shawa, Franciscan Health Indianapolis Hospital, Indianapolis, Indiana, USA.
Princy Kumar, Georgetown University Medical Center, Washington D.C., USA.
Andra Blomkalns, Stanford University School of Medicine, Palo Alto, California, USAand.
Andrew C Adams, Eli Lilly and Company, Indianapolis, Indiana, USA.
Jacob Van Naarden, Eli Lilly and Company, Indianapolis, Indiana, USA.
Kenneth L Custer, Eli Lilly and Company, Indianapolis, Indiana, USA.
Jack Knorr, Eli Lilly and Company, Indianapolis, Indiana, USA.
Gerard Oakley, Eli Lilly and Company, Indianapolis, Indiana, USA.
Andrew E Schade, Eli Lilly and Company, Indianapolis, Indiana, USA.
Timothy R Holzer, Eli Lilly and Company, Indianapolis, Indiana, USA.
Philip J Ebert, Eli Lilly and Company, Indianapolis, Indiana, USA.
Richard E Higgs, Eli Lilly and Company, Indianapolis, Indiana, USA.
Janelle Sabo, Eli Lilly and Company, Indianapolis, Indiana, USA.
Dipak R Patel, Eli Lilly and Company, Indianapolis, Indiana, USA.
Matan C Dabora, Eli Lilly and Company, Indianapolis, Indiana, USA.
Mark Williams, Eli Lilly and Company, Indianapolis, Indiana, USA.
Paul Klekotka, Eli Lilly and Company, Indianapolis, Indiana, USA.
Lei Shen, Eli Lilly and Company, Indianapolis, Indiana, USA.
Daniel M Skovronsky, Eli Lilly and Company, Indianapolis, Indiana, USA.
Ajay Nirula, Eli Lilly and Company, Indianapolis, Indiana, USA.
References
- 1. Cohen MS, Nirula A, Mulligan MJ, et al. ; BLAZE-2 Investigators. . Effect of bamlanivimab vs placebo on incidence of COVID-19 among residents and staff of skilled nursing and assisted living facilities: a randomized clinical trial. JAMA 2021; 326:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Center for Disease Control and Prevention. People at increased risk and other people who need to take extra precautions. Available at: https://wwwcdcgov/coronavirus/2019-ncov/need-extra-precautions/indexhtml?CDC_AA_refVal=https%3A%2F%2Fwwwcdcgov%2Fcoronavirus%2F2019-ncov%2Fneed-extra-precautions%2Fpeople-at-increased-riskhtml2020. Accessed 24 May 2021.
- 3. Williamson EJ, Walker AJ, Bhaskaran K, et al. . Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020; 584:430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang J, Zheng Y, Gou X, et al. . Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis 2020; 94:91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu Y, Yan LM, Wan L, et al. . Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis 2020; 20:656–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Westblade LF, Brar G, Pinheiro LC, et al. . SARS-CoV-2 viral load predicts mortality in patients with and without cancer who are hospitalized with COVID-19. Cancer Cell 2020; 38:661–71.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen P, Nirula A, Heller B, et al. ; BLAZE-1 Investigators. . SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med 2021; 384:229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. US Food and Drug Administration. Convalescent Plasma COVID-19 Letter of Authorization. Available at: https://wwwfdagov/media/141477/download. 2020. Accessed 24 May 2021.
- 9. US Food and Drug Administration. Remdesivir Letter of Authorization. Available at: https://wwwfdagov/media/137564/download. 2020. Accessed 6 June 2021.
- 10. US Food and Drug Administration. Fact sheet for healthcare providers emergency use authorization (EUA) of baricitinib. Available at: https://wwwfdagov/media/143823/download. 2020. Accessed 24 May 2021.
- 11. US Food and Drug Administration. Fact sheet for health care providers emergency use authorization (EUA) of bamlanivimab and etesevimab. Available at: https://wwwfdagov/media/145802/download. 2021; Accessed 10 February 2021.
- 12. Regeneron Pharmaceuticals I. Fact sheet for health care providers: emergency use authorization (EUA) of casirivimab and imdevimab. Available at: https://wwwregeneroncom/sites/default/files/treatment-covid19-eua-fact-sheet-for-hcppdf. 2020; Accessed 8 February 2021.
- 13. US Food and Drug Administration. GSK sotrovimab letter of authorization. Available at: https://wwwfdagov/media/149532/download. 2021; Accessed 3 June 2021.
- 14. Taylor PC, Adams AC, Hufford MM, de la Torre I, Winthrop K, Gottlieb RL.. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat Rev Immunol 2021; 21:382–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiang S, Hillyer C, Du L.. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol 2020; 41:355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shi R, Shan C, Duan X, et al. . A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature 2020; 584:120–4. [DOI] [PubMed] [Google Scholar]
- 17. Jones BE, Brown-Augsburger PL, Corbett KS, et al. . The neutralizing antibody, LY-CoV555, protects against SARS-CoV-2 infection in non-human primates. Sci Transl Med. 2021; eabf1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dougan M, Nirula M, Azizad M, et al. . Bamlanivimab plus etesevimab in mild or moderate COVID-19. N Engl J Med 2021; Online ahead of print: DOI: 10.1056/NEJMoa2102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Centers for Disease Control and Prevention. People at increased risk. Online 2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/index.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fneed-extra-precautions%2Fpeople-at-increased-risk.html. Accessed 23 March 2021.
- 20. Gottlieb RL, Nirula A, Chen P, et al. . Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2021; 325:632–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Firth D. Bias reduction of maximum likelihood estimates. Biometrika 1993; 80:27–38. [Google Scholar]
- 22. Fajnzylber J, Regan J, Coxen K, et al. . SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun 2020; 11:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Magleby R, Westblade LF, Trzebucki A, et al. . Impact of severe acute respiratory syndrome coronavirus 2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019. Clin Infect Dis 2020: ciaa851. doi:10.1093/cid/ciaa851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Planas D, Veyer D, Baidaliuk A, et al. . Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021; 596:276–80. [DOI] [PubMed] [Google Scholar]
- 25. Falcone M, Tiseo G, Valoriani B, et al. . Efficacy of bamlanivimab/etesevimab and casirivimab/imdevimab in preventing progression to severe COVID-19 and role of variants of concern. Infect Dis Ther 2021: 1–10. doi: 10.1007/s40121-021-00525-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Public Health Emergency. Pause in the distribution of bamlanivimab/etesevimab. Available at: https://wwwphegov/emergency/events/COVID19/investigation-MCM/Bamlanivimab-etesevimab/Pages/bamlanivimab-etesevimab-distribution-pauseaspx. 2021. Accessed 9 September 2021.
- 27. US Food and Drug Administration. Emergency use authorization 094. Available at: https://wwwfdagov/media/145801/download. 2021. Accessed 9 September 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.