Opinion statement
Immune checkpoint inhibitors (ICIs) have become an essential part of treatment for many cancer types. These monoclonal antibodies remove a critical negative regulatory signal that allows the immune system to recognize and destroy malignant cells that were previously undetectable. Unfortunately, their use has ushered in a whole new form of drug toxicity whereby the immune system attacks normal tissues in the body, referred to hereafter as immune-related adverse events (irAEs). irAEs are common and can result in treatment discontinuation, hospitalization, and death. When alternative modes of treatment are limited, or considered less efficacious, there may be a desire to resume treatment with ICIs after an irAE. Rechallenge with ICIs carries with it a heightened risk of subsequent toxicity, but with careful consideration and appropriate patient selection, this can be considered a reasonable approach.
Keywords: Immunotherapy, Rechallenge, Immune-related adverse event, PD-1, PD-L1, CTLA-4
Introduction
The number of patients with cancer who are being treated with immunotherapy is growing rapidly due to the potential benefit of durable responses, even in the setting of metastatic disease [1]. Immune checkpoint inhibitors (ICIs) have emerged as one of the primary treatment modalities for metastatic cancer, as monotherapy or in combination with other systemic agents, such as chemotherapy, monoclonal antibodies, targeted therapies, or radiation. Prominent examples of this include lung cancer, melanoma, and renal cell carcinoma, where ICIs have emerged in the front-line setting [2–4]. There is also a rapidly expanding role for ICIs in the adjuvant, neoadjuvant, and maintenance settings [5–7].
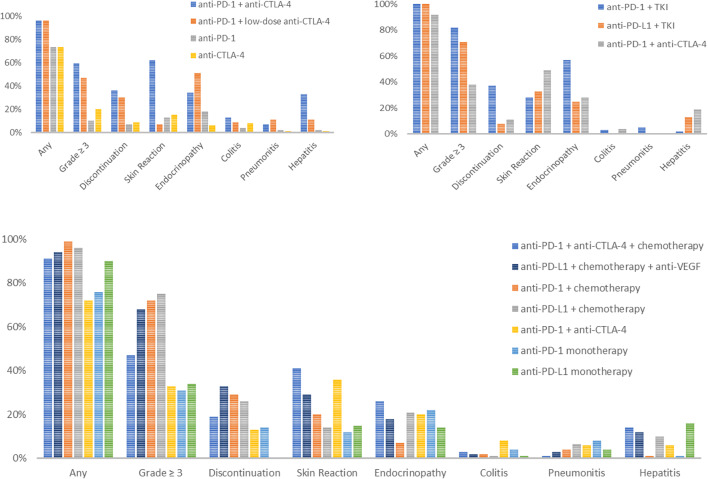
Unfortunately, the cost of such success has been a concomitant increase in the frequency of immune-related adverse events (irAEs) [8]. irAEs are common, problematic, and can be a barrier to further cancer-directed therapies. Figure 1 shows the frequency of treatment-related adverse events (TRAEs) and select irAEs in clinical trials using ICIs in the front-line setting [3, 4, 9–19]. The rate of TRAEs differs between cancer types, the dose of the ICI, the use of dual checkpoint blockade (e.g. anti-CTLA-4 combined with anti-PD-1) versus monotherapy with anti-PD-1, anti-PD-L1, or anti-CTLA-4, and in combination with other therapies, such as chemotherapy, anti-VEGF, or tyrosine-kinase inhibitors (TKIs). In general, the rates and severity of TRAEs increase with combination therapy. There is also an increased rate of treatment discontinuation, ranging between 7 and 14% for ICI monotherapy and 11–36% for anti-PD-1 and anti-CTLA-4 combination therapy [9–12, 15]. These rates are higher when a TKI or chemotherapy is added [4, 18].
Fig. 1.
Treatment-related adverse events for select clinical trials in cutaneous melanoma (A), renal cell carcinoma (B), and non-small cell lung cancer (C).
Fortunately, we now have effective therapies to treat irAEs. Skin rash of mild severity can often be controlled with topical therapy alone [20], anti-diarrheal medications can be used for mild colitis/diarrhea [21], and many of the endocrinopathies can be treated with hormone replacement [22]. As a result, there is an increasing number of patients who experience irAEs of mild severity that can continue ICIs without a period of discontinuation. Systemic immunosuppressive therapies can be used for the more severe and/or disabling irAEs, often with a period of discontinuation [23]. Corticosteroids are most commonly used in this setting.
This article will review the current knowledge and understanding of the how, when, and who to rechallenge with ICIs after a period of discontinuation due to toxicity. Like any treatment decision, the decision to rechallenge with ICIs will depend on the risk/benefit ratio. The focus of this article will be on what is known about rechallenge risk, ways to mitigate this risk, and a suggested approach to weighing the risks and benefits. Additional guidance on the acute management of common irAEs has previously been published [23–26].
Rechallenge with immune checkpoint inhibitor(s) after irAE
Restarting the same immune checkpoint inhibitor(s)
The data that is currently available in the rechallenge setting is all retrospective in nature. No prospective clinical trials have been initiated or published on this topic in the National Institutes of Health registry [27]. The highest level of evidence to date comes from a systematic review and meta-analysis by Zhao et al. (2021) [28•]. Results from this publication and a multitude of cohort studies will be discussed in this section. Most of these studies provide analysis of combined tumor types (referred to as “mixed histology” hereafter) and include different ICI treatment regimens, with a few exceptions that focus on non-small cell lung cancer (NSCLC), renal cell carcinoma (RCC), colorectal cancer (CRC), or melanoma. Here, we have attempted to resolve trends in irAE frequency and type among the different histologies and ICI regimens. Results from select studies are shown in Table 1 [29–41].
Table 1.
Retrospective studies evaluating irAEs after ICI rechallenge
| Rechallenge type | Study | Cancer type | Patients rechallenged | ICI(s) | irAE-2, n (%) | irAE-2 grade ≥3, n (%) | Same irAE, n (%) | Discontinuation ratea | ORR | DCR |
|---|---|---|---|---|---|---|---|---|---|---|
| Restart ICI(s) | Allouchery et al.b | Mixed | 180 | 142 anti-PD-1; 9 anti-PD-L1; 11 anti-CTLA-4; 18 anti-PD-1 + anti-CTLA-4 | 70 (39) | 27/180 (15) | 52/180 (29) | 47/180 (26) | NR | NR |
| Bhatlapenumarthi et al. | Mixed | 27 | 25 anti-PD-1; 2 anti-PD-L1 | 9 (33) | NR | 7/27 (26) | NR | NR | NR | |
| Dolladille et al. | Mixed | 60 | anti-PD-(L)1 + anti-CTLA-4 | NR | NR | 18/60 (30) | NR | NR | NR | |
| 370 | anti-PD-(L)1 | NR | NR | 105/370 (28) | NR | NR | NR | |||
| 22 | anti-CTLA-4 | NR | NR | 7/22 (32) | NR | NR | NR | |||
| Kartolo et al.b | Mixed | 40 | 28 anti-PD-1; 2 anti-CTLA-4; 5 anti-PD-1 + anti-CTLA-4; 5 ICI + chemotherapy | 31 (78) | NR | 19/40 (48) | 8/40 (20) | NR | NR | |
| Simonaggio et al.b | Mixed | 40 | 26 anti-PD-1; 5 anti-PD-L1; 4 anti-PD-1 + anti-CTLA-4; 4 anti-PD-(L)1 + other ICI; 1 other ICI | 22 (55) | 13/40 (33) | 17/40 (43) | NR | NR | NR | |
| Morse et al. | CRC | 25 | anti-PD-1 + low-dose anti-CTLA-4 | 14 (56) | 6/25 (24) | NR | NR | NR | NR | |
| Mouri et al. | NSCLC | 21 | anti-PD-1 | 15 (71) | 1/21 (5) | 9/21 (43) | NR | 15/20c (75) | 18/20c (90) | |
| Niki et al. | NSCLC | 11 | anti-PD-1 | 5 (45) | 0 (0) | NR | 0 (0) | 3 (50) | 4 (67) | |
| Santini et al. | NSCLC | 38 | 24 anti-PD-(L)1; 14 anti-PD-(L)1 + anti-CTLA-4 | 20 (52) | 10/38 (26) | 10/38 (26) | NR | 5/38 (13) | 33/38 (87) | |
| 8 | anti-PD-(L)1 + anti-CTLA-4 | 4 (50) | NR | NR | NR | NR | NR | |||
| Alaiwi et al. | RCC | 36 | 15 anti-PD-(L)1; 11 anti-PD-1 + anti-CTLA-4; 10 anti-PD-(L)1 + anti-VEGF/other | 18 (50) | 7/36 (19) | 6/36 (17) | 10/36 (28) | 6/35c (17) | 30/35c (86) | |
| De-escalation | Dolladille et al. | Mixed | 25 | anti-PD-1 + anti-CTLA-4 → anti-PD-(L)1 | 15 (60) | NR | 11 (44) | NR | NR | NR |
| 11 | anti-PD-1 + anti-CTLA-4 → anti-CTLA-4 | 4 (36) | NR | 2 (18) | NR | NR | NR | |||
| Pollack et al. | Melanoma | 80 | anti-PD-1 + anti-CTLA-4 → anti-PD-1 | 40 (50) | 14/80 (18) | 14/80 (18) | 24/80 (30) | 56/80 (70) | 71/80 (89) | |
| Santini et al. | NSCLC | 6 | anti-PD-1 + anti-CTLA-4 → anti-PD-(L)1 | (54) | NR | NR | NR | NR | NR | |
| Class switch | Abu-Sbeih et al. | Mixed | 64 | anti-CTLA-4 → anti-PD-(L)1 | NR | NR | 17/64 (27) | NR | NR | NR |
| 8 | anti-PD-(L)1 → anti-CTLA-4 | NR | NR | 7/8 (88) | NR | NR | NR | |||
| Menzies et al. | Melanoma | 67 | anti-CTLA-4 → anti-PD-1 | 25 (37) | 14/67 (21) | 2/67 (3) | 8/67 (12) | 27 (40) | NR |
CRC, colorectal cancer; DCR, disease control rate; ICI, immune checkpoint inhibitor; irAEs, immune-related adverse events; irAE-2, irAE after rechallenge; NSCLC, non-small cell lung cancer; NR, not reported; ORR, overall response rate; RCC, renal cell carcinoma
aDiscontinuation rate due to toxicity
bDid not include grade 1 irAE
cOne patient was not evaluable
In mixed histology studies, the risk of developing any irAE after restarting the same regimen was 33–78% [38, 40]. In the largest study that compared anti-PD-(L)1, anti-CTLA-4, and combination therapy, anti-CTLA-4 rechallenge had a slightly higher rate of the same irAE recurring (32%) vs combination therapy (30%) vs anti-PD-(L)1 alone (28%) [39]. It is important to note that trends between different cancer types may be obscured in mixed histology studies. In one study, patients with melanoma had higher rates of second irAEs (47%), followed by NSCLC (36%), and RCC (11%) [41]. Other NSCLC studies have reported a rate of second irAEs between 45 and 71% [33–35], and the single RCC study reported an irAE rate of 50% [32]. These differences in irAE frequency are at least partially explained by variation in grading and reporting, as some studies did not include grade 1 events.
The risk of developing the same irAE in mixed histology studies after restarting the same ICI(s) was 26-48% [37, 38, 40]. The risk of developing a new irAE was 13-30% [37, 41]. Notably, among patients who did experience a second irAE, the majority (61–78%) experienced a recurrence of the same irAE that initially led to ICI discontinuation [38, 40]. Regimens including anti-CTLA-4 were associated with a higher risk of the same irAE recurring [39].
In NSCLC, the risk of developing any irAE after restarting the same ICI(s) was 50–71% [33, 35]. The majority of patients had a recurrence of the same irAE (50–60%) with the remaining 40–50% of patients experiencing a new irAE. The objective response rate (ORR) ranged from 13 to 75%, while the disease control rate (DCR) was 67–90% [33–35]. This is consistent with the pooled ORR and DCR reported in the meta-analysis of 43.1% and 71.9%, respectively [28•].
There was one cohort of RCC patients who restarted the same ICI(s) [32]. The rate of second irAEs was 50%, 33% were recurring irAEs. This is inconsistent with the other studies that reported rates of 61–78%, and may suggest that RCC patients are more likely to develop new irAEs with rechallenge, although more studies are needed to compare these data [38, 40]. The ORR in this cohort was 17% and the DCR was 86% [32]. The ORR and DCR for RCC was similar to what was reported in cohorts with different cancer types.
In the only colorectal cancer (CRC) study available to date, patients mismatch repair-deficient CRC retreated with anti-PD-1 and anti-CTLA-4 combination had a second irAE rate of 56%, 43% of which were grade ≥ 3 [36]. The rates of recurrent versus new irAEs were not reported in this study. Likewise, no data was provided on the ORR or DCR.
The most common recurrent irAEs across studies were colitis (37–60%), arthritis/arthralgias (45–83%), skin reaction (38%), pneumonitis (20–34%), hepatitis (29–60%), and neutropenia (66.6%) [37, 39]. Patients with gastrointestinal irAEs were more likely to have recurrent grade ≥ 2 irAEs after rechallenge [41]. Similarly, a meta-analysis reported that gastrointestinal irAEs were associated with a higher recurrence of high-grade irAEs [28•]. Endocrinopathies were less likely to recur [39, 41]. This is distinct from irAEs seen with initial ICI treatment, which are most commonly dermatologic (7–62%) and endocrine (6–57%), followed by gastrointestinal, hepatic, and pulmonary irAEs (Fig. 1) [42•, 43•, 44•]. Differences in the first versus second instance of endocrine irAEs may be attributed to the fact that patients with initial endocrinopathies may be on active hormone replacement at the time of rechallenge.
Of the patients who experienced a second irAE, up to 33% experienced grade ≥ 3 events, with a pooled incidence of 12% in the meta-analysis [28•,37]. This range was similar to the percentage of patients who had initially experienced grade ≥ 3 irAEs [28•]. None of the second irAEs was more severe than the first irAE, suggesting that rechallenge with the same regimen may be safe [37].
In conclusion, upon rechallenge with the same ICI regimen, it can be expected that between 33 and 78% of patients may experience a subsequent irAE, the majority of which are likely to be the same irAE [38, 40]. This range is consistent with the largest meta-analysis to date, which reported an all-grade irAE rate with rechallenge of 34.2% [28•]. Of note, patients treated with concurrent chemotherapy or TKI were excluded in the meta-analysis. An increased likelihood of recurrence may be expected for patients who experience an initial gastrointestinal irAE [28•, 39, 41]. Less than a third of the second irAEs can be expected to be grade ≥ 3, and it is unlikely that a second irAE will be more severe than the first; this is consistent with the meta-analysis, which reported a high-grade irAE rate of 11.7% [28•]. These rates may be higher in patients treated with regimens including single-agent or combination anti-CTLA-4 [39]. This conclusion may inform clinical decision-making for patients who are unwilling or unable to tolerate the same irAE after rechallenge, especially for patients with an initial gastrointestinal irAE; for these patients, it may be useful to recommend a de-escalation approach or treatment cessation.
De-escalation
De-escalation, defined hereafter as a change from ICI combination therapy to ICI monotherapy, is another approach that can be considered after an irAE has occurred. In three de-escalation studies, resumption of anti-PD-(L)1 monotherapy had a higher rate of overall and recurrent irAEs (50–60% overall, 18–44% recurrent) when compared to resumption of anti-CTLA-4 alone (36% overall, 18% recurring) [31, 33, 39]. This is in contrast with the meta-analysis, which reported that anti-PD-(L)1 rechallenge was associated with a lower recurrence of all-grade irAEs [28•]. Patients with melanoma who switched from combination anti-PD-1 and anti-CTLA-4 to anti-PD-1 monotherapy reported overall irAE rates of 50%, with 18% grade ≥ 3 and 18% having the same irAE [31]. Interestingly, colitis seemed especially unlikely to recur in this cohort, with only 2 patients (6%) experiencing a recurrence; this contrasts with other studies above in which colitis has been reported to recur more frequently with rechallenge of the same ICI(s).
Class switch
Switching from anti-PD-(L)1 to anti-CTLA-4, or vice versa, is another option to consider. In one study of immune-mediated diarrhea and colitis (IMDC), patients switched from anti-PD-(L)1 to anti-CTLA-4 had a recurring irAE rate of 88%, compared to a rate of 27% in a cohort switched from anti-CTLA-4 to anti-PD-(L)1 [29]. Patients with melanoma who switched from anti-CTLA-4 to anti-PD-(L)1 had an overall irAE rate of 37%, the majority (56%) of which were grade ≥ 3, but only 2 irAEs were recurring (3%; arthritis and colitis); this low recurrence rate is unique among the summarized studies [30]. For patients initially treated with anti-PD-(L)1 ICIs, rechallenge with anti-CTLA-4 antibodies had a significantly higher incidence of all-grade irAEs than anti-PD-(L)1 antibody rechallenge, further supporting a restart approach over a class switch for patients with initial anti-PD-(L)1 treatment [28•]. Although there may be distinct differences in irAE rates between cancer types, these may be confounded by the treatment regimen given—i.e., anti-CTLA-4 is more likely to be used in melanoma or RCC vs NSCLC; therefore, the differences in irAE rates may be at least partially attributed to differences in regimen used between cancer types.
Chemotherapy and TKI
Can patients be rechallenged with ICIs in combination with chemotherapy or other drugs? At this time there is very little data on the success of such approaches, all of which is retrospective in nature. For the combination of ICI with chemotherapy, only one study was identified [38]. This study included 6 patients with advanced cancer who were initially treated with an ICI and chemotherapy and then developed grade ≥ 2 irAEs. Five of the patients were then rechallenged with an ICI and chemotherapy. Four of these five patients developed an irAE (80%). In the total rechallenge cohort (n=40), including patients who did not receive chemotherapy, there was a 78% chance of subsequent irAE and a 42% chance of recurrent irAE. The authors concluded that it is relatively safe to rechallenge patients with ICIs. However, it is our opinion that more data is needed to draw any meaningful conclusion on the safety and efficacy of rechallenge with combination ICI and chemotherapy.
ICIs have also been combined with tyrosine-kinase inhibitors (TKIs) in the rechallenge setting. Three separate studies identified a total of 16 patients treated with the combination of ICI and TKI, all of whom had metastatic renal cell carcinoma [32, 45, 46]. Two of the studies reported the rate of irAEs upon rechallenge, which ranged from 38-60% [32, 45]. Only one study reported ORR and DCR, which were 60% and 80% respectively [45]. While it is difficult to draw any strong conclusion from these studies due to the small sample sizes, it is notable that the risk of irAEs upon rechallenge with ICI and TKI does not appear to be any higher than that observed in patients rechallenged without a TKI. The severity of irAEs also did not exceed grade 3 toxicity in the two studies that reported this data [32, 45].
These results are promising, but larger studies are needed to confirm these findings. There will also be interest in studying the combination of ICI and TKI rechallenge across additional cancer types. For example, advanced endometrial cancer, where the combination of ICI and anti-VEGF TKI is now approved in the second-line setting [47, 48].
Rechallenge with concurrent immunosuppression
Our current data on the prevention of irAEs is limited to outcomes observed from case series and retrospective studies. Table 2 summarizes these findings [29, 49, 50]. First-line management of irAEs often involves the use of corticosteroids. Their use in the preventive setting remains to be fully elucidated. In one meta-analysis of 16 studies, patients receiving ICI and steroids for any reason were at increased risk for death (HR = 1.54; 95% CI 1.24–1.91) and disease progression (HR 1.34; 95% CI 1.02–1.76) compared to patients not receiving steroids [51]. The risk of death was the highest in the subset of patients who were receiving corticosteroids for palliative indications (HR 2.5; 95% CI 1.41–4.43) with outcomes likely owing to the overall poor-prognosis of this subgroup. On the other hand, steroids used to mediate irAEs were not associated with worse overall survival (HR 1.08, 95% CI 0.79-1.49).
Table 2.
Studies evaluating irAEs after ICI rechallenge with concurrent IST
| Study | Cancer type | ICI(s) | irAE-1 | Patients rechallenged with IST | IST, n (%) | irAE-2, n (%) | Recurrent irAE, n (%) | New irAE, n (%) | Recurrent irAE grade ≥3, n (%) | Discontinuation ratea | ORR | DCR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kim et al. | Melanoma | anti-PD-1 → anti-CTLA-4 | Arthritis | 1 | Tocilizumab | NR | 0 (0) | NA | NA | NA | NR | NR |
| Abu-Sbeih et al. | Mixed | 47 anti-CTLA-4; 79 anti-PD-(L)1; 41 ICI combination | Colitis | 113 | Corticosteroid, 113 (100) Infliximab or Vedolizumab, 24 (14) | NR | 47 (42) | NR | NR | NR | NR | NR |
| Badran et al. | Mixed | 1 anti-CTLA-4; 2 anti-PD-1; 2 anti-CTLA-4 + anti-PD-1 | Colitis | 5 | Infliximab, 5 (100) | 3 (60) | 1 (20) | 2 (40) | 2 (40) | 1/5 (20) | 20% | 80% |
DCR, disease control rate; ICI, immune checkpoint inhibitor; irAEs, immune-related adverse events; irAE-1, irAE before rechallenge; irAE-2, irAE after rechallenge; IST, immunosuppressive therapy; NA, not applicable; NR, not reported; ORR, overall response rate
aDiscontinuation rate due to toxicity
Cytokine inhibitors are therapeutic options when immune-mediated toxicities are refractory to corticosteroids. Their role in irAE prevention were first studied in colon cancer mouse models where the concurrent use of TNF inhibitors with dual anti-CTLA-4 and PD-1 therapy improved autoimmune colitis and enhanced anti-tumor efficacy [52]. In one case series, five patients were treated concomitantly with infliximab in combination with anti-CTLA-4 and PD-(L)1 therapy or PD-1 monotherapy as secondary prevention for autoimmune colitis [49]. Doses of ICI administered along with infliximab ranged from 2 to 12 doses. In 4 out of 5 patients who had repeat endoscopies 3–4 months after concurrent infliximab and ICI therapy, no acute inflammation was observed endoscopically to suggest colitis recurrence. All but one patient in this case series had disease progression following anti-TNFα and ICI therapy. The incidence of recurrent colitis was 20%. In comparison, the incidence of recurrent IMDC was 34% in one study that examined patients rechallenged with ICI alone [29].
Combining TNFα inhibitors with ICIs is a potential strategy for irAE prevention. Contrary to the efficacy observed in mouse models, anti-TNFα with or without steroids for the treatment of steroid-refractory immunotherapy-related enterocolitis was associated with a significantly decreased OS compared to patients who received steroids alone with a median OS of 17 and 27 months, respectively (HR 1.61; 95% CI, 1.03-1.51) [49]. A phase 1b study, the TICIMEL trial, is currently underway to evaluate the safety, tolerability and clinical outcomes of combined anti-TNFα and ICI therapy in patients with advanced melanoma [53].
Administration of other cytokine inhibitors in addition to anti-TNFα agents have been reported including tocilizumab, an anti-IL6 receptor monoclonal antibody. One case report of a patient with advanced melanoma and refractory Crohn’s disease receiving concurrent therapy with pembrolizumab and tocilizumab experienced a delay in Crohn’s disease exacerbation for at least 16 weeks while retaining an antitumor response [54]. Another small study included 2 patients who received tocilizumab prophylactically with no irAE occurrence [55].
Targeting cytokines may represent a mechanism of preventing irAEs with ongoing studies investigating the use of cytokines as predictive biomarkers for identifying patients at risk for irAEs. For example, increased serum IL-17 concentrations were found in metastatic melanoma patients treated with ipilimumab who developed immunotherapy-related colitis [56, 57]. Serum IL-17 concentrations decreased to levels equivalent to that of patients without colitis following the resolution of colitis, suggesting the correlation between cytokine concentrations and irAE disease status [56–58]. Whether specific cytokine inhibition based on cytokine profiling prevents irAEs in patients on ICIs still has yet to be determined.
Additionally, vedolizumab, a monoclonal antibody that targets α4β7 integrin and inhibits gastrointestinal lymphocyte trafficking, was studied in a small subset of patients receiving concurrent ICI following autoimmune colitis resolution [29, 59]. One out of 8 patients treated with vedolizumab experienced a colitis recurrence while recurrence occurred in 3 out of 6 patients who did not receive vedolizumab [29]. For steroid-refractory IMDC, one retrospective study found that vedolizumab was associated with superior survival outcomes compared to infliximab [60].
Suggested approach to ICI rechallenge
The decision to rechallenge a patient with ICI(s) after toxicity is complex and should take into account the risks and benefits of retreatment, as well as the patient’s preference to resume therapy. The patient may have a strong opinion about ICI rechallenge, particularly if the toxicity was severe and/or had a significant impact on the quality of life. It therefore behooves the treating clinician to elucidate any concerns the patient may have about retreatment, and to actively engage in shared decision making whenever possible. Once a discussion has been initiated, the patient and provider will want to discuss the risks and benefits of retreatment that are categorized below, noting that additional questions/concerns may be of importance.
One of the first questions to consider is whether alternative treatment options exist that have a higher chance of benefiting the patient. While ICI is a useful treatment modality, targeted therapies, chemotherapy, and even local therapies (in the setting of oligoprogressive or oligometastatic disease) have shown efficacy in the second-line and beyond for many cancer types. Careful consideration should be given to the rates of response and toxicity between alternative agents.
As shown in Table 1, the ORR and toxicity of ICI rechallenge can be highly variable, even within the same tumor type. The DCR has been more consistent with ICI rechallenge across tumor types and drug class, ranging between 86 and 90% [31–33, 35], and this should be considered when stable disease is an acceptable outcome. More recently, a systematic review and meta-analysis reported a pooled ORR and DCR after rechallenge of 43.1% and 71.9%, respectively across all cancer types [28•]. There have been no clinical trials to date to provide an accurate comparison of efficacy with alternative treatments. In general, we recommend an alternate treatment over ICI rechallenge when there is a reasonable chance the patient will have a better response to the alternative choice. When the alternatives to ICI rechallenge have a low efficacy rate, the focus should next be on the safety of ICI rechallenge.
Safety will depend on a number of factors including, which organ experienced the initial irAE, the severity of the irAE and how difficult it was to manage, patient-specific factors, and whether the patient will be rechallenged with ICI alone or in combination with other agents. The target organ and severity of the initial irAE should be carefully considered due to the risk of recurrent irAEs, which is reportedly between 17 and 88% [29, 32]. The risk of any irAE is even higher when accounting for new irAEs. A systematic review and meta-analysis reported a pooled incidence of all-grade and ≥ grade 3 irAEs after rechallenge of 34.2% and 11.7%, respectively across all cancer types [28•].
The target organ of the initial irAE becomes particularly relevant when toxicity to that organ has a high risk of morbidity/mortality (e.g. cardiac or CNS toxicity). In these instances, it is generally advised to avoid rechallenge for any toxicity above grade 1 [24]. Conversely, if the irAE manifests as an endocrinopathy, even severe toxicities can be managed with hormone replacement while the ICI is continued. General practice recommendations include holding ICI treatment for toxicities that are grade ≥ 2 until the toxicity has reverted to grade ≤ 1 and permanent discontinuation of ICI(s) for grade 4 toxicities. Additional guideline recommendations on ICI rechallenge for each organ system have been published [24, 26].
Patient-specific factors include, comorbidities, performance status (PS), and expected longevity, each of which can influence the decision to rechallenge. The link between autoimmune disorders and irAEs is well-known, but emerging data has also shown an increased risk of immune-mediated pneumonitis with pre-existing pulmonary disease [61, 62]. Similar relationships may exist between the irAE target organ and the underlying health/function of that organ. This remains an area of active investigation.
Poor PS, defined as Eastern Cooperative Oncology Group (ECOG) PS ≥ 2, has been associated with decreased efficacy of ICIs. Multiple retrospective studies have shown shorter progression free survival (PFS) and OS when the ECOG PS was ≥ 2 [63–67]. ORR was also lower across cancer types, with the exception of advanced urothelial cancer. Patients with an ECOG PS ≥ 2 were also less likely to be referred to hospice and more likely to die in the hospital when they were treated with ICIs [66, 67]. Based on the above, it would be expected that the efficacy of ICI rechallenge would be significantly less if the patient’s PS is ECOG ≥ 2. Many of these patients will qualify for hospice, and the treating physician should consider what the patient’s goals are for end-of-life care. A frank discussion of these goals may impact the decision to rechallenge with ICI(s).
Finally, once a decision has been made to rechallenge, the focus should shift to the when, what, and how. The “when” will be determined by the severity of the initial irAE and the urgency of treatment. Guideline recommendations include holding ICI treatment for toxicities that are grade ≥ 2 until the toxicity has reverted to grade ≤ 1 with few exceptions [24]. If the patient requires urgent treatment, the treating clinician should re-evaluate whether ICI rechallenge is the best available option and if local therapies can be used either before or concurrently with ICI(s).
What ICI to use, either as monotherapy or in combination, should also be carefully considered. There is no randomized data in the rechallenge setting to inform this decision, and even the limited retrospective studies have not done a direct comparison between the different approaches (de-escalation, ICI restart, or class switch). In general, we favor a de-escalation approach when dual-checkpoint blockade was the original treatment modality. This is supported by data in the front-line setting, where multiple phase 3 clinical trials have shown higher rates of irAEs and treatment discontinuation with ICI combinations compared to monotherapy [3, 16]. There is again limited data to compare the efficacy and toxicity of a class switch approach, but the toxicity profile appears to be similar when switching from anti-CTLA-4 to anti-PD-(L)1 compared to a restart of anti-CTLA-4 ICI [29, 30, 39]. On the other hand, switching from anti-PD-(L)1 to anti-CTLA-4 had a much higher toxicity rate [29]. This approach is not recommended at this time.
The “how” refers to the decision of whether to add or continue immunosuppression with the ICI(s), general oversight, and monitoring recommendations. At the present time, there is insufficient data to recommend concurrent immunosuppression with ICI rechallenge. However, multiple small-scale studies suggest there may be improved safety with this approach, and further research is warranted [29, 49, 50]. Ideally, every rechallenge decision should include input from a multidisciplinary team, and a systematic process should be established to review individual cases whenever possible. Close monitoring is recommended. If a subsequent irAE occurs, permanent discontinuation is advised in most instances.
Declarations
Conflict of Interest
Sophia Bylsma declares that she has no conflict of interest. Karen Yun declares that she has no conflict of interest. Sandip Patel receives scientific advisory income from Amgen, AstraZeneca, Bristol-Myers Squibb, Certis, Eli Lilly, Genentech, Illumina, Merck, Pfizer, Rakuten, and Tempus. His university receives research funding from Amgen, AstraZeneca/MedImmune, Bristol-Myers Squibb, Eli Lilly, Fate Therapeutics, Iovance, Merck, Pfizer, Roche/Genentech, and SQZ Biotechnologies. Michael Dennis declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Lung Cancer
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Esfahani K, Roudaia L, Buhlaiga N, Del Rincon SV, Papneja N, Miller JWH., Jr A review of cancer immunotherapy: from the past, to the present, to the future. Curr Oncol (Toronto, Ont) 2020;27(Suppl 2):S87–S97. doi: 10.3747/co.27.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 3.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motzer R, Alekseev B, Rha S, Porta C, Eto M, Powles T, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289–1300. doi: 10.1056/NEJMoa2035716. [DOI] [PubMed] [Google Scholar]
- 5.Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 6.Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810–821. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 7.Choueiri TK, Tomczak P, Park SH, Venugopal B, Ferguson T, Chang Y, et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med. 2021;385(8):683–694. doi: 10.1056/NEJMoa2106391. [DOI] [PubMed] [Google Scholar]
- 8.Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A, Guex-Crosier Y, Kuntzer T, Michielin O, Peters S, Coukos G, Spertini F, Thompson JA, Obeid M. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16(9):563–580. doi: 10.1038/s41571-019-0218-0. [DOI] [PubMed] [Google Scholar]
- 9.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 10.Carlino MS, Menzies AM, Atkinson V, Cebon JS, Jameson MB, Fitzharris BM, et al. Long-term follow-up of standard-dose pembrolizumab plus reduced-dose ipilimumab in patients with advanced melanoma: KEYNOTE-029 part 1B. Clin Cancer Res. 2020;26(19):5086. doi: 10.1158/1078-0432.CCR-20-0177. [DOI] [PubMed] [Google Scholar]
- 11.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob J, Rutkowski P, Lao CD, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–1546. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 12.Hammers HJ, Plimack ER, Infante JR, Rini BI, McDermott DF, Lewis LD, et al. Safety and efficacy of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma: the CheckMate 016 study. J Clin Oncol. 2017;35(34):3851–3858. doi: 10.1200/JCO.2016.72.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103–1115. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, et al. Atezolizumab for first-line treatment of PD-L1–selected patients with NSCLC. N Engl J Med. 2020;383(14):1328–1339. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- 15.Reck M, Delvys Rodríguez-Abreu, Robinson AG, Hui R, Tibor Csőszi, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol 2019;37(7):537-546. [DOI] [PubMed]
- 16.Hellmann MD, Rizvi NA, Goldman JW, Gettinger SN, Borghaei H, Brahmer JR, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 2016;18(1):31–41. doi: 10.1016/S1470-2045(16)30624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.West H, Mccleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (Impower130): a multicentre, randomized, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 18.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 19.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, Finley G, Kelsch C, Lee A, Coleman S, Deng Y, Shen Y, Kowanetz M, Lopez-Chavez A, Sandler A, Reck M, IMpower150 Study Group Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 20.Nadelmann ER, Yeh JE, Chen ST. Management of cutaneous immune-related adverse events in patients with cancer treated with immune checkpoint inhibitors: a systematic review. JAMA Oncol. 2022;8(1):130–138. doi: 10.1001/jamaoncol.2021.4318. [DOI] [PubMed] [Google Scholar]
- 21.Samaan MA, Pavlidis P, Papa S, Powell N, Irving PM. Gastrointestinal toxicity of immune checkpoint inhibitors: from mechanisms to management. Nat Rev Gastroenterol Hepatol. 2018;15(4):222–234. doi: 10.1038/nrgastro.2018.14. [DOI] [PubMed] [Google Scholar]
- 22.Wright JJ, Powers AC, Johnson DB. Endocrine toxicities of immune checkpoint inhibitors. Nat Rev Endocrinol. 2021;17(7):389–399. doi: 10.1038/s41574-021-00484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, Gerber DE, Hamad L, Hansen E, Johnson DB, Lacouture ME, Masters GA, Naidoo J, Nanni M, Perales MA, Puzanov I, Santomasso BD, Shanbhag SP, Sharma R, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. 2021;9(6). [DOI] [PMC free article] [PubMed]
- 24.National Comprehensive Cancer Network. Management of immunotherapy-related toxicities (Version 4.2021). 2021; Available at: https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf. Accessed 1 Feb 2022.
- 25.Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv119–iv142. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 26.Brahmer JR, Lacchetti C, Thompson JA. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of clinical oncology clinical practice guideline summary. J Oncol Pract. 2018;14(4):247–249. doi: 10.1200/JOP.18.00005. [DOI] [PubMed] [Google Scholar]
- 27.NIH. ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/results?cond=&term=rechallenge+immunotherapy&cntry=&state=&city=&dist=. Accessed 1 Feb 2022.
- 28.• Zhao Q, Zhang J, Xu L, Yang H, Liang N, Zhang L, et al. Safety and efficacy of the rechallenge of immune checkpoint inhibitors after immune-related adverse events in patients with cancer: a systemic review and meta-analysis. Front Immunol. 2021;12:730320. 10.3389/fimmu.2021.730320. Systematic review and meta-analysis of clinical outcomes, including rates of irAEs after ICI rechallenge due to toxicity. [DOI] [PMC free article] [PubMed]
- 29.Abu-Sbeih H, Faisal, Ali S, Abdul, Naqash R, Owen DH, et al. Resumption of immune checkpoint inhibitor therapy after immune-mediated colitis. J Clin Oncol 2019;37:2738. [DOI] [PMC free article] [PubMed]
- 30.Menzies AM, Johnson DB, Ramanujam S, Atkinson VG, Wong ANM, Park JJ, McQuade JL, Shoushtari AN, Tsai KK, Eroglu Z, Klein O, Hassel JC, Sosman JA, Guminski A, Sullivan RJ, Ribas A, Carlino MS, Davies MA, Sandhu SK, Long GV. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol. 2017;28(2):368–376. doi: 10.1093/annonc/mdw443. [DOI] [PubMed] [Google Scholar]
- 31.Pollack MH, Betof A, Dearden H, Rapazzo K, Valentine I, Brohl AS, Ancell KK, Long GV, Menzies AM, Eroglu Z, Johnson DB, Shoushtari AN. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol. 2018;29(1):250–255. doi: 10.1093/annonc/mdx642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abou Alaiwi S, Xie W, Nassar AH, Dudani S, Martini D, Bakouny Z, Steinharter JA, Nuzzo PV, Flippot R, Martinez-Chanza N, Wei X, McGregor BA, Kaymakcalan MD, Heng DYC, Bilen MA, Choueiri TK, Harshman LC. Safety and efficacy of restarting immune checkpoint inhibitors after clinically significant immune-related adverse events in metastatic renal cell carcinoma. J Immunother Cancer. 2020;8(1). [DOI] [PMC free article] [PubMed]
- 33.Santini FC, Rizvi H, Plodkowski AJ, Ni A, Lacouture ME, Gambarin-Gelwan M, Wilkins O, Panora E, Halpenny DF, Long NM, Kris MG, Rudin CM, Chaft JE, Hellmann MD. Safety and efficacy of re-treating with immunotherapy after immune-related adverse events in patients with NSCLC. Cancer Immunol Res. 2018;6(9):1093–1099. doi: 10.1158/2326-6066.CIR-17-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niki M, Nakaya A, Kurata T, Yoshioka H, Kaneda T, Kibata K, Ogata M, Nomura S. Immune checkpoint inhibitor re-challenge in patients with advanced non-small cell lung cancer. Oncotarget. 2018;9(64):32298–32304. doi: 10.18632/oncotarget.25949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mouri A, Kaira K, Yamaguchi O, Shiono A, Miura Y, Hashimoto K, Nishihara F, Murayama Y, Kobayashi K, Kagamu H. Clinical difference between discontinuation and retreatment with nivolumab after immune-related adverse events in patients with lung cancer. Cancer Chemother Pharmacol. 2019;84(4):873–880. doi: 10.1007/s00280-019-03926-y. [DOI] [PubMed] [Google Scholar]
- 36.Morse MA, Overman MJ, Hartman L, Khoukaz T, Brutcher E, Lenz H, et al. Safety of nivolumab plus low-dose ipilimumab in previously treated microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer. Oncologist. 2019;24(11):1453–1461. doi: 10.1634/theoncologist.2019-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simonaggio A, Michot JM, Voisin AL, Le Pavec J, Collins M, Lallart A, et al. Evaluation of readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol. 2019;5(9):1310–1317. doi: 10.1001/jamaoncol.2019.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kartolo A, Holstead R, Khalid S, Emack J, Hopman W, Baetz T. Safety of immunotherapy rechallenge after immune-related adverse events in patients with advanced cancer. J Immunother. 2020;44(1):41–48. doi: 10.1097/CJI.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 39.Dolladille C, Ederhy S, Sassier M, Cautela J, Thuny F, Cohen AA, Fedrizzi S, Chrétien B, da-Silva A, Plane AF, Legallois D, Milliez PU, Lelong-Boulouard V, Alexandre J. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol. 2020;6(6):865–871. doi: 10.1001/jamaoncol.2020.0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhatlapenumarthi V, Patwari A, Harb AJ. Immune-related adverse events and immune checkpoint inhibitor tolerance on rechallenge in patients with irAEs: a single-center experience. J Cancer Res Clin Oncol. 2021;147(9):2789–2800. doi: 10.1007/s00432-021-03610-w. [DOI] [PubMed] [Google Scholar]
- 41.Allouchery M, Lombard T, Martin M, Rouby F, Sassier M, Bertin C, Atzenhoffer M, Miremont-Salame G, Perault-Pochat MC, Puyade M. Safety of immune checkpoint inhibitor rechallenge after discontinuation for grade ≥2 immune-related adverse events in patients with cancer. J Immunother Cancer. 2020;8(2). [DOI] [PMC free article] [PubMed]
- 42.• Zhang Y, La B, Liang B, Gu Y. Treatment-related adverse events with PD-1 or PD-L1 inhibitors: a systematic review and meta-analysis. Life (Basel). 2021;11(11):1277. 10.3390/life11111277. Systematic review and meta-analysis of treatment-related adverse events with immune checkpoint inhibitors. [DOI] [PMC free article] [PubMed]
- 43.• Duan J, Cui L, Zhao X, Bai H, Cai S, Wang G, et al. Use of immunotherapy with programmed cell death 1 vs programmed cell death ligand 1 inhibitors in patients with cancer: a systematic review and meta-analysis. JAMA Oncol. 2019;6(3):375-384. Systematic review and meta-analysis of treatment-related adverse events with immune checkpoint inhibitors. [DOI] [PMC free article] [PubMed]
- 44.• Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol. 2019;5(7):1008-1019. Systematic review and meta-analysis of treatment-related adverse events with immune checkpoint inhibitors. [DOI] [PMC free article] [PubMed]
- 45.Siddiqui BA, Gheeya JS, Goswamy R, Bathala TK, Surasi DS, Gao J, Shah A, Campbell MT, Msaouel P, Goswami S, Wang J, Zurita AJ, Jonasch E, Corn PG, Aparicio AM, Siefker-Radtke AO, Sharma P, Subudhi SK, Tannir N. Durable responses in patients with genitourinary cancers following immune checkpoint therapy rechallenge after moderate-to-severe immune-related adverse events. J Immunother Cancer. 2021;9(7):e002850. doi: 10.1136/jitc-2021-002850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ravi P, Mantia C, Su C, Sorenson K, Elhag D, Rathi N, Bakouny Z, Agarwal N, Zakharia Y, Costello BA, McKay RR, Narayan V, Alva A, McGregor BA, Gao X, McDermott DF, Choueiri TK. Evaluation of the safety and efficacy of immunotherapy rechallenge in patients with renal cell carcinoma. JAMA Oncol. 2020;6(10):1606–1610. doi: 10.1001/jamaoncol.2020.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Makker V, Rasco D, Vogelzang NJ, Brose MS, Cohn AL, Mier J, di Simone C, Hyman DM, Stepan DE, Dutcus CE, Schmidt EV, Guo M, Sachdev P, Shumaker R, Aghajanian C, Taylor M. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20(5):711–718. doi: 10.1016/S1470-2045(19)30020-8. [DOI] [PubMed] [Google Scholar]
- 48.FDA. FDA grants regular approval to pembrolizumab and lenvatinib for advanced endometrial carcinoma. 2022; Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-pembrolizumab-and-lenvatinib-advanced-endometrial-carcinoma. Accessed 1 Feb 2022.
- 49.Badran YR, Cohen JV, Brastianos PK, Parikh AR, Hong TS, Dougan M. Concurrent therapy with immune checkpoint inhibitors and TNFα blockade in patients with gastrointestinal immune-related adverse events. J Immunother Cancer. 2019;7(1):226. doi: 10.1186/s40425-019-0711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim ST, Tayar J, Trinh VA, Suarez-Almazor M, Garcia S, Hwu P, Johnson DH, Uemura M, Diab A. Successful treatment of arthritis induced by checkpoint inhibitors with tocilizumab: a case series. Ann Rheum Dis. 2017;76(12):2061–2064. doi: 10.1136/annrheumdis-2017-211560. [DOI] [PubMed] [Google Scholar]
- 51.Petrelli F, Signorelli D, Ghidini M, Ghidini A, Pizzutilo EG, Ruggieri L, Cabiddu M, Borgonovo K, Dognini G, Brighenti M, de Toma A, Rijavec E, Garassino MC, Grossi F, Tomasello G. Association of steroids use with survival in patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Cancers (Basel) 2020;12(3):546. doi: 10.3390/cancers12030546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perez-Ruiz E, Minute L, Otano I, Alvarez M, Ochoa MC, Belsue V, de Andrea C, Rodriguez-Ruiz ME, Perez-Gracia JL, Marquez-Rodas I, Llacer C, Alvarez M, de Luque V, Molina C, Teijeira A, Berraondo P, Melero I. Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature. 2019;569(7756):428–432. doi: 10.1038/s41586-019-1162-y. [DOI] [PubMed] [Google Scholar]
- 53.Institut Claudius Regaud. TNF-inhibitor as immune checkpoint inhibitor for advanced MELanoma - a phase Ib clinical study. Available at: https://clinicaltrials.gov/ct2/show/NCT03293784. Accessed 22 Dec 2021.
- 54.Uemura M, Trinh VA, Haymaker C, Jackson N, Kim DW, Allison JP, Sharma P, Vence L, Bernatchez C, Hwu P, Diab A. Selective inhibition of autoimmune exacerbation while preserving the anti-tumor clinical benefit using IL-6 blockade in a patient with advanced melanoma and Crohn’s disease: a case report. J Hematol Oncol. 2016;9(1):81. doi: 10.1186/s13045-016-0309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dimitriou F, Hogan S, Menzies AM, Dummer R, Long GV. Interleukin-6 blockade for prophylaxis and management of immune-related adverse events in cancer immunotherapy. Eur J Cancer. 2021;157:214–224. doi: 10.1016/j.ejca.2021.08.031. [DOI] [PubMed] [Google Scholar]
- 56.Tarhini AA, Zahoor H, Lin Y, Malhotra U, Sander C, Butterfield LH, Kirkwood JM. Baseline circulating IL-17 predicts toxicity while TGF-β1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J Immunother Cancer. 2015;3(1):39. doi: 10.1186/s40425-015-0081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Callahan MK, Yang A, Tandon S, Xu Y, Subudhi SK, Roman RA, Heine AI, Pogoriler E, Kuk D, Panageas K, Yuan JD, Allison JP, Wolchok JD. Evaluation of serum IL-17 levels during ipilimumab therapy: correlation with colitis. J Clin Oncol. 2011;29(15):2505. doi: 10.1200/jco.2011.29.15_suppl.2505. [DOI] [Google Scholar]
- 58.Kang JH, Bluestone JA, Young A. Predicting and preventing immune checkpoint inhibitor toxicity: targeting cytokines. Trends Immunol. 2021;42(4):293–311. doi: 10.1016/j.it.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 59.Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel J, Sandborn WJ, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369(8):699–710. doi: 10.1056/NEJMoa1215734. [DOI] [PubMed] [Google Scholar]
- 60.Zou F, Faleck D, Thomas A, Harris J, Satish D, Wang X, Charabaty A, Ernstoff MS, Glitza Oliva IC, Hanauer S, McQuade J, Obeid M, Shah A, Richards DM, Sharon E, Wolchok J, Thompson J, Wang Y. Efficacy and safety of vedolizumab and infliximab treatment for immune-mediated diarrhea and colitis in patients with cancer: a two-center observational study. J Immunother Cancer. 2021;9(11):e003277. doi: 10.1136/jitc-2021-003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cortellini A, Buti S, Santini D, Perrone F, Giusti R, Tiseo M, Bersanelli M, Michiara M, Grassadonia A, Brocco D, Tinari N, de Tursi M, Zoratto F, Veltri E, Marconcini R, Malorgio F, Garufi C, Russano M, Anesi C, Zeppola T, Filetti M, Marchetti P, Botticelli A, Antonini Cappellini GC, de Galitiis F, Vitale MG, Sabbatini R, Bracarda S, Berardi R, Rinaldi S, Tudini M, Silva RR, Pireddu A, Atzori F, Chiari R, Ricciuti B, Iacono D, Migliorino MR, Rossi A, Porzio G, Cannita K, Ciciarelli V, Fargnoli MC, Ascierto PA, Ficorella C. Clinical outcomes of patients with advanced cancer and pre-existing autoimmune diseases treated with anti-programmed death-1 immunotherapy: a real-world transverse study. Oncologist. 2019;24(6):e327–e337. doi: 10.1634/theoncologist.2018-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Q, Tang L, Zhou Y, He W, Li W. Immune checkpoint inhibitor-associated pneumonitis in non-small cell lung cancer: current understanding in characteristics, diagnosis, and management. Front Immunol. 2021;12:663986. doi: 10.3389/fimmu.2021.663986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Friedlaender A, Metro G, Signorelli D, Gili A, Economopoulou P, Roila F, Banna G, de Toma A, Camerini A, Christopoulou A, Lo Russo G, Banini M, Galetta D, Jimenez B, Collazo-Lorduy A, Calles A, Baxevanos P, Linardou H, Kosmidis P, Mountzios G, Garassino MC, Addeo A. Impact of performance status on non-small-cell lung cancer patients with a PD-L1 tumour proportion score ≥50% treated with front-line pembrolizumab. Acta Oncol. 2020;59(9):1058–1063. doi: 10.1080/0284186X.2020.1781249. [DOI] [PubMed] [Google Scholar]
- 64.Sehgal K, Gill RR, Widick P, Bindal P, McDonald DC, Shea M, et al. Association of performance status with survival in patients with advanced non–small cell lung cancer treated with pembrolizumab monotherapy. JAMA Netw Open. 2021;4(2):e2037120. doi: 10.1001/jamanetworkopen.2020.37120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pietrantonio F, Loupakis F, Randon G, Raimondi A, Salati M, Trapani D, Pagani F, Depetris I, Maddalena G, Morano F, Corallo S, Prisciandaro M, Corti F, Guarini V, Bocconi A, Marra A, Belli C, Spallanzani A, Fassan M, Lonardi S, Curigliano G, Fucà G, di Bartolomeo M, de Braud F. Efficacy and safety of immune checkpoint inhibitors in patients with microsatellite instability-high end-stage cancers and poor performance status related to high disease burden. Oncologist. 2020;25:803–809. doi: 10.1634/theoncologist.2020-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petrillo LA, El-jawahri A, Nipp RD, Lichtenstein MRL, Durbin SM, Reynolds KL, et al. Performance status and end-of-life care among adults with non–small cell lung cancer receiving immune checkpoint inhibitors. Cancer. 2020;126(10):2288–2295. doi: 10.1002/cncr.32782. [DOI] [PubMed] [Google Scholar]
- 67.Khaki AR, Li A, Diamantopoulos LN, Bilen MA, Santos VV, Esther J, Morales-Barrera R, Devitt M, Nelson A, Hoimes CJ, Shreck E, Assi H, Gartrell BA, Sankin A, Rodriguez-Vida A, Lythgoe M, Pinato DJ, Drakaki A, Joshi M, Isaacsson Velho P, Hahn N, Liu S, Alonso Buznego L, Duran I, Moses M, Jain J, Murgic J, Baratam P, Barata P, Tripathi A, Zakharia Y, Galsky MD, Sonpavde G, Yu EY, Shankaran V, Lyman GH, Grivas P. Impact of performance status on treatment outcomes: a real-world study of advanced urothelial cancer treated with immune checkpoint inhibitors. Cancer. 2019;126(6):1208–1216. doi: 10.1002/cncr.32645. [DOI] [PMC free article] [PubMed] [Google Scholar]