What is chimeric antigen receptor T-cell therapy?
Chimeric antigen receptor (CAR) T-cell therapy is potentially life-changing in patients with haematological malignancies refractory to multiple lines of chemoimmunotherapy and stem cell transplantation. This adoptive cell immunotherapy involves harvesting and engineering patients' own T lymphocytes to express specific antitumour CARs, thereby directing them to target tumour cells. The CAR also has an intracellular, co-stimulatory domain causing in vivo CAR T-cell expansion and persistence. Chimeric antigen receptor T-cells destroy cancer cells but may also induce life-threatening, reversible toxicities that may require support from the critical care team.
The two most widely used products to date are axicabtagene (Yescarta) and tisagenlecleucel (Kymriah) for adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL). Tisagenlecleucel (Kymriah) is used for relapsed/refractory acute lymphoblastic leukaemia (ALL) in children.1, 2, 3 Phase 1 and 2 studies in patients with refractory DLBCL have reported overall response rates (ORRs) of 52–72%, where ORR is defined as the percentage of patients with partial response (reduced disease) plus the percentage of patients with complete response (disease-free), and an overall survival (OS) rate of 52% at 18 months.1,2 The median follow-up duration in these studies was 15.4 months.1 Without CAR T-cell therapy, the median OS in this group is 6.3 months.4 In children with relapsed ALL, the CAR-T-related 3-month ORR is 82% and the 5-yr OS is 61.9%, compared with 5-yr OS of 10% before the introduction of CAR-T therapy.3,5,6
Axicabtagene and tisagenlecleucel are currently approved for use at more than 260 certified treatment centres in 26 countries, with more than 300 patients treated annually in the UK. Currently licenced products target CD19-positive cancer cells with many more CAR T-cell products under investigation (647 clinical trials treating >10,000 patients worldwide). New targets include CD22 in B-cell malignancies and B-cell maturation antigen in multiple myeloma. Exploration of CAR T-cells in solid tumours, autoimmune and degenerative diseases and earlier in treatment pathways is underway.
Giving CAR T-cell therapy
Chimeric antigen receptor T-cell manufacture takes 25–30 days from lymphapheresis. Disease progression and organ failure within this time eventually prevent 22% of previously suitable UK patients from receiving CAR T-cells.
UK centres keep patients in hospital for 10–14 days after the CAR T-cell infusion. This approach allows prompt recognition of early adverse effects and early organ support if required. On discharge, patients must remain within a 1-h drive of the treating centre until Day 28.
Toxicities
Early toxicities from CAR T-cell treatment include cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS).1,2,7 Neutropaenic fevers or sepsis and tumour lysis syndrome can also occur. Late toxicities include prolonged cytopaenia, B-cell aplasia, hypogammaglobulinaemia, infection, and a risk of secondary cancers.1,2
Cytokine release syndrome
After binding to target tumour-cell receptors, CAR T-cells signal via inflammatory cytokines, stimulating further T-cell activity, which destroys cancer cells. An excessive immune response occurs in up to 90% of patients, causing a multisystem disorder (CRS) within 14 days of CAR-T therapy.1,2 Fever is characteristic, correlating with increases in C-reactive protein, tumour necrosis factor-alpha, and interleukins 6, 10, and 2. Hypoxia and hypotension (systolic BP <90 mmHg) can occur.7 Thirty per cent of patients require critical care, with 17% requiring vasopressor drugs and 7% needing tracheal intubation.1,2
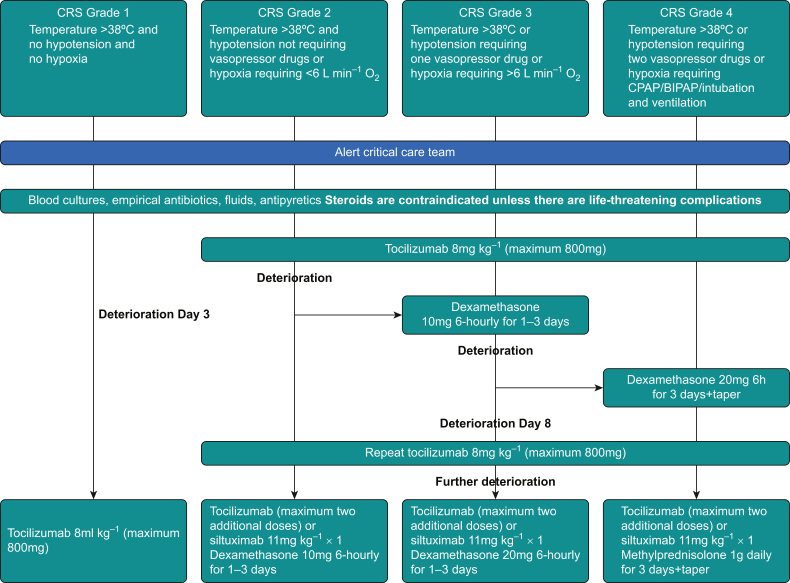
The American Society for Transplantation and Cellular Therapy recommends a graded classification system for CRS, linked to a treatment algorithm for both adults and children by the European Society for Blood and Marrow Transplantation (EBMT) and the American Society of Clinical Oncology (ASCO). Critical care evaluation is advised for Grades 2–4 CRS (Fig 1).7, 8, 9
Figure 1.
Management of cytokine release syndrome (CRS). Adapted from the European Society for Blood and Marrow Transplantation guidelines.8 BIPAP, bilevel positive airway pressure.
Patients who are affected should receive replacement fluids, oxygen and vasopressors when required. The effect of cytokine excess in low-grade CRS can be reduced by giving up to four doses of tocilizumab, a monoclonal IL-6 receptor antagonist. Steroids, which reduce the efficacy of CAR-T therapy, and the other immunomodulators anakinra (an IL-1 receptor antagonist) and siltuximab (a monoclonal antibody targeting IL-6) are used in more challenging cases (Fig 1) and during shortages in supply of tocilizumab.8, 9, 10
Immune effector cell-associated neurotoxicity syndrome
The pathophysiology of CAR T-cell-induced neurotoxicity remains uncertain. Immune effector cell-associated neurotoxicity syndrome occurs in 30–60% of patients and can occur concurrently with CRS.1,2 It may develop more than 30 days after infusion. This means that some patients will present in a critically unwell state to non-CAR-T centres.2
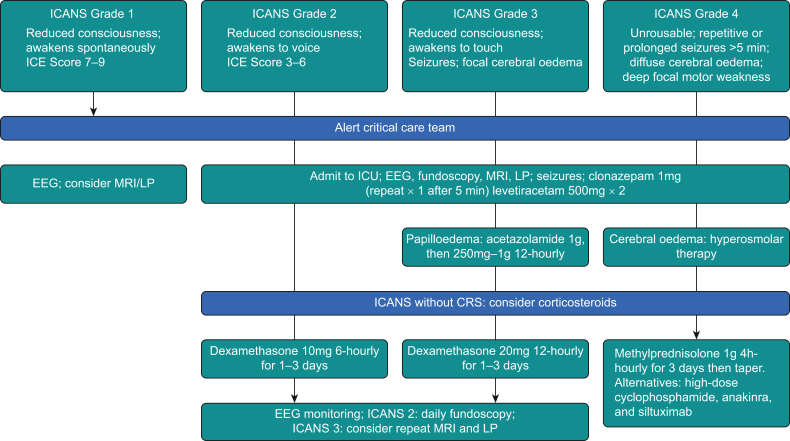
Symptoms of ICANS are varied and sometimes subtle, but the syndrome may cause rapid deterioration.1,2,7 Tremor, inattention, apraxia and lethargy are common. Deterioration in handwriting is an early diagnostic sign and can be used in assessing the response to treatment. Expressive aphasia is characteristic. Clinical diagnosis is aided by the immune effector cell encephalopathy score, performed 8-hourly during the first 28 days.7 Scored out of 10, it assesses orientation, naming of three objects, the ability to follow commands, writing a sentence and counting. Differential diagnoses for ICANS, such as seizures, intra-cerebral haemorrhage, infection and cytotoxic drug effects, must be excluded. Most cases are self-limiting, but progression can lead to diffuse cerebral oedema and death. Steroids form the mainstay of treatment; tocilizumab is reserved for patients with concurrent CRS.8,9 The EBMT guidelines recommend clonazepam and levetiracetam for the treatment of seizures, acetazolamide for the treatment of papilloedema and hyper-osmolar therapy for cerebral oedema.8 Patients with ICANS Grades ≥2 warrant monitoring in a critical care unit. Neuroprotective measures are required in those patients with ICANS Grades ≥3 (Fig 2).7
Figure 2.
Management of chimeric antigen receptor T-cell-related neurotoxicity. Adapted from Société française de greffe de moelle et de thérapie cellulaire recommendations.11 CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; ICE, immune effector cell-associated encephalopathy; LP, lumbar puncture.
Implications for critical care
The growing use of CAR T-cell therapy is likely to increase the demand for ICU beds. Treatment-related mortality remains low (<5%), highlighting the reversible nature of these toxicities.1,12 Many patients require critical care monitoring rather than intervention. Early identification of toxicities and prompt management according to the EBMT and ASCO protocols may reduce the demand for critical care.8,9
Conclusions
Chimeric antigen receptor T-cell therapy offers great hope; yet, there are significant adverse effects. Expanding indications increase the likelihood of patients presenting outside CAR T-cell therapy specialist centres, making it imperative that intensivists are alert to potential complications. Patients who receive CAR T-cell treatment have a predictable and salvageable course of deterioration that usually responds well to intervention. Multidisciplinary platforms integrating pathways between haemato-oncology and critical care teams are needed in an era where cancer immunotherapy is altering perceptions at the interface between end-of-life and critical care.
Declaration of interests
The authors declare that they have no conflicts of interest.
Biographies
Faiuna Haseeb MRCP FFICM MPhil is a specialty registrar in intensive care medicine in the North West region, UK.
Anthony Wilson MSc MRCP FRCA FFICM is a consultant in anaesthesia and intensive care medicine and the critical care lead for CAR-T therapy at Manchester University NHS Foundation Trust.
Eleni Tholouli MRCP FRCPath, MD PhD is the transplant director and director of the GM Haematology-Oncology Pathway Board at Manchester University NHS Foundation Trust, one of 12 expert CAR-T centres in the UK.
Matrix codes: 1A02, 2C01, 3C00
References
- 1.Neelapu S., Locke F.L., Bartlett N.L., et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shuster S.J., Bishop M.R., Tam C.S., et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 3.Maude S.L., Frey N., Shaw P.A., et al. Chimeric antigen receptor T cells for sustained remissions in leukaemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crump M., Neelapu S.S., Farooq U., et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130:1800–1808. doi: 10.1182/blood-2017-03-769620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah N.N., Lee D.W., Yates B., et al. Long-term follow-up of CD19-CAR T-cell therapy in children and young adults with B-ALL. J Clin Oncol. 2021;39:1650–1659. doi: 10.1200/JCO.20.02262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forman S.J., Rowe J.M. The myth of the second remission of acute leukemia in the adult. Blood. 2013;121:1077–1082. doi: 10.1182/blood-2012-08-234492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee D.W., Santomasso B.D., Locke F.L., et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25:625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PubMed] [Google Scholar]
- 8.Yakoub-Agha I., Chabannon C., Bader P., et al. Management of adults and children undergoing chimeric antigen receptor T-cell therapy: best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) Haematologica. 2020;105:297–316. doi: 10.3324/haematol.2019.229781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santomasso B.D., Nastoupil L.J., Adkins S., et al. Management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO guideline. J Clin Oncol. 2021;39:3978–3992. doi: 10.1200/JCO.21.01992. [DOI] [PubMed] [Google Scholar]
- 10.Neelapu S.S., Tummala S., Kebriaei P., et al. Chimeric antigen receptor T-cell therapy—assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornillon J., Hadhoum N., Roth-Guepin G., et al. Management of CAR-T cell-related encephalopathy syndrome in adult and paediatric patients: recommendations of the French Society of Bone Marrow Transplantation and Cellular Therapy (SFGM-TC) Bull Cancer. 2020;107:S12–S17. doi: 10.1016/j.bulcan.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Kuhnl A., Kirkwood A., Roddie C., et al. CD19 CAR-T in less fit patients with R/R high-grade lymphoma. EHA Lib. 2021;325258:EP498. [Google Scholar]