Abstract
Alzheimer's disease (AD) begins with an asymptomatic “preclinical” phase, in which abnormal biomarkers indicate risk for developing cognitive impairment. Biomarker information is increasingly being disclosed in research settings, and is moving toward clinical settings with the development of cheaper and non‐invasive testing. Limited research has focused on the safety and psychological effects of disclosing biomarker results to cognitively unimpaired adults. However, less is known about how to ensure equitable access and robust counseling for decision‐making before testing, and how to effectively provide long‐term follow‐up and risk management after testing. Using the framework of Huntington's disease, which is based on extensive experience with disclosing and managing risk for a progressive neurodegenerative condition, this article proposes a conceptual model of pre‐disclosure, disclosure, and post‐disclosure phases for AD biomarker testing. Addressing research questions in each phase will facilitate the transition of biomarker testing into clinical practice.
Keywords: biomarkers, disclosure, ethics and policy, preclinical Alzheimer's disease
1. INTRODUCTION
Sporadic Alzheimer's disease (AD) is diagnosed with the onset of cognitive impairment, but biomarker changes underlying AD accumulate in the brain possibly decades before symptoms begin. 1 , 2 In this “preclinical” stage of AD (pcAD) abnormal biomarkers of amyloid beta or phosphorylated tau signify an increased risk of developing cognitive impairment. 2 Preclinical biomarker testing can identify individuals for clinical trials, and ultimately may allow earlier diagnosis and initiation of targeted pharmacologic therapies. Amyloid biomarkers such as positron emission tomography (PET) imaging are increasingly being disclosed to cognitively unimpaired participants in research trials. 3 , 4 Disclosure of preclinical biomarkers is currently not recommended for clinical practice. 5 However, recent advances are moving the field significantly closer to routine disclosure of biomarkers in clinical settings. The development of blood‐based biomarkers could allow widespread, inexpensive, and non‐invasive testing. 6 The Food and Drug Administration (FDA) recently approved the first amyloid‐lowering agent for treatment of AD, 7 and other similar drugs will likely be candidates for approval in the near future. 8 These therapies may lead individuals with cognitive complaints or who are otherwise concerned about developing AD to seek information about their amyloid status and access to amyloid‐reducing treatments while they are still in the preclinical phase of AD.
There is an urgent need to address several gaps in current knowledge before biomarker testing for pcAD enters routine clinical practice. 9 Current approaches to biomarker disclosure are largely based on testing and counseling for the apolipoprotein E (APOE) gene, which allow communication of results in 1 to 2 visits with few adverse short‐term outcomes. 10 , 11 , 12 Drawing on experiences with APOE, much of current research on biomarker disclosure has focused on the timepoint of testing, to establish protocols for participant screening, informed consent, and effective communication of biomarker results. 4 , 13 , 14 Evidence shows that biomarker disclosure can generally be done safely in a standardized format, and that participants understand the results. 13 However, there may be additional challenges in actual clinical practice that are absent in research studies, such as delays from limited diagnostic resources or insurance coverage. Follow‐up for clinical populations is likely to be longitudinal and ongoing, rather than predetermined as in a research study. Individuals’ decisions to undergo biomarker testing may also diverge between research and clinical situations, but only limited data exist about decision‐making, which has mainly been based on hypothetical scenarios in non‐representative groups. 15 , 16 , 17 To effectively translate biomarker testing into clinical practice, it will be necessary to move beyond the current approach toward a more comprehensive model that considers multiple timepoints before and after testing, and integrates disclosure with counseling and risk reduction strategies.
To date there are very little data on how to provide personalized counseling, facilitate planning, or lower individuals’ risk for developing AD. 13 Limited information exists about whether testing can ultimately change management, improve outcomes, or have other unforeseen clinical implications. An understanding of the outcomes after testing in diverse populations is needed, given the potential psychosocial impacts of being at increased risk for AD. Participants in biomarker studies are typically recruited through sources that differ from clinical populations, 18 and it is unknown if those populations that are likely to seek diagnosis and treatment will have similarly favorable outcomes in terms of psychological safety. 9 Further, limited data suggest that preclinical AD and symptomatic AD are both stigmatized, 19 but no robust data exist about the effects of stigma on quality of life, employment, or insurance. 20 To ensure equitable access to the benefits of testing, data are also needed about prospective patients’ interest in biomarker testing and availability of specialists, factors likely to influence access to testing and rates of uptake. Interest in biomarker testing may be high in some populations, particularly those with a family history of AD, 16 though more representative studies are lacking. One survey suggests interest may be higher among Blacks than Whites, 21 but relatively little is known about attitudes toward testing among racially and ethnically minoritized or socioeconomically disadvantaged groups.
RESEARCH IN CONTEXT
Systematic review: We reviewed the literature to identify articles that discussed risk disclosure in preclinical Alzheimer's disease (AD). While much of the literature focuses on the timepoint of biomarker testing for preclinical AD, much less is known about attitudes and needs of prospective test takers and clinicians before testing, or about outcomes after testing or effective risk reduction strategies. It is also uncertain if evidence collected from research participants can be generalized to more diverse clinical populations.
Interpretation: We introduce a model identifying clinically relevant phases of care in preclinical AD. This model distinguishes pre‐test, test, and post‐test stages, each of which is associated with different populations and research questions.
Future directions: we highlight a number of important areas in which research is needed to prepare for the use of preclinical biomarker testing in routine clinical practice. Expanding the diversity of research populations and considering aspects relevant to clinical practice will be necessary to identify best practices for biomarker testing.
The current state of knowledge about pcAD has engendered extensive ethical debate. 20 , 22 While risk reduction, planning, and access to clinical trials are potential benefits, the utility of biomarker testing in clinical practice without an effective treatment or a precise prognostic assessment has been questioned. 23 Further, there are concerns that knowledge about risk could lead to negative psychological outcomes, stigmatization, or discrimination. 22 , 24 Ethical principles require fully considering the potential benefits and harms prior to any medical test or invention, to enable individuals to make decisions consistent with their goals and values. 25 The existing evidence base makes such an assessment challenging, underlining the need for research that more closely approximates future clinical use of biomarkers.
Even without a disease‐modifying therapy, non‐invasive biomarker testing is likely to be widely available and of interest to the general public within the near future. 26 The future widespread availability of non‐invasive biomarker testing will necessitate more evidence about the harms and benefits before testing, and about risk reduction strategies and counseling after testing. These issues become more pressing with novel effective treatments. Many drugs currently in the pipeline are most likely to delay onset or slow down neurodegeneration, rather than halt progression or reverse existing damage. 27 For individuals with abnormal biomarkers, such treatments, if effective, could extend the amount of time they spend in the pre‐symptomatic phase, thereby also increasing the number of individuals with pcAD. The net result of widespread preclinical diagnosis may even be to establish a population for whom AD becomes essentially a chronic disease requiring ongoing risk modification, monitoring, and guidance. 28 A comprehensive approach is critical to ensure therapeutic benefits can be maximized, particularly in those populations who are disproportionately affected by dementia and have historically lacked access to care.
To identify gaps in the evidence base about biomarker disclosure and guide future research, we propose a model of biomarker testing for pcAD. This model is based on the experiences with Huntington's disease (HD), a neurodegenerative disorder in which testing can determine an individual's probability for developing the condition. The HD framework encompasses distinct pre‐test, test, and post‐test phases. For each phase, it describes the affected populations and their needs, and identifies key questions for clinical practice and research. The HD framework provides a blueprint for linking testing for neurodegenerative disease with counseling, monitoring, and access to novel therapies and clinical trials, and is widely viewed as the gold standard for genetic risk testing and communication. 11
The HD framework cannot be directly translated to AD, for several reasons: first, HD is a single gene disorder, while AD is a multi‐factorial process, whose underlying pathophysiological changes may never result in symptomatic disease. Second, HD blood tests are performed once to evaluate for a stable genetic sequence. By contrast, pcAD biomarkers (e.g., blood, spinal fluid, imaging) typically evaluate dynamic pathophysiological states that may change over time, and testing may need to be repeated. Third, because the HD gene is transmitted to offspring in an autosomal dominant pattern, reproductive considerations in HD counseling are not relevant to AD. Finally, considerations of disparities among racially and ethnically minoritized populations are less prominent in HD, which tends to affect Whites more often. 29 The prevalence of AD is twice as high among Blacks compared to Whites, 30 as it is among other racially and ethnically minoritized populations. 31
Despite these distinctions, the HD framework provides a comprehensive approach that has been successfully implemented in clinical practice. 11 We draw on and extend this framework, leveraging it to inform a model of care for pcAD that promotes the translation of biomarker testing into clinical practice. While we focus on areas in which research is needed, it is important to note that implementing this model would depend on insurers’ willingness to reimburse elements such as testing or specialized counseling.
We first review the current state of knowledge around testing in pcAD, noting limitations in the available data. Next, we present the HD framework, with lessons learned in each phase of that disease. In the last section we use the HD framework as a guide to provide suggestions for future research in preclinical AD.
2. WHAT IS KNOWN ABOUT DISCLOSURE IN PRECLINICAL AD?
The risk for late‐onset AD, which accounts for >90% of AD cases, 32 is not determined primarily by genetic inheritance but a range of factors. This results in a large pool of potentially at‐risk individuals who might pursue testing. According to survey data conducted largely among White non‐Hispanic populations, ≈two‐thirds of those surveyed would pursue biomarker testing that would inform them of their risk for developing AD, even if no treatment were available. 21 , 33 Other work has shown public interest to be considerably lower. 13 Interest was influenced by the invasiveness of testing, and was generally higher among those with family history of AD. 13 Relatively little is known about attitudes toward biomarker testing among racially and ethnically minoritized populations. Some work has shown a higher interest in biomarker testing among Blacks and Hispanics than Whites, 21 which was not seen in other studies. 34
There has been limited diversity in biomarker research to date, most of which has enrolled non‐Hispanic White populations. 35 Though racially and ethnically minoritized individuals frequently have a higher incidence of dementia, 31 they face a number of disparities, including access to timely diagnosis and adequate support services. 36 The limited inclusiveness of biomarker research raises concerns about the validity of biomarker data in these populations. Data has shown that biomarker changes in AD can vary between Blacks and Whites, and similar biomarker profiles can be associated with divergent cognitive function. 37
To date, it is unknown how frequently biomarker testing of asymptomatic persons is pursued in clinical practice. Surveys conducted prior to the approval of amyloid PET scans for clinical use found that a majority of dementia specialists planned to use them to identify asymptomatic persons at risk for AD dementia, 38 while others would be reluctant to share results for fear of negative psychosocial outcomes. 39 The limited data available suggest that actual practices of disclosure in research settings may vary widely in what is disclosed and how. 3 Patterns of disclosure may similarly diverge in clinical contexts. This was suggested by a survey among German memory centers, where guidelines leave the use of biomarkers to the treating physician's discretion. For patients with subjective memory complaints, normal neuropsychological testing, and abnormal biomarkers, 53% of centers would tell patients they were at increased risk for AD without making a diagnosis of AD, while 13% of centers would formally diagnose AD. 40
Procedures disclosing biomarkers have been developed, though these are only intended for research settings. Recommendations typically include psychological screening and counseling prior to informed consent. Testing is followed by an additional in‐person visit during which results are disclosed by trained providers using standardized language. 4 Amyloid results are typically described as “positive” and “negative,” or “elevated” and “not elevated” levels, given that biomarker data has typically been collected from cross‐sectional cohorts. Additionally, although risks can be estimated on a population scale, 41 it is currently not possible to reliably estimate an individual's risk for developing cognitive symptoms. 42 Psychological outcomes are monitored with short‐term follow‐up after disclosure, and those with higher baseline levels of anxiety or depression are more intensively monitored. 43 While there may be a transient increase in mild‐to‐moderate distress immediately after disclosure, 44 there appears to be a low risk of negative psychological outcomes over 3 to 6 months. 45 Studies in smaller populations have found no significant negative psychological sequelae among amyloid‐positive participants at 12 or 18 months, with 10 participants, 46 and 4 participants, 47 respectively. Recently a limited number of studies have proposed assessing the impact of communicating amyloid and APOE biomarker results together, 11 , 48 but the effects of disclosing multiple sources of risk on psychosocial outcomes are still unknown.
Some data exist on the impacts of knowledge about positive AD biomarkers on quality of life. Prospective trial participants in qualitative focus groups have expressed worry that knowledge about their elevated risk could lead to ongoing anxiety and hyper‐vigilance about cognition, and that others would view them differently. 15 The SOKRATES study is a qualitative substudy in a preclinical AD trial of 50 participants with elevated amyloid versus 30 with not‐elevated amyloid. Findings suggested that those with elevated amyloid levels had a mix of positive and negative outlooks on their future, while those with non‐elevated levels had a more positive outlook overall. 49 In the same study, one quarter of those with an elevated result viewed it as very sensitive information, and were reluctant to share results out of fear of facing discrimination. 49 Some of these concerns can be attributed to AD stigma, or the collectively held beliefs and attitudes that shape how individuals with AD are viewed. 50
Knowledge about risk for AD may also affect cognitive performance. In one nested case control study, individuals who learned of their APOE ε4 carrier status subsequently perceived their memory to be worse, and performed worse on testing, compared to both non‐carriers who learned their status and carriers who did not learn their carrier status. 51 Qualitative research has shown that individuals with elevated amyloid rated their memory worse subjectively. 49
Research participants generally understand the risk assessment communicated to them, but desire more precise prognosis. 52 After disclosure, individuals with elevated amyloid levels may be more likely to make lifestyle changes than those with normal levels, 49 though rigorous controlled studies are lacking. Some evaluation suggests that individuals want to know their risk for AD to make plans for personal and financial arrangements, secure long‐term care, and prepare their family for their future illness. 15 , 16 Currently, no data about best practices for counseling or management are available.
3. WHAT ARE KEY LESSONS FROM THE HD FRAMEWORK?
HD is an autosomal dominant disorder causing movement disorders and progressive cognitive impairment resulting in dementia. Genetic testing for HD has been available since the 1990s, and an HD framework based on extensive research has been developed. The HD framework distinguishes between pre‐genetic test, test, and post‐test phases. This allows the determination of affected populations in each phase, along with an assessment of attitudes and needs among stakeholders. Each phase also entails key issues for clinical practice and research. By identifying those elements relevant to clinical testing, the HD framework can be applied to pcAD to clarify gaps in the current body of evidence and inform future research. We review the HD framework in this section, before applying it to pcAD in the next section.
3.1. HD framework Phase 1(pre‐test): attitudes and access
In HD, risk and age of onset are strongly correlated with the number of CAG repeat expansions on chromosome 4. Individuals may be asymptomatic for many years before they begin developing subtle symptoms in the prodromal stage, and are diagnosed with HD when they manifest motor symptoms. 53 Asymptomatic individuals with a family history of HD can undergo predictive genetic testing to determine whether they are likely to develop HD. Prior to the development of a molecular test for the HD gene, surveys among those at risk because of family history showed a strong interest in predictive genetic testing. After testing became widely available, data show that worldwide only 10% to 20% of those at risk pursue testing. 54
The decision to pursue testing depends on several factors, such as prior knowledge, available therapies, fear of negative psychosocial outcomes, and the attitudes of family members. Sex differences are consistently observed, with women undergoing testing more often than men for unknown reasons. 53 Willingness to recommend HD testing among specialists is generally high, 55 while general practitioners may refer at lower rates, and consider the availability of therapies more when making referrals. 56 Barriers within the health‐care system such as a lack of knowledge among providers or practice patterns have frequently been seen as a major hurdle in referring for testing. 54
3.2. HD framework Phase 2 (test): counseling and disclosure protocols
Guidelines from national and international HD societies outline similar protocols for predictive genetic testing, 57 , 58 which intend to provide as much actionable information as possible to those at risk. 53 Prior to HD testing, trained genetic counselors inform prospective test‐takers about the disease, the test itself, and possible social and emotional consequences. Testing includes psychological evaluation, and takes place across several visits to allow individuals to opt out. Results are disclosed during an in‐person session, in which counseling about prodromal symptoms, the role of specialist management, and research and support groups are provided. This HD disclosure visit is then followed by short‐term follow‐up. 57 , 58 Research has shown that a positive test can cause transient distress but overall there are generally favorable psychological outcomes of those carrying the HD mutation. 54 It has been noted that there may be self‐selection among those who undergo testing, who may have higher levels of emotional health and resourcefulness. 59
Notably, support persons can have as much distress as mutation carriers but may have more difficulty managing this distress than carriers, and may perceive testing to have more negative psychosocial consequences than do carriers. 60 Data also suggest that protocol variations like using fewer counseling resources or telemedicine may remain effective. 53 , 54
3.3. HD framework Phase 3 (post‐test): long‐term outcomes and management
Recommendations for follow‐up after HD testing emphasize counseling and symptom monitoring in specialized clinics. 61 Psychosocial support is provided, and counseling assists individuals in planning, focusing on insurance, future health‐care wishes, and medical–legal issues. Cognitive function is regularly assessed, and symptoms are monitored to detect subtle changes that may require management, or provide reassurance if symptoms are not part of HD. With the onset of clear symptoms, treatment shifts to focus on symptom control, establishing a multi‐disciplinary care team for the patient, and possibly referral to research trials. 61 Outcomes are generally positive, though negative psychological outcomes have been observed 7 to 10 years after testing. 62 Research has examined the effects on employment and challenges for intimate partner and family dynamics. 54 The social and emotional implications of normal results have been explored as well, as some individuals with normal genetic testing have a negative reaction, 53 because of “survivor's guilt” (about not being affected while other family members are), unrealistic expectations about the positive effect of a normal result, or because individuals had already made irreversible decisions based on their assumption they had the disease. 63
4. HOW CAN THE HD FRAMEWORK INFORM RESEARCH IN PRECLINICAL AD?
Following the HD framework, we propose a model for biomarker testing in pcAD that is organized around clinically relevant phases shown in Figure 1. In each phase there are different populations of interest (e.g., those considering testing vs. those who have received a positive test result), which may have implications for management. The model highlights key issues for research and clinical practice in each phase: in Phase 1, these issues are access, patient interest, and decision‐making about testing. In Phase 2, these are counseling, testing, and disclosure protocols for diverse groups. In Phase 3, these are long‐term follow‐up and management to reduce risk of dementia and negative psychosocial outcomes. Based on this model, we identify areas in which further research is needed. Though we focus our discussion on amyloid, currently the most widely used biomarker, this model can incorporate other biomarkers or genetic testing that may be used in the future.
FIGURE 1.
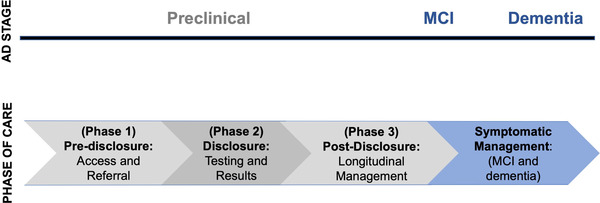
Phases of care in preclinical Alzheimer's disease (AD). MCI, mild cognitive impairment
Importantly, this model for pcAD does not imply that all evidence or research approaches from HD can necessarily be applied to AD. Rather, our model draws on lessons learned from HD to identify elements that will likely be part of a comprehensive approach to counseling, disclosure, and management of patients at risk for neurodegenerative disease. While we highlight several key areas of research need, this list is not exhaustive and several additional important areas could be mentioned.
4.1. Areas of research need in Phase 1 (pre‐test): attitudes and access
4.1.1. Attitudes toward testing among patients and support persons
In the pre‐test phase, more data are needed about knowledge around testing and interest among patients and support persons, as well as appropriate education and recruitment strategies in diverse populations. Data about attitudes toward testing for pcAD come from populations that may not be representative. Underrepresented groups typically have less familiarity with AD, often viewing cognitive decline as a normal or unavoidable part of aging and seeking diagnosis later. 64 There may also be differences among socioeconomic or geographic groups that could influence willingness or perceived ability to seek testing. 65 Without a targeted assessment of attitudes among diverse groups, implementation of widespread testing may funnel toward those who already have high levels of knowledge about AD. Given that biomarker testing will be a prerequisite to receiving disease‐modifying therapy, this has the potential to exacerbate disparities.
It may also be important to understand how attitudes differ between genetic and non‐genetic results. Though APOE testing has not been widely used for risk assessment, it may play an important role in the future. The ε4 allele has been associated with an increased risk for adverse events from bleeding with the amyloid‐lowering agent aducanumab, leading to the recommendation that APOE testing be considered prior to treatment. 66 It is currently unknown how obtaining APOE testing alongside biomarkers such as amyloid influences attitudes toward testing, though prior research has shown lower interest in APOE testing for some groups, such as Blacks compared to Whites. 67
Support persons may also have an important role in influencing desire for testing. Research has indicated that the requirement of having a study partner is an important consideration in deciding to enroll in biomarker research, 68 and study partners of individuals participating in trials disclosing preclinical amyloid results may also desire information about the study participant's amyloid status. 69 These findings suggest that the influence of support persons on attitudes toward testing should be more fully considered.
While amyloid testing is only available in research and clinical settings, genetic testing for AD has been available for many years through direct‐to‐consumer (DTC) testing. The development of blood‐based testing may ultimately lead to DTC testing for AD, 26 in which individuals may be able to obtain diagnosis without formal clinical evaluation or pre‐disclosure education and counseling. These individuals may have different needs when accessing formal health‐care services after DTC testing, and may require additional informational materials and disclosure protocols than patients who have received results in a clinical context. 70
4.1.2. Health‐care capacity and attitudes among referring providers
More data are needed about the role of health‐care systems in the pre‐testing phase. In the future, individuals might be referred directly to specialists for testing, or tested in primary care settings and referred for a positive result. 6 The perceived value of early diagnosis may differ between general practitioners and specialists. Among general practitioners, many do not believe diagnosis at an early symptomatic stage has value, as close to 40% would not or are unsure if they would change their practice even if a disease‐modifying drug was available. 71 Reluctance to diagnose AD may come from general practitioners’ assessment that there is a lack of available support services or the risk of negative psychosocial outcomes, and may negatively influence their willingness to pursue preclinical testing. 24
An evaluation of the US health‐care system's readiness for AD disease‐modifying treatment concluded that there was not enough capacity to evaluate the 15 million individuals currently estimated to be eligible for treatment of mild cognitive impairment (MCI), resulting in unacceptable delays in diagnosis and care. 72 For comparison, in 2017 there were an estimated 46 million individuals in a preclinical stage of AD in the United States. 73 These numbers highlight the potential magnitude of testing, which would require involvement of health‐care providers across disciplines and practice settings. Given the higher incidence of dementia among racially and ethnically minoritized groups and delayed access to dementia diagnosis, limitations in availability of testing may disproportionately affect these populations. Biomarker testing and follow‐up could present significant challenges for referral and evaluation, indicating a need for research to implement sustainable and equitable approaches to preclinical testing, and appropriate coverage of these services by insurance.
Box. 1: Areas of research need in Phase 1
Understand attitudes toward testing in racially/ethnically minoritized, socioeconomically, and geographically diverse groups.
Characterize attitudes toward testing among patients and support persons in distinct referral populations (e.g., primary care vs. direct‐to‐consumer).
Determine knowledge about testing, interest in testing and referral, and provider‐related barriers to testing among general practitioners and others who refer to dementia specialists.
Establish approaches to initial counseling and referral that can be implemented in primary care settings; and gather evidence of effects on outcomes (e.g., rates of testing, safety).
Conduct comprehensive assessments of health systems’ ability to provide accessible, timely diagnoses for large populations of patients.
Identify barriers to access among diverse groups and formulate strategies to minimize disparities.
4.2. Areas of research need in Phase 2 (test): counseling, informed consent, and disclosure protocols
4.2.1. Safety and prognostic outcomes in diverse populations
There is a pressing need for research in more diverse populations to make evidence about safety and prognosis more generalizable. The data used for current prognostic models largely come from non‐Hispanic White study participants, and may not be applicable to other racially and ethnically minoritized groups. 37 Similarly, safety outcomes after disclosure among general clinical populations may not be the same as the participants in biomarker research studies. Research participants may differ in terms of motivation, risk tolerance, and psychological presentation. 45 Studies typically include participants over 65 years of age, with mean ages over 70, 45 , 46 and there is limited evidence about outcomes among younger individuals. Work with diverse groups to better anticipate those populations who may present to clinical practices in the future is critically important. 9
While recommendations about how to disclose amyloid PET biomarker findings in research settings have been published, 4 no guidelines exist for clinical settings. Evidence is needed about the ideal content of counseling, which could include information about potential psychosocial, economic, and legal implications of results. Very little is also known about how patients and their support persons make decisions. Most data come from hypothetical scenarios among affected individuals, which may only incompletely predict behavior, indicating that new methodological approaches are needed to inform decision‐making. While testing has been viewed in the “informed consent” model, perhaps because disclosure has thus far been anchored in clinical trial settings, in widespread clinical practice the counseling around disclosure may shift to an alternative model of “informed choice.” Informed choice is typically used for screening tests, such as for breast or prostate cancer. 74 This approach does not emphasize either risks or benefits, but provides comprehensive information about possible implications of results and attempts to engage patients in shared decision‐making. In conditions in which the underlying pathophysiological changes may never cause symptoms, informed choice enables patients to make decisions appropriate to their situation, balancing prognostic uncertainty, quality of life factors, and potential consequences of knowledge about risk. 75
4.2.2. Evidence‐based counseling and training protocols
To more easily adapt counseling for diverse populations and clinical sites, it may also be helpful to develop abbreviated protocols. As in genetic testing for APOE, broadening geographic availability and reducing the time investment of clinicians who are in short supply will be critical to making disclosure widely accessible. 11 Remote technology may help meet this goal, as preliminary experiences suggest that counseling and disclosure of pcAD biomarkers can safely be done virtually. 76 Current recommendations limit biomarker counseling to experts, 5 and novel protocols are necessary to train different types of providers. Just as HD counseling by trained genetic counselors is reimbursed under established billing codes, appropriate counseling should be reimbursed under newly established billing codes to facilitate high‐quality pre‐test biomarker counseling.
Box. 2: Areas of research need in Phase 2
Gather evidence about prognostic and safety outcomes in clinically representative populations, including in diverse groups with real‐world psychological profiles.
Understand decision‐making among patients and support persons, establish the optimal content of pcAD biomarker counseling for patients and support persons, and determine approaches for ensuring comprehension of results among diverse populations.
Identify best practices for provider training and qualifications, and inform insurance coverage for testing and counseling.
Evaluate the safety and efficacy of disclosure in different clinical settings, including remote or abbreviated protocols.
4.3. Areas of research need in Phase 3 (post‐test): long‐term outcomes and management
4.3.1. Outcomes among individuals not at increased risk for cognitive impairment
Biomarker test results may be normal, or indicate someone is at higher risk for cognitive impairment due to AD. For those with normal results, follow‐up testing may be recommended. These individuals would in the future then re‐enter Phase 1, albeit with higher levels of information about testing and possibly with different needs. While there have been few negative psychological outcomes among individuals not at increased risk for AD, the qualitative SOKRATES study suggested that the lack of an explanation for their cognitive complaints led to frustration for some participants. 49 There may also be other unexpected negative outcomes for a normal result that necessitate follow‐up.
4.3.2. Risk reduction and comprehensive management
Individuals at increased risk will likely need comprehensive, longitudinal management, about which there is a critical lack of evidence. Risk modification is an important potential benefit of preclinical diagnosis, 14 but data are needed about lifestyle changes after biomarker disclosure, including how often changes are implemented, how long changes are sustained, and if cognitive outcomes are improved. Although individuals may correctly recall the risk estimate that was communicated to them, 52 they may evaluate their personal risk differently: individuals with non‐elevated amyloid may over‐estimate their risk based on their own experience, and those with elevated amyloid may minimize their risk as a coping strategy. 4 Understanding individual risk evaluation and how to promote lifestyle changes may have important implications for improving outcomes.
To optimize outcomes, patients may benefit from coordinated care or specialized clinics, which could facilitate non‐pharmacological risk modification and counseling. Interdisciplinary care might include clinicians focusing on aspects of disease progression, and social workers or health psychologists addressing psychosocial needs. Such specialized clinics may be well placed to implement a comprehensive program of care, which could involve elements such as regular reassessment of risk with biomarker and/or cognitive testing (at intervals that still need to be specified), access to counseling resources including medical–legal advice and planning for patients and families, and support for lifestyle changes and risk reduction. Disclosure may also change how medical conditions associated with risk of cognitive decline are managed, 77 and represents an important area in which integrated care could improve outcomes. With the potentially dramatic increase in the number of individuals requiring specialist follow‐up over many years, efficient and effective approaches are needed to prevent straining health system capacity and affordability.
4.3.3. Long‐term planning and psychosocial outcomes for patients and support persons
Ideally, early information about AD risk facilitates long‐term planning by enabling individuals to prepare. Improved planning of personal affairs and increased patient choice about future medical needs are potential benefits of disclosure that can be realized despite the current limitations in prognosis and treatment. In light of biomarkers’ prognostic uncertainty, effective strategies to provide practical guidance to patients are needed. For instance, it may be possible to provide ranges of time to onset of cognitive symptoms rather than precise estimates. This approach has been used with other neurological conditions in which it is difficult to prognosticate exact times of onset or decline. 78
Data about long‐term safety and psychosocial outcomes after disclosure in larger, more diverse cohorts are urgently needed. Outcomes from current studies extend to 18 months, but individuals could be in a preclinical disease state for many years. 2 Little is known about how to minimize “dementia worry,” or the potential negative responses to the perceived threat of developing dementia. 79 Recognizing and managing dementia worry may have implications for serial monitoring of cognition, and for effectively supporting patients without relying on ongoing testing to provide reassurance. Some negative outcomes are likely due to perceived or actual AD stigma, the effects of which remain incompletely understood. In the SOKRATES study, individuals had a number of responses—both positive or negative—to knowledge about their increased risk, such as changing life plans or employment. 49 While emerging evidence suggests that support persons of those undergoing preclinical testing have reported positive experiences, 69 more data are needed about the range of potential effects of disclosure on support persons and family members.
Given the potential effects the stigma of pcAD may have on individuals’ employment and insurance, legal protections and strategies to limit the consequences of disclosure are necessary. 80 Providers and prospective patients have frequently expressed concerns about confidentiality. 15 , 22 In the absence of strong privacy protections, this may encourage incomplete documentation of test results, which could in turn lead to fragmented care and missed opportunities to modify risk. For instance, experience with HD has shown that tests may be paid for out‐of‐pocket rather than through insurance, or results may be omitted or hidden in the medical record to preserve privacy. 81 Regulations in the United States that test results be immediately released to patients electronically are intended to increase patient autonomy, 82 but may require additional strategies to ensure both timely and confidential disclosure.
Differences between types of biomarkers may also be relevant. The Genetic Information Nondiscrimination Act (GINA) prohibits discrimination on the basis of genetic test results, but no such protections currently exist for other biomarkers. 80 In the SOKRATES study, participants reported being more likely to share their APOE status than amyloid results, suggesting that individuals may view the implications of genetic and biomarker information differently. 83 No data about potential differences between biomarkers on stigma, economic, or insurance outcomes exist. Research in these areas is necessary to inform both guidelines for counseling and future legal protections.
Box. 3: Areas of research need in Phase 3
Collect data on outcomes of individuals not at increased risk for cognitive impairment due to AD.
Evaluate if and how biomarker disclosure influences lifestyle changes for brain health.
Formulate approaches to optimize risk reduction among individuals.
Test strategies for interdisciplinary care, and gather data on the effects of comprehensive care on outcomes (psychosocial, lifestyle changes, co‐morbidities, etc.).
Identify approaches to effectively communicate information that is meaningful and actionable for patients, and assess the influence of this counseling on planning and medical–legal outcomes.
Follow larger, diverse cohorts longitudinally to characterize long‐term (> 2 year) psychosocial outcomes among both tested individuals and support persons.
Establish the effects of AD stigma on individuals and family members, and identify effective (informational, legal, educational) strategies to mitigate potential negative effects of disclosure.
5. CONCLUSION
With the approval of the first amyloid targeting therapy for AD and progress toward blood‐based biomarkers, pcAD biomarker disclosure continues to move closer to routine clinical practice. There is an urgent need to expand the conceptual framing of disclosure to prepare for likely real‐world clinical settings in pre‐disclosure, disclosure, post‐disclosure care. Our proposed model, adapted from risk disclosure in HD, offers a framework to identify affected populations and key questions for clinical practice and research. Areas of research need span several domains, including cohort recruitment and retention, clinical outcomes, decision‐making, quality of life, legal, and economic issues. A pressing concern is increasing diversity in AD research, 30 , 35 , 36 an area consistent with the National Institute of Health's focus on addressing health disparities. 84 Multidisciplinary and diverse methodological approaches are likely needed to address these questions.
An important first step toward broadly implementing preclinical biomarker testing will be studies in more diverse populations throughout all three phases. Initial questions for research in each stage of pcAD could include: in Phase 1, to understand differences in pre‐test access, interest, and referral capacity. In Phase 2, the focus could be on increasing the diversity of cohorts and optimizing the content of counseling. In Phase 3, research is needed on strategies for longitudinal non‐pharmacological management of AD as a chronic disease, including counseling on risk reduction and health‐care planning. More information is also needed on cognitive monitoring, and the effects of potential ongoing distress about cognition and possible stigma. Addressing these and other questions will be essential to establishing a comprehensive, sustainable, and equitable model of preclinical AD management.
ACKNOWLEDGMENTS
This project was supported by National Institutes on Aging Awards: RF1 AG057784 (PI Kind, MPI Bendlin); R01 AG070883 (PI Kind, MPI Bendlin); and P30 AG062715 (PI Asthana; Core H/Care Research Core Leader Kind). R01 AG054059 (PI Gleason); R01AG062307 (PI Jacklin, Site Lead Gleason); P30 AG062715 (PI Asthana, Gleason IURG Core Leader); RF1 AG057547 (PI Kantarci, MPI Gleason). R03 AG062975 (PI Clark). Dr. Grill is supported by NIA P30 AG066519, NIA U24 AG057437, and NCATS UL1 TR001414.
Ketchum FB, Chin NA, Grill J, et al. Moving beyond disclosure: Stages of care in preclinical Alzheimer's disease biomarker testing. Alzheimer's Dement. 2022;18:1969–1979. 10.1002/alz.12620
REFERENCES
- 1. Sperling RA, Donohue MC, Raman R, et al. Association of factors with elevated amyloid burden in clinically normal older individuals. JAMA Neurol. 2020;77:735‐745. 10.1001/jamaneurol.2020.0387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jack CR, Bennett DA, Blennow K, et al. NIA‐AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement J Alzheimers Assoc. 2018;14:535‐562. 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roberts JS, Ferber R, Blacker D, Rumbaugh M, Grill JD, for the AGREED Group . Disclosure of individual research results at federally funded Alzheimer's disease research centers. Alzheimers Dement Transl Res Clin Interv. 2021;7:e12213. 10.1002/trc2.12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vanderschaeghe G, Schaeverbeke J, Bruffaerts R, Vandenberghe R, Dierickx K. From information to follow‐up: ethical recommendations to facilitate the disclosure of amyloid PET scan results in a research setting. Alzheimers Dement Transl Res Clin Interv. 2018;4:243‐251. 10.1016/j.trci.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnson KA, Minoshima S, Bohnen NI, et al. Appropriate use criteria for amyloid PET: a report of the amyloid imaging task force, the society of nuclear medicine and molecular imaging, and the Alzheimer's association. J Nucl Med. 2013;54:476‐490. 10.2967/jnumed.113.120618 [DOI] [PubMed] [Google Scholar]
- 6. Hampel H, O'Bryant SE, Molinuevo JL, et al. Blood‐based biomarkers for Alzheimer's disease: mapping the road to the clinic. Nat Rev Neurol. 2018;14:639‐652. 10.1038/s41582-018-0079-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Commissioner O of the . FDA Grants Accelerated Approval for Alzheimer's Drug. FDA; 2021. https://www.fda.gov/news‐events/press‐announcements/fda‐grants‐accelerated‐approval‐alzheimers‐drug (accessed July 2, 2021). [Google Scholar]
- 8. Tolar M, Abushakra S, Hey JA, Porsteinsson A, Sabbagh M. Aducanumab, gantenerumab, BAN2401, and ALZ‐801—the first wave of amyloid‐targeting drugs for Alzheimer's disease with potential for near term approval. Alzheimers Res Ther. 2020;12:95. 10.1186/s13195-020-00663-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. National Academies of Sciences, Engineering, and Medicine . Implications for Behavioral and Social Research of Preclinical Markers of Alzheimer's Disease and Related Dementias: Proceedings of a Workshop—in Brief. Washington (DC): National Academies Press (US); 2021. [PubMed] [Google Scholar]
- 10. Roberts JS, Chen CA, Uhlmann WR, Green RC. Effectiveness of a condensed protocol for disclosing APOE genotype and providing risk education for Alzheimer's disease. Genet Med. 2012;14:742‐748. 10.1038/gim.2012.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Langlois CM, Bradbury A, Wood EM, et al. Alzheimer's prevention initiative generation program: development of an APOE genetic counseling and disclosure process in the context of clinical trials. Alzheimers Dement Transl Res Clin Interv. 2019;5:705‐716. 10.1016/j.trci.2019.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Green RC, Roberts JS, Cupples LA, et al. Disclosure of APOE genotype for risk of Alzheimer's disease. N Engl J Med. 2009;361:245. 10.1056/NEJMoa0809578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Erickson CM, Chin NA, Johnson SC, Gleason CE, Clark LR. Disclosure of preclinical Alzheimer's disease biomarker results in research and clinical settings: why, how, and what we still need to know. Alzheimers Dement Diagn Assess Dis Monit. 2021;13:e12150. 10.1002/dad2.12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Wilde A, van Buchem MM, Otten RHJ, et al. Disclosure of amyloid positron emission tomography results to individuals without dementia: a systematic review. Alzheimers Res Ther. 2018;10. 10.1186/s13195-018-0398-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Milne R, Bunnik E, Diaz A, et al. Perspectives on communicating biomarker‐based assessments of Alzheimer's Disease to cognitively healthy individuals. J Alzheimers Dis. 2018;62:487‐498. 10.3233/JAD-170813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gooblar J, Roe CM, Selsor NJ, Gabel MJ, Morris JC. Attitudes of research participants and the general public regarding disclosure of Alzheimer's disease research results. JAMA Neurol. 2015;72:1484‐1490. 10.1001/jamaneurol.2015.2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Milne R, Diaz A, Badger S, Bunnik E, Fauria K, Wells K. At, with and beyond risk: expectations of living with the possibility of future dementia. Sociol Health Illn. 2018;40:969‐987. 10.1111/1467-9566.12731 [DOI] [PubMed] [Google Scholar]
- 18. Gleason CE, Norton D, Zuelsdorff M, et al. Association between enrollment factors and incident cognitive impairment in Blacks and Whites: data from the Alzheimer's disease center. Alzheimers Dement. 2019;15:1533‐1545. 10.1016/j.jalz.2019.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stites SD, Gill J, Largent EA, et al. The relative contributions of biomarkers, disease modifying treatment, and dementia severity to Alzheimer's stigma: a vignette‐based experiment. Soc Sci Med. 2022;292:114620. 10.1016/j.socscimed.2021.114620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karlawish J. Addressing the ethical, policy, and social challenges of preclinical Alzheimer's disease. Neurology. 2011;77:1487‐1493. 10.1212/WNL.0b013e318232ac1a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wikler EM, Blendon RJ, Benson JM. Would you want to know? Public attitudes on early diagnostic testing for Alzheimer's disease. Alzheimers Res Ther. 2013;5:43. 10.1186/alzrt206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smedinga M, Tromp K, Schermer MHN, Richard E. Ethical arguments concerning the use of Alzheimer's disease biomarkers in individuals with no or mild cognitive impairment: a systematic review and framework for discussion. J Alzheimers Dis. 2018;66:1309‐1322. 10.3233/JAD-180638 [DOI] [PubMed] [Google Scholar]
- 23. Bunnik EM, Richard E, Milne R, Schermer MHN. On the personal utility of Alzheimer's disease‐related biomarker testing in the research context. J Med Ethics. 2018;44:830‐834. 10.1136/medethics-2018-104772 [DOI] [PubMed] [Google Scholar]
- 24. Dubois B, Padovani A, Scheltens P, Rossi A, Dell'Agnello G. Timely diagnosis for Alzheimer's disease: a literature review on benefits and challenges. J Alzheimers Dis. 2016;49:617‐631. 10.3233/JAD-150692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beauchamp TL, Childress JF. Principles of Biomedical Ethics. 5th ed. 5 ed. Oxford University Press; 2001. [Google Scholar]
- 26. Largent EA, Wexler A, Karlawish J. The future is P‐Tau—Anticipating direct‐to‐consumer Alzheimer's disease blood tests. JAMA Neurol. 2021;78:379‐380. 10.1001/jamaneurol.2020.4835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kurz AF, Lautenschlager NT. The ethics of biomarker‐based preclinical diagnosis of Alzheimer's disease. In: Perneczky R, ed. Biomark. Preclin. Alzheimer's Dis. Springer New York; 2018:249‐258. 10.1007/978-1-4939-7674-4_17 [DOI] [Google Scholar]
- 28. Karlawish J, Jack CR, Rocca WA, Snyder HM, Carrillo MC. Alzheimer's disease: the next frontier‐Special Report 2017. Alzheimers Dement J Alzheimers Assoc. 2017;13:374‐380. 10.1016/j.jalz.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 29. Bruzelius E, Scarpa J, Zhao Y, Basu S, Faghmous JH, Baum A. Huntington's disease in the United States: variation by demographic and socioeconomic factors. Mov Disord. 2019;34:858‐865. 10.1002/mds.27653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barnes LL. Biomarkers for Alzheimer's dementia in diverse racial and ethnic minorities—a public health priority. JAMA Neurol. 2019;76:251‐253. 10.1001/jamaneurol.2018.3444 [DOI] [PubMed] [Google Scholar]
- 31. Demirovic J, Prineas R, Loewenstein D, et al. Prevalence of dementia in three ethnic groups: the South Florida program on aging and health. Ann Epidemiol. 2003;13:472‐478. 10.1016/S1047-2797(02)00437-4 [DOI] [PubMed] [Google Scholar]
- 32. Carmona S, Hardy J, Guerreiro R. Chapter 26 ‐ The genetic landscape of Alzheimer's disease. In: Geschwind DH, Paulson HL, Klein C, eds. Handb. Clin. Neurol. Elsevier; 2018:395‐408. 10.1016/B978-0-444-64076-5.00026-0 [DOI] [PubMed] [Google Scholar]
- 33. Caselli RJ, Langbaum J, Marchant GE, et al. Public perceptions of presymptomatic testing for Alzheimer's disease. Mayo Clin Proc. 2014;89:1389‐1396. 10.1016/j.mayocp.2014.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sheffrin M, Stijacic Cenzer I, Steinman MA. Desire for predictive testing for Alzheimer's disease and impact on advance care planning: a cross‐sectional study. Alzheimers Res Ther. 2016;8:55. 10.1186/s13195-016-0223-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Khan MJ, Desaire H, Lopez OL, Kamboh MI, Robinson RAS. Why inclusion matters for Alzheimer's disease biomarker discovery in plasma. J Alzheimers Dis. 2021;79:1327‐1344. 10.3233/JAD-201318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Babulal GM, Quiroz YT, Albensi BC, et al. Perspectives on ethnic and racial disparities in Alzheimer's disease and related dementias: update and areas of immediate need. Alzheimers Dement. 2019;15:292‐312. 10.1016/j.jalz.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gleason CE, Zuelsdorff M, Gooding DC, et al. Alzheimer's disease biomarkers in black and non‐hispanic white cohorts: a contextualized review of the evidence. Alzheimers Dement. 2022;18:1545‐1564. 10.1002/alz.12511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shulman MB, Harkins K, Green RC, Karlawish J. Using AD biomarker research results for clinical care: a survey of ADNI investigators. Neurology. 2013;81:1114‐1121. 10.1212/WNL.0b013e3182a55f4a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Armstrong MJ, Gronseth GS, Day GS, Rheaume C, Alliance S, Mullins CD. Patient stakeholder versus physician preferences regarding amyloid PET testing. Alzheimer Dis Assoc Disord. 2019;33:246‐253. 10.1097/WAD.0000000000000311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schweda M, Kögel A, Bartels C, Wiltfang J, Schneider A, Schicktanz S. Prediction and early detection of Alzheimer's dementia: professional disclosure practices and ethical attitudes. J Alzheimers Dis. 2018;62:145‐155. 10.3233/JAD-170443 [DOI] [PubMed] [Google Scholar]
- 41. Brookmeyer R, Abdalla N. Estimation of lifetime risks of Alzheimer's disease dementia using biomarkers for preclinical disease. Alzheimers Dement. 2018;14:981‐988. 10.1016/j.jalz.2018.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Simrén J, Leuzy A, Karikari TK, et al. The diagnostic and prognostic capabilities of plasma biomarkers in Alzheimer's disease. Alzheimers Dement. 2021;17:1145‐1156. 10.1002/alz.12283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Harkins K, Sankar P, Sperling R, et al. Development of a process to disclose amyloid imaging results to cognitively normal older adult research participants. Alzheimers Res Ther. 2015;7:26. 10.1186/s13195-015-0112-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Burns JM, Johnson DK, Liebmann E, Bothwell R, Morris JK, Vidoni ED. Safety of disclosing amyloid status in cognitively normal older adults. Alzheimers Dement J Alzheimers Assoc. 2017;13:1024‐1030. 10.1016/j.jalz.2017.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Grill JD, Raman R, Ernstrom K, et al. Short‐term psychological outcomes of disclosing amyloid imaging results to research participants who do not have cognitive impairment. JAMA Neurol. 2020;77:1504‐1513. 10.1001/jamaneurol.2020.2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wake T, Tabuchi H, Funaki K, et al. Disclosure of amyloid status for risk of Alzheimer's disease to cognitively normal research participants with subjective cognitive decline: a longitudinal study. Am J Alzheimers Dis Other Demen. 2020;35:1533317520904551. 10.1177/1533317520904551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lim YY, Maruff P, Getter C, Snyder PJ. Disclosure of positron emission tomography amyloid imaging results: a preliminary study of safety and tolerability. Alzheimers Dement. 2016;12:454‐458. 10.1016/j.jalz.2015.09.005 [DOI] [PubMed] [Google Scholar]
- 48. Mozersky J, Hartz S, Linnenbringer E, et al. Communicating 5‐year risk of Alzheimer's disease dementia: development and evaluation of materials that incorporate multiple genetic and biomarker research results. J Alzheimers Dis JAD. 2021;79:559‐572. 10.3233/JAD-200993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Largent EA, Harkins K, Dyck CHvan, Hachey S, Sankar P, Karlawish J. Cognitively unimpaired adults’ reactions to disclosure of amyloid PET scan results. PLOS ONE. 2020;15:e0229137. 10.1371/journal.pone.0229137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stites SD, Rubright JD, Karlawish J. What features of stigma do the public most commonly attribute to Alzheimer's disease dementia? Results of a survey of the U.S. general public. Alzheimers Dement. 2018;14:925‐932. 10.1016/j.jalz.2018.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lineweaver TT, Bondi MW, Galasko D, Salmon DP. Effect of knowledge of APOE genotype on subjective and objective memory performance in healthy older adults. Am J Psychiatry. 2014;171:201‐208. 10.1176/appi.ajp.2013.12121590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mozersky J, Sankar P, Harkins K, Hachey S, Karlawish J. Comprehension of an elevated amyloid positron emission tomography biomarker result by cognitively normal older adults. JAMA Neurol. 2018;75:44‐50. 10.1001/jamaneurol.2017.2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Testa CM, Jankovic J. Huntington disease: a quarter century of progress since the gene discovery. J Neurol Sci. 2019;396:52‐68. 10.1016/j.jns.2018.09.022 [DOI] [PubMed] [Google Scholar]
- 54. Nance MA. Genetic counseling and testing for Huntington's disease: a historical review. Am J Med Genet B Neuropsychiatr Genet. 2017;174:75‐92. 10.1002/ajmg.b.32453 [DOI] [PubMed] [Google Scholar]
- 55. Thies U, Bockel B, Bochdalofsky V. Attitudes of neurologists, psychiatrists, and psychotherapists towards predictive testing for Huntington's disease in Germany. J Med Genet. 1993;30:1023‐1027. 10.1136/jmg.30.12.1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Welkenhuysen M, Evers‐Kiebooms G. General practitioners and predictive genetic testing for late‐onset diseases in flanders: what are their opinions and do they want to be involved? Public Health Genomics. 2002;5:128‐137. 10.1159/000065170 [DOI] [PubMed] [Google Scholar]
- 57. HDSA . HDSA Genetic Testing Protocol for HD. Huntington's Disease Society of America; 2016. [Google Scholar]
- 58. MacLeod R, Tibben A, Frontali M, et al. Recommendations for the predictive genetic test in Huntington's disease. Clin Genet. 2013;83:221‐231. 10.1111/j.1399-0004.2012.01900.x [DOI] [PubMed] [Google Scholar]
- 59. Broadstock M, Michie S, Marteau T. Psychological consequences of predictive genetic testing: a systematic review. Eur J Hum Genet. 2000;8:731‐738. 10.1038/sj.ejhg.5200532 [DOI] [PubMed] [Google Scholar]
- 60. Decruyenaere M, Evers‐Kiebooms G, Boogaerts A, Demyttenaere K, Dom R, Fryns J‐P. Partners of mutation‐carriers for Huntington's disease: forgotten persons? Eur J Hum Genet. 2005;13:1077‐1085. 10.1038/sj.ejhg.5201462 [DOI] [PubMed] [Google Scholar]
- 61. Wild EJ, Tabrizi S. Premanifest and early Huntington's Disease. In: Bates G, Jones L, Tabrizi S, eds. Huntingt. Dis. 4th ed. Oxford University Press; 2014:86‐105. [Google Scholar]
- 62. Timman R, Roos R, Maat‐Kievit A, Tibben A. Adverse effects of predictive testing for Huntington disease underestimated: long‐term effects 7‐10 years after the test. Health Psychol. 2004;23:189. [DOI] [PubMed] [Google Scholar]
- 63. Huggins M, Bloch M, Wiggins S, et al. Predictive testing for Huntington disease in Canada: adverse effects and unexpected results in those receiving a decreased risk. Am J Med Genet. 1992;42:508‐515. 10.1002/ajmg.1320420417 [DOI] [PubMed] [Google Scholar]
- 64. Cooper C, Tandy AR, Balamurali TBS, Livingston G. A systematic review and meta‐analysis of ethnic differences in use of dementia treatment, care, and research. Am J Geriatr Psychiatry Off J Am Assoc Geriatr Psychiatry. 2010;18:193‐203. 10.1097/JGP.0b013e3181bf9caf [DOI] [PubMed] [Google Scholar]
- 65. Innes A, Morgan D, Kostineuk J. Dementia care in rural and remote settings: a systematic review of informal/family caregiving. Maturitas. 2011;68:34‐46. 10.1016/j.maturitas.2010.10.002 [DOI] [PubMed] [Google Scholar]
- 66. Cummings J, Aisen P, Apostolova LG, Atri A, Salloway S, Weiner M. Aducanumab: appropriate use recommendations. J Prev Alzheimers Dis. 2021. 10.14283/jpad.2021.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Roberts JS, Barber M, Brown TM, et al. Who seeks genetic susceptibility testing for Alzheimer's disease? Findings from a multisite, randomized clinical trial. Genet Med. 2004;6:197‐203. 10.1097/01.GIM.0000132688.55591.77 [DOI] [PubMed] [Google Scholar]
- 68. Grill JD, Zhou Y, Elashoff D, Karlawish J. Disclosure of amyloid status is not a barrier to recruitment in preclinical Alzheimer's disease clinical trials. Neurobiol Aging. 2016;39:147‐153. 10.1016/j.neurobiolaging.2015.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Largent EA, Abera M, Harkins K, et al. Family members’ perspectives on learning cognitively unimpaired older adults’ amyloid‐β PET scan results. J Am Geriatr Soc. 2021;n/a. 10.1111/jgs.17362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Messner DA. Informed choice in direct‐to‐consumer genetic testing for Alzheimer's and other diseases: lessons from two cases. New Genet Soc. 2011;30:59‐72. 10.1080/14636778.2011.552300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sannemann L, Müller T, Waterink L, et al. General practitioners’ attitude toward early and pre‐dementia diagnosis of AD in five European countries—A MOPEAD project survey. Alzheimers Dement Diagn Assess Dis Monit. 2021;13:e12130. 10.1002/dad2.12130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liu JL, Hlavka JP, Hillestad R, Mattke S. Assessing the Preparedness of the U.S. Health Care System Infrastructure for an Alzheimer's Treatment. RAND Coporation; 2017. [PMC free article] [PubMed] [Google Scholar]
- 73. Brookmeyer R, Abdalla N, Kawas CH, Corrada MM. Forecasting the prevalence of preclinical and clinical Alzheimer's disease in the United States. Alzheimers Dement. 2018;14:121‐129. 10.1016/j.jalz.2017.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hersch J, Jansen J, Irwig L, et al. How do we achieve informed choice for women considering breast screening? Prev Med. 2011;53:144‐146. 10.1016/j.ypmed.2011.06.013 [DOI] [PubMed] [Google Scholar]
- 75. Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285:2750‐2756. 10.1001/jama.285.21.2750 [DOI] [PubMed] [Google Scholar]
- 76. Erickson CM, Chin NA, Coughlin DM, et al. Virtual disclosure of preclinical Alzheimer's biomarkers: preliminary experiences. J Am Geriatr Soc. 2021;69:2044‐2047. 10.1111/jgs.17184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ding J, Davis‐Plourde KL, Sedaghat S, et al. Antihypertensive medications and risk for incident dementia and Alzheimer's disease: a meta‐analysis of individual participant data from prospective cohort studies. Lancet Neurol. 2020;19:61‐70. 10.1016/S1474-4422(19)30393-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Holloway RG, Gramling R, Kelly AG. Estimating and communicating prognosis in advanced neurologic disease. Neurology. 2013;80:764‐772. 10.1212/WNL.0b013e318282509c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kessler E‐M, Bowen CE, Baer M, Froelich L, Wahl H‐W. Dementia worry: a psychological examination of an unexplored phenomenon. Eur J Ageing. 2012;9:275‐284. 10.1007/s10433-012-0242-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Arias JJ, Karlawish J, Tyler A. Legal consequences and protections: challenges for documenting preclinical biomarkers in patients’ medical records. Alzheimers Dement J Alzheimers Assoc. 2015;11:P282‐3. 10.1016/j.jalz.2015.07.380 [DOI] [Google Scholar]
- 81. Eno CC, Barton SK, Dorrani N, Cederbaum SD, Deignan JL, Grody WW. Confidential genetic testing and electronic health records: a survey of current practices among Huntington disease testing centers. Mol Genet Genomic Med. 2020;8:e1026. 10.1002/mgg3.1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Largent EA, Bradbury AR. Bringing Alzheimer's disease testing and results disclosure into the 21st century cures act. JAMA Neurol. 2022. 10.1001/jamaneurol.2021.4851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Largent EA, Stites SD, Harkins K, Karlawish J. ‘That would be dreadful’: the ethical, legal, and social challenges of sharing your Alzheimer's disease biomarker and genetic testing results with others. J Law Biosci. 2021;8. 10.1093/jlb/lsab004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Alvidrez J, Castille D, Laude‐Sharp M, Rosario A, Tabor D. The National Institute on Minority Health and health disparities research framework. Am J Public Health. 2019;109:S16‐20. 10.2105/AJPH.2018.304883 [DOI] [PMC free article] [PubMed] [Google Scholar]


