Abstract
Purpose
To evaluate the use of QUS for the bone status assessment in children cared because of a chronic disease such as: inherited metabolic disorder, kidney disease and endocrine defect and considered by the attending physician as at specific risk.
Methods
QUS outputs were calculated for each disorder and compared to: sex, age, Tanner stage, Z-score for height, weight and BMI (body mass index).
Results
One-hundred-sixty-eight subjects aged between 3.5 and 18 years met the inclusion criteria. The overall bone quality indexes were under the normal range in all the groups considered. Impairment of bone quality parameters was more evident in the group of patients with inherited metabolic disorders, in which 65% of patients in charge were studied by QUS. Older age and sexual development were associated with less pronounced bone quality impairment, as measured by QUS, in the vast majority of conditions. Overall, the diseases for which the prediction of outcome was the strongest were: hyperphenylalaninemia, nephrotic syndrome and insulin dependent diabetes mellitus.
Conclusions
QUS is capable to provide information on skeletal status in children. Initial evaluation by QUS may allow defining patients with chronic disorders who deserve further, more invasive diagnostic studies. Inherited metabolic disorders warrant specific attention and strict monitoring for their potential effect on bone.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40477-021-00624-5.
Keywords: Bone health, Growth, Pediatric chronic conditions, Puberty, Quantitative ultrasound
Introduction
Bone and mineral disorders not only cause a significant morbidity in the general population [1], but they also significantly affect the quality of life of people affected [2]. This is usually even more pronounced in subjects bearing skeletal abnormalities with early onset [3]. Children bone health results from many anthropological and physiological factors. Their potential impact on adulthood, have attracted many researchers [4, 5].
Furthermore, in children with chronic disorders, either inherited or early acquired, loss of bone health may have a much higher impact, not only on the quality of life, but sometimes even on the prognosis [6]. This lead to several studies addressing this issue in chronic kidney diseases [7], endocrine [8] and, more recently, inherited metabolic disorders [9].
The choice of the optimal method, either imaging or biochemical tests (although these last remain, so far, not-conclusive) is still debated. Dual X-ray absorptiometry (DXA), and Quantitative Computed Tomography (QCT) are the two current reference standards of BMD measurement [10]. However, these methods are costly, clinically based, radiation-associated, and require highly trained operators, thus limiting their friendly use for screening [11]. An additional method for assessing bone mineral content and thus the risk for osteoporosis, is quantitative ultrasound (QUS). In QUS, the attenuation and speed of propagation of a transmitted ultrasound wave describes the physical properties of the examined bone [12, 13]. Advantages of QUS are portability, no need for ionizing radiation, lower cost and higher time effectiveness. Its clinical applications for diagnosis of osteoporosis, fracture risk assessment, treatment initiation, monitoring of treatment and quality control has recently been addressed by the International Society of Clinical Densitometry (ISCD), 2007 Pediatric Position Development Conference [14]. In particular, calcaneal QUS is considered the standard parameter for QUS study of bone health status. The QUS technique in children and adolescents has been used to assess osteopenia and fracture rate in subjects with bone and mineral disorders [15], and steroid-sensitive nephrotic syndrome [16], to monitor uremic osteodistrophy and secondary hyperparathyroidism [17], and in patients with subclinical hypothyroidism [18].
There are a few studies on the simultaneous evaluation of QUS technique in the follow up of different chronic and rare pediatric diseases.
The aim of this study was to assess by QUS the bone status of children and adolescents with selected chronic and rare disorders and to identify those patients who deserved a tailored follow-up.
Methods
Study design
This was a retrospective cohort study, conducted at a single children hospital in the South of Italy. Between December 2016 and December 2019, QUS was made available in the hospital and proposed to the physician in charge for children with kidney disease (KD), inherited metabolic disorders (IMD), or endocrine disorders (ED). The attending physician was responsible for selecting patients considered at possible risk for bone alteration because of the underlying disorder. Patients were eligible for the study, provided they were aged ≤ 18 years, were clinically stable, and informed consent was provided by the patient (when older than 16 years), and by the parents or legal guardian. The study was approved by local institutional review board.
Methods
All patients were assessed for height and weight (in minimal clothing); height-for-age z-scores, weight-for-age z-scores, and body mass index-for-age z-scores (BMI Z-score) were calculated using World Health Organization standards [19].
Well-nourished patients were defined as BMI Z-score between 1 and − 1, obesity as BMI Z-score > 1.64 and short stature as height Z-score lower than − 2, using the WHO charts. Tanner stage was determined by physical examination and patients were categorized in five groups: Tanner 1–5 [20].
The QUS (SONOST 3000; OsteoSys Co., Ltd.) measurement score of the calcaneus region was used to calculate a bone mineral density (BMD) status according to the ISDC Pediatric Official Position (Z-score of − 2.0 or lower was defined as below the expected range for age, and a Z-score above − 2.0 was defined as within the expected range for age) [21]. The machine was calibrated daily according to the manufacturer’s instructions; it provides curves reflecting the structural and mechanical properties of trabecular calcanear bone, no other bones can be examined with this machine. The outputs included the bone quality index (BQI), the broadband ultrasound attenuation (BUA, measured in dB/MHz), the speed of sound (SOS, measured in m/s) (Online Fig. 2, Video 1). BUA reflects bone density and structure by reduction analysis of ultrasound pulse intensity through the bone; SOS expresses speed of ultrasound wave through the bone and reflects bone mineral density. SOS is related to temperature, while BUA is inversely related to temperature. These correlation coefficients (α β) are combined with BUA and SOS to obtain the BQI (BQI = α × SOS + β × BUA) [22].
All QUS tests were performed by the same expert clinician (co-author A.T.).
Statistical analysis
Results were expressed as mean and standard deviation (SD) or median and range according to variable distribution; qualitative variables were expressed as percentages. QUS parameters were calculated for each chronic disorder and compared to growth and pubertal parameters: gender, age, Tanner stage, Z-score for height, weight and BMI.
All eligible patients had at least one QUS measurement, while some had a second or even a third measurement over the observation period. In this case, the first measurement was considered and included in the study.
The Kolmogorov–Smirnov test was utilized to assess the normality of parameter distribution to detect an association between QUS outputs and patients’ parameters, Pearson’s correlation coefficient (Pearson’s r) was used for parameters with normal distribution. Otherwise, for parameters with skewed distribution Spearman’s correlation coefficient (Spearman’s r) was evaluated. All data were analyzed by SPSS22.0 software (SPSS Inc., Chicago, IL, USA) and p < 0.05 was considered as statistically significant.
Results
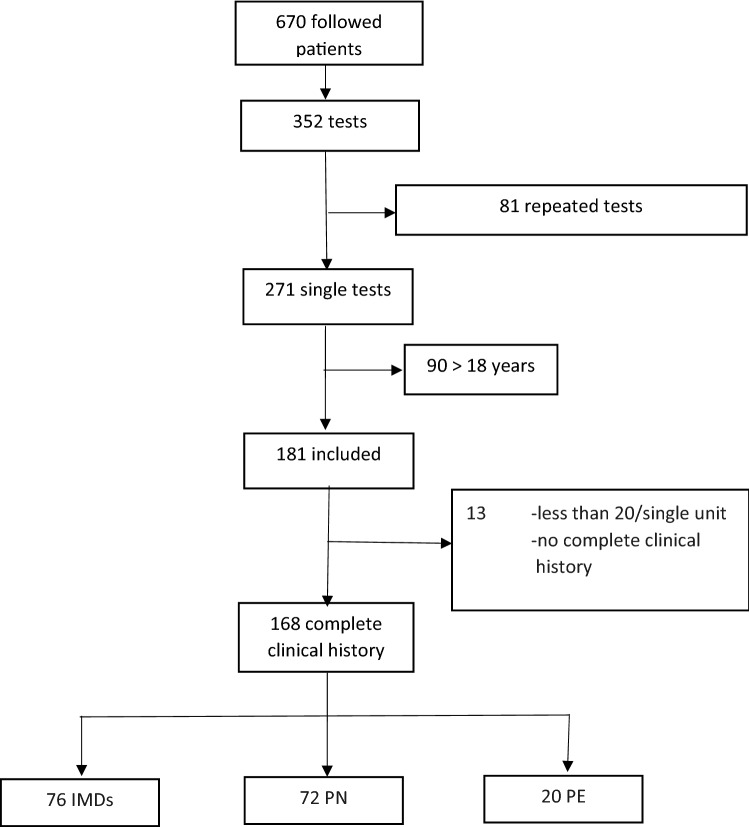
In our intention, the study population comprised all patients followed at our children hospital for chronic and rare diseases, such as: inherited metabolic disorder (IMD); kidney disease (KD); endocrine disease (ED). They underwent QUS for screening of bone status. Figure 1 shows the flow-chart for enrollment of patients.
Fig. 1.
Flow-chart of patients enrollment
Over the 3-year observation time, of the 670 patients with chronic diseases, 271 (40%) underwent at least one QUS assessment; 168 were eligible and were included. Seventy-six patients had an inherited metabolic disorder (IMD), 72 a kidney disease (KD), and 20 an endocrine disease (ED) (Fig. 1).
The median age was 10.7 years (range, 3.5–18 years), 55% were female and 39% were prepubertal. Overall, the Z-score for weight, height and BMI was within the normal range for all patients, with a non-significant trend to lower height, weight and BMI SDS values for the kidney disease group, and higher weight SDS for the endocrine defect group (Table 1).
Table 1.
Characteristics of patients belonging to different groups of conditions
| Inherited metabolic disorders | Kidney disease | Endocrine disorders | All patients | |
|---|---|---|---|---|
| Number of patients | 76 | 72 | 20 | 168 |
| Median age in years (range) | 10.7 (3.5–16.8) | 10.6 (3.7–16.9) | 10.8 (6–18) | 10.7 (3.5–18) |
| Gender F/M | 40/36 | 38/34 | 15/5 | 93/75 |
| Geographic origin | Caucasian | Caucasian | Caucasian | |
| Pubertal stage* | n | n | n | n |
| 1 | 26 | 32 | 8 | 66 |
| 2 | 17 | 17 | 4 | 38 |
| 3 | 12 | 10 | 3 | 25 |
| 4 | 9 | 5 | 4 | 18 |
| 5 | 12 | 8 | 1 | 21 |
| Height Z-score | − 0.4 ± 1.2 | − 0.9 ± 1.6 | − 0.4 ± 1.6 | − 0.5 ± 1.5 |
| Weight Z-score | − 0.17 ± 1 | − 0.5 ± 1.5 | 0.7 ± 1.7 | − 0.2 ± 1.4 |
| BMI Z-score | 0 ± 1 | − 0.7 ± 4 | 1.1 ± 1.5 | 0.4 ± 2.9 |
*By Tanner
Two thirds of the patients (65%) had an inherited metabolic disorder, and 44 had Phenylketonuria (Table 2).
Table 2.
Study population by disease groups and main results of QUS study
| Proportion of patients investigated* | SOS (mean ± SD) |
BUA (mean ± SD) |
BQI (mean ± SD) |
BQI Z-score (mean ± SD) |
|
|---|---|---|---|---|---|
| Inherited metabolic disorders | |||||
| Organic acidemia | 4/10 (40%) | 1501.6 ± 14.2 | 64.9 ± 17.3 | 62.8 ± 17 | − 1.8 ± 1.1 |
| Urea cycle disorders | 6/16 (37%) | 1510.1 ± 16.2 | 60.6 ± 1 | 67.1 ± 1 | − 1.6 ± 0.9 |
| Hereditary carbohydrates defects | 9/14 (64%) | 1446.1 ± 15.5 | 66.6 ± 13.3 | 59.5 ± 14.7 | − 2 ± 0.8 |
| Hyperphenilalaninemia | 6/22 (27%) | 1507.3 ± 15.4 | 77.9 ± 15.1 | 72.2 ± 16 | − 1.1 ± 0.7 |
| Osteogenesis imperfecta | 7/7 (100%) | 1481.8 ± 13.5 | 48.1 ± 11.9 | 44.7 ± 15.4 | − 2.6 ± 0.9 |
| Phenylketonuria | 44/47 (93%) | 1504.2 ± 17.6 | 67.8 ± 15.5 | 65.8 ± 17.3 | − 1.5 ± 0.9 |
| Total | 76/116 (65%) | 1491.8 ± 15.4 | 63.2 ± 12.3 | 61 ± 13.5 | − 1.7 ± 0.8 |
| Kidney diseases | |||||
| Chronic glomerulopathy | 6/82 (7%) | 1504.24 ± 11.7 | 69.97 ± 13.2 | 66.74 ± 13.3 | − 1.49 ± 0.5 |
| Idiopathic hypercalciuria | 14/78 (18%) | 1491.02 ± 13.2 | 55.18 ± 22.5 | 51.16 ± 13.7 | − 2.18 ± 0.7 |
| Chronic renal insufficiency | 12/61 (20%) | 1502.83 ± 15.8 | 64 ± 10.8 | 63.28 ± 13.9 | − 1.9 ± 1 |
| Hereditary rickets | 6/7 (86%) | 1511.19 ± 9 | 74.29 ± 14.2 | 73.52 ± 10.4 | − 1.02 ± 1 |
| Nephrotic syndrome | 15/69 (22%) | 1495.97 ± 16.5 | 55.74 ± 11.8 | 56.22 ± 13.1 | − 1.96 ± 0.5 |
| Inherited tubulopathies | 19/31 (61%) | 1495.90 ± 13.4 | 56.76 ± 17.3 | 55.32 ± 14.4 | − 1.94 ± 0.6 |
| Total | 72/328 (22%) | 1500.19 ± 7.3 | 62.65 ± 8.1 | 61.04 ± 8.3 | − 1.63 ± 0.5 |
| Endocrine disorders | |||||
| Insulin dependent diabetes mellitus | 5/42 (12%) | 1503.77 ± 20.2 | 69.49 ± 18.6 | 66.21 ± 21.6 | − 1.57 ± 0.9 |
| Obesity ( | 8/167 (5%) | 1507.03 ± 23.5 | 61.15 ± 17.3 | 65.13 ± 16.5 | − 1.27 ± 1.1 |
| Turner syndrome | 7/17 (41%) | 1501.06 ± 8.7 | 65.74 ± 13.9 | 62.71 ± 9.6 | − 2.01 ± 0.8 |
| Total | 20/226 (9%) | 1503.95 ± 2.9 | 65.46 ± 4.1 | 64.68 ± 1.8 | − 1.61 ± 0.4 |
*Number of patients investigated/total number of patients in charge with that diagnosis
The mean BQI Z-score felt close to the lower limit of the expected range for age for the three groups analyzed, without statistically significant differences among groups (data not shown). The lowest mean BQI Z-score levels were found in the inherited metabolic disorder group (− 1.7 ± 0.8), although no statistically significant differences were found comparing the BQI Z-score of the three groups: IMD vs KD BQI Z-score p-value: 0.30, IMD vs ED BQI Z-score p-value: 0.33, KD vs ED BQI Z-score p-value: 0.47. For four subgroups, QUS parameters felt under or were equal to the lower limit of the expected range for age (BQI Z-score < − 2): patients with hereditary carbohydrate defects (BQI Z-score: − 2 ± 0.85) had a particular reduction of SOS values; patients affected by osteogenesis imperfecta (BQI Z-score − 2.6 ± 0.9) with a particular reduction of BUA parameters; patients with idiopathic hypercalciuria (BQI Z-score: − 2.18 ± 0.71) had a reduction of both SOS and BUA; patients with Turner Syndrome (BQI Z-score − 2.01 ± 0.83) had a reduction particularly of SOS values (Table 2).
Correlations between QUS parameters, growth and pubertal variables
Linear regression analysis with growth and pubertal parameters revealed a strong correlation of hyperphenylalaninemia (r: 0.88/0.88, p: 0.01/0.02) and Phenylketonuria (PKU) (r: 0.52/0.54, p: 0.0001/0.0001) with age and pubertal stage, for all the three QUS outputs. For patients with carbohydrate disorders, QUS parameters correlated strongly with height (Table 3). Weaker or no correlation was found with height, weight and BMI Z-score for other IMDs.
Table 3.
Correlations of QUS parameters with growth and pubertal variables adjusted for age
| Age | Gender | Tanner stage | Height SDS | Weight SDS | BMI SDS | |
|---|---|---|---|---|---|---|
| SOS | HPE/PKU | HPE/PKU | HPE/HCD | HPE | ||
| CG/IRT | HR | CG/IRT | ||||
| IDDM/obesity/TS | obesity | IDDM/obesity/TS | ||||
| BUA | HPE/PKU | HPE/PKU | UCDs | HCD | HCD | |
| CG/IH/ HR | HR /NS | CG | HR | |||
| IDDM | TS | TS | ||||
| BQI | HPE | UCD/HPE/PKU/OI | HCD/HPE/OI | OI | ||
| CG/IH/NS/HR/IRT | HR | CG/IH/NS/CKD/IRT | HR | NS | ||
| IDDM, obesity | IDDM | Obesity, TS | ||||
| BQI Z-score | HPE, OI | OI | HPE, OI | HCD, HPE, OI | OI | |
| CKD, HR | NS | NS | NS | |||
| IDDM, obesity, TS | IDDM, obesity | IDDM, obesity, TS |
Bold 0.9 < r < 0.7; not in bold 0.69 < r < 0.5
HPE hyperphenylalaninemia, PKU phenylketonuria, HCD hereditary carbohydrate disorders, UCDs urea cycle disorders, OI osteogenesis imperfecta, IRT inherited renal tubulopathy, IH idiopathic hypercalciuria, NS nephrotic syndrome, HR hereditary rickets, CG chronic glomerulopathy, CKD chronic kidney disease, TS turner syndrome, IDDM insulin dependent diabetes mellitus
In the subgroup of kidney diseases, in most of the conditions bone status correlated with age and sexual development for all QUS outputs, with particular strength for age and inherited renal tubulopathies (r: 0.72 p: 0.0001) and sexual development and nephrotic syndrome (r: 0.95 p: 0.0001).
For hereditary rickets, among whom two patients were males affected by X-linked hereditary rickets, gender was the most powerful predictive factor for QUS parameters (r: 0.7 p: 0.0001). Weight and height had little correlation with changes in bone quality with the exception of nephrotic syndrome, particularly for BQI and BQI Z-score.
Also for endocrine disorders, age and pubertal stage showed the strongest correlation with outputs, this finding was particularly true for IDDM (r: 0.9/0.90 p: 0.03/0.0001) and Turner syndrome (r: 0.78/0.75 p: 0.0001/0.0001).
Discussion
Recently, the approach to the diagnosis and monitoring of osteoporosis in children has moved away from a BMD-centric focus to a more functional approach [23], including an evaluation of bone quality indexes related to child age, pubertal development, diet, and physical activity [24]. Indeed, ultrasonography has become, in the last years an easy-to-use and effective mean to speed-up diagnosis in children in many specialist contexts [25–27].
In this study, we reviewed the use of QUS in children with selected groups of chronic disorders in our tertiary-level Children Hospital. Studies in which different pediatric chronic conditions are simultaneously assessed and compared for bone status parameters, are scarce.
The first result is that all three subgroups of patients showed evidence of BQI Z-score at the lower limit of the expected range for age. This is consistent with the inclusion criteria, since only patients with suspected bone health impairment were included in the study. Although this difference did not reach statistical significance, the group of patients with inherited metabolic disorder had a higher level of impairment of bone quality parameters. In this group, the compliance for QUS study was of two thirds. This finding is in keeping with a recent cross-sectional, observational study of the spectrum of microarchitectural bone disease in inborn errors of metabolism: report by Sidhu et al. [9] showing impaired cortical and trabecular bone in comparison to a reference population.
The most frequently predictive parameters of bone quality were age and sexual development, with the increase of which almost all the parameters of QUS improved.
In particular, our patients with Phenylalanine metabolism impairment, 44 with PKU and 6 with hyperphenylalaninemia, had low bone mineral mass, by BQI Z-score close to − 2, in keeping with previous studies [28–30]. This defect is shared by patients with hyperphenylalaninemia, who were protected by a free diet regimen. Yet, interestingly we observed a strongest correlation of QUS parameters with age and pubertal development, suggesting an improvement of bone status with increasing age and after puberty, which is not yet clearly reported in patients with this metabolic disorder, at the best of our knowledge.
Patients with Hereditary carbohydrates defects, especially galactosemia, in our series showed low BQI Z-score (− 2 ± 0.8). This finding is already reported in a meta-analysis based on data from children and adults by van Erven et al. [31], where, in spite of a less severe reported mean BMD-Z score, up to 25% of galactosemia patients were considered to be at risk of a BMD Z score < − 2. Osteogenesis imperfecta, an hereditary connective tissue disorder, showed the lowest BQI Z-score levels (− 2.6 ± 0.9), confirming to be a very severe bone disease with a particular propensity to bone fragility, as already reported in previous studies [32].
Among patients with endocrine disorders, those with Turner syndrome showed the lowest QUS parameters; this is fully expected since osteoporosis is a known feature of these patients, due to intrinsic bone defect exacerbated by hormonal factors [33]. Indeed, estrogen replacement therapy is essential for maintaining bone health in TS as well as in other disorders of sexual development [34]. Patients with insulin dependent diabetes mellitus showed the known improvement of bone parameters over the years, toward the pubertal spurt [35, 36].
Our data in obese patients suggest a trend toward the osteopenia with improving values after puberty. Many studies indicate that the positive effects of body weight on bone mineral density cannot counteract the detrimental effects of obesity on bone quality. However, obese patients may have a not uniform behavior [37], and the exact mechanism underlying their bone deterioration remains unclear [38]. Much is known about bone status of children with kidney disease. Altered bone health induced by chronic inflammation and/or use of glucocorticoids, results in significant morbidity: short stature, bone pain and deformities, fractures, functional complications [7, 39].
In particular, children with Idiopathic hypercalciuria showed the lowest BQI values, which progressively improved with age, possibly due to increment of body mass and reduction in bone resorption, up to almost normalizing in adulthood [40]. Hereditary rickets in our series was associated to a mildly decreased BQI, possibly effect of treatment; regression analysis confirmed significant differences between genders, consistent with the presence of males with the X-linked form in this subgroup.
This study has some limitations. We offered to our colleagues the opportunity to screen by QUS their patients with chronic diseases. We did not include a control group with healthy subjects. Although the total number of patients investigated is relatively high, when the analysis breaks down the population into subgroups, numbers of patients end up in being relatively small. Thus, although the distribution of the results appears quite coherent with available evidences, our findings deserve confirmation on other comparable or even larger patient groups. Furthermore, by study design, we decided not to correlate QUS outputs to laboratory parameters; this because we wanted to explore the use of QUS for screening of patients deserving additional, tailored studies. We were supported, in this design, by the recent study by multiple regression analysis of various laboratory variables, none of which was found to be an independent predictor of BQI [6]. Attempt to correlate calcium intake with bone stiffness was also not successful [24].
Conclusions
Our data suggests that bone impairment is common in children and adolescents with different types of chronic illnesses. This impairment tends to decrease with age, in almost all conditions. QUS confirms as a feasible and affordable method to provide useful information on skeletal status in chronically ill children. Patients with inherited metabolic disorders deserve specific attention and strict monitoring for the potential effect of the underlying disease on bone.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- BQI
Bone quality index
- BUA
Broadband ultrasound attenuation
- EC
Endocrine disease
- IMD
Inherited metabolic disorders
- QUS
Quantitative ultrasound
- KD
Kidney disease
- SOS
Speed of sound
Author contributions
AT performed QUS and wrote the manuscript, MFF, MG and VC made contributions to study sample and interpretation of data; GB made statistical analysis and interpreted data; MA and SP revised critically the manuscript. All authors approved the final version of the manuscript.
Funding
Authors declare no sources of study funding including sponsorship for the described work.
Availability of data and material
The authors confirm that the data supporting the findings of this study are available within the article.
Code availability
N/A.
Declarations
Conflict of interest
No financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
Ethics approval
Ethic approval was obtained from the local ethic commettee.
Consent to participate
Written informed consent was obtained from the participants of the study/their parents.
Consent for publication
Permission for publication was included in the informed consent form.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
4/1/2022
A Correction to this paper has been published: 10.1007/s40477-022-00679-y
References
- 1.NIH Consensus Development Panel on Osteoporosis Prevention Diagnosis, and therapy, March 7–29, 2000: highlights of the conference. South Med J. 2001;94:569–573. [PubMed] [Google Scholar]
- 2.Braithwaite RS, Col NF, Wong JB. Estimating hip fracture morbidity, mortality and costs. J Am Geriatr Soc. 2003;51:364–370. doi: 10.1046/j.1532-5415.2003.51110.x. [DOI] [PubMed] [Google Scholar]
- 3.Rizzoli R, Bianchi ML, Garabédion M, McKay HA, Moreno LA. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and elderly. Bone. 2010;46:294–305. doi: 10.1016/j.bone.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Manifold BM. Bone mineral density in children from anthropological and clinical sciences: a review. Anthropol Rev. 2014;77:111–135. doi: 10.2478/anre-2014-0011. [DOI] [Google Scholar]
- 5.Sopher AB, Fennoy I, Oberfield SE. An update on childhood bone health: mineral accrual, assessment and treatment. Curr Opin Endocrinol Diabetes Obes. 2015;22:35–40. doi: 10.1097/MED.0000000000000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bianchi ML, Leonard MB, Bechtold S, Högler W, Mughal MZ, Schönau E, International Society for Clinical Densitometry et al. Bone health in children and adolescents with chronic diseases that may affect the skeleton: the 2013 ISCD pediatric official positions. J Clin Densitom. 2014;17:281–294. doi: 10.1016/j.jocd.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Kogon AJ, Harshman LA. Chronic kidney disease: treatment of comorbidities I: (nutrition, growth, neurocognitive function, and mineral bone disease) Curr Treat Options Pediatr. 2019;5:78–92. doi: 10.1007/s40746-019-00152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devaraja J, Jacques R, Paggiosi M, Clark C, Dimitri P. Impact of type 1 diabetes mellitus on skeletal integrity and strength in adolescents as assessed by HRpQCT. JBMR Plus. 2020;4:e10422. doi: 10.1002/jbm4.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sidhu K, Ali B, Burt LA, Boyd SK, Khan A. Spectrum of microarchitectural bone disease in inborn errors of metabolism: a cross-sectional, observational study. Orphanet J Rare Dis. 2020;15:251. doi: 10.1186/s13023-020-01521-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guss CE, McAllister A, Gordon CM. DXA in children and adolescents. J Clin Densitom. 2021;24:28–35. doi: 10.1016/j.jocd.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Damilakis J, Adams JE, Guglielmi G, Link TM. Radiation exposure in X-ray-based imaging techniques used in osteoporosis. Eur Radiol. 2010;20:2707–2714. doi: 10.1007/s00330-010-1845-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arpadi SM, Thurman CB, Patel F, Kaufman JJ, Strehlau R, Burke M, et al. Bone quality measured using calcaneal quantitative ultrasonography is reduced among children with HIV in Johannesburg, South Africa. J Pediatr Brief Rep. 2019;215:267–271. doi: 10.1016/j.jpeds.2019.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chin KY, Ima-Nirwana S. Calcaneal quantitative ultrasound as a determinant of bone health status: what properties of bone does it reflect? Int J Med Sci. 2013;10:1778–1783. doi: 10.7150/ijms.6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krieg MA, Barkmann R, Gonnelli S, Stewart A, Bauer DC, Del Rio BL, et al. Quantitative ultrasound in the management of osteoporosis: the 2007 ISCD official positions. J Clin Densitom. 2008;1:163–187. doi: 10.1016/j.jocd.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Baroncelli GI, Federico G, Bertelloni S, Sodini F, De Terlizzi F, Cadossi R, et al. Assessment of bone quality by quantitative ultrasound of proximal phalangeas of the hand and fracture rate in children and adolescents with bone and mineral disorders. Pediatr Res. 2003;54:125–136. doi: 10.1203/01.PDR.0000069845.27657.EB. [DOI] [PubMed] [Google Scholar]
- 16.Aceto G, D'Addato O, Messina G, Carbone V, Cavallo L, Brunetti G, et al. Bone health in children and adolescents with steroid-sensitive nephrotic syndrome assessed by DXA and QUS. Pediatr Nephrol. 2014;29:2147–2155. doi: 10.1007/s00467-014-2834-3. [DOI] [PubMed] [Google Scholar]
- 17.Christoforidis A, Printza N, Gkogka C, Siomou E, Challa A, Kazantzidou E, et al. Comparative study of quantitative ultrasonography and dual-energy X-ray absorptiometry for evaluating renal osteodystrophy in children with chronic kidney disease. J Bone Miner Metab. 2011;29:321–327. doi: 10.1007/s00774-010-0220-1. [DOI] [PubMed] [Google Scholar]
- 18.Di Mase R, Cerbone M, Improda N, Esposito A, Capalbo D, Mainolfi C, et al. Bone health in children with long-term idiopathic subclinical hypothyroidism. Ital J Pediatr. 2012;38:56. doi: 10.1186/1824-7288-38-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization . WHO child growth standards: length/ height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Geneva: World Health Organization; 2006. [Google Scholar]
- 20.Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child. 1976;51:170–179. doi: 10.1136/adc.51.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baim S, Leonard MB, Bianchi ML, Hans DB, Kalkwarf HJ, Langman CB, Rauch F. Official Positions of the International Society for Clinical Densitometry and executive summary of the 2007 ISCD pediatric position development conference. J Clin Densitom. 2008;11:6–21. doi: 10.1016/j.jocd.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Manuals and User Guides for OsteoSys SONOST 3000. Version: 3.03.06, Manufacturer: OsteoSys Co., Ltd. 3F, 308, Byucksan Digital Valley3 Seoul (Korea).
- 23.Högler W, Ward L. Osteoporosis in children with chronic disease. Endocr Dev. 2015;28:176–195. doi: 10.1159/000381045. [DOI] [PubMed] [Google Scholar]
- 24.Cvijetić S, Barić IC, Bolanca S, Juresa V, Ozegović DD. Ultrasound bone measurement in children and adolescents. Correlation with nutrition, puberty, anthropometry, and physical activity. J Clin Epidemiol. 2003;56:591–597. doi: 10.1016/S0895-4356(03)00054-4. [DOI] [PubMed] [Google Scholar]
- 25.Vitale V, Rossi E, Di Serafino M, Minelli R, Acampora C, Iacobellis F, et al. Pediatric encephalic ultrasonography: the essentials. J Ultrasound. 2020;23:127–137. doi: 10.1007/s40477-018-0349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minella R, Minelli R, Rossi E, Cremone G, Tozzi A. Gastroesophageal and gastric ultrasound in children: the state of the art. J Ultrasound. 2021;24:11–14. doi: 10.1007/s40477-020-00471-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brillantino C, Rossi E, Pirisi P, Gaglione G, Errico ME, Minelli R, et al. Pseudopapillary solid tumour of the pancreas in paediatric age: description of a case report and review of the literature. J Ultrasound. 2021 doi: 10.1007/s40477-021-00587-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demirdas S, Coakley KE, Bisschop PH, Hollak CE, Bosch AM, Singh RH. Bone health in phenylketonuria: a systematic review and meta-analysis. Orphanet J Rare Dis. 2015;10:17. doi: 10.1186/s13023-015-0232-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koura HM, Abdallah Ismail N, Kamel AF, Ahmed AM, Saad-Hussein A, Effat LK. A long-term study of bone mineral density in patients with phenylketonuria under diet therapy. Arch Med Sci. 2011;7:493–500. doi: 10.5114/aoms.2011.23417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barat P, Barthe N, Redonnet-Vernhet I, Parrot F. The impact of the control of serum phenylalanine levels on osteopenia in patients with phenylketonuria. Eur J Pediatr. 2002;161:687–688. doi: 10.1007/s00431-002-1091-9. [DOI] [PubMed] [Google Scholar]
- 31.van Erven B, Welling L, van Calcar SC, Doulgeraki A, Eyskens F, Gribben J, et al. Bone health in classic galactosemia: systematic review and meta-analysis. JIMD Rep. 2017;35:87–96. doi: 10.1007/8904_2016_28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunetti G, Papadia F, Tummolo A, Fischetto R, Nicastro F, Piacente L, et al. Impaired bone remodeling in children with osteogenesis imperfecta treated and untreated with bisphosphonates: the role of DKK1, RANKL, and TNF-α. Osteoporos Int. 2016;27:2355–2365. doi: 10.1007/s00198-016-3501-2. [DOI] [PubMed] [Google Scholar]
- 33.Faienza MF, Ventura A, Colucci S, Cavallo L, Grano M, Brunetti G. Bone fragility in turner syndrome: mechanisms and prevention strategies. Front Endocrinol. 2016;7:34. doi: 10.3389/fendo.2016.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertelloni S, Balsamo A, Giordani L, Fischetto R, Russo G, Delvecchio M, et al. 17beta-Hydroxysteroid dehydrogenase-3 deficiency: from pregnancy to adolescence. J Endocrinol Invest. 2009;32:666–670. doi: 10.1007/BF03345738. [DOI] [PubMed] [Google Scholar]
- 35.Bechtold S, Putzker S, Bonfig W, Fuchs O, Dirlenbach I, Schwarz HP. Bone size normalizes with age in children and adolescents with type 1 diabetes. Diabetes Care. 2007;30:2046–2050. doi: 10.2337/dc07-0142. [DOI] [PubMed] [Google Scholar]
- 36.Léger J, Marinovic D, Alberti C, Dorgeret S, Chevenne D, Marchal CL, et al. Lower bone mineral content in children with type 1 diabetes mellitus is linked to female sex, low insulin-like growth factor type I levels, and high insulin requirement. J Clin Endocr Metab. 2006;91:3947–3953. doi: 10.1210/jc.2006-0711. [DOI] [PubMed] [Google Scholar]
- 37.Fintini D, Cianfarani S, Cofini M, Andreoletti A, Ubertini GM, Cappa M, et al. The bones of children with obesity. Front Endocrinol. 2020;24(11):200. doi: 10.3389/fendo.2020.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shapses SA, Pop LC, Wang Y. Obesity is a concern for bone health with aging. Nutr Res. 2017;39:1–13. doi: 10.1016/j.nutres.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tufano A, Minelli R, Di Lascio G, Delicato G, Baffigo G, Signore S. Infected kidney stone progressing to perinephric abscess and thoracic empyema. Arch Ital Urol Androl. 2020 doi: 10.4081/aiua.2020.3.203. [DOI] [PubMed] [Google Scholar]
- 40.Perez-Suarez G, Yanes MIL, de Basoa MCMF, Almeida ES, García Nieto VM. Evolution of bone mineral density in patients with idiopathic hypercalciuria: a 20-year longitudinal study. Pediatr Nephrol. 2021;36:661–667. doi: 10.1007/s00467-020-04754-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
N/A.