Abstract
Purpose
The primary aim was to investigate if treatment guided by serial ultrasound of the inferior vena cava-collapsibility index (IVC-CI) and B-lines on lung ultrasound (LUS) could reduce mortality, readmissions, and length of stay (LOS) in acutely dyspneic patients admitted to a hospital, compared to standard monitoring. The secondary aim was to determine how the changes of B-lines and IVC-CI are correlated to vitals and symptoms.
Methods
A systematic search was conducted on PubMed, Embase, Cochrane, Google Scholar, Web of Science, Scopus, OpenGrey, ProQuest, and databases for ongoing trials. The risk of bias was assessed according to study design.
Results
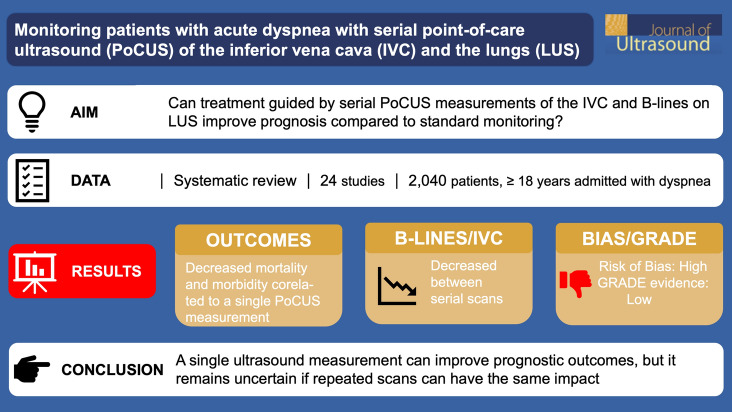
Of the 8258 studies identified, 50 were selected for full-text screening, and 24 studies were chosen for data extraction (19 pre–post-, two non-randomized controlled-, two randomized controlled-, and one retrospective cohort study), covering 2040 patients. Most studies were single-center and had small study populations with only heart failure patients. The risk of bias was high. No studies evaluated how the difference between two ultrasound measurements correlated with the primary outcomes. Seven studies reported that a decline in either B-lines or IVC size, or an increased IVC-CI reduced mortality, readmissions, and LOS when correlated to a single ultrasound measurement. All studies showed changes in the IVC-CI and B-lines, but these were not related to vitals or symptoms.
Conclusion
B-lines and IVC-CI are dynamic variables that change over time and with treatment. A single ultrasound measurement can influence prognostic outcomes, but it remains uncertain if repeated scans can have the same impact.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s40477-021-00622-7.
Keywords: Dyspnea, Focused cardiac ultrasound, Lung ultrasound, Monitoring, Point-of-care ultrasound
Introduction
Acute dyspnea is one of the most frequent reasons for admittance to an emergency department (ED) [1]. Dyspnea is caused by a broad spectrum of conditions, e.g., pneumonia, heart failure, and pulmonary embolism [2]. Patients with dyspnea have high mortality and prolonged length of stay (LOS) compared to patients admitted with other symptoms [3, 4]. Dyspnea is a patient-reported outcome and causes anxiety among patients [5–7].
Ultrasound is used for diagnosis and monitoring and as a prognostic tool in dyspneic patients. Ultrasound of the inferior vena cava (IVC) is used either alone or as a part of focused cardiac ultrasound (FoCUS) or more comprehensive echocardiography to gain information about the patient’s fluid status [8] and to diagnose acute heart failure [9]. Focused lung ultrasound (LUS) is additionally applied to detect pneumothorax, consolidations (e.g., pneumonia), and pleural effusions as well as interstitial syndrome (e.g., lung edema) that is defined sonographically as the presence of multiple B-lines in several scanning zones bilaterally [10].
Studies have investigated the role of monitoring patients admitted with dyspnea with repeated ultrasound of the IVC [11, 12] or LUS [13, 14], either used separately or in combination [15], but the treatment in these studies was not based on a protocol adjusted to the ultrasound findings. Only patients with dyspnea due to heart failure were included, and no studies included all dyspneic patients regardless of the presumptive diagnosis. Furthermore, the studies correlated outcomes to only a single ultrasound of the IVC or B-lines and not to repeated scans. Therefore, it is essential to examine the potential of serial ultrasound assessment in patients with dyspnea due to various causes.
The primary aim of this systematic review was to investigate if treatment guided by ultrasound of B-lines on LUS alone or combined with ultrasound of IVC-collapsibility index (IVC-CI), could reduce mortality, readmissions, and LOS in acutely dyspneic patients admitted to a hospital, when compared to usual care. The secondary aim was to investigate how the dynamic changes of B-lines and IVC-CI are correlated to vital signs and symptoms.
Materials and methods
This systematic review follows the Joanna Briggs Institute methodology for systematic reviews [16] and the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [17, 18] (PRISMA checklist, Supplementary Material 1).
The protocol was registered at the International Prospective Register of Systematic Reviews (PROSPERO), number CRD42018116608, and published to enhance transparency of the research process [19].
Search strategy
A systematic search was performed on PubMed, Cochrane, Embase, Scopus, Web of Science, and Google Scholar. The gray literature was sought in OpenGrey and ProQuest. Ongoing trials were sought in Clinical Trials and the International Clinical Trials Registry Platform (ICTRP). Reference lists of the included studies were searched manually to find additional eligible studies. The inbuilt function “related/similar articles” in PubMed was used to detect further studies. Finally, forward-searching was used in Scopus and Web of Science with the included studies. The general search string, and the specific search strings used in the different databases, are listed in Supplementary Material 2. The last search was run on 17 December 2019, and the snowballing process was completed on 14 January 2020.
Studies were eligible if they included patients 18 years or older with dyspnea who were admitted to a emergency or similar setting and examined with ultrasound of the IVC and/or LUS at least twice during the admission. If patients were admitted with dyspnea mainly due to trauma or were mechanically ventilated, the studies were excluded. This was because patients with trauma or those undergoing mechanical ventilation represent a cohort that can receive a different diagnostic work-up and is typically transferred initially to an intensive care unit or surgical ward. Furthermore, the IVC behaves differently when the patient is ventilated [20]. Outcomes were mortality, readmission, LOS, and changes in ultrasound parameters (IVC-CI and B-lines). Controlled trials (randomized and non-randomized), observational studies (cohort, case–control), case reports, and conference abstracts based on these types of study designs written in English, Danish, Swedish, Norwegian, and German were included for screening. Studies were not excluded solely on the grounds of study type because it narrowed the evidence. Instead, all included studies were critically appraised according to study design.
References were double-checked for duplicates in Endnote V8.2 (Clarivate Analytics, PA, USA) and Covidence (Covidence, Melbourne, Australia). Two reviewers (MDA and NJ) screened all identified studies independently, and any divergences were resolved through discussion and consensus agreement.
Data collection and processing
The data were extracted independently by MDA and NJ according to a predefined data collection form, and disagreements were resolved through discussion, eliminating the need for a third reviewer.
MDA and NJ critically appraised the included studies according to study design with standardized tools for randomized and non-randomized controlled trials provided by the Joanna Briggs Institute [16]. The tools consist of a series of questions and do not provide an overall score. No studies were excluded on the grounds of the appraisal. Instead, a general judgment was based on the strength and limitations of the individual studies. Interrater agreement was assessed with Cohen’s kappa and calculated with STATA V16.1 (StataCorp, TX, USA).
A meta-analysis was planned but was not feasible due to heterogeneity in the study designs, interventions, ultrasound protocols, and scanning intervals.
An overall assessment of the robustness of evidence was made using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach [21]. The GRADE approach rates quality of evidence as high, moderate, low, or very low for each examined outcome.
Results
Study selection
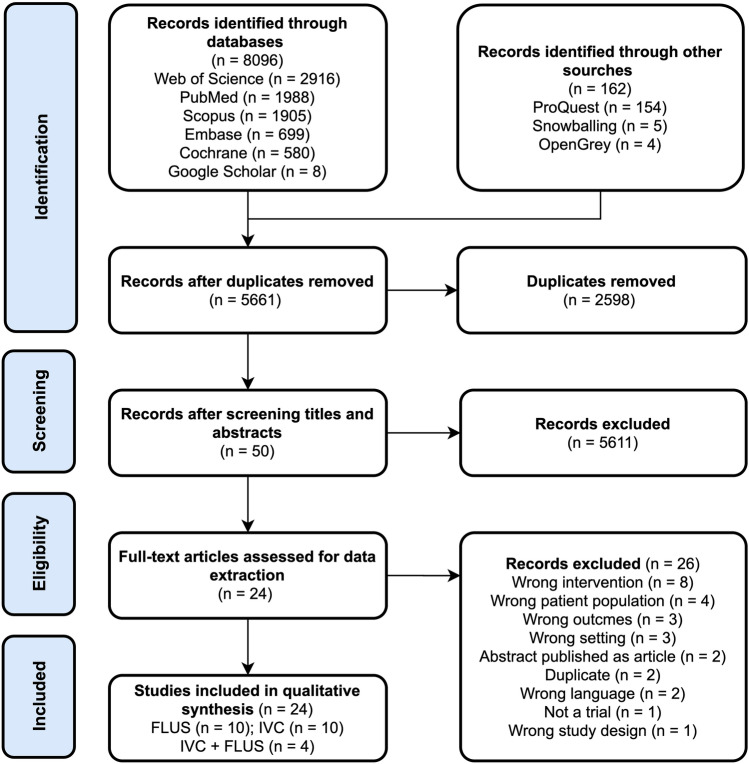
The search identified 8258 studies (Fig. 1). After removing duplicates, 5661 studies underwent screening of titles and abstracts against the eligibility criteria, yielding 50 studies. After the full-text screening, 24 studies met the inclusion criteria. Supplementary Material 3 provides a list of the excluded studies from the full-text screening and the reasons for excluding them. The studies were excluded for various reasons but mainly because ultrasound scanning was not repeated during the admission.
Fig. 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram of the study selection process. IVC inferior vena cava, FLUS focused lung ultrasound
Characteristics of the studies
The characteristics of the studies are presented in Table 1. The 24 included studies comprised 19 pre–post studies [11, 15, 22–38], two non-randomized controlled trials (NRCT) [12, 39], two randomized controlled trials (RCT) [13, 40], and one retrospective cohort study [41]. Seventeen studies were single-center [11–13, 22, 23, 25–28, 30, 32, 34, 36–40], four were multicenter [29, 33, 35, 41], and in three cases the authors did not report the number of sites [15, 24, 31]. Only a few studies reported about funding [15, 23, 28, 32–35, 38]. About half the studies were from either Italy [26, 29, 31, 32, 34, 36, 40, 41], or USA [25, 27, 28, 33, 35].
Table 1.
Characteristics of the included studies
| Study; country; no. of sites | Funding | Design | Cohort | n | Age, years, mean | US protocola | Scanning interval | Mortality | Readmission | LOS | Changes in IVC-CI and B-lines |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies with US of the IVC | |||||||||||
|
Asahi et al. 2016 [11] Japan, 1 site |
NR | Pre–post | AHF, chronic HF | 74 | 75 |
Probe NR Mode NR Meas. point NR |
Admission, 24 h | NR | NR | NR | 72–52% if 1st IVC-CI ≥ 0.5, 28–43% if 1st IVC-CI < 0.5 |
|
Chuen et al. 2009 [22] UK, 1 site |
NR | Pre–post | AHF | 31 | 72 |
Probe NR M-mode Measurement point NR |
0, 0.5, 1, 2, 3 h | NR | NR | NR | ICV-CI 12–14% |
|
Cubo-Romano et al. 2016 [23] Spain, 1 site |
Grants for a medical society in Spain and National Institutes of Health | Pre–post | AHF | 97 | 78 |
Convex Mode NR 2 cm from RA |
< 24 h, discharge |
90 days mortality rate 25.4% if IVCmax ≥ 1.9 cm vs. 3.4% 180 days: 29.3% vs. 3.4% |
90 days readmission rate 30.8% if IVCmax ≥ 1.9 cm vs. 10.7% 180 days, 38.0% vs. 14.3% |
NR | IVC-CI 25.7–33.1% |
|
DeVecchis et al. 2012 [41] Italy, 2 sites |
NR | Retrospective | AHF |
49 34 controls |
77 |
Sector M-mode 3 cm from RA |
Admission, < 48 h | NR | NR | NR | IVC-CI 28.2–30.0% |
|
Fawzi et al. 2012 [24] Egypt, sites NR |
NR | Pre–post | AHF | 30 | 50 | NR | Admission, day 5, day 10 | NR | NR | NR | NRb |
|
Goonewardena et al. 2008 [25] USA, 1 site |
NR | Pre–post | AHF | 75 | 61 |
Probe NR Mode NR 3 cm from RA |
< 12 h, discharge | NR |
30 days: 41% readmitted IVC-CI, at admission was 23% in those readmitted vs. 31% in those not readmitted At discharge 36% vs. 57% |
NR | IVC-CI 27–45% |
|
Guiotto et al. 2010 [26] Italy, 1 site |
NR | Pre–post | AHF | 24 | 72 |
Sector M-mode 3 cm from RA |
Before, after 12 h and at the end of ultrafiltration | NR | NR | NR | IVC-CI 12.5–23.6% |
|
Patnaik et al. 2016, USA [27] USA, 1 site |
NR | Pre–post | AHF | 50 | 68 | NR | < 24 h, daily | NR | NR | No difference in LOS according to IVC-CI | IVC-CI 41.3–40.9% |
|
Tchernodrinski et al. 2014 [28] USA, 1 site |
None | Pre–post | AHF | 70 | 55 |
Sector Mode NR 2 cm from RA |
0.5 h before, 1–2 h, 2–3 h after i.v. furosemid | NR | NR | NR | IVCmax 2.3–0.21 cm |
|
Yavasi et al. 2014 [12] Turkey, 1 site |
NR | NRCT | AHF |
47 50 controls |
67 |
Conex Mode NR Distal to hepatic vein |
Before + after 12 h of Tx | NR | NR | NR |
IVC-CI: 22.80–39.75% Controls: no difference in IVC-CI |
| Studies with LUS | |||||||||||
|
Cortellaro et al. 2017 [29] Italy, 4 sites |
NR | Pre–post | AHF | 41 | 77 |
Convex Transverse/oblique orient 11 zonesc |
Admission, + 3, + 24 h | NR | NR | NR | B-score: 1.59–0.38 points |
|
Daskalov et al. 2016 [30] Bulgaria, 1 site |
NR | Pre–post | AHF | 100 | NR | NR | Admission, 4 ± 2 days | NR | NR | NR | Reduction in B-lines, exact numbers NR |
|
Facchini et al. 2016 [31] Italy, sites NR |
NR | Pre–post | AHF | 50 | 75 |
Probe NR Orient. NR 28 zones Sum B-lines |
Admission, 24 h | NR | NR | NR | B-lines: 53.4–31.7 |
|
Gargani et al. 2015 [32] Italy, 1 site |
One coauthor was consultant for several medical indutries | Pre–post | AHF | 118 | 70 |
Probe NR Orient. NR 28 zones Sum B-lines |
Admission, discharge | 4 died, B-lines relation NR | 14 readmitted with more B-lines at admission + discharge | NR | B-lines: 48–20 |
|
Martindale et al. 2018 [33] USA, 2 sites |
Supported in part by grants from a university and National Institutes of Health | Pre–post | Dyspnea, sBT ≥ 180 mmHg, AHF | 20 | 68 |
Convex Sagittal orient 8 zones Sum B-lines |
< 45 min, after improvement of dyspnea, discharge | NR | NR | NR |
B-lines: 47–8 Correlation between dyspnea (VAS from 0 to 10) and no. of B-lines |
|
Palazzuoli et al. 2018 [34] Italy, 1 site |
One coauthor received grants from medical industry | Pre–post | AHF | 162 | 80 |
Probe NR Orient. NR 8 zones Sum B-lines |
< 12 h, discharge | 10% died, had a higher B-line count at discharge | 28% readmitted, had a higher B-line count at discharge | NR |
B-lines: 31–20 B-lines had a correlation with RR |
|
Platz et al. 2019 [35] USA, 2 sites |
Supported by grants from National Institutes of Health, the British Heart Foundation and several medical indutries | Pre–post | AHF | 349 | 75 |
Sector Sagittal orient 4 zones Sum B-lines |
< 4 days, discharge | 6 months, 36% (0–4 B-lines at discharge) vs. 55% (> 7 B-lines) died/readmitted | 6 month, 36% (0–4 B-lines at discharge) vs. 55% (> 7 B-lines) died/readmitted |
0–3 B-lines: 5 days 4–6 B-lines: 8 days ≥ 7 B-lines: 7 days |
B-lines: 6–4 |
|
Strnad et al. 2016 [13] Slovenia, 1 site |
NR | RCT | AHF | 20 | 81 |
Micro convex Orient. NR 15 zones Sum B-lines |
NR |
CPAP: 58% survied to discharge Control: 75% survied to discharge |
NR |
CPAP: 13 days Control: 7 days |
B-lines: CPAP group, 46.9–29.0 Control, 42.4–43.3 |
|
Vitturi et al. 2011 [36] Italy, 1 site |
NR | Pre–post | Dyspnea | 152 | NR |
Convex Sagittal orient Zones NR Pos. scan: > 8 B-lines |
Admission, 48 h | NR | NR | NR | B-lines decreased, exact no. NR |
|
Volpicelli et al. 2008 [37] Italy, 1 site |
NR | Pre–post | AHF | 81 | 75 |
Convex Sagittal orient 15 zones Sum B-lines |
Admission, discharge | NR | NR | NR | B-lines: 8 positive zones to 0 |
| Studies with both US of the IVC and LUS | |||||||||||
|
Mozzini et al. 2018 [40] Italy, 1 site |
NR | RCT | AHF |
LUS: 60 CXR: 60 |
84 |
LUS: sector Orient. NR Zones NR Sum of B-lines IVC: convex M-mode 2 cm from RA |
LUS: admission, 24 h, 48 h, 72 h, discharge IVC: admission, discharge |
NR | NR |
LUS group: 7 days CXR group: 8 days |
B-lines from 23 to 50% IVC-CI: Inverse association between IVC-CI and no. of B-lines |
|
Spevack et al. 2017 [15] Canada, sites NR |
None | Pre–post | AHF | 50 | 77 |
LUS: convex Oblique orient 8 zones Sum of B-lines IVC: sector mode NR 1–2 cm from RA |
Admission, 1 day, discharge | NR | NR | NR |
IVC-CI 30–25% B-lines: 11–8.3 |
|
Öhman et al. 2018 [39] Finland, 1 site |
NR | NRCT | AHF |
US-guided Tx: 20 Control: 100 |
76 |
LUS: sector Sagittal orient 6 zones Sum B-linesd IVC: sector M- or B-mode 1–2 cm from hepatic vein |
Admission, daily, discharge | 84% patients with resolution of congestion survied at 6 months vs. 61% | 36% patients with resolution of congestion died/readmitted vs. 58% at 6 months | NR | Congestion decreased but no. of B-lines NRe |
|
Öhman et al. 2018 [38] Finland, 1 site |
None | Pre–post | AHF | 60 | 76 |
LUS: sector Sagittal orient 6 zones Sum B-linesd IVC: sector M- or B-mode 1–2 cm from hepatic vein |
0 h, 12 h, 24 h, 48 h, discharge | 84% patients with resolution of congestion survied at 6 months vs. 60% | 37% patients with resolution of congestion died/readmitted vs. 64% at 6 months |
Resolution: 6.16 days Congestion: 7.22 days |
Congestion decreased but no. of B-lines NRe |
AHF acute heart failure, CPAP continuous positive airway pressure, CXR chest X-ray, HF heart failure, IVC-CI inferior vena cava-collapsibility index, IVCmax inferior vena cava max diameter, LOS length of stay, LUS lung ultrasound, NR not reported, NRCT non-randomized controlled trial, orient. orientation, RA right atrium, RCT randomized controlled trial, RR respiratory rate, sBT systolic blood pressure, Tx treatment, US ultrasound, VAS visual analog scale
aProbe type, M- or B-mode (IVC), orientation of the probe (LUS), no. of scanning zones (LUS)
bDiscrepancy between reported IVC-CI in text and table and therefore NR
cScore: 0 points: < 3 B-lines in a zone, 1 point: ≥ 3 B-lines in ≥ 1 zone, 2 points: multiple B-lines
dCongestion definition: 3 ≥ B-lines in ≥ 1 zone bilat. or > 5 mm of pleural fluid bilaterally
eIVC is reported as a categorical scaled IVC index
The included studies involved 2040 patients (1796 subjects vs. 244 controls) with a mean age of 72 years (66% women). The studies had a sample size ranging from 20 to 349 patients with diagnosed or suspected heart failure. In most studies, the treatment intervention strategy was only described in broad terms, e.g., “usual heart failure treatment,” without mentioning specific medications, dosages, or other treatments. Furthermore, the studies did not link the changes in ultrasound findings to a specific treatment algorithm.
The scanning protocols also varied: ten studies with ultrasound of IVC only [11, 12, 22–28, 41], ten with LUS only [13, 29–37], and four with both [15, 38–40]. The ultrasound protocols for IVC scanning were nearly identical with IVC scanning done during a normal respiration cycle in the subcostal view 2 cm from the junction of the IVC to the right atrium. Measurements with both M- and B-mode were used, and the patient was in the supine or semi-supine position. The choice of probe was the phased array. Preset was not described. The minimum scanning interval ranged from 0.5 h to 5 days.
The LUS protocols varied regarding the number of zones scanned (from 4 to 28 zones) and how the B-lines were quantified: (1) sum of B-lines; (2) the number of positive zones (positive zone: 3 ≥ B-lines per zone); (3) a positive scan defined as 8 or more B-lines in total; (4) two different congestion scores—one depending on the number of B-lines in each zone and one depending on the number of B-lines and/or presence of pleural effusions bilaterally. Different types of probes were used (micro convex, convex, sector). The transducer’s orientation was either in the sagittal or oblique plane, and the patient was positioned supine or semi-supine. Preset setting was not reported except for two studies that used the abdominal preset [29, 33]. The time between the two LUS examinations varied from 3 h to 6 days.
Critical appraisal of the studies
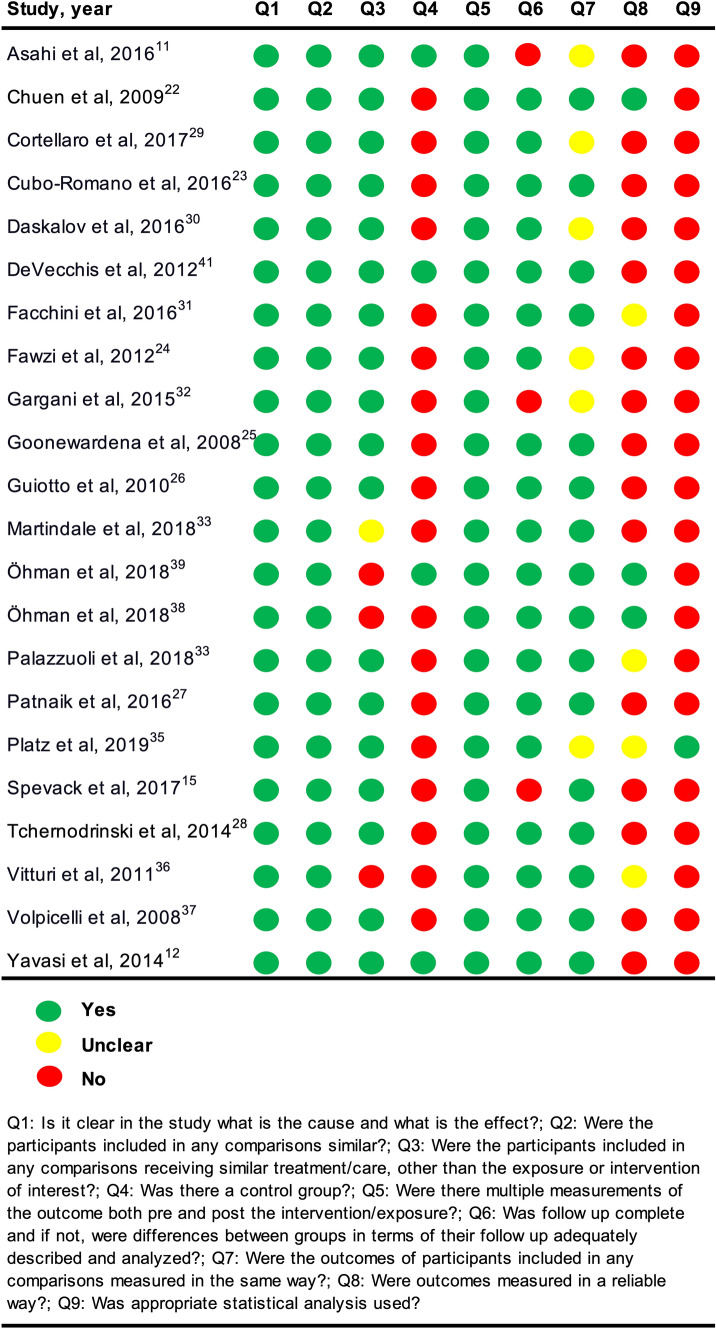
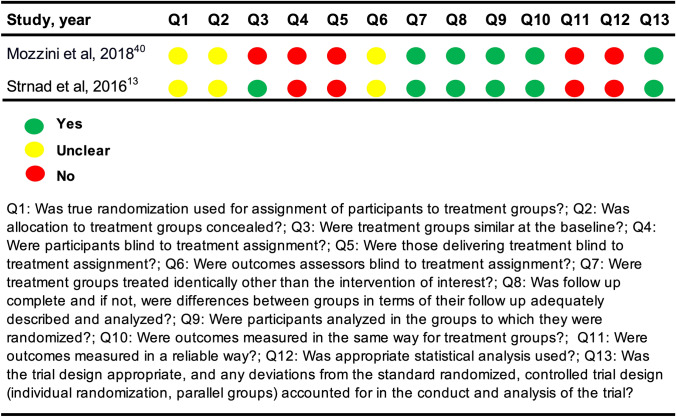
An overview of the critical appraisal is provided in Figs. 2 and 3. Consensus was reached between the two reviewers with a substantial agreement; Cohen’s kappa was 0.69. Responses were either yes, no, or unclear. The option of ‘not applicable’ was not used. The answers “no” and “unclear” were both considered to introduce potential bias. The studies generally failed to test for intra- and intervariability and to report confidence intervals and sample size calculations. In addition, the NRCTs failed to measure outcomes in a reliable way and were missing a control group to compare the intervention against to strengthen the examination of causal validity. In the RCTs, it was unclear if true randomization was used, and it was uncertain if outcome assessors were blinded. According to the predefined critical appraisal tool for the RCT, unblinding of the treating physician or patient should be considered bias. However, this was not considered a bias in our review because we investigated the effect of ultrasound-guided treatment; thus, the physician at some point in time had to be informed of the ultrasound findings in order to react to them.
Fig. 2.
Critical appraisal of the quasi-experimental studies
Fig. 3.
Critical appraisal of the randomized controlled trials
Review findings
No studies reported on how differences between two ultrasound measurements correlated with the primary outcomes—death, LOS, or readmission. However, some of the studies correlated one or several timepoint measurements (e.g., the ultrasound measurement at admission or at discharge) with the outcomes. Seven studies reported on deaths in correlation with point estimates of either IVC [23] or B-lines [13, 32, 34, 35, 38, 39]. Patients with more B-lines had about 1.5-fold increased risk of dying [35, 38, 39] and readmission [32, 34, 35, 38, 39]. A diameter of ≥ 1.9 cm IVCmax was correlated with about 7–9 times higher mortality rate at 3 and 6 months [23]. A larger IVC diameter [23] and/or a smaller IVC-CI [25] correlated with a 2.5-fold increased rate of rehospitalization. No differences were observed in LOS related to IVC-CI [27]. Three studies [35, 38, 40] reported reduced LOS by 1–2 days if the patient had fewer B-lines, but another study [13] showed increased LOS even though there were fewer B-lines compared to a control group. The studies using both IVC and LUS ultrasound did not correlate the combined scanning results with mortality, readmission, or LOS [15, 38–40].
All studies reported variations in B-lines or IVC-CI between two or several timepoints during the admission. A decline in B-lines and an increase in IVC-CI was seen from admission to discharge. Only two studies correlated the findings to vital signs or symptoms. One study reported a positive correlation between dyspnea on a verbal scale from 0 to 10 and the number of B-lines [33]. Another study reported a positive correlation between B-lines and respiratory rate [34].
In the GRADE summary of findings (Tables 2, 3), the outcomes were associated with B-lines and IVC. In general, the evidence was rated as low to very low primarily due to the type of study design, small sample sizes, few events, restriction to patients with heart failure, and no control groups.
Table 2.
Summary of findings using the Grading of Recommendations Assessment, Development and Evaluation approach (GRADE) for outcomes associated with number of B-lines
| Outcomes | No of participants (studies) | Certainty assessment | Comments | |||||
|---|---|---|---|---|---|---|---|---|
| Risk of bias | Inconsisten-cy | Indirectness | Imprecision | Publication bias | Overall certainty of evidence | |||
| Mortality associated with no. of B-lines |
837 (4 pre–post studies, 1 NRCT, 1 RCT) |
Seriousa | Seriousb | Seriousc | Seriousd | None |
⊗◯◯◯ Very low |
The studies suggested that fewer B-lines was associated with better survival |
| Readmission associated with no. of B-lines |
809 (4 pre–post studies, 1 NRCT) |
Seriouse | Seriousb | Seriousc | Seriousf | None |
⊗◯◯◯ Very low |
A lower B-line count at discharge was associated with fewer readmissions |
| LOS associated with no. of B-lines |
549 (2 pre–post studies, 2 RCTs) |
Seriousg | Seriousb | Serioush | Seriousd | None |
⊗◯◯◯ Very low |
A fewer no. of B-lines was associated with a decreased LOS in three studies |
| B-lines associated with vitals |
162 (1 pre–post study) |
Seriousi | Not serious | Seriousj | Seriousk | None |
⊗◯◯◯ Very low |
B-line had a strong correlation with the respiratory rate (Spearmans coefficient 0.75) |
| B-lines associated with degree of dyspnea |
20 (1 pre–post study) |
Seriousl | Not serious | Seriousj | Seriousk | None |
⊗◯◯◯ Very low |
Correlation between dyspnea (on a VAS from 0 to 10) and no. of B-lines, but no correlation between the magnitude of change in sonographic pulmonary edema and VAS scores |
CI confidence interval, LOS length of stay, NRCT non-randomized controlled trial, RCT randomized controlled trial, VAS visual analog scale
aDowngraded due to risk of bias (pre–post studies: no control group, not the same treatment, no sample size calculations. RCT randomization and allocation unclear, no blinding, no sample size calculations)
bDowngraded due to inconsistency (different scanning protocols and outcome measurements)
cDowngraded due to indirectness (only patients with heart failure, different scanning protocols, no control groups, different outcome measurements)
dDowngraded due to imprecision (few events and more events in control group in RCT)
eDowngraded due to risk of bias (no control group, not provided the same treatment, outcomes not measured in a reliable way, no sample size calculations)
fDowngraded due to imprecision (few events)
gDowngraded due to risk of bias (pre–post studies: no control group, no sample size calculations. RCT randomization and allocation unclear, no blinding, no sample size calculations)
hDowngraded due to indirectness (only patients with heart failure, different scanning protocols, small control group, different outcome measurements)
iDowngraded due to risk of bias (no control group, no sample size calculation)
jDowngraded due to indirectness (only patients with heart failure, no control group)
kDowngraded due to imprecision (small sample)
lDowngraded due to risk of bias (no control group)
Table 3.
Summary of findings using the Grading of Recommendations Assessment, Development and Evaluation approach (GRADE) for outcomes associated with IVC-CI
| Outcomes | No. of participants (studies) | Certainty assessment | Comments | |||||
|---|---|---|---|---|---|---|---|---|
| Risk of bias | Inconsisten-cy | Indirectness | Imprecision | Publication bias | Certainty of the evidence | |||
| Mortality associated with IVC-CI | 97 (1 pre–post study) | Not serious | Not serious | Seriousa | Seriousb | None |
⊗⊗◯◯ Low |
Only one pre–post study with a small sample size conducted in a selected population of heart failure patients |
| Readmission associated with IVC-CI | 172 (2 pre–post studies) | Not serious | Seriousc | Seriousa | Seriousb | None |
⊗◯◯◯ Very low |
Only two pre–post studies with a small sample size conducted in a selected population of heart failure patients with different outcomes (IVCmax and IVC-CI) |
| LOS associated with IVC-CI | 50 (1 pre–post study) | Seriousd | Not serious | Seriousa | Seriousb | None |
⊗◯◯◯ Very low |
Only one pre–post study with a small sample size conducted in a selected population of heart failure patients and no differences in LOS associated with IVC-CI |
| IVC-CI associated with vitals | (0 studies) | – | – | – | – | – | – | IVC-CI associated with vitals not reported |
| IVC-CI associated with degree of dyspnea | (0 studies) | – | – | – | – | – | – | IVC-CI associated with degree of dyspnea not reported |
CI confidence interval, IVC-CI inferior vena cava collapsibility index, LOS length of stay
aDowngraded due to indirectness (only patients with heart failure, no control group)
bDowngraded due to imprecision (small sample)
cDowngraded due to inconsistency [uses different outcomes (IVCmax and IVC-CI)]
dDowngraded due to risk of bias (outcomes not measured in a reliable way, no sample size calculations)
Discussion
We found that single LUS or IVC ultrasound alone or in combination have a possible role in monitoring acutely dyspneic patients and may offer prognostic outcomes (death, readmission, LOS) when related to a single ultrasound measurement. However, the association between outcomes and differences between serial ultrasound measurements remains uncertain. Ultrasound parameters (IVC, B-lines) changed during the course of admission, but their correlation to standard measurements was unclear. The studies showed risk of bias, and the body of evidence was low.
The review showed that a larger IVC size or a lower IVC-CI was correlated with higher mortality and more readmissions [23, 25]. However, these prognostic outcomes were only linked to point estimates of IVC-CI at a particular timepoint and not to the differences between two measurements, which was our objective in examining the necessity of repeated ultrasound examinations. Furthermore, the treating clinicians were not instructed on how to react to changes in the IVC.
IVC measurement is usually used in the ED to forecast fluid responsiveness [20]. Two meta-analyses that investigated the role of only one IVC measurement during admission came to opposite conclusions regarding its usefulness to guide fluid administration [42, 43]. It was found to be more reliable in mechanically ventilated patients, which was not the subject of interest in this systematic review. Another meta-analysis suggested cut-offs in the IVC-CI value and IVCmax size to predict if a patient with acute dyspnea is more likely to have acute heart failure than another condition; the heterogeneity and bias across the studies were both high, however [44]. A dynamic structure such as the IVC should have the potential to be followed over time, but the data should be interpreted with caution and always in the clinical context and together with other parameters such as vital signs, physical examination, and blood samples.
A low number of B-lines on LUS was related to better survival, fewer readmissions, and reduced LOS. This is supported by a systematic review investigating the role of B-lines as a prognostic tool in heart failure patients in emergency and outpatient settings [45]. It concluded that the number of B-lines decreased when heart failure was treated, and a high sum of B-lines might identify those with a higher risk of readmission or death. In a study where LUS was compared to low-dose computed tomography as the reference method, B-lines on an 8-zoned LUS could be used to diagnose patients with congestion; ≤ 1 B-line could rule out congestion while ≥ 3 B-lines bilaterally and/or the presence of bilateral pleural effusions could rule in congestion in patients 50 years or older and with suspected heart failure [46]. The evidence thus suggests that B-lines are reasonable to measure upfront in the diagnostic evaluation and later in discharge planning. However, it remains uncertain how far this relates to patients with dyspnea from any cause. B-lines are not always present on the first examination, and they could develop in the course of the admission, e.g., in patients receiving too much fluid. B-lines can also be seen in patients without heart failure, e.g., acute respiratory distress syndrome (ARDS), pulmonary infections, and interstitial lung diseases, underlining the need for studies with broader inclusion criteria [10].
All the included studies showed that ultrasound could provide dynamic measurements that alter over time. This means that treatment effects could theoretically be monitored by the dynamics of the ultrasound parameters. This is time-consuming, however, compared to just asking the patients about symptoms or interpreting the vital signs. Therefore, these ultrasound parameters must be shown to be linked to both symptoms and vital signs to determine if they provide additional clinical information. Furthermore, an ultrasound-based treatment algorithm must be related to the ultrasound variables to investigate the intervention’s effect.
As dyspneic patients have a poor prognosis [3, 4], it is essential to find better ways of monitoring them and guiding their treatment. It is too early to proclaim serial ultrasound as a feasible monitoring tool in the ED. This needs to be examined in a study designed to overcome some of the issues present in most of the included studies in this systematic review. The study has to be powered to answer the research question, with sample size calculated beforehand. Many of the studies had a hard endpoint (e.g., death) that could require a large sample size. Instead, a patient-reported outcome (such as dyspnea on a verbal scale with blinding of the outcome accessor) could be chosen. An evaluation of the effect of serial ultrasound-guided treatment should be based on a treatment protocol, and differences between two (or more) ultrasound measurements (period effect) at different timepoints need to be compared to a control group (treatment effect). Furthermore, the presumptive diagnosis is not always correct [47], and the inclusion of a broader group of patients with dyspnea is desirable, not just heart failure; such a study has already been initiated [48]. Ultrasound should always be interpreted in the clinical context. Instead of using exact estimates like the precise measurement of the IVC and the total number of B-lines, another approach is to use dichotomized variables, e.g., large/small IVC, collapsible/not collapsible IVC, and B-lines present uni- or bilaterally. This method is also more straightforward and faster and can be used by non-specialists with limited training [49, 50].
Limitations
First, we included studies published in the gray literature as well as abstracts with more limited information in order to limit selection bias. This could have led to unjustified heterogeneity, missing data, and reporting bias. Second, only a handful of the included studies reported prognostic outcomes (mortality, readmission, LOS); thus, the evidence is based on a few studies with a high risk of bias, and the results should be interpreted with caution. Third, the studies’ lack of comparability made a meta-analysis inappropriate, and we could not report precise effect estimates or their certainty. However, our qualitative synthesis of the results provided an overview of the literature and current evidence. Fourth, many different tools exist to judge the risk of bias in studies, and it may not be possible for one tool to cover all types of included study designs [51]. We prioritized tools from the same source to ensure consistency, but some of the predefined questions had to be interpreted differently from the original intention, e.g., the importance of blinding of the treating physician.
Conclusion
In conclusion, this systematic review showed that B-lines and IVC are dynamic variables that change over time and treatment. A single ultrasound examination can influence prognostic outcomes in patients with dyspnea, but it remains uncertain if several ultrasound examinations guided by a treatment protocol have the same effectiveness. The nature of the variables could hypothetically be used by the physician to plan patient care, predict clinical deterioration, and adjust treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge Anne Faber Hansen, research librarian, MSci, PhD, from the University Library of Southern Denmark, who contributed to designing the search strategy. We thank Claire Gudex, MD, PhD, from the University of Southern Denmark, for editing the manuscript.
Author contributions
MDA has conceived the study and received inputs and feedback from ATL, NJ, PHG, and CBL. MDA developed the search strategy in cooperation with AFH. MDA and NJ screened the included studies and independently assessed the quality and extracted the data. MDA drafted the manuscript. All co-authors read and approved the final manuscript.
Funding
The study is supported by the Department of Emergency Medicine at Slagelse Hospital and grants from the Naestved, Slagelse, and Ringsted Hospitals’ Research Fund (Grant Number 111.2219) and by a 1-year scholarship from the University of Southern Denmark. The funders have no role in the study’s design, in the collection, analysis, or interpretation of data, in the writing of manuscripts, or in decisions to publish results.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Code availability
Coding is available upon request.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mockel M, Searle J, Muller R, Slagman A, Storchmann H, Oestereich P, et al. Chief complaints in medical emergencies: do they relate to underlying disease and outcome? The Charité Emergency Medicine Study (CHARITEM) Eur J Emerg Med. 2013;20:103–108. doi: 10.1097/MEJ.0b013e328351e609. [DOI] [PubMed] [Google Scholar]
- 2.Kelly AM, Keijzers G, Klim S, Graham CA, Craig S, Kuan WS, et al. An observational study of dyspnea in emergency departments: the Asia, Australia, and New Zealand Dyspnea in Emergency Departments Study (AANZDEM) Acad Emerg Med. 2017;24:328–336. doi: 10.1111/acem.13118. [DOI] [PubMed] [Google Scholar]
- 3.Lindskou TA, Pilgaard L, Søvsø MB, Kløjgård TA, Larsen TM, Jensen FB, et al. Symptom, diagnosis and mortality among respiratory emergency medical service patients. PLoS One. 2019 doi: 10.1371/journal.pone.0213145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ray P, Birolleau S, Lefort Y, Becquemin M-H, Beigelman C, Isnard R, et al. Acute respiratory failure in the elderly: etiology, emergency diagnosis and prognosis. Crit Care. 2006;10:R82. doi: 10.1186/cc4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayen A, Herigstad M, Pattinson KTS. Understanding dyspnea as a complex individual experience. Maturitas. 2013;76:45–50. doi: 10.1016/j.maturitas.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Oxberry SG, Bland JM, Clark AL, Cleland JGF, Johnson MJ. Minimally clinically important difference in chronic breathlessness: Every little helps. Am Heart J. 2012;164:229–235. doi: 10.1016/j.ahj.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Vicent L, Nuñez Olarte JM, Puente-Maestu L, Oliva A, López JC, Postigo A, et al. Degree of dyspnoea at admission and discharge in patients with heart failure and respiratory diseases. BMC Palliat Care. 2017 doi: 10.1186/s12904-017-0208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly N, Esteve R, Papadimos TJ, Sharpe RP, Keeney SA, DeQuevedo R, et al. Clinician-performed ultrasound in hemodynamic and cardiac assessment: a synopsis of current indications and limitations. Eur J Trauma Emerg Surg. 2015;41:469–480. doi: 10.1007/s00068-014-0492-6. [DOI] [PubMed] [Google Scholar]
- 9.Gaskamp M, Blubaugh M, McCarthy LH, Scheid DC. Can bedside ultrasound inferior vena cava measurements accurately diagnose congestive heart failure in the emergency department? A Clin-IQ. J Patient Centered Res Rev. 2016;3:230–234. doi: 10.17294/2330-0698.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38:577–591. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- 11.Asahi T, Nakata M, Higa N, Manita M, Tabata K, Shimabukuro M. Respiratory collapse of the inferior vena cava reflects volume shift and subsequent fluid refill in acute heart failure syndrome. Circ J. 2016;80:1171–1177. doi: 10.1253/circj.CJ-15-1374. [DOI] [PubMed] [Google Scholar]
- 12.Yavasi O, Unluer EE, Kayayurt K, Ekinci S, Saglam C, Surum N, et al. Monitoring the response to treatment of acute heart failure patients by ultrasonographic inferior vena cava collapsibility index. Am J Emerg Med. 2014;32:403–407. doi: 10.1016/j.ajem.2013.12.046. [DOI] [PubMed] [Google Scholar]
- 13.Strnad M, Prosen G, Borovnik LV. Bedside lung ultrasound for monitoring the effectiveness of prehospital treatment with continuous positive airway pressure in acute decompensated heart failure. Eur J Emerg Med. 2016;23:50–55. doi: 10.1097/MEJ.0000000000000205. [DOI] [PubMed] [Google Scholar]
- 14.Via G, Storti E, Gulati G, Neri L, Mojoli F, Braschi A. Lung ultrasound in the ICU: from diagnostic instrument to respiratory monitoring tool. Minerva Anestesiol. 2012;78:1282–1296. [PubMed] [Google Scholar]
- 15.Spevack R, Al Shukairi M, Jayaraman D, Dankoff J, Rudski L, Lipes J. Serial lung and IVC ultrasound in the assessment of congestive heart failure. Crit Ultrasound J. 2017;9:1–7. doi: 10.1186/s13089-017-0062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tufanaru C, Munn Z, Aromataris E et al (2020) Chapter 3: systematic reviews of effectiveness. In: Aromataris E, Munn Z (eds) JBI manual for evidence synthesis. JBI. https://synthesismanual.jbi.global
- 17.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arvig MD, Laursen CB, Jacobsen N, Gæde PH, Lassen AT. Effectiveness of serial focused ultrasound of the lungs and inferior vena cava for monitoring patients with acute dyspnea: a systematic review protocol. JBI Database Syst Rev Implement Rep. 2019;17:2317. doi: 10.11124/JBISRIR-D-19-00027. [DOI] [PubMed] [Google Scholar]
- 20.Via G, Hussain A, Wells M, Reardon R, ElBarbary M, Noble VE, et al. International evidence-based recommendations for focused cardiac ultrasound. J Am Soc Echocardiogr. 2014;27(683):e1–683.e33. doi: 10.1016/j.echo.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Schünemann H, Brożek J, Guyatt G et al (eds) (2013) GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group. http://guidelinedevelopment.org/handbook
- 22.Chuen M, Lip GYH, MacFadyen RJ. Performing repeated noninvasive bedside measures of volume response to intravenous furosemide in acute pulmonary edema: a feasibility assessment. Cardiovasc Ther. 2009;27:89–95. doi: 10.1111/j.1755-5922.2009.00080.x. [DOI] [PubMed] [Google Scholar]
- 23.Cubo-Romano P, Torres-Macho J, Soni NJ, Reyes LF, Rodríguez-Almodóvar A, Fernández-Alonso JM, et al. Admission inferior vena cava measurements are associated with mortality after hospitalization for acute decompensated heart failure: IVC and Mortality in ADHF. J Hosp Med. 2016;11:778–784. doi: 10.1002/jhm.2620. [DOI] [PubMed] [Google Scholar]
- 24.Fawzi S, Rafla SM, El Atroush H, Farouk K, Wilson C. Clinical significance of inferior vena cava index in monitoring patients in acute exacerbation of chronic heart failure. Eur Heart J Cardiovasc Imaging. 2012 doi: 10.1093/ehjci/jes264. [DOI] [Google Scholar]
- 25.Goonewardena SN, Gemignani A, Ronan A, Vasaiwala S, Blair J, Brennan JM, et al. Comparison of hand-carried ultrasound assessment of the inferior vena cava and N-terminal pro-brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure. JACC Cardiovasc Imaging. 2008;1:595–601. doi: 10.1016/j.jcmg.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Guiotto G, Masarone M, Paladino F, Ruggiero E, Scott S, Verde S, et al. Inferior vena cava collapsibility to guide fluid removal in slow continuous ultrafiltration: a pilot study. Intensive Care Med. 2010;36:692–696. doi: 10.1007/s00134-009-1745-4. [DOI] [PubMed] [Google Scholar]
- 27.Patnaik S, Davila C, Lu M, Alhamshari Y, Banerji S, Pressman G. Correlates of serial ultrasound guided volume assessments in patients with acute decompensated heart failure using the Vscan system. J Card Fail. 2016;22:S56. doi: 10.1016/j.cardfail.2016.06.169. [DOI] [Google Scholar]
- 28.Tchernodrinski S, Lucas BP, Athavale A, Candotti C, Margeta B, Katz A, et al. Inferior vena cava diameter change after intravenous furosemide in patients diagnosed with acute decompensated heart failure. J Clin Ultrasound. 2014;43:187–193. doi: 10.1002/jcu.22173. [DOI] [PubMed] [Google Scholar]
- 29.Cortellaro F, Ceriani E, Spinelli M, Campanella C, Bossi I, Coen D, et al. Lung ultrasound for monitoring cardiogenic pulmonary edema. Intern Emerg Med. 2017;12:1011–1017. doi: 10.1007/s11739-016-1510-y. [DOI] [PubMed] [Google Scholar]
- 30.Daskalov I, Petrova V. Reliability of B lines in follow up of patients with acute decompensated left side heart failure. Eur Heart J Acute Cardiovasc Care. 2016;5:40. doi: 10.1177/2048872616663431. [DOI] [Google Scholar]
- 31.Facchini C, Malfatto G, Giglio A, Facchini M, Parati G, Branzi G. Lung ultrasound and transthoracic impedance for noninvasive evaluation of pulmonary congestion in heart failure. J Cardiovasc Med. 2016;17:510–517. doi: 10.2459/JCM.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 32.Gargani L, Pang PS, Frassi F, Miglioranza MH, Dini FL, Landi P, et al. Persistent pulmonary congestion before discharge predicts rehospitalization in heart failure: a lung ultrasound study. Cardiovasc Ultrasound. 2015 doi: 10.1186/s12947-015-0033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martindale JL, Secko M, Kilpatrick JF, deSouza IS, Paladino L, Aherne A, et al. Serial sonographic assessment of pulmonary edema in patients with hypertensive acute heart failure. J Ultrasound Med. 2018;37:337–345. doi: 10.1002/jum.14336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palazzuoli A, Ruocco G, Beltrami M, Nuti R, Cleland JG. Combined use of lung ultrasound, B-type natriuretic peptide, and echocardiography for outcome prediction in patients with acute HFrEF and HFpEF. Clin Res Cardiol. 2018;107:586–596. doi: 10.1007/s00392-018-1221-7. [DOI] [PubMed] [Google Scholar]
- 35.Platz E, Campbell RT, Claggett B, Lewis EF, Groarke JD, Docherty KF, et al. Lung ultrasound in acute heart failure: prevalence of pulmonary congestion and short- and long-term outcomes. JACC Heart Fail. 2019;7:849–858. doi: 10.1016/j.jchf.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vitturi N, Soattin M, Allemand E, Simoni F, Realdi G. Thoracic ultrasonography: a new method for the work-up of patients with dyspnea. J Ultrasound. 2011;14:147–151. doi: 10.1016/j.jus.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volpicelli G, Caramello V, Cardinale L, Mussa A, Bar F, Frascisco MF. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am J Emerg Med. 2008;26:585–591. doi: 10.1016/j.ajem.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 38.Öhman J, Harjola V-P, Karjalainen P, Lassus J. Assessment of early treatment response by rapid cardiothoracic ultrasound in acute heart failure: cardiac filling pressures, pulmonary congestion and mortality. Eur Heart J Acute Cardiovasc Care. 2018;7:311–320. doi: 10.1177/2048872617708974. [DOI] [PubMed] [Google Scholar]
- 39.Öhman J, Harjola VP, Karjalainen P, Lassus J. Focused echocardiography and lung ultrasound protocol for guiding treatment in acute heart failure. Esc Heart Fail. 2018;5:120–128. doi: 10.1002/ehf2.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mozzini C, Di Dio PM, Pesce G, Garbin U, Fratta Pasini AM, Ticinesi A, et al. Lung ultrasound in internal medicine efficiently drives the management of patients with heart failure and speeds up the discharge time. Intern Emerg Med. 2018;13:27–33. doi: 10.1007/s11739-017-1738-1. [DOI] [PubMed] [Google Scholar]
- 41.De Vecchis R, Ariano C, Fusco A, Ciccarelli A, Cioppa C, Giasi A, et al. Ultrasound evaluation of the inferior vena cava collapsibility index in congestive heart failure patients treated with intravenous diuretics: new insights about its relationship with renal function: an observational study. Anatol J Cardiol. 2012;12:391–400. doi: 10.5152/akd.2012.121. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Z, Xu X, Ye S, Xu L. Ultrasonographic measurement of the respiratory variation in the inferior vena cava diameter is predictive of fluid responsiveness in critically ill patients: systematic review and meta-analysis. Ultrasound Med Biol. 2014;40:845–853. doi: 10.1016/j.ultrasmedbio.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 43.Long E, Oakley E, Duke T, Babl F. Does respiratory variation in inferior vena cava diameter predict fluid responsiveness: a systematic review and meta-analysis. Shock. 2017;47:550–559. doi: 10.1097/SHK.0000000000000801. [DOI] [PubMed] [Google Scholar]
- 44.Darwish OS, Mahayni A, Kataria S, Zuniga E, Zhang L, Amin A. Diagnosis of acute heart failure using inferior vena cava ultrasound: systematic review and meta-analysis. J Ultrasound Med. 2020;39:1367–1378. doi: 10.1002/jum.15231. [DOI] [PubMed] [Google Scholar]
- 45.Platz E, Merz AA, Jhund PS, Vazir A, Campbell R, McMurray JJ. Dynamic changes and prognostic value of pulmonary congestion by lung ultrasound in acute and chronic heart failure: a systematic review. Eur J Heart Fail. 2017;19:1154–1163. doi: 10.1002/ejhf.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miger KC, Fabricius-Bjerre A, Maschmann CP, Wamberg J, Winkler Wille MM, Abild-Nielsen AG, et al. Clinical applicability of lung ultrasound methods in the emergency department to detect pulmonary congestion on computed tomography. Ultraschall Med Eur J Ultrasound. 2019 doi: 10.1055/a-1021-1470. [DOI] [PubMed] [Google Scholar]
- 47.Laursen CB, Sloth E, Lassen AT, Christensen RD, Lambrechtsen J, Madsen PH, et al. Point-of-care ultrasonography in patients admitted with respiratory symptoms: a single-blind, randomised controlled trial. Lancet Respir Med. 2014;2:638–646. doi: 10.1016/S2213-2600(14)70135-3. [DOI] [PubMed] [Google Scholar]
- 48.Arvig MD, Lassen AT, Gæde PH, Laursen CB. Monitoring patients with acute dyspnoea with a serial focused ultrasound of the heart and the lungs (MODUS): a protocol for a multicentre, randomised, open-label, pragmatic and controlled trial. BMJ Open. 2020;10:e034373. doi: 10.1136/bmjopen-2019-034373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frederiksen CA, Juhl-Olsen P, Andersen NH, Sloth E. Assessment of cardiac pathology by point-of-care ultrasonography performed by a novice examiner is comparable to the gold standard. Scand J Trauma Resusc Emerg Med. 2013;21:87. doi: 10.1186/1757-7241-21-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swamy V, Brainin P, Biering-Sørensen T, Platz E. Ability of non-physicians to perform and interpret lung ultrasound: a systematic review. Eur J Cardiovasc Nurs. 2019;18:474–483. doi: 10.1177/1474515119845972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeng X, Zhang Y, Kwong JSW, Zhang C, Li S, Sun F, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8:2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Coding is available upon request.