Abstract
Background
As radiology volume from premature patients increases, previously undescribed imaging findings may be identified, posing diagnostic dilemma to the pediatric radiologist.
Objective
The primary goal of our study is to characterize the previously undescribed imaging finding of subependymal echogenicity at the floor of the frontal horns, which we postulate represents normal variant embryologic remnant residual germinal matrix. Furthermore, we hope to equip the pediatric radiologist with diagnostic criteria to distinguish this normal variant from pathology.
Materials and methods
Retrospective review of neonates at our institution over a 10 year period was performed to identify extremely premature infants who received head ultrasounds during their hospital stay. Clinical data from EPIC was collected on these patients in addition to retrospective review of their head ultrasound images.
Results
Literature review of neuroembryology and observed involution of the frontal horn subependymal echogenicity on sequential imaging inform our hypothesis that this imaging finding represents normal variant residual germinal matrix. Two-thirds of the 210 included extremely premature infants demonstrated this finding, which was frequently misinterpreted as grade 1 germinal matrix, intra-choroidal or intra-ventricular hemorrhage. Residual matrix was concomitantly present with additional pathology in 29.4% of the patients.
Conclusion
Previously undescribed subependymal echogenicity at the floor of the frontal horns is favored to represent normal variant embryologic remnant residual germinal matrix. Since this finding may be misinterpreted as germinal matrix, intra-choroidal or intra-ventricular hemorrhage, it is essential for the interpreting radiologist to be aware of this normal variant and not confuse it for pathology.
Keywords: Germinal matrix hemorrhage, Extreme prematurity, Extremely premature infants, Head ultrasound, Intraventricular hemorrhage, Normal variant neuroanatomy
Introduction
Significant medical and technological advances over the last few decades in the realm of pediatric critical care have enabled modern medicine to facilitate extremely premature infants (defined as gestational age under 30 weeks) to survive and thrive to maturity and beyond. Prior to pediatric critical care advances involving CPAP and administration of exogenous surfactant, majority of extremely premature infants didn’t survive the immediate post-partum period [1]. However, in the 1990s with medical and technological advances allowing survival of extremely premature infants, radiologic studies involving this patient population increased, and over the course of the last few years has become mainstay of pediatric radiology volume.
During the precarious period of extreme prematurity immediately after birth, these patients are at significant risk for high morbidity neurologic, cardiopulmonary and gastrointestinal conditions such as germinal matrix hemorrhages, surfactant deficiency and necrotizing enterocolitis [1]. As a result, these patients routinely receive sequential imaging involving head ultrasounds, babygrams, chest and/or abdominal radiographs amongst other imaging studies. Pediatric radiology literature on the imaging findings specific to extremely premature infants is sparse. Given the relatively recent influx of studies from this patient population, normal variant radiologic anatomy in these patients is not well characterized. As a result, normal variant anatomy within this patient population bears the risk of being confused for pathology with significant consequences for the patients, their caregivers/families as well the healthcare system.
Extremely premature infants are at significantly increased risk for neurological complications including germinal matrix hemorrhage, intraventricular hemorrhage, parenchymal hemorrhage, periventricular leukomalacia and hydrocephalus amongst other entities [2]. Head ultrasound is the first line radiographic examination for screening extremely premature patients for neurologic complications and for following extremely premature infants with neurologic complications that have a sonographic correlate. Normal variant neuroanatomy including embryologic remnant neuroanatomy visualized on head ultrasounds of extremely premature infants that is not visualized on head ultrasounds of premature infants (defined as gestational age between 30 and 38 weeks) and term infants (defined as gestational age between 38 and 41 weeks) can be a source of confusion for the pediatric radiologist. Due to the lack of characterization of normal variant neuroanatomy in extreme premature infants, likely benign imaging findings on head ultrasounds may lead to vague reporting and/or be confused for pathology, leading to escalation in the level of intensive care for these patients. Most importantly, diagnostic uncertainty or diagnostic misinterpretation of this nature can cause unnecessary anxiety for the patients’ parents and families regarding the wellbeing of their child in an already stressful situation.
In this study, we review an imaging finding frequently seen on head ultrasounds of extremely premature infants at our institution that can be a source of confusion for the pediatric radiologist. Head ultrasounds performed for extremely premature infants at our institution have frequently demonstrated linear echogenicity at the base of the frontal horns of the lateral ventricles anterior to the caudothalamic groove. This finding has not previously been described and/or characterized in the pediatric radiology literature. Based on review of head ultrasound examinations from several years at our institution, we hypothesize this finding to represent benign normal variant anatomy residual germinal matrix in extremely premature infants that involuted on subsequent sequential imaging examinations. In this study, we will review developmental neuroembryology that informs our hypothesis regarding this finding representing normal variant residual germinal matrix. Additionally, we will demonstrate the spectrum of radiographic appearance of this finding on head ultrasounds as well as illustrate cases where this finding presented a diagnostic dilemma for the pediatric radiologist.
Materials and methods
After obtaining IRB approval, a retrospective cohort study design was performed involving chart reviews from Logician/EPIC, Radiology Information System (RIS) and Picture Archiving and Communication System (PACS). An exhaustive keyword search of the Boston Medical Center EPIC database was performed to identify medical records of extremely premature infants (defined as gestational age under or equal to 30 weeks) who underwent a head ultrasound or brain MRI between 1/1/2007 and 4/8/2018. Every identified patient received a study identification number. An encrypted, password protected mastercode file linking each study subject identification number to the patient’s HIPPA identifiers such as institution specific medical record number was created and stored securely on an institutional computer in the Department of Radiology. A separate encrypted, password protected Excel spreadsheet containing de-identified study identification number for study subjects as well as IRB approved clinical information obtained from their electronic medical chart (as summarized in Table 1) was stored on HIPPA compliant institutional computer in the Department of Radiology.
Table 1.
IRB-approved clinical data collected from electronic medical records through EPIC database search
| Gestational age |
| Birth Weight |
| Gender |
| Ethnicity/race |
| APGAR at birth |
| APGAR at 5 min |
| Multiple births |
|
Medical chart diagnosis of co-morbid conditions (based on ICD-9 and ICD-10 codes) Neurologic abnormality Cardiac abnormality Pulmonary abnormality Gastrointestinal abnormality |
Retrospective RIS review of these identified patients’ radiology reports was performed. Patients who were radiographically diagnosed with grade IV germinal matrix hemorrhage, intra-parenchymal hemorrhage, cerebellar hemorrhage and/or other etiologies such as hypoxic ischemic encephalopathy were excluded. The remainder of the patients with head ultrasounds demonstrating normal examination without evidence of germinal matrix hemorrhage, grade I germinal matrix hemorrhage, grade II germinal matrix hemorrhage, grade III germinal matrix hemorrhage, intraventricular hemorrhage and/or choroidal hemorrhage were included in the study. These inclusion and exclusion criteria are summarized in Table 2.
Table 2.
Inclusion and exclusion criteria for subject selection
| Inclusion criteria | Exclusion criteria |
|---|---|
| Gestational age equal to or under 30 weeks | Grade IV germinal matrix hemorrhage |
| Grade I, II or III germinal matrix hemorrhage | Intraparenchymal hemorrhage |
| Intraventricular hemorrhage or hematoma | Cerebellar hemorrhage |
| Choroidal hemorrhage | Miscellaneous: Hypoxic ischemic encephalopathy, corpus callosum agenesis |
Prior to data collection from included patient subset, two independent teams of investigators (SI; ICA and WC) achieved group consensus on how to identify and characterize the imaging finding of interest during retrospective PACS review, the categories into which imaging findings will be classified based on the degree of concordance and/or discordance between prospectively described radiographic findings and retrospectively identified imaging findings (summarized in Table 3), the protocol for which imaging studies within the patients’ radiology jacket will be retrospectively reviewed based on the above mentioned categorization, and the protocol for determining the size of the finding of interest in millimeters (Fig. 1).
Table 3.
Categorization of examinations based on concordance and/or discordance between the original diagnostic interpretation at the time of the examination and retrospective imaging review during this study
| Category | Prospective imaging interpretation at time of examination | Retrospective imaging interpretation during current study | Protocol for evaluating studies within the radiology jacket |
|---|---|---|---|
| 1 | Normal head US or additional pathology such as grade I germinal matrix hemorrhage | Finding of interest is not present | N/A |
| 2a | Normal head US | Finding of interest is present | N/A |
| 3 | Finding of interest reported as residual germinal matrix | Finding of interest is present | N/A |
| 4a | Grade 1, II or III germinal matrix hemorrhage; intraventricular hemorrhage or hematoma | Finding of interest misinterpreted as pathology | All available radiology studies were carefully reviewed |
| 4b | Grade 1, II or III germinal matrix hemorrhage; intraventricular hemorrhage or hematoma, choroidal hemorrhage or choroidal cysts | Finding of interest is present with additional pathology | All available radiology studies were carefully reviewed |
Fig. 1.
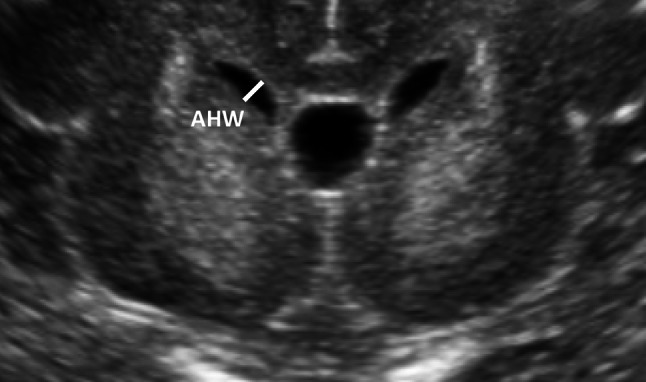
Thickness of the finding of interest (in millimeters) measured in the plane of the anterior horn width (AHW)
Next, the two independent teams of investigators each reviewed half of the subjects included in the study using the pre-established consensus protocol. This phase of the study included PACS based retrospective imaging review of head ultrasounds, retrospective review of any available brain MRI or head CT to determine the existence of any MRI or CT correlate to head ultrasound findings, data on the type of probe (curved probe versus linear probe), data on the type of images (still images versus ciné clips) that demonstrated the finding of interest, as well as the thickness of the finding of interest per the pre-established consensus protocol. Collected data was stored in an encrypted, password protected Excel spreadsheet on HIPPA compliant institutional computer within the department of Radiology.
Official protocol for head ultrasounds at our institution (Boston Medical Center) is summarized in Table 4. Phillips curved array transducer C 8–5 MHz and linear array transducer 12–5 MHz were utilized to obtain images. Prior to 2013, the official protocol included only still images using the curved and linear transducers. During 2013, our institutional protocol for head ultrasounds evolved to include curved probe and linear probe ciné clips. Microsoft Excel was utilized for statistical analysis of demographic data.
Table 4.
Institutional protocol for head ultrasounds
| C 8–5 transducer array (Curved probe) | L 12–5 transducer array (Linear probe) |
|---|---|
| Still coronal images | Still coronal images |
| Still sagittal images | Still sagittal images |
| Coronal ciné clips (after 2013) | Coronal ciné clips (after 2013) |
| Right sagittal ciné clips (after 2013) | Right sagittal ciné clips (after 2013) |
| Left sagittal ciné clips (after 2013) | Left sagittal ciné clips (after 2013) |
| Posterior fossa transmastoid still images |
Scientific literature search was performed and textbooks on embryology as well as fetal anatomy were reviewed to identify possible pathological correlate to the sonographically visualized finding of interest in the frontal horns, with pertinent findings summarized below in the results section.
Results
Summarized are the neuroanatomy and embryology results from our literature search and literature review that informs our hypothesis that the finding of interest represents residual germinal matrix in extremely premature infants. The germinal matrix is the inner most cortical layer during neurodevelopment that houses the neuronal and glial precursor cells [3]. It is T1 hyperintense and T2 hypointense on fetal neuroimaging, similar to the outermost cortical plate. The neuronal precursor cells located within the germinal matrix undergo cortical migration towards their final destination during neurodevelopment in utero, starting at 7 weeks of gestation and culminating around 24 weeks of gestation [4]. As cortex formation progresses during neurodevelopment, the inner most germinal matrix gets increasingly separated and splayed from the outermost cortical plate. Once cortical migration is completed, sulcation of the cortex begins, starting with the sylvian fissure at 18 weeks of gestation and terminating around 34 weeks of gestation.
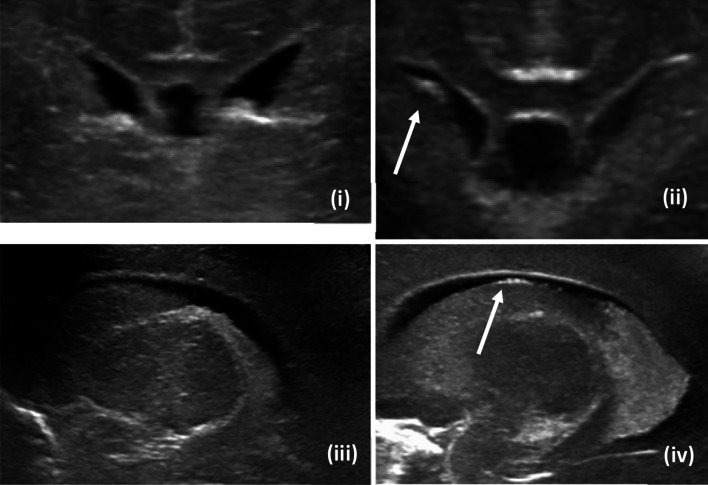
The germinal matrix, given its functionality, is a highly metabolically active as well as highly vascular layer of neuroblasts (Fig. 2). As cortical migration progresses, the germinal matrix gradually regresses during development except for specific regions including the subependymal ventrolateral wall of the lateral ventricles at 24 weeks of gestation, subependymal roof of the temporal and occipital horns as well as the caudothalamic groove until 33 weeks of gestation. Germinal matrix involutes by term [4]. Given that the finding of interest is visualized as linear and/or mildly lobulated echogenicity in the ventrolateral region of the frontal horns, based on the location, we believe this finding to represent residual germinal matrix. Furthermore, we have observed involution of this finding in certain patients on sequential ultrasound examinations (Fig. 3), which further supports our hypothesis that this finding of interest represents regressing germinal matrix in an extremely premature infant. As a result, based on the expected course of neurodevelopment, we postulate the subependymal echogenicity we were visualizing in the frontal horns to represent residual germinal matrix in an extremely premature infant whose brain has not yet completed its normal course of neurodevelopment by birth. From here on, in the remainder of the paper, we will refer to the finding of interest as residual germinal matrix (Figs. 2, 10).
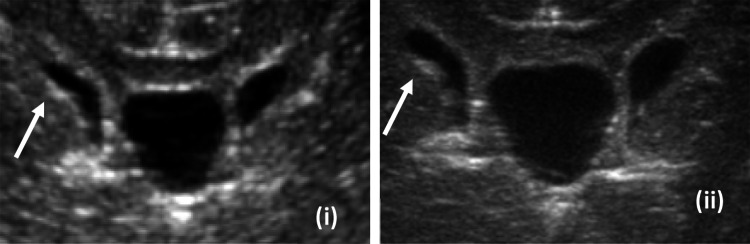
Fig. 2.
Multi plane imaging of residual germinal matrix (i—coronal image of patient without residual matrix, ii—coronal image of patient with residual matrix, iii—sagittal image of patient without residual matrix, iv—sagittal image of patient with residual matrix)
Fig. 3.
Sequential head ultrasound images demonstrating sequential involution of the frontal horn subependymal echogenicity believed to represent regressing residual germinal matrix (i—flagrant bilateral residual germinal matrix on day of life 5, ii—interval decrease in size of bilateral residual germinal matrix on day of life 8, iii—further interval decrease in size of bilateral residual germinal matrix on day of life 12, iv—complete resolution of residual germinal matrix on day of life 29)
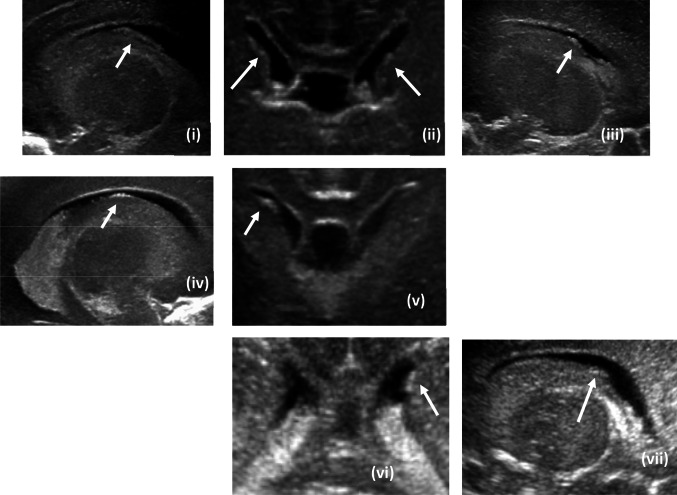
Fig. 10.
Representative head ultrasound images from category 2 (i—sagittal image of right sided residual germinal matrix, ii—coronal image of bilateral residual germinal matrix, iii—sagittal image of left sided residual germinal matrix, iv—sagittal image of the right sided residual germinal matrix in panel v, v—coronal image of right sided residual matrix only, vi—coronal image of left sided residual matrix only, vii—sagittal image of left sided residual matrix visualized in image panel vi)
210 extremely premature infants meeting the inclusion and exclusion criteria based on Table 3 above were identified. Each included patient was assigned a unique subject identification number for the purposes of this study. The determination of misinterpretation of the finding of interest as pathology (category 4a) and/or the determination of the presence of the finding of interest on ultrasounds reported as normal studies (category 3) was made through review of all available serial studies in the case of former (category 4a) and through comprehensive review of the single ultrasound examination under review in the case of latter (category 3) respectively. Primary imaging findings utilized to make this determination included contiguity of the finding of interest with the caudothalamic groove (favoring grade I hemorrhage) and characteristics of evolution of the findings on serial ultrasounds. Specifically, note was made of whether the finding of interest on serial ultrasounds demonstrated egress without changes in echogenicity (favoring normal variant residual matrix) versus involution with changes to echogenicity and homogeneity of echotexture (favoring grade I hemorrhage).
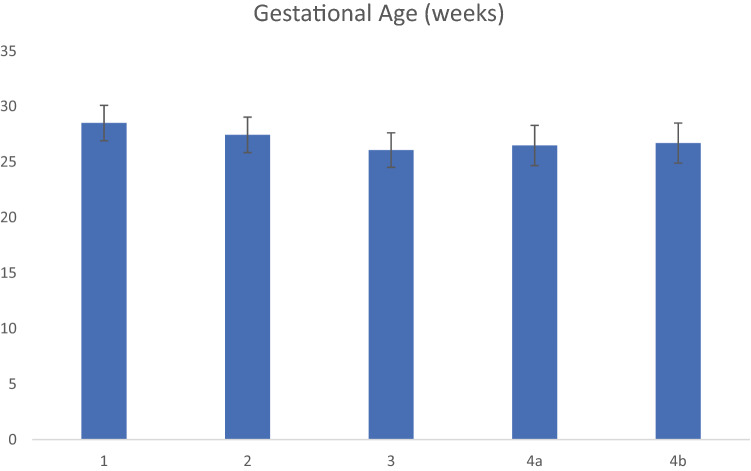
After retrospective PACS imaging review, study subjects’ imaging findings were categorized into several groups (Table 5) per consensus criteria as described in Table 3. 66.2% of the patients in the study cohort (139 out of 210 patients) demonstrated residual germinal matrix while 33.8% of the patients in the study cohort (71 out of 210 patients) did not demonstrate the finding of interest. The presence of residual germinal matrix lead to diagnostic uncertainty and dilemma in 28.8% of the patients with the finding (40 out of 139 patients). Demographics of the patients included in this study are presented by category including gender in Fig. 4, gestational age (in weeks) in Fig. 5 and birth weight (in grams) in Fig. 6. Average APGAR scores of included patient cohort at 1 min and 5 min are presented in Fig. 7. The demographic analysis of gestational age, birth weight and APGAR scores did not demonstrate any statistically significant differences between the various categories.
Table 5.
Categorization of study subjects’ imaging findings based on concordance and/or discordance between prospective and retrospective imaging review
| Category | Number of patients |
|---|---|
| 1 | 70 |
| 2 | 42 |
| 3 | 15 |
| 4a | 39 |
| 4b | 42 |
Fig. 4.
Gender of included patients per category
Fig. 5.
Gestational age (in weeks) of included patients per category
Fig. 6.
Birth weight (in grams) of included patients per category
Fig. 7.
APGAR scores at 1 min and 5 min per category
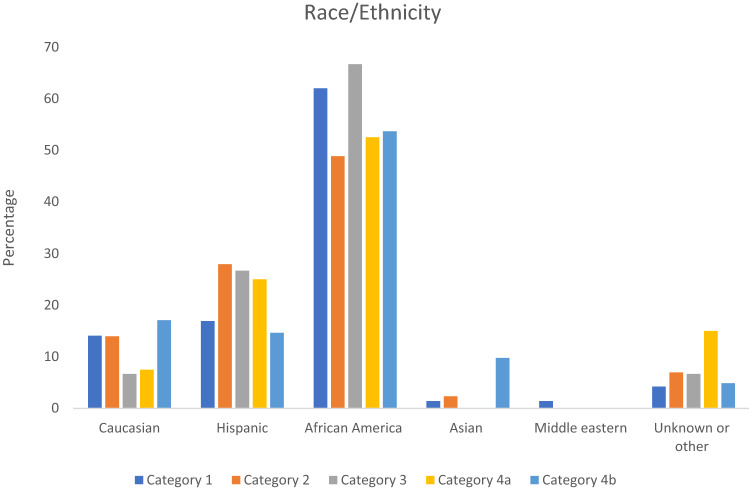
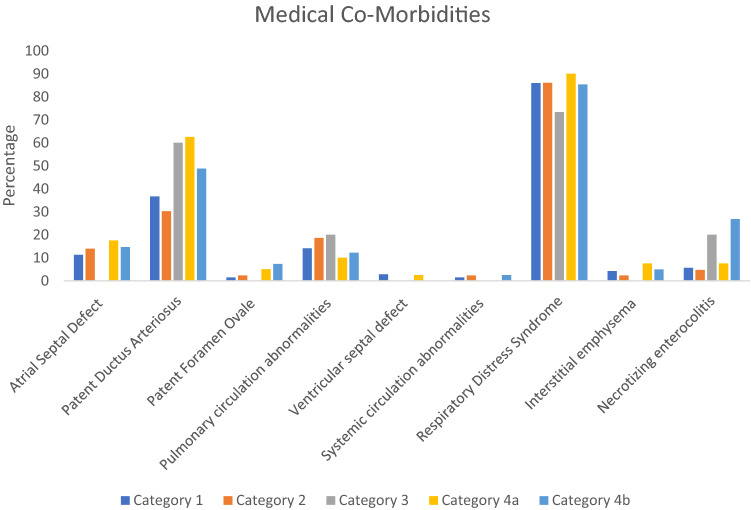
The race/ethnicity of the included patients is summarized in Fig. 8. African American patients were the largest group in all five categories, followed by Hispanic and Caucasian patients. Non-neurologic medical co-morbidities of the included patients are presented in Fig. 9. The predominant cardiac medical co-morbidity was patent ductus arteriosus, with atrial septal defect being the next most common cardiac co-morbidity. In Fig. 9, pulmonary circulation abnormalities included any abnormality involving the right sided circulation including congenital pulmonary artery stenosis, tricuspid atresia, congenital infundibular stenosis and pulmonary artery coarctation. Similarly, systemic circulation abnormalities in Fig. 9 included etiologies such as mitral stenosis or insufficiency and bicuspid aorta. Pulmonary circulation abnormalities were more common than systemic circulation abnormalities across all five categories in the included patient cohort. The predominant pulmonary co-morbidity was respiratory distress syndrome. Necrotizing enterocolitis was the only gastrointestinal co-morbidity in this patient cohort.
Fig. 8.
Race/ethnicity of included patients per category
Fig. 9.
Non-neurologic medical co-morbidities of included patients per category
Table 6 presents pathologies which residual germinal matrix hemorrhage was inaccurately misinterpreted to represent (category 4a), and their respective frequencies. Based on this data, residual germinal matrix was most frequently misinterpreted to represent grade I germinal matric hemorrhage in 34 out of the 40 patients, followed by intra-choroidal hemorrhage in 3 out of the 40 patients and intra-ventricular hemorrhage in 3 out of the 40 patients.
Table 6.
Misinterpretation of residual germinal matrix as various pathology, with associated frequencies
| Grade 1 germinal matrix hemorrhage | 34 |
| Grade 2 germinal matrix hemorrhage | 1 |
| Intraventricular hemorrhage | 1 |
| Intrachoroidal hemorrhage | 3 |
Residual germinal matrix was not mentioned in the formal radiology report in 30.9% (43 out of the 139 patients) who had normal head ultrasounds without any radiographic evidence of pathology (category 2). Residual germinal matrix was alluded to as a possible normal variant in 10.8% (15 out of the 139 patients) who had normal head ultrasounds without any radiographic evidence of pathology (category 3). Residual germinal matrix was concurrently present with additional pathology in 29.4% (41 out of 139) of the patients, summarized in Table 7. Grade 1 germinal matrix hemorrhage was again the most prevalent concurrent pathology in 28 out of the 41 patients, followed by choroid plexus cyst in 5 out of the 41 patients. 3 out of 41 patients presented with grade 4 germinal matrix hemorrhage and 2 out of the 41 patients presented with intra-ventricular hemorrhage (Figs. 10, 11).
Table 7.
Pathology concurrent with residual germinal matrix with frequency
| Grade 1 germinal matrix hemorrhage | 28 |
| Grade 2 germinal matrix hemorrhage | 1 |
| Grade 3 germinal matrix hemorrhage | 3 |
| Choroid plexus cyst | 5 |
| Caudothalamic groove cyst | 1 |
| Intra-ventricular hemorrhage | 2 |
| Intra-choroidal hemorrhage | 1 |
Fig. 11.
Representative head ultrasound images from category 4b with demonstrating bilateral residual germinal matrix with concurrent bilateral intra-choroidal hemorrhage (i—coronal image on DOL 1 demonstrating bilateral residual matrix, ii—coronal image on DOL 7 demonstrating internal ventriculomegaly, interval increase in size and lobulation of bilateral choroid plexus as well echogenicity at the caudothalamic groove representing with bilateral grade III germinal matrix hemorrhage, iii—subependymal residual germinal matrix at the floor of the right frontal horn without contiguity with caudothalamic groove on DOL 1, iv—globular echogenicity at the caudothalamic groove extending anteriorly to involve the previously seen residual matrix consistent with interval development of grade III germinal matrix hemorrhage on DOL 7, v—subependymal residual germinal matrix at the floor of the left frontal horn without continuity with caudothalamic groove on DOL 1, vi—interval development of ill-defined echogenicity at the caudothalamic groove extending anterior to the previously seen residual matrix, consistent with grade III germinal matrix hemorrhage on DOL 7. Please note that hemorrhage within the residual germinal matrix at the subependymal frontal horns in addition to germinal matrix at the caudothalamic groove is not excluded on DOL 7 in the above example. DOL day of life
Given the higher spatial resolution of the linear L12-5 transducer probe compared to the curved C8-5 transducer probe, residual germinal matrix smaller than 1 mm in maximal thickness when measured in the plane of the anterior horn width were significantly more conspicuous and at times only appreciated on linear probe relative to curved probe [5]. The curved probe reliably visualized and detected residual germinal matrix measuring greater than 1 mm in maximal thickness in the anterior horn width (Fig. 12).
Fig. 12.
Representative example demonstrating increased conspicuity of residual germinal matrix less than 1 mm in maximal thickness on linear probe transducer imaging (ii) relative to curved probe transducer imaging (i)
Discussion
The clinical significance of germinal matrix is secondary to its propensity to hemorrhage in premature and low birth weight infants, leading to long term morbidity and mortality depending on its severity including cerebral palsy and mental retardation [6, 7]. Several factors are believed to contribute to the propensity of germinal matrix to hemorrhage including fragile thin walled high-density capillary bed in this region with poor stromal support, physiologic fluctuations in cerebral blood flow and venous congestion within the thalamostriate veins in this region secondary to their sharp U-turn anatomy. Vast majority of the germinal matrix hemorrhages are venous in origin [8]. Germinal matrix hemorrhage (GMH) severity is evaluated based on its extent and includes grade I (limited to the caudothalamic groove), grade II (intraventricular extension without ventriculomegaly), grade III (intraventricular extension with ventriculomegaly) and grade IV (periventricular venous infarcts). It is essential for the pediatric radiologist to differentiate post-hemmorhagic ventriculomegaly that develops as a sequelae of grade II germinal matrix hemorrhage from hydrocephalus associated with grade III germinal matrix hemorrhage (Fig. 13). While ventriculomegaly is visualized in both pathologies, in cases with grade II germinal matrix hemorrhage, post-hemorrhagic hydrocephalus develops after the hemmorhagic insult. In contrast, ventriculomegaly in grade III germinal matrix hemorrhage is visualized concurrently at the same time as visualization of germinal matrix hemorrhage, with hemmorhagic products distending the ventricles. As expected, the morbidity and mortality burden is higher with higher grades of GMH (which are frequently associated with varying degrees of periventricular leukomalacia). Grade III and grade IV patients suffer from long term complications such as developmental delay, cerebral palsy and in some severe cases, death [9–12].
Fig. 13.
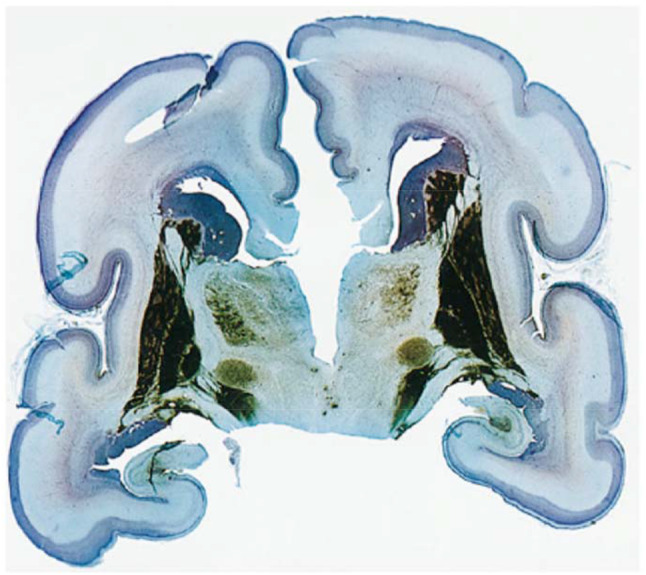
Pathology section from a 24-week old post-conception neonate (body weight 550 g, brain weight 76 g). The section was stained for endogenous alkaline phosphatase and counterstained with cresyl violet acetate and light green. Please note the prominent (blue) germinal matrix over the caudate nucleus (published with permission from AJNR)
Accurately identifying pathology as well as its severity is critical to the clinical function of a pediatric radiologist. To this end, it is extremely crucial for the pediatric radiologist to identify normal variant anatomy like residual germinal matrix as such and not confuse it for pathology, which may ultimately lead to unwarranted follow-up imaging studies and protracted higher level of care during admission. Additionally, while difficult to objectively quantify, the emotional toll of inaccurate diagnosis of “disease” for their newborn can be extremely distressing to the new parents.
While interpreting head ultrasound images from extremely premature patients, it is essential for the pediatric radiologist to recognize that microlobulated or linear echogenicity at the floor of the frontal horns of the lateral ventricles (often times projecting into the ventricles) may represent normal variant residual germinal matrix and not pathology such as intraventricular hematoma. In ambiguous cases, serial follow-up imaging with attention to development of ventriculomegaly may be used to distinguish between intraventricular hemorrhage (which would be expected to lead to communicating hydrocephalus) versus residual germinal matrix (which would not be associated with ventriculomegaly or development of cystic changes). While inaccurately misdiagnosing normal variant residual matrix as intraventricular hemorrhage is suboptimal, misclassifying true intraventricular hemorrhage as normal variant residual matrix that doesn’t need additional follow-up or higher level of patient care can be catastrophic. When in doubt, serial follow-up imaging should be advised to assess for differentiating interval evolutional changes.
Differentiating between intrachoroidal hemorrhage and residual germinal matrix may require carefully evaluating where the echogenic imaging finding is centered. If the imaging finding is centered in the subependymal region of the frontal horns, it is favored to represent residual germinal matrix. If the imaging finding is centered in the choroid plexus within the ventricles, it is favored to represent intrachoroidal hemorrhage. Linear probe transducer with increased spatial resolution as well as ciné clips (from curved and linear probe transducers) in multiple planes (coronal plane, sagittal plane) may prove to be extremely useful in resolving the epicenter of the imaging finding either within the choroid plexus or in the subependymal space.
Furthermore, it is essential for the pediatric radiologist to understand how to distinguish residual germinal matrix from grade 1 germinal matrix hemorrhage, which is the pathology it was most frequently confused with in our study. Grade 1 germinal matrix hemorrhage originates at the caudothalamic groove after which the blood products may migrate in several directions including anteriorly in the subependymal space towards the frontal horns. In contrast, residual germinal matrix originates anterior to the caudothalamic groove and is not located within the caudothalamic groove. Differentiating between these two entities is challenging as grade 1 germinal matrix hemorrhage can occur in the residual germinal matrix and was also coincidentally the most frequently observed concurrent pathology in our patient cohort. Sonographic morphology cannot be utilized to resolve between normal variant residual germinal matrix and grade I hemorrhage because both these entities may demonstrate linear and/or microlobulated morphology. As a rule of thumb, if a linear echogenic finding is seen in the subependymal frontal horns anterior to the caudothalamic groove without contiguity with or increased echogenicity at the caudothalamic groove, it should be favored to represent residual germinal matrix. Frontal horn subependymal echogenicity contiguous with the caudothalamic groove may represent hemorrhagic products of grade I hemorrhage originating from the caudothalamic groove residual matrix that have migrated anteriorly. Such anteriorly migrated hemorrhagic products may mask the presence of normal variant residual germinal matrix located anterior to the caudothalamic groove in a subset of the cases. Additionally, hemorrhage can occur within the normal variant germinal matrix itself. While it is important for the radiologist to be cognizant of all these permutations and combinations between location of residual germinal matrix and hemorrhage within it, in cases of grade I hemorrhage, the clinical significance of which residual germinal matrix it occurred in is likely minimal.
Both germinal matrix hemorrhagic products and residual germinal matrix are expected to egress with time. Some differentiating imaging features that may assist in distinguishing between these two entities include secondary imaging findings suggesting hemorrhagic involution such as the development of parenchymal cystic changes, evolution of echotexture from hyperechoic to isoechoic and eventually hypoechoic, as well as the development of echotexture heterogeneity during involution. When in doubt, serial follow-up imaging should be obtained to help the resolve this diagnostic dilemma. As seen in this study, it was possible to identify both germinal matrix hemorrhage and residual germinal matrix in the same study when these two findings demonstrated spatial separation on imaging.
Majority of the patients in each category, close to 50% or greater in each category (categories I through 4b) were African-American. This finding may in part be explained by epidemiologic studies demonstrating a higher rate of prematurity amongst African-Americans [13]. Regardless, since African-American patients were the majority of the patients in this study by far, the findings and results of this study should be applied to patients from other racial and ethnic backgrounds with caution. Another trend that emerged from the demographic data was the incidence of residual matrix amongst male versus female patients. Male patients were more likely than female patients to demonstrate residual matrix, as evidenced by the gender distribution data subdivided by categories (Fig. 4). This trend may in part be related to higher severity of prematurity amongst male patients when compared to female patients [14].
Based on all the data and evidence presented above, we have postulated that the subependymal echogenicity visualized at the frontal horns is residual germinal matrix that was in the process of evolving and involuting while the patient experienced premature birth. Data from our primary source research and literature search indicate that the pathologic correlate to the questioned radiographic finding is residual germinal matrix. Based on the embryologic development of the brain as well as the natural history of this finding demonstrating involution on serial ultrasound examinations, we postulate our finding to represent normal variant embryologic remnant residual germinal matrix. One of the limitations of our study is the lack of pathology to confirm our hypothesis. While MRI is not frequently performed for premature infants, the few MRI studies available for the patients in our study did not demonstrate a MRI correlate to the sonographic finding of residual matrix, a trend confirmed by other peer reviewed literature [15]. Another limitation of our study is the inherent inter-observer variability in characterizing mild to moderate head ultrasound findings, also reported previously in literature [16]. As a result, it is important to realize that the categorization of patients into categories 1 through 4b based on the degree of concordance or discordance between prospective and retrospective imaging interpretation may have a greater degree of subjectivity associated with it than is evident.
Conclusion
Due to major advances in pediatric critical care and neonatology, the morbidity and mortality burden of extremely premature patients has dramatically decreased over the last few decades and continues to decline. As a greater proportion of these patients present for imaging evaluation, astute observation will reveal radiologic features and imaging findings that have not been previously described in pediatric radiology literature, leading to diagnostic dilemma for the interpreting radiologist. In this study, we present one such finding not previously characterized visualized as echogenicity in the subependymal frontal horns on head ultrasound examinations, which we postulate represents involuting residual germinal matrix inadvertently caught on imaging during its embryologic evolution. We hope that the varied appearance and scope of this sonographic finding presented in this study will assist the interpreting radiologist in identifying this normal variant embryologic remnant when present and avoid confusing it with pathology, which could prove cumbersome at best and deleterious at worst to patient care. Furthermore, we provided practical pointers to assist the interpreting radiologist in differentiating normal variant residual matrix from pathologies it is more frequently confused with including grade 1 germinal matrix hemorrhage, intraventricular hemorrhage and intrachoroidal hemorrhage.
Acknowledgements
We would like to thank Linda Rosen, Clinical Data Warehouse Research Manager at Boston University Office of Human Research Affairs, for assisting in patient identification and data extraction from EPIC.
Author contributions
SI study design, data acquisition, data analysis, retrospective interpretation of imaging, manuscript drafting and preparation, manuscript editing and revision. WC data acquisition, retrospective interpretation of imaging, secondary manuscript editing and revision. ICA study concept and hypothesis, study design, data acquisition, retrospective interpretation of imaging, manuscript editing and revision.
Funding
None.
Availability of data and material
Patient data protected by HIPPAA. Restricted access to patient data for the purposes of research as covered by IRB approval.
Code availability
Not applicable.
Declarations
Conflict of interest
None.
Ethics approval
Institutional Review Board, Boston University Medical Campus and Boston Medical Center.
Consent to participate
Covered under approved IRB.
Consent for publication
Covered under approved IRB.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Glass HC, Costarino AT, Stayer SA, et al. Outcomes for extremely premature infants. Anesth Analg. 2015;120(6):1337–1351. doi: 10.1213/ANE.0000000000000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jarhour IT. Neurodeveopmental outcome after extreme prematurity: a review of literature. Pediatr Neurol. 2015;52920:143–152. doi: 10.1016/j.pediatrneurol.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 3.Guerrini R, Parrini E. Neuronal migration disorders. Neurobiol Dis. 2010;38(2):154–166. doi: 10.1016/j.nbd.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Coady AM (2015) Cranial abnormalities. In: Twinnings text of fetal abnormalities, 3rd edn. Churchill Livingstone Elsevier, China, pp 223–263
- 5.Griffiths DP, Janet M, Larroche J-C, Reeves M. Atlas of the fetal and postnatal brain MRI. 1. Philadelphia: Mosby Elsevier; 2010. Sectional anatomy of the fetal brain; pp. 35–151. [Google Scholar]
- 6.Brouwer AJ, Groenendaal F, Benders MJ, de Vries LS. Early and late complications of germinal matrix-intraventricular haemorrhage in the preterm infant: what is new? Neonatology. 2014;106:296–303. doi: 10.1159/000365127. [DOI] [PubMed] [Google Scholar]
- 7.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–534. doi: 10.1016/S0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 8.Ghazi-Birry HS, et al. Human germinal matrix: venous origin of hemorrhage and vascular characteristics. AJNR Am J Neuroradiol. 1997;18(2):219–229. [PMC free article] [PubMed] [Google Scholar]
- 9.Adams-Chapman I, Hansen NI, Stoll BJ, Higgins R. NICHD research network: neurodevelopmental outcome of extremely low birth weight infants with posthemorrhagic hydrocephalus requiring shunt insertion. Pediatrics. 2008;121:e1167–e1177. doi: 10.1542/peds.2007-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuban K, Sanocka U, Leviton A, Allred EN, Pagano M, Dammann O, Share J, Rosenfeld D, Abiri M, DiSalvo D, Doubilet P, Kairam R, Kazam E, Kirpekar M, Schonfeld S. White matter disorders of prematurity: association with intraventricular hemorrhage and ventriculomegaly. The Developmental Epidemiology Network. J Pediatr. 1999;134:539–546. doi: 10.1016/S0022-3476(99)70237-4. [DOI] [PubMed] [Google Scholar]
- 11.Beaino G, Khoshnood B, Kaminski M, Marret S, Pierrat V, Vieux R, Thiriez G, Matis J, Picaud JC, Rozé JC, Alberge C, Larroque B, Bréart G, Ancel PY. EPIPAGE Study Group: predictors of the risk of cognitive deficiency in very preterm infants: the EPIPAGE prospective cohort. Acta Paediatr. 2011;100:370–378. doi: 10.1111/j.1651-2227.2010.02064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Payne AH, Hintz SR, Hibbs AM, Walsh MC, Vohr BR, Bann CM, Wilson-Costello DE. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network: neurodevelopmental outcomes of extremely low-gestational-age neonates with low-grade periventricular-intraventricular hemorrhage. JAMA Pediatr. 2013;167:451–459. doi: 10.1001/jamapediatrics.2013.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohamed SA, Thota C, Browne PC, Diamond MP, Al-Hendy A. Why is preterm birth stubbornly higher in African-Americans? Obstet Gynecol Int J. 2014;1(3):00019. doi: 10.15406/ogij.2014.01.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shim SY, Cho SJ, Kong KA, Park EA. Gestational age-specific sex difference in mortality and morbidities of preterm infants: a nationwide study. Sci Rep. 2017;7(1):6161. doi: 10.1038/s41598-017-06490-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Intrapiromkul J, Northington F, Huisman TA, Izbudak I, Meoded A, Tekes A. Accuracy of head ultrasound for the detection of intracranial hemorrhage in preterm neonates: comparison with brain MRI and susceptibility-weighted imaging. J Neuroradiol. 2013;40:81–88. doi: 10.1016/j.neurad.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hintz SR, Slovis T, Bulas D, Van Meurs KP, Perritt R, Stevenson DK, Poole WK, Das A, Higgins RD. NICHD Neonatal Research Network: interobserver reliability and accuracy of cranial ultrasound scanning interpretation in premature infants. J Pediatr. 2007;150:592–596. doi: 10.1016/j.jpeds.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Patient data protected by HIPPAA. Restricted access to patient data for the purposes of research as covered by IRB approval.
Not applicable.