Abstract
Background
The aim of this study is to investigate the changes in median nerve and transverse carpal ligament (TCL)-formed carpal arch morphology as possible risk factors for median nerve entrapment in women with type 2 diabetes.
Methods
The distal carpal tunnel was imaged using ultrasound in 30 female subjects (15 with type 2 diabetes, 15 controls). The morphological parameters of the median nerve and carpal arch were derived from the ultrasound images. One-way analysis of variance (ANOVA) was used for statistical analysis.
Results
Diabetic women had an enlarged median nerve area (p < 0.05), salong with a maller carpal arch size, as indicated by a reduced palmar bowing index of the TCL (p < 0.05), and arch area (p < 0.05) than controls. The distance from the median nerve centroid to the volar boundary of the TCL was reduced in diabetic women (p < 0.05) compared to the controls.
Conclusions
Women with type 2 diabetes have reduced available space for the median nerve within the carpal arch due to the enlarged nerve and reduced arch size, making the median nerve more susceptible to entrapment within the tunnel. The current study shows that presence of diabetes increases the risk of median nerve entrapment in women and requires early detection of symptoms to avoid carpal tunnel syndrome.
Keywords: Carpal arch, Carpal tunnel syndrome, Median nerve, Ultrasonography, Type 2 diabetes
Background
The carpal tunnel is a fibro-osseous anatomical compartment formed by the transverse carpal ligament (TCL) and the carpal bones. The carpal tunnel has a passage for the median nerve that runs directly beneath the TCL. The TCL-formed carpal arch influences the space available for the median nerve, with any reduction in the area of the carpal arch making the nerve susceptible to compression. Prolonged median nerve compression may lead to carpal tunnel syndrome (CTS).
Patients with diabetes show higher prevalence of CTS than the general population [1–4]. Clinical and electrophysiological assessments have found that over one-third of patients with type 2 diabetes have CTS [5]. A possible reason for the development of CTS in the diabetic population is changes in the nerves’ morphometry, specifically enlargement of the nerves’ cross-sectional area (CSA) in presence of diabetes [6]. Although previous studies have largely examined diabetes-induced median nerve morphological changes [5, 6], limited investigation has been performed on the space available within the carpal tunnel for the median nerve. A focal examination of the changes in the TCL-formed carpal arch morphology could reveal more information about the higher incidence of nerve entrapment in the diabetic population.
The TCL-formed carpal arch is significantly smaller in women than in men, reducing the space available for the median nerve [7–10]. Furthermore, women with diabetes have been shown to have a significantly enlarged median nerve compared with healthy controls [11]. The reduced available space, in addition to the adverse median nerve morphology, could further increase the risk of median-nerve entrapment in women with diabetes. However, to date, information about how presence of diabetes affects the median nerve and carpal arch morphology in women is scarce.
Therefore, the purpose of the current study is to investigate the effect of type 2 diabetes on the morphology of the median nerve, the TCL-formed carpal arch, and the nerve–arch relationship in women. The morphology was examined using ultrasonography at the narrow distal carpal tunnel. It was hypothesised that women with type 2 diabetes would have (a) an increased median nerve cross-sectional area, (b) a smaller carpal arch area and palmar bowing of the TCL, and (c) a reduced nerve-TCL distance compared with healthy controls.
Patients and Methods
Subjects
Thirty adult women (15 with type 2 diabetes, 15 healthy controls) volunteered to be part of the study. The patients’ age, weight, height, and BMI were 42.3 ± 5 years, 61.4 ± 7.7 kg, 152.1 ± 5.8 cm, and 26.5 ± 2.6 kg/m2 respectively. The healthy controls’ age, weight, height and BMI were 40 ± 4.1 years, 60.9 ± 5.9 kg, 155.6 ± 5.2 cm, and 25.3 ± 3.2 kg/m2 respectively. The average number of years since onset of diabetes in patients was 5.6 ± 2.2 with a range of 4–10 years. Subjects with BMI greater than 30 kg/m2, upper-limb injury history, or musculoskeletal disorders were excluded from participation in the study. Furthermore, a vibration perception test (VPT) was conducted on all patients to exclude subjects with peripheral neuropathy. Patients were recruited from Mother's Care Diabetes Centre, Tamil Nadu, India. The study protocol was approved by Vellore Institute of Technology Review Board. Subjects read and signed a written informed consent form approved by the Institutional Review Board before participating in the experiment.
Experimental Procedures
Each subject was asked to sit with their right hand and wrist stabilised in a supine and anatomically neutral position via a splint. Subjects were asked to keep their shoulder slightly abducted (30°) and the elbow flexed at a right angle. The thumb was secured in a naturally abducted position, while the four digits were stabilised in extension using Velcro® straps.
A portable ultrasonography system with a 128E linear head array probe (Sonoray linear USB probe, Ultraserve Systems, Chennai, TN, India) was utilised for data collection. The probe surface was placed parallel to the palm and was adjusted to image the distal carpal tunnel cross-section containing the two TCL–bone insertion sites (the hook of hamate and ridge of trapezium). A thick ultrasound gel layer maintained the probe at a distance of 2–3 mm from the palm to avoid any compression of the underlying carpal tunnel structure including the median nerve. During the experiment, the ultrasound probe was operated in 2D B-mode imaging (tissue harmonics frequency, 7.5 MHz; gain, 37–43 dB; image depth, 30–40 mm). An ultrasound image containing the TCL, TCL–hamate and TCL–trapezium insertion sites, as well as the median nerve anatomical features at the distal carpal tunnel cross section was captured during each trial. Three ultrasound image collection trials were performed for each subject at the distal carpal tunnel by K.L., who was trained and certified to handle the ultrasound machine and image the carpal tunnel.
Ultrasound Image Processing
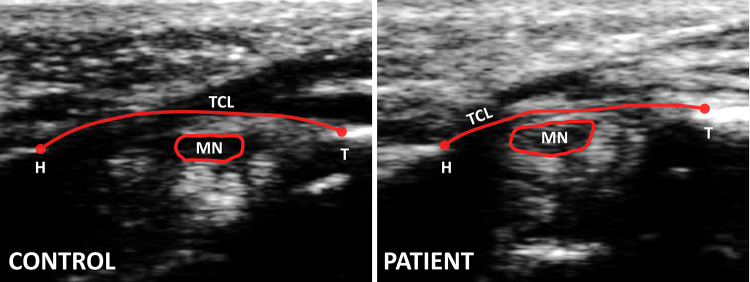
The best image i.e. the one that showed all the anatomical features most distinctly–was chosen from the collection of three ultrasound image trials. The median nerve’s echogenic boundary, the TCL volar boundary, and the two osseous ligament attachment points (hamate and trapezium) were traced manually three times on the best image using a custom LabVIEW program (Fig. 1). The feature points traced on the image were then exported as coordinates in mm using the mm/pixel conversion ratio corresponding to the image. The coordinates were then transformed into a local anatomical coordinate system to correspond with the anatomy. In each ultrasound image, the anatomical coordinate system was defined with the TCL–hamate insertion point as the origin, with the X-axis along the line connecting the two TCL insertion points (hamate and trapezium), and Y-axis perpendicular to the X-axis in the volar direction. The transformed coordinates were used to calculate the outcome measures. The average of the outcome measures from the three tracings was used for statistical analyses. To avoid bias, the authors were blinded to subject group (patients or healthy controls) while tracing each image.
Fig. 1.
Ultrasound images at distal tunnel level for a representative control and patient. The images reflect the tracing of anatomical features: transverse carpal ligament (TCL), hook of hamate (H), ridge of trapezium (T), and echogenic boundary of median nerve (MN)
A custom LabVIEW code was utilised for image processing and calculating the outcome measures. A schematic illustration depicting the outcome parameters is demonstrated in Fig. 2. The median nerve area was calculated by measuring the area within the traced nerve boundary. The carpal arch width was the distance between the two TCL attachment points (hook of hamate and ridge of trapezium), and the arch height was the maximum perpendicular distance between the arch width line and the TCL volar boundary. The palmar bowing index (PBI) was calculated by normalising the arch height to the arch width. The arch area was calculated from the integral of the TCL boundary points along the arch width. The nerve–TCL distance was the perpendicular distance from the centroid of the nerve and the TCL volar boundary.
Fig. 2.
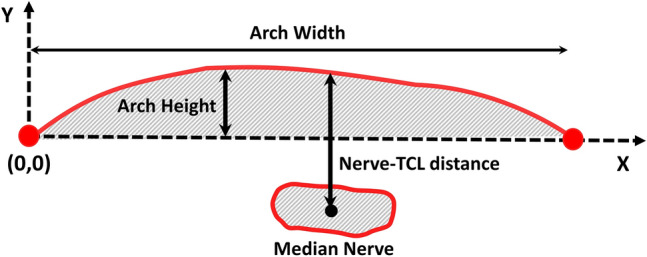
Schematic illustration of carpal arch morphometric parameters in anatomical coordinate system. Arch and nerve areas are represented by shaded regions within the respective anatomical features
Statistical Analysis
Age, body weight, body height, and BMI of the diabetes patients and healthy controls using Student’s t-tests. One-way ANOVAs (α level of 0.05 for significance) were performed to investigate the effects of subject type (patients and healthy controls) on the nerve area, carpal arch parameters (arch width, arch height, PBI, and arch area), and nerve–TCL distance. Post hoc analysis was performed for pairwise comparisons using Bonferroni t tests. All statistical analyses were performed using SigmaPlot 14 (Systat Software Inc, San Jose, CA, USA).
Results
Patients and healthy controls showed no significant differences in age (p = 0.18), weight (p = 0.85), height (p = 0.09) or BMI (p = 0.27). Ultrasonographic examination of the median nerve at the distal tunnel revealed that patients with type 2 diabetes had a median nerve that was enlarged compared to that of healthy controls (Fig. 3A). The median nerve area for the patients (19.9 ± 6.6 mm2) was significantly larger than the nerve area (14.4 ± 2.6 mm2) for healthy controls (p < 0.05).
Fig. 3.
a Median nerve cross-sectional area and b nerve–TCL distance for the two subject groups (Control and Patient). *p < 0.05
Further examination of the distal carpal arch revealed that patients had a smaller carpal arch than healthy controls (Fig. 4). Patients had an arch height (1.3 ± 0.6 mm) that was significantly smaller than the arch height (1.9 ± 0.4 mm) of healthy controls (p < 0.05). However, the arch width (19.9 ± 1.8 mm) for the patients did not show any significant difference compared with the arch width (19.8 ± 1.4 mm) of healthy controls (p = 0.93). The PBI was found to be significantly smaller (p < 0.05) in patients (0.07 ± 0.02) than healthy controls (0.09 ± 0.02). The arch area of patients (17.7 ± 11.8 mm2) was significantly smaller than the arch area (30.8 ± 8.4 mm2) of healthy controls (p < 0.05).
Fig. 4.
a Arch width, b arch height, c palmar bowing index, and d arch area for the two subject groups (Control and Patient). *p < 0.05
Furthermore, the nerve–TCL distance showed that the median nerve was closer to the TCL in patients with type 2 diabetes than in the healthy controls (Fig. 3B). The nerve–TCL distance was significantly smaller in patients (1.9 ± 0.4 mm) compared with healthy controls (2.7 ± 0.5 mm, p < 0.05).
Discussion
The current study performed an ultrasonographic evaluation of the median nerve and carpal arch morphological changes incurred by women with type 2 diabetes. Previous studies have reported that, with the presence of diabetes, the independent high-risk factors for nerve compression are female sex, old age, high BMI, longer duration of diabetes, and neuropathy [11, 13]. In the current study, morphological changes were seen in adult female patients with type 2 diabetes, with an average age of 42 years, normal BMI, an average of 6 years since disease onset and no neuropathy. The results suggest that, even in the absence of other risk factors, onset of type 2 diabetes in females alters the median nerve and carpal arch morphology, increasing their susceptibility to nerve compression.
The ultrasonographic evaluation of the median nerve associated with type 2 diabetes in females has revealed several key findings. The main finding is that females with diabetes have larger median nerve cross-section area compared with controls. The enlarged median nerve, a hallmark consequence of CTS [14], was interestingly found in patients with diabetes and without CTS in the current study, suggesting that even in the absence of CTS or neuropathy, the onset of diabetes still alters the morphology of the median nerve. A similar effect of diabetes was seen in patients with diabetes and CTS, who showed a significantly larger median nerve area compared with patients with idiopathic CTS [1]. A possible mechanism suggested for nerve enlargement in diabetes [15] is the blood–nerve barrier permitting glucose to enter freely into the nerve and causing endoneurial oedema. The oedema subsequently causes the endoneurial hydrostatic pressure to rise and eventually causes myelin changes. In women with type 2 diabetes, an enlarged median nerve makes them more susceptible to nerve entrapment.
The current study also showed that females with type 2 diabetes have a smaller TCL-formed carpal arch compared with controls. The current study showed results similar to previous studies for the carpal arch morphology in the healthy female population [8, 10]. A possible mechanism for a smaller carpal arch in females with diabetes is increased glycosylation of collagen fibres, causing collagen cross-linking and leading to stiffening and thickening of the TCL [16]. A decrease in TCL compliance and an increase in the TCL thickness could lead to reduced palmar bowing and arch area. The reduced palmar bowing, combined with a smaller arch area, leaves little space for the median nerve. The PBI and arch area were found by previous studies to be significantly smaller in healthy females compared with males [8]. The carpal arch morphology in women in general could be a possible cause for women being a high-risk population for median nerve compression within the carpal tunnel [8, 12]. The current findings show that the disadvantageous arch morphology is further worsened in women with the presence of type 2 diabetes.
In addition to an enlarged median nerve and reduced carpal arch size, the nerve–TCL distance also showed a decrease in females with type 2 diabetes compared to controls. The reduced nerve–TCL distance observed in the current study suggests increased physical contact between the nerve and TCL. Additionally, increased nonenzymatic glycosylation of collagen fibres could also lead to accumulation of stiffened fibres in the flexor synovium [17], which could further reduce the space available for the already enlarged nerve, leading to reduced distance between the median nerve and the TCL.
The current study limited the investigation to just Indian women with type 2 diabetes, warranting future studies on larger and more diverse patient populations for deeper understanding of the diabetes induced pathomorphology of the carpal tunnel.
Conclusions
The current study observed an enlarged median nerve, a reduced TCL-formed carpal arch size, and a decreased nerve–TCL distance in women with type 2 diabetes compared with healthy controls. The study extended the previous findings of an enlarged median nerve to the space reduction of the available for the nerve within the TCL-formed carpal arch in women with diabetes. With females in general having a high incidence rate for CTS, keeping watch for nerve compression symptoms at the onset of diabetes would help in early detection and management of the condition.
Acknowledgements
The authors thank Dr. Bharani Gopalan at Mother’s Care Diabetes Center for help in recruiting subjects, Karigiri Hospital for providing the hand splint, and Ultraserve Systems for lending the ultrasound machine. Finally, the authors are grateful to all the study participants.
Abbreviations
- BMI
Body mass index
- CTS
Carpal tunnel syndrome
- CSA
Cross-sectional area
- PBI
Palmar bowing index
- TCL
Transverse carpal ligament
- VPT
Vibration perception test
Author contributions
K.L. and R.S. designed the study. K.L. recruited the subjects and conducted preliminary tests for eligibility. K.L. collected data from the subjects. K.L. and R.S. analyzed the data. K.L. wrote the manuscript with R.S. contributing to revisions. K.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the Vellore Institute of Technology Review Board. Subjects read and signed a written informed consent form approved by the Institutional Review Board before participating in the experiment.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen SF, Huang CR, Tsai NW, Chang CC, Lu CH, Chuang YC, Chang WN. Ultrasonographic assessment of carpal tunnel syndrome of mild and moderate severity in diabetic patients by using an 8-point measurement of median nerve cross-sectional areas. BMC Med Imaging. 2012;12(1):15. doi: 10.1186/1471-2342-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perkins BA, Olaleye D, Bril V. Carpal tunnel syndrome in patients with diabetic polyneuropathy. Diabetes Care. 2002;25(3):565–569. doi: 10.2337/diacare.25.3.565. [DOI] [PubMed] [Google Scholar]
- 3.Dyck PJ, Kratz KM, Karnes JL, Litchy WJ, Klein R, Pach JM, Wilson DM, O’brien PC, Melton L. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology. 1993;43(4):817. doi: 10.1212/WNL.43.4.817. [DOI] [PubMed] [Google Scholar]
- 4.Deal C (1998) The endocrine system. Oxford Textbook of Rheumatology, Oxford
- 5.Thomsen NO, Mojaddidi M, Malik RA, Dahlin LB. Reduced myelinated nerve fibre and endoneurial capillary densities in the forearm of diabetic and non-diabetic patients with carpal tunnel syndrome. Acta Neuropathol. 2009;118(6):785–791. doi: 10.1007/s00401-009-0578-0. [DOI] [PubMed] [Google Scholar]
- 6.Arumugam T, Razali SN, Vethakkan SR, Rozalli FI, Shahrizaila N. Relationship between ultrasonographic nerve morphology and severity of diabetic sensorimotor polyneuropathy. Eur J Neurol. 2016;23(2):354–360. doi: 10.1111/ene.12836. [DOI] [PubMed] [Google Scholar]
- 7.Sassi SA, Giddins G. Gender differences in carpal tunnel relative cross-sectional area: a possible causative factor in idiopathic carpal tunnel syndrome. J Hand Surg (Eur Vol) 2016;41(6):638–642. doi: 10.1177/1753193415625404. [DOI] [PubMed] [Google Scholar]
- 8.Lakshminarayanan K, Shah R, Li ZM. Sex-related differences in carpal arch morphology. PLoS ONE. 2019;14(5):e0217425. doi: 10.1371/journal.pone.0217425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bower JA, Stanisz GJ, Keir PJ. An MRI evaluation of carpal tunnel dimensions in healthy wrists: implications for carpal tunnel syndrome. Clin Biomech. 2006;21(8):816–825. doi: 10.1016/j.clinbiomech.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Monagle K, Dai GU, Chu A, Burnham RS, Snyder RE. Quantitative MR imaging of carpal tunnel syndrome. AJR Am J Roentgenol. 1999;172(6):1581–1586. doi: 10.2214/ajr.172.6.10350293. [DOI] [PubMed] [Google Scholar]
- 11.Comi G, Lozza L, Galardi G, Ghilardi MF, Medaglini S, Canal N. Presence of carpal tunnel syndrome in diabetics: effect of age, sex, diabetes duration and polyneuropathy. Acta Diabetol Latina. 1985;22(3):259–262. doi: 10.1007/BF02590778. [DOI] [PubMed] [Google Scholar]
- 12.Li ZM. Gender difference in carpal tunnel compliance. J Musculoskelet Res. 2005;9(03):153–159. doi: 10.1142/S0218957705001527. [DOI] [Google Scholar]
- 13.Becker J, Nora DB, Gomes I, Stringari FF, Seitensus R, Panosso JS, Ehlers JA. An evaluation of gender, obesity, age and diabetes mellitus as risk factors for carpal tunnel syndrome. Clin Neurophysiol. 2002;113(9):1429–1434. doi: 10.1016/S1388-2457(02)00201-8. [DOI] [PubMed] [Google Scholar]
- 14.Paliwal PR, Therimadasamy AK, Chan YC, Wilder-Smith EP. Does measuring the median nerve at the carpal tunnel outlet improve ultrasound CTS diagnosis? J Neurol Sci. 2014;339(1–2):47–51. doi: 10.1016/j.jns.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 15.Dellon AL, Mackinnon SE. Susceptibility of the diabetic nerve to chronic compression. Ann Plast Surg. 1988;20(2):117–119. doi: 10.1097/00000637-198802000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Fitzgibbons PG, Weiss AP. Hand manifestations of diabetes mellitus. J Hand Surg. 2008;33(5):771–775. doi: 10.1016/j.jhsa.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 17.Rosenbloom AL, Silverstein JH. Connective tissue and joint disease in diabetes mellitus. Endocrinol Metab Clin N Am. 1996;25(2):473–483. doi: 10.1016/S0889-8529(05)70335-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.