Abstract
Background: The treatment of rheumatoid arthritis (RA), a chronic systemic inflammatory autoimmune disease, is based on disease-modifying anti-rheumatic drugs (DMARDs). Typically, it starts with conventional synthetic DMARDs (csDMARDs), and depending on the patient’s response to the treatment and the adverse events experienced, biological DMARDs (bDMARDs) are initiated. bDMARDs are more specific to inflammatory factors than csDMARDs and more efficient in inducing remission and low disease activity. Thus, this study aimed to assess the effectiveness of biological therapy in patients with rheumatoid arthritis in administrative health databases.
Methods: PubMed, Embase, Lilacs, Ovid, Scopus, and Web of Science databases were searched from inception to 21 October 2021, to identify observational studies that evaluated the effectiveness of biological therapy in patients with rheumatoid arthritis using administrative databases and real-world data. The methodological quality was assessed by the methodological index for non-randomized studies (MINORS). A fixed or random-effects model estimated risk ratios with 95% confidence intervals. The analysis was divided into four groups: tumor necrosis factor inhibitors (TNFi) versus non-TNFi; TNFi versus TNFi (adalimumab, etanercept, and golimumab versus infliximab); bDMARDs versus Janus kinase inhibitors (JAKi); and bDMARDs monotherapy versus combination therapy (bDMARDs and MTX).
Results: Twenty-one records were eligible for inclusion in this systematic review and meta-analysis; seven population-based cohorts, eight prospective, and six retrospective cohort studies. Overall, 182,098 rheumatoid arthritis patients were evaluated. In the meta-analysis, lower effectiveness was observed among TNFi users than in non-TNFi (RR: 0.88; 95% CI: 0.81–0.95; p < 0.01; I2 = 94.0%) and bDMARDs than in JAKi (RR: 0.86; 95% CI: 0.79–0.94; p < 0.01; I2 = 93.0%). Higher effectiveness among adalimumab, etanercept, and golimumab than in infliximab (RR: 1.19; 95% CI: 1.05–1.36; p < 0.01; I2 = 96.0%) was found. No significant differences in the effectiveness of bDMARD monotherapy compared to combination therapy (RR: 0.83; 95% CI: 0.68–1.00; p < 0.01; I2 = 81.0%) was observed. E-value analysis indicated that the estimates were not robust against unmeasured confounding.
Conclusion: According to the available real-world data, our results suggest that biological therapy effectively treats patients with rheumatoid arthritis, indicating higher effectiveness with non-TNFi and JAKi than with TNFi.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID#CRD42020190838, identifier CRD42020190838.
Keywords: rheumatoid arthritis, biological therapy, meta-analysis, effectiveness, administrative health databases
1 Introduction
Rheumatoid arthritis (RA) is a chronic systemic inflammatory autoimmune disease that affects the synovial fluid of joints, tendons, and some extra-articular sites, leading to deformity and destruction of joints by bone erosion and cartilage destruction (Guo et al., 2018; Lin et al., 2020). It is estimated that 0.4–1.3% of the world population is affected by the disease, which is two to four times more frequent in women. The age at onset is commonly situated around 30 years, with a peak in the fifth decade of life (Amaya-Amaya et al., 2013; Lin et al., 2020).
Treatment for RA aims to reduce disease activity state, through clinical remission or at least achievement of low disease activity, especially for patients with previous treatment failure. RA treatment is based on disease-modifying anti-rheumatic drugs (DMARDs), typically starting with conventional synthetic DMARDs (csDMARDs) as methotrexate (MTX), hydroxychloroquine, and sulfasalazine, and depending on the patient’s response to the treatment and the adverse events experienced, biological DMARDs (bDMARDs) are initiated to reduce RA symptoms, slow disease progression, and improve physical function (Smolen et al., 2020).
Several bDMARDs have recently emerged in RA management, including TNF-α inhibitors (TNFi) as adalimumab, etanercept, and infliximab; IL-6 receptor antibody, such as tocilizumab; and JAK inhibitors (JAKi) as tofacitinib (Guo et al., 2018; Smolen et al., 2020). However, despite the wide range of biological medicines available, their real-world effectiveness is still under discussion.
There is uncertainty about the effectiveness of TNFi with the first and subsequent uses. In many observational studies, slightly better retention rates and effectiveness have been reported for etanercept than for adalimumab and infliximab, but there is some uncertainty about whether this superiority reflects channeling bias or an actual difference (Lee et al., 2008; Hetland et al., 2010). Consequently, direct evidence of the effectiveness of TNFi is needed to inform clinical and drug reimbursement decision-makers.
Previous systematic reviews and meta-analyses of randomized clinical trials (RCTs) have shown improvement in the remission rates of RA patients with first-line TNFi versus placebo (with or without MTX) (Gulácsi et al., 2019), and better response rates in subjects are exposed to tocilizumab and sarilumab than to adalimumab (Sung and Lee, 2021). Nonetheless, a systematic review and network meta-analysis of 28 RCTs compared the efficacy of csDMARDs, TNFi, non-TNFi, and JAKi with abatacept and found no significant differences between these drugs (Paul et al., 2020).
Although RCTs evaluate the efficacy of treatments in selected groups of patients defined by strict inclusion criteria, the value of these trials in predicting therapeutic effectiveness in “real-world” patients is limited. This systematic review and meta-analysis were designed to complement the knowledge obtained in RCTs and observational studies with primary data by evaluating the real-world effectiveness of TNFi in patients with RA in observational studies with administrative health databases.
Therefore, this systematic review and meta-analysis aimed to assess the real-world effectiveness of biological therapy in patients with rheumatoid arthritis in observational studies with administrative health databases.
2 Methods
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement (Page et al., 2021). The protocol for this systematic review was registered in the International Prospective Register of Systematic Review (PROSPERO) database before starting the literature search (CRD42020190838).
2.1 Eligibility criteria and outcome measures
The PECOS structure was adopted to define the eligibility criteria. Therefore, the population of interest (P) was patients with rheumatoid arthritis, the exposure (E) was the use of biological drugs (adalimumab, certolizumab pegol, etanercept, golimumab, infliximab, abatacept, rituximab, and tocilizumab), the comparator (C) was patients with rheumatoid arthritis unexposed to biological drugs or exposed to different drug classes, the outcome of interest (O) was therapeutic effectiveness, and the study design (S) was observational studies.
Effectiveness was the main outcome of interest for this study. Effectiveness was considered as remission or improvement of disease activity, measured by the Disease Activity Score 28 (DAS28), European Alliance of Associations for Rheumatology (EULAR), Clinical Disease Activity Index (CDAI), or The Simplified Disease Activity Index (SDAI); improvement in functional capacity, measured by the Health Assessment Questionnaire (HAQ); persistence in therapy; or other measures adopted by the studies.
Other outcomes associated with effectiveness explored in this systematic review and meta-analysis were: the reduction of clinical disease activity assessed by ACR70 (70% reduction criteria of the American College of Rheumatology), ACR50 (50% reduction criteria of the American College of Rheumatology), drug withdrawal, and maintenance of remission after withdrawal of the drug.
Observational studies (prospective cohort, retrospective cohort, and case-control) with administrative databases and real-world data were eligible for inclusion. No language or date restrictions were applied. Clinical trials, review articles, case reports, case series, and animal studies were excluded.
2.2 Search strategy
Searches were conducted in Embase, Lilacs, Ovid, PubMed, Scopus, and Web of Science databases to identify studies that assessed the effectiveness of biological therapy in patients with rheumatoid arthritis from inception to 21 October 2021. In addition, grey literature sources were searched (Catálogo de Teses e Dissertações da CAPES and specialized journals) to identify any studies that were not indexed in the databases but might be relevant for inclusion in the present systematic review. Search process details are presented in Supplementary Table S1.
2.3 Study selection and data extraction
Articles’ titles and abstracts were independently evaluated by two reviewers (CCB and LG) for potentially relevant articles using Rayyan (Ouzzani et al., 2016). The studies that met the inclusion criteria in the first stage had their eligibility confirmed by reading the full article. The qualitative and quantitative synthesis included those that met all the inclusion criteria. A third reviewer (DBS) was consulted when the reviewers disagreed on whether an article should be included.
Two reviewers independently extracted the included studies’ details (MJQ and FCA). The extracted data include authors, journal, publication year, country, sample size, effectiveness outcomes, statistical analysis method (including statistical tests and measure of association with confidence intervals), and adjustment variables (confounders).
2.4 Methodological quality assessment
Two reviewers (CTC and MJQ) assessed the methodological quality of the included studies using the methodological index for non-randomized studies (MINORS) (Slim et al., 2003), a validated index to assess the quality of observational studies. This tool contains 12 questions, with a global ideal score for comparative studies of 24 points. The quality assessment of the included studies was measured as follows: 0 to 6 points, very low quality; 7 to 12 points, low quality; 13 to 18 points, moderate quality; and 19 to 24 points, high quality (Pithon et al., 2019).
2.5 Statistical analysis
Data were extracted from eligible studies and arranged in 2 × 2 tables. Risk ratios (RRs) and 95% confidence intervals (95% CI) were calculated by the fixed or the random-effects model, depending on the heterogeneity between the studies. The I2 statistic and Cochran’s Q test were adopted to evaluate heterogeneity and consistency (Higgins, 2003). The random-effects model was applied when heterogeneity was verified (I2 > 50%; p < 0.05). The analysis was divided into four groups: TNFi versus non-TNFi; TNFi versus TNFi (adalimumab, etanercept, and golimumab versus infliximab); bDMARDs versus JAKi; and bDMARD monotherapy versus combination therapy (bDMARDs and MTX). A subgroup analysis by effectiveness measure was conducted. Publication bias was assessed by visual inspection of the funnel plot and statistically using Egger’s tests. A minimum of ten studies were considered to elaborate on this graph and judge the risk of bias associated with missing data (Page et al., 2020). Analyses were carried out with R version 4.1.2 and the “meta” package version 4.13-0 (Balduzzi et al., 2019).
2.6 Sensitivity analysis
Sensitivity analyses were performed, stratifying the analysis by prior use of bDMARDs and no prior use of bDMARDs since bDMARD-naïve patients have a greater response to bDMARDs than those with previous exposure to bDMARDs (Wakabayashi et al., 2011; Mori et al., 2018).
Additionally, an evaluation of how sensitive the estimates from each study were to the effects of unmeasured confounders was performed through the E-value. This measure represents an unmeasured confounder’s strength to make a reported exposure-outcome association statistically non-significant (Mathur and VanderWeele, 2020). Thus, the size of unobserved confounding able to nullify the mean risk ratio was quantified, and the unmeasured confounding strengths sufficient to allow 10% of studies with true RR above or below a threshold to remain statistically significant were calculated for each one of the four groups analyzed.
3 Results
3.1 Selected studies
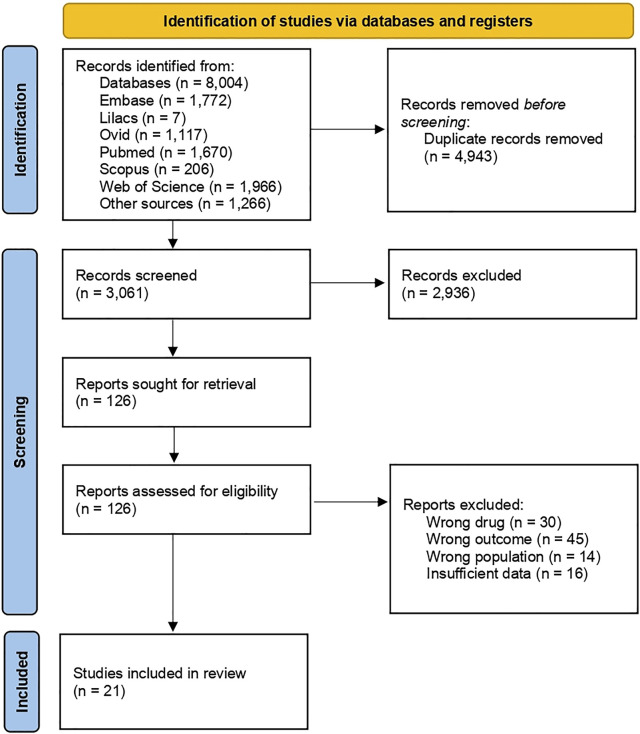
The initial search returned 8,004 records, of which 4,943 were duplicates. After screening titles and abstracts, 126 studies were analyzed regarding inclusion criteria, and 105 were excluded. Afterward, references to the included studies were manually searched to detect relevant articles, but none was identified. Therefore, articles were excluded from analyzing the wrong drug, outcome, and population and from having insufficient data (Figure 1).
FIGURE 1.
Flow chart of search results.
3.2 Study characteristics
Twenty-one records were eligible for inclusion in this systematic review; seven population-based cohorts (Østergaard et al., 2007; Chatzidionysiou et al., 2015; Neovius et al., 2015; Acurcio et al., 2016; Kihara et al., 2017; Choi et al., 2021; Li et al., 2021), eight prospective (Harrold et al., 2015; Lauper et al., 2018; Ebina et al., 2020a, 2020b; Rahman et al., 2020; Curtis et al., 2021; Pappas et al., 2021a, 2021b), and six retrospective cohort studies (Curtis et al., 2015; Yun et al., 2015; Silvagni et al., 2018; Bird et al., 2020; Gharaibeh et al., 2020; Youssef et al., 2020), which are published from 2007 to 2021 (Supplementary Table S2).
Overall, 182,098 rheumatoid arthritis patients were evaluated; the majority were women (67–88%), and the mean age ranged between 48 and 70 years (Supplementary Table S2). Disease duration was between 4 and 13 years. Most studies included patients with moderate to high disease activity, indicating severe rheumatoid arthritis and poor prognosis (Table 1).
TABLE 1.
Characteristics of the included studies.
| Study | Year | Country | Patients | Mean disease duration (years) | Mean disease activity | Prior use of bDMARDs | Current use of steroids | Outcome |
|---|---|---|---|---|---|---|---|---|
| Acurcio | 2016 | Brazil | 76,351 | NR | NR | NR | NR | Medication persistence in the 1st and 2nd year |
| Bird | 2020 | Australia | 1,950 | 8.9–10.0 | NR | No | NR | Medication persistence and improvement and remission in DAS28, CDAI, and SDAI |
| Chatzidionysio | 2014 | Sweden | 7,052 | 8.4–9.9 | DAS28: 4.7–5.1 | Yes | Yes | Improvement, remission, and change in DAS28 and therapy discontinuation in 6 months |
| Choi | 2021 | South Korea | 8,018 | NR | NR | Yes | NR | Drug failure and medication persistence |
| Curtis | 2015 | United States of America | 5,474 | NR | NR | NR | Yes | Effectiveness (high adherence, no increase in biologic dose, no biologic switch, no new DMARD, no new/increased oral glucocorticoid, and ≤1 glucocorticoid injection) |
| Curtis | 2021 | United States of America | 1,270 | 7.3–9.2 | CDAI: 31.5–33.2 | Yes | NR | Change in CDAI at months 6 and 12 |
| Ebina | 2020 (a) | Japan | 3,897 | 4.7–9.2 | DAS28-ESR: 4.1–4.6 | Yes | Yes | Treatment discontinuation |
| Ebina | 2020 (b) | Japan | 221 | 7.8–11.6 | DAS28-CRP: 3.2–3.9 | Yes | Yes | Treatment discontinuation |
| Gharaibeh | 2020 | United States of America | 14,775 | NR | NR | No | Yes | Nonadherence, increased index medication dose, addition of a conventional DMARD, switch of biologic medications, addition of glucocorticoid or increased glucocorticoid dose, and receipt of ≥2 intra-articular injections in 1 year |
| Harrold | 2015 | United States of America | 1,398 | 11.5–13.4 | CDAI: 21.4–22.9 | Yes | Yes | Responsiveness to medication treatment based on improvement in CDAI, modified ACR20 (mACR20), modified ACR50 (mACR50), and modified ACR70 (mACR70) responses at 6th and 12th month |
| Kihara | 2017 | United Kingdom | 2,636 | 4.0–5.0 | DAS28: 6.0–6.2 | Yes | Yes | Change in DAS28, EULAR response, DAS28 remission, change in HAQ score, and proportion of patients who achieved the minimal clinically important difference in HAQ at the 6th month |
| Lauper | 2018 | Czech Republic, Finland, Italy, Norway, Portugal, Romania, Russia, Slovenia, Spain, and Switzerland | 8,308 | 7.9–10.2 | DAS28: 4.0–4.6 | Yes | Yes | Medication persistence, change in CDAI, and DAS28-ESR in 1 year |
| Li | 2021 | Taiwan | 8,663 | NR | NR | No | NR | Treatment discontinuation and switching |
| Neovius | 2015 | Sweden | 9,139 | 12–13 | DAS28: 5.1–5.2 | No | NR | Therapy discontinuation due to any cause (except for pregnancy and remission) and remission in 5 years |
| Østergaard | 2007 | Denmark | 300 | NR | DAS28-CRP: 5.9 | No | NR | DAS28 and EULAR response rates at week 26 and 52 |
| Pappas | 2021 (a) | United States of America | 617 | 8.8 | CDAI: 3.5–3.7 | No | Yes | Medication persistence, discontinuation, and switching |
| Pappas | 2021 (b) | United States of America | 4,816 | 7.1–8.6 | CDAI: 20.4 | No | Yes | Improvement in CDAI and DAS28, remission based on CDAI and DAS28, and change in CDAI, HAQ, and EQ-5D |
| Rahman | 2020 | Canada | 1,577 | 6.5–9.8 | DAS28-CRP: 4.1–5.3 | Yes | Yes | Medication discontinuation, improvement in DAS28 and HAQ-DI, SDAI remission, and low disease activity |
| Silvagni | 2018 | Italy | 4,478 | 5.0 | NR | No | Yes | Medication persistence |
| Youssef | 2020 | Australia | 6,914 | 10.0 | NR | Yes | Yes | Medication persistence |
| Yun | 2015 | United States of America | 14,244 | NR | NR | No | Yes | No switch to a different biologic, high adherence to the index drug, no addition of a new non-biologic DMARD, no biologic dose increase compared with starting, no initiation of glucocorticoids/no increase in dose, and no more than one joint injection on unique days after 3 months of new treatments |
ACR, American College of Rheumatology; CDAI, Clinical Disease Activity Index; DAS28, Disease Activity Score-28; DAS28-CRP, DAS-28C-reactive protein; DAS28-ESR, DAS-28 erythrocyte sedimentation rate; DMARD, disease-modifying anti-rheumatic drug; EULAR, European Alliance of Associations for Rheumatology; HAQ, Health Assessment Questionnaire; HAQ-DI, HAQ Disability Index; NR, not reported; SDAI, Simplified Disease Activity Index.
Ten studies compromised RA patients in second-line therapy (Chatzidionysiou et al., 2015; Harrold et al., 2015; Kihara et al., 2017; Lauper et al., 2018; Ebina et al., 2020a, 2020b; Rahman et al., 2020; Youssef et al., 2020; Choi et al., 2021; Curtis et al., 2021), nine in first-line therapy (Østergaard et al., 2007; Neovius et al., 2015; Yun et al., 2015; Silvagni et al., 2018; Bird et al., 2020; Gharaibeh et al., 2020; Li et al., 2021; Pappas et al., 2021b, 2021a), and two did not report this information (Curtis et al., 2015; Acurcio et al., 2016) (Table 1).
Studies evaluated second-line therapy with tocilizumab versus TNFi (monotherapy or combination therapy with csDMARDs) after the use of at least one bDMARD (Lauper et al., 2018); second-line treatment with bDMARDs and tsDMARD after the use of other bDMARDs and tsDMARD (Youssef et al., 2020); and second and third-line bDMARDs and tsDMARD (Choi et al., 2021). One research included patients with previous therapy with bDMARDs and concurrent DMARDs (Curtis et al., 2021). Another study indicated a proportion of biologic-experienced RA patients of 6.3–19.7% (Rahman et al., 2020).
Five reports evaluated therapy switching, of which one analyzed switching from first TNFi to second TNFi (Chatzidionysiou et al., 2015); one from TNFi to abatacept and other TNFi (Harrold et al., 2015); one from any bDMARD to tocilizumab (Kihara et al., 2017); one from any bDMARD to another bDMARD or tofacitinib (Ebina et al., 2020a); and one from tocilizumab or abatacept after failure to bDMARDs or JAKi (Ebina et al., 2020b). The first four studies did not report the duration of the first therapy. The last one observed mean therapy duration between 16.4 and 26.7 months for tocilizumab and 10.9 and 11.0 months for abatacept.
Furthermore, fourteen records described the proportion of RA patients in current use of steroids (Chatzidionysiou et al., 2015; Curtis et al., 2015; Yun et al., 2015; Harrold et al., 2015; Kihara et al., 2017; Silvagni et al., 2018; Lauper et al., 2018; Ebina et al., 2020a, 2020b; Rahman et al., 2020; Youssef et al., 2020; Gharaibeh et al., 2020; Pappas et al., 2021a, 2021b), which ranged from 9.9 to 78.0%; however, none of these studies did a separate analysis for patients who are currently exposed to steroids and unexposed to steroids.
The 21 studies investigated nine different biological drugs, among them TNFi (etanercept, infliximab, adalimumab, certolizumab pegol, golimumab, and tocilizumab), non-TNFi (rituximab and abatacept), and JAKi (tofacitinib). Additionally, three studies compared bDMARD monotherapy and combination therapy (bDMARDs and MTX).
Regarding the outcomes, most articles analyzed medication persistence, remission, and improvement in disease activity. The studies’ remission and disease activity measures encompassed DAS28, EULAR, CDAI, SDAI, and HAQ.
3.3 Quality of the included studies
According to the MINORS, twenty studies were classified as high quality (Chatzidionysiou et al., 2015; Curtis et al., 2015, 2021; Harrold et al., 2015; Neovius et al., 2015; Yun et al., 2015; Acurcio et al., 2016; Kihara et al., 2017; Lauper et al., 2018; Silvagni et al., 2018; Bird et al., 2020; Ebina et al., 2020a, 2020b; Gharaibeh et al., 2020; Rahman et al., 2020; Youssef et al., 2020; Choi et al., 2021; Li et al., 2021; Pappas et al., 2021a, 2021b) and one as moderate quality (Østergaard et al., 2007). Overall, studies scored between 14 and 24 points (Supplementary Table S3).
3.4 Meta-analysis
3.4.1 TNFi versus non-TNFi
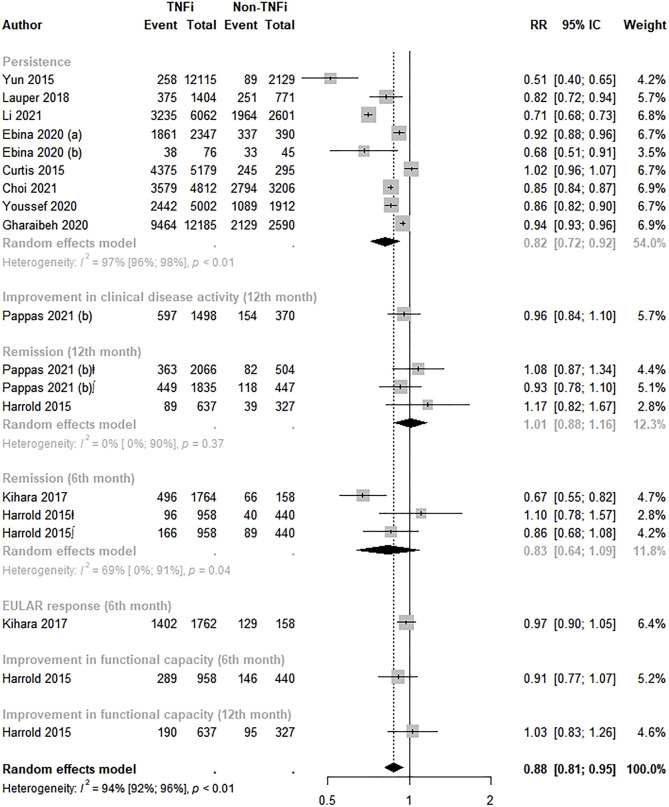
Twelve studies assessed the effectiveness between TNFi and non-TNFi (Curtis et al., 2015; Harrold et al., 2015; Yun et al., 2015; Kihara et al., 2017; Lauper et al., 2018; Ebina et al., 2020a, 2020b; Gharaibeh et al., 2020; Youssef et al., 2020; Choi et al., 2021; Li et al., 2021; Pappas et al., 2021b). A statistically significant lower effectiveness was observed among TNFi users than in non-TNFi users (RR: 0.88; 95% CI: 0.81–0.95; p < 0.01; I2 = 94.0%). The analysis by effectiveness measure revealed lower therapy persistence (RR: 0.82; 95% CI: 0.72–0.92) with TNFi than with non-TNFi drugs (Figure 2). Visual inspection of the funnel plot did not suggest asymmetry (Supplementary Figure S1), and Egger’s test did not indicate publication bias (intercept = −0.01, p = 0.99).
FIGURE 2.
Effectiveness of TNF inhibitors compared to non-TNF inhibitors. TNFi, TNF inhibitors; non-TNFi, non-TNF inhibitors; ⱡ, remission based in CDAI; ∫, remission based in DAS28.
3.4.2 Adalimumab, etanercept, and golimumab versus infliximab
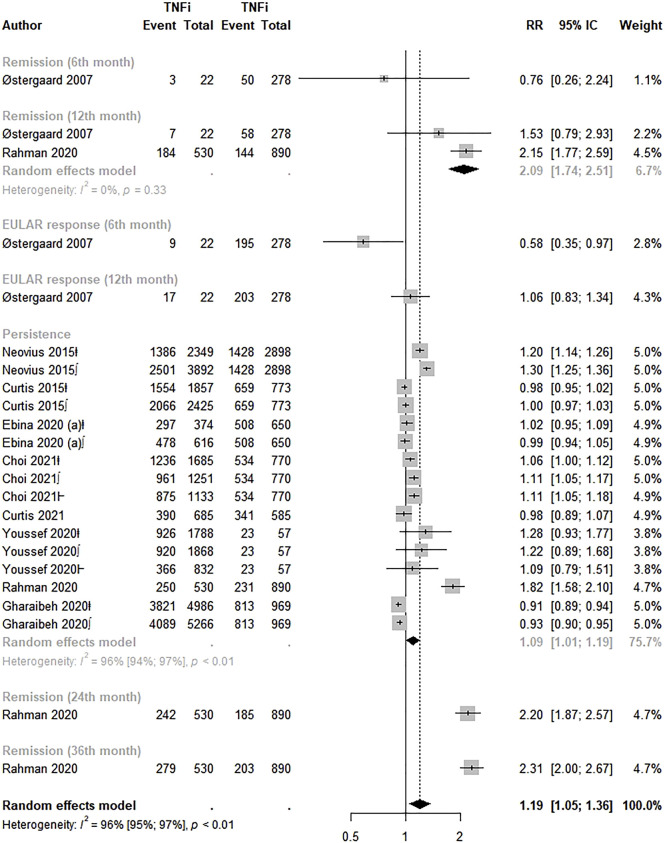
Ten studies evaluated the effectiveness of adalimumab, etanercept, and golimumab versus infliximab (Østergaard et al., 2007; Curtis et al., 2015, 2021; Neovius et al., 2015; Ebina et al., 2020a, 2020b; Gharaibeh et al., 2020; Rahman et al., 2020; Youssef et al., 2020; Choi et al., 2021). Overall, adalimumab, etanercept, and golimumab were 19.0% more effective for rheumatoid arthritis than infliximab (RR: 1.19; 95% CI: 1.05–1.36; p < 0.01; I2 = 96.0%). Higher therapy persistence (RR: 1.09; 95% CI: 1.01–1.19) and remission in the 12th month (RR: 2.09; 95% CI: 1.74–2.51) were pointed out in the analysis by effectiveness measure (Figure 3). Visual inspection of the funnel plot indicated asymmetry, suggesting publication bias (Supplementary Figure S2). Egger’s test indicated publication bias (intercept = 3.97, p = 0.02).
FIGURE 3.
Effectiveness of Adalimumab, Etanercept and Golimumab compared to Infliximab. TNFi, TNF inhibitors; ⱡ, adalimumab; ∫, etanercept; Ⱶ, golimumab.
The analysis by drug showed a significant higher effectiveness of golimumab (RR: 1.57; 95% CI: 1.19–2.08; p < 0.01; I2 = 97.0%) over infliximab. However, the subgroup analysis by effectiveness measure did not reveal statistically significant results for adalimumab, etanercept, or golimumab over infliximab (Supplementary Figure S3).
3.4.3 bDMARDs versus JAKi
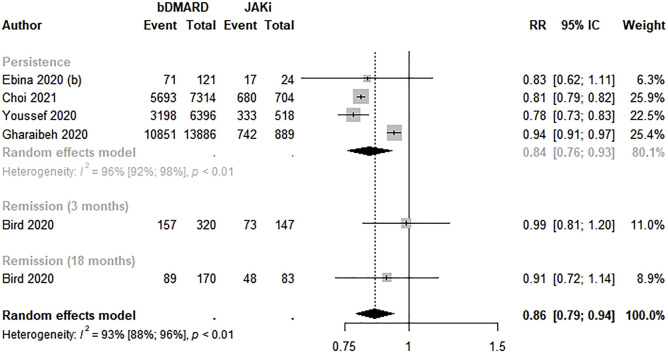
Five studies estimated the effectiveness of bDMARDs compared to JAKi (Bird et al., 2020; Ebina et al., 2020b; Gharaibeh et al., 2020; Youssef et al., 2020; Choi et al., 2021). bDMARDs were 14.0% less effective for rheumatoid arthritis than JAKi (RR: 0.86; 95% CI: 0.79–0.94; p < 0.01; I2 = 93.0%). Regarding the analysis by effectiveness measure, a lower persistence in bDMARD therapy was observed (RR 0.84; 95% CI 0.76–0.93) (Figure 4).
FIGURE 4.
Effectiveness of biological disease-modifying anti-rheumatic drugs compared to Janus kinase inhibitors. bDMARD: biological disease-modifying anti-rheumatic drug; JAKi: Janus kinase inhibitors.
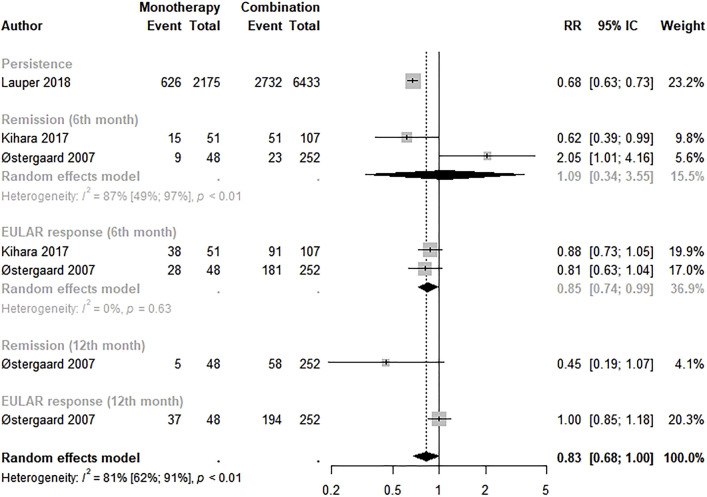
3.4.4 bDMARD monotherapy versus combination therapy
The effectiveness between bDMARD monotherapy and combination therapy was evaluated by three studies (Østergaard et al., 2007; Kihara et al., 2017; Lauper et al., 2018). The meta-analysis revealed a lower effectiveness of bDMARD monotherapy than of combination therapy with borderline statistical significance (RR: 0.83; 95% CI: 0.68–1.00; p < 0.01; I2 = 81.0%). However, a lower EULAR response in the 6th month with statistical significance was observed in bDMARD monotherapy (RR: 0.85; 95% CI: 0.74–0.99) (Figure 5).
FIGURE 5.
Effectiveness of biological disease-modifying anti-rheumatic drugs monotherapy compared to combination therapy. Monotherapy: biological disease-modifying anti-rheumatic drugs monotherapy; combination: biological disease-modifying anti-rheumatic drugs + methotrexate.
3.5 Sensitivity analysis
In analyses by prior use of bDMARDs, a statistically significant lower effectiveness was observed among TNFi users than in non-TNFi users who had never been exposed to biological therapy (RR: 0.86; 95% CI: 0.78–0.95; p < 0.01; I2 = 96.0%), while non-significant differences were observed among biologic-experienced patients (Supplementary Figure S4).
In contrast, a 50.0% higher effectiveness was presented by biologic-experienced subjects exposed to adalimumab, etanercept, and golimumab than to infliximab (RR: 1.50; 95% CI: 1.15–1.95; p < 0.01; I2 = 96.0%). Regarding biologic-naïve patients, there were no significant differences between the drugs (RR: 1.05; 95% CI: 1.00–1.11; p < 0.01; I2 = 94.0%) (Supplementary Figure S5).
In the sensitivity analysis of bDMARDs compared to JAKi, bDMARDs had lower effectiveness than JAKi in biologic-naïve patients (RR: 0.86; 95% CI: 0.79–0.95; p < 0.01; I2 = 95.0%), and non-statistical significance was found among patients with prior use of biologics (Supplementary Figure S6).
Non-significant differences on effectiveness were observed in sensitivity analysis of bDMARD monotherapy compared to combination therapy among patients who had previously been exposed to biologic drugs (RR: 0.87; 95% CI: 0.66–1.14; p < 0.01; I2 = 94.0%) and those who had never been exposed to biologic drugs (RR: 0.89; 95% CI: 0.76–1.04; p < 0.01; I2 = 85.0%) (Supplementary Figure S7).
The sensitivity analysis for unmeasured confounding showed that an unobserved confounder needed to be associated with both TNFi use and effectiveness with a risk ratio of at least 1.65 (95% CI: 1.00–2.82) to reduce to less than 10% the percentage of meaningfully strong true causal effects. For adalimumab, etanercept, and golimumab and the outcome, a risk ratio of at least 1.97 (95% CI: 1.00–3.52) would be necessary to reduce to less than 10% the percentage of meaningfully strong true causal effects, while for bDMARDs and bDMARD monotherapy, the necessary risk ratios should be 1.61 (95% CI: 1.00–2.85) and 2.06 (95% CI: 1.00–4.08), respectively.
4 Discussion
This systematic review and meta-analysis provide a quantitative estimate of the real-world effectiveness of different biological therapies in patients with rheumatoid arthritis in studies using administrative health databases. Real-world effectiveness data provide valuable evidence to support the efficacy findings from randomized controlled trials (RCTs) (Blonde et al., 2018) once trial patients may not represent the real-world RA population.
Overall, this meta-analysis showed statistically significant differences in effectiveness between the biological medicines analyzed. For example, TNFi showed less effectiveness in RA patients than non-TNFi drugs, as well as bDMARDs compared to JAKi, and bDMARD monotherapy compared to combination therapy. In contrast, golimumab showed higher effectiveness than infliximab. However, it is important to highlight the low number of studies included in some analyses.
These findings are similar to the results of efficacy from previous RCTs. The ADACT and AMPLE trials compared the efficacy of tocilizumab versus adalimumab and abatacept versus adalimumab, respectively, and indicated greater effectiveness of non-TNFi over the TNFi analyzed (Gabay et al., 2013; Weinblatt et al., 2013). Regarding JAKi, RCT findings are controversial, pointing to the greater effectiveness of baricitinib over adalimumab (Keystone et al., 2017; Taylor et al., 2017) and lower effectiveness of tofacitinib than of adalimumab (Fleischmann et al., 2017).
The development of drugs to target TNF-α has been one of the most impressive advances in treating inflammatory diseases in the past decade. However, some patients do not tolerate or respond adequately to available TNFi. In these cases, other biologically derived drugs with different action mechanisms may be used, such as abatacept, which is a T-cell co-stimulation inhibitor, and JAKi, which are oral drugs counteracting the activation of cytosolic enzymes presiding over many biologic functions (JAKs) (Smolen et al., 2009; Angelini et al., 2020).
TNF-α is an important cytokine that mediates inflammation and bone degradation in RA through local inflammation and pannus formation, eventually leading to further cartilage erosion and bone destruction. The introduction of TNFi has revolutionized RA treatment options, resulting in the development of further biologic DMARDs (Ma and Xu, 2013). TNFi drugs act by reducing TNF-α levels in RA, restoring the balance in the cytokine system. Many TNFi drugs are available nowadays, including infliximab, adalimumab, etanercept, and golimumab. The first TNFi drug for RA was infliximab, a chimeric human-murine monoclonal antibody that binds with high affinity to soluble and transmembrane forms of TNF-α but not to lymphotoxin. Since the advent of infliximab, genetically engineered molecules employing a slightly different compositional and pharmacodynamic approach have been marketed (Pelechas et al., 2019).
Unlike the present results, where significant effectiveness of adalimumab over infliximab was not observed, ATTEST and AMPLE trials found higher efficacy of adalimumab than infliximab, with a statistically significant odds ratio of ACR20 (OR: 1.73; 95% CI: 1.04–2.87), ACR50 (OR: 1.49; 95% CI: 1.02–2.19), and low disease activity (DAS28) (OR: 2.12; 95% CI: 1.19–3.78) were observed among patients treated with adalimumab (Christensen et al., 2013). However, results from these RCTs reflect a limited population of RA patients, leading to limitations related to small sample size and exclusion criteria that limit generalizability to real-world subjects. So, these differences highlight real-world studies’ importance in investigating drug effects in clinical practice since the effectiveness of drug therapy depends on factors such as adherence to the medication and the outcomes associated with the drug use in different patient populations.
Although significantly higher effectiveness of etanercept over infliximab was not found in the meta-analysis, a retrospective cohort study with data from the CORRONA registry pointed out that patients on etanercept monotherapy experience greater therapy persistence in the 6th and 12th month and are less likely to reintroduce a csDMARD than patients on other TNFi monotherapies. The authors stated that the development of neutralizing anti-drug antibodies to TNFi other than etanercept might contribute to these findings (Pappas et al., 2021a).
Similar to the findings of this systematic review and meta-analysis, a systematic review of sixteen RCTs compared the efficacy of TNFi using Bayesian mixed treatment comparison models and found greater efficacy of golimumab than infliximab by the Health Assessment Questionnaire (HAQ) score (Schmitz et al., 2012). Golimumab is a human anti-TNF-α monoclonal antibody generated and matured in an in vivo system, with high affinity and specificity for human TNF-α, and effectively neutralizes TNF-α bioactivity (Ma and Xu, 2013). Furthermore, this biological drug presents low levels of immunogenicity and a more attractive dosage scheme (every 4 weeks) (Pelechas et al., 2019), which may influence its greater effectiveness than infliximab.
According to previous studies, using combination therapy (bDMARDs and MTX) contributes to a higher persistence of biological therapy in RA patients (Lauper et al., 2018). Similarly, patients treated with higher MTX doses tend to persist in treatment for a longer time (Soliman et al., 2011; Aaltonen et al., 2017). However, in the meta-analysis comparing bDMARD monotherapy to combination therapy, a borderline statistically significant lower effectiveness was found among patients treated exclusively with bDMARDs. This finding may be related to the evaluation of different biological medicines by each study, such as tocilizumab or TNFi (Lauper et al., 2018), tocilizumab (Kihara et al., 2017), and infliximab and etanercept (Østergaard et al., 2007).
Furthermore, the sensitivity analysis revealed lower effectiveness of TNFi versus non-TNFi and bDMARDs versus JAKi in biologic-naïve patients, indicating a possible benefit from non-TNFi and JAKi pharmacotherapy in these subjects. Regarding biologic-experienced subjects, higher effectiveness was observed with adalimumab, etanercept, and golimumab than with infliximab. Given the present findings, adalimumab, etanercept, and golimumab may be effective treatment options for patients with inadequate response to infliximab.
Most of the included studies evaluated as effectiveness measure therapy persistence, remission, and improvement in disease activity. Persistence in therapy is an excellent indirect and composite measure of effectiveness, safety, and tolerability, reflecting the long-term impact on the course of the disease (Silvagni et al., 2018). Twelve studies evaluated the therapy persistence in this systematic review (Curtis et al., 2015; Neovius et al., 2015; Yun et al., 2015; Lauper et al., 2018; Ebina et al., 2020a, 2020b; Gharaibeh et al., 2020; Rahman et al., 2020; Youssef et al., 2020; Choi et al., 2021; Curtis et al., 2021; Li et al., 2021). In addition, the majority of the studies that evaluated the therapy persistence of TNFi in comparison to non-TNFi found significant differences among the therapies, favoring non-TNFi over TNFi (Curtis et al., 2015; Yun et al., 2015; Lauper et al., 2018; Ebina et al., 2020a; Youssef et al., 2020; Choi et al., 2021; Li et al., 2021). The same pattern was observed among articles that assessed persistence among bDMARD and JAKi, showing lower persistence in bDMARD RA patients (Gharaibeh et al., 2020; Youssef et al., 2020; Choi et al., 2021). In contrast, only two articles found significant differences between adalimumab, etanercept, and golimumab versus infliximab, pointing to a higher persistence among RA patients exposed to adalimumab and etanercept than infliximab (Neovius et al., 2015; Rahman et al., 2020).
According to Yun et al. (2014), one in every three patients interrupts their treatments with the first bDMARD in the first year of use due to lack of efficacy and/or adverse events. Nonetheless, treating autoimmune diseases that cause systemic inflammation is vital since there is evidence that the persistence of systemic inflammation leads to a higher risk of death (Listing et al., 2015). Furthermore, RA patients present a higher risk of death due to cardiovascular events when compared to the general population (Zhang et al., 2016).
A critical treatment goal in managing RA patients is the achievement of clinical remission (Ajeganova and Huizinga, 2017). However, only six studies used this outcome as an effectiveness measure (Østergaard et al., 2007; Harrold et al., 2015; Kihara et al., 2017; Bird et al., 2020; Rahman et al., 2020; Pappas et al., 2021b). Furthermore, only one of the included studies observed significant differences among the biological therapies evaluated in clinical remission (Rahman et al., 2020). The prospective cohort used data from the Biologic Treatment Registry Across Canada (BioTRAC) between 2002 and 2017 and evaluated the effectiveness of golimumab and infliximab. The authors observed higher SDAI clinical remission at 12, 24, and 36 months in patients treated with golimumab (34.7, 47.5, and 52.7%, respectively) than in those treated with infliximab (of 16.2, 20.8, and 22.8%, respectively) (Rahman et al., 2020).
The expressive variation in the remission and disease activity measures adopted by the studies included in the present systematic review and meta-analysis, encompassing DAS28, EULAR, CDAI, SDAI, and HAQ, must be highlighted. A treat-to-target strategy is recommended in RA, and for this purpose, regular RA disease activity assessments must be made during routine care. Many RA disease activity measures are available that incorporate data gathered from a combination of sources, including patient-reported measures, provider assessments, laboratory values, and/or imaging modalities; nevertheless, these measures may vary in performance and feasibility (England et al., 2019). Considering these, the American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR) recommend a variety of RA disease activity measures, such as CDAI, DAS28-ESR/CRP, and SDAI, for regular use (England et al., 2019; Smolen et al., 2020).
It is important to emphasize that because of the different immune-modulatory properties of specific drugs and drug classes, biological therapy may be related to several potential adverse events, such as hospitalized infection, solid cancers and lymphoma, cardiovascular diseases, and mortality (Yun et al., 2016). Therefore, the pharmacotherapy selection must consider not only the medicine’s efficacy but also its associated risk.
RA treatment has progressively improved over the last decades due to the contribution of biological therapies and treat-to-target strategies, which aim at the achievement of clinical remission by slowing or stopping the progression of joint destruction and deformity. This process improved therapeutic results and quality of life and reduced patient morbidity and mortality (Bullock et al., 2018; Ho et al., 2019). Furthermore, therapy choice depends on disease severity, the patient’s clinical response, and previously experienced side effects. Although biological medicines improve the likelihood of reaching the treatment target in many RA patients, they are costly, limiting their widespread use and contributing to the inequity of access across countries. Thus, they should be used in an evidence-based manner that accounts for availability and affordability within the local healthcare system (Ho et al., 2019; Smolen et al., 2020).
4.1 Strengths and limitations
This systematic review and meta-analysis present strengths and limitations. This is a comprehensive assessment of the evidence, incorporating all available published studies on the real-world effectiveness of biological therapies in patients with rheumatoid arthritis. Strengths also encompass studies with administrative health databases as inclusion criteria, random-effects meta-analysis to deal with the heterogeneity, and the conduction of sensitivity analysis stratified by prior use of bDMARDs and no prior use of bDMARDs.
A significant limitation is the possibility of findings by chance in the meta-analyses comparing bDMARDS versus JAKi and bDMARD monotherapy versus combination therapy due to the low number of studies included. Meta-analyses of small numbers of studies have limitations that can impact their findings, although they present valid results (Herbison et al., 2011). Also, it was not possible to analyze bDMARDs compared to csDMARDs since only one of the included studies evaluated this (Acurcio et al., 2016).
Also, it was not possible to perform sensitivity analyses by the duration of previous drugs because the included studies did not have this information and by RA patients currently exposed to steroids versus those unexposed to these medicines since none of the included studies reported patients unexposed to steroids.
Another limitation is the high heterogeneity between studies, which persisted after subgroup and sensitivity analyses. This could be justified by several factors such as differences in measures of effectiveness adopted, differences in RA severity and prognosis, and differences in some population characteristics.
The publication bias found in studies that evaluated TNFi compared to TNFi (infliximab) is probably associated with the eligibility criteria adopted, including only observational studies with administrative databases, usually resulting in more extensive studies.
Moreover, raw data were used to perform meta-analyses instead of adjusted measures, considering the variety of association measures and the several combinations of covariates submitted to the adjustment procedures by the studies. So, the type of analysis performed cannot control confounders such as age, gender, ethnicity, education, work, type of health insurance, body mass index (BMI), smoking, comorbidities, and use of drugs that can influence drugs’ effectiveness, such as steroids and NSAIDs.
Although real-world data may not be as rigorous as RCT data because of the string inclusion criteria, data collection, and quality control, it may lead to a better understanding of the effectiveness of biological therapy in a more complex and heterogeneous RA population, which is more representative of clinical practice.
Acknowledgments
We would like to thank the Department of Pharmaceutical Assistance and Strategic Inputs of the Ministry of Health of Brazil, Fiocruz, CIDACS, Institute of Collective Health, Federal University of Bahia (Brazil), Salvador, and the Center for Health Sciences of the Federal University of Recôncavo da Bahia (Brazil).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
CC, MQ, FA, and DS contributed to the study design, data analysis, and data interpretation. CC, MQ, FA, CB, LG, and DS contributed to the study selection, data extraction, and interpretation of data. CC, MQ, FA, CB, LG, DP, SB, WA, EB, JA, RC, MB, and DS were involved in drafting the manuscript and revised it critically.
Funding
This study was financed by the Secretariat of Science, Technology, Innovation, and Strategic Inputs of the Ministry of Health (Brazil) and the Oswaldo Cruz Foundation (Fiocruz).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.927179/full#supplementary-material
References
- Aaltonen K., Joensuu J., Pirilä L., Kauppi M., Uutela T., Varjolahti-Lehtinen T., et al. (2017). Drug survival on tumour necrosis factor inhibitors in patients with rheumatoid arthritis in Finland. Scand. J. Rheumatol. 46, 359–363. 10.1080/03009742.2016.1234641 [DOI] [PubMed] [Google Scholar]
- Acurcio F. A., Machado M. A. A. A., Moura C. S., Ferre F., Guerra A. A., Andrade E. I. G. G., et al. (2016). Medication persistence of disease-modifying antirheumatic drugs and anti-tumor necrosis factor Agents in a cohort of patients with rheumatoid arthritis in Brazil. Arthritis Care Res. 68, 1489–1496. 10.1002/acr.22840 [DOI] [PubMed] [Google Scholar]
- Ajeganova S., Huizinga T. (2017). Sustained remission in rheumatoid arthritis: latest evidence and clinical considerations. Ther. Adv. Musculoskelet. Dis. 9, 249–262. 10.1177/1759720X17720366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaya-Amaya J., Rojas-Villarraga A., Mantilla R. D., Anaya J.-M. (2013). “Rheumatoid arthritis,” in Autoimmunity: From bench to bedside. Editors Anaya J.-M., Shoenfeld Y., Rojas-Villarraga A., Levy R. A., Cervera R. (Bogotá: El Rosario University Press; ). [PubMed] [Google Scholar]
- Angelini J., Talotta R., Roncato R., Fornasier G., Barbiero G., Dal Cin L., et al. (2020). JAK-inhibitors for the treatment of rheumatoid arthritis: A focus on the present and an outlook on the future. Biomolecules 10, 1002. 10.3390/biom10071002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balduzzi S., Rücker G., Schwarzer G. (2019). How to perform a meta-analysis with R: A practical tutorial. Evid. Based. Ment. Health 22, 153–160. 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird P., Littlejohn G., Butcher B., Smith T., da Fonseca Pereira C., Witcombe D., et al. (2020). Real-world evaluation of effectiveness, persistence, and usage patterns of tofacitinib in treatment of rheumatoid arthritis in Australia. Clin. Rheumatol. 39, 2545–2551. 10.1007/s10067-020-05021-7 [DOI] [PubMed] [Google Scholar]
- Blonde L., Khunti K., Harris S. B., Meizinger C., Skolnik N. S. (2018). Interpretation and impact of real-world clinical data for the practicing clinician. Adv. Ther. 35, 1763–1774. 10.1007/s12325-018-0805-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock J., Rizvi S. A. A., Saleh A. M., Ahmed S. S., Do D. P., Ansari R. A., et al. (2018). Rheumatoid arthritis: a brief overview of the treatment. Med. Princ. Pract. 27, 501–507. 10.1159/000493390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzidionysiou K., Askling J., Eriksson J., Kristensen L. E., van Vollenhoven R. (2015). Effectiveness of TNF inhibitor switch in RA: results from the national Swedish register. Ann. Rheum. Dis. 74, 890–896. 10.1136/annrheumdis-2013-204714 [DOI] [PubMed] [Google Scholar]
- Choi S., Ghang B., Jeong S., Choi D., Lee J. S., Park S. M., et al. (2021). Association of first, second, and third-line bDMARDs and tsDMARD with drug survival among seropositive rheumatoid arthritis patients: cohort study in a real world setting. Semin. Arthritis Rheum. 51, 685–691. 10.1016/j.semarthrit.2021.06.002 [DOI] [PubMed] [Google Scholar]
- Christensen R., Tarp S., Furst D. E., Kristensen L. E., Bliddal H. (2013). Efficacy and safety of infliximab or adalimumab, versus abatacept, in patients with rheumatoid arthritis: ATTEST–AMPLE network randomized trial. Int. J. Clin. Rheumtol. 8, 647–655. 10.2217/ijr.13.62 [DOI] [Google Scholar]
- Curtis J. R., Chastek B., Becker L., Quach C., Harrison D. J., Yun H., et al. (2015). Cost and effectiveness of biologics for rheumatoid arthritis in a commercially insured population. J. Manag. Care Spec. Pharm. 21, 318–329. 10.18553/jmcp.2015.21.4.318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis J. R., Chakravarty S. D., Black S., Kafka S., Xu S., Langholff W., et al. (2021). Incidence of infusion reactions and clinical effectiveness of intravenous golimumab versus infliximab in patients with rheumatoid arthritis: the real-world AWARE study. Rheumatol. Ther. 8, 1551–1563. 10.1007/s40744-021-00354-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina K., Hirano T., Maeda Y., Yamamoto W., Hashimoto M., Murata K., et al. (2020a). Drug retention of 7 biologics and tofacitinib in biologics-naïve and biologics-switched patients with rheumatoid arthritis: the ANSWER cohort study. Arthritis Res. Ther. 22, 142. 10.1186/s13075-020-02232-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina K., Hirano T., Maeda Y., Yamamoto W., Hashimoto M., Murata K., et al. (2020b). Drug retention of secondary biologics or JAK inhibitors after tocilizumab or abatacept failure as first biologics in patients with rheumatoid arthritis -the ANSWER cohort study-. Clin. Rheumatol. 39, 2563–2572. 10.1007/s10067-020-05015-5 [DOI] [PubMed] [Google Scholar]
- England B. R., Tiong B. K., Bergman M. J., Curtis J. R., Kazi S., Mikuls T. R., et al. (2019). 2019 update of the American College of Rheumatology recommended rheumatoid arthritis disease activity measures. Arthritis Care Res. 71, 1540–1555. 10.1002/ACR.24042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann R., Mysler E., Hall S., Kivitz A. J., Moots R. J., Luo Z., et al. (2017). Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet 390, 457–468. 10.1016/S0140-6736(17)31618-5 [DOI] [PubMed] [Google Scholar]
- Gabay C., Emery P., van Vollenhoven R., Dikranian A., Alten R., Pavelka K., et al. (2013). Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet 381, 1541–1550. 10.1016/S0140-6736(13)60250-0 [DOI] [PubMed] [Google Scholar]
- Gharaibeh M., Bonafede M., McMorrow D., Maksabedian Hernandez E. J., Stolshek B. S., Hernandez E. J. M., et al. (2020). Effectiveness and costs among rheumatoid arthritis patients treated with targeted immunomodulators using real-world U.S. data. J. Manag. Care Spec. Pharm. 26, 1039–1049. 10.18553/jmcp.2020.26.8.1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulácsi L., Zrubka Z., Brodszky V., Rencz F., Alten R., Szekanecz Z., et al. (2019). Long-term efficacy of tumor necrosis factor inhibitors for the treatment of methotrexate-naïve rheumatoid arthritis: Systematic Literature review and meta-analysis. Adv. Ther. 36, 721–745. 10.1007/s12325-018-0869-8 [DOI] [PubMed] [Google Scholar]
- Guo Q., Wang Y., Xu D., Nossent J., Pavlos N. J., Xu J., et al. (2018). Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 6, 15. 10.1038/S41413-018-0016-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrold L. R., Reed G. W., Kremer J. M., Curtis J. R., Solomon D. H., Hochberg M. C., et al. (2015). The comparative effectiveness of abatacept versus anti-tumour necrosis factor switching for rheumatoid arthritis patients previously treated with an anti-tumour necrosis factor. Ann. Rheum. Dis. 74, 430–436. 10.1136/ANNRHEUMDIS-2013-203936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison P., Hay-Smith J., Gillespie W. J. (2011). Meta-analyses of small numbers of trials often agree with longer-term results. J. Clin. Epidemiol. 64, 145–153. 10.1016/j.jclinepi.2010.02.017 [DOI] [PubMed] [Google Scholar]
- Hetland M. L., Christensen I. J., Tarp U., Dreyer L., Hansen A., Hansen I. T., et al. (2010). Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. 62, 22–32. 10.1002/art.27227 [DOI] [PubMed] [Google Scholar]
- Higgins J. P. T. T., Thompson S. G., Deeks J. J., Altman D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327, 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C. T. K., Mok C. C., Cheung T. T., Kwok K. Y., Yip R. M. L. (2019). Management of rheumatoid arthritis: 2019 updated consensus recommendations from the Hong Kong society of Rheumatology. Clin. Rheumatol. 38, 3331–3350. 10.1007/S10067-019-04761-5 [DOI] [PubMed] [Google Scholar]
- Keystone E. C., Taylor P. C., Tanaka Y., Gaich C., DeLozier A. M., Dudek A., et al. (2017). Patient-reported outcomes from a phase 3 study of baricitinib versus placebo or adalimumab in rheumatoid arthritis: secondary analyses from the RA-BEAM study. Ann. Rheum. Dis. 76, 1853–1861. 10.1136/annrheumdis-2017-211259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara M., Davies R., Kearsley-Fleet L., Watson K. D., Lunt M., Symmons D. P. M. M., et al. (2017). Use and effectiveness of tocilizumab among patients with rheumatoid arthritis: an observational study from the British society for Rheumatology biologics register for rheumatoid arthritis. Clin. Rheumatol. 36, 241–250. 10.1007/s10067-016-3485-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauper K., Nordström D. C., Pavelka K., Hernández M. V., Kvien T. K., Kristianslund E. K., et al. (2018). Comparative effectiveness of tocilizumab versus TNF inhibitors as monotherapy or in combination with conventional synthetic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis after the use of at least one biologic disease-modifying antirheumatic drug: analyses from the pan-European TOCERRA register collaboration. Ann. Rheum. Dis. 77, 1276–1282. 10.1136/ANNRHEUMDIS-2017-212845 [DOI] [PubMed] [Google Scholar]
- Lee Y. H., Woo J. H., Rho Y. H., Choi S. J., Ji J. D., Song G. G., et al. (2008). Meta-analysis of the combination of TNF inhibitors plus MTX compared to MTX monotherapy, and the adjusted indirect comparison of TNF inhibitors in patients suffering from active rheumatoid arthritis. Rheumatol. Int. 28, 553–559. 10.1007/s00296-007-0475-6 [DOI] [PubMed] [Google Scholar]
- Li K.-J. K. J., Chang C.-L. C.-L. L., Hsin C.-Y. Y. C.-Y. Y., Tang C.-H. H. C.-H. (2021). Switching and discontinuation pattern of biologic disease-modifying antirheumatic drugs and tofacitinib for patients with rheumatoid arthritis in taiwan. Front. Pharmacol. 12, 628548. 10.3389/fphar.2021.628548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.-J., Anzaghe M., Schülke S. (2020). Update on the pathomechanism, diagnosis, and treatment options for rheumatoid arthritis. Cells 9, 880. 10.3390/cells9040880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listing J., Kekow J., Manger B., Burmester G.-R., Pattloch D., Zink A., et al. (2015). Mortality in rheumatoid arthritis: the impact of disease activity, treatment with glucocorticoids, TNFα inhibitors and rituximab. Ann. Rheum. Dis. 74, 415–421. 10.1136/annrheumdis-2013-204021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Xu S. (2013). TNF inhibitor therapy for rheumatoid arthritis. Biomed. Rep. 1, 177–184. 10.3892/br.2012.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur M. B., VanderWeele T. J. (2020). Sensitivity analysis for unmeasured confounding in meta-analyses. J. Am. Stat. Assoc. 115, 163–172. 10.1080/01621459.2018.1529598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S., Yoshitama T., Ueki Y. (2018). Tofacitinib therapy for rheumatoid arthritis: A direct comparison study between biologic-naïve and experienced patients. Intern. Med. 57, 663–670. 10.2169/internalmedicine.9341-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neovius M., Arkema E. V., Olsson H., Eriksson J. K., Kristensen L. E., Simard J. F., et al. (2015). Drug survival on TNF inhibitors in patients with rheumatoid arthritis comparison of adalimumab, etanercept and infliximab. Ann. Rheum. Dis. 74, 354–360. 10.1136/ANNRHEUMDIS-2013-204128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østergaard M., Unkerskov J., Linde L., Krogh N. S., Ravn T., Ringsdal V. S., et al. (2007). Low remission rates but long drug survival in rheumatoid arthritis patients treated with infliximab or etanercept: results from the nationwide Danish DANBIO database. Scand. J. Rheumatol. 36, 151–154. 10.1080/03009740601089267 [DOI] [PubMed] [Google Scholar]
- Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. (2016). Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 5, 210. 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M. J., Higgins J. P., Sterne J. A., Jpt H. (2020). “Chapter 13: Assessing risk of bias due to missing results in a synthesis,” in Cochrane handbook for systematic reviews of interventions version 6.1 (updated september 2020). Editors Higgins J., Thomas J., Chandler J., Cumpston M., Li T., Page M. (Cochrane; ). Available at: www.training.cochrane.org/handbook . [Google Scholar]
- Page M. J., McKenzie J. E., Bossuyt P. M., Boutron I., Hoffmann T. C., Mulrow C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst. Rev. 10, n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas D. A., Litman H. J., Lesperance T., Kricorian G., Karis E., Rebello S., et al. (2021a). Persistence on biologic DMARD monotherapy after achieving rheumatoid arthritis disease control on combination therapy: retrospective analysis of corrona registry data. Rheumatol. Int. 41, 381–390. 10.1007/s00296-020-04667-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas D. A., St John G., Etzel C. J., Fiore S., Blachley T., Kimura T., et al. (2021b). Comparative effectiveness of first-line tumour necrosis factor inhibitor versus non-tumour necrosis factor inhibitor biologics and targeted synthetic agents in patients with rheumatoid arthritis: results from a large US registry study. Ann. Rheum. Dis. 80, 96–102. 10.1136/annrheumdis-2020-217209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul D., Fazeli M. S., Mintzer L., Duarte L., Gupta K., Ferri L., et al. (2020). Comparative efficacy and safety of current therapies for early rheumatoid arthritis: A systematic literature review and network meta-analysis. Clin. Exp. Rheumatol. 38, (38, ), 1008–1015. [PubMed] [Google Scholar]
- Pelechas E., Voulgari P., Drosos A. (2019). Golimumab for rheumatoid arthritis. J. Clin. Med. 8, 387. 10.3390/jcm8030387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pithon M. M., Baião F. C. S., Sant Anna L. I. D. de A., Paranhos L. R., Cople Maia L. (2019). Assessment of the effectiveness of invisible aligners compared with conventional appliance in aesthetic and functional orthodontic treatment: A systematic review. J. Investig. Clin. Dent. 10, e12455. 10.1111/jicd.12455 [DOI] [PubMed] [Google Scholar]
- Rahman P., Baer P., Keystone E., Choquette D., Thorne C., Haraoui B., et al. (2020). Long-term effectiveness and safety of infliximab, golimumab and golimumab-IV in rheumatoid arthritis patients from a Canadian prospective observational registry. BMC Rheumatol. 4, 46. 10.1186/s41927-020-00145-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz S., Adams R., Walsh C. D., Barry M., FitzGerald O. (2012). A mixed treatment comparison of the efficacy of anti-TNF agents in rheumatoid arthritis for methotrexate non-responders demonstrates differences between treatments: a bayesian approach. Ann. Rheum. Dis. 71, 225–230. 10.1136/annrheumdis-2011-200228 [DOI] [PubMed] [Google Scholar]
- Silvagni E., Bortoluzzi A., Carrara G., Zanetti A., Govoni M., Scirè C. A., et al. (2018). Comparative effectiveness of first-line biological monotherapy use in rheumatoid arthritis: A retrospective analysis of the RECord-linkage on rheumatic diseases study on health care administrative databases. BMJ Open 8, e021447. 10.1136/BMJOPEN-2017-021447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slim K., Nini E., Forestier D., Kwiatkowski F., Panis Y., Chipponi J., et al. (2003). Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J. Surg. 73, 712–716. 10.1046/j.1445-2197.2003.02748.x [DOI] [PubMed] [Google Scholar]
- Smolen J. S., Kay J., Doyle M. K., Landewé R., Matteson E. L., Wollenhaupt J., et al. (2009). Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor α inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet 374, 210–221. 10.1016/S0140-6736(09)60506-7 [DOI] [PubMed] [Google Scholar]
- Smolen J. S., Landewé R. B. M., Bijlsma J. W. J., Burmester G. R., Dougados M., Kerschbaumer A., et al. (2020). EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 79, 685–699. 10.1136/ANNRHEUMDIS-2019-216655 [DOI] [PubMed] [Google Scholar]
- Soliman M. M., Ashcroft D. M., Watson K. D., Lunt M., Symmons D. P. M., Hyrich K. L. (2011). Impact of concomitant use of DMARDs on the persistence with anti-TNF therapies in patients with rheumatoid arthritis: results from the British society for Rheumatology biologics register. Ann. Rheum. Dis. 70, 583–589. 10.1136/ard.2010.139774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung Y.-K., Lee Y. H. (2021). Comparison of the efficacy and safety of tocilizumab, sarilumab, and sirukumab in comparison with adalimumab as monotherapy in patients with active rheumatoid arthritis: A bayesian network meta-analysis of randomized controlled trials. Int. J. Clin. Pharmacol. Ther. 59, 618–626. 10.5414/CP204017 [DOI] [PubMed] [Google Scholar]
- Taylor P. C., Keystone E. C., van der Heijde D., Weinblatt M. E., del Carmen Morales L., Reyes Gonzaga J., et al. (2017). Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N. Engl. J. Med. 376, 652–662. 10.1056/NEJMoa1608345 [DOI] [PubMed] [Google Scholar]
- Wakabayashi H., Oka H., Nishioka Y., Hasegawa M., Sudo A., Nishioka K., et al. (2011). Do biologics-naïve patients with rheumatoid arthritis respond better to tocilizumab than patients for whom anti-TNF agents have failed? a retrospective study. Clin. Exp. Rheumatol. 29, 314–317. [PubMed] [Google Scholar]
- Weinblatt M. E., Schiff M., Valente R., Van Der Heijde D., Citera G., Zhao C., et al. (2013). Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: findings of a phase IIIb, multinational, prospective, randomized study. Arthritis Rheum. 65, 28–38. 10.1002/ART.37711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef P., Marcal B., Button P., Truman M., Bird P., Griffiths H., et al. (2020). Reasons for biologic and targeted synthetic disease-modifying antirheumatic drug cessation and persistence of second-line treatment in a rheumatoid arthritis dataset. J. Rheumatol. 47, 1174–1181. 10.3899/jrheum.190535 [DOI] [PubMed] [Google Scholar]
- Yun H., Xie F., Delzell E., Chen L., Levitan E. B., Lewis J. D., et al. (2014). Risk of hospitalised infection in rheumatoid arthritis patients receiving biologics following a previous infection while on treatment with anti-TNF therapy. Ann. Rheum. Dis. 74, 1065–1071. 10.1136/annrheumdis-2013-204011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun H., Xie F., Delzell E., Chen L., Yang S., Saag K. G., et al. (2015). The comparative effectiveness of biologics among older adults and disabled rheumatoid arthritis patients in the Medicare population. Br. J. Clin. Pharmacol. 80, 1447–1457. 10.1111/bcp.12709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun H., Xie F., Delzell E., Levitan E. B., Chen L., Lewis J. D., et al. (2016). Comparative risk of hospitalized infection associated with biologic agents in rheumatoid arthritis patients enrolled in medicare. Arthritis Rheumatol. 68, 56–66. 10.1002/art.39399 [DOI] [PubMed] [Google Scholar]
- Zhang J., Xie F., Yun H., Chen L., Muntner P., Levitan E. B., et al. (2016). Comparative effects of biologics on cardiovascular risk among older patients with rheumatoid arthritis. Ann. Rheum. Dis. 75, 1813–1818. 10.1136/annrheumdis-2015-207870 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.