Abstract
In Escherichia coli, the Cpx two-component regulatory system activates expression of protein folding and degrading factors in response to misfolded proteins in the bacterial envelope (inner membrane, periplasm, and outer membrane). It is comprised of the histidine kinase CpxA and the response regulator CpxR. This response plays a role in protection from stresses, such as elevated pH, as well as in the biogenesis of virulence factors. Here, we show that the Cpx periplasmic stress response is subject to amplification and repression through positive and negative autofeedback mechanisms. Western blot and operon fusion analyses demonstrated that the cpxRA operon is autoactivated. Conditions that lead to elevated levels of phosphorylated CpxR cause a concomitant increase in transcription of cpxRA. Conversely, overproduction of CpxP, a small, Cpx-regulated protein of previously unknown function, represses the regulon and can block activation of the pathway. This repression is dependent on an intact CpxA sensing domain. The ability to autoactivate and then subsequently repress allows for a temporary amplification of the Cpx response that may be important in rescuing cells from transitory stresses and cueing the appropriately timed elaboration of virulence factors.
All bacteria require an intact envelope for survival. In gram-negative bacteria, the envelope includes the inner membrane, periplasm, and outer membrane, and it is involved in a large number of diverse structural, physiological, and adaptive processes. These processes generally require specific sets of envelope-associated proteins and include functions such as active and passive transport, virulence factor elaboration, and cell division. Given its essential role, it is not surprising that insults to this compartment are detected by at least two different envelope stress responses in Escherichia coli, the ςE and Cpx signal transduction pathways. Although both pathways cause elevated expression of envelope-localized protein folding and degrading factors in response to misfolded proteins, each response has unique sets of inducing signals and downstream targets, suggesting distinct physiological roles (for a review, see reference 40).
The ςE stress response appears to play an essential role in outer membrane protein (OMP) folding. Activation of the pathway is triggered by misfolded OMPs (30) and leads to elevated production of at least two factors involved in the folding and degradation of such substrates (6, 7, 12, 27), the peptidyl-prolyl-isomerase FkpA (15, 31) and the periplasmic protease DegP (4, 22, 26, 28, 49, 53, 54). Activating signals are transduced mainly by relief of an inhibitory interaction between a membrane-localized anti-sigma factor, RseA, and the transcription factor, ςE, allowing for activation of expression of downstream targets (10, 32).
The Cpx envelope stress response is mediated by a typical two-component regulatory system consisting of the membrane-localized sensor histidine kinase (HK) CpxA and the cytoplasmic response regulator CpxR (RR) (11, 62). CpxA responds to envelope stresses through autophosphorylation, likely at a conserved histidine residue, and subsequent phosphotransfer to CpxR (39). As with other RRs, this phosphorylation probably occurs at a conserved aspartate residue. Phosphorylation allows CpxR to function as a transcriptional activator of genes whose products are involved in protein folding and degradation in the bacterial envelope (6, 7, 9, 36). Footprint analysis suggests that this occurs through binding of phosphorylated CpxR dimers to a conserved direct repeat motif upstream of Cpx-regulated promoters (36). These include the disulfide oxidase DsbA (1, 20); the peptidyl-prolyl-isomerases PpiA (29) and PpiD (9); the protease DegP (22, 26, 28, 53, 54); a small periplasmic protein of unknown function, CpxP (8); and other, as-yet-unidentified regulon members (36).
Discerning the physiological role of the Cpx envelope stress response has not been straightforward, since a variety of envelope perturbations lead to activation of the pathway. It is clear that the Cpx pathway helps protect the cell from potentially toxic, transitory stresses. For example, the Cpx envelope stress response is induced by elevated pH, and cpx mutants exhibit reduced survival in alkaline environments (8, 33). Similarly, activation of the Cpx pathway can rescue the cell from the expression of potentially toxic mutant envelope proteins (4, 49). It is likely that elevated expression of Cpx-regulated factors is necessary under such conditions to maintain proper protein folding in the bacterial envelope and thus the integrity of the cell.
In addition to its role in protection from envelope stress, several observations suggest that the Cpx envelope stress response plays an important role in the virulence of pathogenic organisms. For example, DsbA is required for the correct folding of a number of pathogenic determinants (17, 34, 61, 64) and degP null mutants are avirulent (18). Further, VirF, a transcriptional activator of genes whose products are necessary for host cell invasion by Shigella species, is a member of the Cpx regulon (33). Finally, the Cpx envelope stress response is centrally involved in monitoring and assisting in the assembly of P pili. A number of Cpx-regulated factors, including DsbA and DegP, are required for the assembly of these extracellular appendages on the surface of uropathogenic Escherichia coli and misfolded pilin subunits lead to activation of the Cpx response (17, 19). Thus, one major role of the Cpx envelope stress response appears to be to monitor and assist in the assembly of pili, and possibly other virulence factors.
The appropriately timed activation of the Cpx stress response is therefore important for survival in a number of situations. In light of this, we are interested in how the Cpx pathway senses and responds to envelope stress. Previously, a domain required for sensing envelope stress was identified in the periplasmic region of the CpxA sensor kinase (39). Since activating mutations alter or remove this domain, we proposed that under nonstressed conditions this periplasmic region mediates a negative or downregulatory effect on the kinase, possibly through interactions with a second signaling molecule. Here, we show that elevated expression of the small, periplasmic, Cpx-regulated molecule CpxP leads to downregulation of the Cpx pathway and that this is mediated via the CpxA sensing domain. In the course of these studies, we noted that, in addition to feedback inhibition, the Cpx regulon is subject to autoactivation. Western blot and lacZ fusion analyses demonstrated that the cpxRA operon is itself a member of the Cpx regulon. We propose that the ability to rapidly amplify and subsequently shut down the Cpx signal transduction pathway is important for rescuing cells from potentially toxic, transitory envelope stresses, as well as for mediating the correctly timed biogenesis of virulence factors.
MATERIALS AND METHODS
Bacterial strains.
Bacterial strains used in this study are described in Table 1. Isogenic cpxA* strains were constructed by using P1 transduction (45) and the tightly linked zii::Tn10 marker. Control experiments showed that this marker had no effect on the Cpx signal transduction pathway. All other strains were constructed by standard genetic techniques (45).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype | Source and/or reference |
|---|---|---|
| Strains | ||
| JHC285 | MC4100 lamBA23D zjb::Tn10 | 2 |
| MC4100 | F−araD139 Δ(argF-lac)U169 rpsL 150(Strr) relA1 flbB5301 deoC1 ptsF25 rbsR | 3 |
| TR8 | MC4100 cpxA1::cam | This study; 6 |
| TR10 | MC4100 cpxA24 | This study; 39 |
| TR36 | MC4100 λRS88[cpxP-lacZ] cpxA102 | This study |
| TR48 | MC4100 λRS88[cpxP-lacZ] cpxA101 | 39 |
| TR49 | MC4100 λRS88[degP-lacZ] | 6, 39 |
| TR50 | MC4100 λRS88[cpxP-lacZ] | 8, 39 |
| TR51 | MC4100 cpxR1::spc | This study; 6 |
| TR68 | MC4100 λRS88[cpxP-lacZ] cpxA1::cam | 6, 39 |
| TR143 | MC4100 Φ[lamB-lacZ-phoA] Hyb1-1 [λp1(209)] | This study; 50 |
| TR146 | MC4100 lamBA23D zjb::Tn10 cpxA1::cam | This study |
| TR147 | MC4100 Φ[lamB-lacZ-phoA] cpxA1::cam Hyb1-1 [λp1(209)] | This study |
| TR235 | MC4100 λRS88[cpxR-lacZ] | This study |
| TR237 | MC4100 λRS88[cpxR-lacZ] cpxR1::spc | This study; 6 |
| TR238 | MC4100 λRS88[cpxR-lacZ] cpxA1::cam | This study; 6 |
| TR239 | MC4100 λRS88[cpxR-lacZ] zii::Tn10 | This study |
| TR240 | MC4100 λRS88[cpxR-lacZ] zii::Tn10 cpxA24 | This study; 4 |
| TR241 | MC4100 λRS88[cpxR-lacZ] zii::Tn10 cpxA101 | This study; 4 |
| TR242 | MC4100 λRS88[cpxR-lacZ] zii::Tn10 cpxA711 | This study; 4 |
| TR243 | TR235(pBR322) | This study |
| TR244 | TR235(pLD404) | This study; 49 |
| TR412 | MC4100 λRS88[degP-lacZ] rffA::cam | This study; 5 |
| TR413 | MC4100 λRS88[cpxP-lacZ] rffA::cam | This study; 5 |
| TR493 | MC4100 λRS88[degP-lacZ] ΔcpxP2 metFTn10 | This study |
| TR494 | MC4100 λRS88[cpxP-lacZ] ΔcpxP2 metF Tn10 | This study |
| TR499 | MC4100 λRS88[degP-lacZ] ara−R::Tncam (pBAD18) | This study |
| TR500 | MC4100 λRS88[degP-lacZ] ara−R::Tncam (pND18) | This study |
| TR501 | MC4100 λRS88[cpxP-lacZ] ara−R::Tncam (pBAD18) | This study |
| TR502 | MC4100 λRS88[cpxP-lacZ] ara−R::Tncam(pND18) | This study |
| TR503 | MC4100 λRS88[degP-lacZ] ΔcpxP2 ara−R::Tncam (pBAD18) metF Tn10 | This study |
| TR504 | MC4100 λRS88[degP-lacZ] ΔcpxP2 ara−R::Tncam (pND18) metF Tn10 | This study |
| TR505 | MC4100 λRS88[cpxP-lacZ] ΔcpxP2 ara−R::Tncam (pBAD18) metF Tn10 | This study |
| TR506 | MC4100 λRS88[cpxP-lacZ] ΔcpxP2 ara−R::Tncam (pND18) metF Tn10 | This study |
| Plasmids | ||
| pBAD18 | Cloning vector containing PBAD for arabinose inducible expression of inserts | 13 |
| pBR322 | Cloning vector; parent of pLD404 | New England Biolabs |
| pCMCP | Overexpression vector for a cytoplasmic MBP-CpxP fusion protein | This study |
| pCpxP | CpxP overexpression plasmid | This study |
| pLD404 | NlpE overexpression plasmid | 49 |
| pMal-c2 | Cloning vector for making cytoplasmic MBP fusion proteins; parent of pCMCP | New England Biolabs |
| pMal-p2 | Cloning vector for making periplasmic MBP fusion proteins; parent of pMCP | New England Biolabs |
| pMCP | Overexpression vector for a periplasmic MBP-CpxP fusion protein | This study |
| pND18 | Arabinose inducible NlpE overexpression vector | 6 |
| ptrc99A | Cloning vector; parent of pCpxP | New England Biolabs |
Media and chemicals.
Unless otherwise indicated, all strains were grown on Luria-Bertani (LB) broth or plates at 30°C. cpx* alleles conferring constitutive activation of the Cpx signal transduction cascade confer a number of pleiotropic phenotypes, including resistance to amikacin. Since the strongest cpx* alleles revert at a relatively high rate (38a), these mutant strains were routinely grown in the presence of 3 μg of amikacin (Sigma) per ml to prevent the outgrowth of spontaneous revertants. Strains transformed with plasmids were grown in the presence of 100 μg of ampicillin (Sigma) per ml.
Cell fractionation techniques.
Overnight cultures of the indicated strains were diluted 1:50 into fresh medium and grown to mid-log phase. For whole-cell lysates, 1 ml of cells was pelleted in a microcentrifuge, resuspended in 50 μl of 1× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer (62.5 mM Tris [pH 6.8], 10% glycerol, 5% β-mercaptoethanol, 3% SDS, 0.1% bromophenol blue), and frozen at −20°C until use. Typically, 10 to 20 μl of whole-cell lysate were used in Western blot analysis.
Membranes were prepared from 10-ml cultures of mid-log-phase cells. Bacteria were pelleted in a clinical centrifuge, washed once with 5 ml of lysis buffer (0.1 M KH2PO4, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride [PMSF], 5 mM EDTA, 0.27% β-mercaptoethanol [pH 7.0]), and subjected to one cycle of freeze-thawing. The thawed pellet was resuspended in 1 ml of lysis buffer and sonicated three to four times in 15-s bursts. Whole cells and debris were removed by centrifugation at 6,500 rpm in a microcentrifuge for 10 min. Membranes were isolated from the supernatant by centrifugation at 100,000 rpm for 20 min in a benchtop ultracentrifuge (Beckman). The pellet, containing the membranes, was resuspended in 1 ml of wash buffer (40 mM HEPES, 10% glycerol, 1 mM PMSF, 5 mM EDTA, 0.27% β-mercaptoethanol [pH 8.0]) by sonication and recentrifuged at 100,000 rpm for 20 min. The final membrane pellet was resuspended in 100 μl of wash buffer by sonication and stored at −20°C. Typically, 5 μl of membrane preparation were used in further analysis.
Western blot analysis.
When necessary, an equivalent sample volume of 2× SDS-PAGE loading buffer (125 mM Tris [pH 6.8], 20% glycerol, 10% β-mercaptoethanol, 6% SDS, 0.2% bromophenol blue) was added to samples prior to boiling. Whole-cell lysates and membrane preparations were boiled for 5 min and then electrophoresed on an SDS–10% PAGE minigel system (Bio-Rad) by the technique of Laemmli (23). Proteins were transferred to nitrocellulose membranes employing the methods of Towbin et al. (59). Transfer was carried out at 100 V for 1 h. Nonspecific protein interactions were blocked by incubation in 3% MS (3% powdered milk, 0.9% NaCl, 10 mM Tris-Cl [pH 7.5]) for 1 h at room temperature or overnight at 4°C with shaking. This was followed by a 1-h incubation with a 1:5,000 dilution of polyclonal antisera raised against either a maltose-binding protein (MBP)–CpxR or MBP-CpxA fusion (39) in 3% MS at 37°C. Blots were washed repeatedly by shaking in WS (0.4% Tween, 0.9% NaCl, 10 mM Tris-Cl [pH 7.5]) at room temperature. Secondary anti-rabbit immunoglobulin G-horseradish peroxidase conjugate (Sigma) was used at a 1:10,000 dilution in 3% MS for 1 h at 37°C with shaking to detect immune complexes. After another series of washes in WS, proteins were visualized by using a chemiluminescence kit according to the specifications of the manufacturer (Amersham).
Construction of a single-copy cpxR-lacZ fusion.
The cpxR-lacZ fusion was constructed and integrated into the chromosome in single copy by the technique of Simons et al. (46). Briefly, the cpxR promoter and upstream sequences were PCR amplified from the chromosome of MC4100 with the restriction-tagged primers ERIcpxUP (5′-GGA ATT CCG GCA GCG GTA ACT ATG CG C-3′) and BHIcpxDN (5′-CGG GAT CCC GGG GAA GTC AGC TCT CGG TCA-3′). The resultant PCR product was purified (Qiagen), digested with EcoRI and BamHI (New England Biolabs), and cloned upstream of the promoterless lacZ gene of pRS415 (46). The resultant cpxR-lacZ fusion was recombined onto λRS88 and integrated into the chromosome in single copy at the λatt locus as previously described (46).
Construction of CpxP overexpression vectors.
Three different CpxP overexpression plasmids were used in this study (Table 1). pCpxP contains the entire cpxP open reading frame and translational start site cloned behind the tac promoter of ptrc99A (Pharmacia). The cpxP gene was PCR amplified from the chromosome of MC4100 by using the restriction-tagged primers cpxP5′Eco (5′-GGA ATT CCC TCT CTA TCG TTG AAT CGC G-3′) and 167-7946 (5′-CCC AAG CTT GGG CCG TTC CTT TTG TCC CAA ATG ATG ACC-3′), purified, digested with EcoRI and HindIII, and cloned behind the trc promoter in ptrc99A (Pharmacia).
pMCP encodes an MBP-CpxP fusion protein that is expressed from the tac promoter and localized to the periplasm. It was constructed by PCR amplification of the portion of the cpxP open reading frame encoding the mature protein (i.e., minus the signal sequence) from the chromosome of MC4100 by using the restriction-tagged primers cpxP5′EcoRI (5′-GGA ATT CCC ACG CTG CTG AAG TCG GTT CAG GC-3′) and 167-7946 (see above). The PCR product was purified, digested with EcoRI and HindIII, and cloned into the same sites in the vector pMal-p2 (New England Biolabs) to yield pMCP.
pCMCP is the same as pMCP except that it encodes an MBP-CpxP fusion protein that is localized to the cytoplasm due to a deletion of the malE signal sequence on the parent vector, pMal-c2 (New England Biolabs). It was constructed exactly as pMCP was except that the digested PCR product was cloned into the multiple cloning site of the pMal-c2 vector.
β-Galactosidase assays.
Single colonies of each bacterial strain to be assayed were inoculated into 2-ml overnight cultures of LB broth containing the appropriate antibiotics and grown overnight at 30°C with aeration. The next day, the cultures were diluted 1:40 into the same medium and grown at 30°C to late log phase. β-Galactosidase activity was measured by using a microtiter plate assay (48). All assays were performed in triplicate.
Maltose sensitivity disc assays.
Strains to be assayed were grown to saturation in M63 minimal medium (45) containing 0.4% glycerol. Bacteria were pelleted in a benchtop clinical centrifuge and resuspended in an equal volume of M63 salts solution. Then, 100 μl of cells was mixed with 2.5 ml of molten F-top agar and spread uniformly over the surface of a M63-glycerol plate. A sterile filter paper disc was placed on the surface of the plate and saturated with 10 μl of 10 or 20% maltose. After incubation at 30°C overnight, the maltose sensitivity was measured as the diameter of the zone of growth inhibition surrounding the disc.
Construction of a cpxP null mutant.
A cpxP null was created by transferring an out-of-frame deletion within the cpxP open reading frame encoded on pND27 (8) to the chromosome by using the allelic exchange protocol of Hamilton et al. (14). The resulting strain encodes a truncated CpxP protein that removes approximately two-thirds of the C terminus of the wild-type protein. This allele was shown through diploid analysis to be a loss-of-function mutation (data not shown).
RESULTS
The cpxRA operon is autoactivated.
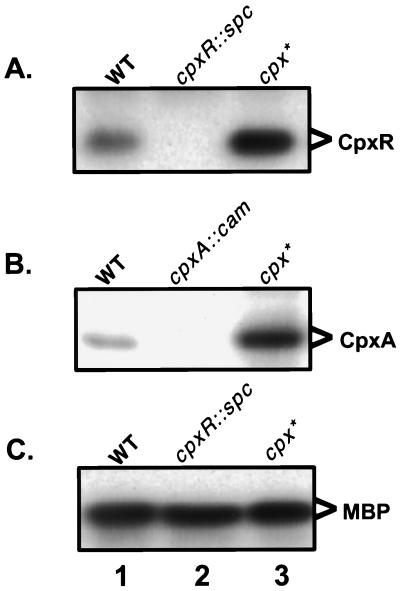
In the course of developing antisera against CpxR and CpxA, we noted that the levels of these proteins were elevated in a cpxA* background (Fig. 1). cpxA* mutations, such as cpxA24, cpxA101, cpxA102, and cpxA711, are a class of dominant alleles that lead to constitutive activation of the Cpx signal transduction pathway (39). Western blots were performed on either whole-cell lysates or membrane fractions derived from wild-type, mutant cpxR, mutant cpxA, and cpxA24 backgrounds utilizing polyclonal antisera directed against either a MBP-CpxR or MBP-CpxA recombinant fusion protein (Fig. 1). CpxR and CpxA were present at low levels in a wild-type background compared to the relatively abundant internal loading control maltose binding protein (MBP). As expected, CpxR and CpxA were absent in the cpxR and cpxA disruption null mutants, respectively. Interestingly, however, the levels of both proteins were markedly elevated in a constitutively activated cpx* background (Fig. 1).
FIG. 1.
Levels of CpxR and CpxA are elevated in a constitutively activated cpx* background. Western blots were performed on whole-cell lysates (A and C) or membrane fractions (B) of MC4100 (lane 1), TR51 (A and C, lane 2), TR8 (B, lane 2), or TR10 (lane 3) bacteria by using polyclonal antisera directed against MBP-CpxR (A and C) or MBP-CpxA (B) fusion proteins.
Inspection of the DNA sequence upstream of the cpxRA operon suggested that the elevated levels of CpxR and CpxA proteins observed in the constitutively activated cpx* background might be due to autoregulation. As first noted by Pogliano et al. (36), a CpxR consensus binding motif of 5′-GTAAA(N5)GTAAA-3′ is found directly upstream of the cpxRA operon. The sequence and spacing of this binding site from the −35 region of the promoter is virtually identical to those of the known Cpx-regulated genes dsbA and ppiA (36).
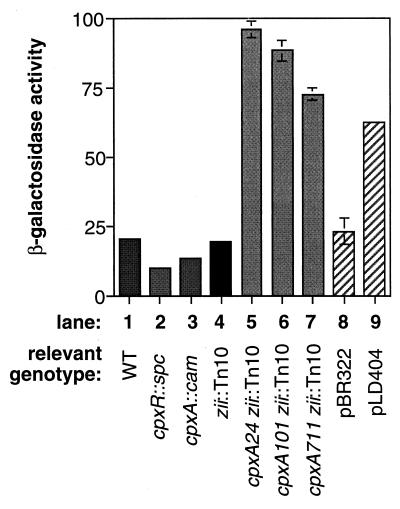
To investigate whether transcriptional autoregulation contributed to the elevated levels of CpxR and CpxA in the cpx* background, transcriptional fusions were constructed between the upstream region of the cpxRA operon and a promoterless lacZ gene and placed in single copy on the chromosome as part of a recombinant λ phage. The cpxR-lacZ operon fusion was placed in several genetic backgrounds and β-galactosidase activity was measured to assess the effect of either deleting or activating the Cpx pathway. Both the cpxR::spc and cpxA::cam disruption null mutations resulted in diminished expression of the cpxR-lacZ fusion (Fig. 2, compare column 1 to columns 2 and 3). Both of these mutations lower the concentration of phosphorylated CpxR in the cell (6), cpxR::spc by eliminating CpxR and cpxA::cam by removing the kinase, CpxA. Interestingly, basal-level expression of the cpxRA operon is not dependent on phosphorylated CpxR, as low levels of expression are seen even in a cpxR mutant background (Fig. 2, column 3).
FIG. 2.
Expression of a cpxR-lacZ operon fusion is autoregulated. Levels of β-galactosidase were assayed in strains lysogenized with a λ phage carrying a cpxR-lacZ operon fusion. Columns: 1, TR235; 2, TR237; 3, TR238; 4, TR239; 5, TR240; 6, TR241; 7, TR242; 8, TR235(pBR322); 9, TR235(pLD404).
In contrast, conditions which lead to elevated levels of phosphorylated CpxR in the cell enhance the expression of the cpxR-lacZ fusion (Fig. 2). In the presence of any of three different constitutively activated cpxA* alleles, cpxR-lacZ expression was enhanced between three- and fivefold (Fig. 2, compare column 1 with columns 5 to 7). Further, the relative increase in cpxR-lacZ expression in these strains correlated with the known strengths of the cpx* alleles (4, 6). More significantly, overproduction of the novel outer membrane lipoprotein, NlpE, a known Cpx-activating signal (49), caused an approximately threefold increase in expression of cpxR-lacZ (Fig. 2, compare columns 8 and 9). Thus, like other members of the Cpx regulon, expression of the cpxRA operon is elevated in response to stresses to the bacterial envelope which are sensed and transduced by the wild-type signal transduction machinery. This is an important distinction from the increase in expression conferred by the cpx* alleles, since these mutant genes confer a large number of pleiotropic phenotypes on the cell (4). Cumulatively, the data lead us to conclude that the cpxRA operon is itself a member of the Cpx regulon and thus subject to transcriptional autoactivation.
Overexpression of CpxP downregulates the Cpx regulon.
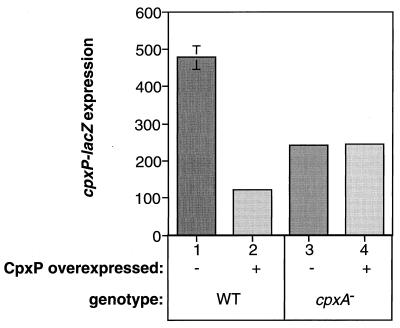
Since alterations to or removal of the CpxA periplasmic sensing domain lead to constitutive activation of the pathway, we have proposed that this region of the HK might mediate a downregulatory or negative effect, under nonstressed conditions, possibly through interaction with another periplasmic signaling molecule (39). This type of interaction occurs in other stress responses and is usually mediated by a regulon member (10, 25, 32, 57, 58). Accordingly, we tested the ability of CpxP, a small periplasmic Cpx-regulated protein of unknown function (8), to repress Cpx-mediated gene expression. The cpxP open reading frame was cloned downstream of the highly expressed, IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible trc promoter on ptrc99 and transformed into cells carrying a cpxP-lacZ fusion in single copy on the chromosome. β-Galactosidase activity in these transformants was approximately fivefold lower than that in the same strain transformed with the parent plasmid (Fig. 3, compare columns 1 and 2). Induction with IPTG caused no further decrease in cpxP-lacZ expression, suggesting that the amount of CpxP expressed from the trc promoter under uninduced conditions is sufficient for the maximum level of repression (data not shown).
FIG. 3.
Overexpression of CpxP downregulates Cpx-mediated gene expression in a CpxA-dependent fashion. β-Galactosidase produced from a cpxP-lacZ fusion was measured as a reporter of Cpx-mediated gene expression in TR50(ptrc99A) (column 1), TR50(pCpxP) (column 2), TR68(ptrc99A) (column 3), and TR68(pCpxP) (column 4).
As a first step towards determining whether CpxP might be a negatively acting signaling molecule for the Cpx regulon, we tested whether this downregulatory effect on Cpx-mediated gene expression was dependent on the HK CpxA. The cpxA1::cam disruption null allele was moved into the cpxP-lacZ reporter strain transformed with either the CpxP overexpression plasmid pCpxP or the parent vector ptrc99, and the β-galactosidase activity was assayed. In the absence of CpxA, cpxP-lacZ expression was decreased approximately twofold relative to the parent wild-type control strain in both transformants (Fig. 3, compare column 1 with columns 3 and 4), and overexpression of CpxP no longer caused diminished Cpx-mediated gene expression (Fig. 3, compare columns 3 and 4). Thus, overexpression of CpxP in a wild-type background leads to repression of Cpx-mediated gene expression in a CpxA-dependent fashion.
CpxP-mediated repression is signaled from the periplasm via the CpxA sensing domain.
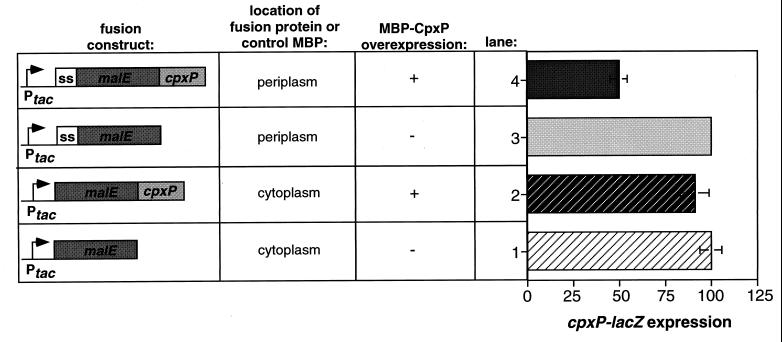
Since CpxP has been localized to the periplasm (8), it seemed probable that its downregulatory effects on the Cpx regulon were also exerted from this compartment. To confirm this supposition, we compared the effects of overexpressing either a cytoplasmic or periplasmic MBP-CpxP fusion protein on cpxP-lacZ expression (Fig. 4). As previously observed with the native CpxP protein, overexpression of a periplasmic MBP-CpxP fusion protein caused a dramatic reduction in the level of cpxP-lacZ expression relative to the vector control (Fig. 4, compare bars 3 and 4). Conversely, overexpression of a MBP-CpxP fusion protein localized to the cytoplasm had no effect on Cpx-mediated gene expression (Fig. 4, compare bars 1 and 2). We conclude that CpxP exerts its repressive effect on Cpx-mediated gene expression from the periplasm.
FIG. 4.
CpxP-mediated repression occurs from the periplasm. cpxP-lacZ expression was assayed as a measure of Cpx-mediated gene expression in strains TR50(pMal-c2) (row 1), TR50(pCMCP) (row 2), TR50(pMal-p2) (row 3), and TR50(pMCP) (row 4). β-Galactosidase activities are expressed as a percentage of the vector control, which was normalized to 100%.
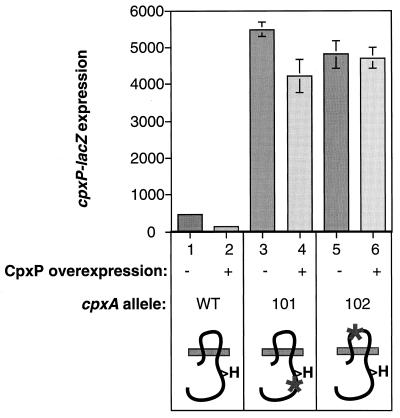
Since CpxP-mediated repression occurs through the sensor kinase CpxA (Fig. 3), we investigated whether the sensing domain of CpxA was required. To test this, we sought to determine whether CpxP overexpression could downregulate Cpx-mediated gene expression in a constitutively activated cpxA* mutant that contains a mutation in the sensing domain which renders it signal blind (39). The cpxP overexpression plasmid pCpxP and the vector control ptrc99A were transformed into reporter strains carrying a cpxP-lacZ fusion on a λ phage and either the wild-type cpxA locus or one of two constitutively activated cpxA* alleles. As expected, in the wild-type background, overexpression of CpxP resulted in a three- to fivefold reduction in Cpx-mediated gene expression (Fig. 5, compare columns 1 and 2). Similarly, CpxP overexpression caused a slight, but reproducible decrease in cpxP-lacZ expression in a strain containing a cpxA* mutation which leaves the sensing domain of CpxA intact (Fig. 5, compare columns 3 and 4). In contrast, CpxP overexpression had no effect on Cpx-mediated gene expression in a strain containing a cpxA* mutation in the sensing domain of CpxA which renders it signal blind (Fig. 5, compare columns 5 and 6). Accordingly, we conclude that the repressive effects of CpxP overexpression are mediated via the sensing domain of CpxA.
FIG. 5.
CpxP-mediated repression requires the sensing domain of CpxA. cpxP-lacZ expression was measured as an indicator of Cpx-mediated gene expression in strains TR50(ptrc99A) (column 1), TR50(pCpxP) (column 2), TR48(ptrc99A) (column 3), TR48(pCpxP) (column 4), TR36(ptrc99A) (column 5), and TR36(pCpxP) (column 6). TR48 contains the constitutively activated cpxA* allele cpxA101, which is still responsive to activating cues. TR36 contains the constitutively activated cpxA* allele cpxA102, which is signal blind and no longer responds to activating stimuli. The diagrams at the bottom of the figure are schematic representations of the CpxA proteins found in the indicated strains. The gray bar represents the inner membrane, with the periplasm located above it and the cytoplasm located below it. The dark, squiggly line depicts the HK CpxA. The “H” is a symbol of the histidine residue that is conserved with all other HKs. Asterisks indicate the locations of cpxA* mutations.
CpxP-mediated repression precludes activation of the Cpx pathway.
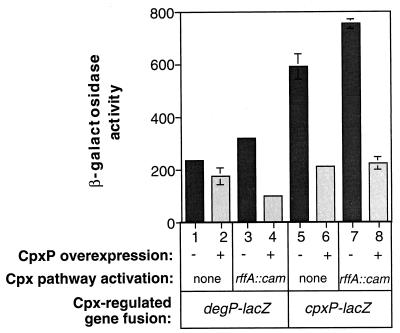
CpxP-mediated repression utilizes the same sensing domain in CpxA that is required for activation (Fig. 5). To gain insight into the role of CpxP as a signaling molecule, we examined the effects of CpxP overexpression in a Cpx-activated background. To do this, the pCpxP overexpression plasmid and the parent vector, ptrc99A, were transformed into wild-type and mutant rffA reporter strains carrying either a degP-lacZ or cpxP-lacZ reporter fusion. The rffA mutation has been shown to activate the Cpx pathway through a buildup of the lipid II intermediate of enterobacterial common antigen synthesis (5).
As previously noted, overexpression of CpxP in the wild-type background caused diminished expression of the Cpx-regulated gene cpxP (Fig. 6, compare columns 5 and 6). Further, overexpression of CpxP also led to a slight, but reproducible, reduction in expression of another Cpx-regulated gene, degP (Fig. 6, compare columns 1 and 2). Although the reason for the difference in the degree of CpxP-mediated repression of cpxP and degP expression is not currently known, we suspect that the degP promoter may be less sensitive, in general, to alterations in the level of phosphorylated CpxR in the cell. In support of this, phosphorylated CpxR seems to have a lower affinity for the degP promoter relative to the cpxP upstream region in mobility shift assays (38a). Accordingly, we would expect any repressive effects of CpxP overexpression on the Cpx signal transduction pathway to be less dramatic at the degP promoter.
FIG. 6.
CpxP-mediated repression precludes activation of the Cpx regulon. The effect of overexpressing CpxP in TR49(ptrc99A) (column 1), TR49(pCpxP) (column 2), TR412(ptrc99A) (column 3), TR412(pCpxP) (column 4), TR50(ptrc99A) (column 5), TR50(pCpxP) (column 6), TR413(ptrc99A) (column 7), and TR413(pCpxP) (column 8) was analyzed by measuring degP-lacZ (columns 1 to 4) or cpxP-lacZ (columns 5 to 8) expression. The rffA::cam mutation, described in the text, was used to activate the Cpx pathway.
As expected, the rffA::cam mutation resulted in elevated expression of the Cpx regulon (Fig. 6, compare columns 1 and 3 and columns 5 and 7). Intriguingly, overexpression of CpxP in this activated background repressed expression of both degP and cpxP to the same level seen in a wild-type background (Fig. 6, compare columns 2 and 4 and columns 6 and 8). Under these conditions, the repressive effect of CpxP overexpression on the degP promoter is obvious. Thus, overexpression of CpxP prevents activation of the Cpx pathway by a known inducing cue.
Overexpression of CpxP does not alleviate envelope stress.
We considered two mechanisms for the downregulatory effects caused by CpxP overexpression. First, it is possible that overexpression simply alleviates the amount of envelope stress that is perceived by the Cpx stress response, thus indirectly downregulating the entire pathway. Alternatively, CpxP may function as a signaling molecule that interacts directly with the periplasmic sensing domain of the HK CpxA to modulate its activity. To distinguish between these possibilities, we asked what effect overexpression of CpxP would have in strains containing one of two normally toxic envelope proteins: LamBA23D or LamB-LacZ-PhoA, commonly known as the tribrid. lamBA23D encodes a signal sequence mutation in the gene for the maltoporin LamB, which effectively tethers this OMP to the inner membrane upon passage through the secretion machinery, and leads to toxicity upon induction with maltose (2). The tribrid protein forms large, toxic disulfide bonded aggregates in the periplasm upon secretion which are also manifest by sensitivity to maltose (50). Activation of the Cpx pathway or overexpression of the Cpx regulon member DegP can alleviate these toxicities (4, 49). Therefore, if CpxP overexpression downregulates the Cpx regulon by alleviating envelope stress, this would be reflected in a diminished maltose sensitivity of strains encoding these toxic envelope proteins.
To test this prediction, we transformed pCpxP and the parent vector ptrc99A into strains containing either lamBA23D or lamB-lacZ-phoA and measured maltose sensitivity (Table 2). We found that in both cases, overexpression of CpxP either worsened or had no effect on the toxicity of these proteins (Table 2). Thus, the envelope stress conferred by these mutant proteins is not alleviated in any way by overexpression of CpxP and, in fact, is worsened in some cases. Further, these effects occurred in a CpxA-dependent fashion (Table 2), exactly like the downregulatory effects previously observed (Fig. 3). The simplest explanation for these observations is that the overexpression of CpxP represses the Cpx regulon through an interaction with the HK CpxA. These data strongly suggest that the repressive effects of CpxP overexpression are not due to an alleviation of envelope stress but rather to a downregulation of the regulon that is mediated through the HK CpxA.
TABLE 2.
CpxP overexpression and maltose sensitivity results
| Genotype | Strain | CpxP over-expression | Maltose sensitivity (zone of clearing [mm]) |
|---|---|---|---|
| lamBA23D | JHC285(ptrc99A) | − | 39 |
| JHC285(pCpxP) | + | 45 | |
| lamBA23D cpxA1::cam | TR146(ptrc99A) | − | 44 |
| TR146(pCpxP) | + | 44 | |
| lamB-lacZ-phoA | TR143(ptrc99A) | − | 31 |
| TR143(pCpxP) | + | 31 | |
| lamB-lacZ-phoA cpxA1::cam | TR147(ptrc99A) | − | 32 |
| TR147(pCpxP) | + | 33 |
Analysis of cpxP null mutants.
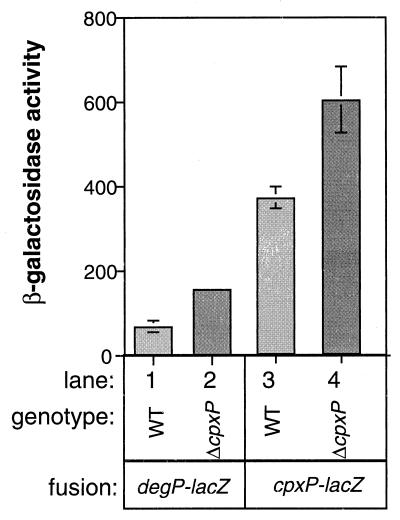
Our analysis of CpxP overexpression suggested that it is involved in repression of the Cpx envelope stress response. Accordingly, we predicted that loss of cpxP should lead to activation of the pathway. Analysis of existing cpxP null alleles that contain large insertions (8) was complicated by the fact that these mutations have cis-acting negative effects on the expression of the divergently transcribed cpxRA operon (data not shown). To circumvent these effects, we created a cpxP loss-of-function mutant which lacks approximately two-thirds of the C terminus of the wild-type protein. For reasons we do not understand, this mutation has much less severe cis-acting effects than those conferred by the cpxP insertions (data not shown). Strains containing such a mutation expressed slightly elevated levels of both degP-lacZ and cpxP-lacZ reporter constructs (Fig. 7), confirming that CpxP does indeed exert a negative effect on the Cpx regulon.
FIG. 7.
A cpxP deletion causes activation of the Cpx pathway. β-Galactosidase activity was assayed in TR49 (column 1), TR493 (column 2), TR50 (column 3), and TR494 (column 4).
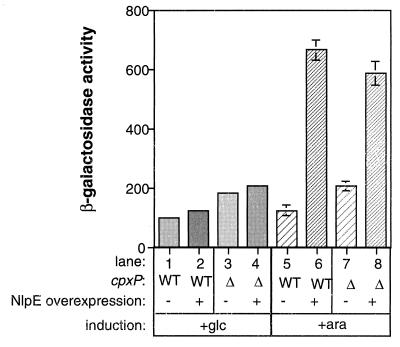
Previously, we proposed that titration of such a negatively acting molecule during times of envelope stress could be responsible for activation of the Cpx pathway (39). However, analysis of the cpxP null suggested that this is not sufficient for full activation of the stress response, since only a mild stimulation of the Cpx pathway was observed (Fig. 7). To analyze the role of CpxP titration in activation of the Cpx regulon, we measured the ability of cpxP mutants to respond to a known activating signal, NlpE overexpression. As previously noted, overexpression of NlpE in a wild-type strain carrying a cpxP-lacZ reporter resulted in a five- to sevenfold increase in β-galactosidase expression (Fig. 8, compare columns 5 and 6). Similarly, the strain carrying the cpxP mutation was able to mount a response not significantly different from that of the wild-type strain when NlpE was overexpressed (Fig. 8, compare columns 6 and 8). Thus, while removal of CpxP leads to a mild derepression of the regulon, it is neither necessary nor sufficient for full activation of the pathway.
FIG. 8.
cpxP mutants can still respond to activating cues. The ability of wild-type and mutant cpxP bacteria to respond to activating signals was monitored by measuring β-galactosidase activity from a cpxP-lacZ fusion contained in single copy on the chromosome of strains TR50(pBAD18) (columns 1 and 5), TR50(pND18) (columns 2 and 6), TR494(pBAD18) (columns 3 and 7), and TR494(pND18) (columns 4 and 8). The Cpx pathway was activated by addition of 0.2% arabinose (columns 5 to 8) to induce overexpression of NlpE from plasmid pND18 (columns 6 and 8). As a control the same strains were transformed with the parent plasmid, pBAD18 (columns 1, 3, 5, and 7) and the experiment was carried out in the presence of 0.4% glucose instead of arabinose (columns 1 to 4).
DISCUSSION
In the present study, we have demonstrated that activation of the Cpx envelope stress response is a tightly regulated event controlled by both autoamplification and feedback inhibition mechanisms. The cpxRA operon, which encodes the signal transduction apparatus, is itself a member of the Cpx regulon and, thus, is subject to autoactivation. Further, the extent of the response appears to be self-limiting, since high-level production of a second regulon member, CpxP, leads to repression of the pathway. Interestingly, the cpxP open reading frame is found directly upstream of and in the opposite orientation to the cpxRA operon, suggesting that these three regulatory genes may have evolved together in E. coli into the autoregulatory circuit they constitute. We suspect that this circuitry allows for high-level activation of the pathway for a discrete period of time, a feature which may be important for protecting the cell from transitory stresses and correctly timing the elaboration of virulence factors.
The cpxRA operon is autoactivated.
Levels of the RR CpxR and the HK CpxA correlate with the amount of phosphorylated CpxR present in the cell (Fig. 1 and 2). This, coupled with the observation that a perfect CpxR consensus binding sequence exists upstream of the cpxRA promoter (36), provides evidence that the cpxRA operon is autoactivated. By analogy with OmpR, one of the most closely related RRs to CpxR, we speculate that the mechanism of transcription activation occurs by binding of phosphorylated CpxR dimers to the direct repeats of the binding site followed by an interaction between CpxR and RNA polymerase that facilitates transcription (44, 47). Work is currently under way to test this hypothesis.
Possible functions of autoactivation.
Autoregulation is a common feature of many regulatory pathways that control stress responses or developmental programs. In these systems, the rapid amplification of the response conferred by autoregulation seems to function to commit the cell to the response once the environment dictates its initiation. Perhaps the best known example is the genetic switch that determines lysis and lysogeny of phage λ (for a review, see reference 37). In this system, if the cI repressor is made, it autoactivates its own expression, leading to repression of lytic functions and commitment to lysogeny.
Similarly, numerous two-component systems that control developmental or differentiative processes in bacteria are autogenously activated. For instance, the PhoPQ, BvgAS, and VirAG two-component regulators that control expression of virulence factors required for survival in the host of Salmonella, Bordetella, and Agrobacterium species, respectively, are all subject to autoamplification (42, 43, 51, 52, 63). Further, sporulation in Bacillus subtilis (55, 56), competence in Bacillus (60) and Streptococcus species (35), and antibiotic production in numerous gram-positive organisms (for a recent review, see reference 21) are all controlled by two-component regulators that mediate their own upregulation when the environment signals initiation of these developmental processes. Here, the high levels of phosphorylated RR needed to initiate the developmental processes are not present until a threshold level of stimulus signals autoactivation and thus amplification of the signal transduction apparatus. In this manner, autoactivation ensures that these energetically expensive differentiative programs are not initiated inappropriately and allows for a rapid response or “switch” once the appropriate conditions prevail.
Recently, the Cpx envelope stress response has been implicated in the elaboration of virulence factors, in addition to its role in protecting the envelope from stress (17–19, 34, 61, 64). Specifically, it appears that activation of the Cpx pathway is critical for the correctly timed expression and assembly of P pili on the surface of uropathogenic E. coli (16, 17, 19). Thus, one function of autoactivation may be to commit the cell to virulence factor production once an appropriate host environment is sensed, thus ensuring successful infection and survival.
Another feature of autoregulation may be to allow for an extra level of control. If Cpx-regulated promoters have various affinities for phosphorylated CpxR (as, for example, do OmpR regulon members), then the combined ability to influence phosphorylated CpxR levels enzymatically through the action of the HK CpxA, as well as transcriptionally, by autoactivation, allows for a wider range of phosphorylated CpxR levels and potentially for the expression of different Cpx regulon members at different stages of pathway activation. Such a control feature could allow for different responses to mild as opposed to severe stress and ensure that complex developmental programs, such as the elaboration of adhesive organelles, are not initiated prematurely. Indeed, phosphorylated CpxR does demonstrate differential binding affinity for the degP and cpxP promoters (38a), suggesting that this may be an important feature of Cpx gene regulation.
CpxP mediates feedback inhibition of the Cpx envelope stress response.
The Cpx envelope stress response is also subject to a second form of autoregulation: feedback inhibition. We have shown that overexpression of CpxP, a Cpx-regulated periplasmic protein of previously unknown function (8), leads to downregulation of the Cpx pathway in a CpxA-dependent fashion (Fig. 3 to 7).
This observation was initially made while testing a model we proposed for CpxA sensation of envelope stress (39). Since constitutively active cpx* mutations in the sensing domain of CpxA confer a signal blind phenotype, we suggested that envelope stress is sensed through titration of a negatively acting periplasmic molecule from this region in the presence of inducing cues. This model predicts that overexpression of such a signaling molecule should downregulate the pathway, while deletion would both upregulate the stress response and preclude a further response to activating signals. CpxP fulfills two of these criteria: overexpression dampens the Cpx response, while its deletion causes a slight activation of the pathway (Fig. 3 to 8). However, since inducing stimuli are still clearly sensed in the absence of cpxP (Fig. 8), there must be a further requirement(s) for perceiving envelope stress than simple titration of CpxP.
Functional similarities between RseAB of the ςE stress response and CpxAP of the Cpx stress response.
The functional similarities between the RseAB signal transduction machinery of the ςE envelope stress response and the CpxAP apparatus of the Cpx response are remarkable. Transduction of envelope stress by the ςE pathway is mediated by the relief of a negative interaction between ςE and a membrane-bound anti-sigma factor, RseA (10, 32). Similarly, transduction of envelope stress by the Cpx pathway requires altered interactions between the membrane-bound HK CpxA and the cytoplasmic transcriptional regulator CpxR (39). Like CpxP, overexpression of the accessory, periplasmic, signaling molecule RseB can downregulate the ςE response, presumably via a direct interaction with RseA (10, 32). Further, deletion of rseB leads to elevated expression of ςE-regulated genes. However, like CpxP, rseB is not essential for responding to envelope stress; rseB null bacteria can still activate the ςE pathway in response to appropriate inducing signals.
Possible functions of feedback inhibition.
What could be the function of these accessory regulatory factors if they play, at best, a relatively minor role in sensing envelope stress? One possibility is that they function to fine-tune the regulatory responses. For example, titration of RseB or CpxP by inducing signals could “prime” RseA or CpxA for activation. Alternatively, we suggest that they function primarily as shutoff molecules. Like the Cpx pathway, the ςE stress response is subject to autoactivation (10, 32, 38, 41). Accordingly, rapid amplification of either pathway in response to transitory envelope stresses could lead to an imbalance in envelope folding factors that is likely to be detrimental to the cell (31). Since RseB and CpxP are also ςE and Cpx regulated, respectively, increased expression of these negative regulatory molecules acts as a safety valve, allowing for feedback inhibition and rapid shutoff of the pathway in question upon alleviation of the envelope stress.
A model for CpxP-mediated repression.
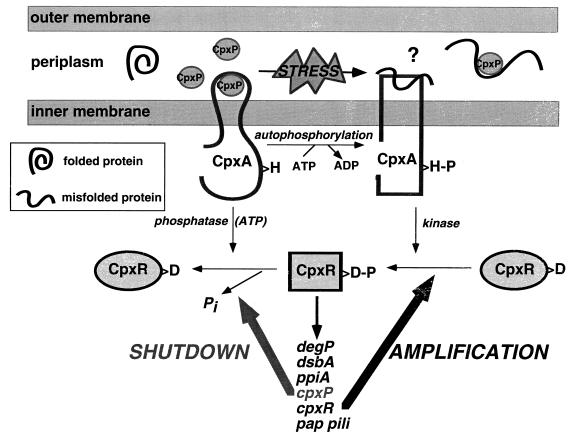
The repressive effect of CpxP overexpression is mediated from the periplasm (Fig. 4) and requires the sensing domain of CpxA (Fig. 5). Although at this time we cannot rule out an indirect mechanism, the simplest explanation for the negative effects of CpxP on Cpx-mediated gene expression invokes a direct interaction between CpxP and CpxA that alters the enzymatic activities of the HK. We propose that under nonstressed conditions CpxP interacts with this domain, causing a decrease in the kinase/phosphatase ratio of CpxA (Fig. 9), thus maintaining the pathway in an off state. Activating signals would lead to a titration of CpxP and some other, undefined event, that elevates the kinase/phosphatase ratio of CpxA and turns on the pathway (Fig. 9). Misfolded proteins may interact directly with the sensing domain of CpxA to manifest activation. Alternatively, perhaps levels of a second periplasmic folding factor are used as an indicator of envelope stress. Upon alleviation of envelope stress, free levels of CpxP would be elevated and bind to CpxA, turning off the response.
FIG. 9.
Model for activation and repression of the Cpx regulon. Under nonstressed conditions, CpxP (circles) interacts with the sensing domain of CpxA (central portion of periplasmic domain of CpxA), maintaining the kinase in an off state (thick, black, curvy line). In response to envelope stress, CpxP is titrated away from the sensing domain, perhaps by preferential binding to misfolded proteins (squiggly line), which may also bind to the sensing domain of CpxA and activate the kinase (rectangular form). Activation leads to rapid amplification of the pathway through autoactivation of the cpxRA operon. Upon alleviation of envelope stress, high levels of free CpxP exist in the periplasm which rebind the sensing domain of CpxA and shutoff the response by downregulating the ratio of kinase to phosphatase activity. H, the histidine residue in CpxA that is conserved with those of other HKs; H-P, phosphorylation of this residue; D, aspartate residue in CpxR that is conserved with those of other RRs; D-P, phosphorylation of this residue. The square form of CpxR symbolizes the phosphorylated form that is competent for transcriptional activation of the downstream regulon members.
Other functions of CpxP.
Intriguingly, in other stress responses that are subject to feedback inhibition, the shutoff molecules also have stress-combative functions. For example, the heat shock response can be downregulated through the feedback inhibition of ςH activity by cytoplasmic chaperones (24, 25, 57, 58). During times of stress these molecules function to interact with and refold denatured proteins. However, upon alleviation of stress, the free chaperones are proposed to bind to and inhibit ςH activity. Similarly, we think it likely that CpxP serves some other function in addition to that of a shutoff mechanism for the Cpx envelope stress response. Although at present we cannot say what this role is, given the function of other regulon members, it seems likely that CpxP will be involved in protein folding and/or degradation in the bacterial envelope. Accordingly, we propose that CpxP is titrated away from the sensing domain of CpxA through preferential binding to some substrate present during times of envelope stress (Fig. 9). This factor could be any one of a number of possibilities, including misfolded proteins or even another Cpx regulon member. Levels of both would increase during envelope stress, titrating CpxP and allowing activation of the Cpx pathway. Conversely, levels of these putative CpxP substrates would decrease upon alleviation of envelope stress, releasing free CpxP, which could then bind to and repress CpxA. Elucidation of the other roles of CpxP awaits further study.
Alternatively, perhaps synthesis, secretion, and folding of CpxP serve as a time-dependent mechanism for system shutoff, much as the synthesis and secretion of small peptide molecules serve to signal high cell density in the regulation of competence and sporulation in gram-positive bacteria (for a review, see reference 21). In this manner, levels of mature, folded CpxP could be monitored by the Cpx pathway as an indirect measure of folding factor levels in the envelope. Further study of the elements required for proper folding of CpxP will yield more insight into this alternative.
The Cpx stress response is a regulatory pathway for sensing and responding to misfolded proteins in the bacterial envelope (4, 7, 36, 39, 49). Recently, it has become clear that this stress response is also intimately involved in complex developmental processes such as the assembly of adhesive organelles (16, 17, 19). In this study we have demonstrated the presence of a tightly controlled autoamplification and feedback inhibition circuit which could effectively function to flip the Cpx response on and off very rapidly. We propose that such a switch mechanism is critical for allowing the correctly timed elaboration of virulence factors and rescuing the cell from potentially toxic, transitory envelope stresses.
REFERENCES
- 1.Bardwell J C, McGovern K, Beckwith J. Identification of a protein required for disulfide bond formation in vivo. Cell. 1991;67:581–589. doi: 10.1016/0092-8674(91)90532-4. [DOI] [PubMed] [Google Scholar]
- 2.Carlson J H, Silhavy T J. Signal sequence processing is required for the assembly of LamB trimers in the outer membrane of Escherichia coli. J Bacteriol. 1993;175:3327–3334. doi: 10.1128/jb.175.11.3327-3334.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casadaban M J. Transposition and fusion of lac genes to selected promoters in Escherichia coli using bacteriophages lambda and Mu. J Mol Biol. 1976;104:541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- 4.Cosma C L, Danese P N, Carlson J H, Silhavy T J, Snyder W B. Activation of the Cpx two-component signal transduction pathway in Escherichia coli suppresses envelope associated stresses. Mol Microbiol. 1995;18:491–505. doi: 10.1111/j.1365-2958.1995.mmi_18030491.x. [DOI] [PubMed] [Google Scholar]
- 5.Danese P N, Oliver G R, Barr K, Bowman G D, Rick P D, Silhavy T J. Accumulation of the enterobacterial common antigen lipid II biosynthetic intermediate stimulates degP transcription in Escherichia coli. J Bacteriol. 1998;180:5875–5884. doi: 10.1128/jb.180.22.5875-5884.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danese P N, Snyder W B, Cosma C L, Davis L J, Silhavy T J. The Cpx two-component signal transduction pathway of Escherichia coli regulates transcription of the gene specifying the stress-inducible periplasmic protease, DegP. Genes Dev. 1995;9:387–398. doi: 10.1101/gad.9.4.387. [DOI] [PubMed] [Google Scholar]
- 7.Danese P N, Silhavy T J. The ςE and the Cpx signal transduction systems control the synthesis of periplasmic protein-folding enzymes in Escherichia coli. Genes Dev. 1997;11:1183–1193. doi: 10.1101/gad.11.9.1183. [DOI] [PubMed] [Google Scholar]
- 8.Danese P N, Silhavy T J. CpxP, a stress-combative member of the Cpx regulon. J Bacteriol. 1998;180:831–839. doi: 10.1128/jb.180.4.831-839.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dartigalongue C, Raina S. A new heat-shock gene, ppiD, encodes a peptidyl-prolyl isomerase required for folding of outer membrane proteins in Escherichia coli. EMBO J. 1998;17:3968–3980. doi: 10.1093/emboj/17.14.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Las Penas A, Connolly L, Gross C A. The ςE-mediated response to extracytoplasmic stress in Escherichia coli is transduced by RseA and RseB, two negative regulators of ςE. Mol Microbiol. 1997;24:373–385. doi: 10.1046/j.1365-2958.1997.3611718.x. [DOI] [PubMed] [Google Scholar]
- 11.Dong J S, Iuchi S, Kwan H S, Lu Z, Lin E C C. The deduced amino-acid sequence of the cloned cpxR gene suggests the protein is the cognate regulator for the membrane sensor, CpxA, in a two-component signal transduction system of Escherichia coli. Gene. 1993;136:227–230. doi: 10.1016/0378-1119(93)90469-j. [DOI] [PubMed] [Google Scholar]
- 12.Erickson J W, Gross C A. Identification of the ςE subunit of Escherichia coli RNA polymerase: a second alternate ς factor involved in high-temperature gene expression. Genes Dev. 1989;3:1462–1471. doi: 10.1101/gad.3.9.1462. [DOI] [PubMed] [Google Scholar]
- 13.Guzman L, Barondess J J, Beckwith J. FtsL, an essential cytoplasmic membrane protein involved in cell division in Escherichia coli. J Bacteriol. 1992;174:7717–7728. [PMC free article] [PubMed] [Google Scholar]
- 14.Hamilton C M, Aldea M, Washburn B K, Babitzke P, Kushner S R. New method for generating deletions and gene replacements in Escherichia coli. J Bacteriol. 1989;171:4617–4622. doi: 10.1128/jb.171.9.4617-4622.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horne S M, Young K D. Escherichia coli and other species of the Enterobacteriaceae encode a protein similar to the family of Mip-like FK506-binding proteins. Arch Microbiol. 1995;163:357–365. doi: 10.1007/BF00404209. [DOI] [PubMed] [Google Scholar]
- 16.Hung, D. L., T. L. Raivio, C. H. Jones, T. J. Silhavy, and S. J. Hultgren. Unpublished observations.
- 17.Jacob-Dubuisson F, Pinkner J, Xu X, Striker R, Padmanhaban A, Hultgren S J. PapD chaperone function in pilus biogenesis depends on oxidant and chaperone-like activities of DsbA. Proc Natl Acad Sci USA. 1994;91:11552–11556. doi: 10.1073/pnas.91.24.11552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson K, Charles I, Dougan G, Pickard D, O’Gaora P, Costa G, Ali T, Miller I, Hormaeche C. The role of a stress-response protein in Salmonella typhimurium virulence. Mol Microbiol. 1991;5:401–407. doi: 10.1111/j.1365-2958.1991.tb02122.x. [DOI] [PubMed] [Google Scholar]
- 19.Jones C H, Danese P N, Pinkner J S, Silhavy T J, Hultgren S J. The chaperone-assisted membrane release and folding pathway is sensed by two signal transduction systems. EMBO J. 1997;21:6394–6406. doi: 10.1093/emboj/16.21.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamitani S, Akiyama Y, Ito K. Identification and characterization of an Escherichia coli gene required for the formation of correctly folded alkaline phosphatase, a periplasmic enzyme. EMBO J. 1992;11:57–62. doi: 10.1002/j.1460-2075.1992.tb05027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleerebezem M, Quadri L E N, Kuipers O P, de Vos W M. Quorum sensing by peptide pheromones and two-component signal-transduction systems in gram-positive bacteria. Mol Microbiol. 1997;24:895–904. doi: 10.1046/j.1365-2958.1997.4251782.x. [DOI] [PubMed] [Google Scholar]
- 22.Kolmar H, Waller P R, Sauer R T. The DegP and DegQ periplasmic endoproteases of Escherichia coli: specificity for cleavage sites and substrate conformation. J Bacteriol. 1996;178:5925–5929. doi: 10.1128/jb.178.20.5925-5929.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Liberek K, Galitski T P, Zylicz M, Georgopoulos C. The DnaK chaperone modulates the heat shock response of Escherichia coli by binding to the ς32 transcription factor. Proc Natl Acad Sci USA. 1992;89:3516–3520. doi: 10.1073/pnas.89.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liberek K, Georgopoulos C. Autoregulation of the Escherichia coli heat shock response by the DnaK and DnaJ heat shock proteins. Proc Natl Acad Sci USA. 1993;90:11019–11023. doi: 10.1073/pnas.90.23.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipinska B, Fayet O, Baird L, Georgopoulos C. Identification, characterization, and mapping of the Escherichia coli htrA gene, whose product is essential for bacterial growth only at elevated temperatures. J Bacteriol. 1989;171:1574–1584. doi: 10.1128/jb.171.3.1574-1584.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipinska B, Sharma S, Georgopoulos C. Sequence analysis and regulation of the htrA gene of Escherichia coli: a ς32-independent mechanism of heat-inducible transcription. Nucleic Acids Res. 1988;16:10053–10067. doi: 10.1093/nar/16.21.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipinska B, Zylicz M, Georgopoulos C. The HtrA (DegP) protein, essential for Escherichia coli survival at high temperatures, is an endopeptidase. J Bacteriol. 1990;172:1791–1797. doi: 10.1128/jb.172.4.1791-1797.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Walsh C T. Peptidyl-prolyl cis-trans-isomerase from Escherichia coli: a periplasmic homolog of cyclophilin that is not inhibited by cyclosporin A. Proc Natl Acad Sci USA. 1990;87:4028–4032. doi: 10.1073/pnas.87.11.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mecsas J, Rouviere P E, Erickson J W, Donohue T J, Gross C A. The activity of ςE, an Escherichia coli heat-inducible ς-factor, is modulated by expression of outer membrane proteins. Genes Dev. 1993;7:2618–2628. doi: 10.1101/gad.7.12b.2618. [DOI] [PubMed] [Google Scholar]
- 31.Missiakas D, Betton J-M, Raina S. New components of protein folding in extracytoplasmic compartments of Escherichia coli SurA, FkpA and Skp/OmpH. Mol Microbiol. 1996;21:871–884. doi: 10.1046/j.1365-2958.1996.561412.x. [DOI] [PubMed] [Google Scholar]
- 32.Missiakas D, Mayer M P, Lemaire M, Georgopoulos C, Raina S. Modulation of the Escherichia coli ςE (RpoE) heat-shock transcription-factor activity by the RseA, RseB and RseC proteins. Mol Microbiol. 1997;24:355–371. doi: 10.1046/j.1365-2958.1997.3601713.x. [DOI] [PubMed] [Google Scholar]
- 33.Nakayama S-I, Watanabe H. Involvement of cpxA, a sensor of a two-component regulatory system, in the pH-dependent regulation of expression of Shigella sonnei virF gene. J Bacteriol. 1995;177:5062–5069. doi: 10.1128/jb.177.17.5062-5069.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peek J A, Taylor R K. Characterization of a periplasmic thiol:disulfide interchange protein required for the functional maturation of secreted virulence factors of Vibrio cholerae. Proc Natl Acad Sci USA. 1992;89:6210–6214. doi: 10.1073/pnas.89.13.6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pestova E V, Havarstein L S, Morrison D A. Regulation of competence for genetic transformation in Streptococcus pneumoniae by an auto-induced peptide pheromone and a two-component regulatory system. Mol Microbiol. 1996;21:853–862. doi: 10.1046/j.1365-2958.1996.501417.x. [DOI] [PubMed] [Google Scholar]
- 36.Pogliano J, Lynch A S, Belin D, Lin E C C, Beckwith J. Regulation of Escherichia coli cell envelope proteins involved in protein folding and degradation by the Cpx two-component system. Genes Dev. 1997;11:1169–1182. doi: 10.1101/gad.11.9.1169. [DOI] [PubMed] [Google Scholar]
- 37.Ptashne M. A genetic switch, gene control and phage λ. Blackwell Scientific Publications; 1986. /Cell Press, Cambridge, Mass. [Google Scholar]
- 38.Raina S, Missiakas D, Georgopoulos C. The rpoE gene encoding the ςE (ς24) heat shock sigma factor of Escherichia coli. EMBO J. 1995;14:1043–1055. doi: 10.1002/j.1460-2075.1995.tb07085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38a.Raivio, T. L., and T. J. Silhavy. Unpublished observations.
- 39.Raivio T L, Silhavy T J. Transduction of envelope stress in Escherichia coli by the Cpx two-component system. J Bacteriol. 1997;179:7724–7733. doi: 10.1128/jb.179.24.7724-7733.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raivio T L, Silhavy T J. The ςE and Cpx regulatory pathways: overlapping but distinct envelope stress responses. Curr Opin Microbiol. 1999;2:159–165. doi: 10.1016/S1369-5274(99)80028-9. [DOI] [PubMed] [Google Scholar]
- 41.Rouviere P E, De Las Penas A, Mecsas J, Lu C Z, Rudd K E, Gross C A. rpoE, the gene encoding the second heat-shock sigma factor, ςE, in Escherichia coli. EMBO J. 1995;14:1032–1042. doi: 10.1002/j.1460-2075.1995.tb07084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roy C R, Miller J F, Falkow S. Autogenous regulation of the Bordetella pertussis bvgABC operon. Proc Natl Acad Sci USA. 1990;87:3763–3767. doi: 10.1073/pnas.87.10.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scarlato V, Prugnola A, Arico B, Rappuoli R. Positive transcriptional feedback at the bvg locus controls expression of virulence factors in Bordetella pertussis. Proc Natl Acad Sci USA. 1990;87:6753–6757. doi: 10.1073/pnas.87.17.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharif R T, Igo M M. Mutations in the alpha subunit of RNA polymerase that affect the regulation of porin gene transcription in Escherichia coli K-12. J Bacteriol. 1993;175:5460–5468. doi: 10.1128/jb.175.17.5460-5468.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1984. [Google Scholar]
- 46.Simons R W, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusion. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 47.Slauch J M, Russo F D, Silhavy T J. Suppressor mutations in rpoA suggest that OmpR controls transcription by direct interaction with the α subunit of RNA polymerase. J Bacteriol. 1991;173:7501–7510. doi: 10.1128/jb.173.23.7501-7510.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slauch J M, Silhavy T J. cis-Acting ompF mutations that result in OmpR-dependent constitutive expression. J Bacteriol. 1991;173:4039–4048. doi: 10.1128/jb.173.13.4039-4048.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snyder W B, Davis L J B, Danese P N, Cosma C L, Silhavy T J. Overproduction of NlpE, a new outer membrane lipoprotein, suppresses the toxicity of periplasmic LacZ by activation of the Cpx signal transduction pathway. J Bacteriol. 1995;177:4216–4223. doi: 10.1128/jb.177.15.4216-4223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Snyder W B, Silhavy T J. β-Galactosidase is inactivated by intermolecular disulfide bonds and is toxic when secreted to the periplasm of Escherichia coli. J Bacteriol. 1995;177:953–963. doi: 10.1128/jb.177.4.953-963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soncini F C, Vescovi E G, Groisman E. Transcriptional autoregulation of the Salmonella typhimurium phoPQ operon. J Bacteriol. 1995;177:4364–4371. doi: 10.1128/jb.177.15.4364-4371.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stachel S E, Zambryski P C. VirA and VirG control the plant-induced activation of the T-DNA transfer process of A. tumefaciens. Cell. 1986;46:325–333. doi: 10.1016/0092-8674(86)90653-7. [DOI] [PubMed] [Google Scholar]
- 53.Strauch K L, Beckwith J. An Escherichia coli mutation preventing degradation of abnormal periplasmic proteins. Proc Natl Acad Sci USA. 1988;85:1576–1580. doi: 10.1073/pnas.85.5.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strauch K L, Johnson K, Beckwith J. Characterization of degP, a gene required for proteolysis in the cell envelope and essential for growth of Escherichia coli at high temperature. J Bacteriol. 1989;171:2689–2696. doi: 10.1128/jb.171.5.2689-2696.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strauch M A, Trach K A, Day J, Hoch J A. SpoOA activates and represses its own synthesis by binding at its dual promoters. Biochimie. 1992;74:619–626. doi: 10.1016/0300-9084(92)90133-y. [DOI] [PubMed] [Google Scholar]
- 56.Strauch M A, Wu J J, Jonas R H, Hoch J A. A positive feedback loop controls transcription of the spoOF gene, a component of the sporulation phosphorelay in Bacillus subtilis. Mol Microbiol. 1993;7:967–974. doi: 10.1111/j.1365-2958.1993.tb01188.x. [DOI] [PubMed] [Google Scholar]
- 57.Straus D B, Walter W A, Gross C A. The activity of ς32 is reduced under conditions of excess heat shock protein production in Escherichia coli. Genes Dev. 1989;3:2003–2010. doi: 10.1101/gad.3.12a.2003. [DOI] [PubMed] [Google Scholar]
- 58.Straus D, Walter W, Gross C. DnaK, DnaJ, and GrpE heat shock proteins negatively regulate heat shock gene expression by controlling the synthesis and stability of ς32. Genes Dev. 1990;4:2202–2209. doi: 10.1101/gad.4.12a.2202. [DOI] [PubMed] [Google Scholar]
- 59.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Sinderen D, Venema G. comK acts as an autoregulatory switch in the signal transduction route to competence in Bacillus subtilis. J Bacteriol. 1994;176:5762–5770. doi: 10.1128/jb.176.18.5762-5770.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Watarai M, Tobe T, Yoshikawa M, Sasakawa C. Disulfide oxidoreductase activity of Shigella flexneri is required for release of Ipa proteins and invasion of epithelial cells. Proc Natl Acad Sci USA. 1995;92:4927–4931. doi: 10.1073/pnas.92.11.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weber R F, Silverman P J. The Cpx proteins of Escherichia coli K-12: structure of the CpxA polypeptide as an inner membrane component. J Mol Biol. 1988;203:467–476. doi: 10.1016/0022-2836(88)90013-7. [DOI] [PubMed] [Google Scholar]
- 63.Winans C. Transcriptional induction of an Agrobacterium regulatory gene at tandem promoters by plant-released phenolic compounds, phosphate starvation, and acidic growth media. J Bacteriol. 1990;172:2433–2438. doi: 10.1128/jb.172.5.2433-2438.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang H Z, Donnenberg M S. DsbA is required for stability of the type IV pilin of enteropathogenic Escherichia coli. Mol Microbiol. 1996;21:787–797. doi: 10.1046/j.1365-2958.1996.431403.x. [DOI] [PubMed] [Google Scholar]