Abstract
Objective
Adjuvant chemotherapy with trastuzumab improves the postoperative life expectancy of women with early-stage breast cancer. Although trastuzumab is reportedly cardiotoxic, quantification based on real-world evidence is lacking. Therefore, in this study, we aimed to analyse trastuzumab cardiotoxicity using a nationwide claim-based database.
Methods
In this retrospective study, we used data from a nationwide claims database (Japan Medical Data Center, Tokyo, Japan) under the universal healthcare system. Women with breast cancer who underwent initial surgery were included. Patients with recurrent or advanced-stage breast cancer, with a history of heart failure, receiving neoadjuvant chemotherapy or a preoperative history of less than 6 months were excluded. Propensity score (PS) was calculated using logistic regression based on age, cardiovascular risk factors, radiotherapy and concomitant anthracyclines (AC).
Results
We identified 12 060 eligible patients (mean age 50.8±8.56 years) between January 2010 and December 2019. After 1:2 PS matching (trastuzumab users, TZ, n=1005; non-users, NT, n=2010), Cox proportional hazards model analysis showed that the rate of heart failure development within 18 months postoperative was significantly higher in the TZ group than in the NT group (adjusted HR 2.28, 95% CI 1.38 to 3.77). Baseline cardiac evaluation in the combined AC/TZ cases was 27.2% preoperative, 66.0% pre-AC and 86.6% pre-TZ, respectively.
Conclusion
Trastuzumab cardiotoxicity remained relevant in the claim-based analysis adjusted for AC effects. Further collaborative studies in cardio-oncology with real-world data are warranted to improve the rate of baseline cardiovascular risk assessment in patients with cancer scheduled for cardiotoxic cancer treatment.
Keywords: HEART FAILURE; Cardiomyopathies; RISK FACTORS; Echocardiography; Pharmacology, Clinical
WHAT IS ALREADY KNOWN ON THIS TOPIC
Trastuzumab, a postoperative adjuvant chemotherapy agent used to treat human epidermal growth factor receptor 2-positive early-stage breast cancer, prolongs patients’ life expectancy. However, a baseline assessment of cardiac function is required because of the known complications of trastuzumab-induced cardiotoxicity, including heart failure.
WHAT THIS STUDY ADDS
Heart failure had a higher cumulative rate with the combination of trastuzumab and anthracycline than with trastuzumab alone. However, patients on the combination therapy tended to have a lower baseline cardiovascular risk assessment rate.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
In the new interdisciplinary field of cardio-oncology, there is a lack of clinical evidence as a basis for guideline development. Therefore, real-world evidence studies using various data sources and methodologies, including insurance claim databases, electronic health records and patient registries, are warranted.
Introduction
Breast cancer (BC) is the most prevalent type of cancer in women.1 Trastuzumab, a humanised monoclonal antibody, plays an essential role in treating patients with human epidermal growth factor receptor 2 (HER2)-positive BC, as it significantly reduces mortality in metastatic2 and adjuvant settings.3 However, trastuzumab-induced cardiotoxicity is a concern that must be addressed while administering the drug.4 5 Currently, adjuvant and neoadjuvant chemotherapy with trastuzumab is the standard treatment for patients with HER2-positive early-stage BC (ESBC) and is usually administered preoperatively or postoperatively for 1 year.3 Early discontinuation of trastuzumab due to symptomatic heart failure can affect life expectancy,6 and practice guidelines recommend baseline cardiovascular risk assessment and regular monitoring depending on the level of risk.7 8 In contrast, there are concerns that cancer treatment may be unnecessarily interrupted due to overdiagnosis of cardiac dysfunction,9 and low rates of preoperative echocardiography have been reported in real-world situations.10 Therefore, strategies for preventing, diagnosing, and treating trastuzumab-induced cardiotoxicity must be optimised for improved patient outcomes.
Cardio-oncology is a new interdisciplinary field encompassing cardiology and oncology.11 However, in developing evidence-based guidelines, the shared goal of improving patient outcomes has highlighted challenges related to traditional research approaches.7 9 12 For instance, patients with cancer have been excluded from major cardiovascular studies, while many cancer clinical trials have not fully validated cardiovascular end points. Moreover, cancer and cardiovascular disease share common risk factors, such as smoking and obesity, presumably because of similar underlying mechanisms, including chronic inflammation.13 14 One of the most promising new methodologies in regulatory science is real-world evidence (RWE), a new concept recently proposed by the US Food and Drug Administration.15 By definition, real-world data (RWD) are data routinely collected from a variety of sources, including insurance claim databases, patient registries, electronic medical records and social networking services. Moreover, RWE is clinical evidence derived from RWD analysis. Therefore, obtaining RWE from relevant RWD to serve evidence-based practice guidelines is one of the most critical challenges in cardio-oncology.16
Japan Medical Data Center (JMDC) is one of the most promising RWD sources because this health insurance claim database takes advantage of anonymous data linkage, universal coverage and fee-for-service (FFS) payment systems.17–19 The anonymous linkage algorithm of this claim database enables the provision of a patient-centred relational database, including monthly billing receipts collected from hospitals, clinics and pharmacies, with codes for diagnosis, drugs, surgeries and diagnostic tests.17 Under the national universal health insurance system, JMDC has grown to enrol approximately 8.4 million insured subscribers registered by 2020, although there is known ‘healthy-worker bias’.18 In addition, the FFS payment system allows for a low rate of missing values, especially for expensive drugs and surgical procedures.20 However, to the best of our knowledge, no study in Japan has applied insurance claims databases to cardio-oncology. Therefore, in this study, we analysed trastuzumab cardiotoxicity using the nationwide claim-based database JMDC to provide reliable and relevant RWD in the field of cardio-oncology.16
Methods
Data source
In this retrospective cohort study, we used deidentified individual-level data obtained from JMDC, Tokyo, Japan.17–19 The data provided by the JMDC have characteristics that are less likely to have outliers and time-series inconsistencies because the consistency of the data has been confirmed through an external review process.18 In addition, the data are standardised with codes provided by the JMDC, such as International Classification of Diseases (ICD-10) and Anatomical Therapeutic Chemical Classification (ATC).17 In this study, standard codes provided by the JMDC are indicated in square brackets.
Patient selection
The subjects were women with a diagnosis code of BC who underwent a breast surgery between January 2010 and December 2019. Based on monthly insurance claims, codes for diagnoses, tests, prescriptions and surgeries were anonymously linked to each patient from multiple providers. Recurrent and advanced cases were excluded to focus on those with ESBC. Patients with a history of heart failure, receiving neoadjuvant chemotherapy or with preoperative records less than 6 months old were also excluded to minimise the influence on trastuzumab cardiotoxicity.
Treatment strategies for BC
To delineate treatment strategies for ESBC,13 we summarised the data of patients prescribed anthracyclines, taxanes or antitumour hormone antagonists at least once, and at least one dose of radiation therapy. In addition, subjects prescribed trastuzumab at least once were included in the exposed group. Finally, claims data for mammography, breast ultrasound, needle biopsy procedures and diagnostic testing for oestrogen receptor and HER2 were collected.
Cardiovascular risk factors
Preoperative cardiovascular risk factors,13 including diabetes mellitus, hypertension and dyslipidaemia, were defined using combinations of diagnosis and treatment in the same monthly billing receipt. Furthermore, as claim-based databases used in Japan include the category ‘tentative diagnosis’ for reimbursement, diagnosis and prescription were combined for specificity.21 Additionally, age 50 years and older was included as an explanatory variable, as cardiovascular risk factors and cancer treatment strategies change dynamically during menopause.13
Baseline cardiac assessment and patient outcomes
As an indicator for baseline cardiac assessment, at least one order for echocardiography was adopted because the specificity of the test order, as opposed to the diagnosis code, is high in the Japanese FFS health insurance.21 In this claim-based analysis, we defined congestive heart failure (CHF) based on a combination of diagnosis and prescriptions on the same monthly receipt. The primary outcome of this study was the development of CHF within 18 months postsurgery. We focused on the first 1.5 years based on previous reports on trastuzumab cardiotoxicity.22–24 The other outcomes were also evaluated.
We set the cut-off date as the end of September 2020 and censored at the end of follow-up, at the time of withdrawal, or at 18 months after surgery.
Statistical analyses
Baseline characteristics are summarised using mean values (SD) for continuous data and counts (percentage; %) for categorical data. Intergroup comparisons were performed using crude analysis and propensity score (PS) matching.25 PS was generated using logistic regression, with the dependent variable being trastuzumab treatment. Independent variables were selected as potential confounding factors based on the results of a previous study,24 and included age at surgery (>50 years), sequential anthracyclines, concomitant hormone antagonists and preoperative cardiovascular risk factors (diabetes, hypertension and dyslipidaemia). PS matching was performed using the greedy pair algorithm with a 1:2 ratio without replacements and 0.2 as the calliper width.25 Standardised differences were used to assess residual differences in the subset of matched patients.
The rates of CHF during the first 18 months after BC surgery with and without trastuzumab exposure were compared. We used Cox hazards regression models to examine the relationship between the development of CHF and exposure to trastuzumab. In addition, we calculated the cumulative rate of development of CHF using the Kaplan-Meier method and compared with and without trastuzumab use using the log-rank test. All analyses were performed using SAS Windows, V.9.4 (SAS Institute, Cary, North Carolina).
Patient and public involvement
Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Results
Data source and patient selection
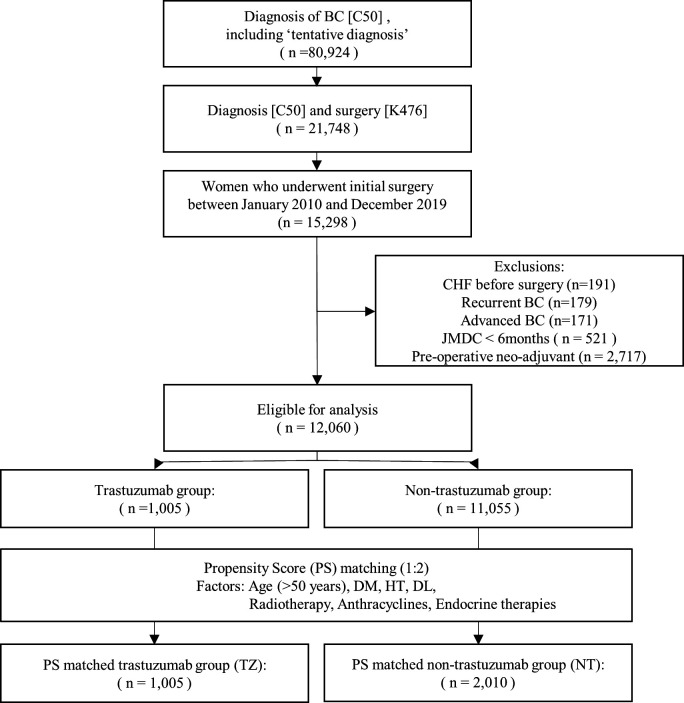
At the time of data collection, the JMDC database contained 80 924 BC cases, including tentatively diagnosed cases. Among those, 21 748 patients had undergone surgery for BC. After excluding patients with data recorded for less than 6 months in the JMDC, 15 298 women with ESBC who underwent their first surgery between January 2010 and December 2019 were selected. Patients with a history of heart failure and metastatic or recurrent BC were excluded, yielding 12 060 patients. Figure 1 shows the flowchart of patient selection. As the JMDC is a claims database using the FFS insurance system in Japan, there were no missing data other than withdrawals during the study period.
Figure 1.
Flowchart of patient selection. BC, breast cancer; DL, dyslipidaemia; DM, diabetes mellitus; HT, hypertension; JMDC, Japan Medical Data Center database.
Baseline patient characteristics
Table 1 summarises the baseline characteristics of the patients before and after PS matching.
Table 1.
Patient characteristics and treatment strategies of crude and propensity score (PS)-matched cohorts
| Crude | After PS match | |||||||||
| TZ | NT | Total | TZ | NT | Total | |||||
| (N=1005) | (N=11 055) | (N=12 060) | P value | (N=1005) | (N=2010) | (N=3015) | P value | |||
| Age | ||||||||||
| N | 1005 | 11 055 | 12 060 | 1005 | 2010 | 3015 | ||||
| Mean (SD) | 51.1 (8.90) | 50.8 (8.53) | 50.8 (8.56) | 0.2746 | * | 51.1 (8.90) | 51.4 (8.71) | 51.3 (8.77) | 0.3454 | * |
| Median | 51 | 50 | 50 | 51 | 51 | 51 | ||||
| (IQR) | (45.0, 57.0) | (45.0, 56.0) | (45.0, 56.0) | (45.0, 57.0) | (45.0, 57.0) | (45.0, 57.0) | ||||
| Cardiovascular risk factors | ||||||||||
| DM(E10-E14, A10) | 26 (2.6%) | 225 (2.0%) | 251 (2.1%) | 0.2407 | † | 26 (2.6%) | 57 (2.8%) | 83 (2.8%) | 0.6939 | † |
| HT(I10-I15, C03, C07-C09) | 68 (6.8%) | 661 (6.0%) | 729 (6.0%) | 0.3162 | † | 68 (6.8%) | 149 (7.4%) | 217 (7.2%) | 0.5171 | † |
| DL(E78, C10) | 36 (3.6%) | 465 (4.2%) | 501 (4.2%) | 0.3424 | † | 36 (3.6%) | 73 (3.6%) | 109 (3.6%) | 0.945 | † |
| Baseline assessments | ||||||||||
| Ehocardiography(160 072 510) | 286 (28.5%) | 2175 (19.7%) | 2461 (20.4%) | <0.0001 | † | 286 (28.5%) | 464 (23.1%) | 750 (24.9%) | 0.0013 | † |
| MMG(170 026 910) | 911 (90.6%) | 10 364 (93.7%) | 11 275 (93.5%) | 0.0001 | † | 911 (90.6%) | 1899 (94.5%) | 2810 (93.2%) | <0.0001 | † |
| US_Br(160 165 010) | 994 (98.9%) | 10 938 (98.9%) | 11 932 (98.9%) | 0.9147 | † | 994 (98.9%) | 1987 (98.9%) | 2981 (98.9%) | 0.9029 | † |
| CNB(D410) | 727 (72.3%) | 7747 (70.1%) | 8474 (70.3%) | 0.1332 | † | 727 (72.3%) | 1444 (71.8%) | 2171 (72.0%) | 0.7742 | † |
| ER(160 060 350) | 881 (87.7%) | 9445 (85.4%) | 10 326 (85.6%) | 0.0542 | † | 881 (87.7%) | 1737 (86.4%) | 2618 (86.8%) | 0.341 | † |
| HER2(160 173 550) | 863 (85.9%) | 9017 (81.6%) | 9880 (81.9%) | 0.0007 | † | 863 (85.9%) | 1689 (84.0%) | 2552 (84.6%) | 0.1863 | † |
| Treatment strategies | ||||||||||
| Radiotherapy(M000, M001) | 486 (48.4%) | 6019 (54.4%) | 6505 (53.9%) | 0.0002 | † | 486 (48.4%) | 960 (47.8%) | 1446 (48.0%) | 0.7571 | † |
| Systemic therapy(L01, L02) | 1005 (100.0%) | 4822 (43.6%) | 5827 (48.3%) | <0.0001 | † | 1005 (100.0%) | 1449 (72.1%) | 2454 (81.4%) | <0.0001 | † |
| Alkyl(L01A) | 738 (73.4%) | 2385 (21.6%) | 3123 (25.9%) | <0.0001 | † | 738 (73.4%) | 1233 (61.3%) | 1971 (65.4%) | <0.0001 | † |
| Anti Metab(L01B) | 230 (22.9%) | 820 (7.4%) | 1050 (8.7%) | <0.0001 | † | 230 (22.9%) | 455 (22.6%) | 685 (22.7%) | 0.8779 | † |
| Vinca(L01C1) | 6 (0.6%) | 24 (0.2%) | 30 (0.2%) | 0.0206 | † | 6 (0.6%) | 12 (0.6%) | 18 (0.6%) | 1.0000 | † |
| Taxan(L01C2) | 780 (77.6%) | 2109 (19.1%) | 2889 (24.0%) | <0.0001 | † | 780 (77.6%) | 997 (49.6%) | 1777 (58.9%) | <0.0001 | † |
| Anthra(L01D) | 591 (58.8%) | 1594 (14.4%) | 2185 (18.1%) | <0.0001 | † | 591 (58.8%) | 1182 (58.8%) | 1773 (58.8%) | 1.0000 | † |
| Trastuzumab(L01XC03) | 1005 (100.0%) | 0 (0.0%) | 1005 (100.0%) | 0 (0.0%) | ||||||
| Hormone Anti(L02B) | 651 (64.8%) | 8032 (72.7%) | 8683 (72.0%) | <0.0001 | † | 651 (64.8%) | 1310 (65.2%) | 1961 (65.0%) | 0.8289 | † |
| SERM(L02B1) | 388 (38.6%) | 5486 (49.6%) | 5874 (48.7%) | <0.0001 | † | 388 (38.6%) | 841 (41.8%) | 1229 (40.8%) | 0.0885 | † |
| Aroma I(L02B3) | 327 (32.5%) | 3370 (30.5%) | 3697 (30.7%) | 0.1765 | † | 327 (32.5%) | 646 (32.1%) | 973 (32.3%) | 0.8256 | † |
| AntiEmet(A04A) | 831 (82.7%) | 2681 (24.3%) | 3512 (29.1%) | <0.0001 | † | 831 (82.7%) | 1271 (63.2%) | 2102 (69.7%) | <0.0001 | † |
| CSF(L03A1) | 455 (45.3%) | 1359 (12.3%) | 1814 (15.0%) | <0.0001 | † | 455 (45.3%) | 713 (35.5%) | 1168 (38.7%) | <0.0001 | † |
| Trastuzumab use | ||||||||||
| Duration (months) | ||||||||||
| Mean (SD) | 11.7 (2.98) | 11.7 (2.98) | ||||||||
| Median | 13 | 13 | ||||||||
| (IQR) | (12.0, 13.0) | (12.0, 13.0) | ||||||||
| First dose after surgery (months) | ||||||||||
| Median | 4 | 4 | ||||||||
| (IQR) | (2.0, 5.0) | (2.0, 5.0) | ||||||||
*Equal variance two sample t-test.
† Chi-square p value.
Alkyl, alkylating agents (eg, cyclophosphamide [L01AA01]); Anthra, anthracyclines (eg, doxorubicin [L01DB01], epirubicin [L01DB03]); AntiEmet, antiemetics; Anti Metab, antimetabolites (eg, 5-fluorouracil [(L01BC02]), capecitabine [L01BC06]); Aroma_I, aromatase inhibitors (eg, anastrozole [L02BG03], letrozole [L02BG04]); CNB, needle biopsy; CSF, colony-stimulating factors; DL, dyslipidaemia; DM, diabetes mellitus; ER, oestrogen receptor; HER2, human epidermal growth factor receptor 2; HT, hypertension; MMG, mammography; NT, non-users; SEAM, anti-oestrogens (eg, tamoxifen [L02BA01], toremifene [L02BA02], fulvestrant [L02BA03]); Taxan(s), (eg, paclitaxel [L01CD01], docetaxel [L01CD02]); TZ, trastuzumab users; US_Br, breast ultrasound; Vinca, vinca alkaloids.
Before matching, the mean age was 50.8±8.56 years. Trastuzumab was prescribed in 1005 cases (8.3%) and anthracycline in 2185 (18.1%) cases; preoperative mammography was performed in 11 275 cases (93.5%) and breast ultrasound in 11 932 (98.9%) cases. A needle biopsy was performed in 8474 (70.3%) cases, and echocardiography was recorded in 2461 (20.4%) cases. The number of cases with the preoperative cardiovascular risk factors such as diabetes mellitus, hypertension and dyslipidaemia were 251 (2.1%), 729 (6.0%) and 501 (4.2%), respectively.
There were 3015 patients in the 1:2 PS matching for trastuzumab administration (trastuzumab use group; TZ, n=1005 vs no trastuzumab use group; NT, n=2010), and covariates were balanced between the groups after PS matching. The mean age was 51.3±8.77 years (TZ 51.1±8.90 years vs NT 51.4±8.71 years).
Preoperative cardiovascular risk of diabetes, hypertension and dyslipidaemia was noted in 83 (2.8%) (TZ 26 (2.6%), NT 57 (2.8%)), 217 (7.2%) (TZ 68 (6.8%), NT 149 (7.4%)) and 109 (3.6%) (TZ 36 (3.6%), NT 73 (3.6%)) cases, respectively.
Treatment strategy for BC
Preoperative diagnosis of ESBC, systemic therapies and endocrine therapy are summarised in table 1. The mean duration of trastuzumab therapy was 11.7 months (SD: 2.98). The time lag between the surgery and the first dose of trastuzumab had a median of 4.0 (IQR: 2.0–5.0) months. Radiotherapy was performed in 1446 cases (48.0%) (TZ 486 (48.4%) vs NT 960 (47.8%)).
Baseline cardiac assessment and patient outcomes
Table 2 shows the cumulative incidence in the PS-matched population with age, cardiovascular risk factors and treatment strategies, including anthracyclines. Within 18 months after surgery, the number of CHF cases was 115 (1.0%), with 31 all-cause mortalities (0.3%).
Table 2.
Patient outcomes of crude and propensity score (PS)-matched cohorts
| Crude | After PS match | |||||||
|
TZ (N=1005) |
NT (N=11 055) |
Total (N=12 060) |
P value |
TZ (N=1005) |
NT (N=2010) |
Total (N=3015) |
P value | |
| Months after surgery | 0.0881* | 0.2018* | ||||||
| N | 1005 | 11 055 | 12 060 | 1005 | 2010 | 3015 | ||
| Mean (SD) | 36.9 (25.15) | 35.5 (25.59) | 35.6 (25.55) | 36.9 (25.15) | 35.7 (25.67) | 36.1 (25.50) | ||
| Median | 30 | 29 | 29 | 30 | 29 | 29 | ||
| (IQR) | (18.0, 50.0) | (16.0, 49.0) | (16.0, 49.0) | (18.0, 50.0) | (16.0, 50.0) | (17.0, 50.0) | ||
| DM(E10-E14, A10) | 10 (1.0%) | 89 (0.8%) | 99 (0.8%) | 0.5228* | 10 (1.0%) | 20 (1.0%) | 30 (1.0%) | 1.0000* |
| HT(I10-I15, C03, C07-C09) | 55 (5.5%) | 317 (2.9%) | 372 (3.1%) | <.0001* | 55 (5.5%) | 90 (4.5%) | 145 (4.8%) | 0.2287* |
| DL(E78, C10) | 50 (5.0%) | 239 (2.2%) | 289 (2.4%) | <.0001* | 50 (5.0%) | 62 (3.1%) | 112 (3.7%) | 0.0097* |
| Diuretics(C03) | 205 (20.4%) | 784 (7.1%) | 989 (8.2%) | <.0001* | 205 (20.4%) | 299 (14.9%) | 504 (16.7%) | 0.0001* |
| Frosemide(C03C A01) | 178 (17.7%) | 628 (5.7%) | 806 (6.7%) | <.0001* | 178 (17.7%) | 266 (13.2%) | 444 (14.7%) | 0.0011* |
| Spironolactone(C03DA01) | 61 (6.1%) | 225 (2.0%) | 286 (2.4%) | <.0001* | 61 (6.1%) | 105 (5.2%) | 166 (5.5%) | 0.3372* |
| Tolvaptan(C03XA01) | 3 (0.3%) | 8 (0.1%) | 11 (0.1%) | 0.0230* | 3 (0.3%) | 3 (0.1%) | 6 (0.2%) | 0.3860* |
| BB(C07) | 29 (2.9%) | 173 (1.6%) | 202 (1.7%) | 0.0018* | 29 (2.9%) | 31 (1.5%) | 60 (2.0%) | 0.0128* |
| Carvedirol(C07AG02) | 7 (0.7%) | 26 (0.2%) | 33 (0.3%) | 0.0074* | 7 (0.7%) | 9 (0.4%) | 16 (0.5%) | 0.3755* |
| Bisoprorol(C01AA05) | 1 (0.1%) | 6 (0.1%) | 7 (0.1%) | 0.5687* | 1 (0.1%) | 2 (0.1%) | 3 (0.1%) | 1.0000* |
| RAS(C09) | 45 (4.5%) | 304 (2.7%) | 349 (2.9%) | 0.0018* | 45 (4.5%) | 63 (3.1%) | 108 (3.6%) | 0.0614* |
| Captopril(C09AA01) | 2 (0.2%) | 16 (0.1%) | 18 (0.1%) | 0.6696* | 2 (0.2%) | 3 (0.1%) | 5 (0.2%) | 0.7516* |
| Enarapril(C09AA02) | 5 (0.5%) | 15 (0.1%) | 20 (0.2%) | 0.0070* | 5 (0.5%) | 3 (0.1%) | 8 (0.3%) | 0.0797* |
| Amiodarone(C01BD01) | 1 (0.1%) | 5 (0.0%) | 6 (0.0%) | 0.4601* | 1 (0.1%) | 1 (0.0%) | 2 (0.1%) | 0.6170* |
| Digoxin(C07AB07) | 17 (1.7%) | 78 (0.7%) | 95 (0.8%) | 0.0007* | 17 (1.7%) | 16 (0.8%) | 33 (1.1%) | 0.0259* |
| Death | 19 (1.9%) | 111 (1.0%) | 130 (1.1%) | 0.0092* | 19 (1.9%) | 53 (2.6%) | 72 (2.4%) | 0.2058* |
| Death (18mo) | 4 (0.4%) | 27 (0.2%) | 31 (0.3%) | 0.3566* | 4 (0.4%) | 10 (0.5%) | 14 (0.5%) | 0.7048* |
| CHF | 44 (4.4%) | 132 (1.2%) | 176 (1.5%) | <.0001* | 44 (4.4%) | 48 (2.4%) | 92 (3.1%) | 0.0027* |
| CHF (18mo) | 33 (3.3%) | 82 (0.7%) | 115 (1.0%) | <.0001* | 33 (3.3%) | 28 (1.4%) | 61 (2.0%) | 0.0005* |
| Events/n | 33/1005 | 82/11055 | 115/12060 | <.0001† | 33/1005 | 28/2010 | 61/3015 | 0.0009† |
| HR (95% CI) | 4.32 (2.88 to 6.47) | Reference | 2.28 (1.38 to 3.77) | Reference | ||||
*Chi-square p value.
†Logrank p value.
BB, beta-blocking agents; CHF, congestive heart failure; Death, all-cause deaths; Death (18 months), all-cause deaths within 18 months post-surgery; DL, dyslipidaemia; DM, diabetes mellitus; RAS, angiotensin-converting enzymes or angiotensin II receptor blocking agents.
After PS matching, the number of patients for whom CHF therapy was initiated after surgery and included diuretics, beta-blockers and angiotensin-converting enzyme inhibitors or receptor blockers was 504 (16.7%) (TZ 205 (20.4%) vs NT 299 (14.9%)), 60 (2.0%) (TZ 29 (2.9%) vs NT 31 (1.5%)) and 108 (3.6%) (TZ 45 (4.5%) vs NT 63 (3.1%)), respectively. The number of patients for whom CHF therapy was initiated within 18 months was 61 (2.0%) (TZ 33 (3.3%) vs NT 28 (1.4%)), with 18 all-cause deaths within 18 months (0.5%) (TZ 4 (0.4%) vs NT 10 (0.5%)).
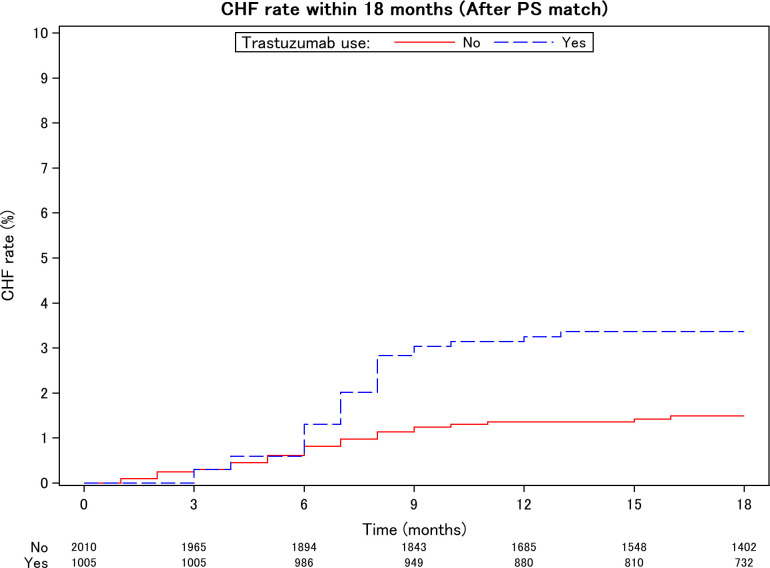
The HR of CHF in the trastuzumab group was 2.28 (95% CI 1.38 to 3.77) in the PS-matched population. In the crude population, the HR was 4.32 (95% CI 2.88 to 6.47), exhibiting the same trend. The cumulative rate of CHF with trastuzumab is shown in the Kaplan-Meier curve in figure 2. As a subgroup analysis, because the known cardiotoxicity of concomitant anthracyclines (AC) may modify the effect of trastuzumab, we calculated the HR and Kaplan-Meier curve using AC (online supplemental table 1, figure 1).
Figure 2.
Cumulative incidence of postoperative heart failure within 18 months among women with early-stage breast cancer after propensity score (PS) matching. Cumulative incidence of postoperative heart failure within 18 months between groups was significantly different (log-rank test p=0.0009, HR=2.28 (95% CI: 1.38 to 3.77). CHF, congestive heart failure.
openhrt-2022-002053supp001.pdf (169.1KB, pdf)
The number of patients with preoperative echocardiography was 750 (24.9%) (TZ 286 (28.5%) vs NT 464 (23.1%)) (table 1). Within the trastuzumab with anthracycline combination group (n=591), the percentage of baseline cardiac assessment with echocardiography (%echocardiography) was 27.2% (preoperatively), 66.0% (pre-AC) and 86.6% (pre-TZ) (table 3).
Table 3.
Timing of baseline cardiac assessment with echocardiography
| Anthracyclines | Total | ||
| No | Yes | ||
| (N=414) | (N=591) | (N=1005) | |
| Pre-ope | 125 (30.2%) | 161 (27.2%) | 286 (28.5%) |
| Pre-AC | 390 (66.0%) | ||
| Pre-TZ | 323 (78.0%) | 512 (86.6%) | 835 (83.1%) |
Pre-AC, period from surgery to anthracycline prescription; Pre-ope, preoperative; Pre-TZ, period from surgery to trastuzumab prescription.
Discussion
To the best of our knowledge, this is the first RWD study in Japan to investigate trastuzumab cardiotoxicity using a nationwide claim-based database. A significant increase in the incidence of heart failure following adjuvant chemotherapy was observed, although there is room for improvement in baseline cardiovascular risk assessment.
One of the most important deliverables of this study is the RWD on ethnic factors of trastuzumab cardiotoxicity for the new interdisciplinary field of cardio-oncology.26 Early studies in Japan, a country with a traditionally low incidence of cardiovascular disease, showed low trastuzumab cardiotoxicity.27 A population-based study in Taiwan also suggested that cardiotoxicity may be lower in East Asia.28 In contrast, our previous study conducted in a Japanese teaching hospital identified a mild decrease in left ventricular ejection fraction (LVEF) in 26% of the examined cases, severe decreases in LVEF in 13%, symptomatic heart failure in 6% and premature termination of cancer treatment in 2% in patients undergoing adjuvant trastuzumab therapy.24 In our current RWD study, trastuzumab cardiotoxicity remained statistically significant after adjusting for the effect of AC administration. The advantages of using claims data from FFS payment systems under universal healthcare systems are low rates of missing values and high generalisability.20 Thus, our combined results support that all patients require optimum care with cancer therapeutic-related cardiac dysfunction in mind, regardless of ethnicity.
To maximise the specificity of our definitions of heart failure and cardiotoxicity, we employed a combination of diagnosis (ICD-10) and prescription (ATC) rather than diagnosis codes alone. The key to increasing the specificity of JMDC derives from a report by Fujihara et al,21 who showed that diagnosis codes alone in the Japanese insurance claims database might be insufficient to identify heart failures. In their validation study using intensive chart review as the gold standard, ‘tentative diagnosis’ codes for billing purposes increased false-positive rates. In addition, false-negative rates were higher with diuretics or cardioprotective drugs without heart failure codes. In our current study, a combination of diagnosis and prescription codes allowed us to quantify the clinical course of the patients consistently with our previous chart review.24 In addition, interhospital aggregation using the JMDC anonymised link system increased the sensitivity by revealing real-world risks of heart failure recorded outside cancer centres. Thus, we believe that our algorithms to identify heart failure and cardiotoxicity in this claim-based analysis are clinically relevant.
RWE requires validations from several perspectives, including temporality and biological gradient.16 We focused on heart failure at 18 months postsurgery, as adjuvant trastuzumab is associated with a temporal risk of cardiotoxicity during the treatment period but not thereafter.22 23 In addition, our previous study24 showed that most incidences of heart failure occurred during trastuzumab therapy (mean 12 months), although it was confounded by the concomitant use of anthracyclines. We used a large insurance claim database for the present investigation to allow PS matching using age, cardiovascular risk factors and treatment strategies, including anthracyclines and antitumour hormone therapy. Heart failure within 18 months remained associated with trastuzumab after adjustment for potential confounders (HR=2.28 (CI 1.38 to 3.77)) (table 2). Even after adjusting for confounding factors, trastuzumab and heart failure were associated during the initial 1.5 years of treatment (figure 2), after which no independent association was observed. The timing of the median first dose of trastuzumab (table 1), a median of 4 months (IQR: 2–5 months), was consistent with the steep rise in the cumulative rate of CHF from 6 to 9 months (figure 2). In this study, we could not evaluate the dose-dependency of trastuzumab because the cumulative dose could be either a cause or a result of cardiotoxicity. However, the combination group, in particular, exhibited a stronger trend and higher increase in the cumulative rate of cardiotoxicity than those in the other groups, suggesting a biological gradient in cardiotoxicity (online supplemental figure 1). Therefore, our RWD study is consistent with our previous chart review24 and other studies.22 23
We also detected a suboptimal baseline cardiovascular risk assessment as a quality indicator for improvement from this RWD study. Although our Japanese claims database analysis is consistent with the previously known cardiotoxicity, there is room for improving the rates of baseline echocardiography for patients receiving trastuzumab, especially with AC. In our study, especially in the combination anticancer therapy group (n=591), 66.0% (pre-AC) and 86.6% (Pre-TZ) of the patients underwent echocardiography, which raises the concern that prophylactic decision-making may have been delayed (table 3). The low compliance in Japan may be due to the insufficient allocation of cardiologists to cancer centres.25 Alternatively, it may be because of the global concern that frequent imaging and biomarker measurements for cardiac function may lead to an unnecessary interruption of cancer treatment.9 As cardiac monitoring is expected to support the completion of trastuzumab-based therapy effectively, clinical validations in new biomarkers29 and imaging modalities30 for monitoring cardiac function during cancer treatment are underway. Therefore, the latest guidelines recommend at least baseline cardiovascular risk assessment in patients with cancer scheduled to undergo cardiotoxic cancer treatment.31 We believe that future RWD studies in cardio-oncology should help narrow the gap between guidelines and practice by efficiently providing data to improve patient outcomes.16
Study limitations
As with any observational study, ours had some limitations. First, RWE requires validations from several perspectives, including biological gradient, temporality, strength, consistency, specificity and validity.16 However, there may be unknown temporality factors in trastuzumab beyond our focus of 18 months. Furthermore, the effect of unmeasured confounders remains even after the PS matching. Therefore, randomised controlled trials remain the gold standard. Second, the JMDC has a ‘healthy-worker bias’ with a high proportion of employed people and a low prevalence of hypertension.18 However, robust research needs ‘triangulation’ with many lines of evidence.16 Therefore, RWD from claims databases need to be interpreted using other data sources with different characteristics, such as randomised controlled trials and patient registries. Third, although we leveraged our previous study24 to increase clinical relevance, the terms ‘heart failure’ and ‘cardiotoxicity’ used in this claim-based analysis should be interpreted with caution. Although we matched diagnosis, prescription and surgery codes on the same monthly insurance claim receipts to exclude ‘tentative diagnosis’ and increase specificity, the sensitivity might have been compromised. Therefore, clinical validation for heart failure diagnosis with imaging,30 biomarkers29 and other data sources16 is needed to support RWD studies. Fourth, the JMDC does not include all factors related to intrinsic or extrinsic ethnic factors. Therefore, a fit-for-purpose design is needed to evaluate the gene, environment and gene–environment interactions. Finally, although echocardiography is the guideline-recommended modality for baseline cardiovascular risk assessment,31 32 it is far from ideal as a risk stratification tool for cancer outcomes. Therefore, there is a need for cardio-oncology studies to evaluate the rate of cancer treatment completion and, ultimately, overall survival.
Conclusions
We found an increased risk of heart failure development in women who underwent adjuvant trastuzumab therapy compared with the control group matched with risk factors and the effect of anthracyclines based on Japanese claims data. Notably, the RWD revealed a lower implementation of baseline cardiac assessment in the patients with a higher risk of trastuzumab cardiotoxicity due to AC use. Therefore, further studies applying this new methodology to improve quality indicators in the emerging field of cardio-oncology are warranted.
Acknowledgments
We thank Kiyoshi Matsuoka, MS, for the help with biostatistics, Shiro Matsuya, MS, for the excellent assistance as a data scientist, and Editage (https://authorservices.bmj.com/) for English language editing.
Footnotes
Twitter: @hiro_stat, @ksase
Contributors: HO designed the claim-based analysis, wrote the statistical analysis plan, analysed the data and drafted the manuscript. AS and CS analysed the breast cancer-related data and drafted the manuscript. SM and SU performed data collection, analysed all cardiovascular endpoints and critically reviewed the manuscript. SK designed the claim-based analysis, analysed the data, drafted the manuscript, and is the guarantor of the study. All authors provided final approval of the manuscript.
Funding: This study was partially supported by JSPS/MEXT (KAKENHI 18K12134 for HO and KS, 20K08427 for HO, SU and KS), MHLW (20FA1018 for KS and HO and 20KC2009 for KS), and AMED (20ck0106633h0001 for HO and KS).
Competing interests: AS received lecture fees from Chugai and Eli-Lilly and received research grant from Chugai, AstraZeneca, Daiichi Sankyo, Eisai and Taiho. NY is an employee and a shareholder of Pfizer. SU received grants from Kowa, Bayer, Bristol-Myers Squibb, Pfizer, Daiichi Sankyo, and Takeda and received lecture fees from Eisai, Novartis, MSD, and Boelinger Ingelheim. CS received research grant from Eli-Lilly. KS received lecture fees from Daiichi Sankyo. All of the above are unrelated to the submitted work. All other authors have no conflicts with any industry.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The Institutional Review Board of Juntendo University approved the protocol of this study (JM#2021009). The study protocol followed the Ethical Guidelines for Medical Research Involving Human Subjects (Ministry of Health, Labour, and Welfare of Japan) and the World Medical Association Declaration of Helsinki. The need for informed consent was waived in this observational study as the data were already anonymised.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783–92. 10.1056/NEJM200103153441101 [DOI] [PubMed] [Google Scholar]
- 3.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005;353:1659–72. 10.1056/NEJMoa052306 [DOI] [PubMed] [Google Scholar]
- 4.Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol 2002;20:1215–21. 10.1200/JCO.2002.20.5.1215 [DOI] [PubMed] [Google Scholar]
- 5.Rosen SD. Trastuzumab induced cardiomyopathy: wider implications for cardio-oncology. Heart 2013;99:599–600. 10.1136/heartjnl-2012-303495 [DOI] [PubMed] [Google Scholar]
- 6.Rushton M, Lima I, Tuna M, et al. Impact of Stopping Trastuzumab in Early Breast Cancer: A Population-Based Study in Ontario, Canada. J Natl Cancer Inst 2020;112:1222–30. 10.1093/jnci/djaa054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armenian SH, Lacchetti C, Barac A, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of clinical oncology clinical practice guideline. J Clin Oncol 2017;35:893–911. 10.1200/JCO.2016.70.5400 [DOI] [PubMed] [Google Scholar]
- 8.Curigliano G, Lenihan D, Fradley M, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol 2020;31:171–90. 10.1016/j.annonc.2019.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dang CT, Yu AF, Jones LW, et al. Cardiac surveillance guidelines for trastuzumab-containing therapy in early-stage breast cancer: getting to the heart of the matter. J Clin Oncol 2016;34:1030–3. 10.1200/JCO.2015.64.5515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subar M, Lin W, Chen W, et al. Lack of uniformity in cardiac assessment during trastuzumab therapy. Breast J 2011;17:383–90. 10.1111/j.1524-4741.2011.01101.x [DOI] [PubMed] [Google Scholar]
- 11.Lenneman CG, Sawyer DB. Cardio-oncology: an update on cardiotoxicity of cancer-related treatment. Circ Res 2016;118:1008–20. 10.1161/CIRCRESAHA.115.303633 [DOI] [PubMed] [Google Scholar]
- 12.Levis BE, Binkley PF, Shapiro CL. Cardiotoxic effects of anthracycline-based therapy: what is the evidence and what are the potential harms? Lancet Oncol 2017;18:e445–56. 10.1016/S1470-2045(17)30535-1 [DOI] [PubMed] [Google Scholar]
- 13.Mehta LS, Watson KE, Barac A, et al. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American heart association. Circulation 2018;137:e30–66. 10.1161/CIR.0000000000000556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Boer RA, Hulot J-S, Tocchetti CG, et al. Common mechanistic pathways in cancer and heart failure. A scientific roadmap on behalf of the translational research Committee of the heart failure association (HFA) of the European Society of cardiology (ESC). Eur J Heart Fail 2020;22:2272–89. 10.1002/ejhf.2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sherman RE, Anderson SA, Dal Pan GJ, et al. Real-world evidence - what is it and what can it tell us? N Engl J Med 2016;375:2293–7. 10.1056/NEJMsb1609216 [DOI] [PubMed] [Google Scholar]
- 16.Ohtsu H, Shimomura A, Sase K. Real-world evidence in cardio-oncology: what is it and what can it tell us? JACC CardioOncol 2022;4:95–7. 10.1016/j.jaccao.2022.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura S, Sato T, Ikeda S, et al. Development of a database of health insurance claims: standardization of disease classifications and anonymous record linkage. J Epidemiol 2010;20:413–9. 10.2188/jea.JE20090066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagai K, Tanaka T, Kodaira N, et al. Data resource profile: JMDC claims database sourced from health insurance societies. J Gen Fam Med 2021;22:118–27. 10.1002/jgf2.422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujioka I, Ohtsu H, Yonemoto N, et al. Association between prenatal exposure to antidepressants and neonatal morbidity: an analysis of real-world data from a nationwide claims database in Japan. J Affect Disord 2022;310:60–7. 10.1016/j.jad.2022.04.103 [DOI] [PubMed] [Google Scholar]
- 20.Behrendt C-A, Debus ES, Mani K, et al. The strengths and limitations of claims based research in countries with fee for service reimbursement. Eur J Vasc Endovasc Surg 2018;56:615–6. 10.1016/j.ejvs.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 21.Fujihara K, Yamada-Harada M, Matsubayashi Y, et al. Accuracy of Japanese claims data in identifying diabetes-related complications. Pharmacoepidemiol Drug Saf 2021;30:594–601. 10.1002/pds.5213 [DOI] [PubMed] [Google Scholar]
- 22.Goldhar HA, Yan AT, Ko DT, et al. The temporal risk of heart failure associated with adjuvant trastuzumab in breast cancer patients: a population study. J Natl Cancer Inst 2016;108. 10.1093/jnci/djv301. [Epub ahead of print: 16 10 2015]. [DOI] [PubMed] [Google Scholar]
- 23.Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 2017;389:1195–205. 10.1016/S0140-6736(16)32616-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shinano H, Miyazaki S, Miura K, et al. Risk profiling of cancer treatment-related cardiovascular disorders in breast cancer patients who received adjuvant chemotherapy with trastuzumab. Circ Rep 2020;2:235–42. 10.1253/circrep.CR-19-0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011;10:150–61. 10.1002/pst.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oka T, Akazawa H, Sase K, et al. Cardio-Oncology in Japan. JACC CardioOncol 2020;2:815–8. 10.1016/j.jaccao.2020.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishihara M, Mukai H, Nagai S, et al. Cardiac safety of trastuzumab as adjuvant treatment for Japanese patients with early breast cancer. Int J Clin Oncol 2009;14:431–5. 10.1007/s10147-009-0898-z [DOI] [PubMed] [Google Scholar]
- 28.Chien H-C, Kao Yang Y-H, Bai JPF. Trastuzumab-related cardiotoxic effects in Taiwanese women: a nationwide cohort study. JAMA Oncol 2016;2:1317–25. 10.1001/jamaoncol.2016.1269 [DOI] [PubMed] [Google Scholar]
- 29.Pudil R, Mueller C, Čelutkienė J, et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: a position statement from the Cardio-Oncology Study group of the heart failure association and the Cardio-Oncology Council of the European Society of Cardiology. Eur J Heart Fail 2020;22:1966–83. 10.1002/ejhf.2017 [DOI] [PubMed] [Google Scholar]
- 30.Čelutkienė J, Pudil R, López-Fernández T, et al. Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: a position statement on behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the Cardio-Oncology Council of the European Society of Cardiology (ESC). Eur J Heart Fail 2020;22:1504–24. 10.1002/ejhf.1957 [DOI] [PubMed] [Google Scholar]
- 31.Lyon AR, Dent S, Stanway S, et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur J Heart Fail 2020;22:1945–60. 10.1002/ejhf.1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Baat EC, Naaktgeboren WR, Leiner T, et al. Update in imaging of cancer therapy-related cardiac toxicity in adults. Open Heart 2021;8:e001506. 10.1136/openhrt-2020-001506 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2022-002053supp001.pdf (169.1KB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available.