Abstract
Introduction
The perioperative period is high risk for older adults. Depression and anxiety are common perioperative problems, frequently coexisting with cognitive impairment. Older patients with these conditions are more likely than younger patients to experience postoperative delirium, long hospital stays, poor quality of life and rehospitalisation. These experiences can, in turn, exacerbate anxiety and depressive symptoms. Despite these risks, little is known about how to treat perioperative anxiety and depression among older adults.
Methods and analysis
We designed a feasibility study of a perioperative mental health intervention bundle to improve perioperative mental health, specifically depression and anxiety. The overarching goals of this study are twofold: first, to adapt and refine an intervention bundle comprised of behavioural activation and medication optimisation to meet the needs of older adults within three surgical patient populations (ie, orthopaedic, oncological and cardiac); and second, to test the feasibility of study procedures and intervention bundle implementation. Quantitative data on clinical outcomes such as depression, anxiety, quality of life, delirium, falls, length of stay, hospitalisation and pain will be collected and tabulated for descriptive purposes. A hybrid inductive–deductive thematic approach will be employed to analyse qualitative feedback from key stakeholders.
Ethics and dissemination
The study received approval from the Washington University Institutional Review Board. Results of this study will be presented in peer-reviewed journals, at professional conferences, and to our perioperative mental health advisory board.
Trial registration number
Keywords: MENTAL HEALTH, GERIATRIC MEDICINE, SURGERY
Strengths and limitations of this study
This perioperative mental health intervention bundle comprised of behavioural activation and medication optimisation will be the first of its kind focused on improving cognitive and mental health of older patients who undergo surgery and manage their symptoms of depression and anxiety along the perioperative continuum.
This study will iteratively adapt and test the feasibility of implementing a patient-centred perioperative mental health intervention bundle with psychological and pharmacological optimisation components.
Our approach will provide feasibility data on whether we can: (1) enrol patients, (2) collect and refine data collection methods, (3) implement the intervention bundle within the perioperative context, (4) tailor the intervention bundle for the three surgical cohorts and (5) determine whether a future randomised effectiveness-implementation trial of the intervention bundle in the perioperative setting is feasible.
Introduction
Americans undergo an average of nine surgeries in their lifetime.1 Over 51 million surgeries are performed in the USA each year, with older adults representing approximately half of all surgical patients.2 The perioperative period—encompassing preoperative (before surgery), intraoperative (during surgery) and postoperative (after surgery) phases—is a high-risk and vulnerable time for older patients. Older patients are at increased risk for postoperative morbidity and mortality compared with younger adults.3–10 Anxiety and depression in older surgical patients increase the risk of postoperative complications, including short-term functional dependence and falls,11 postoperative delirium,12 opioid misuse,13 14 decreased quality of life15 and readmission. A meta-analysis of over 200 000 patients undergoing cardiac surgery revealed significantly increased mortality risk among individuals with perioperative depression and anxiety.16
There have been efforts to reduce perioperative risks in older adults by optimising physical health prior to surgery (prehabilitation), implementing protocolised pathways during the surgical hospitalisation and also promoting postoperative rehabilitation (eg, enhanced recovery). However, no corresponding perioperative interventions have been developed to address cognitive and mental health and well-being. In other words, we lack effective interventions tailored for older surgical patients, in spite of the high prevalence of depression and anxiety in this population,17 frequent co-occurring cognitive impairment and detrimental impact on surgical recovery.18
In our prior needs assessment interview study with older surgical adults diagnosed with anxiety and depression and their treating clinicians,18 we found that older surgical patients had varying care experiences, depending on their symptoms in the perioperative setting. Fear and uncertainty leading into the surgery and poor management of their depression and anxiety medications postoperatively were of key concern. Clinicians treating this population similarly noted that patients have a fear of surgery, experience acute pain, and can suffer from postoperative neurocognitive disorders. They were also worried that central nervous system active medications could worsen outcomes, yet many patients reported taking these medications at a subtherapeutic dose of medications for mental health, suggesting a need to optimise their dosage. However, clinicians reported concerns that stopping these medications could lead to withdrawal symptoms, but maintaining them could worsen their cognitive and mental health impairment.
Patients and clinician stakeholders emphasised the need for a perioperative intervention bundle to address these issues and argued for a bundle encompassing psychological components that are behavioural, simple, interactive and engaging, and pharmacotherapy components that can minimise the risk of psychiatric medication withdrawal symptoms and improper dosages during perioperative care. They also recommended that such a mental health intervention bundle would be effective if it started preoperatively to assist with preparation for surgery and continued postoperatively to enhance recovery after surgery.18
Researchers have examined the use of counselling, cognitive–behavioral therapy (CBT), and other psychological treatments (eg, relaxation, mindfulness and supportive therapy) to promote the mental well-being of younger surgical patients.19–21 For example, Li and colleagues20 found that a psychological intervention provided to patients with cancer throughout the perioperative period was associated with decreased depressive symptoms and anxiety postoperatively. Similarly, research with patients undergoing orthopaedic surgery suggests that perioperative psychological education and counselling improved both psychological function,21 22 as well as physical function.21 In addition, studies suggest that a combination of pharmacological and psychotherapy is more effective at treating anxiety and depression in older adults than monotherapy23–28 and may be considered more acceptable to older adults,25 suggesting the need for an intervention bundle that combines psychotherapeutic and pharmacological treatment. Older adults often take many medications, and in the perioperative period, there is heightened risk of adverse drug reactions and drug–drug interactions.29 Medication optimisation and deprescription can help with reduction or elimination of potentially inappropriate medications (such as benzodiazepines), in conjunction with appropriate antidepressant dosing and continuation across outpatient and inpatient care transitions.
Our team has previously demonstrated the effectiveness of CBT and behavioural activation for depression in medically ill populations,30–35 and for anxious older adults with comorbid depression,28 and also of medication optimisation and deprescription for older adults36 in the perioperative setting.37 Informed by our prior work (see table 1 for features, clinical evidence and rationale), we propose to develop a perioperative mental health intervention bundle (hereafter referred to as the intervention bundle) encompassing two integrated components: behavioural activation (psychotherapy) and medication optimisation and deprescription (pharmacotherapy) for older surgical patients with anxiety and depression.
Table 1.
Details on adapted perioperative mental health bundle components
| Intervention bundle | Behavioural activation | Medication optimisation |
| Target | Patients | Clinicians and patients |
| Interventionist | Perioperative wellness partner. | Perioperative wellness partner follows an algorithm for medication optimisation and works alongside with pharmacists and a geriatric psychiatrist. |
| Description | A behavioural intervention helping depressed and anxious patients by engaging them in reinforcing activities or activities that are meaningful and guided by their personal values.66 | A pharmacological intervention to adjust suboptimal doses of antidepressants, ensure continuation of antidepressants during transitions of care and deprescribe medications that are harmful to older adults.67 68 |
| Features | Flexible component of cognitive–behavioral therapy (CBT) and standalone treatment in which the therapist helps a patient generate a list of pleasant, reinforcing activities and cocreates action plans. Patient-centred treatment, in which the patient chooses the modality (ie, which activities to engage in). |
Medication optimisation consists of a simple set of principles: identify the patient’s likely need for a medication adjustment, advise their provider to make the adjustment, and assess response29 69 70 Additionally, it involves a review of current medications (including over the counter) for those that are eligible for deprescribing, including strong centrally acting anticholinergic and antihistaminergic drugs and benzodiazepines. |
| Rationale for including | Comparative efficacy and non-inferiority trials have shown that behavioural activation is about as effective as comprehensive CBT, and it can be delivered by less highly trained staff. Trials in medically ill patients have emphasised behavioural activation because it complements medically indicated physical activation and exercise goals, and it is feasible and acceptable.31 |
Medication optimisation is a cardinal rule in treatment guidelines for depression.71 Antidepressants are often prescribed at subtherapeutic doses and then not adjusted for response.72 Suboptimal dosing is a main reason for these drugs’ low effectiveness in the real world.73 Strong centrally acting anticholinergic and antihistaminergic drugs and benzodiazepines are ‘low-hanging fruit’ for deprescribing as their harms outweigh benefits.74 They are harmful perioperatively, increasing falls and delirium.75–78 |
| Core active components |
|
|
| Modifiable components |
|
|
Developing the intervention bundle: adaptation process prior to feasibility evaluation
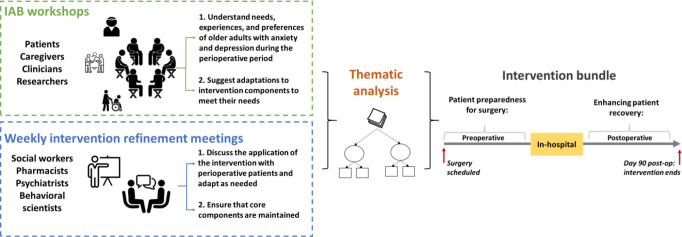
In preparation for this study, we organised an internal advisory board (IAB), comprised of older surgical patients, their caregivers, clinicians and researchers, to propose initial adaptations to the intervention bundle, informed by a collaborative planning approach. This approach integrates community-based participatory research with intervention mapping to guide intervention planning, implementation and evaluation.38 39 Intervention mapping is a step-by-step process that uses activities (eg, group discussions) and tools (eg, logic models) to develop a roadmap to inform the adaptation and implementation of interventions and has been used in a range of interventions and health issues.40 The IAB members participated in three workshop sessions, which provided us with an interactive forum to garner their perspectives and experiences with mental healthcare management and its impact on preparation before surgery and recovery after surgery. The sessions were moderated by an experienced qualitative researcher and focused on two key goals: (1) ascertain needs and design requirements for an intervention bundle to address the barriers associated with effective perioperative mental healthcare management and (2) suggest modifications to an intervention bundle to align with older surgical patient care pathways. We also held weekly meetings with interventionists including social workers, pharmacists, psychiatrists and behavioural scientists to refine and adapt the intervention bundle, based on the IAB input such that our bundle components integrates within the perioperative context and address needs of older adults. Transcripts of these sessions and the weekly meetings were thematically analysed to inform our preimplementation adaptations to ensure effectiveness, feasibility, acceptability and overall satisfaction of the intervention bundle.
Findings from this work pointed to three major design requirements and adaptations: first, the intervention bundle be initiated prior to surgery and continued after surgery to cover two phases (see figure 1): preoperative: focusing on improving patient preparedness for surgery, and postoperative: focusing on enhancing recovery (see section on components of the intervention bundle; appendices A and B for the detailed SOPs). Second, the term medication optimisation was suggested for the pharmacological component. Third, the term, perioperative wellness partner was formulated to refer to interventionists.
Figure 1.
Adaptation process of the perioperative mental health intervention bundle. IAB, internal advisory board.
Interventionists
The interventionists, referred to as perioperative wellness partners are masters-level clinicians trained in behavioural activation using the material developed by Puspitasari and colleagues.41 They will deliver the intervention bundle with oversight from study team members with knowledge in both medications and systems of care for perioperative management, including pharmacists, a psychologist, a geriatric psychiatrist and a licenced clinical social worker.
Components of the intervention bundle
Behavioural activation
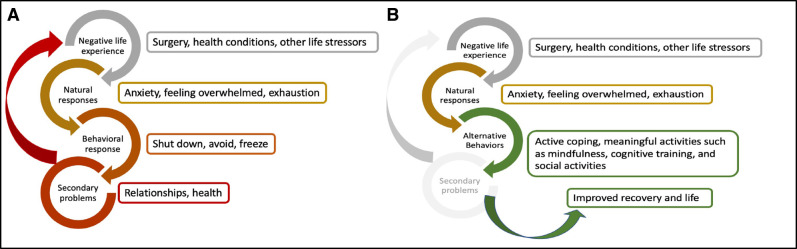
Figure 2 presents a model of behavioural activation for surgery. Behavioural activation will be practised according to Kanter’s Behavioral Activation for Depression.42 Behavioural activation as the core intervention allows for uniformity across participants yet enough flexibility for the actual components of behavioural activation to be individually adapted based on patient preferences. In addition to the core components of behavioural activation (table 1), study participants in collaboration with their perioperative wellness partner will be able to adapt the intervention by choosing activities, per their preference, with demonstrated benefit in improving depression and anxiety symptoms in older surgical patients.43 44 The behavioural activation process will be guided by the Behavioural Activation Standard Operating Procedure (BA SOP) (online supplemental appendix A), which will be adapted and calibrated as needed during the feasibility study.
Figure 2.
Behavioural activation model for the perioperative setting. (A) Behavioral activation – symptom cycles. (B) Behavioral activation – interrupting symptom cycles.
bmjopen-2022-062398supp001.pdf (91KB, pdf)
Medication optimisation
Patient antidepressant medications will be reviewed with the patient by the perioperative wellness partner, and based on the decision algorithm, are optimised by our study team of interventionists including a psychiatrist, psychologist and pharmacists. The medication optimisation process will be guided by the Medication Optimisation Standard Operating Procedure (MO SOP) (online supplemental appendix B), which will be adapted as needed during the feasibility study.
bmjopen-2022-062398supp002.pdf (120.1KB, pdf)
In this paper, we present a protocol for a prospective study to further adapt and test the feasibility of implementing our intervention bundle to reduce anxiety and depressive symptoms in older surgical patients undergoing cardiac, oncological and orthopaedic surgeries at a large academic medical centre. Towards this end, we will use frameworks from implementation science to capture the nuances and complexities unique to each patient population/setting that will inform our adaptation and implementation of the mental health intervention bundle. The Consolidated Framework for Implementation Research (CFIR)45 is a well-operationalised, multilevel determination framework derived from theory that will help us identify the determinants (ie, barriers and facilitators) that affect the implementation process across the three settings and populations. The framework has 39 constructs across five domains: intervention characteristics, inner setting, outer setting, characteristics of individuals and implementation process, which help elucidate the context and factors that affect implementation and intervention bundle evaluation. The Framework for Reporting Adaptations and Modifications-Expanded (FRAME)46 will allow us to systematically track all adaptations to the flexible components of the intervention bundle to ensure the feasibility, fit and relevance in older surgical patients, without compromising its core components.
Study objectives
The study objectives are summarised below:
Examine the feasibility of implementing a patient-centred intervention bundle for older surgical patients with clinically significant symptoms of depression and/or anxiety.
Iteratively test and adapt the intervention bundle and the implementation plan to make it patient centred, in response to the needs/demands of older surgical patients with clinically significant symptoms of depression and/or anxiety along the preoperative and postoperative phases.
Identify multiple stakeholder perspectives and experiences with the intervention bundle with specific emphasis on its implementation barriers, enablers and implementation strategies to ensure its reach, uptake and sustainability in perioperative settings.
Demonstrate the fidelity, acceptability and appropriateness of the intervention bundle delivery for older surgical patients with clinically significant symptoms of depression and/or anxiety.
Assess the feasibility of study procedures including patient recruitment, screening, outcome assessments and intervention materials for older patients.
Following this study, we will evaluate the effectiveness and implementation potential of our adapted intervention bundle using a randomised controlled trial.
Methods
Study design and approach
A mixed methods (quant+qual) approach supported by a parallel convergent study design will be followed; this will allow us to collect quantitative and qualitative data simultaneously) and merge the data in order to compare and interpret together.47 Quantitative data on anxiety and depression, quality of life, in-hospital delirium incidence, postdischarge falls, medications, length of stay, all-cause rehospitalisation, pain, patient experience and shared decision making will be collected. Qualitative surveys and interviews will help us to assess participants’ feedback and experiences about factors affecting implementation and use of the bundle.
Study participants and recruitment procedure
Patient participants include older adults undergoing cardiac, orthopaedic or oncology surgery receiving treatment at a large teaching hospital serving a catchment area including both urban and rural patients in a Midwest state in the USA. We will also invite their caregivers to participate in this study. Table 2 provides information about the expected enrolment numbers, inclusion criteria and exclusion criteria of participants.
Table 2.
Enrolment, inclusion criteria and exclusion criteria by type of participant
| Participant type | Expected enrolment | Inclusion criteria | Exclusion criteria* |
| Patients | 8–10 cardiac surgery patients. 8–10 orthopaedic surgery patients. 8–10 oncological surgery patients. |
|
|
| Caregivers | 24–30 caregivers will be recruited alongside patient participants. |
|
|
*Patients may meet any one or more of the exclusion criteria to become ineligible to participate.
†Patients must meet all eligibility criteria to participate.
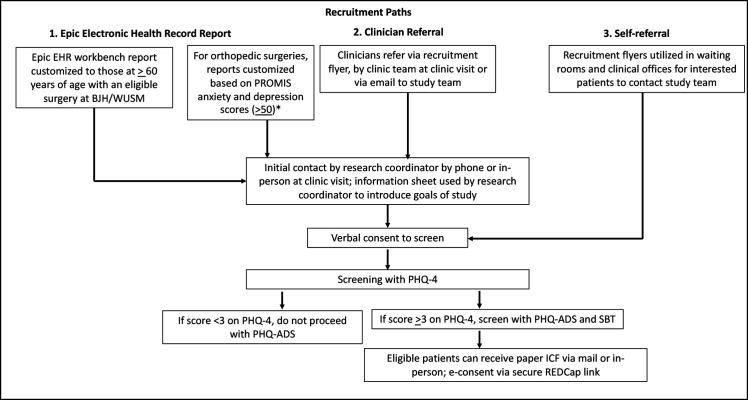
Patient participants will be recruited via three paths: Epic Electronic Health Record (EHR) report, clinician referral and self-referral (figure 3).
Figure 3.
Patient recruitment paths.
With the patients’ consent, caregiver participants will be recruited via two paths: patient referral to either contact the study team or share the caregiver’s phone number such that the study team will contact caregivers by phone or mail and invite them.
Assessment measures
At enrolment, a research coordinator will administer a battery of assessments to characterise patient participants and their current condition.
Patient baseline measures
Demographics
The following characteristics will be collected: age, sex, race/ethnicity, education level, employment status, psychiatric diagnosis, substance use and psychotropic medications.
Medical history of comorbidities
Patient medical history and comorbidities will also be collected.
Pain
The Brief Pain Invento (BPI) is a well-validated 11-item measure of pain severity and interference in pain,48–53 including after orthopaedic,54 oncological55 and cardiac surgery.56 Three questions from the BPI will be used to assess pain, including whether the patient is diagnosed with chronic pain, whether they experience pain daily in the past 3 months and if they have been experiencing pain in the past week related or unrelated to their surgery.
Short Blessed Test (SBT)
The SBT, sometimes called the Orientation-Memory-Concentration Test, is a six-item scale frequently used to assess dementia within patients across three dimensions: orientation, registration and attention. The SBT has demonstrated good test-retest reliability.57
Ultra-Brief Confusion Assessment Method (UB-CAM)
This two-item test58 is used for a quick assessment of delirium using items from the 3 min diagnostic interview for confusion assessment method (3D-CAM).59 Patients are asked to state the day of the week and months of the year backwards. If the UB-CAM is positive, the assessment continues with the full 3D-CAM.
Medication list
The research coordinator and perioperative wellness partner will review the patients’ medications from the EHR and confirmed with the patient at the initial intervention visit, capturing the medication name, dose, units, frequency, start date, stop date and indication, where appropriate.
Intervention adaptation measures
Intervention fidelity
Data related to intervention fidelity to examine the extent to which an intervention bundle is carried out by our perioperative wellness partner as intended and consistently across different settings, and patients will be tracked as adherence to core components of the intervention bundle, quality of delivery and participant responsiveness.60 All sessions will be audio recorded and reviewed by the supervising perioperative wellness partner (ie, trained in intervention bundle and is fidelity certified). Additionally, all intervention sessions will be reviewed and rated for fidelity by a team of researchers with training in the intervention bundle (licenced social worker and research assistant). Written and/or verbal feedback will be shared with our wellness partners.
Adaptations to the intervention bundle and its implementation
Adaptations are thoughtful and deliberate alterations to the flexible components of the intervention bundle, the format or delivery of the intervention bundle by perioperative wellness partners in order to improve its fit or effectiveness in a given context.46 Other changes may happen to the delivery of the intervention. Data on such adaptations and modifications will be collected during: (A) weekly case review intervention meetings where wellness partners will report on any changes they made to the intervention content and delivery method and their underlying rationale to implement that change and (B) periodic reflection meetings61 led by implementation scientists with the wellness partners where they will be asked to reflect on any modifications made deliberately and proactively, in response to unanticipated challenges in a given session or context.
The Behavioral Activation for Depression Scale – Short Form,62 or BADS-SF, is our measure of target engagement. The BADS-SF is a nine-item questionnaire derived from the original BADS63 questionnaire that consists of 25 items across four subscales: activation, avoidance/rumination, work/school impairment, and social impairment. It is frequently used to measure changes in behavioural activation levels following treatment.
Outcome measures for feasibility study
Outcomes for the feasibility study and their timepoints are provided in table 3. We will be assessing the reach of our study and our intervention bundle (ie, primary outcome), the feasibility of collecting depression and anxiety outcome planned for our randomised control trial (ie, secondary outcome) and implementation potential of intervention bundle and other outcomes such as quality of life and readmissions (ie, exploratory outcomes).
Table 3.
Feasibility study outcomes and potential study primary and secondary outcomes for planned randomised controlled trial (RCT).
| Outcomes | Specific measure: description | Source | Timepoint |
| Reach (primary) |
Reach of the study: patients who agreed to participate in the study out of total eligible to participate. Reach of the intervention bundle: patients who completed the interventions out of patients who agreed to participate in the pilot. |
Electronic health record and research data warehouse | End of study |
| Completeness of planned RCT primary outcome data collection at specified timepoints (secondary) |
Defined as a percentage of instrument or data fields completed for: Patient Health Questionnaire Anxiety and Depression Scale79: 16-item scale with components of the Patient Health Questionnaire-9 and Generalised Anxiety Disorder Scale (collected at baseline, 1 month, 3 months) | Research data warehouse | End of study |
| Implementation potential (exploratory) |
Acceptability, appropriateness and feasibility of the interventions: the acceptability of intervention measure, the intervention appropriateness measure and the feasibility of intervention measure.80 Each survey has four items in a Likert scale ranging from completely disagree to completely agree. | Surveys | End of study |
| Completeness of planned RCT secondary outcomes data collection at specified timepoints (exploratory) |
We will be measuring the completeness of data collection for the following potential secondary outcomes for the planned RCT secondary outcomes:
|
Research data warehouse | End of study |
Note: the surveys and questionnaires will be administered via email or research coordinators over the telephone.
Data related to participant recruitment, retention and assessments will be collected to help us ascertain if any modifications to the study procedures need to be made to inform sample size estimates and power calculations in subsequent randomised controlled trial studies.
To obtain participant perspectives on the intervention bundle, we will conduct semistructured interviews with patients and caregivers, and the topics of discussions will be guided by the CFIR constructs. The interviews will explore the participants’ perceptions, attitudes and experiences with the intervention bundle, intervention bundle acceptability and detailed accounts of participants’ experiences after the intervention has been stopped with regards to intervention sustainability and maintenance. These insights will inform whether the intervention bundle needs to be changed or adapted before our future trials. Interviews will be conducted via Zoom or telephone and will be digitally recorded and transcribed verbatim.
Data management and analysis plan
Data management
This study will be conducted under appropriate Washington University Institutional Review Board guidance and use only Institutional Review Board-approved study procedures and instruments. A unique patient number will be assigned at enrolment and used wherever possible on the case report forms to identify data, minimising use of patients’ names or personal identifiers in data.
Data analysis
Quantitative data collected for the outcome measures for the feasibility study will be tallied and summarised using descriptive statistics. Completion of data collection will be described as a percentage of the instruments completed. The primary outcome of anxiety and depression for the planned RCT will be tabulated for descriptive purposes.
Fidelity and adaptation data will be analysed using open coding and the FRAME analytic framework to help track any adaptations to intervention bundle and delivery.46 Interview data will be analysed using an inductive–deductive thematic analysis.64 After reading the transcripts multiple times for familiarity, research team members will openly code using data-driven codes and then using CFIR-driven codes. Codes will be compared across the data to identify repeated and interrelated concepts and categories, and subthemes will be formed. Similar subthemes will be grouped over multiple rounds of review to generate overarching themes out of significant patterns between interviews.
Patient and public involvement
In preparation for this study, we organised an internal advisory board with surgical patients, caregivers, clinicians (eg, physicians, nurses, pharmacists and social workers) and institutional leaders focused on patient experience to adapt our intervention bundle. Through the internal advisory board meetings, we sought to ensure that the intervention bundle facilitates patient preparedness for surgery during the preoperative period and enhances recovery during the postoperative period, coordinating and communicating with inpatient clinicians and to evaluate whether the intervention methods are practical and appropriate for the patient populations and clinicians, without affecting perioperative workflow.
Ethics and dissemination
Participant consents
Patients who meet all eligibility criteria and provide written informed consent will be enrolled into the study. Patient consent will be obtained via a paper collected by mail or in person or by secure REDCap link to e-consent. Caregivers (participating in semistructured interviews via Zoom/phone or in-person) will be consented verbally or with a written consent, depending on participant convenience.
Harms
This study involves minimal risk to subjects. Unlikely but potential risks include errors in medication recommendations; however, this risk is mitigated by the utilisation of a multidisciplinary group of experts agreeing on the recommendation and ongoing check points throughout the intervention process to ensure recommendations are correct and free from error. Additional risks include medication withdrawal symptoms as a result of the intervention recommendation and breach of confidentiality. The risk of medication withdrawal (ie, from benzodiazepines) is mitigated by slowly tapering rather than stopping these medications. If a participant endorses suicidal ideation, intent or plan, the coordinator and perioperative wellness partners are trained to follow an operationalised protocol (see online supplemental appendices A and B) that has been developed to manage high-risk participants in other studies of depressed participants potentially at risk for suicide. This protocol has already been used successfully by members of the research team to manage acutely suicidal patients. Patients will be encouraged to check with their physician if there is any question about the safety of any physical activities that are included in the behavioural activation plan. It is possible that the participant may feel uncomfortable completing the surveys or participating in the study sessions. The study sessions and interview can be discontinued at any time, and the patient may refuse to answer any questions that he or she does not wish to answer.
We will monitor for breaches of confidentiality and other adverse events on an ongoing basis. Once we become aware of a reportable adverse event, the event will be reported to Human Research Protection Office and Quality Assurance and Safety Monitoring Committee (QASMC) according to institutional guidelines. This study does not require QASMC audit or submission of DSM reports. Should any unexpected serious adverse events occur, our study protocol will be modified to prevent other similar events.
Internal auditing for data quality
The methodology core team meets biweekly with the research coordinators and data manager to review the study report on study enrolment, recruitment, monitor data quality and discuss the study progress and any issues raised by the participants.
Data safety and monitoring plan
The specific monitoring plan for this investigation is commensurate with the risks and the size and complexity of the studies planned. Given the nature of the protocol, the risks are likely limited to breach of confidentiality.
Dissemination
The feasibility study results will be disseminated at scientific meetings and peer-reviewed publications. Additionally, the results will be presented to our perioperative mental health internal and external advisory board consisting of patients, clinicians, nationally recognised researchers (psychiatry, health services and pain medicine) and hospital administrator stakeholder groups to determine which components of the intervention and its delivery to preserve, which adaptations to carry forward and how to advance with the randomised controlled trials. The Washington University Centre for Perioperative Mental Health website (https://perioperativewellness.wustl.edu/) will be used to introduce the intervention bundle to patients and clinicians alike. To accelerate the dissemination efforts, the Centre will use online communication channels including the Centre’s webpage, popular news media, social media, webinars and patient and family community networks. As per the National Institute of Mental Health sponsor guidelines, we will also be sharing the deidentified data to ClinicalTrials.gov.
Trial status
This study is registered in Clinical Trials Registry NCT05110690. Recruitment commenced during the last week of November 2021, and the enrolment is expected to conclude in March 2023.
Discussion
To our knowledge, the proposed perioperative mental health bundle will be the first of its kind to assist older surgical patients in managing their perioperative mental health. Current interventions need to be adapted and tested for older surgical patients, who face additional unique challenges such as frailty, multimorbidity with co-occurring cognitive and physical impairments, and polypharmacy that can also impact their mental health and well-being.11 65
The study protocol will adapt the intervention bundle comprised of behavioural activation and medication optimisation and provide evidence on the feasibility of testing the bundle as a potential intervention for anxiety and depression in older surgical patients. In addition, this study will provide feasibility data on implementing the bundle successfully within perioperative settings. Despite the empirical evidence available on behavioural activation and medication optimisation, there are unique challenges to using and implementing these interventions for the perioperative population of older adults, in perioperative settings notable for their complexity. To the best of our knowledge, the protocol is the first to adapt and examine the feasibility of the intervention bundle within the perioperative setting for older surgical patients. We will identify components of our intervention bundle and its delivery mechanism that can be common across the three different surgical populations, and also components that are unique for a particular population, and further for a particular patient based on their surgical pathways.
Results from this mixed method study will inform the following: first, findings related to experiences in participating in the intervention bundle, along with intervention fidelity and adaptation tracking, will allow us to finalise modifications to our initial ‘in-progress’ intervention bundle, resulting in a more patient-centred bundle that meets the needs and preferences of our diverse patients. Second, findings will determine if the components of the intervention bundle are feasible to be delivered in three very different settings in terms of dose, timing and duration of intervention. Third, findings will lead to an adapted intervention standard of procedure (SOP) that can guide the delivery of the intervention bundle by perioperative wellness partners and one that can be tested for fidelity in our future effectiveness-implementation RCT. Fourth, findings will offer an initial roadmap for adapting and implementing patient-centred mental health interventions that are likely to be accepted and used by multiple stakeholders in the future. The intervention bundle adaptations performed in this study can be flexible enough and tailored based on patient needs/surgical conditions in diverse surgical settings while also maintaining the core components of the bundle, leading to a higher potential for scalability and sustainability in the long term. Use of the implementation science frameworks offers us the lens to examine the feasibility and acceptability of the intervention bundle ahead of time, in order to accelerate the translation of the intervention bundle to usual care. Lastly, the study will inform the design and conduct of the planned randomised controlled trials in the three surgical cohorts. This study provides us with an opportunity to identify and address unanticipated challenges with our study procedures including recruitment methods, engagement strategies, study design flaws and outcome measurement challenges.
This study comes with several limitations, similar to other feasibility trials. First, the sample sizes will be small as the proposed study focuses only on the evaluation of feasibility and implementation potential of the intervention bundle, thereby limiting the ability to detect changes in outcomes. Second, the study does not include a control condition, and hence we will not be making any conclusions about the intervention bundle effectiveness. Third, results from this study are specific to our study setting and population at an academic medical centre and may not be generalised to other non-academic settings. Nevertheless, this study will demonstrate whether it is feasible to: (1) recruit, (2) implement the intervention bundle in the perioperative period and (3) track the outcomes of interest, prior to conducting an RCT with a comparison group that will determine the efficacy of patient-centred intervention bundle in the three different surgical populations.
Supplementary Material
Acknowledgments
We would like to acknowledge and thank all of our Center for Perioperative Mental Health team members, including Rebecca Aslakson, Ryan Calfee, Laura Carpenter, Jennifer Carron, Renée El-Gabalawy, Margie Grant, Chet Hammill, Simon Haroutounian, Sharon Inouye, Thomas Kannampallil, Avi Klein, Kunal Kotkar, Benjamin Kozower, Alex Kronzer, Matthew Lauer, Muhammad Faraz Masood, Sherry McKinnon, J. Phillip Miller, Jay Piccirillo, Beth Prusaczyk, Charles Reynolds III, Marissa Rhea, Mayola Rowser, Marilyn Schallom, Julie Schweiger, Elizabeth Stuart, Kyle Stumbaugh, Terri Swider, Wilberforce Tumwesige, Heidi Tymkew, Lei Yang and Michael Yingling. We would also like to acknowledge and thank the members of our Internal Advisory Board for their review of our intervention bundle and their constructive feedback.
Footnotes
JA and KJH contributed equally.
Contributors: EL and MSA conceived the study idea; JA and KJH drafted the study protocol; JA, KJH, EL, KEF, BRTP, RCW, TAC, AAB, MP and MSA participated in the adaptation of the intervention; all authors edited the protocol and approved the final version of the protocol submission.
Funding: This research was supported in part by a grant from the National Institute of Mental Health (P50MH122351).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Lee PHU, Gawande AA. The number of surgical procedures in an American lifetime in 3 states. J Am Coll Surg 2008;207 https://www.journalacs.org/article/S1072-7515(08)00774-6/abstract 10.1016/j.jamcollsurg.2008.06.186 [DOI] [Google Scholar]
- 2.Yang R, Wolfson M, Lewis MC. Unique aspects of the elderly surgical population: an anesthesiologist's perspective. Geriatr Orthop Surg Rehabil 2011;2:56–64. 10.1177/2151458510394606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bufalari A, Ferri M, Cao P, et al. Surgical care in octogenarians. Br J Surg 2005;83:1783–7. 10.1002/bjs.1800831239 [DOI] [PubMed] [Google Scholar]
- 4.Etzioni DA, Liu JH, O'Connell JB, et al. Elderly patients in surgical workloads: a population-based analysis. Am Surg 2003;69:961. [PubMed] [Google Scholar]
- 5.Hamel MB, Henderson WG, Khuri SF, et al. Surgical outcomes for patients aged 80 and older: morbidity and mortality from major noncardiac surgery. J Am Geriatr Soc 2005;53:424–9. 10.1111/j.1532-5415.2005.53159.x [DOI] [PubMed] [Google Scholar]
- 6.Massarweh NN, Legner VJ, Symons RG, et al. Impact of advancing age on abdominal surgical outcomes. Arch Surg 2009;144:1108–14. 10.1001/archsurg.2009.204 [DOI] [PubMed] [Google Scholar]
- 7.Turrentine FE, Wang H, Simpson VB, et al. Surgical risk factors, morbidity, and mortality in elderly patients. J Am Coll Surg 2006;203:865–77. 10.1016/j.jamcollsurg.2006.08.026 [DOI] [PubMed] [Google Scholar]
- 8.Polanczyk CA, Marcantonio E, Goldman L, et al. Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Ann Intern Med 2001;134:637–43. 10.7326/0003-4819-134-8-200104170-00008 [DOI] [PubMed] [Google Scholar]
- 9.Finlayson EV, Birkmeyer JD, Hanover N. Operative mortality with elective surgery in older adults. Eff Clin Pract 2001;4:172–7. [PubMed] [Google Scholar]
- 10.Chung J-Y, Chang W-Y, Lin T-W, et al. An analysis of surgical outcomes in patients aged 80 years and older. Acta Anaesthesiol Taiwan 2014;52:153–8. 10.1016/j.aat.2014.09.003 [DOI] [PubMed] [Google Scholar]
- 11.Radinovic KS, Markovic-Denic L, Dubljanin-Raspopovic E, et al. Effect of the overlap syndrome of depressive symptoms and delirium on outcomes in elderly adults with hip fracture: a prospective cohort study. J Am Geriatr Soc 2014;62:1640–8. 10.1111/jgs.12992 [DOI] [PubMed] [Google Scholar]
- 12.Leung JM, Sands LP, Mullen EA, et al. Are preoperative depressive symptoms associated with postoperative delirium in geriatric surgical patients? J Gerontol A Biol Sci Med Sci 2005;60:1563–8. 10.1093/gerona/60.12.1563 [DOI] [PubMed] [Google Scholar]
- 13.Chang Y-P, Compton P. Opioid misuse/abuse and quality persistent pain management in older adults. J Gerontol Nurs 2016;42:21–30. 10.3928/00989134-20161110-06 [DOI] [PubMed] [Google Scholar]
- 14.Cochran G, Rosen D, McCarthy RM, et al. Risk factors for symptoms of prescription opioid misuse: do older adults differ from younger adult patients? J Gerontol Soc Work 2017;60:443–57. 10.1080/01634372.2017.1327469 [DOI] [PubMed] [Google Scholar]
- 15.Alattas SA, Smith T, Bhatti M, et al. Greater pre-operative anxiety, pain and poorer function predict a worse outcome of a total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 2017;25:3403–10. 10.1007/s00167-016-4314-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takagi H, Ando T, Umemoto T, et al. Perioperative depression or anxiety and postoperative mortality in cardiac surgery: a systematic review and meta-analysis. Heart Vessels 2017;32:1458–68. 10.1007/s00380-017-1022-3 [DOI] [PubMed] [Google Scholar]
- 17.Mirani SH, Areja D, Gilani SS, et al. Frequency of depression and anxiety symptoms in surgical hospitalized patients. Cureus 2019;11:e4141. 10.7759/cureus.4141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abraham J, Meng A, Siraco S, et al. A qualitative study of perioperative depression and anxiety in older adults. Am J Geriatr Psychiatry 2020;28:1107–18. 10.1016/j.jagp.2020.02.010 [DOI] [PubMed] [Google Scholar]
- 19.Zhao L, Ma L, Chen X, et al. Psychological nursing intervention improve the mental health status of young patients with lung cancer surgery during the perioperative period. Medicine 2021;100:e26736. 10.1097/MD.0000000000026736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Z, Zhang H, Zhang H. Psychological intervention on mental health of perioperative patients with cancer. Chinese Mental Health Journal. 2002. [Google Scholar]
- 21.Richard HM, Nguyen DC, Podeszwa DA, et al. Perioperative interdisciplinary intervention contributes to improved outcomes of adolescents treated with hip preservation surgery. J Pediatr Orthop 2018;38:254–9. 10.1097/BPO.0000000000000816 [DOI] [PubMed] [Google Scholar]
- 22.Geng X, Wang X, Zhou G, et al. A randomized controlled trial of psychological intervention to improve satisfaction for patients with depression undergoing TKA: a 2-year follow-up. J Bone Joint Surg Am 2021;103:567–74. 10.2106/JBJS.20.00169 [DOI] [PubMed] [Google Scholar]
- 23.Bottino CMC, Barcelos-Ferreira R, Ribeiz SRI. Treatment of depression in older adults. Curr Psychiatry Rep 2012;14:289–97. 10.1007/s11920-012-0281-z [DOI] [PubMed] [Google Scholar]
- 24.Gonçalves DC, Byrne GJ. Interventions for generalized anxiety disorder in older adults: systematic review and meta-analysis. J Anxiety Disord 2012;26:1–11. 10.1016/j.janxdis.2011.08.010 [DOI] [PubMed] [Google Scholar]
- 25.Hanson AE, Scogin F. Older adults' acceptance of psychological, pharmacological, and combination treatments for geriatric depression. J Gerontol B Psychol Sci Soc Sci 2008;63:P245–8. 10.1093/geronb/63.4.P245 [DOI] [PubMed] [Google Scholar]
- 26.Jayasekara R, Procter N, Harrison J, et al. Cognitive behavioural therapy for older adults with depression: a review. J Ment Health 2015;24:168–71. 10.3109/09638237.2014.971143 [DOI] [PubMed] [Google Scholar]
- 27.Reynolds CF, Lenze E, Mulsant BH. Assessment and treatment of major depression in older adults. Handb Clin Neurol 2019;167:429–35. 10.1016/B978-0-12-804766-8.00023-6 [DOI] [PubMed] [Google Scholar]
- 28.Wetherell JL, Petkus AJ, White KS, et al. Antidepressant medication augmented with cognitive-behavioral therapy for generalized anxiety disorder in older adults. Am J Psychiatry 2013;170:782–9. 10.1176/appi.ajp.2013.12081104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kok RM, Reynolds CF. Management of depression in older adults: a review. JAMA 2017;317:2114–22. 10.1001/jama.2017.5706 [DOI] [PubMed] [Google Scholar]
- 30.Carney RM, Freedland KE, Steinmeyer BC, et al. Collaborative care for depression symptoms in an outpatient cardiology setting: a randomized clinical trial. Int J Cardiol 2016;219:164–71. 10.1016/j.ijcard.2016.06.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cowan MJ, Freedland KE, Burg MM, et al. Predictors of treatment response for depression and inadequate social support--the ENRICHD randomized clinical trial. Psychother Psychosom 2008;77:27–37. 10.1159/000110057 [DOI] [PubMed] [Google Scholar]
- 32.Davidson KW, Bigger JT, Burg MM. Centralized, stepped, patient preference-based treatment for patients with post-acute coronary syndrome depression: CODIACS vanguard randomized controlled trial. JAMA Intern Med 2013;173:997–1004. 10.1001/jamainternmed.2013.915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freedland KE, Carney RM, Rich MW, et al. Cognitive behavior therapy for depression and self-care in heart failure patients: a randomized clinical trial. JAMA Intern Med 2015;175:1773–82. 10.1001/jamainternmed.2015.5220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freedland KE, Skala JA, Carney RM, et al. Treatment of depression after coronary artery bypass surgery: a randomized controlled trial. Arch Gen Psychiatry 2009;66:387–96. 10.1001/archgenpsychiatry.2009.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lustman PJ, Griffith LS, Freedland KE, et al. Cognitive behavior therapy for depression in type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med 1998;129:613–21. 10.7326/0003-4819-129-8-199810150-00005 [DOI] [PubMed] [Google Scholar]
- 36.Cristancho P, Lenard E, Lenze EJ, et al. Optimizing outcomes of treatment-resistant depression in older adults (optimum): study design and treatment characteristics of the first 396 participants randomized. The American Journal of Geriatric Psychiatry 2019;27:1138–52. 10.1016/j.jagp.2019.04.005 [DOI] [PubMed] [Google Scholar]
- 37.Wildes TS, Mickle AM, Ben Abdallah A, et al. Effect of Electroencephalography-Guided anesthetic administration on postoperative delirium among older adults undergoing major surgery: the engages randomized clinical trial. JAMA 2019;321:473–83. 10.1001/jama.2018.22005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cabassa LJ, Druss B, Wang Y, et al. Collaborative planning approach to inform the implementation of a healthcare manager intervention for Hispanics with serious mental illness: a study protocol. Implementation Science 2011;6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cabassa LJ, Gomes AP, Meyreles Q, et al. Using the Collaborative intervention planning framework to adapt a health-care manager intervention to a new population and provider group to improve the health of people with serious mental illness. Implementation Science 2014;9:1–11. 10.1186/s13012-014-0178-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernandez ME, Ruiter RA, Markham CM. Theory-and evidence-based health promotion program planning. intervention mapping: Frontiers Media SA 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puspitasari AJ, Kanter JW, Busch AM. A randomized controlled trial of an online, modular, active learning training program for behavioral activation for depression. J Consult Clin Psychol 2017;85:814–25. 10.1037/ccp0000223 [DOI] [PubMed] [Google Scholar]
- 42.Kanter JW, Bowe WM, Baruch DE. Behavioral activation for depression. Treatment of Depression in Adolescents and Adults: Clinician’s Guide to Evidence-Based Practice 2011;4:113. [Google Scholar]
- 43.Farhang M, Miranda-Castillo C, Rubio M, et al. Impact of Mind-body interventions in older adults with mild cognitive impairment: a systematic review. Int Psychogeriatr 2019;31:643–66. 10.1017/S1041610218002302 [DOI] [PubMed] [Google Scholar]
- 44.Lenze EJ, Hickman S, Hershey T, et al. Mindfulness-based stress reduction for older adults with worry symptoms and co-occurring cognitive dysfunction. Int J Geriatr Psychiatry 2014;29:991–1000. 10.1002/gps.4086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Damschroder LJ, Aron DC, Keith RE, et al. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009;4:50. 10.1186/1748-5908-4-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stirman SW, Baumann AA, Miller CJ. The frame: an expanded framework for reporting adaptations and modifications to evidence-based interventions. Implementation Science 2019;14:1–10. 10.1186/s13012-019-0898-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Creswell JW, Clark VLP. Designing and conducting mixed methods research. SAGE publications, 2017. [Google Scholar]
- 48.Chen Y-W, HajGhanbari B, Road JD, et al. Reliability and validity of the brief pain inventory in individuals with chronic obstructive pulmonary disease. Eur J Pain 2018;22:1718–26. 10.1002/ejp.1258 [DOI] [PubMed] [Google Scholar]
- 49.Cleeland C, Ryan K. Pain assessment: global use of the brief pain inventory. Singapore: Annals, Academy of medicine, 1994. [PubMed] [Google Scholar]
- 50.Lapane KL, Quilliam BJ, Benson C, et al. One, two, or three? constructs of the brief pain inventory among patients with non-cancer pain in the outpatient setting. J Pain Symptom Manage 2014;47:325–33. 10.1016/j.jpainsymman.2013.03.023 [DOI] [PubMed] [Google Scholar]
- 51.Song C-Y, Lin S-F, Huang C-Y, et al. Validation of the brief pain inventory in patients with low back pain. Spine 2016;41:E937–42. 10.1097/BRS.0000000000001478 [DOI] [PubMed] [Google Scholar]
- 52.Lindberg MF, Miaskowski C, Rustøen T, et al. The impact of demographic, clinical, symptom and psychological characteristics on the trajectories of acute postoperative pain after total knee arthroplasty. Pain Med 2017;18:124–39. 10.1093/pm/pnw080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shvartzman P, Friger M, Shani A, et al. Pain control in ambulatory cancer patients--can we do better? J Pain Symptom Manage 2003;26:716–22. 10.1016/s0885-3924(03)00220-3 [DOI] [PubMed] [Google Scholar]
- 54.Kapstad H, Rokne B, Stavem K. Psychometric properties of the brief pain inventory among patients with osteoarthritis undergoing total hip replacement surgery. Health Qual Life Outcomes 2010;8:148–8. 10.1186/1477-7525-8-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tittle MB, McMillan SC, Hagan S, eds. Validating the brief pain inventory for use with surgical patients with cancer. Oncology Nursing Forum, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Gjeilo KH, Stenseth R, Wahba A, et al. Validation of the brief pain inventory in patients six months after cardiac surgery. J Pain Symptom Manage 2007;34:648–56. 10.1016/j.jpainsymman.2007.01.010 [DOI] [PubMed] [Google Scholar]
- 57.Ball LJ, Bisher GB, Birge SJ. A simple test of central processing speed: an extension of the short Blessed test. J Am Geriatr Soc 1999;47:1359–63. 10.1111/j.1532-5415.1999.tb07440.x [DOI] [PubMed] [Google Scholar]
- 58.Fick DM, Inouye SK, Guess J, et al. Preliminary development of an ultrabrief two-item bedside test for delirium. J Hosp Med 2015;10:645–50. 10.1002/jhm.2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marcantonio ER, Ngo LH, O'Connor M, et al. 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med 2014;161:554–61. 10.7326/M14-0865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carroll C, Patterson M, Wood S, et al. A conceptual framework for implementation fidelity. Implement Sci 2007;2:40. 10.1186/1748-5908-2-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Finley EP, Huynh AK, Farmer MM, et al. Periodic reflections: a method of guided discussions for documenting implementation phenomena. BMC Med Res Methodol 2018;18:1–15. 10.1186/s12874-018-0610-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manos RC, Kanter JW, Luo W. The behavioral activation for depression scale-short form: development and validation. Behav Ther 2011;42:726–39. 10.1016/j.beth.2011.04.004 [DOI] [PubMed] [Google Scholar]
- 63.Kanter JW, Mulick PS, Busch AM, et al. The behavioral activation for depression scale (bads): psychometric properties and factor structure. J Psychopathol Behav Assess 2007;29:191–202. 10.1007/s10862-006-9038-5 [DOI] [Google Scholar]
- 64.Ritchie J, Lewis J. Qualitative research practice: a guide for social science students and researchers. London: Sage Publications, 2003. [Google Scholar]
- 65.King A, Bartley J, Johanson DL, et al. Components of preoperative anxiety: a qualitative study. J Health Psychol 2019;24:1897–908. 10.1177/1359105317709512 [DOI] [PubMed] [Google Scholar]
- 66.Puspitasari AJ, Kanter JW, Busch AM, et al. A randomized controlled trial of an online, modular, active learning training program for behavioral activation for depression. J Consult Clin Psychol 2017;85:814–25. 10.1037/ccp0000223 [DOI] [PubMed] [Google Scholar]
- 67.Lenze EJ, Lenard E, Bland M, et al. Effect of enhanced medical rehabilitation on functional recovery in older adults receiving skilled nursing care after acute rehabilitation: a randomized clinical trial. JAMA Netw Open 2019;2:e198199-e. 10.1001/jamanetworkopen.2019.8199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ajam Oughli H, Lavretsky H, Karp J, et al. Optimizing Outcomes of Treatment - Resistant Depression in Older Adults (OPTIMUM): Study Design and Sample. The American Journal of Geriatric Psychiatry 2021;29:S33–4. 10.1016/j.jagp.2021.01.028 [DOI] [PubMed] [Google Scholar]
- 69.Trivedi MH, Daly EJ. Measurement-based care for refractory depression: a clinical decision support model for clinical research and practice. Drug Alcohol Depend 2007;88 Suppl 2:S61–71. 10.1016/j.drugalcdep.2007.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rush AJ, Thase ME. Improving depression outcome by patient-centered medical management. Am J Psychiatry 2018;175:1187–98. 10.1176/appi.ajp.2018.18040398 [DOI] [PubMed] [Google Scholar]
- 71.Bao Y, Post EP, Ten TR, et al. Achieving effective antidepressant pharmacotherapy in primary care: the role of depression care management in treating late-life depression. J Am Geriatr Soc 2009;57:895–900. 10.1111/j.1532-5415.2009.02226.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang PS, Berglund P, Kessler RC. Recent care of common mental disorders in the United States : prevalence and conformance with evidence-based recommendations. J Gen Intern Med 2000;15:284–92. 10.1046/j.1525-1497.2000.9908044.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang PS, Schneeweiss S, Brookhart MA, et al. Suboptimal antidepressant use in the elderly. J Clin Psychopharmacol 2005;25:118–26. 10.1097/01.jcp.0000155819.67209.e5 [DOI] [PubMed] [Google Scholar]
- 74.Williams S, Miller G, Khoury R, et al. Rational deprescribing in the elderly. Ann Clin Psychiatry 2019;31:144–52. [PubMed] [Google Scholar]
- 75.Clegg A, Young JB. Which medications to avoid in people at risk of delirium: a systematic review. Age Ageing 2011;40:23–9. 10.1093/ageing/afq140 [DOI] [PubMed] [Google Scholar]
- 76.Ensrud KE, Blackwell TL, Mangione CM, et al. Central nervous system-active medications and risk for falls in older women. J Am Geriatr Soc 2002;50:1629–37. 10.1046/j.1532-5415.2002.50453.x [DOI] [PubMed] [Google Scholar]
- 77.Foy A, O'Connell D, Henry D, et al. Benzodiazepine use as a cause of cognitive impairment in elderly hospital inpatients. J Gerontol A Biol Sci Med Sci 1995;50:M99–106. 10.1093/gerona/50A.2.M99 [DOI] [PubMed] [Google Scholar]
- 78.Lenze EJ, Iaboni A, Wetherell JL. Benzodiazepines in older adults: definite harms, doubtful benefits. (In response to: Benzodiazepine use and risk of Alzheimer’s disease: case-control study. Billioti de Gage, et al.). The BMJ Online 2014. http://www.bmj.com/content/349/bmj.g5205/rr/777842 [Google Scholar]
- 79.Kroenke K, Wu J, Yu Z, et al. Patient health questionnaire anxiety and depression scale: initial validation in three clinical trials. Psychosom Med 2016;78:716–27. 10.1097/PSY.0000000000000322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weiner BJ, Lewis CC, Stanick C, et al. Psychometric assessment of three newly developed implementation outcome measures. Implementation Sci 2017;12:108. 10.1186/s13012-017-0635-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-062398supp001.pdf (91KB, pdf)
bmjopen-2022-062398supp002.pdf (120.1KB, pdf)